Abstract
INTRODUCTION
This study examined the relationship between blood‐brain‐barrier permeability (BBBp), measured by cerebrospinal fluid/serum albumin ratio (QAlb), and cognitive decline progression in a clinical cohort.
METHODS
This prospective observational study included 334 participants from the BIODEGMAR cohort. Cognitive decline progression was defined as an increase in Global Deterioration Scale and/or Clinical Dementia Rating scores. Associations between BBBp, demographics, and clinical factors were explored.
RESULTS
Male sex, diabetes mellitus, and cerebrovascular burden were associated with increased log‐QAlb. Vascular cognitive impairment patients had the highest log‐QAlb levels. Among the 273 participants with valid follow‐up data, 154 (56.4%) showed cognitive decline progression. An 8% increase in the hazard of clinical worsening was observed for each 10% increase in log‐QAlb.
DISCUSSION
These results suggest that increased BBBp in individuals with cognitive decline may contribute to clinical worsening, pointing to potential targeted therapies. QAlb could be a useful biomarker for identifying patients with a worse prognosis.
Keywords: blood‐brain barrier, cerebrovascular disease, cognition, dementia, vascular dementia
1. BACKGROUND
Cognitive impairment (CI) and dementia, including Alzheimer's disease (AD), are major causes of disability and dependency worldwide, affecting millions of people and their families. 1 The impact of these conditions on quality of life is substantial and represents a significant public health challenge. The blood‐brain barrier (BBB) plays a crucial role in maintaining brain homeostasis and health by regulating the exchange of molecules into and out of the brain parenchyma. 2 Disruption of the BBB may contribute to the development and progression of CI and dementia. 3 Vascular risk factors and cerebrovascular pathology are common in all stages of CI and dementia and have been shown to affect BBB integrity. 4 , 5 , 6 Previous studies reported an increase in BBB permeability (BBBp) in individuals with CI. 3 , 7 , 8 , 9 Moreover, cerebrovascular pathology and BBB dysfunction have been linked to AD pathophysiology. 10 , 11 The cerebrospinal fluid (CSF)/serum albumin ratio (QAlb) is a widely used parameter for measuring BBBp with respect to large molecules. 12 , 13 , 14 Due to its reproducibility and relative ease of determination in clinical laboratories, it may be useful in studies of cognitive decline and other neurological diseases. 12 , 15 , 16 , 17 , 18 , 19 , 20 However, its clinical utility has not been well established.
The primary objective of this study was to assess the association between BBBp (as measured by QAlb) and the risk of progression of cognitive decline in a clinical cohort of individuals with cognitive decline at different stages, from subjective cognitive decline to severe dementia. Our secondary objective was to explore the independent relationship between BBBp and demographic, clinical, vascular, etiologic, and radiological factors.
By investigating the relationship between BBBp and cognitive decline progression in a real‐world clinical cohort, this study aimed to increase knowledge on the underlying mechanisms of CI and dementia and to identify potential biomarkers that may be useful in patient management.
RESEARCH IN CONTEXT
Systematic review: The authors conducted a comprehensive literature review using traditional sources (eg, PubMed) to explore blood‐brain‐barrier permeability (BBBp) and albumin quotient (QAlb) in cognitive decline. Despite numerous publications on this topic, few have investigated their significance in real‐world memory clinic populations. Relevant publications are cited throughout the manuscript.
Interpretation: In this longitudinal observational study in a memory clinic population, we found that increased BBBp was associated with cognitive decline progression. This suggests that QAlb could serve as a potential biomarker for identifying individuals at higher risk of cognitive decline progression and that increased BBBp may be linked to clinical worsening.
Future directions: Additional research should focus on understanding the underlying mechanisms of increased BBBp and developing effective therapeutic strategies targeting BBB dysfunction in patients with cognitive decline. Future studies should investigate the potential role of QAlb as a prognostic biomarker in clinical settings, which could lead to improved patient assessment and management.
2. METHODS
2.1. Participants and study design
We conducted a prospective observational study of patients consecutively included in the BIODEGMAR cohort 21 between September 2017 and September 2021. The BIODEGMAR is an observational longitudinal study that enrolls individuals with memory complaints or cognitive decline admitted at the Cognitive Decline and Movement Disorders Unit, Department of Neurology, Hospital del Mar (Barcelona, Spain). As a clinical cohort of a memory unit, the BIODEGMAR cohort includes participants with a high heterogeneity in demographics, comorbidities, and disease presentations, reflecting a real‐world scenario. Inclusion criteria: (i) subjects evaluated in the Cognitive Decline and Movement Disorders Unit at Hospital del Mar included in DEGMAR register (see eMethods in Supplement 1); (ii) signed informed consent; (iii) meet diagnostic criteria for the following syndromes and conditions (see ‘clinical diagnostic’). Exclusion criteria: (i) ≥80 years old; (ii) any contraindication for lumbar puncture; (iii) do not agree with study procedures. The procedures of the BIODEGMAR study include a clinical visit, an extensive neuropsychological evaluation, magnetic resonance imaging (MRI), APOE genotyping, a lumbar puncture for CSF collection, and blood sampling. 22 A comprehensive description of the BIODEGMAR cohort, the inclusion and exclusion criteria, study procedures, the core AD CSF biomarkers measurements, and cutoffs determination can be found in the eMethods in the Supplement 1.
2.2. Sociodemographic and clinical data
The sociodemographic and clinical information was collected using an extensive structured questionnaire. 21 Briefly, we collected sociodemographic information including sex, age, years of education, and vascular risk factors such as hypertension, diabetes mellitus type 2 (DM), hyperlipidemia, and active smoking habit.
2.3. Clinical diagnosis
Participants in the BIODEGMAR cohort with a Global Deterioration Scale (GDS) > 1 22 and the following clinical diagnosis were included in the current study: subjective cognitive complaints (n = 21) 23 ; mild cognitive impairment syndrome (MCI; n = 110) 24 ; AD‐type dementia (possible, probable, and atypical presentation; n = 120) 25 ; behavioral variant Frontotemporal Dementia (n = 12) 26 ; primary progressive or progressive aphasia (logopenic, progressive non fluent, and semantic variants; n = 17) 27 ; Lewy body dementia (LBD; n = 9) 28 ; corticobasal syndrome (CBS) 29 and progressive supranuclear palsy (PSP) (n = 8) 30 ; vascular cognitive impairment and dementia (VCID; n = 12) 31 ; and cerebral amyloid angiopathy (CAA; n = 11). 32 Individuals with other causes of dementia or with an unspecified clinical diagnosis were included as “other” (n = 9).
