Abstract
INTRODUCTION
Compared to males, females have an accelerated trajectory of cognitive decline in Alzheimer's disease (AD). The neurobiological factors underlying the more rapid cognitive decline in AD in females remain unclear. This study explored how sex‐dependent alterations in hippocampal connectivity over 2 years are associated with cerebrovascular and amyloid pathologies in normal aging.
METHODS
Thirty‐three females and 21 males 65 to 93 years of age with no cognitive impairment performed a face‐name associative memory functional magnetic resonance imaging (fMRI) task with a 2‐year follow‐up. We acquired baseline carbon 11‐labeled Pittsburgh compound B ([11C]PiB) positron emission tomography (PET) and T2‐weighted fluid‐attenuated inversion recovery (T2‐FLAIR) MRI to quantify amyloid β (Aβ) burden and white matter hyperintensity (WMH) volume, respectively.
RESULTS
Males had increased hippocampal‐prefrontal connectivity over 2 years, associated with greater Aβ burden. Females had increased bilateral hippocampal functional connectivity, associated with greater WMH volume.
DISCUSSION
These findings suggest sex‐dependent compensatory mechanisms in the memory network in the presence of cerebrovascular and AD pathologies and may explain the accelerated trajectory of cognitive decline in females.
Keywords: Alzheimer's disease, amyloid beta, cerebral small vessel disease, hippocampal functional connectivity, neural compensation, sex, white matter hyperintensity
1. BACKGROUND
There is limited understanding of the significant changes that occur in the brain during the years leading up to the onset of cognitive impairment in Alzheimer's disease (AD). Amyloid β (Aβ) plaques are a pathological hallmark of AD and accumulate early in the cascade before the initial onset of cognitive decline. 1 , 2 , 3 Aβ deposition is commonly observed in the brains of older adults without cognitive impairment at autopsy 4 and in vivo, 5 , 6 suggesting that individuals may have varying resilience to AD pathology and resistance to clinical decline. Thus considerable research has focused on neural processes that allow older adults to maintain normal cognition despite Aβ accumulation.
Functional magnetic resonance imaging (fMRI) has been proposed as a putative biomarker to probe the functional integrity of brain networks supporting cognitive domains such as memory. 7 Studies have consistently found AD subjects to have decreased hippocampal activation during memory fMRI tasks. 8 , 9 , 10 In contrast, studies have frequently observed increased hippocampal activation and functional connectivity in cognitively normal subjects who are at risk for AD due to genetic predisposition 11 , 12 or high Aβ deposition. 13 , 14 , 15 The increased activation/functional connectivity observed early in AD progression is postulated to be the recruitment of extra neural resources to allow people to maintain normal cognition despite the neurotoxic effect of Aβ deposition. 14 However, it is unclear when hippocampal hyperactivity/hyperconnectivity occurs along the cascade of AD and how this biomarker varies among individuals and populations. Because most studies have been cross‐sectional, there is a growing need for longitudinal analyses to compare changes in biomarkers of Aβ deposition and fMRI activation/connectivity over time in older adults without cognitive impairment.
Sex is an important factor when studying AD risk and progression. Roughly two‐thirds of individuals diagnosed with AD in the United States are females, and the longer life span of females does not fully explain this disparity. 16 Studies have reported marked sex differences in the trajectories of cognitive decline and disease progression. 17 For example, data from the Alzheimer's Disease Neuroimaging Initiative (ADNI) study displayed that female participants with mild cognitive impairment (MCI) experienced cognitive deterioration faster than males with MCI over 1 year, 18 and this rate doubled over 8 years. 19 Few studies have examined whether these sex‐dependent differences in AD progression are reflected by the fMRI biomarker of hippocampal activity/connectivity.
Recently our group reported two cross‐sectional studies of a cognitively unimpaired cohort, investigating the sex‐by‐Aβ interaction on hippocampal connectivity 20 and the white matter hyperintensity (WMH)‐by‐Aβ interaction on hippocampal connectivity 21 during associative memory encoding. We found that, compared to males with high Aβ burden, females with high Aβ burden had higher local hippocampal–medial temporal lobe (MTL) connectivity and lower hippocampal–prefrontal connectivity. 20 This pattern of connectivity is similar to what we and others have found in relation to cerebral small vessel disease (cSVD). 21 , 22 Specifically, in individuals with high SVD burden (evidenced by WMH volume), greater Aβ burden was associated with greater local hippocampal–parahippocampal connectivity and lower hippocampal–prefrontal connectivity, similar to what we found in females with pre‐clinical AD.
RESEARCH IN CONTEXT
Systematic review: The authors reviewed the literature using traditional (e.g., PubMed) sources and meeting abstracts and presentations. We and others have previously published on the interactive effects of sex, cerebral amyloid β (Aβ) burden, and white matter hyperintensity (WMH) burden on hippocampal connectivity observed through functional magnetic resonance imaging (fMRI) memory tasks. These relevant citations are cited appropriately.
Interpretation: Our findings support our previous cross‐sectional studies to suggest that there are sex‐dependent compensatory mechanisms in the memory network in the presence of cerebrovascular and Aβ pathologies. These findings may explain the accelerated trajectory of cognitive decline in females.
Future directions: This study observed sex‐dependent hippocampal connectivity changes over a 2‐year period. Future longitudinal studies should examine hippocampal connectivity changes over a longer period of and with multiple follow‐up visits. Additional studies should incorporate females during all stages of menopause to further elucidate the effects of estrogen on hippocampal connectivity.
The neurobiological mechanisms driving different rates of AD progression between the sexes remains to be fully elucidated. The present study uses fMRI to investigate sex differences in the 2‐year change in hippocampal functional connectivity during associative memory encoding in cognitively unimpaired older adults. As a post hoc analysis, we examine whether sex‐dependent changes in hippocampal connectivity are driven by cerebral Aβ burden, indexed by [11C]PiB PET (Pittsburgh compound‐B positron emission tomography), or cSVD burden, assessed using T2‐FLAIR (fluid‐attenuated inversion recovery) MRI to quantify WMH volume. Considering the sex differences observed in the prevalence, incidence, and progression of AD, we hypothesized that males and females would have different patterns of hippocampal connectivity alterations over a 2‐year period. Based on our previous studies, we hypothesized that males would have a larger increase in hippocampal–prefrontal connectivity, whereas females would have a larger increase in bilateral hippocampal connectivity. We further hypothesized that any observed changes in hippocampal connectivity would be associated more with AD and cSVD pathology for males and females, respectively.
