Abstract
INTRODUCTION
Depressive symptoms are among early behavioral changes in Alzheimer's disease (AD); however, the relationship between neurodegeneration and depressive symptoms remains inconclusive. To better understand this relationship in preclinical AD, we examined hippocampal volume and depressive symptoms in cognitively unimpaired carriers of the presenilin‐1 (PSEN1) E280A mutation for autosomal dominant AD.
METHODS
A total of 27 PSEN1 mutation carriers and 26 non‐carrier family members were included. Linear regression was used to test the relationship between hippocampal volume and 15‐item Geriatric Depression Scale.
RESULTS
Carriers and non‐carriers did not differ in depressive symptoms or hippocampal volume. Within carriers, lower hippocampal volume was associated with greater depressive symptoms, which remained significant after adjusting for age and cognition. This relationship was not significant in non‐carriers.
DISCUSSION
Hippocampal neurodegeneration may underlie depressive symptoms in preclinical autosomal dominant AD. These findings provide support for the utility of targeting depressive symptoms in AD prevention.
Highlights
We compared unimpaired autosomal dominant Alzheimer's disease (AD) mutation carriers and non‐carriers.
Carriers and non‐carriers did not differ in severity of depressive symptoms.
In carriers, hippocampal volume was inversely associated with depressive symptoms.
Depressive symptoms may be a useful target in AD prevention.
Keywords: autosomal dominant Alzheimer's disease, depression, hippocampus, preclinical, presenilin‐1
1. BACKGROUND
Depressive symptoms are prevalent in older adults and are among the early behavioral changes observed in Alzheimer's disease (AD). 1 , 2 , 3 Experience of depressive symptoms may increase an individual's risk for AD or reflect the early pathological changes of AD (i.e., a prodromal marker). 4 , 5 , 6 , 7 , 8 Greater depressive symptoms have been associated with pathological hallmarks of disease progression, including higher amyloid beta (Aβ) 9 , 10 and medial temporal lobe tau 11 in cognitively normal older adults. Increases in depressive symptoms have also been associated with worsening cognition in older adults with high Aβ. 12 To that end, it is important to understand the mechanisms through which depression and AD are related.
One proposed mechanism by which depression and dementia are related is through hippocampal degeneration. The hippocampus is one of the earliest sites affected by AD pathology. 13 Decreased hippocampal volume is evident prior to clinical dementia onset and occurs more rapidly as the disease progresses. 14 , 15 Separately, clinical depression has been associated with smaller hippocampal volumes in younger and middle‐aged adults, 16 , 17 though this association may be strongest in older adults. 18 In support of this common mechanism, clinical depression has been associated with smaller hippocampal volumes in both cognitively impaired 19 and unimpaired older adults. 20 In cognitively unimpaired older adults with depression, lower hippocampal volume was associated with longitudinal episodic memory decline. 20 The inverse relationship between hippocampal volume and depressive symptoms has also been reported consistently in cognitively healthy adults experiencing a range of depressive symptoms, including those at subthreshold levels. 21 , 22 , 23 , 24 , 25 However, other studies have found no or marginal associations between symptoms in the subthreshold range and hippocampal volume. 18 , 26 Importantly, the mechanisms underlying the association between hippocampal volume and depressive symptoms may be multifactorial and include both AD and age‐related processes. 21
Studying the relationship between depressive symptoms and hippocampal volume in younger, cognitively unimpaired adults with autosomal dominant AD (ADAD) can provide unique insight into early behavioral changes in the preclinical and prodromal stages of AD that are associated with incipient neurodegeneration, apart from age‐related co‐morbidities. The largest kindred with ADAD due to a single mutation, presenilin‐1 (PSEN1) E280A, resides in Antioquia, Colombia. Carriers of this mutation are genetically determined to develop dementia, with a median age of onset of mild cognitive impairment (MCI) at 44 years and dementia at 49 years. 27 PSEN1 E280A carriers with depressive symptoms may have a faster clinical progression than those without depressive symptoms, 8 , 28 and carriers have been found to have decreased hippocampal volumes beginning approximately 6 years prior to MCI diagnosis. 15 The association between hippocampal volume and depressive symptoms in ADAD remains in need of further exploration.
In this study, we aimed to (1) examine differences in depressive symptoms and hippocampal volume between cognitively unimpaired PSEN1 E280A mutation carriers and non‐carriers, and (2) examine the relationship between depressive symptoms and hippocampal volume in preclinical ADAD. We hypothesized that cognitively unimpaired mutation carriers would have greater depressive symptoms and lower hippocampal volume than non‐carriers prior to dementia onset, and that greater depressive symptoms would be associated with lower hippocampal volume in mutation carriers. Such findings would provide further support of the shared underlying mechanism between hippocampal‐related neurodegeneration and depression specific to AD, outside of age‐related processes, and thus have implications for approaches aimed at early detection, intervention, and prevention of AD.
2. METHODS
2.1. Participants
A total of 53 cognitively unimpaired individuals from a Colombian kindred with a high prevalence of the PSEN1 E280A mutation for ADAD were included in the study, including 27 mutation carriers and 26 age‐matched non‐carrier family members. Mutation carriers from this cohort have a median onset of MCI at age 44 years (95% confidence interval [CI]: 43–45) and of dementia at age 49 years (95% CI: 49–50). 27 All participants were unaware of their own genetic status, but all participants had a parent who was a mutation carrier. Only participants living in the metropolitan area of the Aburra Valley, within 105 miles of the University of Antioquia, were invited to participate in the study. Potential participants were screened in advance for the presence of neurologic and psychiatric disorders, drug use, and eligibility to undergo magnetic resonance imaging (MRI).
