Abstract
All retroviruses contain two copies of genomic RNA that are linked noncovalently. The dimeric RNA of human immunodeficiency virus type 1 (HIV-1) undergoes rearrangement during virion maturation, whereby the dimeric RNA genome assumes a more stable conformation. Previously, we have shown that the packaging of the HIV-1 polymerase (Pol) proteins reverse transcriptase (RT) and integrase (IN) is essential for the generation of the mature RNA dimer conformation. Analysis of HIV-1 mutants that are defective in processing of Pol showed that these mutant virions contained altered dimeric RNA conformation, indicating that the mature RNA dimer conformation in HIV-1 requires the correct proteolytic processing of Pol. The HIV-1 Pol proteins are multimeric in their mature enzymatically active forms; RT forms a heterodimer, and IN appears to form a homotetramer. Using RT and IN multimerization defective mutants, we have found that dimeric RNA from these mutant virions has the same stability and conformation as wild-type RNA dimers, showing that the mature enzymatically active RT and IN proteins are dispensable for the generation of mature RNA dimer conformation. This also indicated that formation of the mature RNA dimer structure occurs prior to RT or IN maturation. We have also investigated the requirement of Pol for RNA dimerization in both Mason-Pfizer monkey virus (M-PMV) and Moloney murine leukemia virus (MoMuLV) and found that in contrast to HIV-1, Pol is dispensable for RNA dimer maturation in M-PMV and MoMuLV, demonstrating that the requirement of Pol in retroviral RNA dimer maturation is not conserved among all retroviruses.
All retroviruses contain two strands of RNA that bind together noncovalently to form dimers. The evolutionary conservation of genomic RNA dimerization illustrates its importance in the retroviral life cycle (reviewed in references 19, 51, and 57). The dimeric RNA structure is one of the conformations that human immunodeficiency virus type 1 (HIV-1) genomic RNA assumes, and different structural motifs of retroviral genomic RNA are required at distinct stages of virus production. Hence, RNA structures are thought to be important regulatory elements of HIV-1 replication (12).
Dimeric RNA from several different retroviruses has been visualized by electron microscopy and was shown to interact near the 5′ end of the genome at a region designated the dimer linkage structure (8, 9, 33, 44). In HIV-1, a region important for RNA dimerization termed the dimerization initiation site (DIS) was identified through the use of short, in vitro-transcribed RNA strands (14, 34, 43, 47, 65). The DIS is located within the 5′ untranslated region of the genome and forms a hairpin structure (65). Mutations that prevent formation of the DIS loop self-complementary region prevent the RNA dimerization of in vitro-transcribed RNA strands (47, 48, 65). Dimerization of genomic RNA is critical for the strand transfer of newly synthesized viral cDNA and template switching during reverse transcription (4, 5, 10), and probably one of the most important functions of genomic RNA dimerization is that it enables the recombination of retroviral genomes during reverse transcription (4, 5, 71). Regions of the genome involved in RNA dimerization have also been shown to have increased recombination, such as the dimer linkage structure of murine leukemia virus (40) and the DIS in HIV-1 (4, 5). Therefore, the dimeric conformation of RNA may regulate recombination events, which are important in terms of viral fitness, enabling increased genetic variability and rescue of reverse transcription in the event of strand breakage.
HIV-1 RNA structural motifs have been shown to have critical regulatory roles at multiple steps of the viral life cycle, including translation control of HIV-1 Gag and Gag-polymerase (Pol) polyproteins via an RNA stem-loop to induce ribosomal frameshifting (28) and transcriptional regulation through the trans activation response element RNA structure (11). In addition, the HIV-1 5′ untranslated region contains multiple RNA structural motifs that are required for packaging of genomic RNA (37) and reverse transcription of viral genomic RNA (6, 7, 38). It has been proposed that HIV-1 genomic RNA can also assume alternative conformations that serve as a switch between a translation-competent mRNA and a dimerization and packaging-competent genomic RNA (27). It is thought that the long-distance interaction (LDI) formed by the translation-competent RNA masks the DIS, preventing dimerization. Conversely, the branched multiple hairpin (BMH) structure exposes the DIS promoting dimerization and packaging while occluding the Gag start codon (1). In vitro evidence suggests that the viral nucleocapsid (NC) protein mediates the switch from LDI to BMH (27), and this illustrates the interplay between viral proteins and RNA conformation in regulating steps in the replication cycle.
HIV-1 dimeric RNA undergoes structural changes during virion assembly, packaging, and release. In vitro studies have led to a model of RNA dimerization known as the kissing loop model, whereby two RNA strands initially interact via their respective DIS stem-loop structures. The kissing loop complex is then converted into a more stable extended duplex (35, 42). It is generally assumed that the conversion of the kissing loop interaction (loose dimer) to the extended duplex can be achieved by 55°C heat treatment (35, 42) or by the addition of NC protein at physiological temperature (41, 42). The extended duplex is often referred to as the tight dimer. This two-step process of RNA dimerization may have relevance in vivo, as there is evidence to suggest genomic RNA undergoes structural changes during virion particle maturation (13, 16, 17, 45, 66, 67). Thus, the loose dimers observed in vitro are thought to be analogous to genomic RNA dimers from immature viral particles while the tight dimers represent dimeric RNA from mature viral particles; however, direct evidence to support this view is currently lacking (51).
The conversion of immature HIV-1 virions to the mature infectious viral particle requires proteolytic processing of the precursor proteins Gag and Gag-Pol by the viral protease (32, 56). Proteolytic processing of the 55-kDa-precursor protein Gag by the viral protease generates matrix (MA), capsid (CA), p2 spacer peptide, NC, p1 spacer peptide, and p6Gag. The 160-kDa Gag-Pol polyprotein is cleaved into MA, CA, p2, NC, p6Pol, the viral protease (PR), reverse transcriptase (RT) and integrase (IN) (20). The HIV-1 Gag and Gag-Pol polyproteins are encoded by overlapping reading frames and are synthesized at different rates. During synthesis of the Gag polyprotein, the ribosome occasionally stalls and slips back 1 nucleotide into the Pol reading frame generating the Gag-Pol polyprotein. This ribosomal frameshifting event results in a 20:1 ratio of Gag to Gag-Pol (28), and maintenance of the Gag:Gag-Pol ratio is critical for RNA dimerization and viral infectivity (60).
Formation of the mature RNA dimer conformation observed in infectious retroviral particles requires proteolytic processing of viral proteins. We have previously demonstrated that in HIV-1, the Pol proteins RT and IN are essential for generating the mature RNA dimer conformation in the virion (61). Proteolytic processing of the Gag polyprotein is required for the mature RNA dimer structure, specifically the primary cleavage site in Gag (p2/nucleocapsid), whereas abolishing processing at the secondary (MA/CA and p1/p6) and tertiary (CA/p2) cleavage sites does not alter RNA dimer stability (62). While it is clear that in HIV-1, the Pol proteins are required for mature RNA dimer conformation, it is not known whether RT and IN exert their effects as part of the polyprotein complex or as their mature processed subunits.
In HIV-1, multimerization of RT and IN is essential for their efficient catalytic activity. HIV-1 RT is expressed as part of the Pr160Gag-Pol precursor, which undergoes proteolytic processing to produce the RT heterodimer formed by the association of the p66 and p51 subunits. This RT heterodimer is the form of the enzyme biologically active with both polymerase and RNase H activity (55, 70), while the minimal nuclear IN complex is thought to be a homotetramer (36). Mutations that prevent formation of these dimeric and tetrameric active RT or IN protein complexes have been well characterized and generate noninfectious virions (30). It is not known whether the catalytic activity of these mature Pol proteins is a requirement for the mature RNA dimer conformation in HIV-1.
In addition to HIV-1, other retroviral RNA genomes undergo conformational changes during virion maturation, including avian leukosis virus (ALV), Rous sarcoma virus (RSV), and Moloney murine leukemia virus (MoMuLV) (16, 17, 45, 66, 67). This maturation of virion genomic RNA requires proteolytic processing of the viral polyproteins by the viral protease (16, 17, 45, 66, 67). While the mature HIV-1 RNA dimer structure requires RT and IN, it is not known whether this requirement for Pol proteins is conserved among all retroviruses (61).
In this study, we investigated the contribution of RT and IN in generating the mature HIV-1 RNA dimer conformation. RNA dimer analysis of mutants defective in Pol processing revealed that the correct processing of Pol is critical for RNA dimer maturation. We have also exploited mutations shown to inhibit RT dimerization (18, 69) and IN multimerization (30) and found no change in RNA dimer conformation from that of wild-type (WT) dimeric RNA. This demonstrates that the mature RT and IN are dispensable for generating the mature RNA dimer conformation in HIV-1, suggesting that the mature RNA dimer structure is formed prior to maturation of RT or IN into their multimeric complexes. To determine whether Pol is required for the mature RNA dimer conformation in other retroviruses, we generated RT- and IN-deficient mutants for two retroviruses, Mason-Pfizer monkey virus (M-PMV, a type D retrovirus) and MoMuLV (a type-C simple retrovirus). Analysis of virion genomic RNA in mutants lacking RT and IN revealed that Pol is not required for the mature RNA dimer conformation in either M-PMV or MoMuLV.
