Abstract
INTRODUCTION
Isolated/idiopathic rapid eye movement sleep behavior disorder (iRBD) is a powerful early predictor of dementia with Lewy bodies (DLB) and Parkinson's disease (PD). This provides an opportunity to directly observe the evolution of prodromal DLB and to identify which cognitive variables are the strongest predictors of evolving dementia.
METHODS
IRBD participants (n = 754) from 10 centers of the International RBD Study Group underwent annual neuropsychological assessment. Competing risk regression analysis determined optimal predictors of dementia. Linear mixed‐effect models determined the annual progression of neuropsychological testing.
RESULTS
Reduced attention and executive function, particularly performance on the Trail Making Test Part B, were the strongest identifiers of early DLB. In phenoconverters, the onset of cognitive decline began up to 10 years prior to phenoconversion. Changes in verbal memory best differentiated between DLB and PD subtypes.
DISCUSSION
In iRBD, attention and executive dysfunction strongly predict dementia and begin declining several years prior to phenoconversion.
Highlights
Cognitive decline in iRBD begins up to 10 years prior to phenoconversion.
Attention and executive dysfunction are the strongest predictors of dementia in iRBD.
Decline in episodic memory best distinguished dementia‐first from parkinsonism‐first phenoconversion.
Keywords: dementia with Lewy bodies, evolution, Parkinson's disease, pre‐diagnostic, prodromal stage, REM sleep behavior disorder
1. BACKGROUND
Isolated/idiopathic rapid eye movement (REM) sleep behavior disorder (iRBD) is a parasomnia characterized by loss of REM sleep muscle atonia and dream‐enactment behavior. It is also a powerful indicator of underlying synucleinopathy: the vast majority (>80%) of individuals with iRBD will ultimately develop an overt neurodegenerative disease, including Parkinson's disease (PD), dementia with Lewy bodies (DLB), and multiple system atrophy (MSA), with a phenoconversion rate of 6%‐8% per year. 1 Those with iRBD are at an approximately equal risk of developing DLB or PD, and when classified according to the initial phenoconversion event (parkinsonism‐first vs. dementia‐first), show remarkably similar rates of progression in motor and non‐motor clinical variables. 1 , 2 , 3 In fact, the only robust differentiating clinical marker between parkinsonism‐first and dementia‐first phenoconverters is cognitive dysfunction itself. 4 , 5 Early differentiation between the two would allow clinicians and patients to anticipate and plan for the consequences of progressive cognitive decline. Importantly, if the underlying pathomechanisms that drive neurodegeneration are substantially different between PD and DLB, 6 , 7 reliably distinguishing iRBD subjects destined to phenoconvert to dementia‐first from parkinsonism‐first presentations might enable a more precise selection of subjects for future targeted therapies.
Patterns of cortical and subcortical brain atrophy can be observed in iRBD, even among those without mild cognitive impairment (MCI). 8 , 9 , 10 Therefore, the neurodegenerative processes that underlie DLB begin well before the time of phenoconversion. 11 However, it is uncertain how early in the prodromal disease course cognitive dysfunction can be detected and consequently, how long before diagnosis those destined to develop DLB can be identified. Moreover, it remains unclear which specific cognitive tests are most useful for detecting early cognitive dysfunction, and whether a distinct and evolving pattern of cognitive dysfunction exists in iRBD. Previous analyses of neuropsychological testing over the course of the prodromal disease have been limited to relatively small or single‐center studies with consequent heterogeneity between results. 4 , 5 , 12 , 13 , 14 , 15
In the present study, we combine the prospective experience of 10 centers of the International RBD Study Group representing six countries to: (1) determine the predictive value of baseline neuropsychological testing on the development of dementia in iRBD; (2) assess how neuropsychological testing in iRBD evolves up to the point of phenoconversion; (3) determine how progression differs between prodromal sub‐types; and (4) assess at what timepoint can individual neuropsychological tests identify an individual destined to develop dementia.