2.4. Core AD CSF biomarkers
Core AD CSF biomarkers (Aβ42/40, p‐tau181 and t‐tau) were measured at the Laboratori de Referència de Catalunya with Lumipulse, Fujirebio. 21 AD was biologically defined (b‐AD) according to CSF amyloid beta (Aβ) 42/p‐tau181 ratio < 10.25, 33 regardless of clinical diagnostic. Extensive information about core CSF AD biomarkers and cutoffs are included in eMethods Supplement 1.
2.5. QAlb (CSF/serum albumin)
Serum albumin was measured by colorimetric method using bromocresol green. CSF albumin was measured by the immunoturbidimetry method. Both serum and CSF albumin measurements were performed with Cobas‐Hitachi automated reagents and systems (Roche Diagnostics GmbH). Higher levels of QAlb point to increased BBBp. 13
2.6. Neuroimaging and cerebrovascular burden (CVB)
Brain MRI was performed in all participants, except in cases with contraindication (eg, pacemaker, MRI‐incompatible aneurysm clips, and claustrophobia). In case of contraindication for MRI, head CT scan was performed as part of the clinical diagnostic process. Structural MRI scans were acquired on 1.5 T (General Electric Signa Explorer) or 3T (Philips Achieva). The imaging protocol included T1‐ and T2‐weighted sequences, high‐resolution T1 3D, diffusion‐weighted images, FLAIR imaging, gradient echo, and/or ven‐Bold sequences. CVB was defined as the presence of extensive white matter hyperintensities as a subcortical and/or periventricular Fazekas’ score 34 higher than 1 and/or presence of any brain vascular infarct or hemorrhagic lesion including microbleeds. 35
2.7. Follow‐up and neuropsychological controls
A clinical visit after lumbar puncture was performed (2 to 8 weeks). Follow‐up visits, including clinical and neuropsychological evaluations, were conducted yearly (12 ± 2 months). According to clinical and neuropsychological evaluations, Global Deterioration Scale (GDS) and Clinical Dementia Rating (CDR; global score) 36 were assessed to determine the cognitive decline stage. The endpoint for longitudinal analyses was progression of cognitive decline, which was defined as a binary variable, with patients undergoing any increase in GDS and/or CDR scales during the follow‐up being considered as progressors and those without increase in these scales as nonprogressors. Other clinical visits were performed at a neurologist's discretion depending on clinical necessities. Clinical records were reviewed to check on the possible progression of cognitive decline before annual visits. The present study followed up patients until September 2022. We only included those subjects with GDS and CDR scores lower than 6 and 3, respectively, where a GDS score of 6 indicates a severe dementia stage and a CDR of 3 is the highest possible. We defined a minimum time of follow‐up of 12 months. All cases were reviewed at the conclusion of follow‐up to confirm cognitive decline progression and rule out fluctuations of symptoms (n = 5).
2.8. Standard protocol approvals, registrations, and patient consent
The DEGMAR register and the BIODEGMAR study were approved by the Drug Research Ethics Committee (CEIm), Barcelona (CEIm PSMAR, project code 2018/7805I). All participants (and/or a representative when appropriate), provided written informed consent approved by our local ethics committee (CEIm PSMAR).
2.9. Statistical analysis
2.9.1. Sample characteristics
In the descriptive analyses, we present data as medians (interquartile range) for continuous variables and as frequencies (percentage) for categorical variables. As we show in Figure 1, QAlb levels had a left‐skewed distribution, and we applied the loge transform, which is used henceforth as the variable of interest (log‐QAlb).
FIGURE 1.
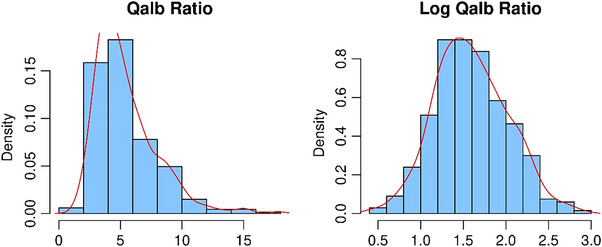
Distribution of Qalb levels before (left) and after (right) loge transformation.
2.9.2. Cross‐sectional association between BBBp and main clinical factors
We first conducted a series of univariate analyses to assess which clinical factors or diagnoses were associated with QAlb at the baseline visit (cross‐sectional analysis, N = 334). To that end, we used Spearman's correlation, t, or ANOVA tests, as appropriate.
2.9.3. Longitudinal effect of BBBp on risk of cognitive decline progression
In the second part of the analysis, we focused on a subset of patients with valid follow‐up data (longitudinal analysis, N = 273). These patients were followed up until September 2022, and we registered the incidence of cognitive decline progression. Longitudinal data were right censored at the third year of follow‐up to avoid an excessive number of censored patients. We first used Kaplan‐Meier curves to observe which clinical variables were associated with the progression of cognitive decline within the follow‐up, and significance levels were estimated using the log‐rank test. The cognitive decline progression rate is presented as 1 (survival function) and 95% confidence intervals (CIs). For the univariate analysis, continuous variables were split into tertiles (post hoc comparisons were corrected for family‐wise error with Bonferroni adjustment). We subsequently built multivariate Cox regression models to estimate the effect of QAlb on clinical progression after adjusting for potential confounders that were selected according to the univariate analysis (p ≤ .1) or previous literature. Therefore, the scope model was fully adjusted for age, sex, education, hypertension, diabetes, hyperlipidemia, b‐AD, CDR scale, cerebrovascular burden, and log‐QAlb. Variables were selected via a forward stepwise algorithm, and the metric of interest was the Akaike information criterion. We chose to adjust this multivariate model only for the baseline CDR instead of both the CDR and GDS scores to avoid having to deal with collineary. For the last model, we checked Schoenfeld and deviance residuals to detect potential violations, discard outliers, and confirm the proportional hazard assumption. We set the α value at 0.05. A team of two biostatisticians reviewed all statistical analyses (JJ‐B, A.O.). All the statistical analyses were done with R software (R version 4.2.1; Copyright (c) 2022 The R Foundation for Statistical Computing, Vienna, Austria).
3. RESULTS
3.1. Participant characteristics
From September 22, 2017 to September 29, 2021, a total of 360 individuals were included in the BIODEGMAR cohort, 350 with GDS > 1. For this study, we excluded 11 participants with undetermined plasma or CSF albumin levels, three cases of rapidly progressive dementia caused by Creutzfeldt‐Jakob disease (CJD), one case with a disseminated neoplastic disease, and one case with an active systemic inflammatory disease.
3.2. Sample characteristics
Table 1 shows the main demographic, clinical, and radiological characteristics of the sample (N = 334); the median age was 74 years (Q1‐Q3: 69 to 77), and 195 (58.4%) were females. Median QAlb was 4.75 mg/g (Q1‐Q3: 3.66 to 6.58).
TABLE 1.