2. METHODS
2.1. Participants
This study included 54 cognitively normal older adults (mean age ± SD at baseline: 74.58 ± 6.09 years, range: 65–93 years, 33/54 (61.1%) female). The mean follow‐up interval was 2.43 ± 0.82 years. Inclusion and exclusion criteria were described in detail previously. 20 Briefly, inclusion criteria were: 65 of years of age or older, an education greater than or equal to 12 years, and fluent in English. Exclusion criteria were: prior diagnosis of MCI or dementia, history of a major psychiatric or neurological condition, conditions affecting cognition or cognitive test performance, and MR contraindications. To rule out individuals meeting criteria for MCI 23 , 24 , 25 or dementia (according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition [DSM‐IV]) 26 and to ensure participants had normal cognition, a comprehensive neuropsychological battery was administered to all participants during each visit. The testing battery included assessments of memory, visuospatial construction, language, and attention and executive functions, as described previously. 13 The Human Use Subcommittee of the Radioactive Drug Research Committees and the institutional review board of the University of Pittsburgh approved all studies.
2.2. Image acquisition and processing
2.2.1. MRI acquisition
MR scanning was performed on a 3T Siemens Trio scanner with 12‐channel head coil at the University of Pittsburgh Magnetic Resonance Research Center. T1‐weighted structural images were acquired axially using a magnetization‐prepared rapid gradient echo sequence (T1w MPRAGE): repetition time (TR) = 2300 ms, echo time (TE) = 3.4 ms, flip angle (FA) = 9°, field of view (FOV) = 240 × 256 mm 2 , matrix = 240 × 256, slice thickness/gap = 1/0 mm, and 160 slices. T2‐weighted FLAIR images were acquired axially (T2w FLAIR): TR = 9160 ms, TE = 90 ms, inversion time (TI) = 2500 ms, FA = 150°, FOV = 256 × 212 mm2, matrix = 256 × 212, slice thickness/gap = 3/0 mm, and 48 slices. Whole‐brain fMRI data were acquired axially using gradient‐echo echo‐planar imaging sequence with the following parameters: TR = 2 seconds, TE = 32 ms, FA = 90°, FOV = 256 × 256 mm2, acquisition matrix 128 × 128, slice thickness/gap = 4/0 mm (voxel size = 2 × 2 × 4 mm3), and 28 axial slices.
Task fMRI data were collected while participants performed a face‐name associative encoding task. Participants are presented with face‐name association pairs and then asked to respond to each face‐name association by pressing a button designated to whether they think the face and name fit each other. They are instructed to try to remember the association for a later recognition test. 27 During each block, the subjects are instructed to press a button to signal that they have memorized the association, and this encoding time is recorded. This task is a mixed block/event‐related design task where there are alternating blocks of novel face‐name associations, familiar face‐name pairs, and fixation. The task consisted of two experimental blocks and two control blocks lasting a total of 267 s. Each run consists of four, 48‐s blocks where each block presents eight faces for 5 s with 1 s of intermittent fixation. There is a 25‐s fixation period between each block. A recognition test is carried out at the end of the scanning session, in which each face is presented with two names, and participants are asked to indicate which name was paired with the face during the fMRI task. The participants were trained and thus familiar with these two face–name pairs in the pre‐scan session.
2.2.2. [11C]Pittsburgh compound‐B (PiB) PET imaging
[11C]Pittsburgh compound‐B ([11C]PiB) was synthesized in accordance with previously described methods. 28 PET imaging was performed as described previously. 21 Briefly, PET imaging was performed using a Siemens/CTI ECAT HR+ scanner (Siemens Medical Solutions, Knoxville, TN) operating in three‐dimensional (3D) imaging mode. Subjects were injected intravenously with 15 mCi of high specific activity [11C]PiB (> 2 Ci/μmol at end‐of‐synthesis) before being positioned in the scanner. Following a 10–15 min transmission scan using rotating 68Ge/68Ga rod sources for the purpose of attenuation correction of PET emission data, a 20‐min emission scan was acquired (4 × 5 min frames) beginning 50 min after injection. PET emission data were reconstructed using 3D filtered back projection with Fourier rebinning, and standard corrections for photon attenuation, scatter, and radioactive decay were applied.
2.3. MR data processing
2.3.1. Gray matter and white matter hyperintensity segmentation
T1w MPRAGE images were oriented manually such that the axial planes were oriented parallel to the plane intersecting the anterior and posterior commissure (AC‐PC). MPRAGE images were processed using FreeSurfer (version 7.1.1, https://surfer.nmr.mgh.harvard.edu/) for automated cortical surface reconstruction and cortical volume estimation on the T1w image. WMHs on T2 FLAIR images were segmented using an automated method based on our previous work. 29 Cerebral and cerebellar WM was segmented on the T1w imaged and then mapped to the T2w FLAIR image space using SPM12 (Welcome Trust Center for Neuroimaging (http://www.fil.ion.ucl.ac.uk/spm/) and FreeSurfer. Because there were very few lesions in the cerebellum in our sample, the mean and SD of cerebellar WM were used to Z‐transform the T2w FLAIR image (Z‐T2w FLAIR). Using Z‐T2w FLAIR images, voxels were identified as WMH if the z‐score was ≥2 and within the cerebral WM mask. The total WMH volume (WMHV) was normalized by the intracranial volume (ICV) [WMHV = WMH/ICV] and log‐transformed for analysis.
2.3.2. Functional connectivity
Preprocessing on fMRI scans was performed in SPM12 for slice‐timing correction, motion correction, image co‐registration and normalization, and spatially smoothed (an isotropic 8 mm‐full width at half maximum [FWHM] Gaussian filter). Left and right hippocampus seeds based on the automated anatomic labeling (AAL) atlas 30 were used to estimate functional connectivity between hippocampus and voxels in the brain via generalized psychophysiological interaction (gPPI) analysis. 31 Using singular value decomposition (SVD), principal time series (i.e., the eigenvariate) was generated for the left and right hippocampus, respectively. The following parameters were included in the design matrix: principal time series of the seed region (left or right hippocampus), task conditions (experimental condition: novel face‐name pairs and control condition: familiar repeated face–name pairs), interaction variables (seed time series x task condition), and motion parameters. We computed for each participant at each time point its functional connectivity map (left or right hippocampus) during associative encoding (i.e., novel face–name pairs vs familiar face–name pairs).