MATERIALS AND METHODS
DNA plasmids.
The full-length wild-type HIV-1 plasmid referred to as NL4.3 was obtained from the AIDS Research and Reference Reagent Program, Division of AIDS, National Institutes of Health. Additional HIV-1 constructs were generated using stitch PCR mutagenesis whereby sequence-specific primers were used to introduce a desired mutation (22). The region of interest was then amplified and subcloned back into the NL4.3 backbone using relevant restriction sites. An additional NotI site was introduced into amino acids 461 through 463 of reverse transcriptase without altering the amino acid coding sequence. This NL4.3 RNase H (NotI) construct had wild-type levels of infectivity (data not shown) and was also used as a wild-type backbone for subcloning. The HIV-1 protease-defective PR(−) has a D25N amino acid substitution (nucleotides GAT to AAT) at the active site of the enzyme, preventing protease activity and generating immature virions. HIV-1 GagUAA contains an additional termination codon, which has been engineered immediately after the Gag coding region within the Pol reading frame, which did not alter the Gag protein sequence but prevented translation of functional PR, RT, and IN. The introduced GagUAA termination codon is located at amino acid 15 of PR, where nucleotides ATA have been changed to TAA, within the pol reading frame. HIV-1 PRUAG has a termination codon (TAG) inserted immediately after the final amino acid of the PR coding region, preventing translation of RT and IN proteins. The mutations in HIV-1 PR(−), GagUAA, and PRUAG were subcloned into the NL4.3 backbone using ApaI and BclI restriction sites. HIV-1 RTUAG contains a termination codon (TAG) immediately after the RT coding region, preventing translation of IN. The mutation in HIV-1 RTUAG was subcloned into NL43 using NotI and PflmI sites. The HIV-1 Pol processing-defective mutants (PDMs) contain additional amino acids (threonine [nucleotides ACG] and arginine [nucleotides CGT]) inserted between the P1 and P1′ residues of the RT p51-RNaseH and/or RT-IN cleavage sites in the Pol polyprotein. Pol PDM 1 contains Thr and Arg codons that were inserted at the p51-RNase H cleavage site. Pol PDM 2 contains Thr and Arg codons inserted at the RT-IN cleavage site. Pol PDM 1 and 2 mutations were subcloned into the NL4.3 RNase H (NotI) backbone using AgeI with NotI and NotI with PflmI, respectively. Pol PDM 3 contains Thr and Arg codon insertions at both cleavage sites and was generated by subcloning the Pol PDM 1 AgeI -NotI insert into the Pol PDM 2 construct using AgeI and NotI restriction sites.
The HIV-1 RT and IN proteins form multimeric complexes in their mature forms. HIV-1 RT or IN multimerization mutants were generated by the introduction of amino acid substitutions using stitch PCR mutagenesis. Mutations L234A (18) or W401A (69) in RT and V260E (30) in IN have been reported to prevent multimerization of RT or IN and abolish their respective enzymatic activities. L234A (CTC to GCC) or W401A (TGG to GCG) was subcloned into NL4.3 using BstZ17I and AgeI or AgeI and NotI sites, respectively. V260E (GTG to GAG) was constructed using NotI and PflmI restriction sites. Once individual clones had been generated, they were used to construct double (L234A and W401A [LW], L234A and V260E [LV], and W401A and V260E [WV]) and triple (L234A, W401A, and V260E [LWV]) Pol multimerization mutant clones.
Mason-Pfizer monkey virus.
WT pSHRM15 and PR(−) were kindly provided by Scott Parker and Eric Hunter. M-PMV PR(−) contains a D26N substitution (nucleotides GAT to AAT) at the active site of the PR enzyme abolishing protease activity. M-PMV GagUAA has an additional stop codon introduced 12 nucleotides after the WT Gag termination codon replacing nucleotides GGG to TAA. The introduced GagUAA termination codon is situated downstream of the first frameshift site in the Gag-Pro reading frame and prevents translation of full-length M-PMV PR, RT, and IN. M-PMV PRUAA contains a termination codon downstream of the PR coding sequence at amino acid 54 of RT (nucleotides GCA to TAA). The introduced PRUAA stop codon is 24 nucleotides after the second frameshift site within the Pr180Gag-Pro-Pol RT reading frame preventing translation of M-PMV RT and IN. Amplified regions containing the desired mutation were subcloned back into the pSHRM15 backbone using the BsgI and PmlI restriction sites.
MoMuLV. MoMuLV WT pNCA was kindly provided by Stephen Goff. MoMuLV mutant clones were generated by stitch PCR mutagenesis. MoMuLV PR(−) contains a D32L substitution (nucleotides GAT to CTT) at the active site of PR preventing protease activity. The readthrough event that produces MoMuLV Gag-Pol is dependant on a bipartite signal consisting of a purine-rich region immediately downstream from the Gag TAG codon (15, 25), followed by a RNA pseudoknot structure (25). MoMuLV GagUAA contains an additional termination codon at amino acid 18 of the PR gene (nucleotides ATA to TAA) downstream of the readthrough RNA stem-loop structure, which prevents translation of full-length PR, RT, and IN. MoMuLV PRUAG and MoMuLV RTUAG contain additional termination codons (TAG) inserted immediately after the PR and RT coding regions, respectively, preventing the translation of downstream sequences. The MoMuLV PR(−)-, GagUAA-, and PRUAA-amplified regions were subcloned into the pNCA backbone using the NruI and BclI restriction sites, while MuMoLV RTUAA was subcloned using SalI and HindIII. All regions amplified by stitch PCR were sequenced to confirm that the desired mutations were present and to ensure that no additional mutations were introduced.
Virus production.
293T cells (1.5 × 106 cells) were plated onto 10-cm-diameter plates (Nunc) and maintained for 30 h in 7 ml of Dulbecco's modified Eagle medium (Gibco) containing 10% fetal bovine serum (P.A. Biological Co.), 100 U/ml of penicillin, and 100 μg/ml of streptomycin. The production of WT and mutant HIV-1 viral particles was achieved by transfection of 10 μg of proviral DNA into 293T cells by a calcium phosphate method as previously described (39). An enhanced green fluorescent protein (Clontech) reporter plasmid (2 μg) was cotransfected with the HIV-1 plasmids to determine transfection efficiency. Cells were washed twice with phosphate-buffered saline 10 h posttransfection and maintained in fresh Dulbecco's modified Eagle medium. Supernatants were collected 36 h posttransfection and centrifuged at 3,000 rpm (Beckman GS-6R centrifuge) for 30 min at 4°C to remove cellular debris.
Virion purification and protein analysis.
Clarified supernatants from transfected cells were purified and concentrated by ultracentrifugation through a 20% (wt/vol) sucrose cushion using a Beckman L-90 ultracentrifuge (SW28 rotor) at 26,500 rpm for 1 h at 4°C. Virion pellets were resuspended in 250 μl TE buffer (10 mM Tris, 1 mM EDTA), pH 8.0, and 10-μl samples taken for virion protein analysis.
Analysis of virion protein profile.
Virion samples (10 μl) were mixed with an equal volume of 2× Tris-buffered saline (TBS) lysis buffer containing 0.01% of Nonidet P-40, 20 mM phenylmethylsulfonyl fluoride, 1 μM pepstatin, and 1 μM leupeptin. Equal amounts of virion protein (normalized by the transfection efficiency of enhanced green fluorescent protein) were mixed with 3 μl of sample buffer (100 mM Tris [pH 6.8], 3% sodium dodecyl sulfate [SDS], 33% glycerol, 0.03% bromophenol blue, 5 mM β-mercaptoethanol), incubated for 10 min at 95°C, and resolved by SDS-10% polyacrylamide gel electrophoresis (PAGE). Resolved proteins were transferred to a nitrocellulose membrane (Amersham) for Western blot analysis. The membrane was blocked by washing three times in 5% skim milk dissolved in 1× TBS containing 0.3% Tween 20 and then was probed overnight with pooled HIV-1-seropositive patient sera. After another three washes in TBS-Tween 20 containing 5% skim milk, the membrane was incubated with anti-human horseradish peroxidase-conjugated secondary antibody (Dako) for 2 h at room temperature. An enhanced chemiluminescence technique was used for visualization of HIV-1 proteins present in the cellular lysates (Amersham).
Analysis of virion RNA dimerization.