Main characteristics of sample (N = 334).
| Age | 74.0 [69.0; 77.0] |
| Sex, Female | 195 (58.4%) |
| Education, years | 8.00 [6.00; 10.0] |
| Hypertension | 186 (55.7%) |
| Diabetes mellitus | 72 (21.6%) |
| Hyperlipidemia | 167 (50.0%) |
| Active smoker | 28 (9.36%) |
| Cerebrovascular burden | 121 (38.2%) |
| b‐AD | 217 (65.0%) |
| ApoE‐ε4 carrier | 114 (45.8%) |
| GDS score | |
| 2 | 27 (8.08%) |
| 3 | 128 (38.3%) |
| 4 | 125 (37.4%) |
| 5 | 41 (12.3%) |
| 6 | 13 (3.89%) |
| CDR score | |
| 0 | 26 (7.78%) |
| 0.5 | 127 (38.0%) |
| 1 | 86 (25.7%) |
| 2 | 78 (23.4%) |
| 3 | 17 (5.09%) |
Note: Values represent medians (interquartile range) or numbers (percentage) according to type of each variable. Missing data: education, five cases; smoking, 35 cases; cerebrovascular burden, 17 cases; ApoE polymorphism: 85 cases.
Abbreviations: b‐AD, biologically defined AD (according to abnormal CSF Aβ42/p‐Tau181 ratio; CDR, clinical dementia rating scale; GDS, global deterioration scale.
3.3. Cross‐sectional association between BBBp and main clinical factors
We first analyzed which of the clinical variables included in Table 1 were associated with log‐QAlb levels and found that males presented higher permeability levels than females (Figure 2A). Similarly, the presence of DM and CVB was associated with increased log‐QAlb (Figure 2A). On the other hand, clinical staging according to GDS or CDR did not affect log‐QAlb levels (Figure 2B). Neither age (r = 0.02, p = 0.655) nor years of education (r = 0.06, p = 0.250) were significantly correlated with BBBp as measured by log‐QAlb. We finally compared how log‐QAlb levels differed between subjects according to their clinical diagnosis, finding global significant differences across groups (F (9324) = 2.89, p = 0.003). Post hoc comparisons revealed that VCID patients had the highest levels of log‐QAlb and were significantly different compared to the AD‐type dementia group, which presented the lowest levels of log‐QAlb together with the progressive aphasia group (Figure 3).
FIGURE 2.
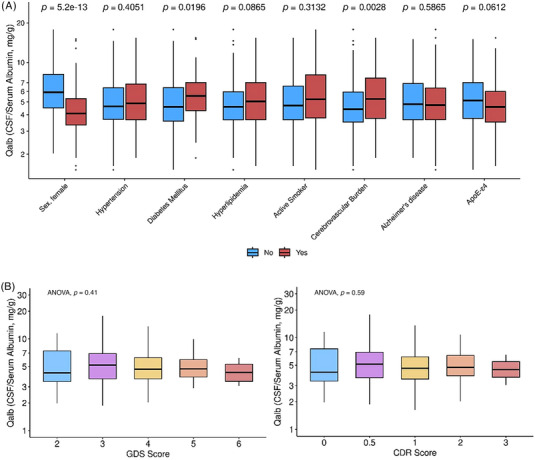
Cross‐sectional differences in levels of Qalb for main clinical variables. (A) Differences in Qalb levels for main vascular and cognitive impairment risk factors (p values represent pairwise t test contrasts). (B) Illustration of how Qalb differs according to clinical stage both for GDS and CDR scales; p values were obtained with ANOVA tests. In both panels, the y axis (Qalb levels) were log‐scaled.
FIGURE 3.
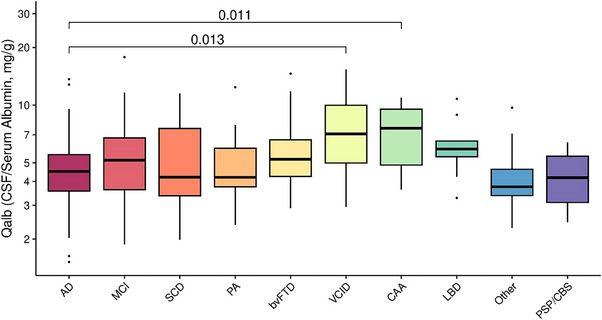
Qalb levels by clinical diagnosis. Groups were compared with pairwise t tests; square brackets indicate which groups are significantly different at p < 0.05. AD, Alzheimer's disease; bvFTD, behavioral variant frontotemporal dementia; LBD, Lewy body dementia; MCI, mild cognitive impairment; PA, progressive aphasia; PSP/CBS, progressive supranuclear palsy and/or corticobasal syndrome; SCD, subjective cognitive complaints; VCID, vascular cognitive impairment and dementia.
3.4. Longitudinal effect of BBBp on risk of cognitive decline progression
Among 334 cases, we followed up with 273 (81.7%) patients within 3 years (median follow‐up [Q1 to Q3]: 17 months [12.5 to 29]), representing 5675 patient‐months. A total of 61 participants were excluded from this analysis, 48 participants because they did not have a minimum follow‐up of 12 months and 13 participants due to advanced dementia stage at baseline. Twenty‐nine participants were censored due to loss of follow‐up (n = 18) or death (n = 11).
We detected 154 (56.4%) patients showing progression of cognitive decline, which represents an event rate of 65.7% in the whole sample at the end of the follow‐up (95% CI: 58.6% to 71.7%, Figure 4A). In Table 2 we show the effect of the main clinical variables on the risk of cognitive decline progression. As we visually represent in Figure 4B, patients with higher BBBp presented an increased rate of progression at the end of the follow‐up (log‐rank p value = .020). In post hoc pairwise comparisons, we found that this result was driven by patients with log‐QAlb ≤ 3.61 (first tertile: cognitive decline progression rate at 3 years = 54%) as compared to patients with log‐QAlb > 6.62 (third tertile: cognitive decline progression rate at 3 years = 73%, log‐rank p valueBonferroni = .02). An AD core biomarker profile in CSF (b‐AD), ApoE‐ε4 carrier status, and higher GDS or CDR score were all associated with progression of cognitive decline as well (Table 2).
FIGURE 4.
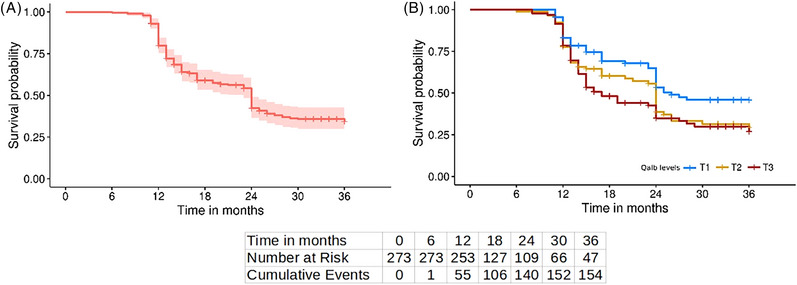
Survival curve of sample. Both survival curves show likelihood of cognitive decline progression within follow‐up, as an increment in GFS and/or CDR scales. (A) Survival curve of sample, where light red shade represents 95% confidence intervals, and vertical lines are censored patients. (B) Same survival curve by Qalb levels, split into tertiles (T1, blue; T2, yellow; and T3, red). At bottom of both figures are the number of patients at risk of progression at the beginning of each time point and the cumulative number of events at the end of the same time point.
