Abstract
INTRODUCTION
The U.S. study to protect brain health through lifestyle intervention to reduce risk (U.S. POINTER) is conducted to confirm and expand the results of the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) in Americans.
METHODS
U.S. POINTER was planned as a 2‐year randomized controlled trial of two lifestyle interventions in 2000 older adults at risk for dementia due to well‐established factors. The primary outcome is a global cognition composite that permits harmonization with FINGER.
RESULTS
U.S. POINTER is centrally coordinated and conducted at five clinical sites (ClinicalTrials.gov: NCT03688126). Outcomes assessments are completed at baseline and every 6 months. Both interventions focus on exercise, diet, cognitive/social stimulation, and cardiovascular health, but differ in intensity and accountability. The study partners with a worldwide network of similar trials for harmonization of methods and data sharing.
DISCUSSION
U.S. POINTER is testing a potentially sustainable intervention to support brain health and Alzheimer's prevention for Americans. Impact is strengthened by the targeted participant diversity and expanded scientific scope through ancillary studies.
Keywords: aging, Alzheimer's disease, clinical trial, cognition, cognitive training, diet, exercise, lifestyle intervention, non‐pharmacological, prevention, risk modification
1. BACKGROUND
The most recent report of the Lancet Commission 1 summarizes the extensive evidence that treating modifiable risk factors may prevent or delay up to 40% of dementia cases. 1 Moreover, multidomain interventions targeting a combination of risk factors may be more effective than single‐component interventions in reducing risk for cognitive decline. 2 , 3 , 4 , 5 Although treating individual lifestyle components (e.g., cognitive stimulation 6 ) may provide some benefits, large clinical trials examining the effects of single‐domain lifestyle interventions on risk for Alzheimer's disease and related dementia (ADRD) have, thus far, yielded variable and inconclusive results. 2 The multidomain approach is used increasingly to examine the effects of simultaneously addressing multiple risk factors to increase cognitive resilience and protect against cognitive decline. 7 , 8 , 9 , 10 , 11 , 12 This approach allows for additive and/or synergistic effects between individual domains and provides flexibility for intervention tailoring to meet person‐specific needs (e.g., more intensive cardiovascular risk reduction) and/or challenges that can jeopardize long‐term adherence to single‐domain interventions (e.g., joint pain with physical activity, reduced access to healthier foods). The results of the population‐based 2‐year Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) demonstrated that a multidomain intervention of physical activity, nutritional guidance, cognitive training, social activities, and management of cardiovascular disease (CVD) risk factors improved global cognition in older adults who were cognitively unimpaired but at increased risk for decline. 10 These results are promising, but need to be replicated and confirmed in heterogeneous cohorts in other countries with regard to culture, race, ethnicity, and socioeconomic status that could potentially affect adherence and cognitive response to the intervention.
The U.S. study to protect brain health through lifestyle intervention to reduce Risk (U.S. POINTER) is a large, multi‐site randomized controlled trial investigating whether a FINGER‐like 2‐year multidomain lifestyle intervention—adapted to American culture and delivered within the community—can protect or improve cognition in a diverse and representative population of older Americans at risk for cognitive decline and dementia. U.S. POINTER is one of several lifestyle intervention studies now being conducted across the globe as part of a collaborative international network referred to as World‐Wide FINGERS. 13 Here we describe the study's design, multi‐layered support infrastructure, targeted population, screening and enrollment procedures, and the interventions that were developed and expanded from FINGER, but also from the evolving science around cognitive assessment, recruitment, and retention of representative cohorts, and sustained behavior change in older adults.
2. METHODS
The goal of U.S. POINTER is to investigate the effects of random assignment to one of two lifestyle interventions for 2 years on cognitive function in 2000 older adults without significant cognitive impairment but at risk for decline and dementia due to well‐established risk factors. Both interventions were designed to target specific lifestyle behaviors that have been linked to brain health and yet differ in format and intensity. The interventions were modeled on FINGER and adapted for American culture and delivery in the community—in collaboration with community partners. The cognitive battery and primary outcome were selected to permit head‐to‐head comparisons with FINGER and other ongoing nonpharmacological and pharmaceutical trials. Ancillary studies were solicited to meaningfully expand scientific scope using rich parent trial resources. Regulatory oversight is provided by a single institutional review board (sIRB) of record at Wake Forest University School of Medicine. The trial is registered on ClinicalTrials.gov (NCT03688126).
RESEARCH IN CONTEXT
Systematic review. The literature was reviewed using standard sources (e.g., PubMed). Several other multidomain lifestyle intervention trials have been completed or are in progress and are referenced in the article.
Interpretation. The article describes the study design and methods of the largest lifestyle intervention study conducted to date. U.S. study to protect brain health through lifestyle intervention to reduce risk (U.S. POINTER) is a large multi‐site 2‐year randomized controlled trial testing the cognitive effects of two lifestyle interventions that differ in intensity, structure, and accountability in older adults at risk for cognitive decline, including Alzheimer's disease and related dementia (ADRD). Distinguishing characteristics of U.S. POINTER design relative to other similar trials are described.
Future directions. U.S. POINTER partners with the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) investigators to advance lessons learned for multi‐domain lifestyle trials, adapts the interventions to American culture, and leverages community partnerships for intervention delivery to test a model of feasibility and sustainability. The study design includes innovative components that may inform the conduct of future nonpharmacological trials.
2.1. Trial planning
U.S. POINTER—from inception and design to delivery—has progressed as a full partnership between academia and health care systems, and the Alzheimer's Association. The study team also leveraged additional expertise from multiple academic and community advisors on grassroots outreach and engagement, cultural diversity in lifestyle practices, achieving equipoise in messaging about group assignment and sustaining behavior change. The study was conceptualized and funded by the Alzheimer's Association. The Association identified and partnered with academic leadership to develop the study design and methods, with input from their scientific advisory board.
2.2. Infrastructure to support the trial
U.S. POINTER is overseen by the executive leadership (trial Principal Investigators [PIs], Association PIs, Coordinating Center), a steering committee and other committees. Intervention delivery at the site is provided through a partnership between the clinic and the local Alzheimer's Association Chapter. The structure and contributions of the Coordinating Center, Committees, and Clinical Sites that have been instrumental for development and rollout of the trial are described below.
Coordinating Center . The Coordinating Center (CC), housed at Wake Forest University School of Medicine, consists of an Administrative and Clinical Operations Coordination Center (ACOCC) and a Data Coordination Center (DCC). The two branches of the CC provide central oversight and quality control of clinical operations, intervention delivery, outcomes assessments, participant safety, and data management, reporting, data analyses, and data sharing.
Committees . The Steering Committee—comprising executive leadership, site principal investigators (PIs), CC directors and representatives, Association Chapter leads (community partners), committee leads, project managers, and ancillary study PIs and co‐investigators—provides leadership and establishes scientific and administrative policies. Other committees provide direction for specific components of the study and include Recruitment; Outcomes; Clinical Operations; Intervention Oversight; Safety; Data Management; Data Analysis; and Emerging Science, Presentations & Publications.
Clinical Sites . The Alzheimer's Association, in collaboration with academic leadership (includes the CC), selected five clinical sites based on geographic and ethnocultural diversity for recruitment, site capacity to enroll and follow 400 participants, expertise conducting large AD clinical trials, and the Association's local leadership capacity to oversee the intervention. Sites include Wake Forest University School of Medicine (NC), University of California Davis (CA), Rush Medical Center and Advocate Health (IL), Baylor College of Medicine and Kelsey Research Foundation (TX), and Brown University/Butler Hospital and The Miriam Hospital (RI).
3. RESULTS
3.1. Overview
The study design and methods are described below, and the protocol is provided as a Supplement. The primary objective of U.S. POINTER is to test whether random assignment to one of two lifestyle intervention groups for 2 years can protect or improve cognitive function in 2000 older Americans who are at risk for cognitive decline associated with ADRD. Both interventions focus on exercise, diet, cognitive/social stimulation, and cardiovascular risk reduction, but they differ in intensity, accountability, and format. Outcomes assessments are completed at baseline and months 6, 12, 18 and 24, and the primary outcome is a global cognition composite score that will allow harmonization with FINGER and other trials. Following completion of the baseline assessment, eligible participants are assigned randomly to one of two intervention groups and placed into “Teams” of 10–15 participants within these groups. Participants progress through the 2‐year interventions with their assigned Teams and facilitators. Both groups receive results of laboratory blood tests and blood pressure and weight measurements following each clinic visit (Figure 1). The Self‐Guided (SG) intervention consists of group meetings three times in Year 1 and two times in Year 2 to provide education and support and encourage healthy lifestyle practices. The Structured (STR) intervention consists of regular facilitated group meetings, and a structured program of aerobic exercise, resistance training, and stretching completed primarily at a participating community facility (e.g., YMCA), dietary counseling to support adherence to the MIND diet (Mediterranean‐DASH Intervention for Neurodegenerative Delay), computer‐based cognitive training, cognitively and socially challenging activities, and regular guideline‐based health coaching and goal‐setting to support self‐management of cardiometabolic health.