2.3.3. Second‐level analyses
Hippocampal functional connectivity maps were entered into Statistical Parametric Mapping (SPM, https://www.fil.ion.ucl.ac.uk/spm/) to test for the interaction effect of sex by time (MRI scan at baseline and follow‐up) on hippocampal connectivity during associative memory encoding. To test if the association between hippocampal connectivity and baseline versus follow‐up varies by sex, an analysis of variance (ANOVA) model was used with main effects of sex and time, as well as the interaction of time by sex. To control for multiple comparisons, joint height and extent thresholds were determined via Monte Carlo simulations (10,000 iterations) with an a priori medial temporal and frontal lobe mask (AlphaSim, AFNI) for a corrected p < 0.05. An initial cluster‐forming threshold of p < 0.005 was used, and clusters with an extended threshold of p < 0.05 familywise errors were considered to be statistically significant.
For post hoc analyses, the mean beta values were extracted from the significant region of interest (ROI) masks that had a significant sex‐by‐time interaction effect (p < 0.001). We then performed multivariate linear regression analysis to find correlations with connectivity change (follow‐up minus baseline). We tested two models to examine if there was a significant sex x Aβ or sex x WMHV interaction with the linear regression models. Both models were controlled for age at baseline, and WMHV or Aβ was a control variable when not testing for interaction effect. Linear regression analysis was performed using R (version 4.2.1 https://www.R‐project.org).
Analyses of variances were performed in R to examine the effects of sex and time points [baseline vs follow‐up], and the interaction of sex x time on neurocognitive data (Supplementary Table 1). In addition, we performed linear regression models in R to examine the effects of baseline Aβ load (PiB standardized uptake value ratio [SUVR]), sex, and Aβ × sex on changes (follow‐up minus baseline) in neurocognitive performance scores. We tested these effects, controlling for age at baseline.
2.3.4. [11C]PiB PET data processing and analyses
Processing of [11C]PiB PET images was performed as described previously. 21 Briefly, PET images were corrected for interframe motion and summed into a single PET image corresponding to the 50‐ to 70‐min post‐injection interval. The summed PET image was co‐registered and resliced to the space of the T1w MPRAGE MR image using the normalized mutual information algorithm implemented in PMOD software (PMOD technologies, Zurich, Switzerland). T1 MPRAGE MR images were processed using FreeSurfer (version 5.3) to parcellate into a set of ROIs. The ROIs generated were then used to sample radioactivity concentrations in the summed PET images as described previously. 32 , 33 Regional [11C]PiB PETSUVR outcomes were calculated using cerebellar gray matter as reference for nine composite regions 34 (anterior cingulate, posterior cingulate, insula, superior frontal cortex, orbitofrontal cortex, lateral temporal cortex, parietal cortex, precuneus, and ventral striatum), as well as a global [11C]PiB SUVR based on a volume‐weighted average of these nine composite regions (GBL9), as described previously. 33 Participants were classified as Aβ positive or Aβ negative using SUVR cutoffs determined by a sparse k‐means clustering analysis. 35
3. RESULTS
3.1. Participant characteristics
Table 1 provides demographics and clinical characteristics stratified by sex, whereas Table S1 provides neurocognitive data stratified by sex. Males had a significantly higher number of years of education compared to females, but they did not significantly differ on age, race, PiB(+)%, or Mini‐Mental State Examination (MMSE) scores.
TABLE 1.
Demographic variables and clinical characteristics by sex (N = 54 a ).
| Characteristic | Group; Mean (SD) | Statistical test | ||
|---|---|---|---|---|
| Male, n = 20 | Female, n = 34 | t‐test/chi‐square | p‐value | |
| Age at baseline, years b | 74.5 (5.45) | 74.6 (6.52) | t52 = −0.078 | 0.94 |
| Education, years | 15.8 (2.36) | 14.2 (2.56) | t52 = 2.18 | 0.034 |
|
Race/ethnicity, n (%) |
χ2 = 1.72 | 0.42 | ||
| White | 18 (90.0%) | 26 (76.5%) | ||
| Black | 2 (10.0%) | 7 (20.6%) | ||
| Asian | 0 (0.00%) | 1 (2.9%) | ||
|
APOE genotype, n (%) c |
χ2 = 4.11 | 0.13 | ||
| ε2/ε3 | 2 (10.0%) | 3 (0.9%) | ||
| ε3/ε3 | 10 (50.0%) | 24 (75.0%) | ||
| ε3/ε4 | 8 (40.0%) | 4 (12.5%) | ||
| ε4/ε4 | 0 (0.0%) | 1 (3.1%) | ||
|
Baseline PiB(+), n (%) d |
3 (9.09%) | 4 (21.1%) | χ2 = 1.62 | 0.44 |
| Baseline MMSE score | 29.0 (1.21) | 28.8 (1.11) | t52 = 0.65 | 0.53 |
Abbreviations: APOE, apolipoprotein E; MMSE, Mini–Mental State Examination; PiB, Pittsburgh compound‐B; SUVR, standardized uptake value ratio.
Unless otherwise indicated.
Age range 65−93 years.
APOE genotyping was available for 52 of 54 participants (32 female and 20 male).
Baseline PiB SUVR data were available for 48 of 54 participants (33 female and 19 male).
TABLE 2.
Sex‐by‐time ANOVAs for hippocampal functional connectivity during the face–name associative memory fMRI task controlling for age.
| Brain Region | Brodmann area (BA) | Peak MNI coordinates (x,y,z) | Size (mm3) | β Value of ROI Time 1 Mean (SD) | β Value of ROI Time 2 Mean (SD) | Sex* Time F‐value |
Sex* Time p‐value |
|---|---|---|---|---|---|---|---|
| Left hippocampal functional connectivity | |||||||
| ACC | BA 24 | −2, 33, 10 | 1128 | Male: −0.22 (1.54) Female: 0.32 (0.92) | Male: 0.50 (1.28) Female: −0.61 (1.30) | F = 10.80 | p = 0.0014** |
| MFG | BA 10, 32 | –4, 56, 14 | 3504 | Male: −0.089 (2.24) Female: 0.93 (1.27) | Male: 0.85 (1.77) Female: −0.38 (1.64) | F = 12.89 | p = 0.00052*** |
| Hippocampus | N/A | 32, −30, −8 | 1408 | Male: 0.47 (0.91) Female: −0.48 (1.22) | Male: −0.20 (0.67) Female: 0.34 (0.98) | F = 12.06 | p = 0.00077*** |
| Right hippocampal functional connectivity | |||||||
| MFG | BA 10, 32 | −6,52,10 | 1608 | Male: −0.50 (1.95) Female: 0.17 (1.17) | Male: 0.61 (1.86) Female: −0.55 (1.25) | F = 12.43 | p = 0.00064*** |
Abbreviations: ACC, anterior cingulate cortex; ANOVA, analysis of variance; BA, Brodmann area; L, left; MFG, middle frontal gyrus; MNI, Montreal Neurologic Institute; R, right.