This study was approved by the institutional review board of the University of Antioquia in Colombia and was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki. All participants completed informed consent prior to the initiation of any study procedures. Participants included a representative sample of adults living in Antioquia, Colombia and were not excluded on the basis of sex or gender.
2.2. Clinical assessments
Neuropsychological tests were administered by a trained psychologist, blind to carrier status. Clinical assessments were administered in Spanish within 1 year of neuroimaging (mean = 157 ± 119 days). Cognitive impairment was determined via the Functional Assessment Staging Test (FAST), which ranges from 1 (normal) to 7 (severe dementia). 29 A score of 1 indicates no difficulties, either subjectively or objectively. A score of 2 indicates that there may be some subjective memory concerns that are in the normal range for aging adults. A score of 3 or above, which resulted in exclusion from the study, indicates that memory problems are affecting performance at work and/or other life activities. Additionally, all cognitively unimpaired carriers and non‐carriers had a Mini‐Mental State Examination (MMSE) 30 score of at least 24/30. Cognitive assessments also included the Consortium to Establish a Registry for Alzheimer's Disease (CERAD) word list delayed recall. 31 Subjective memory complaints were obtained via the Subjective Memory Complaints Questionnaire. 32
RESEARCH IN CONTEXT
Systematic review: The authors reviewed the literature using traditional sources (e.g., PubMed). The association between depression and Alzheimer's disease (AD) dementia has been widely studied, but few studies have reported on the underlying mechanisms in relation to neurodegeneration and depressive symptoms in preclinical autosomal dominant AD.
Interpretation: We examined the association between hippocampal volume and depressive symptoms in cognitively unimpaired carriers of the presenilin‐1 E280A mutation for autosomal dominant AD. Within mutation carriers, lower hippocampal volume was associated with greater depressive symptoms. No relationship was observed in non‐carriers. Our findings demonstrate an association between hippocampal volume and depressive symptoms in preclinical autosomal dominant AD, without the confounds of normal aging.
Future directions: Future studies should examine the progression of depressive symptoms in conjunction with markers of AD pathology, including amyloid beta, tau, and hippocampal neurodegeneration to determine whether depressive symptoms precede or exacerbate pathological changes.
To measure depressive symptoms, participants completed the Spanish version of the 15‐item Geriatric Depression Scale (GDS). 33 , 34 The GDS is a self‐reported measure of depressive symptoms, consisting of 15 yes/no questions, for which higher scores indicate greater depressive symptoms. The GDS is the standard instrument to measure depressive symptoms in this kindred, 28 chosen as a comparable scale to studies of late‐onset AD and for its appropriateness for groups with early stages of cognitive impairment.
2.3. Structural MRI
Structural data were acquired on a 1.5T Philips (Best) Achieva MRI scanner at the Instituto de Alta Tecnología Médica in Medellin, Colombia. For each participant, two high‐resolution T1‐weighted structural MRI scans were collected to examine hippocampal volumes (3D fast field echo, repetition time 2530 ms, echo time 3.39 ms, flip angle 7°, field of view 256 × 256, voxel size 1.0 × 1.0 × 1.0 mm, 176 slices). Automatic shimming procedures were performed. Hippocampal volumes were automatically estimated using FreeSurfer 35 (v. 4.5). Left and right hippocampal volumes were averaged to create a single metric for each participant. For each participant, hippocampal volumes were normalized by dividing by the individual's intracranial volume and then multiplying that proportion by the mean intracranial volume of their group (carrier/non‐carrier).
2.4. Statistical analyses
All analyses and visualizations were conducted in R (v. 4.0.5), and a two‐tailed alpha of 0.05 was used to determine statistical significance. Group differences between carriers and non‐carriers were assessed using independent two‐sample t tests (Levene test was used for examining equality of variances) for normally distributed continuous variables and Mann–Whitney U tests for non‐normally distributed continuous variables. Because hippocampal volume was normalized within group separately for carriers and non‐carriers, we compared hippocampal volume across groups using both normalized and unadjusted values. Chi‐square tests were used for categorical variables. Linear regressions were used to examine GDS and normalized hippocampal volume separately as a function of age in carriers and non‐carriers. Linear regressions were calculated with GDS as the dependent variable and with normalized hippocampal volume as the predictor separately for carriers and non‐carriers. Models were repeated additionally including demographic covariates (age, sex, and education). Sensitivity analyses were run to examine the effect of cognitive covariates (MMSE, word list recall, subjective memory complaints). Standardized beta weights from regressions are reported in the text. To account for non‐normality of GDS scores, analyses including GDS score were also run using non‐parametric Spearman rank correlation.
3. RESULTS
3.1. Sample characteristics
Demographics and clinical characteristics of the sample are shown in Table 1. Carriers and non‐carriers did not differ in age (t = 0.55, P = 0.586), education (t = 0.36, P = 0.724), sex (χ2 = 2.64, P = 0.105), or MMSE scores (W = 434, P = 0.085). Carriers had lower CERAD delayed recall scores (t = 2.81, P = 0.007) and higher subjective memory complaints (t = −2.45, P = 0.018). Carriers and non‐carriers did not differ in GDS scores (W = 374, P = 0.681; Figure 1A), hippocampal volume (unadjusted: t = 1.70, P = 0.097; Figure 1B; normalized: t = 1.91, P = 0.063), or intracranial volume (t = 1.72, P = 0.091).