FIGURE 1.
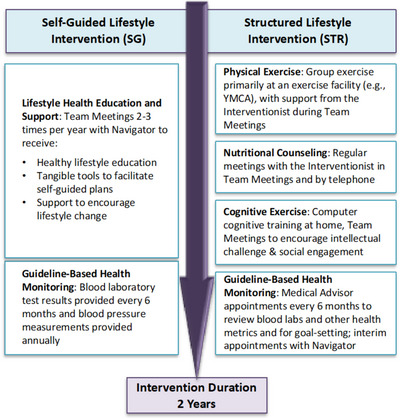
Overview of intervention arms.
3.2. Interventions and delivery
Infrastructure to Support Intervention Delivery and Oversight. The clinics and local Alzheimer's Association at the site share responsibility for intervention delivery and oversight. A trained facilitator is assigned to each Team of participants. For the STR group, the facilitators include an Interventionist (content specialist on exercise, diet, or brain health; clinic employee) and a Navigator (counseling/coaching expertise; Association employee). SG groups are facilitated by a Navigator (different from STR Navigator). Intervention oversight is monitored regularly at the site and centrally by the Intervention Oversight Committee (IOC). Objective and self‐report metrics are tracked to assess intervention adherence and sustainability over time.
Team Meetings . Team Meetings for both intervention groups are led by facilitators using a semi‐structured format to encourage participant satisfaction with the assigned intervention and retention in the trial through interactive discussions, education that can be readily applied, positive reinforcement for success, and social cohesion. Participants were recruited in waves by zip code to form Teams that could meet at community facilities within their neighborhood. Missed meetings due to illness or travel are completed in‐person (majority), via web conferencing, or, in rare cases, by telephone.
Self‐Guided Lifestyle Intervention . The SG intervention encourages participants to develop an individualized healthy lifestyle program that best suits their needs, priorities, and schedules, and broadly targets physical and cognitive activity, diet, social engagement, and cardiovascular health. A SG group was used rather than a wait list or usual care to mimic the general standard of care in community settings, and to increase equipoise of perceived benefit by the participant. Navigators facilitate Team Meetings at baseline and months 3, 9, 15, and 21 (plus graduation celebration at month 24) to provide guideline‐based lifestyle health education and encouragement. SG participants receive semi‐annual health monitoring and test results at the clinic (e.g., lipids, hemoglobin A1c [HbA1c], blood pressure, weight).
Structured Lifestyle Intervention . The Structured Intervention provides participants with intensive structure and coaching support to encourage increased physical exercise, adherence to the MIND diet, increased intellectual and social challenges, and regular monitoring of cardiometabolic health. Individual domains of the STR intervention are introduced serially over the first 4 months to progressively acclimate participants to the relevant activities. STR Team Meetings, facilitated by the Intervention Team, are held weekly during the first 4 months, every other week in months 5 and 6, and monthly thereafter. The STR Team Meetings rely on principles of social cognitive theory, 14 self‐determination theory, 15 and group dynamics 16 , 17 to encourage behavior change and maximize intervention adherence and retention in the trial by providing education, self‐regulation skills, and positive reinforcement, and by addressing barriers, leveraging group processes, and promoting self‐efficacy to meet intervention goals. Adherence is tracked for the STR group using only participant self‐report activity and diet logs, and objective data from an activity monitor (Fitbit) and cognitive training electronic records (BrainHQ).
Physical Exercise. STR physical exercise includes aerobic exercise, resistance training, and stretching/balance/range of motion activities that align with standard American College of Sports Medicine (ACSM) recommendations 18 and protocols tested in smaller‐scale clinical trials. 19 , 20 , 21 , 22 The program targets moderate‐to‐high intensity aerobic exercise (70%–80% of heart rate reserve) for 30–35 minutes, four times/week; resistance training with weight machines, free weights, and/or resistance bands for 15–20 minutes, two times/week; and stretching/balance/range of motion activities for 10–15 minute, two times/week. Guidelines and resources are provided to assist participants in completing these activities. Participants are encouraged to attend group classes at a participating exercise facility (e.g., YMCA) and use the facility's equipment as needed. Onboarding of the exercise program is gradual (slowly increasing frequency and duration over 7 weeks) to build self‐efficacy and stamina, and to reduce risk of injury. The STR Intervention Team assists participants by identifying appropriate classes and strategies to meet their exercise goals, addressing concerns, encouraging adherence tracking, and providing ongoing coaching and support as needed.
Diet. STR diet involves nutritional counseling to encourage adherence to the MIND diet, a hybrid of the Mediterranean and DASH diets with selected modifications based on the most compelling evidence in the diet‐dementia field. 23 , 24 , 25 , 26 , 27 , 28 The MIND diet is rich in dark green leafy vegetables, berries, whole grains, unsaturated fats including extra‐virgin olive oil, nuts and fish, and low in saturated fat. 23 , 24 , 25 , 26 , 27 The Intervention Team provides nutrition education and guidance to help participants incorporate these foods into dietary plans, and uses behavior change principles 14 , 15 to encourage adherence through regular calls. Weekly food logs are reviewed with the Interventionist to assess dietary adherence and address challenges, and to calculate MIND diet scores.
Cognitive Exercise. STR cognitive exercise includes computer‐based cognitive training and home‐based cognitively and socially challenging activities. The Team Meetings also provide cognitive stimulation and social engagement. Web‐based BrainHQ (Posit Science) was selected for cognitive training given the results of the Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE) trial showing moderate‐strength evidence for cognitive benefit and maintained everyday function with regular training. 6 , 29 , 30 BrainHQ tasks train speed and accuracy of information processing in vision and audition. Participants are asked to complete training at least 3 times per week for 30 minutes. Task completion and performance metrics are tracked through BrainHQ and regularly uploaded to the study database.
Health Coaching. STR health coaching encourages self‐monitoring and care aimed at reducing cardiovascular and metabolic risk in older adults. Participants meet with a study Medical Advisor every 6 months to review blood pressure, weight, and blood laboratory results, and to recalibrate goals for improving health status.
3.3. Study population
U.S. POINTER targeted enrollment of 2000 older adults without significant cognitive impairment who largely represent the U.S. demographics with regard to (self‐reported) gender, race and ethnicity, and region. Participants were recruited through the electronic health records (EHRs) and grassroots outreach in the community. Extensive resources were invested to reach underserved communities to ensure their representation in the trial with culturally appropriate recruitment materials, and by leveraging established and new community partnerships and targeted outreach tactics. Key partners for grassroots recruitment efforts included leaders of Black Church communities at the NC and RI sites, community health clinic providers at the TX and IL sites, the Alpha Kappa Alpha Sorority and Mexican Consulate at the CA site, and local chapters of AARP at multiple sites. Additional details about the recruitment strategy will be presented in a separate publication. From trial conception, U.S. POINTER has prioritized diversity not only in participants but also in investigators and staff, and an environment that promotes equity and inclusion. For example, it is expected that site staff and co‐investigator demographics reflect those of their community, celebration of cultural diversity is a key theme in site team‐building activities, transparent conversations about implicit (or explicit) bias are encouraged, and cultural awareness and sensitivity trainings are provided to ensure inclusiveness in participant interactions.
Eligibility . Sensitivity to detect 2‐year differences in cognitive function depends on inclusion of a cohort at risk for decline. Key inclusion criteria include age 60 to 79 years; not a regular exerciser (as per a modified Telephone Administered Physical Activity questionnaire); MIND diet screener score < 9.5 (indicating poor diet); and two or more of the following: first‐degree family history of memory impairment, African American/Black or Native American race, Hispanic ethnicity, older age (≥ 70 years), and at least mild elevation in systolic blood pressure (BP ≥125 mmHg), low‐density lipoprotein (LDL ≥115 mg/dL) cholesterol, or glycated hemoglobin (HbA1c ≥6.0%). Exclusions include history of significant neurological or psychiatric disorder, recent or current alcohol or substance abuse, regular use of cognition‐altering medications (e.g., narcotics, antipsychotics, medications for Parkinson's or Alzheimer's disease), residence in an assisted living facility or nursing home, and significant cardiovascular, lung, renal or other organ disease, or recent malignancy. In addition, individuals were excluded with significant cognitive impairment as per self‐report, the modified Telephone Interview for Cognitive Status (TICSm < 32), or the Clinical Dementia Rating Scale (CDR > 0.5). If CDR‐Sum of Boxes >1, continued eligibility required lead (central) neuropsychologist approval indicating no cognitive impairment.