*p < 0.05; **p < 0.01, ***p < 0.001.
3.2. Neurocognitive outcomes
Table S1 summarizes ANOVA test results of the main and interactions effects of sex and time (baseline vs follow‐up) with their interactions on the 16 different neurocognitive measures in visual spatial construction, language, attention, executive, and memory domains. After adjusting for multiple comparisons via Bonferroni correction (p = 0.05/16 = 0.003), the only significant effects that remained were the main effect of sex on Boston Naming Test and Stroop color‐word test, where females scored higher than men. Detailed uncorrected statistical results are shown in Table S1, which may suggest a possible effect but would need to be tested a priori. When analyzing performance in face–name fMRI task via ANOVA (not shown), females had a longer mean time to encode during novel condition compared to males (p = 0.046), and there was no change in performance from baseline to follow‐up (p = 0.94). We observed no statistically significant difference in performance for mean time to encode during control condition for sex (p = 0.077) or time (p = 0.22).
Table S2 summarizes the linear regression results of the main and interaction effects of sex and baseline Aβ with their interactions on the change in 16 different neurocognitive measures in visual spatial construction, language, attention, executive, and memory domains. The main effect of sex that withstood Bonferroni multiple comparisons correction for the 48 different tests (0.05/16 = 0.003) was on changes in Word List Learning Trials and Digit Symbol. The Aβ × sex interaction effects that survived correction for multiple comparisons were on changes in Logical Memory Story delayed recall and the Boston Naming Test. In the two significant Aβ × sex interactions observed, increasing Aβ was associated with a significant decrease in neuropsychological test performance change in females but not in males. Detailed uncorrected statistical results are shown in Table S2.
3.3. Functional connectivity
Table 2 summarizes the interaction effects of sex and time (baseline vs follow‐up) on hippocampal functional connectivity during associative encoding for each ROI that survived multiple comparisons analysis. We observed significant sex‐by‐time interactions for left hippocampal functional connectivity with right hippocampus, anterior cingulate (ACC), and middle frontal gyrus (MFG), respectively. We observed males to have an increased left hippocampal functional connectivity from time 1 to time 2 in the MFG and ACC, whereas females had increased left hippocampal functional connectivity in the right hippocampus. Furthermore, we observed males to have increased right functional connectivity in the MFG. Additional ROIs were found for right hippocampal functional connectivity in the ACC and left hippocampus, but its clusters did not survive an extended threshold of p < 0.05 familywise errors. Of note, we reported previously on cross‐sectional results showing hippocampal functional connectivity in the MFG; ACC were sex‐dependent and Aβ‐dependent. 20 , 21 We observed no significant associations between the four ROI's functional connectivity correlated with the subjects’ mean time to encode during control and novel conditions (not shown).
3.4. Left hippocampus‐right hippocampus functional connectivity change
Figure 1A and Table 3 demonstrate a significant interaction between sex and baseline WMHV with a change in left–right hippocampal functional connectivity (follow‐up minus baseline). To better understand the interaction between sex and hippocampal functional connectivity change, we extracted mean functional connectivity from regions with significant sex‐by‐time interaction (left hippocampus with MFG, ACC and right hippocampus, and right hippocampus with MFG). The mean ROI change in functional connectivity was plotted against baseline WMHV and then a linear regression model was tested controlling for baseline age and Aβ load (adjusted r2 = 0.35). Specifically, left–right hippocampal functional connectivity change had a significant sex‐by‐WMHV interaction effect (p = 0.029), in that females had a positive association between functional connectivity change and baseline WMHV but males had no significant association. We did not observe a significant sex‐by‐WMHV interaction effect for any other significant regions. The sex‐by‐WMHV interaction remained significant when additionally controlling for race (p = 0.023, not shown) or Aβ status (p = 0.028, not shown), but was not significant when additionally controlling for apolipoprotein E APOE ε4 status (p = 0.19, not shown).
FIGURE 1.
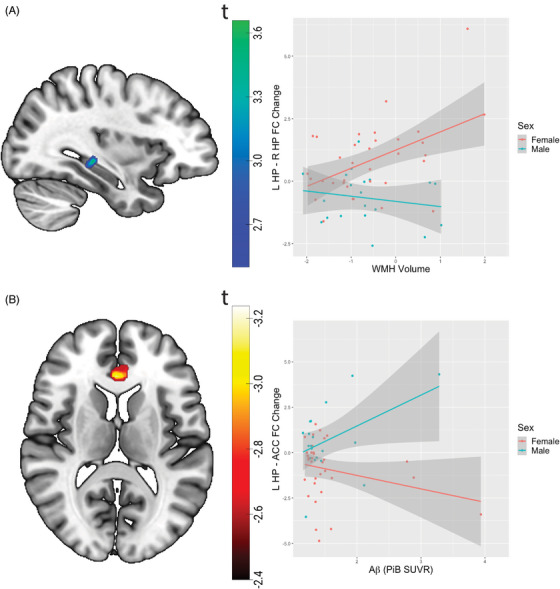
Sex differences in the 2‐year change in hippocampal functional connectivity is associated with baseline Aβ deposition and WMH load. (A) Sex × time (baseline vs follow‐up) interactions on left hippocampus–right hippocampus functional connectivity during associative memory encoding is shown on the left. On the right, the 2‐year change (follow‐up minus baseline) in bilateral hippocampal functional connectivity is associated with greater WMHV for females but not males. (B) Sex × time interactions on left hippocampus–ACC functional connectivity during associative memory encoding is shown on the left. On the right, the 2‐year change (follow‐up minus baseline) in hippocampus–ACC functional connectivity is associated with greater Aβ deposition (PiB SUVR) for males but not females. L, left; R, right; Aβ, amyloid beta; ACC, anterior cingulate cortex; HP, hippocampus; PiB, Pittsburgh compound‐B; SUVR, standardized uptake value ratio; WMHV, white matter hyperintensity volume.
TABLE 3.
