Abstract
BACKGROUND
White matter hyperintensities (WMHs) are associated with cognitive decline and progression to mild cognitive impairment (MCI) and dementia. It remains unclear if sex differences influence WMH progression or the relationship between WMH and cognition.
METHODS
Linear mixed models examined the relationship between risk factors, WMHs, and cognition in males and females.
RESULTS
Males exhibited increased WMH progression in occipital, but lower progression in frontal, total, and deep than females. For males, history of hypertension was the strongest contributor, while in females, the vascular composite was the strongest contributor to WMH burden. WMH burden was more strongly associated with decreases in global cognition, executive functioning, memory, and functional activities in females than males.
DISCUSSION
Controlling vascular risk factors may reduce WMH in both males and females. For males, targeting hypertension may be most important to reduce WMHs. The results have implications for therapies/interventions targeting cerebrovascular pathology and subsequent cognitive decline.
Highlights
Hypertension is the main vascular risk factor associated with WMH in males
A combination of vascular risk factors contributes to WMH burden in females
Only small WMH burden differences were observed between sexes
Females’ cognition was more negatively impacted by WMH burden than males
Females with WMHs may have less resilience to future pathology
Keywords: cognition, older adults, sex differences, white matter hyperintensities
1. INTRODUCTION
Evidence for cerebral small vessel disease (CSVD) can appear as white matter hyperintensities (WMHs) observed on T2‐weighted or FLAIR magnetic resonance images (MRI) or as hypointensities on T1‐weighted MRIs. Although WMHs are commonly observed in cognitively unimpaired older adults 1 their presence has been linked to cognitive deterioration in healthy older adults, 2 and increased risk for progression to mild cognitive impairment (MCI) 3 and dementia. 4 CSVD is associated with various vascular risk factors such as hypertension, diabetes, high body mass index (BMI), tobacco smoking, and alcohol consumption. 5 , 6 , 7 , 8 , 9 , 10 These risk factors are known to affect females differently than males. 11 For example, females who are obese and have hypertension are more likely to suffer a stroke than males. 11 Furthermore, females are more likely to be treated for hypertension, but their high blood pressure (BP) is less likely to be managed effectively than males. 11 Although males may have more risk factors early in life, after menopause females may be at a higher risk than males. 12 For example, although males tend to have increased BP early in life, females have a steeper increase in BP that remains higher and often less controlled than males in mid life. 11 , 13 CSVD risk factors exclusive to females include gestational diabetes and preeclampsia. 14
Previously reported sex‐related differences in WMH burden have been inconsistent with some studies reporting greater WMH burden in females, 15 , 16 , 17 , 18 some reporting no sex differences, 10 , 19 while others reporting increased WMH burden in males. 20 In addition, several studies have noted that vascular risk factors such as hypertension are more strongly associated with WMH development in males than females, 15 , 21 , 22 whereas BMI has shown an association with increased WMHs for both males and females 23 and smoking with higher WMHs in females. 15
These sex‐related differences in WMH load have implications for the treatment and mitigation of cognitive decline because many of the underlying CSVD risk factors are potentially treatable or modifiable (e.g., hypertension, BMI, smoking, alcohol consumption, type 2 diabetes). Therefore, identifying which risk factors are most likely to result in the accumulation of WMHs could help reduce their impact on cognition and the subsequent conversion to dementia may be mitigated. Our goals are thus to determine if sex: (1) influences progression in WMH, (2) influences which vascular risk factors are associated with WMH burden, (3) interacts with risk factors to influence WMH burden, and (4) influences the relationship between WMH burden and cognition.
RESEARCH IN CONTEXT
Systematic review: The authors reviewed the literature using traditional sources (e.g., PUBMED). While there have been numerous reports examining the relationship between white matter hyperintensities (WMHs), risk factors, and cognition, few studies have examined sex differences.
Interpretation: Our findings suggest that hypertension drives WMH progression in males whereas WMH progression in females is driven by a combination of many risk factors (e.g., hypertension, smoking, diabetes). Even when presented with the same amount of WMH as males, females exhibit more cognitive change associated with WMHs than males.
Future directions: While small WMH burden differences were observed between sexes, females’ cognition was more negatively impacted by the same amount of WMH burden as males. These findings suggest that females may have less resilience to future dementia‐related pathology. Future interventions should target hypertension in males but many risk factors in female to help reduce cognitive decline and progression to dementia.
2. METHODS
2.1. Alzheimer's disease neuroimaging initiative
Data used in the preparation of this article were obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). The ADNI was launched in 2003 as a public‐private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial MRI, positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of MCI and early AD. The study received ethical approval from the review boards of all participating institutions. Written informed consent was obtained from participants or their study partner. Participants were selected only from all ADNI Cohorts (ADNI‐1, ADNI‐GO, ADNI‐2, and ADNI‐3).
2.2. Participants
Full participant inclusion and exclusion criteria are available at www.adni‐info.org. All participants were between the ages of 55 and 90 at baseline, with no evidence of depression. Cognitively healthy older adults exhibited no evidence of memory decline, as measured by the Wechsler Memory Scale and no evidence of impaired global cognition as measured by the Mini Mental Status Examination (MMSE) or Clinical Dementia Rating (CDR). MCI participants scored between 24 and 30 on the MMSE, 0.5 on the CDR, and abnormal scores on the Wechsler Memory Scale. Dementia was defined as participants who had abnormal memory function on the Wechsler Memory Scale, an MMSE score between 20 and 26, and a CDR of 0.5 or 1.0 and a probable AD clinical diagnosis according to the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association criteria. 24
Participants were included if they had MRIs from which WMHs could be extracted, Hachinski scores, as well as completed information for all vascular risk factors (i.e., BMI, BP, history of hypertension, diabetes, stroke, smoking, alcohol abuse). A total of 2119 participants with 9847 timepoints were included in this study. There were 987 female participants with a total of 4332 timepoints (average of 4.4 follow‐ups) and 1132 male participants with a total of 5515 timepoints (average of 4.9 follow‐ups).
2.3. Structural MRI acquisition and processing
All scans were downloaded from the ADNI website (see http://adni.loni.usc.edu/methods/mri‐tool/mri‐analysis/ for the detailed MRI acquisition protocol). T1w scans for each participant were pre‐processed through our standard longitudinal pipeline 25 based on open access MINC tools available at https://bic‐mni.github.io/ including noise reduction using mincnlm, 26 intensity inhomogeneity correction using nu_correct, 27 and intensity normalization into range [0–100] using volume_pol command. The pre‐processed images were then linearly (nine parameters: three translation, three rotation, and three scaling) 28 registered to the ADNI average template (based on 150 ADNI participants; 50 NC, 50 MCI, and 50 AD 29 ) using bestlinreg_s2 MRI linear registration tool. 25
2.4. WMH measurements
A previously validated WMH segmentation technique was employed to generate participant WMH measurements. 4 , 30 This technique has been validated in ADNI in which a library of manual segmentations based on 50 ADNI participants (independent of those studied here) was created. The technique has also been validated in other multi‐center studies such as the Parkinson's Markers Initiative 31 and the National Alzheimer's Coordinating Center. 32 WMHs were automatically segmented using the T1w contrasts, along with a set of location and intensity features obtained from a library of manually segmented scans in combination with a random forest classifier to detect the WMHs in new images. 30 , 33 The intensity features include voxel intensity, the probability of a specific intensity value being a WMH (PWMH) or non‐WMH (Pnon‐WMH), as well as the ratio of these two probabilities. Additional spatial features include a spatial WMH probability map indicating the probability of a voxel at a specific location being a WMH and the average intensity of a non‐WMH voxel at that specific voxel location. WMH volumes were segmented in the native space to ensure that any blurring or partial volume effects caused by the resampling process did not impact the segmentation accuracy. Following the segmentation, the WMH maps were linearly registered to the template space to calculate the normalized WMH volumes. WMH load was defined as the volume of all voxels as WMH in the standard space (in mm3) and are normalized for head size. The volumes of the WMHs for frontal, parietal, temporal, and occipital lobes as well as the entire brain were calculated based on regional masks from the Hammers atlas. 30 , 34 The quality of the registrations and WMH segmentations was visually verified by an experienced rater (author M.D.), blinded to diagnostic group.