3.4. Recruitment and screening
Recruitment was completed using a sequential multi‐step screening process to maximize outreach without overburdening clinic resources (Figure 2). EHR and grassroots strategies moved progressively through the site's catchment area by zip code to identify candidates who could attend intervention Team Meetings within targeted neighborhoods. The three‐phase screening process is described below; additional details are provided in the protocol (Supplement).
FIGURE 2.
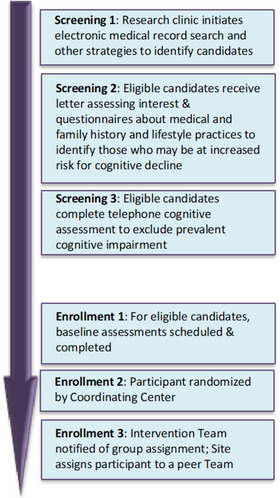
Stages of recruitment and enrollment.
Screening 1: Initial Contact . The EHR of each site's health care network was used to identify study candidates meeting preliminary inclusion criteria in targeted neighborhoods, prioritizing individuals from racial and ethnic minoritized groups. In parallel, widespread national and local multimedia (including social media) was used for outreach together with extensive grassroots efforts that leveraged community partnerships and boots‐on‐the‐ground engagement tactics.
Screening 2: Eligibility Questionnaires . Screening 1 responders were provided with more information about the study, and questionnaires about medical history, physical activity, and diet composition to permit assessment of eligibility.
TABLE 1.
Schedule of events.
| Visit Name/Month | Baseline | Month 6 | Month 12 | Month 18 | Month 24 |
|---|---|---|---|---|---|
| Informed consent for enrollment, HIPAA | X | ||||
| Demographics, brief physical and neurological exam, ECG, medical history | X | ||||
| Unmasked intake interview | X | X | X | X | |
| Medication review, weight measurement | X | X | X | X | X |
| Vital signs, waist circumference | X | X | X | ||
| Fasting clinical blood labs | |||||
| Comprehensive metabolic panel, hemoglobin/hematocrit | X | X | |||
| Glucose, HbA1c, lipid panel | X | X | X | ||
| Non‐fasting clinical blood labs | |||||
| HbA1c, lipid panel | X | X | |||
| Blood Collection for APOE genotyping and DNA extraction and storage | X | ||||
| Fasting blood collection for banking | X | X | X | ||
| AE monitoring at clinic | X | X | X | X | |
| POINTER modified neuropsychological test battery (Free and Cued Selective Reminding Test, Story Recall, Visual Paired Associates, Number Span, Word Fluency, Digit Symbol Substitution Test, Trail‐Making test) | X | X | X | X | X |
| Digital cognition technologies clock drawing test, BrainHQ assessment, MMSE | X | X | X | X | X |
| C‐3: Cogstate detection, identification, one back, one‐card learning, face name associative memory exam, behavioral pattern separation of objects | X | X | X | ||
| CDR, IADL, ECog, SF‐36, EQ5D, CFI | X | X | X | ||
| Sleep questionnaire | X | X | X | X | X |
| Lifestyle questionnaires (physical activity, sitting habits, rush food frequency, cognitive activity), sleep questionnaire, GDS | X | X | X | X | X |
| 400 Meter Walk Test, SPPB | X | X | X | ||
| Randomization | X | ||||
| Obtain participant feedback and exit interview | X |
Abbreviations. AE, adverse event; APOE, apolipoprotein E, C‐3: Cogstate Battery; CDR, Clinical Dementia Rating, CFI: Cognitive Function Index; DNA, deoxyribonucleic acid; ECG, electrocardiography; ECog, Everyday Cognition; GDS, Geriatric Depression Scale; HbA1c, glycated hemoglobin; HIPAA, Health Information Portability and Accountability Act; IADL, independent activities of daily living; MMSE, Mini‐Mental Status Exam; SF‐36 and EQ5D, quality of life scales; SPPB, Short Physical Performance Battery.
Screening 3: Telephone Cognitive Screening . Screening 2–eligible candidates completed the TICSm to exclude low performers (i.e., score <32) with cognitive impairment. The TICSm assesses orientation, attention, language, word recall, and abstraction. The screening test has been validated for administration to older adults, correlates highly with other measures of global cognitive function, and has excellent sensitivity and specificity for differentiating older adults with and without dementia and for identifying mild cognitive impairment (MCI). 31 TICSm scores were adjusted to minimize bias against subgroups using (1) published algorithms 32 and (2) the results of percentile regression analyses on pooled scores for 4000 adults (60–79 years) from three large Wake Forest–coordinated studies (WHIMS‐ECHO, COSMOS‐Mind, WHIMSY). TICSm adjustments applied include: +1 point if age ≥75 years; +4 points if education < high school; +3 points if Native American or Alaska Native, Asian or Asian American, Native Hawaiian or Pacific Islander, Black or African American, North African or Middle Eastern, Hispanic or Latin); maximum adjustment was +5 points and total adjusted TICSm score was capped at 50 points. To reduce waiting time between baseline and intervention initiation, participants were advanced to the baseline assessment in batches based on availability to join the next set of planned SG and STR Team Meetings based on time, date, and location.
3.5. Clinic study visits
Eligible candidates following Screening 3 continued to the baseline visit. The schedule of events for each clinic visit is provided in Table 1 and summarized below. Participants are compensated $75 for completion of baseline and annual clinic assessments, and $25 for the month 6 and 18 assessments.
Baseline . Baseline procedures included informed consent, physical and neurological exams, fasting blood collection for clinical labs and banking in the study repository, apolipoprotein E (APOE) genotyping, outcomes assessments, completion of questionnaires by participants and study partners (when available), functional assessments (Short Physical Performance Battery, 400 meter walk), and randomization. Following randomization, participants signed a behavioral contract to confirm their willingness to participate in the assigned intervention.
Months 6, 12, 18, 24 . Each follow‐up visit starts with an intake interview by unmasked (to group assignment) personnel to review intervention progress and medications, and to query change in medical status, medical procedures, and adverse events (includes hospitalizations). Outcomes assessments to collect cognitive and functional outcomes and physical measurements at each visit are administered and data‐entered by personnel masked to intervention assignment. Fasting blood is collected for clinical labs and banking in the repository at months 12 and 24, and under non‐fasting conditions for clinical labs at months 6 and 18. At every visit, participants receive clinical laboratory and health metric results (e.g., blood pressure, weight). At month 24, clinical personnel meet with participants to review their study achievements and 2‐year clinical laboratory findings, obtain feedback about their experiences, and discuss individual plans to continue U.S. POINTER lifestyle activities post‐intervention.
3.6. Outcomes assessments
Cognitive Outcomes . Primary, secondary, and exploratory cognitive outcomes are listed in Table 2. The primary outcome is a composite measure of global cognition (referred to as the POINTER modified Neuropsychological Test Battery [PmNTB]) comprising averaged and equally weighted composites of memory, executive function, and processing speed derived from pre‐specified scores of the test battery administered during clinic visits. The PmNTB was modeled on the FINGER outcome and includes additional contemporary tests with documented sensitivity to early cognitive changes in older adults 33 , 34 and that will allow harmonization with other large trials (e.g., A4, AHEAD 3‐45, EXERT). Specifically, the PmNTB includes a longer word list (Free and Cued Selective Reminding Test) and additional tests of executive function (number sequencing, phonemic fluency), and excludes the Boston Naming Test (includes culturally inappropriate and biased stimuli 35 ) and the Stroop Test (standardized test administration can be challenging as per study team experience). By combining individual test scores, the composite is expected to provide greater statistical power than individual tests. Alternate forms of tests most susceptible to practice effects (Free and Cued Selective Reminding Test, Story Recall, Visual Paired Associates, Digit Symbol Substitution Test) are administered in the order of A‐B‐C‐A‐B across visits. Additional tests are administered every 6 months (Digital Clock Drawing, BrainHQ Assessment, Mini‐Mental Status Exam) or annually (C‐3 Cogstate battery). Key secondary outcomes include domain‐specific composite scores for memory, executive function, and processing speed. Additional details about each cognitive test are provided in the protocol (Supplement).
TABLE 2.