TABLE 1.
Sample characteristics.
| Carriers (n = 27) | Non‐carriers (n = 26) | P‐value | |
|---|---|---|---|
| Age (years) | 35.93 ± 8.18 (24–53) | 37.04 ± 6.48 (24–48) | 0.586 |
| Sex (female/male) | 19F / 8M | 23F / 3M | 0.105 |
| Education (years) | 10.19 ± 3.26 (4–17) | 10.58 ± 4.67 (3–20) | 0.724 |
| GDS‐15 | 2.11 ± 2.61 (0–11) | 2.85 ± 3.54 (0–13) | 0.681 |
| Mini‐Mental State Examination | 28.74 ± 1.87 (24–30) | 29.69 ± 0.55 (28–30) | 0.085 |
| CERAD delayed recall | 5.70 ± 2.49 (1–9) | 7.38 ± 1.79 (4–10) | 0.007 |
| Subjective memory complaints | 14.56 ± 9.70 (1–35) | 9.12 ± 6.11 (3–24) | 0.018 |
| Intracranial volume (mm3) |
1.41 × 106 ± 1.08 × 105 (1.20 × 106 − 1.64 × 106) |
1.35 × 106 ± 1.28 × 105 (1.05 × 106 − 1.57 × 106) |
0.091 |
| Hippocampal volume (mm3) | 3866.85 ± 568.80 (2622–4883) | 4089.94 ± 370.92 (3402–4734) | 0.097 |
Note. Group differences in age, education, CERAD delayed recall, subjective memory complaints, hippocampal volume, and intracranial volume were conducted using an independent sample t test. Group differences in GDS‐15 and Mini Mental State Examination scores were conducted using Mann–Whitney U tests. Group differences in sex were conducted using chi‐square tests. Means ± standard deviation given for continuous measures with observed range in parentheses.
Abbreviations: CERAD: Consortium to Establish a Registry for Alzheimer's Disease; GDS‐15: Geriatric Depression Scale (15‐item).
FIGURE 1.
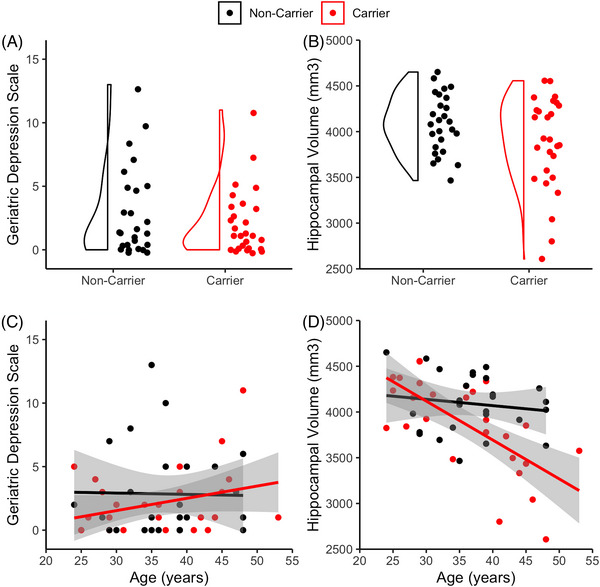
Depressive symptoms and hippocampal volume in PSEN1 carriers and non‐carriers. Distribution of (A) Geriatric Depression Scale scores and (B) normalized hippocampal volume by group. Scatterplot with estimated regression line and standard error bands of (C) Geriatric Depression Scale scores and (D) normalized hippocampal volume by age in carriers (red) and non‐carriers (black). PSEN1, presenilin‐1
As a secondary analysis to examine differences in GDS and hippocampal volume, we examined each variable as a function of age, which serves as a proxy for disease progression in mutation carriers (Figure 1C‐D). In carriers, smaller hippocampal volume was associated with increasing age (β = −0.68, P < 0.001). GDS was not associated with age in carriers (β = 0.30, P = 0.124, rho = 0.23, P = 0.242). Age was not associated with either hippocampal volume (β = −0.14, P = 0.493) or GDS (β = −0.02, P = 0.927; rho = 0.02, P = 0.926) in non‐carriers.
3.2. Hippocampal volume is a predictor of depressive symptoms in PSEN1 E280A carriers
To assess the association between hippocampal volume and depressive symptoms, we regressed GDS score on normalized hippocampal volume separately in carriers and non‐carriers. In carriers, lower hippocampal volume was associated with higher GDS score (β = −0.54, P = 0.003; Figure 2A), which remained significant after adjusting for age, sex, and education (Table 2) and after adjusting for time between measurements (hippocampal volume β = −0.50, P = 0.006). There was no significant relationship between hippocampal volume and GDS in non‐carriers in the simple regression (β = −0.18, P = 0.383; Figure 2B), nor when including demographic covariates (β = −0.12, P = 0.568) or time between measurements (β = −0.16, P = 0.443).
FIGURE 2.
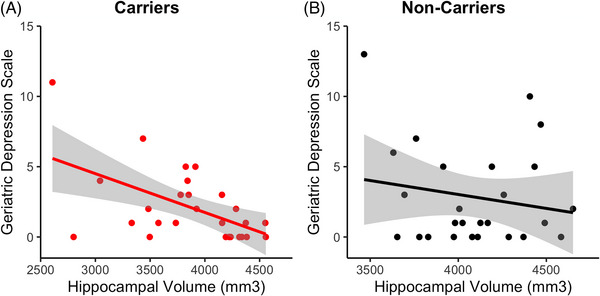
Association between hippocampal volume and depressive symptoms in carriers and non‐carriers. Scatterplots showing Geriatric Depression Scale scores plotted by normalized hippocampal volume in (A) carriers and (B) non‐carriers with regression lines and standard error bands.