In addition, deep and periventricular (PV) WMHs were also obtained through a previously validated technique that started by segmenting the ventricles on the T1w images with a patch‐based label fusion segmentation technique. 35 , 36 People with AD were included in the training library to ensure that this technique could accurately segment the larger ventricles observed in people with AD. All ventricle segmentations were visually inspected (by author M.D.); those that did not pass quality control were excluded from the PV and deep WMH analyses (N = 9). The ventricle mask was dilated by 8 mm and applied to the WMH labels to calculate PV WMH and deep WMH volumes, that is, all voxels inside the dilated ventricular mask were taken as PV WMH, and all remaining voxels outside the dilated ventricular mask were taken as deep WMH. The distinction between PV and deep WMH was made since PV and deep WMHs have been shown to be associated with different risk factors, etiologies, and clinical presentations. 37 , 38 A recent study also reported greater levels of differences in deep WMHs between males and females. 16 The analyses were repeated using PV and deep WMHs measures as well as the global and lobar measures of WMHs.
2.5. Independent vascular risk factors
Both systolic BP and history of hypertension were included in the statistical analyses because they represent different measures of hypertension. History of hypertension refers to a history of high BP throughout life (identified at baseline as a dichotomous variable), while systolic and diastolic BP refers to current continuous BP measures at each time of testing. BMI was a continuous measure calculated from the participants height and weight at each visit. History of smoking was used to identify people who previously or currently smoke tobacco products (identified at baseline as a dichotomous variable). ADNI guidelines excluded participants with alcohol or substance abuse/dependence within the past 2 years (identified using DSM IV criteria). Therefore, the participants who endorsed alcohol abuse exhibited alcohol abuse at least 2 years prior to study start date according to the DSM IV criteria. History of stroke was also identified at baseline (as a dichotomous variable). Diabetic status at baseline was determined using medication information. Medication lists were downloaded from ADNI, and medications prescribed to manage diabetes were identified. This list was then used as a proxy to determine which participants had diabetes (diabetes = 1 or no diabetes = 0). History of hypertension, smoking, history of stroke, alcohol abuse, and diabetes were dichotomous measures (i.e., present = 1 and absent = 0). BP and BMI values were continuous values time matched to the closest MRI visit with a 6‐month maximum difference cut‐off.
2.6. Vascular composite
We developed a vascular composite (VC) score to examine whether vascular risk factors, when combined, influenced WMH load in males and females. The VC is a sum of questions regarding vascular conditions, diabetes, 0–1; alcohol abuse, 0–1; smoking history, 0–1; high systolic BP, 0–1; high diastolic BP, 0–1; and high BMI, 0–1; where 0 represents no and 1 represents yes, as well as Hachinski score, 0–4. It should be noted that while someone can obtain up to 18 on the Hachinski score, ADNI restricted people who had 4 or less to ensure participants included were associated with primarily Alzheimer's disease related pathology. Following the National Institute of Health and National Institute on Aging guidelines for older adults, high systolic blood pressure was identified as a measure of ≥130 and high diastolic BP was identified as a measure of ≥80. 39 The VC score was used as a continuous measure ranging from 0 (endorsing no conditions) to 10 (endorsing all conditions and maximum Hachinski score).
The Hachinski score is a tool used to identify vascular dementia and vascular pathology with higher scores representing higher vascular risk pathology. 40 This measure includes history of hypertension and history of stroke which is why these independent variables were not included in the composite. The Hachinski score was included in the VC because of its relationship with vascular pathology which is known to be associated with WMH burden. See supplementary document for the ADNI datasheets used to extract participant information.
2.7. Cognitive measures
For the purposes of the current study, specific cognitive tests were chosen to examine multiple cognitive domains known to be associated with WMHs. Global cognition, 41 , 42 executive functioning, 42 , 43 memory, 44 and functional status 15 have all been previously observed to be associated with WMH burden. For those reasons, we examined the relationship between WMHs and global cognition (CDR‐SB), executive functioning (Trail‐Making Test Part B [TMT‐B]), Rey Auditory Verbal Learning Test (RAVLT), and functional status in abilities to complete activities of daily living (Functional Activities Questionnaire [FAQ]). Cognitive test scores were time matched to the closest MRI visit with a 6‐month maximum difference cutoff.
2.8. Statistical analysis
Independent sample t‐tests and chi‐squared analysis (with Bonferroni correction) were completed on demographic and clinical information using MATLAB R2019b. WMH volumes were log‐transformed to achieve a more normal distribution. Linear mixed effects models were used to investigate the association between longitudinal WMH load (whole brain and subregions: frontal, temporal, parietal, occipital, deep, and periventricular) and vascular risk factors. Left and right lobes were summed for regional WMH values. All continuous values (including log‐transformed WMH volumes) were z‐scored within the population prior to the analyses. All p‐values are reported as raw values with significance determined by false discovery rate (FDR) correction at 0.05. 45
A multi‐step strategy was used for analysis. The first step evaluates if longitudinal WMH progression rates differ between males and females. Baseline diagnosis, education, APOE4 status (defined as either 0 or 1; 0 = no ɛ4 alleles and 1 = 1 or 2 ɛ4 alleles), sex, and age were included as covariates. The interaction of interest was Sex:Age, contrasting rate of change (i.e., progression of WMHs) with increased age in males against females. The model was run separately for each WMH load measure (total, then frontal, temporal, parietal, occipital, deep and PV).
| (1) |
These analyses were completed to examine sex effects on the influence of the VC on longitudinal WMHs. Following the Canadian Institutes of Health Research, institute of gender and health recommendations for sex and gender in the analysis of secondary data from human participants, 46 we ran males and females separately (Equation 2) and then together with an interaction term (Equation 3). The mixed effects model also included age, education, APOE4 status, and baseline diagnosis (contrasting MCI and AD against normal controls [NC]), as covariates. Participant ID was included as a categorical random effect. The model was run separately for each WMH load (total, then frontal, temporal, parietal, occipital, deep and PV).
| (2) |
The follow‐up model was used to determine whether the VC interacted with sex to influence longitudinal WMH burden in males versus females. The interaction of interest was VC:Sex (contrasting WMH burden in relation to the VC for males against females). The model was run separately for each WMH load (total and then frontal, temporal, parietal, occipital, and also deep and PV).
| (3) |
A second set of analyses was run to examine the influence of individual vascular risk factors on longitudinal WMHs, running the following model separately for males and females (Equation 4) and then with an interaction term for the factors found significant in equation 4 (Equation 5):
| (4) |
To determine whether the significant vascular risk factors interacted with sex to influence longitudinal WMH, a secondary level analysis was completed using linear mixed effects and including the significant risk factors from the fourth model (i.e., Equation 4). The interactions of interest were Systolic:Sex and Hypertension:Sex (contrasting WMH burden in relation to the risk factors for males against females) in Equation (5). The model also included age, education, APOE4 status, and baseline diagnosis (contrasting MCI and AD against NC) as covariates. Participant ID was included as a categorical random effect. The model was run separately for each WMH load (total, then frontal, temporal, parietal, occipital, deep and PV).
| (5) |
A final analysis was completed using linear mixed effects to examine whether there was an association between sex and longitudinal WMH load on cognition (for three cognitive domains: global cognition, memory, and executive functioning). The interaction of interest was WMH:Sex (contrasting the difference in cognition in relation to WMH for males against females). The model also included age, education, APOE4 status, baseline diagnosis (contrasting MCI and AD against NC), and VC as covariates. Participant ID was included as a categorical random effect. The models were run separately for each WMH load (total, then frontal, temporal, parietal, and occipital, deep and PV).
| (6) |
3. RESULTS
3.1. Demographics
Table 1 presents demographic and clinical characteristics of study participants. Males were older than females (74.2y vs. 72.3y; t = 6.24, p < 0.001) and had higher education (16.4y vs. 15.6y; t = 7.46, p < 0.001). In terms of risk factors, males had a greater proportion of diabetes (10% vs. 5% participants, x2= 13.19, p < 0.001), hypertension (51% vs. 44%, x2= 9.03, p = 0.002), smoking (39% vs. 28%, x2= 25.85, p < 0.001), and alcohol abuse (6% vs. 2%, x2= 14.21, p < 0.001). When looking at diagnostic status, males represented more MCI participants (40% vs. 31%, x2= 11.09, p < 0.001) and fewer cognitively healthy older adults (42% vs. 54%, x2= 34.91, p < 0.001) than females.
TABLE 1.