Post hoc linear regression analysis of change in hippocampal functional connectivity for significant ROIs.
| Sex‐by‐Time | β | SE | 95% CI for β | p‐value |
|---|---|---|---|---|
| Change in left hippocampus–right hippocampus functional connectivity | ||||
| Age at baseline | −0.043 | 0.032 | (−0.11, 0.021) | 0.19 |
| Global PiB | −0.010 | 0.33 | (−0.77, 0.57) | 0.76 |
| Sex | −2.13 | 0.46 | (−3.07, −1.20) | 3.2e‐05*** |
| WMH volume | 0.82 | 0.23 | (0.36, 1.28) | 7.8e‐04*** |
| Sex by WMH volume | −0.97 | 0.43 | (−1.84, −0.11) | 0.029* |
| Change in left hippocampus–ACC functional connectivity | ||||
| Age at baseline | −0.0040 | 0.042 | (−0.088, 0.080) | 0.92 |
| Global PiB | −0.57 | 0.51 | (−1.58, 0.46) | 0.27 |
| Sex | −1.89 | 1.48 | (−4.87, 1.09) | 0.21 |
| WMH volume | −0.42 | 0.27 | (−0.96, 0.12) | 0.13 |
| Sex by Global PiB | 2.21 | 0.93 | (0.35, 4.07) | 0.021* |
Abbreviations: ACC, anterior cingulate cortex; PiB, Pittsburgh compound‐B; SUVR, standardized uptake value ratio; WMH, white matter hyperintensity.
*p < 0.05; ***p < 0.001.
3.5. Left hippocampus–ACC functional connectivity change
Figure 1B and Table 3 demonstrate a significant interaction between sex and Aβ in relation to change in left hippocampus functional connectivity with ACC (follow‐up minus baseline). We extracted mean functional connectivity from regions with significant sex‐by‐time interaction, and the mean ROI change in functional connectivity was plotted against baseline Aβ burden. In a linear regression model controlling for age and baseline WMHV (adjusted r2 = 0.25), we observed a significant sex‐by‐Aβ interaction (p = 0.021), in that males had a positive association between left hippocampal–ACC connectivity change and Aβ burden, whereas females had no significant association. We did not observe a significant sex‐by‐Aβ interaction effect for any other significant regions. The sex‐by‐Aβ interaction remained significant when additionally controlling for race (p = 0.020, not shown), Aβ status (p = 0.029, not shown), or APOE ε4 status (p = 0.030, not shown).
4. DISCUSSION
Using a face–name associative memory fMRI task in a normal aging cohort, this study investigated sex differences in the 2‐year change in hippocampal functional connectivity during associative memory encoding in older adults without cognitive impairment. As a post hoc analysis, we then investigated whether sex‐dependent changes in hippocampal functional connectivity are associated with Aβ burden or WMHV. Our main finding is that males and females have different 2‐year alterations in hippocampal functional connectivity, and that these alterations are differentially associated with the presence of baseline white matter damage and Aβ burden. Specifically, in females but not males, greater WMHV was accompanied by increased bilateral hippocampal functional connectivity. In males but not females, greater Aβ burden was associated with increased functional connectivity change between left hippocampus and ACC. As a secondary finding, we observed a significant sex‐by‐Aβ interaction effect in relation to change in neuropsychological test performance. Specifically, a faster decrease in memory performance was associated with increasing Aβ load in females but not males, further suggesting that females could not compensate as well for the neurotoxic effect of Aβ on the memory network. This study builds upon two earlier cross‐sectional studies from our laboratory that examined the effect of sex and WMH on Aβ‐related alterations to hippocampal functional connectivity, respectively. 20 , 21 Notably, we observed the same prefrontal regions to have altered functional connectivity with the hippocampus. In summary, we observed sex‐dependent alterations in hippocampal connectivity, which are linked to cerebrovascular and amyloid pathologies in normal aging over a 2‐year period.
Our findings in this longitudinal analysis suggest that compensation of the memory network in the presence of Aβ and white matter disruption is sex dependent. Differences observed in memory network alterations may relate to different aging trajectories of steroid hormones between males and females, particularly estradiol. 36 Mounting evidence in animal research supports the role of estradiol in structural and synaptic plasticity of the hippocampus 37 , 38 , 39 , 40 , 41 and the prefrontal cortex. 42 , 43 , 44 , 45 , 46 Moreover, extensive human neuroimaging studies further implicate estrogen in the regulation of memory circuitry. 47 , 48 , 49 , 50 , 51 , 52 , 53 In particular, a recent study demonstrated postmenopausal females who had no history of menopausal hormone therapy use had enhanced bilateral hippocampal connectivity relative to both premenopausal and perimenopausal females as well as males in a verbal memory fMRI task. 54 This study also found that greater alterations in hippocampal functional connectivity were associated with lower endogenous estradiol concentrations. Similarly, our study found an increase in bilateral hippocampal connectivity for females but not males. Although our study did not have menopausal status available, all females were likely postmenopausal based on their age. With this information, the dramatic drop in estrogen levels during the menopause transition may render the female hippocampal circuitry particularly vulnerable to synaptic plasticity loss. A decrease in synaptic plasticity may impair the ability of elderly females to compensate for the additional insults associated with Aβ accumulation. This may explain why we observe that older adult males, but not females, show increased hippocampal–prefrontal functional connectivity change in the presence of Aβ burden. This poor compensation could then explain why only females were found to have an association between decreased memory performance with increasing Aβ burden. These results are in agreement with our previous cross‐sectional study that found that hippocampal–prefrontal functional connectivity is increased with higher Aβ burden in cognitively normal males but not females. 20
The hippocampus–prefrontal cortex connection is of vital importance for the memory network 55 , 56 and appears to be altered during normal aging and the progression of AD. During memory tasks, studies have consistently shown that older subjects recruit the prefrontal cortex to compensate for an under‐recruitment of the MTL compared to younger subjects. 57 , 58 , 59 In AD, fMRI observations of hippocampal hyperactivity/hyperconnectivity during memory encoding is evidence of the recruitment of extra neural resources to maintain normal cognition despite Aβ deposition 14 and has been postulated to mark the initial onset of memory impairment and predict more rapid memory decline. 7 , 60 Indeed, Aβ burden has been associated with alterations in hippocampal–prefrontal cortex functional connectivity at rest 61 , 62 , 63 , 64 and during memory tasks. 15 , 20 , 21 When taken together, our findings suggest that males were able to compensate for the neurotoxic effects of Aβ by increasing engagement of prefrontal regions during associative memory encoding. Furthermore, given the potential deleterious effects of the menopause transition on hippocampal neuroplasticity, females may be less able to recruit prefrontal regions. These findings have important implications for our understanding and treatment of potentially distinct progressions from normal cognition, MCI, and ultimately AD in males versus females.