2. METHODS
2.1. Study participants
Details of this International RBD Study Group cohort have been described previously. 16 This study included those centers that performed systematic and longitudinal cognitive testing. Baseline and longitudinal participant data were collected between November 2004 and February 2022, with the majority of participants (77.8%) recruited after 2014. All study participants were included based on the following criteria: (1) polysomnogram‐confirmed iRBD according to standard criteria 17 ; (2) aged 30 years and older; (3) underwent baseline clinical and neuropsychological testing with at least one follow‐up; and (4) were without parkinsonism or dementia at baseline. Parkinsonism was defined as bradykinesia plus at least one of rigidity or rest tremor 18 ; Dementia was defined as cognitive impairment on screening testing (Mini‐Mental State Examination [MMSE] < 28 or Montreal Cognitive Assessment [MoCA] < 26) with significant functional impact related to cognitive deficit. 19 The baseline timepoint was set to the first visit at which cognitive testing was performed (8.2% of included participants had a separate baseline visit at a mean of 1.2 years before neuropsychologic testing, five of whom were known to have phenoconverted at a later date). Ethics approval was obtained from the local institutional boards of each center with participant consent in accordance with the Declaration of Helsinki.
2.2. Neuropsychological testing and clinical measurements
This study combined results from multiple centers, each of which used their own specific testing protocols. Therefore, a pragmatic approach to data collection and harmonization was used. Neuropsychological tests were pooled based upon similar testing methodologies; for inclusion each test had to be administered by at least three study centers, involve ≥30% of the total study population, and have available published normative scores to enable comparisons between centers. Characteristics of the included neuropsychological tests by the study center are shown in Table S1.
Additional demographic and clinical data included standardized symptom assessment and motor examination using a harmonized measurement for the Movement Disorders Society Universal Parkinson's Disease Rating Scale (MDS‐UPDRS) part II and III sub‐scales. 16 The presence of baseline depression as a comorbidity was assessed either by formal clinical diagnosis, meeting the threshold for moderate depression by standardized questionnaire, or by the use of antidepressant medication.
RESEARCH IN CONTEXT
Systematic review: The literature was reviewed using PubMed searches for isolated/idiopathic rapid eye movement sleep behavior disorder (iRBD) and cognition. Previous longitudinal analyses of neuropsychological testing over the course of prodromal dementia with Lewy bodies (DLB) have been limited to relatively small or single‐center studies.
Interpretation: Our findings demonstrate that the decline in cognitive function in iRBD can be detected very early in the disease course. The pattern of cognitive dysfunction begins with attention and executive dysfunction, followed later by dysfunction in the memory and visuospatial domains.
Future directions: These findings could enable the early differentiation of iRBD subjects that will go on to develop dementia. This could form the groundwork for prodromal cognitive screening to identify subjects for targeted preventative trials in the early stages of the neurodegenerative process, when they are more likely to be beneficial.
3. Statistical analyses
Statistical analyses were performed using R (version 4.2.2).
3.1. Data preprocessing
For neuropsychological test comparisons, raw scores were converted to z‐scores using available normative data (Table S1). To minimize the effect of outliers, z‐scores were winsorized to −3.5 and 3.5, which affected 2.6% of the data. MCI was determined using Level I PD‐MCI criteria: subjective cognitive complaints (Table S1) with either impairment on global cognitive abilities (MoCA cutoff: <26/30 or MMSE cutoff <28/30) or impairment (z‐score ≤ −1.5) in at least two not highly related/correlated tests. 20
3.2. Assessment of neuropsychological testing performance
To combine neuropsychological testing from multiple centers, a meta‐analysis of single means approach was used. The inverse variance method for pooling and the Hartung‐Knapp method for random effects (study center) was used to generate weighted mean z‐scores and confidence intervals for each neuropsychological test. 21 To determine if iRBD participants scored below normal performance, normative z‐scores for the entire cohort and stratified by phenoconversion status were compared to the expected normative means. A negative z‐score with the exclusion of zero from the 95% confidence interval indicated reduced cognitive performance, whereas a z‐score ≤ −1.5 was set as the threshold for impaired test performance.