Demographic and clinical information for study participants
| Females (n = 987) | Males (n = 1132) | |
|---|---|---|
| Age | 72.3 ± 7.2 | 74.2 ± 7.1 a |
| Education | 15.6 ± 2.7 | 16.4 ± 2.8 a |
| APOE ϵ4+ | 440 (45%) | 512 (45%) |
| Baseline diagnosis | ||
| NC | 424 (54%) | 346 (42%) a |
| MCI | 398 (31%) | 579 (40%) a |
| AD | 165 (15%) | 207 (18%) |
| BMI | 26.6 ± 5.5 | 27.2 ± 4.1 |
| Systolic BP | 134.3 ± 17.7 | 134.7 ± 17.2 |
| Diastolic BP | 73.9 ± 10.2 | 75.1 ± 9.6 |
| Hypertension | 435 (44%) | 574 (51%) a |
| Diabetes | 54 (5%) | 111 (10%) a |
| Stroke | 8 (1%) | 19 (2%) |
| Smoking | 276 (28%) | 436 (39%) a |
| Alcohol abuse | 21 (2%) | 61 (6%) a |
| Hachinski score | ||
| 0 | 509 (51.5%) | 517 (46%) |
| 1 | 419 (42.5%) | 540 (48%) |
| 2 | 33 (3.3%) | 44 (4%) |
| 3 | 24 (2%) | 25 (2%) |
| 4 | 2 (< 1%) | 6 (< 1%) |
Notes: Values are represented as mean ± standard deviation or as the amount of the sample and percentage of sample with that trait.
Abbreviations: AD, Alzheimer's disease; APOE, apolipoprotein E; BMI, body mass index; BP, blood pressure; MCI, mild cognitive impairment; NC, normal controls.
Males had statistically (p < 0.05) increased age, higher education, more MCI participants and fewer NCs, and higher rates of hypertension, diabetes, smoking, and alcohol abuse.
3.2. Progression of WMHs
WMH progression between males and females differed in all regions and measures except temporal and parietal regions (Equation 1). Males had lower WMH progression with increased age in total brain (β = −0.03, SE = 0.01, t = −2.38, p = 0.01), frontal region (β = −0.07, SE = 0.02, t = −4.35, p < 0.001), and deep brain (β = −0.12, SE = 0.03, t = −4.38, p < 0.001). Conversely, males had increased progression of WMHs in the occipital region (β = 0.11, SE = 0.02, t = 5.26, p < 0.001) (see Figure 1A to see progression in deep and occipital WMHs where the sex difference was largest).
FIGURE 1.
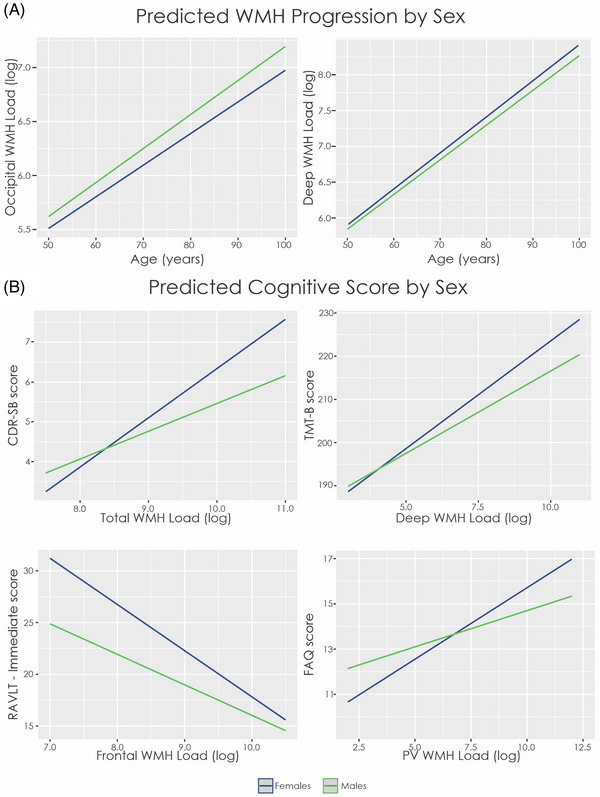
Estimated WMH progression and cognitive change over time by sex. (A) Males have more WMH volume than females in occipital regions, and this difference increases over time indicating a greater progression of WMH accumulation over time (note use of log scale for volume). Males have less WMH volume than females in deep WMH, and this difference increases with time. (B) In all plots, females have a steeper slope than males indicating a stronger relationship between cognitive test scores and WMH volume. It should be noted that in all cognitive domains except RAVLT‐ immediate, higher values represent lower performance. Notes: CDR‐SB, Clinical Dementia Rating – Sum of Boxes; FAQ, Functional Activities Questionnaire; PV, periventricular; RAVLT, Rey Auditory Verbal Learning Test; TMT‐B, Trail Making Test – Part B; WMH, white matter hyperintensity
3.3. Vascular composite on WMHs
Table 2 and Table S1 summarizes the influence of the VC score on male and females (Equations 2 and 3), for that reason the β and SE are presented in the table and not in text. For males, age (t belongs to [14.68 – 61.21], p < 0.001), MCI diagnosis (t belongs to [2.71 – 4.82], p < 0.006), and AD diagnosis (t belongs to [4.90 – 6.05], p < 0.001) were associated with increased WMHs in all regions except deep WMH. Further, APOE positivity was associated with increased WMH burden in the parietal (t = 2.49, p = 0.01) and occipital regions (t = 2.90, p = 0.004). Importantly, an increased VC score was associated with increased total, frontal, temporal, and parietal WMH burden (t belongs to [2.69 – 3.76], p < 0.007).
TABLE 2.
Linear mixed effects showing the vascular composite score and significant individual risk factors on WMH burden
| Males | Total | Frontal | Temporal | Parietal | Occipital | Deep | Periventricular |
|---|---|---|---|---|---|---|---|
| VC (Equation 2) |
β = 0.02, SE < 0.01, t = 3.63, p < 0.001 |
β = 0.02, SE < 0.01, t = 3.76, p < 0.001 |
β = 0.02, SE < 0.01, t = 2.69, p = 0.007 |
β = 0.02, SE < 0.01, t = 3.20, p = 0.001 |
β = 0.01, SE < 0.01, t = 1.41, p = 0.16 |
β = 0.03, SE = 0.02, t = 2.06 p = 0.039 |
β = 0.34, SE = 0.02, t = 2.00, p = 0.046 |
| Systolic BP (Equation 4) |
β = 0.01, SE < 0.01, t = 2.32, p = 0.020 |
β < 0.01, SE < 0.01, t = 2.14, p = 0.032 |
β < 0.01, SE < 0.01, t = 1.09, p = 0.27 |
β = 0.01, SE < 0.01, t = 1.71, p = 0.09 |
β = 0.02, SE < 0.01, t = 2.69, p = 0.007 |
β < −0.01, SE = 0.01, t = −0.49, p = 0.63 |
β = 0.01, SE = 0.02, t = 0.81, p = 0.42 |
| History Of hypertension (Equation 4) |
β = 0.27, SE = 0.05, t = 5.18, p < 0.001 |
β = 0.32, SE = 0.05, t = 6.09, p < 0.001 |
β = 0.17, SE = 0.05, t = 3.20, p = 0.001 |
β = 0.23, SE = 0.06, t = 3.19, p = 0.001 |
β < 0.01, SE = 0.06, t = 0.13, p = 0.89 |
β = 0.19, SE = 0.06, t = 3.19, p = 0.001 |
β = 0.15, SE = 0.05, t = 3.26, p = 0.001 |
| Females | Total | Frontal | Temporal | Parietal | Occipital | Deep | Periventricular |
|---|---|---|---|---|---|---|---|
| VC (Equation 2) |
β = 0.04, SE < 0.01, t = 4.05, p < 0.001 |
β = 0.04, SE < 0.01, t = 3.79, p < 0.001 |
β = 0.04, SE = 0.01, t = 3.49, p < 0.001 |
β = 0.03, SE < 0.01, t = 2.80, p = 0.005 |
β = 0.04, SE = 0.01, t = 3.15, p = 0.002 |
β = 0.04, SE = 0.03, t = 2.68, p = 0.007 |
β = 0.05, SE = 0.02, t = 2.73 p = 0.006 |
| Systolic BP (Equation 4) |
β < 0.01, SE < 0.01, t = 0.95, p = 0.34 |
β < 0.01, SE < 0.01, t = 0.43, p = 0.67 |
β < 0.01, SE < 0.01, t = 1.19, p = 0.23 |
β < 0.01, SE < 0.01, t = 1.09, p = 0.27 |
β = 0.01, SE < 0.01, t = 0.20, p = 0.84 |
β = 0.03, SE = 0.01, t = 2.33, p = 0.019 |
β = 0.03, SE = 0.02, t = 2.25, p = 0.024 |
| History Of hypertension (Equation 4) |
β = 0.12, SE = 0.06, t = 2.10, p = 0.037 |
β = 0.14, SE = 0.06, t = 2.44, p = 0.015 |
β = 0.04, SE = 0.06, t = 0.70, p = 0.49 |
β = 0.10, SE = 0.06, t = 1.82, p = 0.07 |
β = −0.02, SE = 0.01, t = −0.28, p = 0.78 |
β = 0.17, SE = 0.06, t = 2.92, p = 0.003 |
β = 0.14, SE = 0.05, t = 2.72, p = 0.006 |
Notes: Main effects of age, education, APOE status, and diagnosis can be found in supplementary material.