TABLE 2.
Effect of main clinical variables and Qalb on risk of clinical decline progression (N = 273).
| Variable | CDP rate (95% CI) | P | Variable | CDP rate (95% CI) | P |
|---|---|---|---|---|---|
| Age, years | QAlb, mg/g | ||||
| Tertile 1 [50, 69] | 0.56 (0.45;0.67) | 0.1599 | Tertile 1 [1.52, 3.61] | 0.54 (0.43;0.65) | 0.0199 |
| Tertile 2 [70, 77] | 0.73 (0.63;0.83) | Tertile 2 [3.61, 6.62] | 0.71 (0.6;0.82) | ||
| Tertile 3 [78, 84] | 0.69 (0.56;0.82) | Tertile 3 [6.62, 17.80] | 0.73 (0.62;0.84) | ||
| Sex, female | GDS score | ||||
| Yes | 0.65 (0.55;0.75) | 0.3338 | 2 | 0.19 (0.02;0.36) | <0.0001 |
| No | 0.67 (0.59;0.75) | 3 | 0.62 (0.52;0.72) | ||
| Education, years | 4 | 0.77 (0.67;0.87) | |||
| Tertile 1 [0, 6] | 0.64 (0.53;0.75) | 0.2833 | 5 | 0.84 (0.7;0.98) | |
| Tertile 2 [7, 10] | 0.76 (0.66;0.86) | CDR score | |||
| Tertile 3 [11, 20] | 0.56 (0.44;0.68) | 0 | 0.2 (0.02;0.38) | 0.0001 | |
| Hypertension | 0.5 | 0.62 (0.52;0.72) | |||
| Yes | 0.66 (0.57:0.75) | 0.7579 | 1 | 0.76 (0.65;0.87) | |
| No | 0.65 (0.55;0.75) | 2 | 0.8 (0.67;0.93) | ||
| Diabetes | CVB | ||||
| Yes | 0.73 (0.6;0.86) | 0.2950 | Yes | 0.76 (0.66;0.86) | 0.1217 |
| No | 0.63 (0.56;0.7) | No | 0.64 (0.55;0.73) | ||
| Hyperlipidemia | b‐AD | ||||
| Yes | 0.71 (0.62;0.8) | 0.3878 | Yes | 0.8 (0.73;0.87) | <0.0001 |
| No | 0.6 (0.5;0.7) | No | 0.38 (0.27;0.49) | ||
| Active smoker | ApoE‐ε4 | ||||
| Yes | 0.7 (0.51;0.89) | 0.1535 | Yes | 0.77 (0.68;0.86) | 0.0008 |
| No | 0.66 (0.59;0.73) | No | 0.51 (0.41;0.61) |
Note: Values represent rate of clinical progression at third year of follow‐up according to Kaplan‐Meier estimator (1 − survival rate) and 95% confidence intervals for each variable and group. Significance levels were calculated using log‐rank test. Continuous variables (Qalb, age, and education) were split into tertiles. Missing data: education, three cases; smoking, 25 cases; cerebrovascular burden, 14 cases; ApoE polymorphism, 61 cases.
Abbreviations: b‐AD, biologically defined AD (according to abnormal CSF Aβ42/p‐Tau181 ratio); CI, confidence interval; CDP, clinical decline progression; CDR, clinical dementia rating scale; CVB, cerebrovascular burden; CDR, clinical dementia rating scale; log‐QAlb, log‐scaled CSF/serum albumin ratio.
After adjusting for variables that were associated with clinical worsening in the univariate analysis or that have been described to increase the risk of cognitive decline (age, sex, years of education, hypertension, diabetes, hyperlipidemia, b‐AD, CDR scale, and CVB), we analyzed the independent effect of BBBp on this progression. Variables were selected for inclusion in the final regression model using a forward stepwise algorithm, and only sex, b‐AD, CDR, and log‐QAlb were found to be significant predictors of cognitive decline progression in our cohort. As we show in Table 3, for each 10% increase in log‐QAlb levels we observed an 8% increase in the hazard of clinical worsening (95% CI = 1.04 to 1.13, p < 0.001). Similarly, female sex was associated with increased risk of cognitive decline progression such that females showed a 1.63‐fold increased hazard of progression at the end of the follow‐up as compared to males (Table 3).
TABLE 3.
Cox regression models showing effect of BBBp on risk of cognitive decline progression (N = 273).
| HR (95% CI) | P | |
|---|---|---|
| Sex, female | 1.63 (1.12;2.36) | 0.0099 |
| Log‐QAlb, 10% increase in Qalb | 1.08 (1.04;1.13) | 0.0003 |
| Alzheimer's disease | 2.09 (1.39;3.13) | 0.0004 |
| CDR Score = 0.5 | 2.92 (1.05;8.16) | 0.0408 |
| CDR Score = 1 | 3.23 (1.13;9.21) | 0.0286 |
| CDR Score = 2 | 4.67 (1.62;13.47) | 0.0043 |
Note: The dependent variable was the incidence of CDP within 3 years, and values represent hazard ratios, 95% confidence intervals, and p values. Variables were selected via a forward stepwise algorithm, where the metric of interest was the Akaike information criterion and the scope model was fully adjusted for age, sex, education, hypertension, diabetes, hyperlipidemia, Alzheimer's disease, CDR scale, cerebrovascular burden, and log‐Qalb. In this table we show the final model at the last iteration.
Abbreviations: BBBp, blood‐brain‐barrier permeability; CI, confidence interval; CDR, clinical dementia rating scale; HR, hazard ratio; log‐QAlb, log‐scaled CSF/serum albumin ratio.
4. DISCUSSION
In our study, we found that QAlb was positively associated with male sex, DM, and CVB. We did not observe any association with AD CSF profile, nor with CI severity. Notably, we found that increased BBBp was independently associated with progression of cognitive decline.