3.3. Survival analysis
To determine the risk of dementia according to baseline performance on individual cognitive tests or domains, a competing risk regression analysis was used with dementia‐first phenoconversion as the event of interest and parkinsonism‐first phenoconversion as the competing risk (note that phenoconverters termed “dementia” vs. “dementia‐first” are the same; for readability, we reserve the longer “dementia‐first” terminology primarily for comparisons against parkinsonism‐first phenoconversion). A competing risk analysis was selected over traditional methods since those phenoconverting to a parkinsonism‐first phenotype were effectively censored at the time of diagnosis (i.e., they cannot then go on to develop a dementia‐first phenotype). Those without follow‐up assessments were excluded. The time of baseline cognitive testing was used as the start time and the time between testing and phenoconversion or censoring was used as the follow‐up time. The onset of phenoconversion was estimated to be the half‐way point between the prior ‘disease‐free’ assessment and the visit in which phenoconversion was diagnosed. Those not phenoconverting to overt synucleinopathy were censored at their last visit. The overall regression model included covariates for sex, age, years of education, depression, and study center. The full model was compared to a set of candidate models created by successively adding covariates on the basis of their significance in the full model and selecting the model with the smallest Bayesian information criteria (BIC) value. 22 , 23 Because of insufficient sample size, data from follow‐up times beyond 10 years were excluded from the analysis (1.6% of participants had data beyond 10 years, all from the Montreal cohort). An assessment of Schoenfeld residuals against time did not show violations of the assumption of locally constant means.
3.4. Progression of cognitive performance
To assess the evolution of cognitive performance, the year of phenoconversion was set as Year 0, and all preceding cognitive measurements plotted backward from that point (i.e., Year −1, Year −2, etc. up to Year −10). For participants not known to have phenoconverted, Year 0 was set as the most recent cognitive assessment. If a test was administered only once in a participant, that cognitive test was excluded from the longitudinal analysis. Linear mixed‐effect modeling (LMEM) fit by restricted maximum likelihood was used to estimate the annual progression rate of each cognitive test with the participant (random slopes and intercepts) and study center (random intercepts) as random effects, year of assessment, sex, age, education, and depression as fixed effects, and phenoconversion status as an interacting term. 24 Linearity was observed when examining the fitted residuals of the model for neuropsychological testing. The estimated marginal means and estimated marginal trends were calculated and used to determine annual progression rates for each neuropsychological test subdivided by phenoconversion status. 25 Contrast comparisons were used to determine statistical differences in annual progression rate between phenoconversion types. 25 Predictor effects plots were generated to provide graphical summaries of the fitted LMEMs to the raw data. 26
4. RESULTS
4.1. Overall outcomes
Detailed baseline demographics for each center are shown in Table S2 and summarized in Table 1. Data were collected from a total of 948 participants from 10 centers in six countries, from which 191 were excluded due to lack of follow‐up and a further three were excluded due to phenoconversion to MSA. Thus, analyses were performed on a total of 754 participants with a mean follow‐up time of 3.3 ± 2.4 years, or a total of 2488 person‐years of follow‐up. The mean age at baseline neuropsychological testing was 67.4 ± 8.2 years, 20.3% were female, and time from iRBD diagnosis was 1.5 ± 2.6 years.
TABLE 1.
Baseline demographics and clinical variables by phenoconversion status.