Cognitive outcomes.
| Cognitive outcomes | Tests |
|---|---|
| Primary Global Composite Score (POINTER modified Neuropsychological Test Battery, PmNTB) | Memory: Free & Cued Selective Reminding Test; Immediate & Delayed Story Recall; Immediate & Delayed Visual Paired Associates |
| Executive Function/Processing Speed: Number Span Forward, Backward, Sequencing; Word Fluency by Letter (F, A, S); Word Fluency by Category (Animals, Vegetables, Fruits); Digit Symbol Substitution Test; Trail Making Parts A & B | |
| Secondary Domain‐Specific Composite Scores | Memory: Free & Cued Selective Reminding Test; Story Recall; Visual Paired Associates; Cogstate One‐Card Learning, Face Name Associative Memory Exam & Behavioral Pattern Separation of Objects |
| Executive Function: Number Span; Word Fluency; Digit Symbol Substitution; Trail‐Making Part B; Digital Clock Drawing Test (planning/organization); Cogstate One‐Back | |
| Processing Speed: Trail‐Making Part A; Digit Symbol Substitution; Digital Clock Drawing (speed/efficiency); Cogstate Detection & Identification | |
| Exploratory Measures | Clinical Dementia Rating Scale—Sum of Boxes |
| Mini‐Mental Status Exam | |
| C‐3 (Cogstate battery composite score) | |
| Digital Clock Drawing Test (composite score) | |
| BrainHQ Assessment (for target engagement) |
Clinical and Behavioral Outcomes . Assessments of clinical status and behavioral function include the CDR and self‐report questionnaires about cognitive concerns, everyday cognition, activities of daily living, mood, sleep quality, lifestyle exposures (physical and cognitive activity, sitting habits, diet composition and vitamin intake via food frequency questionnaire), physical function and frailty, and perceived quality of life. The CDR is administered to the participant and the study partner. Identification of a study partner is strongly encouraged but not required in order to reduce barriers to participation. When a study partner is not available, the study clinician provides input that is considered in the score. For these cases, the site neuropsychologist reviews source documents to confirm that scoring was appropriate. Specific instruments and assessment frequency are listed in Table 1; additional details are provided in the protocol (Supplement).
3.7. Masking
All site investigators and clinic staff are masked to intervention assignment except for the study clinician (oversees medical safety and adverse events [AEs]) and intervention delivery personnel who are masked to outcomes data. In the CC, ACOCC investigators and staff are masked to intervention assignment and outcomes with two exceptions: outcomes oversight personnel are unmasked to outcomes, and intervention oversight personnel are unmasked to group assignment. DCC investigators and staff are unmasked to outcomes and group assignment. Masked personnel have restricted access to segments of the study database and are excluded from discussions that disclose masked information. In the clinic, participants are reminded by unmasked personnel to “keep the secret” and refrain from sharing information about their assigned group with other personnel. In the event of unmasking of the examiner or others involved in outcomes assessment or data entry, an alternate plan for data collection at future clinic visits is deployed to ensure continued high data fidelity.
3.8. Adaptations during COVID‐19
In response to coronavirus disease 2019 (COVID‐19) social distancing mandates in the United States, in‐person activities (clinic visits, Team Meetings, exercise at community facilities) were paused from March 2020 to July 2020. During the pause, sites maintained regular contact with participants to encourage continued participation in intervention activities and to provide support as needed. When study activities restarted on July 13, 2020, Team Meetings were conducted via web conferencing (participants were provided with devices as needed). In‐person Team Meetings were resumed in September 2021.
3.9. Safety monitoring and reporting
Participants were required to receive primary care provider (PCP) authorization to enroll and are monitored for safety while in the study. At baseline, participants receive physical and neurological exams, clinical laboratory tests, and resting electrocardiography (ECG). Participants with clinically significant findings are referred for PCP clearance before enrollment. The study clinician reviews, evaluates, and reports all AEs and serious adverse events (SAEs) to the Safety Committee. For U.S. POINTER, AEs are defined as clinically relevant unfavorable or unintended health events that occur during intervention delivery, intervention‐related activities, or outcomes assessments. As defined per Federal Regulation, SAEs include death, a life‐threatening adverse experience, inpatient hospitalization, or persistent or significant disability/incapacity. Potential AEs are described to participants during the informed consent process, queried and documented during clinic visits, and can be reported by participants between visits during telephone contacts and Team Meetings. An external Data and Safety Monitoring Board (DSMB) monitors and reviews participant safety on an ongoing basis and makes recommendations regarding trial conduct.
3.10. Intervention monitoring and reporting
Intervention fidelity is guided by recommendations of the National Institutes of Health (NIH) Behavior Change Consortium 36 and is assessed on its delivery, receipt, and enactment. Prior to participant contact, Interventionists and Navigators are trained on intervention protocol delivery by the IOC and then certified following central review and approval of a submitted video recording of a facilitated mock Team Meeting. Interventionists and Navigators are re‐certified annually by the IOC through in‐person site visits that include live observation of Team Meetings to ensure consistent inter‐site intervention delivery, to enhance quality of Interventionist and Navigator skillsets, and to confirm participants’ ability to receive, understand, and integrate intervention information for sustained behavior change. Team Meeting attendance (both groups) and STR adherence metrics for each intervention domain (self‐report logs, Fitbit data, BrainHQ data) are captured dynamically in the study's web‐based tracking system via nightly downloads through vendor‐specific application programming interfaces (APIs). Data are summarized in reports and reviewed by the IOC; challenges are addressed during monthly meetings with each site.
3.11. Cognitive outcomes monitoring and reporting
The CC has primary responsibility for the training of examiners and monitoring data quality, alongside site study neuropsychologists. The initial certification process is extensive, and re‐certification is required at 6 months and annually thereafter. Monitoring includes central review of audio and source documents for 10% of all administrations, and site neuropsychologist review of audio and source documents for 10% of administrations at the site. Once per month, assessments to be monitored are centrally selected at random, and stratified by examiner. In addition, 50% of source documents are checked by peer examiners.
3.12. Data monitoring and management
The DCC oversees data collection and standardization, data management, data transfer, and quality control analyses. The sites use a secure password‐protected website to enter data collected during contacts and maintain appropriate medical and research records, in compliance with regulatory and institutional requirements and guidelines for protecting participant confidentiality. The study website also includes a fully integrated data management system with tools to track, monitor, and schedule study activities in real‐time. Study data are regularly monitored, reviewed, and evaluated using several approaches, including central review of source documents by the ACOCC and the IOC, and by the DCC with logic checks of database entries to enhance data accuracy, completeness, and consistency.
3.13. Power and sample size determination
The targeted enrollment of N = 2000 participants was chosen to provide a minimum of 85% power for its primary inference. The goal of 85% power, rather than the more stringent goal of 90% power, was chosen because U.S. POINTER is being conducted in the context of several other similar trials in the Worldwide FINGER network, including FINGER. Thus, U.S. POINTER will not be viewed as a stand‐alone assessment of a multidomain intervention for which a high independent degree of evidence is required, but as an important contributor to a broader assessment of efficacy. The choice to target 85%, rather than 90%, power also conserves resources and provides a more efficient trial. Power is based on the primary outcome and does not consider secondary cognitive outcomes which, per protocol, will be reported using 95% confidence intervals (CIs) rather than inference.
To project statistical power, longitudinal sequences of cognitive test scores based on data from the Women's Health Initiative Study of Cognitive Aging (WHISCA) trial 37 were simulated using their longitudinal covariance of the WHISCA composite outcome from its battery of test scores. Although different from the test battery that U.S. POINTER uses, the WHISCA battery was expected to provide benchmark covariances that, as from potentially less precise measures, may lead to power estimates that are lower (i.e., more conservative) than what will be achieved by U.S. POINTER. Databases of N = 2000 longitudinal sequences of data corresponding to the WHISCA data points were simulated from a multi‐normal distribution. Missing data were randomly culled to accumulate at a rate of 2.5% per 6 months. Inference was based on a two‐tailed Wald test (type 1 error set to be 0.05) of slopes from a random effects linear model, as specified in the statistical analysis plan for the trial.
The FINGER trial observed an intervention effect of 0.03 SD per year for its composite cognitive outcome, 10 which we applied to our simulated databases. This effect is projected to be both cost‐effective 38 and to correspond to clinically important benefits in related outcomes. 39 It also corresponds to the effect seen in the Cocoa Supplement and Multivitamin Outcomes Study of the Mind trial (COSMOS‐Mind), which was linked to significant benefits on cognitive aging. 40
This approach projected a statistical power of 89.7% for the targeted intervention, with N = 2000 randomized participants allocated equally between the two arms of the trial. We project 83.7% power for an intervention effect of 0.0275 SD per year and 93.9% power for an intervention effect of 0.325 SD per year. One interim power projection is specified in the U.S. POINTER protocol to re‐assess power using observed on‐trial longitudinal covariances and attrition rates once sufficient data have been acquired to estimate these cohort characteristics. This interim analysis will make no use of (and be masked from) any outcome data by arm.