4.1. Cross‐sectional association between BBBp and main clinical factors
Associations between QAlb, male sex, 20 , 37 , 38 and vascular risk factors were previously described. 18 , 19 , 20 , 39 , 40 In our study, although only the association of log‐QAlb with DM reached statistical significance, QAlb was positively associated with all vascular risk factors (HTA, DM, hyperlipidemia, and being active smoker). Moreover, patients with CVB showed higher QAlb levels, in line with previous studies. 39 , 40 In addition, we found higher Qalb levels in patients with a clinical diagnosis of VCID, as previously reported in similar studies. 16 , 17 , 39
On the other hand, clinical staging according to GDS or CDR was not associated with QAlb, neither age nor b‐AD. To the best of our knowledge, no recent studies evaluated BBBp and CI syndrome caused by diverse etiologies at several stages, as we did in this study. Although most participants were classified as being between prodromal and mild to moderate dementia stages, observing a lower number of participants with subjective cognitive complaints (SCD) or advanced dementia stages (27 GDS 2, 13 GDS6), we find these numbers sufficiently robust. Our results might suggest that increased BBBp reaches a limit in mild dementia stages. However, it is important to point out that clinical diagnoses at each functional stage are diverse. Thus, we cannot rule out a relationship between QAlb and GDS or CDR scores within each specific group. Previous works showed a positive association of QAlb with aging. 38 , 41 In our study, we found no association, probably because of a relative short range of age distribution in our sample, which included patients visiting at a memory unit with a low number of young patients. We found no association between biologically defined AD (as AD CSF profile regardless of clinical diagnosis) and QAlb. Previous studies described altered BBBp in AD patients, from preclinical to dementia stages. 41 These findings were consistent with results obtained in animal models that proposed amyloidosis as a potential factor contributing to increased BBBp. 8 , 42 However, recent studies do not support this hypothesis when BBBp is measured through determination of QAlb, which indicates increased BBBp to large molecules. A meta‐analysis by Olson et al. 12 suggested that there was no significant change in QAlb levels in AD compared to other neurological diseases. 15 Three additional studies 16 , 18 , 19 reported that this association was more likely related to the well‐known coexistence of AD and vascular risk factors rather than being an AD‐specific pathophysiology. Our results support these findings, although we cannot rule out an association of increased BBBp and AD pathophysiology within the AD continuum, as our cohort did not include a cognitively unimpaired population.
4.2. Longitudinal data and associations of BBBp with increased risk of cognitive decline progression
Multiple studies have analyzed possible predictors that identify subjects at higher risk of clinical worsening, most of them in the AD continuum assessing AD pathology‐related fluid biomarkers as well as imaging biomarkers such as the dementia conversion‐related pattern (ADCRP) on [18F] Fluorodeoxyglucose Positron Emission Tomography (FDG PET). 43 , 44 , 45 , 46 , 47 Our results show factors already described as strongly associated with progression of cognitive decline such as b‐AD (abnormal Aβ42/p‐Tau181 ratio), APOE‐ε4 carrier status, and female sex. 48 Abnormal levels of core AD CSF biomarkers are related to a higher risk of progression to dementia among SCD and MCI patients. 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 Interestingly, our results show an association between female sex and worse clinical prognosis, in line with previous evidence, 57 although this effect might be modulated by sociodemographic aspects, suggesting a role of modifiable risk factors. 57 Despite the growing interest in sex differences in cognitive decline, particularly in the AD continuum, there is a need for further epidemiological and clinical data to achieve a deeper understanding of this topic. Finally, our data showed a higher risk of cognitive decline progression as CDR increased. In recent years, few studies have compared the risk of clinical progression across different stages of cognitive impairment. A recent study 58 proposed a model of disease progression in the AD continuum, although it did not include the progression risk within dementia stages. A previous work showed that the risk of institutionalization and death increases with age and severity stage. 59 In our opinion, a better knowledge of prognostic factors during dementia stages of cognitive decline is needed, as patient management and the need of support change notably.
The results of our study show that increased BBBp, which is associated with vascular risk factors and CVB, is independently associated with a worse prognosis in individuals with cognitive decline. QAlb has been associated with a poorer prognosis in other neurological diseases such as spinal amyotrophic lateral sclerosis or multiple sclerosis. 60 , 61 To the best of our knowledge, this is the first time that QAlb has been related to the progression of cognitive decline. Moreover, the association between increased BBBp and clinical progression was observed independently of b‐AD. These results highlight the role of vascular pathophysiological mechanisms in neurodegenerative, vascular, and mixed etiologies of cognitive impairment. 4 , 10 , 11 Of note, other fluid and imaging biomarkers of BBB alterations have provided valuable insights into the contributions of vascular dysfunction to cognitive decline and AD in recent years. Dynamic contrast‐enhanced MRI (DCE‐MRI) provides a method to estimate the index of BBB permeability (Ktrans) at the voxel level. 9 Using this technique, previous studies found that BBB disruption started at the early stages of the disease in AD‐signature regions of brain such as the hippocampus and the parahippocampal gyrus, 9 , 62 finding differences between patients with a CDR of 0 and 0.5 independently of Tau and Aβ markers. Moreover, the soluble platelet‐derived growth factor β (sPDGFRβ), which is a marker of pericyte injury, 9 , 63 , 64 , 65 , 66 is increased during the AD continuum, including early stages, 64 and correlates with age and BBB breakdown as measured by Ktrans and Qalb. 63 , 67 Interestingly, patients with progressive MCI show higher CSF sPDGFRβ levels as compared to non‐progressors. 63 Moreover, baseline levels of sPDGFRβ in APOE‐ε4 carriers predicted future cognitive decline after controlling for Aβ and Tau status. 68 Altogether, Qalb, Ktrans, and sPDGFRβ might represent different aspects of physiopathological mechanisms involved in BBB disruption, which encourages study of their complementarity as markers of vascular dysfunction in cognitive impairment.
Our results may have important implications for the clinical management of patients with cognitive decline. Given the paradigm shift that the arrival of disease‐modifying therapies may entail soon, novel biomarkers for a better characterization of patients are needed. The AT(N) system 69 might incorporate novel biomarkers of other pathophysiological mechanisms such as synaptic loss, neuroimmune dysregulation, or BBB dysfunction, 62 , 70 evolving toward an ATX(N) system 69 , 71 in which vascular dysfunction would be represented as ‘V.’ 11 , 71 Our findings point to the possible use of QAlb as a vascular biomarker of BBB alteration in the ATV(N) system, 71 providing important clinical insight on this regard besides a better characterization. 62 , 70 In individuals in the AD continuum, it might identify those individuals at higher risk of a more aggressive disease course. In addition to this, QAlb might serve as a prognostic biomarker among other neurodegenerative, vascular, and mixed etiologies beyond the AD continuum. Identifying AD‐negative individuals at high risk of clinical worsening through this marker holds clinical relevance, as there is a lack of prognostic biomarkers in these groups of patients. However, further studies focusing on the potential role of Qalb as a prognostic biomarker in other more homogeneous clinical cohorts with a larger number of patients is needed.
Our study is not free of limitations. First, as an observational study, our results do not establish causality, and the possibility of confounding factors cannot be ruled out. Additionally, as our sample size was relatively small, it would be important to investigate whether our findings are generalizable to other populations. It is also important to note that our sample consisted primarily of individuals with clinical diagnoses within the AD continuum, and the relatively limited number of participants with other diagnoses may limit the generalizability of our results. However, our cohort reflects the characteristics of a real‐world clinical memory unit, with a higher heterogeneity of demographic, comorbidities, and clinical presentations compared to research cohorts.