| Total | Unconverted (A) | Parkinsonism‐first (B) | Dementia‐first (C) | p‐Value | Post‐hoc | |
|---|---|---|---|---|---|---|
| n | 754 | 584 | 96 | 74 | – | |
| Baseline age (years) | 67.4 (8.2) | 66.5 (8.2) | 69.0 (7.3) | 72.5 (6.8) | <0.001 | A < B*; B < C** |
| Female (%) | 20.3 | 19.2 | 21.9 | 27 | 0.263 | – |
| Education (years) | 13.2 (4.3) | 13.6 (4.1) | 12.4 (4) | 11.5 (5.1) | <0.001 | A > B*; B > C** |
| iRBD duration (years) | 1.5 (2.6) | 1.2 (2.1) | 2.3 (3.0) | 2.5 (4.4) | <0.001 | A < B = C** |
| Follow‐up (years) | 3.3 (2.4) | 3.4 (2.3) | 2.9 (2.6) | 2.8 (2.6) | 0.030 | ns |
| Depression (%) | 26.1 | 24.0 | 30.2 | 37.8 | 0.018 | A < C* |
| MMSE | 27.8 (2.1) | 28.0 (1.8) | 28.0 (1.8) | 26.1 (3.2) | <0.001 a | A = B > C** |
| MoCA | 25.7 (2.9) | 26.0 (2.8) | 25.7 (2.1) | 22.6 (3.3) | <0.001 a | A = B > C** |
| MDS‐UPDRS‐I | 7.8 (6.2) | 7.5 (6.0) | 8.1 (5.9) | 9.3 (8.2) | 0.157 a | – |
| MDS‐UPDRS‐II | 2.5 (3.7) | 2.3 (3.7) | 2.9 (3.5) | 3.2 (4.2) | 0.029 a | ns |
| MDS‐UPDRS‐III | 3.9 (4.5) | 3.3 (3.8) | 5.4 (5.2) | 6.8 (6.4) | <0.001 a | A < B = C** |
| MCI (%) | 29.8 | 25.3 | 30.2 | 58.0 | <0.001 | A = B < C** |
Note: Statistical differences were calculated using analysis of covariance with Tukey post‐hoc testing for continuous variables and χ2 test with post‐hoc pairwise testing for categorical variables.
Abbreviations: MCI, mild cognitive impairment; MDS‐UPDRS, Movement Disorders Society Unified Parkinson's Disease Rating Scale; MMSE, Mini‐Mental State Examination; MoCA, Montreal Cognitive Assessment; ns, no significance.
Adjusted by age, sex, education, and depression.
p < 0.05.
p ≤ 0.001.
During follow‐up, 170 participants (22.5%) phenoconverted to a defined neurodegenerative disease, including 96 developing parkinsonism‐first and 74 developing dementia‐first phenotypes. The baseline characteristics of phenoconverted participants are summarized in Table 1. Dementia‐first phenoconverters were significantly older than both parkinsonism‐first phenoconverters and non‐converters. Similarly, the proportion of participants with MCI at baseline was greater in those who developed a dementia phenotype (dementia‐first = 58.0%, parkinsonism‐first = 30.2%, unconverted = 25.3%, p < 0.001), with an overall baseline frequency of 30.2%.
4.2. Baseline neuropsychological performance
Pooled results of baseline neuropsychological testing showed an overall high level of between‐center variability (Figure 1A and Figure S1), with the median I2 from all comparisons of 91% (indicating that 91% of the variability in the final estimate was the result of between‐center variability). Relatively low variability was observed with the Word list recognition (I2 = 23%) and Digit span backward tasks (I2 = 41%). Despite an overall high degree of heterogeneity, lower than expected cognitive performance (i.e., mean and 95% confidence interval [CI] ≤ zero) in the whole iRBD cohort was observed in the Trail Making Test (TMT) B (z‐score = −0.76) and TMT B‐A subscore (z‐score = −0.67).
FIGURE 1.
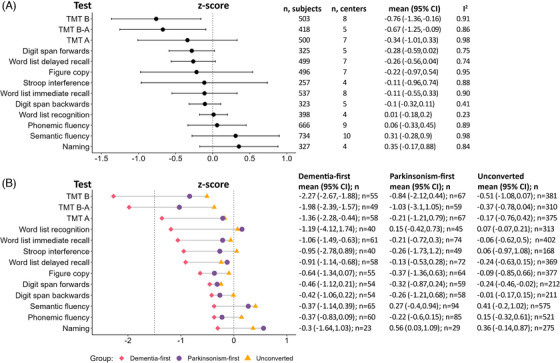
Neuropsychological testing in iRBD at baseline. (A) Pooled results for each neuropsychological test in all iRBD participants using a meta‐analysis of single means. Detailed results by study center are shown in Figure S1. (B) Mean testing results sub‐grouped by phenoconversion status. TMT, trail making test.