3.14. Data analysis
The primary efficacy analysis of the global cognition composite will include all randomized participants using the intent‐to‐treat approach. To construct the composite, (1) scores for each constituent test are converted to z‐scores by dividing the differences between individual scores from the cohort‐wide mean at baseline by the cohort‐wide standard deviation at baseline, (2) z‐scores are transformed so that positive scores reflect better performance, (3) z‐scores are averaged by cognitive domain provided that ≥50% of the scores per domain are available (otherwise, domain score is missing), and (4) the mean z‐score across cognitive domains is re‐normalized by subtracting it from the cohort‐wide mean at baseline and dividing this difference by the cohort‐wide SD at baseline. Secondary domain‐specific composite outcomes will be analyzed using a similar approach. Multiple imputation will be used to allow C‐3 scores (Cogstate battery, which is not administered at months 6 and 18) to contribute to secondary composite outcomes throughout follow‐up. Differences in intervention response by APOE genotype and other ADRD risk factors (e.g., family history of memory impairment) will be assessed.
Inference is based on a random effects linear model, 41 with the dependent variable consisting of composite scores measured from baseline through the 24‐month assessment. Covariates include site (sole stratification factor) and clinic visit by age interaction to control for potentially nonlinear factors (e.g., learning effects; different outcomes assessors) that may systematically affect both intervention groups and vary by age. The fixed effects are intervention assignment and its interaction with follow‐up time as a continuous variable. Models will be fitted with restricted maximum likelihood to adjust for baseline differences among participants. Longitudinal correlations between measures collected over time within individual participants will be parameterized using an unstructured model. If this model results in non‐convergence, a first‐order autocorrelation model will be used instead. The significance of the intervention will be determined based on a Wald test for the interaction between intervention assignment and time from randomization. Limiting the number of covariates is recommended for clinical trials of U.S. POINTER's size. 42 Addressing differences among individuals through restricted maximum likelihood rather than models for random effects does not require assumptions about their distribution and treats differences as nuisance parameters.
An overview of AEs, including the number and percentage of participants who died, had SAEs, and discontinued due to AEs, will be provided. A comparison between intervention arms will be performed using Fisher's exact tests. Summaries of AEs by system organ class will be provided for (1) pre‐existing conditions, (2) possibly or probably related AEs, and (3) SAEs.
3.15. NIH‐Funded ancillary studies to U.S. POINTER
Four ancillary studies leverage rich main‐trial resources for cost‐efficiency to significantly increase the breadth and depth of scientific investigation and impact.
POINTER‐Neuroimaging (NIH/NIA [National Institute on Aging] 1R01AG062689; PI: Landau). This ancillary study examines treatment effects on pathophysiology related to AD and cerebrovascular disease in more than 1000 parent trial participants using (1) positron emission tomography (PET) to measure amyloid beta and tau burden at baseline and month 24, and (2) magnetic resonance imaging (MRI) to measure brain morphometry, white matter hyperintensities and microstructural integrity, and cerebral blood flow at baseline and months 12 and 24. The study will also examine the roles of these PET and MRI markers at baseline in cognitive response to treatment.
POINTER‐Neurovascular (NIH/NIA 1R01AG066910; PIs: Brinkley, Shaltout). This ancillary study examines treatment effects on cerebral blood flow regulation, which plays an important role in the pathophysiology of brain aging and renders the brain more susceptible to damaging effects of comorbid vascular conditions and cognitive decline. The study assesses aortic, carotid, and cerebral hemodynamics at baseline and months 12 and 24 in more than 450 parent trial participants, and their associations with cognition and outcomes obtained through other ancillary studies (e.g., imaging).
POINTER‐zzz (NIH/NIA 5R01AG064440; PIs: Hayden, Baker). This ancillary study conducts in‐home assessments to examine treatment effects on objective measures of sleep quality in more than 750 parent trial participants. At baseline and months 12 and 24, sleep‐disordered breathing is measured using oximetry, and other sleep exposures are measured using actigraphy (e.g., duration, fragmentation, total activity). POINTER‐zzz will examine intervention effects on sleep as they relate to cognition and outcomes collected through other ancillary studies.
POINTER‐Microbiome (NIH/NIA U19AG063744 [PI: Kaddurah‐Daouk, Knight, Mazmanian] Project 2; PIs: Keshavarzian, Craft; POINTER ancillary study; PIs: Baker, Espeland). This ancillary study examines gut microbiome composition and function, and blood and fecal metabolomes as they relate to pre‐defined AD metabolic signatures, cognitive function, brain imaging structure and function, and other ancillary study and parent trial outcomes. Metagenomic and metabolomic technologies are used to profile in‐home collected fecal samples and stored plasma samples from more than 750 parent trial participants at baseline and month 24.
4. DISCUSSION
A key goal of U.S. POINTER is to confirm and expand the FINGER study findings in a diverse and representative cohort of older American adults who differ in their demographics, lifestyle, culture, and health status relative to older Finnish adults. A second key goal is to adapt the multidomain intervention to American culture in a community‐based setting that involves strategic community partnerships to promote adherence and sustainability. U.S. POINTER leveraged lessons learned from FINGER and other similar studies to strengthen the design, relied on contemporary methods of recruitment science to reach and enroll a representative cohort, and added ancillary studies to maximize overall scientific impact.
Achieving appropriate representation of the U.S. population with regard to race, ethnicity, education, older age, gender, and general geographic regions was a priority for the study. Recruitment through the EHR prioritized contact of individuals from traditionally underrepresented groups with targeted search criteria and the use of culturally responsive recruitment mailings. The study team also developed and deployed an extensive grassroots outreach initiative tailored to leverage strengths at each site with regard to expertise, trusted community networks, and established collaborations with key community ambassadors to engage and recruit participants who may be unreachable through the EHR. U.S. POINTER interventions were designed to “meet people where they are,” and to learn from participants from different cultures who may have different values, needs, resource access, and life challenges. The intervention educational content is iteratively adapted to be responsive to these differences, which is essential for sustainability of the intervention, and ultimately, generalizability of trial results.
U.S. POINTER eligibility criteria were designed to enrich the cohort for cognitive decline risk, including CVD indicated by mild elevations in systolic BP, LDL cholesterol, or HbA1c. Data collected from our 2000‐person at‐risk and representative cohort will provide a rich resource to examine lifestyle components that have already been shown to reduce CVD and diabetes risk (exercise, diet, health monitoring). 43 , 44 In the United States, health disparities in CVD risk factors (e.g., hypertension, diabetes, obesity, poor diet, reduced access to preventive health care) known to contribute to the substantially higher risk of adverse health outcomes in Black and Hispanic Americans compared to white Americans may also contribute to the higher prevalence of ADRD in these groups. 45 The large, well‐characterized U.S. POINTER cohort is enriched for AD and CVD risk and thus will provide an unprecedented data resource of outcomes spanning cognition, physical function, specimen and brain imaging AD biomarkers, sleep quality, peripheral‐ and neurovascular function, and the gut microbiome.
Developing effective, translatable, and scalable strategies to promote and sustain participant adherence and intervention delivery is integral to the study's concept and design. Intervention delivery in U.S. POINTER relies on a range of established strategies guided by key tenets of social cognitive and self‐determination theories to motivate and support sustainable behavior change. Behavioral changes required to ensure adherence to the study interventions are fostered through intrinsic motivation, belief that change is possible, means to implement behavioral changes, and ongoing support and reinforcement. Long‐term behavioral change is fundamental to achieving this goal and requires a hefty investment by the individual and ongoing genuine support by the community. It is important to note that U.S. POINTER—from inception and design to delivery—has progressed as a partnership that included academia, associated health care systems, and the Alzheimer's Association, with critical guidance and support from multiple community organizations and advisors to ensure the adaptability and sustainability of a community‐based intervention program.