In conclusion, our study highlights the potential clinical utility of QAlb for identifying individuals at higher risk of progression of cognitive decline, suggesting that increased BBBp may contribute to clinical worsening and represent a potential therapeutic target. Further studies are needed to explore the underlying mechanisms of increased BBBp and to develop effective therapeutic strategies targeting BBB dysfunction in patients with CI.
CONFLICT OF INTEREST STATEMENT
A.P.‐P. has served on advisory boards for Schwabe Farma Iberica. M.S‐C. has served as a consultant and on advisory boards for Roche Diagnostics International Ltd. and has given lectures at symposia sponsored by Roche Diagnostics, S.L.U. and Roche Farma, S.A. All other coauthors declare no competing interests. Author disclosures are available in the supporting information.
CONSENT STATEMENT
All human subject participants in the study provided informed consent.
Supporting information
Supporting information
Supporting information
ACKNOWLEDGMENTS
The authors would like to express their most sincere gratitude to the BIODEGMAR participants and relatives at Hospital del Mar (Barcelona, Spain) without whom this research would not have been possible. The authors also thank all the staff at the Cognitive Decline and Movement Disorders Unit of the Department of Neurology at Hospital del Mar, as well as the entire team of the Neurology Department, the nursing, assistant, and administrative staff in the outpatient and day care units in Hospital del Mar, and the staff at IMIM (Hospital del Mar Research Institute) who supported this project. The authors also wish to extend their appreciation to our knowledgeable and skilled Ñoldor Joan J‐B, whose generous contributions and collaborative spirit have been invaluable to our team. Finally, the authors also thank the collaboration of the staff at Laboratori de Referència de Catalunya. This project was funded in part by the Spanish Institute of Health Carlos III (project reference AC20/00001, PI PI21/00194) and European Research Area Net (ERANET) ERA‐CVD_JTC2020‐015.
Puig‐Pijoan A, Jimenez‐Balado J, Fernández‐Lebrero A, et al. Risk of cognitive decline progression is associated to increased blood‐brain‐barrier permeability: A longitudinal study in a memory unit clinical cohort. Alzheimer's Dement. 2024;20:538–548. 10.1002/alz.13433
Joan Jiménez‐Balado and Albert Puig‐Pijoan are first authors.
Albert Puig‐Pijoan will be handling the correspondence: apuig@psmar.cat
Contributor Information
Albert Puig‐Pijoan, Email: apuig@psmar.cat.
Angel Ois, Email: aois@psmar.cat.
REFERENCES
- 1. World Health Organization (WHO) . Dementia. https://www.who.int/news‐room/fact‐sheets/detail/dementia 2023.
- 2. Chow BW, Gu C. The molecular constituents of the blood–brain barrier. Trends Neurosci. 2015;38:598‐608. doi: 10.1016/j.tins.2015.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sweeney MD, Sagare AP, Zlokovic BV. Blood‐brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol. 2018;14:133‐150. doi: 10.1038/nrneurol.2017.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Snyder HM, Corriveau RA, Craft S, et al. Vascular contributions to cognitive impairment and dementia including Alzheimer's disease. Alzheimers Dement. 2015;11:710‐717. https://doi.org10.1016/j.jalz.2014.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Toledo JB, Arnold SE, Raible K, et al. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer's Coordinating Centre. Brain. 2013;136:2697‐2706. doi: 10.1093/brain/awt188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. 2022 Alzheimer's disease facts and figures. Alzheimer's Dement. 2022;18:700‐789. doi: 10.1002/alz.12638 [DOI] [PubMed] [Google Scholar]
- 7. Nehra G, Bauer B, Hartz AMS. Blood‐brain barrier leakage in Alzheimer's disease: from discovery to clinical relevance. Pharmacol Ther. 2022;234. doi: 10.1016/j.pharmthera.2022.108119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rosenberg GA. Blood‐brain barrier permeability in aging and Alzheimer's disease. J Prev Alzheimers Dis. 2014;1:138‐139. doi: 10.14283/jpad.2014.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nation DA, Sweeney MD, Montagne A, et al. Blood‐brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med. 2019;25:270‐276. doi: 10.1038/S41591-018-0297-Y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Iturria‐Medina Y, Sotero RC, Toussaint PJ, et al. Early role of vascular dysregulation on late‐onset Alzheimer's disease based on multifactorial data‐driven analysis. Nat Commun. 2016;7:11934. doi: 10.1038/ncomms11934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sweeney MD, Montagne A, Sagare AP, et al. Vascular dysfunction‐The disregarded partner of Alzheimer's disease. Alzheimers Dement. 2019;15:158‐167. doi: 10.1016/J.JALZ.2018.07.222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Olsson B, Lautner R, Andreasson U, et al. CSF and blood biomarkers for the diagnosis of Alzheimer's disease: a systematic review and meta‐analysis. Lancet Neurol. 2016;15:673‐684. doi: 10.1016/S1474-4422(16)00070-3 [DOI] [PubMed] [Google Scholar]
- 13. Reiber H, Peter JB. Cerebrospinal fluid analysis: disease‐related data patterns and evaluation programs. J Neurol Sci. 2001;184:101‐122. doi: 10.1016/S0022-510X(00)00501-3 [DOI] [PubMed] [Google Scholar]
- 14. Wallin A, Kapaki E, Boban M, et al. Biochemical markers in vascular cognitive impairment associated with subcortical small vessel disease—A consensus report. BMC Neurol. 2017;17. doi: 10.1186/s12883-017-0877-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Giacopuzzi Grigoli E, Solca F, Milone I, et al. Cerebrospinal fluid/serum albumin quotient (Q‐Alb) is not increased in Alzheimer's disease compared to neurological disease controls: a retrospective study on 276 patients. Neurolog Sci. 2022. doi: 10.1007/s10072-022-06530-w [DOI] [PubMed] [Google Scholar]
- 16. Musaeus CS, Gleerup HS, Høgh P, Waldemar G, Hasselbalch SG, Simonsen AH. Cerebrospinal fluid/plasma albumin ratio as a biomarker for blood‐brain barrier impairment across neurodegenerative dementias. J Alzheimer's Dis. 2020;75:429‐436. doi: 10.3233/JAD-200168 [DOI] [PubMed] [Google Scholar]
- 17. Skillbäck T, Delsing L, Synnergren J, et al. CSF/serum albumin ratio in dementias: a cross‐sectional study on 1861 patients. Neurobiol Aging. 2017;59:1‐9. https://doi.org10.1016/j.neurobiolaging.2017.06.028 [DOI] [PubMed] [Google Scholar]