TABLE 2.
Standardized regression output predicting Geriatric Depression Scale score in carriers and non‐carriers, adjusting for demographic variables.
| Carriers | Non‐carriers | |||
|---|---|---|---|---|
| Predictor | β | P | β | P |
| Hippocampal volume (normalized) | −0.64 | 0.014 | −0.12 | 0.568 |
| Age | −0.12 | 0.671 | −0.29 | 0.227 |
| Sex | 0.15 | 0.444 | 0.29 | 0.151 |
| Education | −0.02 | 0.930 | −0.43 | 0.090 |
Similar results were obtained when using Spearman correlation to account for the non‐normality of the GDS scores (carriers, no covariates: rho = −0.49, P = 0.010; carriers, with covariates: rho = −0.45, P = 0.028; non‐carriers, no covariates: rho = −0.05, P = 0.801; non‐carriers, with covariates: rho = 0.05, P = 0.831). Relationships between GDS and hippocampal volume were also consistent using log‐transformed GDS scores in linear regression models and in median‐based regression models (data not shown).
A post hoc linear regression model was run in all participants to examine whether the relationship between hippocampal volume and GDS differs by group. There were no main effects of hippocampal volume (β = −0.28, P = 0.290) or group (β = 0.30, P = 0.836) nor an interaction between group and hippocampal volume (β = −0.50, P = 0.722) on GDS.
3.3. The relationship between hippocampal volume and depressive symptoms is independent of memory performance
A series of sensitivity analyses were conducted adjusting for the cognitive measures within carriers. Hippocampal volume remained a significant predictor of GDS after adjusting for MMSE total score (β = −0.50, P = 0.018), CERAD word list delayed recall (β = −0.48, P = 0.018), and subjective memory complaints (β = −0.49, P = 0.007). The objective and subjective cognitive measures were not significant predictors of GDS score (MMSE: β = −0.10, P = 0.609; recall: β = −0.14, P = 0.470; subjective memory complaints: β = 0.28, P = 0.109).
3.4. Influence of clinical versus subthreshold levels of depressive symptoms
Seven participants (13.21%; two carriers, five non‐carriers) had scores greater than 5, indicative of presence of depressive symptoms in the clinical range, of at least mild or greater severity. To explore the relative influence of participants with depressive symptoms in the clinical versus those with subthreshold scores, we categorized participants as having no depression (0 to 1 symptoms), subthreshold scores (2 to 5 symptoms), and depressive symptoms in the clinical range (6 or more symptoms), deriving cutoff values from a meta‐analysis of studies examining subthreshold levels of depression. 36
Using these values, 15 PSEN1 E280A carriers were classified as having no depression, 10 were classified as having scores in the subthreshold range, and 2 having scores in the range of clinical depression. Hippocampal volume significantly differed between groups (Kruskal–Wallis X2 = 6.19, P = 0.045; Supplementary Figure 1). Post hoc pairwise comparisons were not statistically significant, but numeric differences were in the hypothesized direction, such that carriers with no depression had numerically higher hippocampal volume than those with subthreshold scores (uncorrected P = 0.129) or scores in the range of clinical depression (uncorrected P = 0.059). Further, those with subthreshold scores had numerically higher hippocampal volume than those with scores in the range of clinical depression (uncorrected P = 0.061). To address the small sample size of these groups, we additionally compared participants with no depression (n = 15, GDS scores 0–1) to those with subthreshold scores and scores in the range of clinical depression (n = 12, GDS scores 2 or above), finding that those with no depression had significantly higher hippocampal volume than those with scores in the subthreshold and clinical depression ranges (Wilcoxon W = 49, P = 0.048). Within the subgroup of carriers with subthreshold scores and scores in the range of clinical depression, higher hippocampal volume was associated with lower GDS score (β = −0.74, P = 0.006). Regressions were not run in the no depression subgroup due to low score range (0–1) in this group.
4. DISCUSSION
The association between depression and dementia has been well established, 1 , 2 , 4 , 37 yet the underlying mechanisms of this association remain uncertain. Previous literature in non‐demented older adults has shown converging evidence between depressive symptoms and hippocampal neurodegeneration, 21 , 22 , 23 , 24 , 25 though these studies are complicated by non‐AD, age‐related changes in brain pathology. Therefore, we sought to examine depressive symptoms and their association with hippocampal volume in preclinical ADAD mutation carriers. Because the average age of the current sample was in the mid‐30s and the mutation carriers will develop AD dementia, it is unlikely that our findings are driven predominantly by age‐related changes, thus allowing us to approach this timely question from a novel lens. [Correction added on November 14, 2023, after first online publication: The preceding paragraph was missing and the following paragraph was duplicated and this has now been corrected.]
There were no statistically significant differences in hippocampal volume between carriers and non‐carriers, but there was a trend in the hypothesized direction, such that carriers had numerically smaller hippocampal volumes than non‐carriers. Lower hippocampal volume was inversely associated with age within the carrier group, suggesting that carriers closer to the estimated age of clinical onset are likely driving these differences, whereas younger carriers may not have yet experienced significant levels of hippocampal atrophy. Significant hippocampal volume reductions in this kindred are evident on average approximately 6 years prior to the onset of MCI (age 38), 15 which is slightly older than the average age of our sample.