Abbreviations: History of hypertension, current or past history of high blood pressure, reported as 0 (no history of high blood pressure) or 1 (history of high blood pressure); Systolic BP, current systolic blood pressure, reported as a continuous measure; VC, vascular composite.
Bold values represent results that remained significant after correction for multiple comaprisons.
For females, age (t belongs to [9.11 – 51.04], p < 0.001) and AD diagnosis (t belongs to [2.88 – 5.94], p < 0.005) were associated with increased WMHs in all regions except deep WMHs. MCI was associated with increased WMH load compared to NCs in all regions except occipital and deep WMH load (t belongs to [3.17 – 3.74], p < 0.001). APOE status was associated with increased WMH burden in only the occipital region (t = 2.88, p < 0.004). Importantly, an increased VC score was associated with increased WMH burden at all regions and measures (t belongs to [2.50 – 4.05], p < 0.05).
3.4. Interaction of vascular composite with sex on WMHs
In Equation 3, male sex was significantly associated with lower WMH accumulation over time in the frontal region (t = −3.57, p < 0.001) and deep regions (t = −3.58, p < 0.001) but with increased WMH accumulation over time in the temporal (t = 2.79, p = 0.005) and occipital regions (t = 5.23, p < 0.001). The interaction Sex:VC was not significant in any region (see Table 3).
TABLE 3.
Models from Equations 3 and 5 showing the main effect of sex WMHs and interaction between sex and each significant risk factor
| Interaction models | Total | Frontal | Temporal | Parietal | Occipital | Deep | Periventricular |
|---|---|---|---|---|---|---|---|
| Sex (Male) (Equation 3) |
β = −0.04, SE = 0.04, t = −1.05, p = 0.29 |
β = −0.14, SE = 0.04, t = −3.57 p < 0.001 |
β = 0.11, SE = 0.04, t = 2.79, p = 0.005 |
β = 0.03, SE = 0.04, t = 0.73, p = 0.46 |
β = 0.22, SE = 0.04, t = 5.23, p < 0.001 |
β = −0.19, SE = 0.05, t = −3.58, p < 0.001 |
β < 0.01, SE = 0.03, t = 0.11, p = 0.92 |
| Sex (male):VC (Equation 3) |
β = −0.01, SE = 0.01, t = −0.83 p = 0.40 |
β = −0.01, SE = 0.01, t = −0.52, p = 0.60 |
β = −0.01, SE = 0.01, t = −1.03, p = 0.30 |
β < −0.01, SE = 0.01, t = −0.05, p = 0.96 |
β = −0.02, SE = 0.02, t = −1.40, p = 0.16 |
β = −0.01, SE = 0.03, t = −0.48, p = 0.63 |
β = −0.02, SE = 0.03, t = −0.71, p = 0.48 |
| Sex (male): Systolic BP (Equation 5) |
β < 0.01, SE < 0.01, t = 1.06, p = .29 |
β < 0.01, SE < 0.01, t = 0.86, p = .39 |
β < 0.01, SE < 0.01, t = 0.31, p = .76 |
β < 0.01, SE < 0.01, t = 1.19, p = .24 |
β = 0.02, SE < 0.01, t = 2.18, p = .029 |
β = −0.03, SE = 0.01, t = −2.23, p = .026 |
β = −0.02, SE = 0.02, t = −1.66, p = .24 |
| Sex (male): History of Hypertension (Equation 5) |
β = 0.15, SE = 0.07, t = 1.94, p = 0.052 |
β = 0.22, SE = 0.09, t = 2.47, p = 0.014 |
β = 0.13, SE = 0.08, t = 1.65, p = 0.09 |
β = 0.12, SE = 0.08, t = 1.61, p = 0.11 |
β = 0.07, SE = 0.08, t = 0.82, p = 0.41 |
β < −0.01, SE = 0.01, t = −0.06, p = 0.95 |
β = 0.03, SE = 0.07, t = 0.38, p = 0.70 |
Notes: The colon (:) denotes the interaction between sex and each risk factor, vascular composite, systolic blood pressure, and history of hypertension. In all analysis we contrasted males against females, with females as the reference category.
Abbreviations: History of hypertension, current or past history of high blood pressure, reported as 0 (no history of high blood pressure) or 1 (history of high blood pressure); Systolic BP, current systolic blood pressure, reported as a continuous measure; VC, vascular composite.
Bold values represent results that remained significant after correction for multiple comaprisons.
3.5. Individual risk factors on WMHs
Table 2 presents the output for the independent association for each individual risk factor on WMH burden in males and females separately (Equation 4). Neither BMI, diastolic BP, diabetes, tobacco smoking, nor alcohol abuse were significantly associated with WMH load in males or females (and are not shown in Table 2). Systolic BP and hypertension were associated with WMH load in both males and females. More specifically, increased systolic BP was associated with total (t = 2.32, p = 0.020) and occipital (t = 2.69, p = 0.007) WMHs in males. History of hypertension was associated with increased total, frontal, temporal, parietal, deep, and periventricular WMHs in males (t belongs to [3.19 – 6.09], p < 0.005). In females, systolic BP was associated with deep (t = 2.33, p = 0.019) and periventricular (t = 2.25, p = 0.025) WMHs, while history of hypertension was associated with increased frontal, deep, and periventricular WMHs (t belongs to [2.44 – 2.72], p < 0.05).
3.6. Interaction of risk factors with sex on WMHs
Further analysis with Equation 5 shows that males with a history of hypertension had increased WMH burden in the frontal region compared to females (t = 2.47, p = 0.014) and males with a high systolic BP had increased WMH burden in the occipital region (t = 2.18, p = 0.029). On the other hand, females with high systolic BP had increased rates of deep WMH burden compared to males (t = 2.23, p = 0.026, see Table 3). To ensure that differences in demographics and diagnostic group distributions did not impact our results, the models were repeated matching participant groups by age and diagnostic status. Similar results were obtained when matching the samples based on age and diagnostic status.
3.7. Cognitive outcomes
Table 4 presents the estimates from the linear mixed effects model for change in cognition associated with WMHs for males and females (Equation 6). Increased WMH burden was associated with higher values (i.e., worse global cognition) on the CDR‐SB at all regions and measures (t belongs to [3.23–17.72], p < 0.001) for females (see Figure 1B). This finding demonstrates that increased WMH burden is associated with worse global cognition in females. The Male Sex by WMH interaction revealed that males had a less steep slope than females at all regions and measures except deep WMHs (t belongs to [−2.43 – −4.56], p < 0.02), indicating that females have increased change in global cognition compared to males, that is, associated with WMHs.
TABLE 4.
Linear mixed effects model showing the interactions between sex and WMHs for each cognitive domain.