Virion pellets resuspended in 250 μl TE, pH 8.0, were lysed by the addition of 2× RNA lysis buffer (50 mM Tris [pH 7.5], 2.5 mM EDTA [pH 8.0], 50 mM NaCl, 1% SDS, yeast tRNA at 50 μg/ml, proteinase K at 100 μg/ml) and incubated for 30 min at room temperature for proteinase K digestion. The RNA was then phenol-chloroform extracted three times and isolated for melting curve analysis as previously described (16, 17). Similar amounts of genomic RNA were used to analyze the stability of the virion RNA dimer in each preparation. Samples were heated at indicated temperatures for a period of 10 min and then chilled on ice. Dimeric and heat-denatured monomeric RNAs were separated by electrophoresis in a 1% native agarose gel in 0.5× Tris-borate-EDTA buffer. Samples were transferred overnight onto a nitrocellulose Hybond N membrane (Amersham). The membrane containing the RNA samples was air dried for 2 h at room temperature and exposed to UV light for 90 s to induce cross-linking. Blocking of the membrane was performed for 1 h at 42°C with 10 ml of hybridization buffer (40 ml of hybridization buffer contained 8 ml of 5× SSPE [750 mM NaCl, 63 mM NaH2PO4, 0.5 mM EDTA], 20 ml of deionized formamide, 4 g of dextran sulfate, 400 μl of salmon sperm DNA [10 mg/ml], 3 ml of 20% SDS, and 5 ml of double-distilled H2O). Dimeric and monomeric RNAs were incubated overnight with a 32P-labeled riboprobe (pGEM7zHIV-1), which was complementary to the 5′ end of the HIV-1 genomic RNA sequences, as previously described (60). The radioactive riboprobe was synthesized by linearizing pGEM7z HIV-1 with BamHI, followed by T7 RNA polymerase-directed in vitro transcription (Promega) in the presence of [α-32P]CTP (Amersham). After being probed, the membrane was washed once for 30 min with 1× SSC-0.1% SDS buffer and twice for 30 min with 0.2× SSC-0.1% SDS buffer. The results were visualized by autoradiography.
RESULTS
WT HIV-1 RNA dimer structure requires the presence of RT and IN.
Using a Vpr- and Nef-defective HIV-1 construct, HXB2-BH10, we have previously shown that an RT- and IN-negative mutant exhibits an altered dimeric virion RNA conformation (61), and this defect can be rescued by supplementing RT and IN into the virion particle by using Vpr-RT-IN fusion protein (61). In this study, we used a different strain of HIV-1, NL4.3, which expressed a functional Vpr and Nef to verify the requirement of RT and IN in the formation of WT virion RNA dimer conformation. HIV-1 NL4.3 WT and four HIV-1 mutants [PR(−), GagUAA, PRUAG, RTUAG] were constructed (Fig. 1A). HIV-1 PR(−) immature virions were expected to contain uncleaved viral Gag and Gag-Pol precursor proteins, due to a D25N mutation at the active site of the viral protease. HIV-1 GagUAA, HIV-1 PRUAG, and HIV-1 RTUAG virion particles were designed to contain various truncated versions of the Gag-Pol polyprotein, which was the result of the insertion of a termination codon downstream of the stated gene.
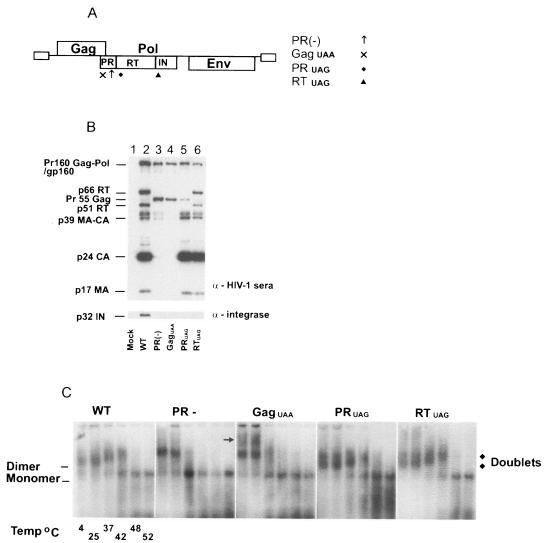
FIG. 1.
HIV-1 Pol proteins RT and IN are required for RNA dimer maturation. (A) Schematic representation of HIV-1 WT and mutant proviral DNA constructs. HIV-1 WT pDRNL4.3 was used as a control. PR(−) has a D25N substitution at the active site of PR ↑↑ preventing proteolytic activity. GagUAA has a termination codon × inserted immediately after the Gag coding region. PRUAG has a termination codon ⧫ inserted at the end of PR. RTUAG has a termination codon inserted at the end of the RT ▴ coding sequence. The UAA/G insertions prevent translation of proteins located downstream. (B) Western blot analysis was performed on purified virions. Proteins were resolved by 10% Tris-glycine SDS-PAGE and then probed with pooled sera from HIV-1-infected individuals or anti-integrase monoclonal antibodies as described in Materials and Methods. (C) Virion RNA was resuspended in RNA dimerization buffer and heat denatured for 10 min at the indicated temperatures. Dimers and monomers were electrophoresed in a 1% native agarose gel and Northern analysis performed using an HIV-1-specific riboprobe.
Western blot analyses of virion protein profiles were consistent with the mutations found in these HIV-1 particles (Fig. 1B). The HIV-1 WT control revealed the proteolytic processing of the Pr55Gag polyprotein into p17 matrix and p24 capsid proteins and the cleavage of Pr160Gag-Pol into p66 RT, p51 RT, and p32 IN (Fig. 1B, lane 2). Both HIV-1 PR(−) and HIV-1 GagUAA had no proteolytic activity; thus, no cleavage products of Gag or Gag-Pol were present in these virions (Fig. 1B, lanes 3 and 4). HIV-1 PRUAG contained a truncated Gag-Pol that lacked RT and IN (Fig. 1B, lane 5), while HIV-1 RTUAG had a similar protein profile as the WT control except for the absence of IN (Fig. 1B, lane 6).
The virion genomic RNA was isolated as previously described (16, 17, 60-62), and the virion RNAs were subjected to heat treatment at various temperatures to dissociate the dimeric RNA genome into monomeric form prior to electrophoresis. The WT dimeric RNA genomes of HIV-1 migrated as a single band in a native agarose gel (Fig. 1C, WT). Prior to complete dissociation into monomeric RNA at 48°C, heat treatment of WT dimeric RNA at increasing temperatures generated characteristic reduced electrophoretic mobilities (Fig. 1C, WT temperatures of 4, 25, 37, and 42°C). Consistent with previous reports (16, 60), the immature HIV-1 RNA dimers in PR(−) virions were less stable and dissociated into monomers at 37°C [Fig. 1C, PR(−)]. The instability of the dimeric RNA in the PR(−) mutant was also evident by the presence of monomeric RNA at 4 and 25°C [Fig. 1C, PR(−)]. In contrast to WT, dimeric RNA isolated from PR(−) had altered mobility [Fig. 1C, compare WT and PR(−)]. Virion particles that lacked Gag-Pol proteins, i.e., HIV-1 GagUAA, contained RNA dimers with similar stabilities to HIV-1 PR(−) as a significant amount of dimeric RNA had dissociated into monomers at 37°C (Fig. 1C, GagUAA). In agreement with our previous report (61), HIV-1 GagUAA contained an additional subset of dimeric RNA with a slower mobility than the dimeric RNA found in PR(−) immature virions (Fig. 1C, GagUAA indicated by →). Both HIV-1 PRUAG and HIV-1 RTUAG virions had RNA dimer profiles distinct from those of WT, PR(−), or GagUAA. Dimeric RNAs from HIV-1 PRUAG and RTUAG have stabilities similar to those of HIV-1 WT RNA dimers, being completely dissociated into monomeric RNA at 48°C. However, in contrast to the HIV-1 WT RNA dimers, the dimeric RNA derived from HIV-1 PRUAG and HIV-1 RTUAG particles migrated as diffuse “doublets,” which were clearly visible at lower temperatures (Fig. 1C, PRUAG and RTUAG, 4 and 25°C). The altered RNA dimer conformation seen in the PRUAG and RTUAG mutants confirmed our previous report that HIV-1 RT and IN are required for the formation of the mature WT RNA dimer conformation (61), and this requirement is not HIV-1 strain dependant.
The correct proteolytic processing of Gag-Pol is required for WT RNA dimer conformation.
In HIV-1, proteolytic processing of the retroviral polyproteins is required for virion particle maturation (31, 56) and also facilitates the rearrangement of virion genomic RNA into a more stable conformation (16). Processing of Gag occurs in a sequential manner (54), with cleavage of p2/NC in Gag, the first site cleaved in both Gag and Gag-Pol (52, 53), being critical for generating the WT RNA dimer conformation (62). While mutations at the Gag regions (p2/NC, CA/p2) of Gag-Pol have little effect on RNA dimer stability (62), the contribution of proteolytic processing of Pol regions within Gag-Pol to RNA dimer conformation has not been investigated. HIV-1 Pol processing-defective mutants were constructed by insertion of threonine and arginine residues at the cleavage junctions between the p51-RNase H or RT-IN cleavage sites to create Pol PDM 1 and Pol PDM 2, respectively. Pol PDM 3 contained Thr and Arg insertions in both p51-RNase H and RT-IN cleavage sites (Fig. 2A).