Several other lifestyle intervention trials are testing various implementation strategies in cohorts with different risk profiles (reviewed 46 ). 7 , 8 , 9 , 10 , 11 , 12 U.S. POINTER differs from other studies most notably in its “high touch” approach during recruitment (i.e., grassroots outreach) and intervention delivery to engage and retain underserved communities and provide essential support of a cohort at high risk for cognitive decline. U.S. POINTER includes weekly (first 4 months) and then monthly accountability for adherence, and leverages peer support to encourage behavior change. The large U.S. POINTER cohort is enriched for risk based on multiple factors, which may increase the power to detect a protective effect and provides numerous outcomes through the parent trial and its four ancillary studies. Although a more diverse cohort may yield different results than in other trials, AD risk is higher for racially and ethnically minoritized communities and therefore these groups critically need to be included in these and other studies to better understand the impact. The COVID‐19 pandemic may also affect the consistency of U.S. POINTER findings relative to previous reports. The study was in full swing as of March 2020, when the pandemic transitioned to an American health crisis, and in response, study procedures were temporarily modified to reduce person‐to‐person contact. Preliminary findings, however, suggest that U.S. POINTER may have fared well as retention rates remained high (98%) even after a 4‐month study pause, 47 and intervention adherence remained high (> 80%) while Team Meetings were temporarily held using a virtual format. 48
U.S. POINTER is one of several lifestyle intervention studies of a collaborative international network referred to as World‐Wide FINGERS. 13 Within the network, study teams collaborate to share protocols to harmonize methods, outcomes, and data analyses. 49 The U.S. POINTER protocol has been shared with several countries, and was largely adopted by the Latin American (LatAm‐FINGERS) team, which allows for extensive data harmonization. 50 Considering the diversity of cultures and a vast array of demographic and other population characteristics across the globe (socioeconomic, access to health resources and care, environment, safety, etc.), rigid prescriptive lifestyle interventions to protect brain health may not show comparable efficacy for all people. International cross‐cultural comparisons in study design, outcomes, intervention adoption, and cognitive response are essential to inform the development of trials that best incorporate appropriate practices and priorities while supporting healthier lifestyle exposures for cognition in older adults. We anticipate that the findings from U.S. POINTER and other World‐Wide FINGERS studies could have tremendous impact for hundreds of millions of at‐risk individuals around the world.
From a public health perspective, U.S. POINTER's multidomain intervention approach is modeled on standard health care recommendations, which may ultimately facilitate adoption in clinical care. Engaging older Americans where they live, work, and play will be essential if we are to identify an accessible and sustainable healthy lifestyle intervention to protect cognitive function and prevent ADRD in the diverse communities that are integral to the fabric of our U.S. population.
AUTHOR CONTRIBUTIONS
Clinical sites . North Carolina – Wake Forest University School of Medicine: Jo Cleveland, MD (PI); Jeff D. Williamson, MD, MHS (Co‐PI); Leslie Teague, MS; Justin Johnson, MS; Mackenzie Anderson; Philicia Armstrong, MSW; Derrick Barnes, Margaret Brown; Sarah Brown; Elizabeth Chmelo, MS; Tiffany Cummings, PsyD (deceased); Elizabeth Dahl; Carlo Davids, MS; Rebecca Davis; Abbie Eaton, PhD, MMS; Mary Ellenburg; Ana Glover; Anallely Hernandez Laguna; Carolyn Higgins; Christiana Higgs, MS; Benjamin Hutchison; Ansley Jewell; Rachel Kiger; Michelle Lewis; Kristy Lievense; Marie Liquete, MS; Kristi Long; Beth Lovette; Brittney McDermott; Melisa Ramirez‐Pineda; Andrea Rivis, MS; Cristian Rivis; Sam Rogers, PA‐C; Bonnie Sachs, PhD; Krissi Shook; Lindsay Tysinger; Eileen Weston; Malcom Williams; Kelvin L. Williams, PhD, D.Min; Dixie Yow; Ezequiel Zamora, MD; Christine Zecca. Northern California – University of California, Davis: Rachel A. Whitmer, PhD; Sarah Tomaszewski Farias, PhD; Oanh Meyer, PhD; Anna Garzon, MHA; Kellie Holley; Hollie Adams; Raquel Alto; Ashley Balley, MPH; Carmen Benavides; Jennifer Cartan, MA; Casey Castro; Manesy Ceja Cevallos; Michelle Chan, PhD; Carolina Chernyetsky; Evelyn Cordero; Fawn Cothran; Jennifer DeGuzman; Mayra Diaz; Jessica Famula; Alice Fisher; Marisela Flores; Martha Forloines, PhD; Jessica Geltz; Parvaneh Gerami, FNP; Jessica Holscher; Jorge Hurtado; Emily Kostner; Elisa Lee; Edward Lingayo Jr; Tyler McConnell; Macaria Mendoza, MS; Tara Miskovich; Elias Ortiz; Amber Pippins; Magaly Quinteros; Roberto Ramos; Sarina Rodriguez; Ashley Romo; Lindsay Ruiz Graham; Sandra Ruiz‐White; Vanessa Sanchez; Ellen Thomas; Erica Tutuwan; Itzel Vargas; Hillary Vossler; Kelly Wallace; Derron Yu. Chicagoland – Rush School of Medicine: Christy C. Tangney, PhD; Martha Clare Morris, ScD; Neelum Aggarwal, MD; Meera Sotor, MPH; Kristie Miller; Elizabeth Arrvizu; Nancy Barrett; Patricia Butler; Melanie Chavin, MS; Miriam De la Torre; Mindy Dershem, MS; Pankaja Desai, PhD; Sarah Ehlers; Tiffini Funches; Jazmin Garcia; Crystal Glover, PhD; Sarah Graef, DC; Thomas M. Holland, MD; Leah Johnston; Courtney Jost; Kaitlin Koncilja; Kristin Krueger, PhD; Heidi Langdon, MS; Brittany Mabry; Mercedes Maceyras; Daniel Madock; Mia McClintic; Miguel Montero; Melissa Morales‐Perez; Brooke Nanni, MPH; Radhika Patel; Tanvi Polavarapu; Cristina Quiroz; Nancy Rainwater, MBA; Terrianne Reynolds, MPH; Chartay Robinson; Nikita Sawlani; Lisa Tam; Jennifer Ventrelle, MS; Genesis Villalobos; Michelle Villanueva. Chicagoland – Advocate Health: Darren R. Gitelman, MD; Masun Jackson, MS; Zeyad Alaswad, MD; Claudia Amador; Carlos Corado; Maha Haroon; Elizabeth Hartman, PhD; Megon Holldorf; Margaret Konieczny, MSN; Chauncey Lawson‐Weinert; Viet Le; Grace Lucenti; Anthony McCormack, MD; Elizabeth Omotoye, MPH; Olivia Preissle; Tawny Pyszka; Mary Schmidt; Samuel Streeter; Evelyn Torres; Sherri Velez; Kathryn Waitzman, MEd. Houston – Baylor College of Medicine and Kelsey Research Foundation: Valory N. Pavlik, PhD; Melissa M. Yu, MD; Ashley S. Alexander, MHSA; Michele York, PhD; Sydney O'Connor, MA; Rose Trevino‐Whitaker, MPH; Ruchi Aggarwal, MD; Sayo Awosika‐Olumo, MD, PhD; Talitha Baszile; Jeffrey Bishop, PA‐C; Regan Brooks; Maricela Caceres; Vanessa Cardenas; Maria Chaudhary, MS; Valerie Coffman; Jackielynn Cruz; Edina Dervisefendic; Anna Duron; Jacob Faircloth; Jennifer Garrett; Denise Gibson; Cesar Gonzalez; Latrel Grant; Beck Hill; Kristin Ijeh, MS; Nallely Infante; Chi‐Ying Lin, MD, MPH; Elizabeth Lipscomb, RN; Leah Logan; Erica Lonquich; Patricia Lynch; Bobby Marker; Crystal Martinez‐Busarow; Milena Olivar; Nat Pacini, MA; Arely Perez, MS; Courtney Rice, DNP; Monica Rodriguear, MA; Demetrio Selman; Kaila Sevilla; Akash Shah; Hannah Shields; Raven Small; Jackie Soto; Juan Toledo, MD, PhD; John Valenta, PhD; Shayla Yonce. New England‐Rhode Island – Butler Hospital and Miriam Hospital: Stephen Salloway, MD; Stephen Correia, PhD; Rena R. Wing, PhD; Meghan Riddle, MD; Tyler Rosenholm; Samuel Slezak, MS; Kathryn Demos, PhD; KayLoni Olson, PhD; Kelsey Adams; Roseurys Almonte Nova; Nicole Amichetti; Kirsten Annis; Kathryn Beaulieu; Sarah Benjamin, MS; Sarah Bica; Joni Bloom; Courtney Bodge, PhD; Gregory Brunson; Brian Castelluccio, PhD; Monique Coley, PA; Karleen Coppola; Ashleigh Cregan; Katherine Daneault; Linda Davidge; Brittany Dawson, MS; Sarah Deforest; Lyndsay DeMatteo, MSN, Elizabeth DiGregorio; Caitlin Egan, MS; Sara Eksuzian; Sheina Emrani, PhD; Melanie Faust, MS, FNP‐C; Joslynn Faustino; Angel Garcia; Daisy Garcia; Tiffany Giampa; Brooke Huemann; Shauna Hyde, MS; Athena Lavoie; Athene Lee, PhD; Heather Maloney; Bill Menard; Andrea Nanos, MSN; Greg Pappas; Bryanne Peets; Dominique Popescu; Erin Poyant; Ariana Rafanelli; Vivian Ramos; Eliza Rego; Corinne Roma; Lorrance Saraiva; Sydney Saunders; Tara Tang; Gina Tonini; Priscilla Villa, MS; James Weir.