Contrary to our hypotheses, carriers and non‐carriers did not differ in self‐reported depressive symptoms. In a study of individuals with ADAD due to various mutations from the Dominantly Inherited Alzheimer's Network (DIAN) cohort, the odds of experiencing depressive symptoms was greater in mildly cognitively symptomatic ADAD mutation carriers relative to non‐carriers but not in asymptomatic carriers, suggesting the behavioral changes arise in prodromal rather than preclinical disease stages. 3 In PSEN1 E280A carriers, depression onset most commonly occurred after the onset of MCI or dementia. 28 Similarly, older adults with subjective cognitive decline or objective cognitive impairment have been shown to have greater depressive symptoms than cognitively unimpaired older adults. 38 We did not find an increase in depressive symptoms with age in the current sample; however, all included participants were young and cognitively unimpaired. Depressive symptoms, including those below the clinical threshold, should be further examined in PSEN1 E280A carriers with MCI and early dementia, as well as longitudinally in cognitively unimpaired carriers, to determine when such behavioral symptoms manifest. Depressive symptoms can also be influenced by many non–disease‐specific factors, including lifestyle or social support, which may diminish the relationship between depressive symptoms and hippocampal volume observed in a preclinical sample.
Members of this kindred have a complex history with all participants having a parent affected by AD at a young age and knowledge that they might carry this mutation. Because carriers and non‐carriers were blind to their genetic status and had similar, low depressive symptom scores, we do not believe this has a significant impact on the current results. A study of ADAD mutation carriers from the DIAN cohort found no difference in depressive symptoms, hippocampal volume, or clinical disease progression as a function of awareness of mutation status. 39 Rates of depression in our sample (approximately 13% of participants with scores in the clinical range) is similar to that observed in the older adult population in Colombia, though estimates vary based on age, sex, and other health factors. 40 , 41 , 42 Future studies should consider possible differences between ADAD kindreds and older adult sporadic AD populations that may influence the association between depression and dementia or the generalizability of results between populations.
Although carriers did not have elevated depressive symptoms relative to non‐carriers, we found that lower hippocampal volume was associated with greater depressive symptoms in cognitively unimpaired PSEN1 E280A carriers. These results were independent of memory or global cognitive deficits, and no relationship was observed in the non‐carriers. This study is among the first to demonstrate a relationship between neurodegeneration and depressive symptoms in preclinical ADAD. These findings expand on prior reports of an association between depressive symptoms and hippocampal volume in dementia 19 and cognitively unimpaired older adults. 20 , 21 , 22 , 23 , 24 , 25 The association between hippocampal atrophy and depressive symptoms in the general older adult population may reflect various causes because hippocampal atrophy is not specific to AD processes. 43 , 44 In ADAD, however, because all mutation carriers are genetically determined to develop AD dementia by midlife, observed hippocampal atrophy is very likely to be associated with disease progression rather than typical aging or other processes. The extent to which these findings are specific to the hippocampus versus larger regional or whole‐brain measures 26 should be further examined, as well as alternative methods to quantify hippocampal atrophy (e.g., hippocampal integrity) that may be more sensitive than volume. 45 Finally, although we considered overall quantity of depressive symptoms in our models, specific symptoms may drive the relationship between hippocampal volume and depression in AD. Apathy in particular has been independently associated with hippocampal volume in cognitively unimpaired 21 and impaired 46 older adults.
Longitudinal studies, including additional measures of pathology, are needed to clarify the role of depressive symptoms as a risk factor and/or prodrome of AD dementia. Depressive symptoms have been associated with medial temporal lobe tau in cognitively unimpaired older adults. 11 Additionally, increases in depressive symptoms over 1 year were associated with higher Aβ burden, 9 and worsening depressive symptoms, in the presence of Aβ, are associated with declining cognition. 12 Yet, other studies have reported no association between low gray matter volume in depressed older adults and Aβ. 47 , 48 Studies including multiple pathologies across several years with rigorous phenotyping of depressive symptoms (i.e., depressive symptoms vs. major depressive disorder) will be instrumental in disentangling effects of risk or early behavioral manifestations. Recently it was reported that PSEN1 E280A carriers with clinical depression prior to MCI onset had faster progression to dementia. 28 Taken together with our findings in the range of subthreshold to clinical depressive symptoms, prevention and treatment of early depressive symptoms, even prior to reaching clinical levels, should be prioritized. This raises the possibility of interventions targeting depression to slow progression to dementia in ADAD. Indeed, depression has been identified by the Lancet Commission as an important modifiable risk factor for dementia prevention. 49 Whether depressive symptoms are a disease risk factor, behavioral manifestation, or both, multimodal treatments for disease‐modifying therapies, pharmacological antidepressants, and psychosocial and lifestyle interventions will be critical to develop and administer early.
This study has several limitations. Although our participants are carriers of a single mutation with a well‐characterized disease trajectory, the sample size is relatively small. Follow‐up studies in a larger sample of both ADAD and sporadic AD populations are needed to determine the generalizability of these results. In particular, although we found significant effects of hippocampal volume on depressive symptoms in mutation carriers and not in non‐carriers, we did not find evidence that the association between hippocampal volume and depressive symptoms differed by group. It is likely that we had inadequate power to detect an interactive effect, but further analysis is needed in larger samples to clarify these relationships. Psychiatric history was also not available for these participants, though this history may influence hippocampal volume and should be accounted for in future investigations. Additionally, the range of depressive symptoms was low, and we were unable to examine whether specific symptoms or symptom clusters were driving our results. Finally, our data were cross‐sectional, and therefore, we are unable to determine whether depressive symptoms predate or are a consequence of hippocampal atrophy. Future longitudinal studies in this kindred that incorporate amyloid and tau measurements will provide more insight into the temporal ordering of behavioral and pathological change in preclinical ADAD.