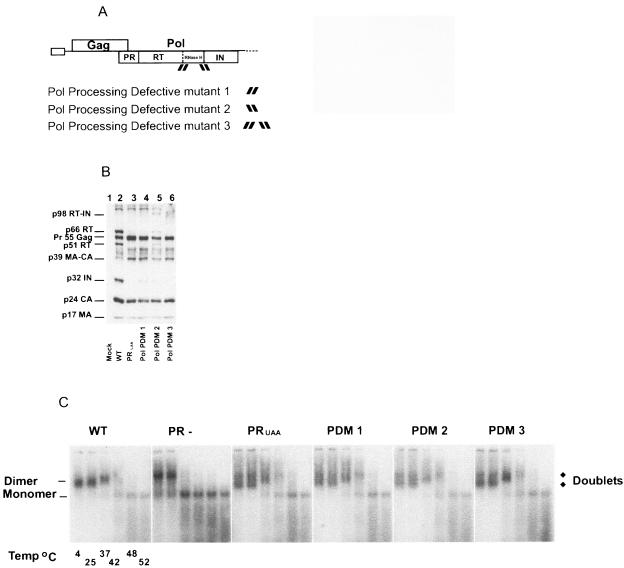
FIG. 2.
The impact of Pol-processing mutations on HIV-1 virion protein composition and genomic RNA dimer maturation. (A) Schematic representation of HIV-1 and Pol PDMs 1, 2, and 3 is given. Constructs were generated by insertion of Thr and Arg codons into the indicated cleavage sites by PCR mutagenesis. Pol PDM 1 had a Thr and Arg insertion between the RT p51-RNase H cleavage site indicated by [ ] Pol PDM 2 contains Thr and Arg at the RT-IN cleavage site indicated by [
] Pol PDM 2 contains Thr and Arg at the RT-IN cleavage site indicated by [ ] Pol PDM 3 contained Thr and Arg insertions at both p51-RNase H and RT-IN cleavage sites indicated by [
] Pol PDM 3 contained Thr and Arg insertions at both p51-RNase H and RT-IN cleavage sites indicated by [ ]. (B) Western blot analysis was performed on WT, PRUAG (lacking in Pol proteins), and Pol PDMs 1, 2, and 3. Proteins from purified virions were resolved by 10% Tris-glycine SDS-PAGE and then probed with pooled sera from HIV-1-infected individuals. (C) Virion RNA was resuspended in RNA dimerization buffer and heat denatured for 10 min at the indicated temperatures. Dimers and monomers were electrophoresed in a 1% native agarose gel and probed with an HIV-1-specific riboprobe as described in Materials and Methods.
]. (B) Western blot analysis was performed on WT, PRUAG (lacking in Pol proteins), and Pol PDMs 1, 2, and 3. Proteins from purified virions were resolved by 10% Tris-glycine SDS-PAGE and then probed with pooled sera from HIV-1-infected individuals. (C) Virion RNA was resuspended in RNA dimerization buffer and heat denatured for 10 min at the indicated temperatures. Dimers and monomers were electrophoresed in a 1% native agarose gel and probed with an HIV-1-specific riboprobe as described in Materials and Methods.
Virion protein profiles of the HIV-1 Pol PDMs revealed different Gag-Pol processing defects for each mutant. WT and all Pol PDMs displayed similar Pr55Gag processing into p24 capsid and p17 matrix (Fig. 2B, lanes 2 and 4 through 6). However, Gag-Pol processing of these mutants was altered such that Pol PDM 1 had low levels of IN and an undetectable amount of RT, Pol PDM 2 had low levels of p66 and p51 RT subunits and a small quantity of IN, and Pol PDM 3 had low levels of RT and IN (Fig. 2B, lanes 4 through 6, respectively).
To ensure that the Pol PDMs were defective in processing and that the virion packaging of Pol was not affected, these PDMs have also been cloned into protease-negative constructs. If these processing-defective mutations affect viral Gag-Pol packaging but not Gag-Pol processing, one would expect that these PR(−) PDMs will have a defect in GagPol packaging. Western analysis of virion Gag and Gag-Pol in these PR(−) PDMs indicated that packaging was not affected in any of these three PDMs, implying that these mutants are truly defective in Pol processing (data not shown).
In contrast to the WT RNA dimer conformation (Fig. 2C, WT), the RNA dimer profiles of Pol PDMs 1, 2, and 3 (Fig. 2C, PDMs 1, 2, and 3) were indistinguishable from those of the PRUAG dimers, where dimeric RNA doublets were clearly visible at 4°C and 25°C (Fig. 2C, PRUAG). These data suggested that the formation of the HIV-1 WT RNA dimer conformation requires the correct proteolytic processing of Gag-Pol or the stability of the Pol processing intermediate.
HIV-1 WT RNA dimer conformation occurs prior to formation of the mature RT heterodimer or IN multimer.
Whether HIV-1 RT and IN are required to be in their mature multimeric forms for the production of WT RNA dimer conformation is unknown. By taking advantage of amino acid substitutions known to prevent formation of the mature RT heterodimer (L234A [18], W401A [69], or the IN tetramer V260E [30]), we were able to generate RT or IN mutants that were defective in RT heterodimerization or IN multimerization. The L234A mutation is located within the highly conserved primer grip region of p66 RT, at a distance from the dimer interface (29), and it is thought that heterodimer formation is probably prevented through indirect conformational changes in the p66 subunit (73). The W401A site is located within the dimer interface between the p66 and p51 subunits (69). The hydrophobic cluster containing the W401A site is located within a tryptophan repeat motif which is highly conserved among primate lentiviral reverse transcriptases (3). The W401A substitution prevents both RT heterodimer formation and reverse transcriptase activity in vitro (69). The minimum HIV-1 IN complex required to perform integration is proposed to be a homotetramer (36, 72) or even an octomer (21, 36). The V260E mutation within the C-terminal domain of IN prevents the multimerization of IN, and this mutant is unable to support viral integration (30).
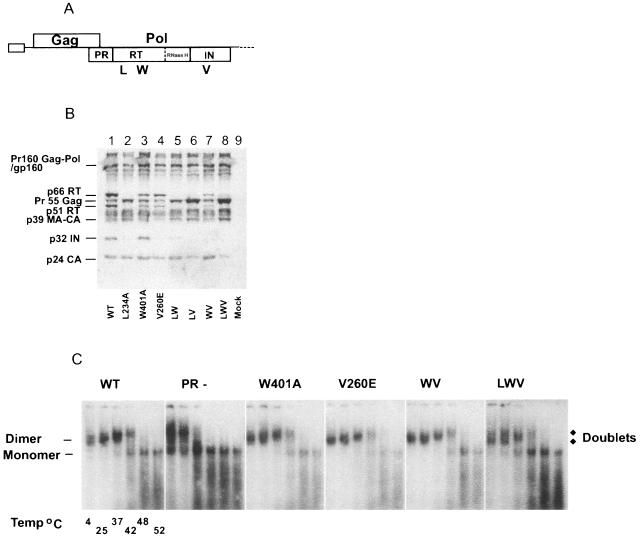
Western blot analysis revealed that HIV-1 virions containing the single RT L234A substitution had undetectable RT or IN, indicating possible Gag-Pol instability (Fig. 3B, lane 2). This was consistent with a previous report showing that mutation at the L234 site reduces the stability of the Gag-Pol precursor, resulting in a 20-fold decrease of RT and IN found in mutant virions (76). As Gag-Pol packaging is required for forming the WT RNA dimer conformation, most of the mutants containing L234A were excluded from RNA dimer analysis, except the triple LWV mutant that was used as a negative control. The RT W401A mutant had a similar virion protein profile in comparison with WT HIV-1 (Fig. 3B, lanes 1 and 3), whereas the IN V260E mutant profile contained both RT subunits but no IN was detected (Fig. 3B, lane 4). This indicated that the p66 and p51 RT monomers were stable, while multimerization of IN might be required to stabilize the mature IN in the virion. The combination of RT W401A and IN V260E mutations (WV) resulted in the same RT and IN defective protein profiles as the single V260E mutant (Fig. 3B, lanes 4 and 7). Although W401A and V260E mutations prevent the multimerization of RT and IN, respectively, they are unlikely to prevent Gag-Pol interaction, shown by the presence of correctly processed Gag and RT subunits in the virion protein profile for both single and double mutants (Fig. 3B, lanes 3, 4, and 7).
FIG. 3.