The classic brain “disconnection” model where structural disconnection can lead to functional disruption may explain our observation of WMHV association with bilateral hippocampal functional connectivity change in females but not in males. The different patterns of functional connectivity change we observed may relate to white matter disruption. Studies have demonstrated that WMHs disrupt white matter tracts traversing the lesions, 65 , 66 as well as cause damage to normal‐appearing white matter close to the lesions. 67 Hence, several studies have observed higher WMH load to be associated with reduced long‐range functional connectivity. 68 , 69 , 70 In the context of this study, WMH burden has been shown to degrade the microstructural integrity of the fornix and cingulum bundle, which are the two major white matter tracts associated with distal hippocampal connections. 65 , 71 , 72 We have reported previously in a cross‐sectional study that WMH severity moderated the effect of Aβ load on hippocampal connectivity during an associative memory fMRI task. 21 In this study, we observed that greater Aβ load was accompanied by increasing hippocampal–prefrontal connectivity only in subjects with low WMH burden, whereas greater Aβ load in subjects with high WMH burden was associated with an increase in local hippocampal connectivity. 21 Our previous study suggests that subjects with high WMH burden have relatively disconnected white matter tracts and cannot efficiently recruit distant prefrontal regions, instead recruiting more locally. Our current longitudinal study appears to agree with this finding, as we observed that increased bilateral hippocampal functional connectivity change was associated with greater WMH burden in females but not males. Notably, our previous cross‐sectional study had a cohort that was 67% female, which could partially explain this observation. Another study reported that the same degree of cognitive impairment is associated with greater structural white matter damage in cognitively normal older males compared to females, suggesting that males may have greater compensatory mechanisms to preserve cognition in the presence of white matter disruption. 73 Combined, our findings suggest that, in females, white matter damage exacerbates the ability of the memory network to compensate for Aβ accumulation and instead recruit locally as evidenced by increased bilateral hippocampal functional connectivity.
Several important limitations of this study should be considered. We were not able to measure sex hormones or explore how functional connectivity is related to levels of estradiol, testosterone, or other sex steroids. Given the age of the cohort, it is likely that all of the females were postmenopausal, and therefore inferences about the menopause transition cannot be made here. Current medications were recorded and no participants were on systemic hormone therapy at the time of the scans. This study has a relatively modest sample size (N = 54), which may not have sufficient power to detect more subtle changes than the ones found. For example, we did not have a significant number of participants who were APOE ε4 positive (N = eight males, five females) and thus could not effectively test for the effect of APOE ε4 status on hippocampal connectivity change in the different ROIs. Similarly, race is a known risk factor in disease progression, yet our study contained 90% and 77% White males and females, respectively. Although there was no statistically significant difference, our sample had a higher proportion of Black females compared to males and a lower proportion of APOE ε4+ females compared to males. Race and APOE ε4 most likely contribute to the same pathways that caused the observed alterations in the memory network. Future studies should investigate how APOE ε4 status, race, and sex independently and synergistically alter memory circuitry at the early stages of AD. In addition, males and females may have Aβ‐related connectivity changes in brain regions other than the hippocampus, yet the present work only evaluated hippocampal functional connectivity and as such could not investigate sex‐dependent changes in other brain regions that may relate to cognition. Our longitudinal analysis examined 2‐year changes of hippocampal functional connectivity, yet WMH and Aβ could interact on a different timescale; thus a 2‐year interval between serial MRI scans may not capture the full effect. Furthermore, the blood oxygen level–dependent (BOLD) signal changes in hippocampal functional connectivity could be of non‐neural origin and not be reflective of compensatory measures. Finally, our study design and sample size did not allow us to capture how age potentially contributes to altered hippocampal connectivity in cognitively unimpaired older adults. Although the turning point appears to be menopause, age exacerbates cerebrovascular damage and thus could contribute to higher bilateral hippocampal connectivity in females. Age is also associated with higher Aβ accumulation, which could contribute to higher hippocampal–frontal connectivity in males. Future studies with a larger sample size should examine the effect of the age continuum on the observed alterations in hippocampal connectivity.
In conclusion, using a face–name associative memory fMRI task in older adults without cognitive impairment, the present study investigated sex differences in the 2‐year change in hippocampal functional connectivity during associative memory encoding. We observed males to have an increased functional connectivity change between hippocampus and prefrontal regions, and furthermore that greater hippocampus–ACC functional connectivity change was associated with increased Aβ burden. In addition, we observed females to have no association between hippocampus–ACC connectivity change and Aβ burden. Instead, we observed females to have increased bilateral hippocampal functional connectivity change that was associated with greater WMH burden. These findings may represent an early indication of a homeostatic response in the memory network that potentially explains some of the sex differences in AD trajectories.
AUTHOR CONTRIBUTIONS
Minjie Wu is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
CONFLICTS OF INTEREST STATEMENT
Thurston: Astellas, Bayer, Hello Therapeutics, Happify Health (advisory board). All other authors declare no competing interests. Author disclosures are available in the supporting information.
CONSENT STATEMENT
Written informed consent was obtained from all study participants.
PRIOR PRESENTATION
Schweitzer N, Li J, Lopresti B, Klunk WE, Snitz B, Tudorascu D, Cohen A, Kamboh M I, Halligan E, Villemagne VL, Iordanova B, Aizenstein H, Wu M. Risk architecture for altered hippocampal connectivity in normal aging differs between men and women. 2023 AAIC Annual Conference 16–20 July 2023.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
This work was supported by the National Institute on Aging (R01AG067018 to Wu, and RF1AG025516 to Aizenstein, Villemagne); the National Institute of Neurological Disorders and Stroke (RF1NS116450 to Iordanova); and the National Heart, Lung, and Blood Institute (K24HL123565 to Thurston).