| Total | Frontal | Temporal | Parietal | Occipital | Deep | Periventricular | |
|---|---|---|---|---|---|---|---|
| CDR‐SB (Equation 6) | |||||||
| WMH |
β = 0.34, SE = 0.02, t = 17.40, p < 0.001 |
β = 0.34, SE = 0.02, t = 17.72, p < 0.001 |
β = 0.19, SE = 0.02, t = 10.45, p < 0.001 |
β = 0.27, SE = 0.02, t = 14.18, p < 0.001 |
β = 0.24, SE = 0.02, t = 14.34, p < 0.001 |
β = 0.06, SE = 0.02, t = 3.23 p = .001 |
β = 0.06, SE = 0.02, t = 5.25 p < 0.001 |
| Sex (Male):WMH |
β = −0.11, SE = 0.02, t = −4.42, p < 0.001 |
β = −0.11, SE = 0.02, t = −4.56 p < 0.001 |
β = −0.10, SE = 0.02, t = −4.12 p < 0.002 |
β = −0.09, SE = 0.02, t = −3.62 p < 0.001 |
β = −0.06, SE = 0.02, t = −3.26 p = 0.001 |
β < 0.01, SE = 0.02, t = 0.22 p = 0.82 |
β = −0.06, SE = 0.02, t = −2.55 p = 0.01 |
| Sex (Male):VC |
β < −0.01, SE = 0.02, t = −0.15, p = 0.88 |
β < −0.01, SE = 0.02, t = −0.34, p = 0.73 |
β < −0.01, SE = 0.02, t = −0.19, p = 0.85 |
β < −0.01, SE = 0.02, t = −0.20, p = 0.84 |
β < −0.01, SE = 0.02, t = −0.27, p = 0.79 |
β < −0.01, SE = 0.02, t = −0.41, p = 0.68 |
β < −0.01, SE = 0.02, t = −0.42, p = 0.68 |
| TMT‐B (Equation 6) | |||||||
| WMH |
β = 0.23, SE = 0.02, t = 10.02, p < 0.001 |
β = 0.20, SE = 0.03, t = 8.75, p < 0.001 |
β = 0.16, SE = 0.02, t = 7.32, p < 0.001 |
β = 0.22, SE = 0.03, t = 9.62, p < 0.001 |
β = 0.21, SE = 0.02, t = 9.22, p < 0.001 |
β = 0.07, SE = 0.02, t = 2.82 p = 0.005 |
β = 0.04, SE = 0.02, t = 2.52 p = 0.012 |
| Sex (Male):WMH |
β = −0.05, SE = 0.03, t = −0.02, p = 0.98 |
β = 0.02, SE = 0.03, t = 0.78, p = 0.44 |
β = −0.04, SE = 0.03, t = −1.25, p = 0.21 |
β = −0.07, SE = 0.03, t = −2.34, p = 0.019 |
β = −0.08, SE = 0.02, t = −2.45, p = 0.014 |
β = −0.07, SE = 0.03, t = −2.67 p = 0.007 |
β = −0.01, SE = 0.02, t = −0.29 p = 0.77 |
| Sex (Male):VC |
β = 0.02, SE = 0.03, t = 0.83, p = 0.41 |
β = 0.02, SE = 0.03, t = 0.61, p = 0.54 |
β = 0.03, SE = 0.03, t = 0.91, p = 0.36 |
β = 0.02, SE = 0.03, t = 0.87, p = 0.38 |
β = 0.03, SE = 0.03, t = 0.91, p = 0.36 |
β = 0.02, SE = 0.03, t = 0.67, p = 0.50 |
β = 0.02, SE = 0.03, t = 0.66, p = 0.50 |
| RAVLT‐ Immediate (Equation 6) | |||||||
| WMH |
β = −0.24, SE = 0.02, t = −12.15, p < 0.001 |
β = −0.22, SE = 0.02, t = −11.64, p < 0.001 |
β = −0.15, SE = 0.02, t = −8.17, p < 0.001 |
β = −0.22, SE = 0.02, t = −11.07 p < 0.001 |
β = −0.14, SE = 0.02, t = −8.89, p < 0.001 |
β = 0.02, SE = 0.02, t = 1.21 p = 0.22 |
β = −0.04, SE = 0.01, t = −3.59 p < 0.001 |
| Sex (Male):WMH |
β = 0.06, SE = 0.02, t = 2.55 p = 0.01 |
β = 0.06, SE = 0.02, t = 2.63, p = 0.008 |
β = 0.04, SE = 0.02, t = 1.79, p = 0.07 |
β = 0.06, SE = 0.02, t = 2.33, p = 0.02 |
β = 0.02, SE = 0.02 t = 1.00, p = 0.31 |
β = −0.02, SE = 0.02, t = −0.74 p = 0.46 |
β = 0.03, SE = 0.01, t = 1.82 p = 0.07 |
| Sex (Male):VC |
β < 0.01, SE = 0.02, t = 0.11, p = 0.90 |
β < 0.01, SE = 0.02, t = 0.22, p = 0.82 |
β < 0.01, SE = 0.02, t = 0.10, p = 0.92 |
β < 0.01, SE = 0.02, t = 0.13, p = 0.89 |
β < 0.01, SE = 0.02, t = 0.09, p = 0.92 |
β < 0.01, SE = 0.02, t = 0.36, p = 0.72 |
β < 0.01, SE = 0.02, t = 0.20, p = 0.85 |
| RAVLT‐ Learning (Equation 6) | |||||||
| WMH |
β = −0.13, SE = 0.02, t = −5.68, p < 0.001 |
β = −0.11, SE = 0.02, t = −4.99, p < 0.001 |
β = −0.08, SE = 0.02, t = −3.69, p < 0.001 |
β = −0.13, SE = 0.02, t = −6.00, p < 0.001 |
β = −0.09, SE = 0.02, t = −4.65, p < .001 |
β = 0.02, SE = 0.02, t = 0.95 p = 0.34 |
β = −0.07, SE = 0.02, t = −4.30 p < 0.001 |
| Sex (Male):WMH |
β = 0.04, SE = 0.03, t = 1.57, p = 0.11 |
β = 0.03, SE = 0.03, t = 1.23, p = 0.22 |
β = 0.02, SE = 0.03, t = 0.73, p = 0.47 |
β = 0.05, SE = 0.03, t = 1.88, p = 0.06 |
β = 0.03, SE = 0.03 t = 1.26, p = 0.21 |
β = −0.03, SE = 0.03, t = −1.02 p = 0.30 |
β = 0.07, SE = 0.02, t = 3.47 p < 0.001 |
| Sex (Male):VC |
β = −0.05, SE = 0.03, t = −1.83, p = 0.07 |
β = −0.05, SE = 0.03, t = −1.72, p = 0.08 |
β = −0.05, SE = 0.03, t = −1.78, p = 0.075 |
β = −0.05, SE = 0.03, t = −1.89, p = 0.058 |
β = −0.05, SE = 0.03, t = −1.79, p = 0.073 |
β = −0.04, SE = 0.03, t = −1.54, p = 0.12 |
β = −0.05, SE = 0.03, t = −1.79, p = 0.072 |
| RAVLT‐ Perc_Forgetting (Equation 6) | |||||||
| WMH |
β = 0.09, SE = 0.02, t = 3.59, p < 0.001 |
β = 0.09, SE = 0.02, t = 3.55, p < 0.001 |
β = 0.04, SE = 0.02, t = 1.75, p = 0.08 |
β = 0.07, SE = 0.02, t = 2.17, p = 0.003 |
β = 0.06, SE = 0.02, t = 2.73, p = 0.006 |
β < 0.01, SE = 0.02, t = −0.01 p = 0.99 |
β = 0.01, SE = 0.02, t = 0.67 p = 0.51 |
| Sex (Male):WMH |
β = −0.03, SE = 0.03, t = −0.90, p = 0.36 |
β = −0.03, SE = 0.03, t = −1.00, p = 0.32 |
β = −0.01, SE = 0.03, t = −0.05, p = 0.96 |
β = −0.01, SE = 0.03, t = −0.46, p = 0.64 |
β = −0.02, SE = 0.03, t = −0.72, p = 0.47 |
β = 0.03, SE = 0.03, t = 1.03 p = 0.30 |
β = −0.04, SE = 0.02, t = −1.58 p = 0.11 |
| Sex (Male):VC |
β < 0.01, SE = 0.03, t = 0.17, p = 0.86 |
β < 0.01, SE = 0.03, t = 0.11, p = 0.92 |
β < 0.01, SE = 0.03, t = 0.17, p = 0.87 |
β < 0.01, SE = 0.03, t = 0.18, p = 0.86 |
β < 0.01, SE = 0.03, t = 0.20, p = 0.84 |
β < −0.01, SE = 0.03, t = −0.05, p = 0.96 |
β < −0.01, SE = 0.03, t = 0.16, p = 0.87 |
| FAQ (Equation 6) | |||||||
| WMH |
β = 0.33, SE = 0.02, t = 16.46, p < 0.001 |
β = 0.23, SE = 0.02, t = 16.13, p < 0.001 |
β = 0.20, SE = 0.02, t = 10.31, p < 0.001 |
β = 0.29, SE = 0.02, t = 14.10, p < 0.001 |
β = 0.24, SE = 0.02, t = 13.78, p < 0.001 |
β = −0.03, SE = 0.02, t = −1.69 p = 0.091 |
β = 0.06, SE = 0.01, t = 4.82 p < 0.001 |
| Sex (Male):WMH |
β = −0.06, SE = 0.03, t = −2.40, p = 0.02 |
β = −0.06, SE = 0.03, t = −1.98 p = 0.047 |
β = −0.07, SE = 0.02, t = −2.96 p = 0.003 |
β = −0.06, SE = 0.03, t = −2.46 p = 0.014 |
β = −0.02, SE = 0.02, t = −0.72 p = 0.47 |
β = −0.07, SE = 0.02, t = −3.21 p = 0.001 |
β = −0.07, SE = 0.02, t = −4.43 p = 0.001 |
| Sex (Male):VC |
β < 0.01, SE = 0.02, t = 0.24, p = 0.81 |
β = −0.01, SE = 0.02, t = −0.46, p = 0.64 |
β < 0.01, SE = 0.02, t = 0.15, p = 0.88 |
β < 0.01, SE = 0.02, t = 0.24, p = 0.81 |
β < 0.01, SE = 0.02, t = 0.14, p = 0.88 |
β = −0.01, SE = 0.02, t = −0.46, p = 0.65 |
β < −0.01, SE = 0.02, t = −0.29, p = 0.77 |
Notes: The colon (:) denotes the interaction between sex and the vascular composite or sex and white matter hyperintensities. In all analyses, we contrasted males against females, with females as the reference category.