In conclusion, we found that low hippocampal volume is associated with greater depressive symptoms in PSEN1 E280A mutation carriers, more than a decade prior to the estimated clinical onset of dementia. This association is independent of memory deficits and unlikely to be associated with age‐related changes. These findings provide support for the potential utility of targeting depressive symptoms in early AD identification and prevention.
CONFLICT OF INTEREST STATEMENT
S.L. has nothing to disclose. F.L. has nothing to disclose. A.B. has nothing to disclose. J.T.F.F. has nothing to disclose. D.M. has nothing to disclose. J.E.M. has nothing to disclose. A.G. has nothing to disclose. P.V. has nothing to disclose. B.H. has nothing to disclose. G.A.M. has served as site principal investigator for AD clinical trials sponsored by Eli Lilly and Company, Eisai Inc., and Genentech. Y.T.Q. serves as a consultant for Biogen. J.R.G. has nothing to disclose. Author disclosures are available in the supporting information.
CONSENT STATEMENT
This study was approved by the institutional review board of the University of Antioquia in Colombia and was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki. All participants completed informed consent prior to the initiation of any study procedures.
Supporting information
Supplementary Information
ICMJE DISCLOSURE FORM
ACKNOWLEDGMENTS
The authors thank the PSEN1 Colombian families for contributing their valuable time and effort, without which this study would not have been possible. The authors thank the research staff of the Group of Neuroscience of Antioquia for their help coordinating study visits. Stephanie Langella was supported by an Alzheimer's Association Research Fellowship (AARF‐22‐920754). Francisco Lopera was supported by an Anonymous Foundation, and the Administrative Department of Science, Technology and Innovation (Colciencias Colombia;111565741185). Joshua T. Fox‐Fuller was supported by an NRSA fellowship from the National Institute on Aging (F31AG062158). Bernard Hanseeuw was funded through the Belgian National Fund for Scientific Research (FNRS #CCL40010417, Welbio #40010035). Gad A. Marshall was supported by grants from the National Institute on Aging (R01 AG071074, R01 AG067021, R01 AG053184, R21 AG064413, R42 AG069629, R21 AG070877). Yakeel T. Quiroz was supported by grants from the National Institute on Aging (R01 AG054671, RF1AG077627), the Alzheimer's Association, and Massachusetts General Hospital ECOR. JRG was funded through NIH/NIA K23 AG058805, NIH/NIA R01AG078191; MGH Rappaport Fellowship. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Langella S, Lopera F, Baena A, et al. Depressive symptoms and hippocampal volume in autosomal dominant Alzheimer's disease. Alzheimer's Dement. 2024;20:986–994. 10.1002/alz.13501
Drs Yakeel T. Quiroz and Jennifer R. Gatchel are co‐senior authors.
REFERENCES
- 1. Blazer D, Williams CD. Epidemiology of dysphoria and depression in an elderly population. Am J Psychiatry. 1980;137(4):439‐444. doi: 10.1176/ajp.137.4.439 [DOI] [PubMed] [Google Scholar]
- 2. Geda YE, Roberts RO, Knopman DS, et al. Prevalence of neuropsychiatric symptoms in mild cognitive impairment and normal cognitive aging: population‐based study. Arch Gen Psychiatry. 2008;65(10):1193‐1198. doi: 10.1001/archpsyc.65.10.1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ringman JM, Liang L‐J, Zhou Y, et al. Early behavioural changes in familial Alzheimer's disease in the Dominantly Inherited Alzheimer Network. Brain. 2015;138(4):1036‐1045. doi: 10.1093/brain/awv004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dafsari FS, Jessen F. Depression—an underrecognized target for prevention of dementia in Alzheimer's disease. Transl Psychiatry. 2020;10(1):160. doi: 10.1038/s41398-020-0839-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Steenland K, Karnes C, Seals R, Carnevale C, Hermida A, Levey A. Late‐life depression as a risk factor for mild cognitive impairment or Alzheimer's disease in 30 US Alzheimer's disease centers. J Alzheimers Dis. 2012;31(2):265‐275. doi: 10.3233/JAD-2012-111922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li G, Wang LY, Shofer JB, et al. Temporal relationship between depression and dementia: findings from a large community‐based 15‐year follow‐up study. Arch Gen Psychiatry. 2011;68(9):970‐977. doi: 10.1001/archgenpsychiatry.2011.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Panza F, Frisardi V, Capurso C, et al. Late‐life depression, mild cognitive impairment, and dementia: possible continuum? Am J Geriatr psychiatry Off J Am Assoc Geriatr Psychiatry. 2010;18(2):98‐116. doi: 10.1097/JGP.0b013e3181b0fa13 [DOI] [PubMed] [Google Scholar]
- 8. Mejía S, Giraldo M, Pineda D, Ardila A, Lopera F. Nongenetic factors as modifiers of the age of onset of familial Alzheimer's disease. Int psychogeriatrics. 2003;15(4):337‐349. doi: 10.1017/s1041610203009591 [DOI] [PubMed] [Google Scholar]