Coordinating Center . Administrative and Clinical Operations Coordination Center: Laura D. Baker, PhD; Nancy Woolard; Sharon Wilmoth; Amber Adkins Thro; Rebecca Badgio, MS; Brad Caudle; Taylor Dannemiller; Leslie Gordineer; Allison Heinrich, MS; Marcus A. Hill, MPH; Cara Johnson, MA; Jeffrey Katula, PhD; Katelyn King; Desiree Lopez, MSW; Kate Papp, PhD; Margaret Scales, MA; Wilson Sommerville, PhD; Benjamin J. Williams, MD, PhD. Data Coordination Center: Mark A. Espeland, PhD; Iris Leng, MD, PhD; Laura Lovato, MS; Julia Spell; Scott Rushing; Debbie Felton; Megan Adkisson; Julissa Almonte; Bobby Amoroso; Ryan Barnard, MS; Daniel Beavers, PhD; Shyh‐Huei Chen, PhD; Danielle Cunio; Yitbarek Demesie, MS; Katie Garcia, MS; Sarah Gaussoin, MS; KaShawna Guy, MPH; Darrin Harris; Marjorie Howard, MS; Christopher McLouth, PhD; John Nichols; Jing Su, PhD; Jennifer Talton, MS; Jennifer Walker; Jack White; James Willard, MAS.
Alzheimer's Association. Maria C. Carrillo, PhD; Heather M. Snyder, PhD; Susan Antkowiak; Claire E. Day; Richard Elbein; Ann Marie McDonald, MBA, MEd; Terrianne Reynolds, MPH; Carl Hill, PhD; Courtney Kloske, PhD; Katherine L. Lambert; Heidi Langdon, MS; Olivia Matongo; Emily Meyers, PhD; Joanne Pike, DrPh; April Ross, PhD; Rebecca Edelmayer, PhD.
Karolinska Institute. Miia Kivipelto, MD, PhD; Tiia Ngandu, MD, PhD; Alina Solomon, MD, PhD.
University of Southern California, Alzheimer's Therapeutic Research Institute. Robert Rissman, PhD; Sara Abdel‐Latif; Louise Monte, MS; Rema Ramen, PhD.
Vanderbilt University Medical Center. Consuela Wilkins, MD; Tiffany Israel, MSSW.
University of Wisconsin – Madison. Gina Green Harris, MS, PhD.
Posit Science Corporation. Mouna Attarha, PhD; Henry W. Mahncke, PhD.
Concept and design: Baker, Snyder, Espeland, Whitmer, Kivipelto, Woolard, Katula, Papp, Ventrelle, Graef, Hill, Rushing, Spell, Lovato, Felton, Williams, Ghadimi Nouran, Raman, Ngandu, Solomon, Wilmoth, Cleveland, Williamson, Lambert, Tomaszewski Farias, Day, Tangney, Gitelman, Matongo, Reynolds, Pavlik, Yu, Alexander, Elbein, McDonald, Salloway, Wing, Antkowiak, Carrillo.
Acquisition, analysis, or interpretation of data: Baker, Snyder, Espeland, Whitmer, Kivipelto, Woolard, Katula, Papp, Ventrelle, Graef, Hill, Rushing, Spell, Lovato, Felton, Williams, Ghadimi Nouran, Raman, Ngandu, Solomon, Wilmoth, Cleveland, Williamson, Lambert, Tomaszewski Farias, Day, Tangney, Gitelman, Matongo, Reynolds, Pavlik, Yu, Alexander, Elbein, McDonald, Salloway, Wing, Antkowiak, Carrillo.
Statistical analysis: N/A.
Manuscript development: Baker, Snyder, Espeland, Whitmer, Kivipelto, Woolard, Katula, Papp, Ventrelle, Graef, Hill, Rushing, Spell, Lovato, Felton, Williams, Ghadimi Nouran, Raman, Ngandu, Solomon, Wilmoth, Cleveland, Williamson, Lambert, Tomaszewski Farias, Day, Tangney, Gitelman, Matongo, Reynolds, Pavlik, Yu, Alexander, Elbein, McDonald, Salloway, Wing, Antkowiak, Carrillo. The authors also wish to thank Elizabeth A. Finch, Rose Li and Associates for their assistance with draft development.
CONFLICT OF INTEREST STATEMENT
U.S. POINTER was conceived and developed in partnership with the Alzheimer's Association. Drs. Snyder and Carrillo, full‐time employees of the Alzheimer's Association, have contributed to the study design, trial oversight, manuscript preparation, and the decision to submit the article for publication. The authors have no conflicts to report. Author disclosures are available in the Supporting Information.
CONSENT STATEMENT
All study participants provided written, informed consent. Study partners provided written consent if questionnaires were completed in the clinic, and verbal consent if by telephone. The study was approved by the single institutional review board of record at Wake Forest University School of Medicine and is conducted in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGEMENTS
U.S. POINTER is supported through funding provided by the Alzheimer's Association (POINTER‐19‐611541). Alzheimer's Association investigators (Snyder, Carrillo) participate as indicated above.
Baker LD, Snyder HM, Espeland MA, et al. Study design and methods: U.S. study to protect brain health through lifestyle intervention to reduce risk (U.S. POINTER). Alzheimer's Dement. 2024;20:769–782. 10.1002/alz.13365
[Correction added on 16 October 2023, after first online publication: The affiliation for Darren R. Gitelman has been corrected.]