Abbreviations: CDR‐SB, Clinical Dementia Rating ‐ Sum of Boxes; FAQ, Functional Activities Questionnaire; Perc‐Forgetting, Percent forgetting; RAVLT, Ray's Auditory Verbal Learning test; TMT‐B, Trail Making Test‐B; VC, vascular composite; WMH, white matter hyperintensity.
Bold values represent results that remained significant after correction for multiple comaprisons.
When examining executive functioning, there was an association between WMHs and TMT‐B score in females. Increased TMT‐B scores was associated with increased WMH burden in females at all regions and measures (t belongs to [2.52 – 10.02], p < 0.05). The Male Sex by WMH interaction revealed that males had a less steep slope than females in parietal, occipital, and deep WMHs region (t belongs to [−2.34 – −2.67], p < 0.02), indicating that females have increased change in executive functioning compared to males, that is, associated with WMHs in those two regions.
An association between the RAVLT and WMHs was observed. Both RAVLT immediate (t belongs to [−3.59 – −12.15], p < 0.001) and learning (t belongs to [−3.69 – 5.68], p < 0.05) were significantly associated with WMHs in females at all regions and measures except deep WMHs. For RAVLT percent forgetting, a significant association between WMHs was observed in females for total, frontal, parietal, and occipital WMHs (t belongs to [2.17 – 3.59], p < 0.01). That is, increased WMHs were associated with worse memory performance in females. The Male Sex by WMH interaction for RAVLT immediate score was significant for total, frontal, and temporal WMH burden (t belongs to [2.33 –2.63], p < 0.05). The interaction between Male Sex and WMHs was also significant for RAVLT learning and periventricular WMHs (t = 3.47, p = 0.001). That is, males had a less steep slope compared to females, indicating that more WMH accumulation is needed (in males) for the same change in RAVLT immediate and learning score as females.
The FAQ was also observed to be associated with WMHs. The relationship between WMHs and the FAQ was significant for females at all regions except deep WMHs (t belongs to [4.82 – 16.46], p < 0.001). The Male Sex by WMH interaction revealed that males had a less steep slope than females in total, temporal, parietal, deep, and periventricular WMH progression (t belongs to [−2.40 – −4.43], p < 0.05). These findings suggest that, for females, higher scores (i.e., worse functional abilities) is impacted by increased WMHs, this relationship does not occur in males for total, temporal, parietal, deep, or periventricular WMH progression.
To ensure our results were not influenced by a different proportion of males and females in the different diagnostic categories (NC, MCI, and AD), we re‐ran all analysis that were completed on males and females together (Equations (1), (3), (5) and 6) including the interaction Sex:Diagnosis. The model outputs did not differ from that of the current results, indicating that sex‐related diagnostic differences did not influence the outcomes observed in this study.
4. DISCUSSION
This study examined if sex affects the association between WMHs and vascular risk factors and the association between WMHs and cognition. The findings show that history of hypertension (past and current high BP) and current high systolic BP are the main independent factors associated with WMHs in males. In females, few associations were observed with individual factors, with the strongest associations between WMHs and the VC. Despite observing sex differences in risk factors, the sex by vascular risk factor interaction did not reveal different rates of WMH change in males and females due to vascular risk factors. We also observed a strong association between WMHs and cognition in females in all domains (global cognition, executive functioning, memory, and functional activities). In males, this association was less steep compared to females (for global cognition, executive functioning, some memory scores, and functional activities), indicating more WMH accumulation is needed in males to see the same decline.
Compared to females, males demonstrated similar, increased, and decreased WMHs depending on the regions observed. These findings are consistent with previous reports indicating both increased 16 , 17 , 18 and decreased 20 WMHs in females relative to males. While female parietal WMHs did not differ from males, females exhibited lower WMHs in only the occipital region relative to males. Consistent with previous studies, females showed increased WMHs compared to males in deep 15 , 16 and frontal 15 followed by total WMHs. When examining the interaction between risk factors and sex, the VC did not interact with sex to influence rate of WMH accumulation. When examining the risk factors independently, a few interactions with systolic BP and hypertension influenced the rate of WMH progression. Relative to females, males with history of hypertension exhibited increased WMH progression in the frontal region and males with high systolic BP had increased WMH progression in the occipital region. Females with high systolic BP showed increased deep WMH progression compared to males. Examination by region is essential in these studies as frontal WMHs are associated with more vascular processes while parietal WMHs are associated with AD. 47 Therefore, examining WMHs in a regional approach may provide increased specificity in disease classification. Despite the differences in the independent risk factors and composite score in males and females, the findings here suggest that the different risk factors contribute only slightly to differences in WMHs in males and females. It is possible that other sex‐specific risk factors not available to be examined in this study such as preeclampsia and menopause may contribute to sex differences in WMHs. Future research should further explore the relationship between these risk factors and WMH progression.
Consistent with previous research, WMHs were associated with lower cognition. 2 , 41 , 42 More specifically, change in global cognition and executive functioning was associated with WMHs in all regions and measures for females. Males had less change in global cognition that was associated with the same amount of WMH burden than females. With respect to executive functioning, males had a less steep slope in parietal and occipital regions, indicating less cognitive change in those regions associated with WMHs. Previous research also observed that WMHs are associated with change in memory performance in NCs, MCI, and AD. 44 Similarly, we observed that lower memory performance (i.e., high scores in RAVLT percent forgetting, and low scores in RAVLT immediate and learning) was associated with increased WMH accumulation in females. Males showed less change in RAVLT immediate scores associated with total, frontal, and parietal WMHs than females. With respect to RAVLT learning, the only sex difference was periventricular WMHs, with males showing less change associated with WMH load than females. No difference in RAVLT percent forgetting scores were observed between males and females. Taken together, immediate memory appears to be more strongly associated with WMHs in females compared to males. This finding indicates that WMH progression is associated with short‐term memory (i.e., RAVLT immediate) differently in males and females but the relationship between WMHs and long‐term memory (i.e., RAVLT percent forgetting) is similar in males and females. Previous research has reported that WMHs are associated with lower functional capacity and physical health, particularly in females. 15 The current study supports this finding, with females showing a stronger relationship between higher scores on the FAQ (i.e., lower ability to independently perform activities of daily living) and WMHs. On the other hand, males had a less steep slope compared to females in total, frontal, parietal, and occipital regions. That is, functional performance was less affected by WMH accumulation in males than females.
Several factors could explain why females’ WMH burden is more affected by a VC score than individual factors than males. Males tend to have higher prevalence of risk factors throughout life while females tend to have an increase in these risk factors later in life, after menopause. 11 , 13 , 48 Therefore, it is possible that because females have these risk factors for a shorter amount of time, more factors are needed to see the same effect as males. Interestingly, our findings also showed that even when females exhibit the same amount of WMH burden they experience more cognitive decline than males. Previous research observed that females require less pathology to be diagnosed with clinical AD, 49 exhibit steeper cognitive decline, and increased rates of brain atrophy compared to males. 48 This increased decline in females may be associated with postmenopausal factors such as reductions in the protective effect of estrogen. 48 , 49 Further research into the mechanisms underlying sex differences is needed to improve our understanding of the relationship between WMHs and risk factors in males and females. This research would help inform health policy and the tailoring of targeted interventions.
There are a few limitations of the current study. While history of smoking and alcohol abuse were examined, we did not examine past versus current use or severity of use as the information was unavailable. Future research should examine number of alcoholic drinks and amount of tobacco smoking as continuous measures to determine if increased severity has a larger impact on WMH burden. Another potential issue in the current dataset is that risk factors and conditions were selected from datasheets provided by ADNI. It is possible that some information (e.g., medications for diabetes, smoking) may have been missing or not comprehensive for all people, impacting participant classifications. However, with our large sample size, it is unlikely that a few misplaced participants would impact the results. It should also be noted that ADNI selected highly educated individuals with limited vascular pathology; therefore, the findings here may not be generalizable to people of lower education and may underrepresent the influence of vascular risk factors that might be expected in the general population with greater levels of risk factors and pathology. Lastly, because of sample size limitations, we only assessed linear relationships between variables, future studies with sufficiently large sample sizes should investigate the possible nonlinearity of these relationships.