The effect of HIV-1 Pol multimerization-defective mutants on RNA dimer maturation. (A) Schematic representation of HIV-1 proviral DNA constructs containing amino acid substitutions at the indicated sites that prevent RT dimerization is given: L234A (L) and W401A (W) or IN multimerization V260E (V). Further combinations of these substitutions were also generated. (B) Western blot analysis was performed on purified virions. Proteins were resolved by 10% Tris-glycine SDS-PAGE and then probed with pooled sera from HIV-1-infected individuals. (C) RNA dimer analysis was performed on WT, PR(−) (which contains an inactive protease), W401A, and V260E single mutants; the W401A and V260E double mutant (WV); and the L234A, W401A, and V260E triple mutant (LWV). Virion RNA was resuspended in RNA dimerization buffer and heat denatured for 10 min at the indicated temperatures. Dimers and monomers were electrophoresed in a 1% native agarose gel and probed with an HIV-1-specific riboprobe.
As expected, the LWV triple multimerization mutant (Fig. 3C, LWV) had an RNA dimer conformation different from WT and similar to PRUAG (Fig. 1C, PRUAG), which is most likely attributed to the lack of RT or IN in these virions. RNA dimer analysis of single multimerization mutants RT W401A, IN V260E, and double multimerization mutant RT W401A and IN V260E (WV) revealed RNA dimer profiles indistinguishable from those of HIV-1 WT (Fig. 3C, WT, W401A, V260E, WV). These results indicated that generation of the WT RNA dimer conformation was independent of RT dimerization or the presence of mature IN, implying that the requirement of Pol for WT RNA dimer structure occurs prior to RT or IN maturation.
The requirement of RT and IN in WT RNA dimer structure is not conserved among all retroviruses.
We have used M-PMV, a prototype of type D retroviruses that assembles intracellularly, and MoMuLV, a prototype of a simple type C retrovirus, to determine if RT and IN proteins are required for WT RNA dimer conformation in other retroviruses by using a series of M-PMV (Fig. 4A) and MoMuLV (Fig. 4B) mutants. M-PMV and MoMuLV protease-negative mutants were generated by an amino acid substitution at the active site of the viral protease. Truncated versions of Pol were generated for each virus by introducing additional termination codons within the Pol gene to prevent the translation of downstream protein sequences. M-PMV and MoMuLV GagUAA mutants were unable to translate PR, RT, or IN proteins, whereas M-PMV PRUAA and MoMuLV PRUAG mutants lacked RT or IN proteins. The MoMuLV RTUAG mutant produced no IN protein. The cleavage site between the M-PMV RT and IN proteins has not been identified; consequently an RTUAA mutant was not generated for M-PMV.
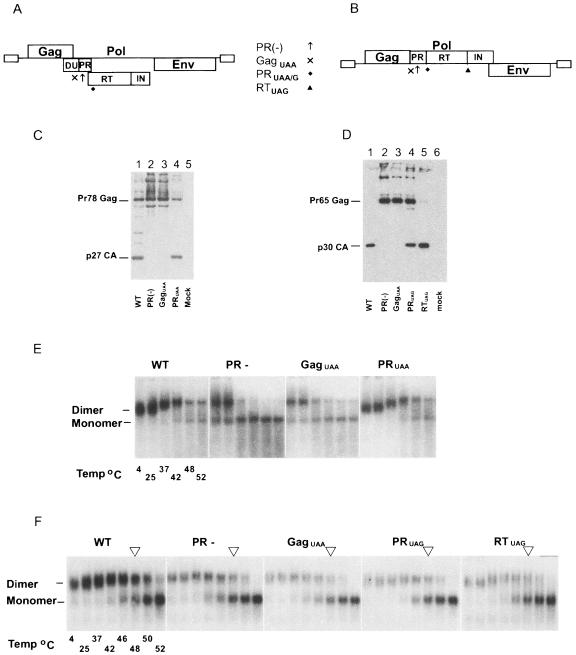
FIG. 4.
Mason-Pfizer monkey virus and Moloney murine leukemia virus do not require RT and IN for WT RNA dimer conformation. (A and B) Schematic representation of M-PMV and MoMuLV proviral DNA constructs, respectively. PR(−) contained an amino acid substitution at the active site of PR (↑↑), preventing proteolytic activity. GagUAA contained termination codon in the PR reading frame immediately after Gag (X), preventing translation of PR or the Pol proteins. PRUAA and PRUAG had termination codons at the ends of their respective PR (⧫) coding regions. MoMuLV RTUAG had a termination codon end of RT (▴), preventing translation of the IN enzyme. Western blot analysis was performed on purified virions for M-PMV (C) and MoMuLV (D). Proteins were resolved by 10% Tris-glycine SDS-PAGE and then probed with appropriate anti-Gag antibody. Virion RNA was resuspended in RNA dimerization buffer and heat denatured for 10 min at the indicated temperatures. Dimers and monomers were electrophoresed in a 1% native agarose gel and probed with a M-PMV (E) or MoMuLV (F)-specific riboprobe.
Western analysis of M-PMV WT and PRUAA virions (Fig. 4C, lanes 1 and 4) revealed normal proteolytic processing of the Gag precursor into capsid. MoMuLV WT virions contained the Gag cleavage products, and MoMuLV PRUAG virions contained partially processed Gag as well as capsid, implicating the presence of MoMuLV PR in the MoMuLV PRUAG mutant (Fig. 4D, lanes 1 and 4). The MoMuLV RTUAG mutant (Fig. 4D, lane 5) revealed normal Gag processing. Virion protein profiles of M-PMV PR(−) and GagUAA (Fig. 4C, lanes 2 and 3) and MoMuLV PR(−) and GagUAA (Fig. 4D, lanes 2 and 3) revealed an absence of proteolytic processing as expected. Both M-PMV PRUAA and MoMuLV PRUAG mutants are unlikely to express RT or IN, whereas the MoMuLV RTUAG mutant is predicted to lack IN.
M-PMV and MoMuLV do not require Pol proteins for WT RNA dimer conformation.
Both M-PMV WT and M-PMV PRUAA RNA dimers dissociated into monomers after heat treatment at 48°C (Fig. 4E, WT and PRUAA). As M-PMV PRUAA was not expected to express RT and IN, these data suggested that Pol is dispensable for WT RNA dimer conformation in this virus. M-PMV PR(−) and GagUAA RNA dimers were less stable than WT and had altered electrophoretic mobilities [Fig. 4E, PR(-) and GagUAA], which is consistent with reports on other PR(−) retroviral species such as HIV-1 (16), MoMuLV (17), RSV (45), and ALV (66) where proteolytic processing of viral precursor proteins is critical for WT RNA dimer structure and stability. Similar to WT HIV-1 dimeric RNA, both M-PMV WT and M-PMV PRUAA RNA dimers exhibited characteristic reduced electrophoretic mobilities when the samples were treated at 4°C, 25°C, 37°C, and 42°C. It appeared that the MoMuLV WT dimeric RNA was fractionally more stable than MoMuLV PR(−), GagUAA, PRUAG, and RTUAG RNA dimers shown by the majority of WT RNA remaining dimeric post-heat treatment at 48°C (Fig. 4F, see arrows at 48°C for each panel). In contrast to HIV-1 and M-PMV, the difference in electrophoretic mobility between MoMuLV WT and mutant virion RNA dimers was more subtle than that for RNA dimers seen with HIV-1 and M-PMV. These data do not support the requirement of Pol in the production of WT RNA dimers in M-PMV and MoMuLV.
DISCUSSION
We have shown that the requirement for RT and IN in the formation of the mature HIV-1 RNA dimer conformation is not strain dependant. We have also demonstrated that the correct proteolytic processing of the HIV-1 Pol proteins is essential for RNA dimer maturation. However, an enzymatically active RT or IN is not required for RNA dimer conformation, which suggests that RNA dimer maturation is completed prior to formation of the mature multimeric RT or IN proteins. RT and IN are not required for WT RNA dimer conformation in M-PMV and Mo-MuLV, showing that the requirement of Pol for generating the mature RNA dimer structure is not conserved among all retroviruses.
Our NL43 HIV-1 dimeric RNA from WT, PR(−), GagUAA, and PRUAG virions exhibits the same stability and mobility profiles as those of our previously published HxB2-BH10 constructs (61). Our previous report utilized a Vpr-supplement system to provide Vpr-RT, Vpr-IN, or Vpr-RT-IN in trans to an RT- and IN- deficient mutant (PRUAA). Only supplementation with Vpr-RT-IN was able to restore RNA dimer conformation to that of the WT (61). Consistent with our previous report, our HIV-1 RTUAG mutant is insufficient to yield a WT RNA dimer structure in the absence of IN (61). As the Gag/Gag-Pol ratio is important for RNA dimerization (60, 63), we utilized the RTUAG system to ensure that our previously observed impact of Pol protein in RNA dimerization was not biased by the altered levels of RT or IN that were introduced into the virion via the Vpr supplement system (75).