- 18. Lin Z, Sur S, Liu P, et al. Blood‐brain barrier breakdown in relationship to Alzheimer and vascular disease. Ann Neurol. 2021;90:227‐238. doi: 10.1002/ANA.26134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Janelidze S, Hertze J, Nägga K, et al. Increased blood‐brain barrier permeability is associated with dementia and diabetes but not amyloid pathology or APOE genotype. Neurobiol Aging. 2017;51:104‐112. doi: 10.1016/J.NEUROBIOLAGING.2016.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Skillbäck T, Blennow K, Zetterberg H, et al. Sex differences in csf biomarkers for neurodegeneration and blood‐brain barrier integrity. Alzheimers Dement: diagn, Assess Dis Monit 2021;13. doi: 10.1002/dad2.12141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Puig‐Pijoan A, García‐Escobar G, Fernández‐Lebrero A, et al. The CORCOBIA study: cut‐off points of Alzheimer's disease CSF biomarkers in a clinical cohort. Neurología (English Edition). doi: 10.1016/j.nrleng.2022.05.002. n.d.. [DOI] [PubMed] [Google Scholar]
- 22. The Global Deterioration Scale for assessment of primary degenerative dementia. American J Psyc. 1982;139:1136‐1139. doi: 10.1176/ajp.139.9.1136 [DOI] [PubMed] [Google Scholar]
- 23. Jessen F, Amariglio RE, van Boxtel M, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimer's Dement. 2014;10:844‐852. https://doi.org10.1016/j.jalz.2014.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's Dement. 2011;7:270‐279. doi: 10.1016/j.jalz.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's Dement. 2011;7:263‐269. doi: 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456‐2477. doi: 10.1093/brain/awr179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gorno‐Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006. doi: 10.1212/WNL.0b013e31821103e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies. Neurology. 2017;89:88. doi: 10.1212/WNL.0000000000004058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Armstrong MJ, Litvan I, Lang AE, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology. 2013;80:496. doi: 10.1212/WNL.0b013e31827f0fd1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Höglinger GU, Respondek G, Stamelou M, et al. Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov Disord. 2017;32:853‐864. https://doi.org10.1002/mds.26987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Skrobot OA, Black SE, Chen C, et al. Progress toward standardized diagnosis of vascular cognitive impairment: guidelines from the Vascular Impairment of Cognition Classification Consensus Study. Alzheimers Dement. 2018;14:280‐292. doi: 10.1016/J.JALZ.2017.09.007 [DOI] [PubMed] [Google Scholar]
- 32. Greenberg SM, Charidimou A. Diagnosis of Cerebral Amyloid Angiopathy. Stroke. 2018;49:491‐497. doi: 10.1161/STROKEAHA.117.016990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ashton NJ, Puig‐Pijoan A, Milà‐Alomà M, et al. Plasma and CSF biomarkers in a memory clinic: head‐to‐head comparison of phosphorylated tau immunoassays. Alzheimer's Dement. 2022. doi: 10.1002/alz.12841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fazekas F, Chawluk J, Alavi A, Hurtig H, Zimmerman R. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. American J Roentgenol. 1987;149:351‐356. doi: 10.2214/ajr.149.2.351 [DOI] [PubMed] [Google Scholar]
- 35. Thrippleton MJ, Backes WH, Sourbron S, et al. Quantifying blood‐brain barrier leakage in small vessel disease: review and consensus recommendations. Alzheimer's Dement. 2019;15:840‐858. doi: 10.1016/j.jalz.2019.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Morris JC. The Clinical Dementia Rating (CDR). Neurology. 1993;43:2412‐2413. doi: 10.1212/WNL.43.11.2412-a [DOI] [PubMed] [Google Scholar]
- 37. Parrado‐Fernández C, Blennow K, Hansson M, Leoni V, Cedazo‐Minguez A, Björkhem I. Evidence for sex difference in the CSF/plasma albumin ratio in ∼20 000 patients and 335 healthy volunteers. J Cell Mol Med. 2018;22:5151‐5154. https://doi.org10.1111/jcmm.13767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Castellazzi M, Morotti A, Tamborino C, et al. Increased age and male sex are independently associated with higher frequency of blood–cerebrospinal fluid barrier dysfunction using the albumin quotient. Fluids Barriers CNS. 2020;17:14. doi: 10.1186/s12987-020-0173-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kettunen P, Bjerke M, Eckerström C, et al. Blood‐brain barrier dysfunction and reduced cerebrospinal fluid levels of soluble amyloid precursor protein‐β in patients with subcortical small‐vessel disease. Alzheimers Dement (Amst). 2022;14. doi: 10.1002/DAD2.12296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Farrall AJ, Wardlaw JM. Blood‐brain barrier: ageing and microvascular disease–systematic review and meta‐analysis. Neurobiol Aging. 2009;30:337‐352. doi: 10.1016/J.NEUROBIOLAGING.2007.07.015 [DOI] [PubMed] [Google Scholar]
- 41. Davies DC, Hardy JA. Blood brain barrier in ageing and Alzheimer's disease. Neurobiol Aging. 1988;9:46‐48. doi: 10.1016/S0197-4580(88)80017-4 [DOI] [PubMed] [Google Scholar]
- 42. Biron KE, Dickstein DL, Gopaul R, Jefferies WA. Amyloid triggers extensive cerebral angiogenesis causing blood brain barrier permeability and hypervascularity in Alzheimer's disease. PLoS One. 2011;6:e23789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Clark C, Lewczuk P, Kornhuber J, et al. Plasma neurofilament light and phosphorylated tau 181 as biomarkers of Alzheimer's disease pathology and clinical disease progression. Alzheimers Res Ther. 2021;13. doi: 10.1186/S13195-021-00805-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xiao Z, Wu W, Ma X, et al. Plasma Aβ42/Aβ40 and p‐tau181 predict long‐term clinical progression in a cohort with amnestic mild cognitive impairment. Clin Chem. 2022;68:1552‐1563. doi: 10.1093/CLINCHEM/HVAC149 [DOI] [PubMed] [Google Scholar]
- 45. Honey MIJ, Verberk IMW, Gouda M, et al. Plasma glial fibrillary acidic protein, neurofilament light, phosphorylated‐tau‐181 and amyloid β 42/40 as prognostic biomarkers for clinical progression to dementia in individuals with subjective cognitive decline and mild cognitive impairment. Alzheimer's Dement. 2022;18. doi: 10.1002/alz.064620 [DOI] [Google Scholar]