Although T1w images typically measure hypointensities, we used T1w images for segmentation of WMHs because of the consistent availability of T1w MRI contrasts across all ADNI cohorts. ADNI1 includes T1w and T2w/PD images, ADNI2/GO includes T1w and axial 2D FLAIR images, and ADNI3 includes T1w and sagittal 3D FLAIRs. The resolution of the images from these scans were very different (e.g., T2w/PD, 1*1*3 mm3 and FLAIRs 0.85*0.85*5 mm3 and 1*1*1.2 mm3), making it unreliable to combine WMH volumes from the different study cohorts when using multi‐contrast segmentations. It is important to note that our previous work has established that our T1w‐based segmentation method holds very strong correlations the multicontrast T1w and T2w or FLAIR based WMHs segmentations (r = 0.97, p < 0.0001) and have similar relationships with clinical/cognitive scores as the multicontrast WMH segmentations. 4 , 28 In a previous paper, we also compared group differences using T1w only WMH measures in the same subsample of participants that had T2w/PD (ADNI1) and FLAIR (ADNI2) sequences. We observed that the group differences using the T1w‐based WHM segmentations sequences were similar to that of the T1+T2w/PD and T1+FLAIR segmentations. 50 The method employed here using only T1w images has the reliability and validity to accurately measure WMHs and is consistent across ADNI sub‐cohorts.
Many underlying cerebrovascular disease causes are preventable and/or treatable. Identifying which factors are associated with WMHs could enable timely interventions to mitigate cognitive decline progression and conversion to dementia. The findings presented here show that vascular risk factors are largely associated with WMH development in both males and females. From a clinical standpoint, controlling vascular risk factors (e.g., alcohol consumption, history of hypertension, smoking, high BMI, diabetes, alcohol consumption, and Hachinski score) are important to reduce WMH burden in both males and females. In males, these interventions should emphasize the importance of reducing hypertension throughout life to control WMHs. Developing targeted interventions may reduce deterioration in cognitive functioning and dementia progression. Importantly, the current study also observed that females exhibited increased cognitive decline compared to males even when presenting with the same amount of WMHs. These findings may improve the development of interventions to slow cognitive decline and dementia by encouraging researchers/clinicians to examine males and females separately.
CONFLICT OF INTEREST STATEMENT
The authors declare no competing interests. Author disclosures are available in the supporting information.
CONSENT STATEMENT
Written informed consent was obtained from participants or their study partner.
Supporting information
ICMJE DISCLOSURE FORM
Table S1
Supporting Information
ACKNOWLEDGMENTS
Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH‐12‐2‐0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer's Association; Alzheimer's Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol‐Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann‐La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California. Alzheimer's Disease Neuroimaging Initiative; This research was supported by a grant from the Canadian Institutes of Health Research. Dr. Morrison is supported by a postdoctoral fellowship from Canadian Institutes of Health Research, Funding Reference Number: MFE‐176608. Dr. Dadar reports receiving research funding from the Healthy Brains for Healthy Lives (HBHL), Alzheimer Society Research Program (ASRP), and Douglas Research Centre (DRC). Dr. Collins reports receiving research funding from Canadian Institutes of Health research, Funding Reference Number: PJT‐165921, the Canadian National Science and Engineering Research Council, Brain Canada, the Weston Foundation, and the Famille Louise & André Charron.
Morrison C, Dadar M, Collins DL. Sex differences in risk factors, burden, and outcomes of cerebrovascular disease in Alzheimer's disease populations. Alzheimer's Dement. 2024;20:34–46. 10.1002/alz.13452
Data used in preparation of this article were obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp‐content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf
REFERENCES
- 1. Rhodius‐Meester HFM, Benedictus MR, Wattjes MP, et al. MRI visual ratings of brain atrophy and white matter hyperintensities across the spectrum of cognitive decline are differently affected by age and diagnosis. Front Aging Neurosci. 2017;9(MAY):1‐12. doi: 10.3389/fnagi.2017.00117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Morrison C, Dadar M, Villeneuve S, Collins DL. White matter lesions may be an early marker for age‐related cognitive decline. NeuroImage Clin. 2022;35(January):103096. doi: 10.1016/j.nicl.2022.103096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boyle PA, Yu L, Fleischman DA, et al. White matter hyperintensities, incident mild cognitive impairment, and cognitive decline in old age. Ann Clin Transl Neurol. 2016;3(10):791‐800. doi: 10.1002/acn3.343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dadar M, Maranzano J, Ducharme S, Collins DL. White matter in different regions evolves differently during progression to dementia. Neurobiol Aging. 2019;76:71‐79. doi: 10.1016/j.neurobiolaging.2018.12.004 [DOI] [PubMed] [Google Scholar]
- 5. van der Flier WM, Skoog I, Schneider JA, et al. Vascular cognitive impairment. Nat Rev Dis Prim. 2018;4(1):1‐16. [DOI] [PubMed] [Google Scholar]
- 6. Abraham HMA, Wolfson L, Moscufo N, Guttmann CRG, Kaplan RF, White WB. Cardiovascular risk factors and small vessel disease of the brain: blood pressure, white matter lesions, and functional decline in older persons. J Cereb Blood Flow Metab. 2016;36(1):132‐142. doi: 10.1038/jcbfm.2015.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tamura Y, Araki A. Diabetes mellitus and white matter hyperintensity. Geriatr Gerontol Int. 2015;15:34‐42. doi: 10.1111/ggi.12666 [DOI] [PubMed] [Google Scholar]
- 8. Habes M, Sotiras A, Erus G, et al. White matter lesions spatial heterogeneity, links to risk factors, cognition, genetics, and atrophy. Neurology. 2018;91(10):E964‐E975. doi: 10.1212/WNL.0000000000006116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grueter BE, Schulz UG. Age‐related cerebral white matter disease (Leukoaraiosis): a review. Postgrad Med J. 2012;88(1036):79‐87. doi: 10.1136/postgradmedj-2011-130307 [DOI] [PubMed] [Google Scholar]
- 10. Zhuang FJ, Chen Y, He WB, Cai ZY. Prevalence of white matter hyperintensities increases with age. Neural Regen Res. 2018;13(12):2141‐2146. doi: 10.4103/1673-5374.241465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kumar A, McCullough L. Cerebrovascular disease in women. Ther Adv Neurol Disord. 2021;14:1‐22. doi: 10.1177/1756286420985237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Appelman Y, van Rijn BB, ten Haaf ME, Boersma E, Peters SAE. Sex differences in cardiovascular risk factors and disease prevention. Atherosclerosis. 2014;241(1):211‐218. doi: 10.1016/j.atherosclerosis.2015.01.027 [DOI] [PubMed] [Google Scholar]
- 13. Ji H, Kim A, Ebinger JE, et al. Sex differences in blood pressure trajectories over the life course. JAMA Cardiol. 2020;5(3):255‐262. doi: 10.1001/jamacardio.2019.5306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carpenter MW. Gestational diabetes, pregnancy hypertension, and late vascular disease. Diabetes Care. 2007;30(2):S246‐S250. doi: 10.2337/dc07-s224 [DOI] [PubMed] [Google Scholar]
- 15. Sachdev PS, Parslow R, Wen W, Anstey KJ, Easteal S. Sex differences in the causes and consequences of white matter hyperintensities. Neurobiol Aging. 2009;30(6):946‐956. doi: 10.1016/j.neurobiolaging.2007.08.023 [DOI] [PubMed] [Google Scholar]
- 16. Alqarni A, Jiang J, Crawford JD, et al. Sex differences in risk factors for white matter hyperintensities in non‐demented older individuals. Neurobiol Aging. 2021;98:197‐204. doi: 10.1016/j.neurobiolaging.2020.11.001 [DOI] [PubMed] [Google Scholar]