REFERENCES
- 1. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the lancet commission. Lancet. 2020;396(10248):413‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. National Academies of Sciences E, Medicine, Health, Medicine D, Board on Health Sciences P, Committee on Preventing D, Cognitive I . In Downey A, Stroud C, Landis S, Leshner AI, eds. Preventing Cognitive Decline and Dementia: A Way Forward. National Academies Press (US); 2017. [PubMed] [Google Scholar]
- 3. Committee on the Public Health Dimensions of Cognitive A, Board on Health Sciences P, Institute of M . The National academies collection: reports funded by National Institutes of Health. In Blazer DG, Yaffe K, Liverman CT, eds. The National academies collection: reports funded by National institutes of health. Cognitive Aging: Progress in Understanding and Opportunities for Action. National Academies Press (US); 2015. [PubMed] [Google Scholar]
- 4. Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population‐based perspective. Alzheimers Dement. 2015;11(6):718‐726. [DOI] [PubMed] [Google Scholar]
- 5. WHO Guidelines Approved by the Guidelines Review Committee . In: Risk Reduction of Cognitive Decline and Dementia: WHO Guidelines. World Health Organization; 2019. [PubMed] [Google Scholar]
- 6. Ball K, Berch DB, Helmers KF, et al. Advanced cognitive training for I, vital elderly study G. Effects of cognitive training interventions with older adults: a randomized controlled trial. JAMA. 2002;288(18):2271‐2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vellas B, Carrie I, Gillette‐Guyonnet S, et al. Mapt study: a multidomain approach for preventing Alzheimer's disease: design and baseline data. J Prev Alzheimers Dis. 2014;1(1):13‐22. [PMC free article] [PubMed] [Google Scholar]
- 8. Richard E, Van den Heuvel E, Moll van Charante EP, et al. Prevention of dementia by intensive vascular care (PreDIVA): a cluster‐randomized trial in progress. Alzheimer Dis Assoc Disord. 2009;23(3):198‐204. [DOI] [PubMed] [Google Scholar]
- 9. Yaffe K, Barnes DE, Rosenberg D, et al. Systematic Multi‐Domain Alzheimer's Risk Reduction Trial (SMARRT): study protocol. J Alzheimers Dis. 2019;70(s1):S207‐S220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at‐risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255‐2263. [DOI] [PubMed] [Google Scholar]
- 11. Zulke A, Luck T, Pabst A, et al. AgeWell.de ‐ study protocol of a pragmatic multi‐center cluster‐randomized controlled prevention trial against cognitive decline in older primary care patients. BMC Geriatr. 2019;19(1):203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim S, McMaster M, Torres S, et al. Protocol for a pragmatic randomised controlled trial of body brain life‐general practice and a lifestyle modification programme to decrease dementia risk exposure in a primary care setting. BMJ Open. 2018;8(3):e019329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kivipelto M, Mangialasche F, Snyder HM, et al. World‐Wide FINGERS network: a global approach to risk reduction and prevention of dementia. Alzheimers Dement. 2020;16(7):1078‐1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bandura A. Self‐Efficacy: The Exercise of Control. Freeman; 1997. [Google Scholar]
- 15. Ryan RM, Deci EL. Self‐Determination Theory: Basic Psychological Needs in Motivation, Development, and Wellness. 1st ed. The Guilford Press; 2018. [Google Scholar]
- 16. Rejeski WJ, Brawley LR, Ambrosius WT, et al. Older adults with chronic disease: benefits of group‐mediated counseling in the promotion of physically active lifestyles. Health Psychol. 2003;22(4):414‐423. [DOI] [PubMed] [Google Scholar]
- 17. Rejeski WJ, Focht BC. Aging and physical disability: on integrating group and individual counseling with the promotion of physical activity. Exerc Sport Sci Rev. 2002;30(4):166‐170. [DOI] [PubMed] [Google Scholar]
- 18. Piercy KL, Troiano RP, Ballard RM, et al. The physical activity guidelines for Americans. JAMA. 2018;320(19):2020‐2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lautenschlager NT, Cox KL, Flicker L, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA. 2008;300(9):1027‐1037. [DOI] [PubMed] [Google Scholar]
- 20. Baker LD, Frank LL, Foster‐Schubert K, et al. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol. 2010;67(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Colcombe SJ, Erickson KI, Scalf PE, et al. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61(11):1166‐1170. [DOI] [PubMed] [Google Scholar]
- 22. Voss MW, Prakash RS, Erickson KI, et al. Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Front Aging Neurosci. 2010;2:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Joseph JA, Shukitt‐Hale B, Willis LM. Grape juice, berries, and walnuts affect brain aging and behavior. J Nutr. 2009;139(9):1813S‐1817S. [DOI] [PubMed] [Google Scholar]
- 24. Morris MC, Tangney CC, Wang Y, et al. MIND diet slows cognitive decline with aging. Alzheimers Dement. 2015;11(9):1015‐1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morris MC, Tangney CC, Wang Y, Sacks FM, Bennett DA, Aggarwal NT. MIND diet associated with reduced incidence of Alzheimer's disease. Alzheimers Dement. 2015;11(9):1007‐1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Scarmeas N, Stern Y, Tang MX, Mayeux R, Luchsinger JA. Mediterranean diet and risk for Alzheimer's disease. Ann Neurol. 2006;59(6):912‐921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Smith PJ, Blumenthal JA, Babyak MA, et al. Effects of the dietary approaches to stop hypertension diet, exercise, and caloric restriction on neurocognition in overweight adults with high blood pressure. Hypertension. 2010;55(6):1331‐1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Devore EE, Kang JH, Breteler MM, Grodstein F. Dietary intakes of berries and flavonoids in relation to cognitive decline. Ann Neurol. 2012;72(1):135‐143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Willis SL, Tennstedt SL, Marsiske M, et al. Long‐term effects of cognitive training on everyday functional outcomes in older adults. JAMA. 2006;296(23):2805‐2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shah TM, Weinborn M, Verdile G, Sohrabi HR, Martins RN. Enhancing cognitive functioning in healthly older adults: a systematic review of the clinical significance of commercially available computerized cognitive training in preventing cognitive decline. Neuropsychol Rev. 2017;27(1):62‐80. [DOI] [PubMed] [Google Scholar]
- 31. Duff K, Shprecher D, Litvan I, Gerstenecker A, Mast B, investigators E. Correcting for demographic variables on the modified telephone interview for cognitive status. Am J Geriatr Psychiatry. 2014;22(12):1438‐1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Knopman DS, Roberts RO, Geda YE. Validation of the telephone interview for cognitive status‐modified in subjects with normal cognition, mild cognitive impairment, or dementia. Neuroepidemiology. 2010;34(1):34‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Papp KV, Rentz DM, Orlovsky I, Sperling RA, Mormino EC. Optimizing the preclinical Alzheimer's cognitive composite with semantic processing: the PACC5. Alzheimers Dement (N Y). 2017;3(4):668‐677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Papp KV, Rofael H, Veroff AE, et al. Sensitivity of the Preclinical Alzheimer's Cognitive Composite (PACC), PACC5, and Repeatable Battery for Neuropsychological Status (RBANS) to amyloid status in preclinical Alzheimer's disease ‐Atabecestat phase 2b/3 EARLY clinical trial. J Prev Alzheimers Dis. 2022;9(2):255‐261. [DOI] [PubMed] [Google Scholar]
- 35. Byrd DA, Rivera Mindt MM, Clark US, et al. Creating an antiracist psychology by addressing professional complicity in psychological assessment. Psychol Assess. 2021;33(3):279‐285. [DOI] [PubMed] [Google Scholar]
- 36. Bellg AJ, Borrelli B, Resnick B, et al. Treatment fidelity workgroup of the NIHBCC. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH behavior change consortium. Health Psychol. 2004;23(5):443‐451. [DOI] [PubMed] [Google Scholar]
- 37. Espeland MA, Brunner RL, Hogan PE, et al. Women's health initiative study of cognitive aging study G. Long‐term effects of conjugated equine estrogen therapies on domain‐specific cognitive function: results from the women's health initiative study of cognitive aging extension. J Am Geriatr Soc. 2010;58(7):1263‐1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wimo A, Handels R, Antikainen R, et al. Dementia prevention: the potential long‐term cost‐effectiveness of the FINGER prevention program. Alzheimers Dement. 2022. [DOI] [PubMed] [Google Scholar]
- 39. Lehtisalo J, Rusanen M, Solomon A, et al. Effect of a multi‐domain lifestyle intervention on cardiovascular risk in older people: the FINGER trial. Eur Heart J. 2022;43(21):2054‐2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Baker LD, Manson JE, Rapp SR, et al. Effects of cocoa extract and a multivitamin on cognitive function: a randomized clinical trial. Alzheimers Dement. 2023;19(4):1308‐1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS System for Mixed Models. SAS Institute, Inc.; 1996. [Google Scholar]
- 42. Grizzle JE. A note on stratifying versus complete random assignment in clinical trials. Control Clin Trials. 1982;3(4):365‐368. [DOI] [PubMed] [Google Scholar]
- 43. Tuomilehto J, Lindstrom J, Eriksson JG, et al. Finnish diabetes prevention study G. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343‐1350. [DOI] [PubMed] [Google Scholar]
- 44. Gaede P, Lund‐Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358(6):580‐591. [DOI] [PubMed] [Google Scholar]
- 45. Alzheimer's Association . Alzheimer's disease facts and figures. Alzheimers Dement. 2022;18(4):700‐789. [DOI] [PubMed] [Google Scholar]
- 46. Bott NT, Hall A, Madero EN, et al. Face‐to‐Face and digital multidomain lifestyle interventions to enhance cognitive reserve and reduce risk of Alzheimer's disease and related dementias: a review of completed and prospective studies. Nutrients. 2019;11(9):2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Baker LD, Espeland MA, Whitmer RA, et al. U.S. POINTER: lessons learned about delivery of a multi‐domain lifestyle intervention during the COVID‐19 pandemic. Alzheimers Dement. 2021;17(Suppl 10):e055289. [Google Scholar]
- 48. Katula JA, Ventrelle J, King K, et al. Adherence to in‐person vs. virtual delivery of a multi‐domain behavioral intervention to prevent cognitive decline: the U.S. POINTER study. Alzheimers Dement: Am J Alzheimer's Dis. 2022;18:e061543. [Google Scholar]
- 49. Rohr S, Kivipelto M, Mangialasche F, Ngandu T, Riedel‐Heller SG. Multidomain interventions for risk reduction and prevention of cognitive decline and dementia: current developments. Curr Opin Psychiatry. 2022;35(4):285‐292. [DOI] [PubMed] [Google Scholar]
- 50. Crivelli L, Calandri IL, Suemoto CK, et al. Latin American Initiative for Lifestyle Intervention to Prevent Cognitive Decline (LatAm‐FINGERS): study design and harmonization. Alzheimers Dement. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information