- 46. Dansson HV, Stempfle L, Egilsdóttir H, et al. Predicting progression and cognitive decline in amyloid‐positive patients with Alzheimer's disease. Alzheimers Res Ther. 2021;13. doi: 10.1186/S13195-021-00886-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sörensen A, Blazhenets G, Schiller F, Meyer PT, Frings L. Amyloid biomarkers as predictors of conversion from mild cognitive impairment to Alzheimer's dementia: a comparison of methods. Alzheimers Res Ther. 2020;12. doi: 10.1186/S13195-020-00721-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mielke MM, Aggarwal NT, Vila‐Castelar C, et al. Consideration of sex and gender in Alzheimer's disease and related disorders from a global perspective. Alzheimer's Dement. 2022;18:2707‐2724. doi: 10.1002/alz.12662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between CSF biomarkers and incipient Alzheimer's disease in patients with mild cognitive impairment: a follow‐up study. Lancet Neurol. 2006;5:228‐234. doi: 10.1016/S1474-4422(06)70355-6 [DOI] [PubMed] [Google Scholar]
- 50. Visser PJ, Verhey F, Knol DL, et al. Prevalence and prognostic value of CSF markers of Alzheimer's disease pathology in patients with subjective cognitive impairment or mild cognitive impairment in the DESCRIPA study: a prospective cohort study. Lancet Neurol. 2009;8:619‐627. doi: 10.1016/S1474-4422(09)70139-5 [DOI] [PubMed] [Google Scholar]
- 51. Shaw LM, Vanderstichele H, Knapik‐Czajka M, et al. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403‐413. https://doi.org10.1002/ana.21610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mattsson N, Zetterberg H, Hansson O, et al. CSF biomarkers and incipient alzheimer disease in patients with mild cognitive impairment. JAMA. 2009;302:385‐393. doi: 10.1001/jama.2009.1064 [DOI] [PubMed] [Google Scholar]
- 53. Hansson O, Seibyl J, Stomrud E, et al. CSF biomarkers of Alzheimer's disease concord with amyloid‐β PET and predict clinical progression: a study of fully automated immunoassays in BioFINDER and ADNI cohorts. Alzheimer's Dement. 2018;14:1470‐1481. https://doi.org10.1016/j.jalz.2018.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wallin ÅK, Blennow K, Andreasen N, Minthon L. CSF biomarkers for Alzheimer's disease: levels of β‐Amyloid, tau, phosphorylated tau relate to clinical symptoms and survival. Dement Geriatr Cogn Disord. 2006;21:131‐138. doi: 10.1159/000090631 [DOI] [PubMed] [Google Scholar]
- 55. Hertze J, Minthon L, Zetterberg H, Vanmechelen E, Blennow K, Hansson O. Evaluation of CSF biomarkers as predictors of Alzheimer's disease: a clinical follow‐up study of 4.7 years. J Alzheimer's Dis. 2010;21:1119‐1128. doi: 10.3233/JAD-2010-100207 [DOI] [PubMed] [Google Scholar]
- 56. Elias‐Sonnenschein LS, Viechtbauer W, Ramakers IHGB, Verhey FRJ, Visser PJ. Predictive value of <em>APOE</em>‐ε4 allele for progression from MCI to AD‐type dementia: a meta‐analysis. J Neurol, Neurosurg Psychiatry. 2011;82:1149. doi: 10.1136/jnnp.2010.231555 [DOI] [PubMed] [Google Scholar]
- 57. Levine DA, Gross AL, Briceño EM, et al. Sex differences in cognitive decline among US adults. JAMA Netw Open. 2021;4:e210169. doi: 10.1001/jamanetworkopen.2021.0169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kim KW, Woo SY, Kim S, et al. Disease progression modeling of Alzheimer's disease according to education level. Sci Rep. 2020;10:16808. doi: 10.1038/s41598-020-73911-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Davis M, O`Connell T, Johnson S, et al. Estimating Alzheimer's disease progression rates from normal cognition through mild cognitive impairment and stages of dementia. Curr Alzheimer Res. 2018;15:777‐788. doi: 10.2174/1567205015666180119092427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Uher T, Horakova D, Tyblova M, et al. Increased albumin quotient (QAlb) in patients after first clinical event suggestive of multiple sclerosis is associated with development of brain atrophy and greater disability 48 months later. Mult Scler J. 2015;22:770‐781. doi: 10.1177/1352458515601903 [DOI] [PubMed] [Google Scholar]
- 61. Assialioui A, Domínguez R, Ferrer I, Andrés‐Benito P, Povedano M. Elevated cerebrospinal fluid proteins and albumin determine a poor prognosis for spinal amyotrophic lateral sclerosis. Int J Mol Sci. 2022;23. doi: 10.3390/ijms231911063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Montagne A, Barnes SR, Sweeney MD, et al. Blood‐brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85:296‐302. doi: 10.1016/J.NEURON.2014.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wang J, Fan DY, Li HY, et al. Dynamic changes of CSF sPDGFRβ during ageing and AD progression and associations with CSF ATN biomarkers. Mol Neurodegener. 2022;17. doi: 10.1186/S13024-021-00512-W [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lv X, Zhang M, Cheng Z, et al. Changes in CSF sPDGFRβ level and their association with blood–brain barrier breakdown in Alzheimer's disease with or without small cerebrovascular lesions. Alzheimers Res Ther. 2023;15:51. doi: 10.1186/s13195-023-01199-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sweeney MD, Sagare AP, Pachicano M, et al. A novel sensitive assay for detection of a biomarker of pericyte injury in cerebrospinal fluid. Alzheimers Dement. 2020;16:821‐830. doi: 10.1002/ALZ.12061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sagare AP, Sweeney MD, Makshanoff J, Zlokovic BV. Shedding of soluble platelet‐derived growth factor receptor‐β from human brain pericytes. Neurosci Lett. 2015;607:97‐101. doi: 10.1016/j.neulet.2015.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cicognola C, Mattsson‐Carlgren N, van Westen D, et al. Associations of CSF PDGFRβ with aging, blood‐brain barrier damage, neuroinflammation, and Alzheimer disease pathologic changes. Neurology. 2023;101:e30‐e39. doi: 10.1212/WNL.0000000000207358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Montagne A, Nation DA, Sagare AP, et al. APOE4 leads to blood‐brain barrier dysfunction predicting cognitive decline. Nature. 2020;581:71‐76. doi: 10.1038/S41586-020-2247-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Jack CR, Bennett DA, Blennow K, et al. NIA‐AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14:535‐562. doi: 10.1016/J.JALZ.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Barisano G, Montagne A, Kisler K, Schneider JA, Wardlaw JM, Zlokovic BV. Blood‐brain barrier link to human cognitive impairment and Alzheimer's Disease. Nat Cardiovasc Res. 2022;1:108‐115. doi: 10.1038/S44161-021-00014-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hampel H, Cummings J, Blennow K, Gao P, Jack CR, Vergallo A. Developing the ATX(N) classification for use across the Alzheimer disease continuum. Nat Rev Neurol. 2021;17:580‐589. doi: 10.1038/s41582-021-00520-w [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Supporting information