- 17. Bonberg N, Wulms N, Dehghan‐Nayyeri M, Berger K, Minnerup H. Sex‐specific causes and consequences of white matter damage in a middle‐aged cohort. Front Aging Neurosci. 2022;14(May):810296. doi: 10.3389/fnagi.2022.810296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fatemi F, Kantarci K, Graff‐Radford J, et al. Sex differences in cerebrovascular pathologies on FLAIR in cognitively unimpaired elderly. Neurology. 2018;90(6):e466‐e473. doi: 10.1212/WNL.0000000000004913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wen W, Sachdev PS, Li JJ, Chen X, Anstey KJ. White matter hyperintensities in the forties: their prevalence and topography in an epidemiological sample aged 44‐48. Hum Brain Mapp. 2009;30(4):1155‐1167. doi: 10.1002/hbm.20586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Geerlings MI, Appelman APA, Vincken KL, et al. Brain volumes and cerebrovascular lesions on MRI in patients with atherosclerotic disease. The SMART‐MR study. Atherosclerosis. 2010;210(1):130‐136. doi: 10.1016/j.atherosclerosis.2009.10.039 [DOI] [PubMed] [Google Scholar]
- 21. Assareh AA, Mather KA, Crawford JD, et al. Renin‐angiotensin system genetic polymorphisms and brain white matter lesions in older Australians. Am J Hypertens. 2014;27(9):1191‐1198. doi: 10.1093/ajh/hpu035 [DOI] [PubMed] [Google Scholar]
- 22. Filomena J, Riba‐Llena I, Vinyoles E, et al. Short‐term blood pressure variability relates to the presence of subclinical brain small vessel disease in primary hypertension. Hypertension. 2015;66(3):634‐640. doi: 10.1161/HYPERTENSIONAHA.115.05440 [DOI] [PubMed] [Google Scholar]
- 23. Lampe L, Zhang R, Beyer F, et al. Visceral obesity relates to deep white matter hyperintensities via inflammation. Ann Neurol. 2019;85(2):194‐203. doi: 10.1002/ana.25396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tierney MC, Fisher RH, Lewis AJ, et al. The NINCDS‐ADRDA work group criteria for the clinical diagnosis of probable alzheimer's disease: a clinicopathologic study of 57 cases. Neurology. 1988;38(3):359‐364. doi: 10.1212/wnl.38.3.359 [DOI] [PubMed] [Google Scholar]
- 25. Aubert‐Broche B, Fonov VS, García‐Lorenzo D, et al. A new method for structural volume analysis of longitudinal brain MRI data and its application in studying the growth trajectories of anatomical brain structures in childhood. Alzheimers Assoc Int Conf. 2011;82:393‐402. doi: 10.1016/j.neuroimage.2013.05.065 [DOI] [PubMed] [Google Scholar]
- 26. Coupe P, Yger P, Prima S, Hellier P, Kervrann C, Barillot C. An optimized blockwise nonlocal means denoising filter for 3‐D magnetic resonance images. IEEE Trans Med Imaging. 2008;27(4):425‐441. doi: 10.1109/TMI.2007.906087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in mri data. IEEE Trans Med Imaging. 1998;17(1):87‐97. doi: 10.1109/42.668698 [DOI] [PubMed] [Google Scholar]
- 28. Dadar M, Fonov VS, Collins DL. A comparison of publicly available linear MRI stereotaxic registration techniques. Neuroimage. 2018;174(March):191‐200. doi: 10.1016/j.neuroimage.2018.03.025 [DOI] [PubMed] [Google Scholar]
- 29. Fonov V, Coupé P, Eskildsen S, et al. Atrophy specific MRI brain template for Alzheimer's disease and mild cognitive impairment. Alzheimer's Dement. 2011;7(4):S58. [Google Scholar]
- 30. Dadar M, Pascoal TA, Manitsirikul S, et al. Validation of a regression technique for segmentation of white matter hyperintensities in Alzheimer's disease. IEEE Trans Med Imaging. 2017;36(8):1758‐1768. [DOI] [PubMed] [Google Scholar]
- 31. Dadar M, Fereshtehnejad SM, Zeighami Y, Dagher A, Postuma RB, Collins DL. White matter hyperintensities mediate impact of dysautonomia on cognition in Parkinson's disease. Mov Disord Clin Pract. 2020;7(6):639‐647. doi: 10.1002/mdc3.13003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Anor CJ, Dadar M, Collins DL, Tartaglia MC. The longitudinal assessment of neuropsychiatric symptoms in mild cognitive impairment and Alzheimer's disease and their association with white matter hyperintensities in the National Alzheimer's coordinating center's uniform data set. Biol Psychiatry Cogn Neurosci Neuroimaging. 2021;6(1):70‐78. doi: 10.1016/j.bpsc.2020.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dadar M, Misquitta K, Anor CJ, et al. NeuroImage Performance comparison of 10 different classification techniques in segmenting white matter hyperintensities in aging. NeuroImage. 2017;157(May):233‐249. doi: 10.1016/j.neuroimage.2017.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hammers A, Allom R, Koeep M, et al. Validation of T1w‐based segmentations of white matter hyperintensity volumes in large‐scale datasets of aging. Hum Brain Mapp. 2003;19:224‐247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Coupé P, Manjón JV, Fonov V, Pruessner J, Collins DL. NeuroImage patch‐based segmentation using expert priors : application to hippocampus and ventricle segmentation. Neuroimage. 2011;54(2):940‐954. doi: 10.1016/j.neuroimage.2010.09.018 [DOI] [PubMed] [Google Scholar]
- 36. Manera AL, Dadar M, Collins DL, Ducharme S. Ventricular features as reliable differentiators between bvFTD and other dementias. NeuroImage Clin. 2022;33:102947. doi: 10.1016/j.nicl.2022.102947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Smith CD, Johnson ES, Van Eldik LJ, et al. Peripheral (deep) but not periventricular MRI white matter hyperintensities are increased in clinical vascular dementia compared to Alzheimer's disease. Brain Behav. 2016;6(3):1‐11. doi: 10.1002/brb3.438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Armstrong NJ, Mather KA, Sargurupremraj M, et al. Common genetic variation indicates separate causes for periventricular and deep white matter hyperintensities. Stroke. 2020;51(7):2112‐2121. doi: 10.1161/STROKEAHA.119.027544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. National Institutes of Health . High Blood Pressure and Older Adults. Accessed July 28, 2023. https://www.nia.nih.gov/health/high‐blood‐pressure‐and‐older‐adults#:~:text=For%20older%20adults%2C%20often%20the,stiffening%20of%20the%20major%20arteries
- 40. Hachinski V, Oveisgharan S, Romney AK, Shankle WR. Optimizing the hachinski ischemic scale. Arch Neurol. 2012;69(2):169‐175. doi: 10.1001/archneurol.2011.1698 [DOI] [PubMed] [Google Scholar]
- 41. Dadar M, Camicioli R, Duchesne S, Collins DL. The temporal relationships between white matter hyperintensities, neurodegeneration, amyloid beta, and cognition. Alzheimer's Dement Diagnosis, Assess Dis Monit. 2020;1(June):e12091. doi: 10.1002/dad2.12091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kloppenborg RP, Geerlings MI, Presence and progression of white matter hyperintensities and cognition. 2014;(May). doi: 10.1212/WNL.0000000000000505 [DOI] [PubMed]
- 43. Morrison C, Dadar M, Villeneuve S, Collins DL. White matter hyperintensities may be an early marker for age‐related cognitive decline. bioRxiv. Published online 2021. [DOI] [PMC free article] [PubMed]
- 44. Kamal F, Morrison C, Moranzano J, Zeighami Y, Dadar M, Topographical differences in white matter hyperintensity burden and cognition in aging, MCI, and AD. medRXIV. Published online 2022. [DOI] [PMC free article] [PubMed]
- 45. Benjamini Y, Hochberg Y, Benjamini YoavHY. Controlling the false discovery rate: a practical and powerful approach to mutliple testing. J R Stat Soc Ser B. 1995;57(1):289‐300. [Google Scholar]
- 46. Canadian Institutes of Health Research . Sex and Gender Training Modules. Accessed July 28, 2023. https://cihr‐irsc.gc.ca/e/49347.html
- 47. McAleese KE, Walker L, Graham S, et al. Parietal white matter lesions in Alzheimer's disease are associated with cortical neurodegenerative pathology, but not with small vessel disease. Acta Neuropathol. 2017;134:459‐473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ferretti MT, Iulita MF, Cavedo E, et al. Sex differences in Alzheimer disease — The gateway to precision medicine. Nat Rev Neurol. 2018;14(8):457‐469. doi: 10.1038/s41582-018-0032-9 [DOI] [PubMed] [Google Scholar]
- 49. Barnes LL, Wilson RS, Bienias JL, Schneider JA, Evans DA, Bennett DA. Sex differences in the clinical manifestations of Alzheimer disease pathology. Arch Gen Psychiatry. 2005;62(6):685‐691. doi: 10.1001/archpsyc.62.6.685 [DOI] [PubMed] [Google Scholar]
- 50. Kamal F, Morrison C, Moranzano J, Zeighami Y, Dadar M. White matter hyperintensity trajectories in patients with progressive and stable mild cognitive impairment. Neurology. 2023;101(8):e815‐e824. 10.1212/WNL.0000000000207514 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ICMJE DISCLOSURE FORM
Table S1
Supporting Information