Schweitzer N, Li J, Thurston RC, et al. Sex‐dependent alterations in hippocampal connectivity are linked to cerebrovascular and amyloid pathologies in normal aging. Alzheimer's Dement. 2024;20:914–924. 10.1002/alz.13503
DATA AVAILABILITY STATEMENT
The data sets generated during and/or analyzed in the current study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Jack CR, Jr., Wiste HJ, Lesnick TG, et al. Brain β‐amyloid load approaches a plateau. Neurology. 2013;80:890‐896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Villemagne VL, Burnham S, Bourgeat P, et al. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: a prospective cohort study. Lancet Neurol. 2013;12:357‐367. [DOI] [PubMed] [Google Scholar]
- 3. Landau SM, Fero A, Baker SL, et al. Measurement of longitudinal β‐amyloid change with 18F‐Florbetapir PET and standardized uptake value ratios. J Nucl Med. 2015;56:567‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bennett DA, Schneider JA, Arvanitakis Z, et al. Neuropathology of older persons without cognitive impairment from two community‐based studies. Neurology. 2006;66:1837‐1844. [DOI] [PubMed] [Google Scholar]
- 5. Aizenstein HJ, Nebes RD, Saxton JA, et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol. 2008;65:1509‐1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mintun MA, Larossa GN, Sheline YI, et al. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67:446‐452. [DOI] [PubMed] [Google Scholar]
- 7. Sperling R. Potential of functional MRI as a biomarker in early Alzheimer's disease. Neurobiol Aging. 2011;32(1):S37‐43. Suppl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Golby A, Silverberg G, Race E, et al. Memory encoding in Alzheimer's disease: an fMRI study of explicit and implicit memory. Brain. 2005;128:773‐787. [DOI] [PubMed] [Google Scholar]
- 9. Grön G, Bittner D, Schmitz B, Wunderlich AP, Riepe MW. Subjective memory complaints: objective neural markers in patients with Alzheimer's disease and major depressive disorder. Ann Neurol. 2002;51:491‐498. [DOI] [PubMed] [Google Scholar]
- 10. Hämäläinen A, Pihlajamäki M, Tanila H, et al. Increased fMRI responses during encoding in mild cognitive impairment. Neurobiol Aging. 2007;28:1889‐1903. [DOI] [PubMed] [Google Scholar]
- 11. Bookheimer SY, Strojwas MH, Cohen MS, et al. Patterns of brain activation in people at risk for Alzheimer's disease. N Engl J Med. 2000;343:450‐456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bondi MW, Houston WS, Eyler LT. Brown GG. fMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer disease. Neurology. 2005;64:501‐508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Edelman K, Tudorascu D, Agudelo C, et al. Amyloid‐beta deposition is associated with increased medial temporal lobe activation during memory encoding in the cognitively normal elderly. Am J Geriatr Psychiatry. 2017;25:551‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Elman JA, Oh H, Madison CM, et al. Neural compensation in older people with brain amyloid‐β deposition. Nat Neurosci. 2014;17:1316‐1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Oh H, Jagust WJ. Frontotemporal network connectivity during memory encoding is increased with aging and disrupted by beta‐amyloid. J Neurosci. 2013;33:18425‐18437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. 2023 Alzheimer's disease facts and figures. Alzheimer's & Dementia.n/a. [DOI] [PubMed]
- 17. Ferretti MT, Iulita MF, Cavedo E, et al. Sex differences in Alzheimer disease—the gateway to precision medicine. Nat Rev Neurol. 2018;14:457‐469. [DOI] [PubMed] [Google Scholar]
- 18. Holland D, Desikan RS, Dale AM, McEvoy LK. Higher rates of decline for women and apolipoprotein E epsilon4 carriers. AJNR Am J Neuroradiol. 2013;34:2287‐2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lin KA, Choudhury KR, Rathakrishnan BG, Marks DM, Petrella JR, Doraiswamy PM. Marked gender differences in progression of mild cognitive impairment over 8 years. Alzheimers Dement (N Y). 2015;1:103‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu M, Thurston RC, Tudorascu DL, et al. Amyloid deposition is associated with different patterns of hippocampal connectivity in men versus women. Neurobiol Aging. 2019;76:141‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu M, Schweitzer N, Iordanova BE, et al. Pre‐clinical AD small vessel disease is associated with altered hippocampal connectivity and atrophy. The American Journal of Geriatric Psychiatry. 2023;31:112‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. De Marco M, Manca R, Mitolo M, Venneri A. White matter hyperintensity load modulates brain morphometry and brain connectivity in healthy adults: a neuroplastic mechanism? Neural Plast. 2017;2017:4050536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183‐194. [DOI] [PubMed] [Google Scholar]
- 24. Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment–beyond controversies, towards a consensus: report of the International working group on mild cognitive impairment. J Intern Med. 2004;256:240‐246. [DOI] [PubMed] [Google Scholar]
- 25. Wolk DA, Price JC, Saxton JA, et al. Amyloid imaging in mild cognitive impairment subtypes. Ann Neurol. 2009;65:557‐568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bell CC. DSM‐IV: diagnostic and statistical manual of mental disorders. Jama. 1994;272:828‐829. [Google Scholar]
- 27. Sperling RA, Bates JF, Chua EF, et al. fMRI studies of associative encoding in young and elderly controls and mild Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2003;74:44‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wilson AA, Garcia A, Jin L, Houle S. Radiotracer synthesis from [(11)C]‐iodomethane: a remarkably simple captive solvent method. Nucl Med Biol. 2000;27:529‐532. [DOI] [PubMed] [Google Scholar]
- 29. Wu M, Rosano C, Butters M, et al. A fully automated method for quantifying and localizing white matter hyperintensities on MR images. Psychiatry Res. 2006;148:133‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage. 2002;15:273‐289. [DOI] [PubMed] [Google Scholar]
- 31. Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218‐229. [DOI] [PubMed] [Google Scholar]
- 32. Fischl B, Van Der Kouwe A, Destrieux C, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11‐22. [DOI] [PubMed] [Google Scholar]
- 33. Sullivan KJ, Liu A, Chang CCH, et al. Alzheimer's disease pathology in a community‐based sample of older adults without dementia: the MYHAT neuroimaging study. Brain Imaging Behav. 2021;15:1355‐1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lopresti BJ, Klunk WE, Mathis CA, et al. Simplified quantification of Pittsburgh Compound B amyloid imaging PET studies: a comparative analysis. J Nucl Med. 2005;46:1959‐1972. [PubMed] [Google Scholar]
- 35. Cohen AD, Mowrey W, Weissfeld LA, et al. Classification of amyloid‐positivity in controls: comparison of visual read and quantitative approaches. Neuroimage. 2013;71:207‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Radaghdam S, Karamad V, Nourazarian A, Shademan B, khaki‐khatibi F, Nikanfar M. Molecular mechanisms of sex hormones in the development and progression of Alzheimer's disease. Neuros Lett. 2021;764:136221. [DOI] [PubMed] [Google Scholar]
- 37. Woolley CS, McEwen BS. Estradiol regulates hippocampal dendritic spine density via an N‐methyl‐D‐aspartate receptor‐dependent mechanism. J Neurosci. 1994;14:7680‐7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McEwen BS. Sex, stress and the hippocampus: allostasis, allostatic load and the aging process. Neurobiol Aging. 2002;23:921‐939. [DOI] [PubMed] [Google Scholar]