Having established that Pol is critical for generating the mature RNA dimer conformation, we speculated on the biological significance of this RNA structure in retroviral replication. The altered RNA conformation seen in mature retroviral particles including HIV-1, ALV, RSV, MoMuLV, and additionally, M-PMV (16, 17, 45, 66, 67) is likely to be a prerequisite for subsequent steps in the viral replication cycle. The importance of the rearrangement of the genomic RNA dimers during virion maturation is supported by the fact that it is a conserved feature in all in vivo RNA dimer maturation studies thus far (16, 17, 45, 66, 67). The obvious candidate for requiring a specific RNA conformation is to support the synthesis of viral cDNA from the genomic RNA template. This process is particularly likely, considering that the factors required for RNA dimer conformation, such as genomic RNA, RT, and IN (74), are all part of the ribonucleoprotein reverse transcription complex. In addition, it has been established that RNA dimerization is required for the first strand transfer step of reverse transcription (10).
Processing of the HIV-1 viral polyproteins during virion maturation is a highly regulated event, and alteration of this process can cause severe defects in virion assembly, RNA dimerization, and infectivity. Until now, the importance of the proteolytic processing of Pol in RNA dimer conformation has not been investigated. By disrupting Pol processing through the introduction of Thr and Arg residues into Pol cleavage junctions, we showed that the correct processing of the HIV-1 Pol proteins RT and IN is essential in generating mature RNA dimers that exhibit the same stability and mobility as those of WT RNA. The doublets seen in PRUAG mutants may represent a heterogeneous population of dimeric RNA or an intermediate immature conformation that occurs prior to formation of the WT mature conformation. Processing of Gag-Pol may induce a rearrangement of genomic RNA that positions the genomic RNA, RT, and IN into the ribonucleoprotein initiation complex. As enzymatically active RT or IN is not required for RNA dimer maturation, the formation of the mature RNA dimer conformation is likely to occur prior to maturation of the RT or IN protein complexes.
The biological necessity of the mature WT RNA dimer conformation may also be related to the effects of RNA dimerization on the synthesis of viral cDNA during reverse transcription. HIV-1 DIS mutations that impair RNA dimerization have also been shown to reduce DNA synthesis around the second strand transfer step, though the mechanism for this is unknown (49, 64). DIS mutants are capable of forming dimeric virion RNA, but these dimers have an altered conformation to that of the WT (23). The finding that HIV-1 DIS is required for viral infectivity in a T-cell line (49) but not in peripheral blood mononuclear cells suggests the involvement of a novel host cell factor in peripheral blood mononuclear cells that is able to compensate for the role of DIS in RNA dimerization to support HIV-1 replication (23). Perhaps the requirement of this host cell factor may in part regulate the RNA dimeric structure to support the synthesis of viral cDNA during infection.
Although the DIS is thought to be one of the primary regions involved in RNA dimerization (34, 47, 65), other as yet undefined regions of the genomic RNA are also likely to be involved. Studies showing DIS mutants with WT RNA stability in vivo suggest that other regions are involved in the dimerization of genomic RNA (2, 23, 58, 59). In addition, internal regions of genomic RNA can negatively affect RNA dimerization (59). This may be to prevent the formation of an incorrectly folded dimeric RNA structure. The altered RNA conformation seen in WT mature virions as opposed to that in immature virions may be reflective of dimerization occurring at additional regions within the genomic RNA.
The genomic RNA dimer conformation in M-PMV has not been investigated previously; here, we show for the first time that the WT RNA dimer conformation in M-PMV requires proteolytic processing of the viral polyproteins, consistent with other retroviruses (16, 17, 45, 66, 67). Our MoMuLV WT RNA dimers are more stable than dimeric RNA from PR(−) mutants when subjected to melting point analysis, which is consistent with previous reports (17). The limitations of our RNA dimer analysis technique made it difficult to clearly differentiate between MoMuLV WT and mutant RNA dimer profiles. The requirement of RT and IN for WT RNA dimer conformation in HIV-1 is not conserved among all retroviruses, namely, M-PMV and MoMuLV. This may be a reflection of the requirements of complex (HIV-1) versus simple (M-PMV and MoMuLV) retroviruses. Alternatively, the lack of requirement of Pol for RNA dimer structure in MoMuLV and M-PMV may reflect the limitations of our current mobility-based system for the analysis of RNA dimer structure. Development of more direct analysis of virion RNA dimer structure will be essential to define the significance of RNA dimer conformation in retroviral replication. A chemical-based RNA probing technique has recently been described to dissect the secondary structure of the HIV-1 viral RNA genome (50), and such technique will undoubtedly provide important clues in defining the precise RNA structure of the dimeric viral RNA genome.
The importance of RNA dimer conformation in mediating events in the viral life cycle is illustrated by the proposed LDI to BMH switch (26, 27, 46). These two different RNA conformations formed within the 5′ leader region are thought to regulate the progression from a translation-competent mRNA to the dimerization and packaging-competent genomic RNA (12). The relevance of these in vitro-derived structures needs to be analyzed in an in vivo system, and thus the need for ultrastructural studies on full-length genomic RNA. RNA regions involved in dimerization in the virion could potentially be identified through the use of the RNA cross-linking agent psoralen, which forms covalent bonds between dimerized RNA strands when exposed to long-wave UV light (68) and then subsequent visualization of the dimeric RNA by electron microscopy as previously described (24). Analysis of RNA ultrastructure by electron microscopy would provide a direct means of analyzing the interacting regions of HIV-1 RNA and provide insights into RNA dimer structure. Elucidation of the dimeric structure of HIV-1 RNA would also aid in determining the role of RNA conformation in reverse transcription and other steps in the viral replication cycle.
Acknowledgments
We thank Stephen P. Goff for providing the MoMuLV proviral DNA construct and Scott Parker and Eric Hunter for providing the M-PMV DNA constructs. We also thank Melissa Hill for critical review of the manuscript.
Penelope Buxton is a recipient of the Australian Postgraduate Award scholarship. Gilda Tachedjian is a recipient of an NHMRC RD Wright Research Fellowship. Johnson Mak is a recipient of a Monash Logan Fellowship and a Pfizer Senior Research Fellowship. This work was supported in part by project grants from the NHMRC.
REFERENCES
- 1.Abbink, T. E., and B. Berkhout. 2003. A novel long distance base-pairing interaction in human immunodeficiency virus type 1 RNA occludes the Gag start codon. J. Biol. Chem. 278:11601-11611. [DOI] [PubMed] [Google Scholar]
- 2.Andersen, E. S., S. A. Contera, B. Knudsen, C. K. Damgaard, F. Besenbacher, and J. Kjems. 2004. Role of the trans-activation response element in dimerization of HIV-1 RNA. J. Biol. Chem. 279:22243-22249. [DOI] [PubMed] [Google Scholar]
- 3.Baillon, J. G., N. T. Nashed, A. Kumar, S. H. Wilson, and D. M. Jerina. 1991. A leucine zipper-like motif may mediate HIV reverse transcriptase subunit binding. New Biol. 3:1015-1019. [PubMed] [Google Scholar]
- 4.Balakrishnan, M., P. J. Fay, and R. A. Bambara. 2001. The kissing hairpin sequence promotes recombination within the HIV-I 5′ leader region. J. Biol. Chem. 276:36482-36492. [DOI] [PubMed] [Google Scholar]
- 5.Balakrishnan, M., B. P. Roques, P. J. Fay, and R. A. Bambara. 2003. Template dimerization promotes an acceptor invasion-induced transfer mechanism during human immunodeficiency virus type 1 minus-strand synthesis. J. Virol. 77:4710-4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beerens, N., and B. Berkhout. 2002. The tRNA primer activation signal in the human immunodeficiency virus type 1 genome is important for initiation and processive elongation of reverse transcription. J. Virol. 76:2329-2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beerens, N., F. Groot, and B. Berkhout. 2001. Initiation of HIV-1 reverse transcription is regulated by a primer activation signal. J. Biol. Chem. 276:31247-31256. [DOI] [PubMed] [Google Scholar]