- 9. Babulal GM, Ghoshal N, Head D, et al. Mood changes in cognitively normal older adults are linked to Alzheimer disease biomarker levels. Am J Geriatr Psychiatry. 2016;24(11):1095‐1104. doi: 10.1016/j.jagp.2016.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Donovan NJ, Locascio JJ, Marshall GA, et al. Longitudinal association of amyloid beta and anxious‐depressive symptoms in cognitively normal older adults. Am J Psychiatry. 2018;175(6):530‐537. doi: 10.1176/appi.ajp.2017.17040442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gatchel JR, Donovan NJ, Locascio JJ. Depressive symptoms and tau accumulation in the inferior temporal lobe and entorhinal cortex in cognitively normal older adults: a pilot study. J Alzheimer's Dis. 2017;59(3):975‐985. doi: 10.3233/JAD-170001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gatchel JR, Rabin JS, Buckley RF, et al. Longitudinal association of depression symptoms with cognition and cortical amyloid among community‐dwelling older adults. JAMA Netw Open. 2019;2(8):e198964. doi: 10.1001/jamanetworkopen.2019.8964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sengoku R. Aging and Alzheimer's disease pathology. Neuropathology. 2020;40(1):22‐29. doi: 10.1111/neup.12626 [DOI] [PubMed] [Google Scholar]
- 14. Fox NC, Warrington EK, Freeborough PA, et al. Presymptomatic hippocampal atrophy in Alzheimer's disease: a longitudinal MRI study. Brain. 1996;119:2001‐2007. [DOI] [PubMed] [Google Scholar]
- 15. Fleisher AS, Chen K, Quiroz YT, et al. Associations between biomarkers and age in the presenilin 1 e280a autosomal dominant Alzheimer disease kindred. JAMA Neurol. 2015;72(3):316. doi: 10.1001/jamaneurol.2014.3314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Videbech P, Ravnkilde B. Reviews and overviews hippocampal volume and depression : a meta‐analysis of MRI studies. Hippocampal Vol Depress A meta‐Analysis MRI Stud. 2004;161:1957‐1966. [DOI] [PubMed] [Google Scholar]
- 17. MacQueen G, Frodl T. The hippocampus in major depression: evidence for the convergence of the bench and bedside in psychiatric research. Mol Psychiatry. 2011;16(3):252‐264. doi: 10.1038/mp.2010.80 [DOI] [PubMed] [Google Scholar]
- 18. Brown ES, Hughes CW, McColl R, Peshock R, King KS, Rush AJ. Association of depressive symptoms with hippocampal volume in 1936 adults. Neuropsychopharmacology. 2014;39(3):770‐779. doi: 10.1038/npp.2013.271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Steffens DC, Payne ME, Greenberg DL, et al. Hippocampal volume and incident dementia in geriatric depression. Am J Geriatr Psychiatry. 2002;10(1):62‐71. doi: 10.1097/00019442-200201000-00008 [DOI] [PubMed] [Google Scholar]
- 20. O'Brien JT, Lloyd A, McKeith I, Gholkar A, Ferrier N. A longitudinal study of hippocampal volume, cortisol levels, and cognition in older depressed subjects. Am J Psychiatry. 2004;161(11):2081‐2090. doi: 10.1176/appi.ajp.161.11.2081 [DOI] [PubMed] [Google Scholar]
- 21. Donovan NJ, Hsu DC, Dagley AS, et al. Depressive symptoms and biomarkers of Alzheimer's disease in cognitively normal older adults. J Alzheimer's Dis. 2015;46(1):63‐73. doi: 10.3233/JAD-142940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhou H, Li R, Ma Z, Rossi S, Zhu X, Li J. Smaller gray matter volume of hippocampus/parahippocampus in elderly people with subthreshold depression: a cross‐sectional study. BMC Psychiatry. 2016;16(1):219. doi: 10.1186/s12888-016-0928-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. O'Shea DM, Dotson VM, Woods AJ, et al. Depressive symptom dimensions and their association with hippocampal and entorhinal cortex volumes in community dwelling older adults. Front Aging Neurosci. 2018;10. doi: 10.3389/fnagi.2018.00040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Szymkowicz SM, Woods AJ, Dotson VM, et al. Associations between subclinical depressive symptoms and reduced brain volume in middle‐aged to older adults. Aging Ment Health. 2019;23(7):819‐830. doi: 10.1080/13607863.2018.1432030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Touron E, Moulinet I, Kuhn E, et al. Depressive symptoms in cognitively unimpaired older adults are associated with lower structural and functional integrity in a frontolimbic network. Mol Psychiatry. 2022;27(12):5086‐5095. doi: 10.1038/s41380-022-01772-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pink A, Przybelski SA, Krell‐Roesch J, et al. Cortical thickness and depressive symptoms in cognitively normal individuals: the mayo clinic study of aging. J Alzheimer's Dis. 2017;58(4):1273‐1281. doi: 10.3233/JAD-170041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Acosta‐Baena N, Sepulveda‐Falla D, Lopera‐Gómez CM, et al. Pre‐dementia clinical stages in presenilin 1 E280A familial early‐onset Alzheimer's disease: a retrospective cohort study. Lancet Neurol. 2011;10(3):213‐220. doi: 10.1016/S1474-4422(10)70323-9 [DOI] [PubMed] [Google Scholar]