- 39. Brinton RD. Estrogen‐induced plasticity from cells to circuits: predictions for cognitive function. Trends Pharmacol Sci. 2009;30:212‐222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hara Y, Park CS, Janssen WG, Roberts MT, Morrison JH, Rapp PR. Synaptic correlates of memory and menopause in the hippocampal dentate gyrus in rhesus monkeys. Neurobiol Aging. 2012;33:421. e17‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu F, Day M, Muñiz LC, et al. Activation of estrogen receptor‐beta regulates hippocampal synaptic plasticity and improves memory. Nat Neurosci. 2008;11:334‐343. [DOI] [PubMed] [Google Scholar]
- 42. Dumitriu D, Hao J, Hara Y, et al. Selective changes in thin spine density and morphology in monkey prefrontal cortex correlate with aging‐related cognitive impairment. J Neurosci. 2010;30:7507‐7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hao J, Rapp PR, Leffler AE, et al. Estrogen alters spine number and morphology in prefrontal cortex of aged female rhesus monkeys. J Neurosci. 2006;26:2571‐2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Morrison JH, Brinton RD, Schmidt PJ, Gore AC. Estrogen, menopause, and the aging brain: how basic neuroscience can inform hormone therapy in women. J Neurosci. 2006;26:10332‐10348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rapp PR, Morrison JH, Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci. 2003;23:5708‐5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang AC, Hara Y, Janssen WG, Rapp PR, Morrison JH. Synaptic estrogen receptor‐alpha levels in prefrontal cortex in female rhesus monkeys and their correlation with cognitive performance. J Neurosci. 2010;30:12770‐12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shaywitz SE, Shaywitz BA, Pugh KR, et al. Effect of estrogen on brain activation patterns in postmenopausal women during working memory tasks. Jama. 1999;281:1197‐1202. [DOI] [PubMed] [Google Scholar]
- 48. Grigorova M, Sherwin BB, Tulandi T. Effects of treatment with leuprolide acetate depot on working memory and executive functions in young premenopausal women. Psychoneuroendocrinology. 2006;31:935‐947. [DOI] [PubMed] [Google Scholar]
- 49. Dumas JA, Kutz AM, Naylor MR, Johnson JV, Newhouse PA. Increased memory load‐related frontal activation after estradiol treatment in postmenopausal women. Horm Behav. 2010;58:929‐935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Epperson CN, Amin Z, Ruparel K, Gur R, Loughead J. Interactive effects of estrogen and serotonin on brain activation during working memory and affective processing in menopausal women. Psychoneuroendocrinology. 2012;37:372‐382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shanmugan S, Epperson CN. Estrogen and the prefrontal cortex: towards a new understanding of estrogen's effects on executive functions in the menopause transition. Hum Brain Mapp. 2014;35:847‐865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hampson E, Morley EE. Estradiol concentrations and working memory performance in women of reproductive age. Psychoneuroendocrinology. 2013;38:2897‐2904. [DOI] [PubMed] [Google Scholar]
- 53. Jacobs EG, Weiss B, Makris N, et al. Reorganization of functional networks in verbal working memory circuitry in early midlife: the impact of sex and menopausal status. Cereb Cortex. 2017;27:2857‐2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jacobs EG, Weiss BK, Makris N, et al. Impact of sex and menopausal status on episodic memory circuitry in early midlife. J Neurosci. 2016;36:10163‐10173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Simons JS, Spiers HJ. Prefrontal and medial temporal lobe interactions in long‐term memory. Nat Rev Neurosci. 2003;4:637‐648. [DOI] [PubMed] [Google Scholar]
- 56. Preston AR, Eichenbaum H. Interplay of hippocampus and prefrontal cortex in memory. Curr Biol. 2013;23:R764‐R773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Morcom AM, Good CD, Frackowiak RS, Rugg MD. Age effects on the neural correlates of successful memory encoding. Brain. 2003;126:213‐229. [DOI] [PubMed] [Google Scholar]
- 58. Gutchess AH, Welsh RC, Hedden T, et al. Aging and the neural correlates of successful picture encoding: frontal activations compensate for decreased medial‐temporal activity. J Cogn Neurosci. 2005;17:84‐96. [DOI] [PubMed] [Google Scholar]
- 59. Dennis NA, Daselaar S, Cabeza R. Effects of aging on transient and sustained successful memory encoding activity. Neurobiol Aging. 2007;28:1749‐1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Huijbers W, Mormino EC, Schultz AP, et al. Amyloid‐β deposition in mild cognitive impairment is associated with increased hippocampal activity, atrophy and clinical progression. Brain. 2015;138:1023‐1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mormino EC, Smiljic A, Hayenga AO, et al. Relationships between β‐amyloid and functional connectivity in different components of the default mode network in aging. Cereb Cortex. 2011;21:2399‐2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sperling RA, Laviolette PS, O'Keefe K, et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63:178‐188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lim HK, Nebes R, Snitz B, et al. Regional amyloid burden and intrinsic connectivity networks in cognitively normal elderly subjects. Brain. 2014;137:3327‐3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sheline YI, Raichle ME, Snyder AZ, et al. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol Psychiatry. 2010;67:584‐587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Reginold W, Itorralba J, Luedke AC, et al. Tractography at 3T MRI of corpus callosum tracts crossing white matter hyperintensities. AJNR Am J Neuroradiol. 2016;37:1617‐1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Reginold W, Sam K, Poublanc J, Fisher J, Crawley A, Mikulis DJ. Impact of white matter hyperintensities on surrounding white matter tracts. Neuroradiology. 2018;60:933‐944. [DOI] [PubMed] [Google Scholar]
- 67. Maillard P, Fletcher E, Lockhart SN, et al. White matter hyperintensities and their penumbra lie along a continuum of injury in the aging brain. Stroke. 2014;45:1721‐1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Schaefer A, Quinque EM, Kipping JA, et al. Early small vessel disease affects frontoparietal and cerebellar hubs in close correlation with clinical symptoms—a resting‐state FMRI study. J Cereb Blood Flow Metab. 2014;34:1091‐1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Langen CD, Zonneveld HI, White T, et al. White matter lesions relate to tract‐specific reductions in functional connectivity. Neurobiol Aging. 2017;51:97‐103. [DOI] [PubMed] [Google Scholar]
- 70. Quandt F, Fischer F, Schröder J, et al. Higher white matter hyperintensity lesion load is associated with reduced long‐range functional connectivity. Brain Comm. 2020;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. He J, Wong VS, Fletcher E, et al. The contributions of MRI‐based measures of gray matter, white matter hyperintensity, and white matter integrity to late‐life cognition. AJNR Am J Neuroradiol. 2012;33:1797‐1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Yatawara C, Lee D, Ng KP, et al. Mechanisms linking white matter lesions, tract integrity, and depression in Alzheimer Disease. Am J Geriatr Psychiatry. 2019;27:948‐959. [DOI] [PubMed] [Google Scholar]
- 73. O'Dwyer L, Lamberton F, Bokde AL, et al. Sexual dimorphism in healthy aging and mild cognitive impairment: a DTI study. PLoS One. 2012;7:e37021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Data Availability Statement
The data sets generated during and/or analyzed in the current study are available from the corresponding author upon reasonable request.