- 8.Bender, W., Y. H. Chien, S. Chattopadhyay, P. K. Vogt, M. B. Gardner, and N. Davidson. 1978. High-molecular-weight RNAs of AKR, NZB, and wild mouse viruses and avian reticuloendotheliosis virus all have similar dimer structures. J. Virol. 25:888-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bender, W., and N. Davidson. 1976. Mapping of poly(A) sequences in the electron microscope reveals unusual structure of type C oncornavirus RNA molecules. Cell 7:595-607. [DOI] [PubMed] [Google Scholar]
- 10.Berkhout, B., A. T. Das, and J. L. van Wamel. 1998. The native structure of the human immunodeficiency virus type 1 RNA genome is required for the first strand transfer of reverse transcription. Virology 249:211-218. [DOI] [PubMed] [Google Scholar]
- 11.Berkhout, B., and K. T. Jeang. 1989. trans activation of human immunodeficiency virus type 1 is sequence specific for both the single-stranded bulge and loop of the trans-acting-responsive hairpin: a quantitative analysis. J. Virol. 63:5501-5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berkhout, B., M. Ooms, N. Beerens, H. Huthoff, E. Southern, and K. Verhoef. 2002. In vitro evidence that the untranslated leader of the HIV-1 genome as an RNA checkpoint that regulates multiple functions through conformational changes. J. Biol. Chem. 277:19967-19975. [DOI] [PubMed] [Google Scholar]
- 13.Canaani, E., K. V. D. Helm, and P. Duesberg. 1973. Evidence for the 30-40S RNA as precursor of the 60-70S RNA of Rous sarcoma virus. Proc. Natl. Acad. Sci. USA 70:401-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clever, J. L., M. L. Wong, and T. G. Parslow. 1996. Requirements for kissing-loop-mediated dimerization of human immunodeficiency virus RNA. J. Virol. 70:5902-5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng, Y. X., H. Yuan, A. Rein, and J. G. Levin. 1992. Bipartite signal for read-through suppression in murine leukemia virus mRNA: an eight-nucleotide purine-rich sequence immediately downstream of the gag termination codon followed by an RNA pseudoknot. J. Virol. 66:5127-5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu, W., R. J. Gorelick, and A. Rein. 1994. Characterization of human immunodeficiency virus type 1 dimeric RNA from wild-type and protease-defective virions. J. Virol. 68:5013-5018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu, W., and A. Rein. 1993. Maturation of dimeric viral RNA of Moloney murine leukemia virus. J. Virol. 67:5443-5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghosh, M., P. S. Jacques, D. W. Rodgers, M. Ottman, J.-L. Darlix, and S. F. J. Le Grice. 1996. Alteration to the primer grip of p66 HIV-1 reverse transcriptase and their consequences for template-primer utilization. Biochemistry 35:8553-8562. [DOI] [PubMed] [Google Scholar]
- 19.Greatorex, J. S. 2004. The retroviral RNA dimer linkage: different structures may reflect different roles. Retrovirology 1:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henderson, L. E., M. A. Bowers, R. C. Sowder II, S. A. Serabyn, D. G. Johnson, J. W. Bess, Jr., L. O. Arthur, D. K. Bryant, and C. Fenselau. 1992. Gag proteins of the highly replicative MN strain of human immunodeficiency virus type 1: posttranslational modifications, proteolytic processings, and complete amino acid sequences. J. Virol. 66:1856-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heuer, T. S., and P. O. Brown. 1998. Photo-cross-linking studies suggest a model for the architecture of an active human immunodeficiency virus type 1 integrase-DNA complex. Biochemistry 37:6667-6678. [DOI] [PubMed] [Google Scholar]
- 22.Higuchi, R. 1990. Recombinant PCR, p. 177-183. In M. A. Innis, D. H. Gelfand, J. J. Sninsky, and T. J. White (ed.), PCR protocols: a guide to methods and applications. Academic Press, Inc., New York, N.Y.
- 23.Hill, M. K., M. Shehu-Xhilaga, S. M. Campbell, P. Poumbourios, S. M. Crowe, and J. Mak. 2003. The dimer initiation sequence stem-loop of human immunodeficiency virus type 1 is dispensable for viral replication in peripheral blood mononuclear cells. J. Virol. 77:8329-8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Höglund, S., A. Öhagen, J. Goncalves, A. T. Panganiban, and D. Gabuzda. 1997. Ultrastructure of HIV-1 genomic RNA. Virology 233:271-279. [DOI] [PubMed] [Google Scholar]
- 25.Honigman, A., D. Wolf, S. Yaish, H. Falk, and A. Panet. 1991. cis Acting RNA sequences control the gag-pol translation readthrough in murine leukemia virus. Virology 183:313-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huthoff, H., and B. Berkhout. 2002. Multiple secondary structure rearrangements during HIV-1 RNA dimerization. Biochemistry 41:10439-10445. [DOI] [PubMed] [Google Scholar]
- 27.Huthoff, H., and B. Berkhout. 2001. Two alternating structures of the HIV-1 leader RNA. RNA 7:143-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacks, T., M. D. Power, F. R. Masiarz, P. A. Luciw, P. J. Barr, and H. E. Varmus. 1988. Characterization of ribosomal frameshifting in HIV-1 gag-pol expression. Nature 331:280-283. [DOI] [PubMed] [Google Scholar]
- 29.Jacobo-Molina, A., J. Ding, R. G. Nanni, A. D. Clark, Jr., X. Lu, C. Tantillo, R. L. Williams, G. Kamer, A. L. Ferris, P. Clark, A. Hizi, S. H. Hughes, and E. Arnold. 1993. Crystal structure of human immunodeficiency virus type 1 reverse transcriptase complexed with double-stranded DNA at 3.0 A resolution shows bent DNA. Proc. Natl. Acad. Sci. USA 90:6320-6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalpana, G. V., A. Reicin, G. S. Cheng, M. Sorin, S. Paik, and S. P. Goff. 1999. Isolation and characterization of an oligomerization-negative mutant of HIV-1 integrase. Virology 259:274-285. [DOI] [PubMed] [Google Scholar]
- 31.Kaplan, A. H., J. A. Zack, M. Knigge, D. A. Paul, D. J. Kempf, D. W. Norbeck, and R. Swanstrom. 1993. Partial inhibition of the human immunodeficiency virus type 1 protease results in aberrant virus assembly and the formation of noninfectious particles. J. Virol. 67:4050-4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kohl, N. E., E. A. Emini, W. A. Schleif, L. J. Davis, J. C. Heimbach, R. A. Dixon, E. M. Scolnick, and I. S. Sigal. 1988. Active human immunodeficiency virus protease is required for viral infectivity. Proc. Natl. Acad. Sci. USA 85:4686-4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kung, H. J., S. Hu, W. Bender, J. M. Bailey, N. Davidson, M. O. Nicolson, and R. M. McAllister. 1976. RD-114, baboon, and woolly monkey viral RNA's compared in size and structure. Cell 7:609-620. [DOI] [PubMed] [Google Scholar]
- 34.Laughrea, M., and L. Jette. 1994. A 19-nucleotide sequence upstream of the 5′ major splice donor is part of the dimerization domain of human immunodeficiency virus 1 genomic RNA. Biochemistry 33:13464-13474. [DOI] [PubMed] [Google Scholar]
- 35.Laughrea, M., and L. Jetté. 1996. Kissing-loop model of HIV-1 genomic dimerization: HIV-1 RNAs can assume alternative dimeric forms, and all sequences upstream or downstream of hairpin 248-271 are dispensable for dimeric formation. Biochemistry 35:1589-1598. [DOI] [PubMed] [Google Scholar]
- 36.Lee, S. P., J. Xiao, J. R. Knutson, M. S. Lewis, and M. K. Han. 1997. Zn2+ promotes the self-association of human immunodeficiency virus type-1 integrase in vitro. Biochemistry 36:173-180. [DOI] [PubMed] [Google Scholar]
- 37.Lever, A., H. Göttlinger, W. Haseltine, and J. Sodroski. 1989. Identification of a sequence required for efficient packaging of human immunodeficiency virus type 1 RNA into virions. J. Virol. 63:4085-4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liang, C., X. Li, L. Rong, P. Inouye, Y. Quan, L. Kleiman, and M. A. Wainberg. 1997. The importance of the A-rich loop in human immunodeficiency virus type 1 reverse transcription and infectivity. J. Virol. 71:5750-5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mak, J., M. Jiang, M. A. Wainberg, M.-L. Hammarskjold, D. Rekosh, and L. Kleiman. 1994. Role of Pr160gag-pol in mediating the selective incorporation of tRNALys into human immunodeficiency virus type 1 particles. J. Virol. 68:2065-2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mikkelsen, J. G., A. H. Lund, K. D. Kristensen, M. Duch, M. S. Sorensen, P. Jorgensen, and F. S. Pedersen. 1996. A preferred region for recombinational patch repair in the 5′ untranslated region of primer binding site-impaired murine leukemia virus vectors. J. Virol. 70:1439-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muriaux, D., H. De Rocquigny, B. Roques, and J. Paoletti. 1996. NCp7 activates HIV-1Lai RNA dimerization by converting a transient loop-loop complex into a stable dimer. J. Biol. Chem. 271:33686-33692. [DOI] [PubMed] [Google Scholar]
- 42.Muriaux, D., P. Fosse, and J. Paoletti. 1996. A kissing complex together with a stable dimer is involved in the HIV- 1Lai RNA dimerization process in vitro. Biochemistry 35:5075-5082. [DOI] [PubMed] [Google Scholar]