- 28. Acosta‐Baena N, Lopera‐Gómez CM, Jaramillo‐Elorza MC, et al. Early depressive symptoms predict faster dementia progression in autosomal‐dominant Alzheimer's disease. J Alzheimers Dis. 2023. doi: 10.3233/JAD-221294 [DOI] [PubMed] [Google Scholar]
- 29. Reisberg B. Functional assessment staging (FAST). Psychopharmacol Bull. 1988;24(4):653‐659. [PubMed] [Google Scholar]
- 30. Folstein MF, Folstein SE, McHugh PR. Mini‐mental state. J Psychiatr Res. 1975;12(3):189‐198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 31. Aguirre‐Acevedo DC, Gómez RD, Moreno S, et al. [Validity and reliability of the CERAD‐Col neuropsychological battery]. Rev Neurol. 2007;45(11):655‐660. doi: 10.33588/rn.4511.2007086 [DOI] [PubMed] [Google Scholar]
- 32. Youn JC, Kim KW, Lee DY, et al. Development of the subjective memory complaints questionnaire. Dement Geriatr Cogn Disord. 2009;27(4):310‐317. doi: 10.1159/000205512 [DOI] [PubMed] [Google Scholar]
- 33. Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res;17(1):37‐49. doi: 10.1016/0022-3956(82)90033-4 [DOI] [PubMed] [Google Scholar]
- 34. Bacca AM, González A, Uribe Rodríguez AF. Validación de la Escala de Depresión de Yesavage (versión reducida) en adultos mayores colombianos. Pensam Psicológico. 2005;1(5):53‐64. ISSN 1657‐8961, Vol 1, No 5, 2005, págs 53‐64. [Google Scholar]
- 35. Fischl B. FreeSurfer. Neuroimage. 2012;62:774‐781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rodríguez MR, Nuevo R, Chatterji S, Ayuso‐Mateos JL. Definitions and factors associated with subthreshold depressive conditions: a systematic review. BMC Psychiatry. 2012;12:181. doi: 10.1186/1471-244X-12-181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Diniz BS, Butters MA, Albert SM, Dew MA. Late‐life depression and risk of vascular dementia and Alzheimer's disease: systematic review and meta‐analysis of community‐based cohort studies. Br J Psychiatry. 2013;202(5):329‐335. doi: 10.1192/bjp.bp.112.118307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Moulinet I, Touron E, Mézenge F, et al. Depressive symptoms have distinct relationships with neuroimaging biomarkers across the Alzheimer's clinical continuum. Front Aging Neurosci. 2022;14(June):1‐12. doi: 10.3389/fnagi.2022.899158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Aschenbrenner AJ, James BD, McDade E, et al. Awareness of genetic risk in the Dominantly Inherited Alzheimer Network (DIAN). Alzheimer's Dement. 2020;16(1):219‐228. doi: 10.1002/alz.12010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Salazar AM, Plata SJ, Reyes MF, et al. Prevalencia y factores de riesgo psicosociales de la depresión en un grupo de adultos mayores en Bogotá. Acta Neurológica Colomb. 2015;31(2):176‐183. doi: 10.22379/2422402225 [DOI] [Google Scholar]
- 41. Segura‐Cardona A, Hernández‐Calle J, Cardona‐Arango D, Segura‐Cardona A, Muñoz‐Rodríguez D, Jaramillo‐Arroyave D. Depresión en el adulto mayor: un estudio en tres ciudades de Colombia. Salud Uninorte. 2019;34(2):409‐419. doi: 10.14482/sun.34.2.362.29 [DOI] [Google Scholar]
- 42. Segura Cardona A, Cardona Arango D, Segura Cardona Á, Garzón Duque M. Riesgo de depresión y factores asociados en adultos mayores. Antioquia, Colombia. 2012. Rev Salud Pública. 2015;17(2):184‐194. doi: 10.15446/rsap.v17n2.41295 [DOI] [PubMed] [Google Scholar]
- 43. Anand KS, Dhikav V. Hippocampus in health and disease: an overview. Ann Indian Acad Neurol. 2012;15(4):239‐246. doi: 10.4103/0972-2327.104323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fotuhi M, Do D, Jack C. Modifiable factors that alter the size of the hippocampus with ageing. Nat Rev Neurol. 2012;8(4):189‐202. doi: 10.1038/nrneurol.2012.27 [DOI] [PubMed] [Google Scholar]
- 45. Bruno D, Ciarleglio A, Grothe MJ, et al. Hippocampal volume and integrity as predictors of cognitive decline in intact elderly. Neuroreport. 2016;27(11):869‐873. doi: 10.1097/WNR.0000000000000629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Banning LCP, Ramakers IHGB, Köhler S, et al. The association between biomarkers and neuropsychiatric symptoms across the Alzheimer's disease spectrum. Am J Geriatr Psychiatry. 2020;28(7):735‐744. doi: 10.1016/j.jagp.2020.01.012 [DOI] [PubMed] [Google Scholar]
- 47. De Winter F‐L, Emsell L, Bouckaert F, et al. No association of lower hippocampal volume with Alzheimer's disease pathology in late‐life depression. Am J Psychiatry. 2017;174(3):237‐245. doi: 10.1176/appi.ajp.2016.16030319 [DOI] [PubMed] [Google Scholar]
- 48. Takamiya A, Vande Casteele T, Koole M, et al. Lower regional gray matter volume in the absence of higher cortical amyloid burden in late‐life depression. Sci Rep. 2021;11(1):15981. doi: 10.1038/s41598-021-95206-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413‐446. doi: 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
ICMJE DISCLOSURE FORM