- 43.Muriaux, D., P.-M. Girard, B. Bonnet-Mathonière, and J. Paoletti. 1995. Dimerization of HIV-1Lai RNA at low ionic strength. An autocomplementary sequence in the 5′ leader region is evidenced by an antisense oligonucleotide. J. Biol. Chem. 270:8209-8216. [DOI] [PubMed] [Google Scholar]
- 44.Murti, K. G., M. Bondurant, and A. Tereba. 1981. Secondary structural features in the 70S RNAs of Moloney murine leukemia and Rous sarcoma viruses as observed by electron microscopy. J. Virol. 37:411-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oertle, S., and P. F. Spahr. 1990. Role of the gag polyprotein precursor in packaging and maturation of Rous sarcoma virus genomic RNA. J. Virol. 64:5757-5763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ooms, M., H. Huthoff, R. Russell, C. Liang, and B. Berkhout. 2004. A riboswitch regulates RNA dimerization and packaging in human immunodeficiency virus type 1 virions. J. Virol. 78:10814-10819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paillart, J.-C., R. Marquet, E. Skripkin, B. Ehresmann, and C. Ehresmann. 1994. Mutational analysis of the bipartite dimer linkage structure of human immunodeficiency virus type 1 genomic RNA. J. Biol. Chem. 269:27486-27493. [PubMed] [Google Scholar]
- 48.Paillart, J.-C., E. Skripkin, B. Ehresmann, C. Ehresmann, and R. Marquet. 1996. A loop-loop “kissing” complex is the essential part of the dimer linkage of genomic HIV-1 RNA. Proc. Natl. Acad. Sci. USA 93:5572-5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paillart, J. C., L. Berthoux, M. Ottmann, J. L. Darlix, R. Marquet, B. Ehresmann, and C. Ehresmann. 1996. A dual role of the putative RNA dimerization initation site of human immunodeficiency virus type 1 in genomic RNA packaging and proviral DNA synthesis. J. Virol. 70:8348-8354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paillart, J. C., M. Dettenhofer, X. F. Yu, C. Ehresmann, B. Ehresmann, and R. Marquet. 2004. First snapshots of the HIV-1 RNA structure in infected cells and in virions. J. Biol. Chem. 279:48397-48403. [DOI] [PubMed] [Google Scholar]
- 51.Paillart, J. C., M. Shehu-Xhilaga, R. Marquet, and J. Mak. 2004. Dimerization of retroviral RNA genomes: an inseparable pair. Nat. Rev. Microbiol. 2:461-472. [DOI] [PubMed] [Google Scholar]
- 52.Pettit, S. C., S. Gulnik, L. Everitt, and A. H. Kaplan. 2003. The dimer interfaces of protease and extra-protease domains influence the activation of protease and the specificity of GagPol cleavage. J. Virol. 77:366-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pettit, S. C., M. D. Moody, R. S. Wehbie, A. H. Kaplan, P. V. Nantermet, C. A. Klein, and R. Swanstrom. 1994. The P2 domain of human immunodeficiency virus type 1 Gag regulates sequential proteolytic processing and is required to produce fully infectious virions. J. Virol. 68:8017-8027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pettit, S. C., N. Sheng, R. Tritch, S. Erickson-Vitanen, and R. Swanstrom. 1998. The regulation of sequential processing of HIV-1 Gag by the viral protease. Adv. Exp. Med. Biol. 436:15-25. [DOI] [PubMed] [Google Scholar]
- 55.Restle, T., B. Muller, and R. S. Goody. 1990. Dimerization of human immunodeficiency virus type 1 reverse transcriptase. J. Biol. Chem. 265:8986-8988. [PubMed] [Google Scholar]
- 56.Ross, E. K., T. R. Fuerst, J. M. Orenstein, T. O'Neill, M. A. Martin, and S. Venkatesan. 1991. Maturation of human immunodeficiency virus particles assembled from the gag precursor protein requires in situ processing by gag-pol protease. AIDS Res. Hum. Retrovir. 7:475-483. [DOI] [PubMed] [Google Scholar]
- 57.Russell, R. S., C. Liang, and M. A. Wainberg. 2004. Is HIV-1 RNA dimerization a prerequisite for packaging? Yes, no, probably? Retrovirology 1:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sakuragi, J.-I., A. Iwamoto, and T. Shioda. 2002. Dissociation of genome dimerization from packaging functions and virion maturation of human immunodeficiency virus type 1. J. Virol. 76:959-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sakuragi, J.-I., and A. T. Panganiban. 1997. Human immunodeficiency virus type 1 RNA outside the primary encapsidation and dimer linkage region affects RNA dimer stability in vitro. J. Virol. 71:3250-3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shehu-Xhilaga, M., S. M. Crowe, and J. Mak. 2001. Maintenance of the Gag/Gag-Pol ratio is important for human immunodeficiency virus type 1 RNA dimerization and viral infectivity. J. Virol. 75:1834-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shehu-Xhilaga, M., M. Hill, J. A. Marshall, J. Kappes, S. M. Crowe, and J. Mak. 2002. The conformation of the mature dimeric human immunodeficiency virus type 1 RNA genome requires packaging of Pol protein. J. Virol. 76:4331-4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shehu-Xhilaga, M., H. G. Kraeusslich, S. Pettit, R. Swanstrom, J. Y. Lee, J. A. Marshall, S. M. Crowe, and J. Mak. 2001. Proteolytic processing of the p2/nucleocapsid cleavage site is critical for human immunodeficiency virus type 1 RNA dimer maturation. J. Virol. 75:9156-9164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shehu-Xhilaga, M., J.-Y. Lee, S. M. Campbell, J. A. Marshall, S. M. Crowe, and J. Mak. 2002. Overexpression and incorporation of GagPol precursor does not impede packaging of HIV-1 tRNA(Lys3) but promotes intracellular budding of virus-like particles. J. Biomed. Sci. 9:697-705. [DOI] [PubMed] [Google Scholar]
- 64.Shen, N., L. Jetté, C. Liang, M. A. Wainberg, and M. Laughrea. 2000. Impact of human immunodeficiency virus type 1 RNA dimerization on viral infectivity and of stem-loop B on RNA dimerization and reverse transcription and dissociation of dimerization from packaging. J. Virol. 74:5729-5735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Skripkin, E., J.-C. Paillart, R. Marquet, B. Ehresmann, and C. Ehresmann. 1994. Identification of the primary site of the human immunodeficiency virus type 1 RNA dimerization in vitro. Proc. Natl. Acad. Sci. USA 91:4945-4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stewart, L., G. Schatz, and V. M. Vogt. 1990. Properties of avian retrovirus particles defective in viral protease. J. Virol. 64:5076-5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stoltzfus, C. M., and P. N. Snyder. 1975. Structure of B77 sarcoma virus RNA: stabilization of RNA after packaging. J. Virol. 64:1161-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Swanstrom, R., L. M. Hallick, J. Jackson, J. E. Hearst, and J. M. Bishop. 1981. Interaction of psoralen derivatives with the RNA genome of Rous sarcoma virus. Virology 113:613-622. [DOI] [PubMed] [Google Scholar]
- 69.Tachedjian, G., H. E. Aronson, M. de los Santos, J. Seehra, J. M. McCoy, and S. P. Goff. 2003. Role of residues in the tryptophan repeat motif for HIV-1 reverse transcriptase dimerization. J. Mol. Biol. 326:381-396. [DOI] [PubMed] [Google Scholar]
- 70.Telesnitsky, A., and S. P. Goff. 1997. Reverse transcription and the generation of retroviral DNA, p. 121-160. In J. M. Coffin, S. H. Hughes, and H. E. Varmus (ed.), Retroviruses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y. [PubMed]
- 71.Temin, H. M. 1991. Sex and recombination in retroviruses. Trends Genet. 7:71-74. [DOI] [PubMed] [Google Scholar]
- 72.Wang, J. Y., H. Ling, W. Yang, and R. Craigie. 2001. Structure of a two-domain fragment of HIV-1 integrase: implications for domain organization in the intact protein. EMBO J. 20:7333-7343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wohrl, B. M., R. Krebs, S. H. Thrall, S. F. Le Grice, A. J. Scheidig, and R. S. Goody. 1997. Kinetic analysis of four HIV-1 reverse transcriptase enzymes mutated in the primer grip region of p66. Implications for DNA synthesis and dimerization. J. Biol. Chem. 272:17581-17587. [DOI] [PubMed] [Google Scholar]
- 74.Wu, X., H. Liu, H. Xiao, J. A. Conway, E. Hehl, G. V. Kalpana, V. Prasad, and J. C. Kappes. 1999. Human immunodeficiency virus type 1 integrase protein promotes reverse transcription through specific interactions with the nucleoprotein reverse transcription complex. J. Virol. 73:2126-2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu, X., H. Liu, H. Xiao, J. A. Conway, E. Hunter, and J. C. Kappes. 1997. Functional RT and IN incorporated into HIV-1 particles independently of the Gag/Pol precursor protein. EMBO J. 16:5113-5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yu, Q., M. Ottmann, C. Pechoux, S. LeGrice, and J.-L. Darlix. 1998. Mutations in the primer grip of human immunodeficiency virus type 1 reverse transcriptase impair proviral DNA synthesis and virion maturation. J. Virol. 72:7676-7680. [DOI] [PMC free article] [PubMed] [Google Scholar]