When stratifying baseline scores by phenoconversion status (Figure 1B), a general trend of reduced cognitive performance was observed in dementia‐first phenoconverters relative to parkinsonism‐first and unconverted participants. This was most clear in tasks of attention, executive function, and learning/memory. Impaired cognitive performance (i.e., z‐score mean and 95% CI ≤ −1.5) was found in dementia‐first but not parkinsonism‐first and unconverted participants in the TMT B (dementia‐first = −2.27, parkinsonism‐first = −0.84, unconverted = −0.51) and TMT B‐A (dementia‐first = −1.98, parkinsonism‐first = −1.03, unconverted = −0.37). Likewise, reduced cognitive performance (i.e., z‐score mean and 95% CI ≤ zero) was observed in dementia‐first participants in the TMT A (dementia‐first = −1.36, parkinsonism‐first = −0.21, unconverted = −0.17), Word list immediate recall (dementia‐first = −1.06, parkinsonism‐first = −0.21, unconverted = −0.06), and Word list delayed recall (dementia‐first = −0.91, parkinsonism‐first = −0.13, unconverted = −0.24).
4.3. Predictors of dementia outcome
Survival analysis using baseline neuropsychological testing is illustrated in Table 2 and Figures 2 and 3. On competing risk regression analysis, a variety of cognitive tests had reduced performance at baseline which were significantly predictive of dementia. The strongest predictors (tests that predicted dementia with hazard ratio [HR] > 3 and that were more frequently impaired in dementia phenoconverters) included: (1) attention domain: any impaired test (HR = 4.65), TMT A (HR = 3.82), Stroop interference (HR = 5.62); (2) executive domain: any impaired test (HR = 6.05), TMT B (HR = 4.62), TMT B‐A (HR = 7.86), Semantic fluency (HR = 4.14); (3) memory domain: any impaired test (HR = 3.26), Word list immediate recall (HR = 4.06). Baseline MCI was associated with a milder increased risk of dementia (HR = 2.74). Although impaired baseline scores in Digit span backward (HR = 5.14), Phonemic fluency (HR = 2.35), Figure copy (HR = 2.07), and Naming (HR = 6.17) were all predictive of dementia, no significant difference was found between dementia‐first versus parkinsonism‐first phenoconverters.
TABLE 2.
Baseline prediction of dementia phenoconversion in iRBD.
| Percent abnormal a | |||||
|---|---|---|---|---|---|
| Test | HR (95% CI) | Parkinsonism‐first | Dementia‐first | p‐Value | |
| Attention domain (any test) | 4.65 (2.49, 8.7) | 26.5 | 64.1 | <0.001 | |
| TMT A | 3.82 (1.91, 7.64) | 15.2 | 42.1 | 0.003 | |
| Stroop interference | 5.62 (2.29, 13.82) | 6.1 | 35.0 | 0.003 | |
| Digit span backward | 5.14 (1.38, 19.17) | 0.0 | 9.4 | 0.073 | |
| Digit span forward | 1.93 (0.97, 3.85) | 12.1 | 22.6 | 0.245 | |
| Executive domain (any test) | 6.05 (2.97, 12.33) | 36.2 | 73.1 | <0.001 | |
| TMT B | 4.62 (2.32, 9.21) | 33.3 | 64.8 | 0.003 | |
| TMT B‐A | 7.86 (3.19, 19.41) | 17.9 | 59.4 | 0.003 | |
| Phonemic fluency | 2.35 (1.06, 5.19) | 9.4 | 19.0 | 0.189 | |
| Semantic fluency | 4.14 (2.19, 7.84) | 8.6 | 28.6 | 0.004 | |
| Memory domain (any test) | 3.26 (1.81, 5.86) | 16.4 | 53.3 | <0.001 | |
| Word list immediate recall | 4.06 (2.01, 8.16) | 11.0 | 35.0 | 0.003 | |
| Word list delayed recall | 2.18 (1.08, 4.39) | 11.3 | 36.8 | 0.003 | |
| Word list recognition | 1.88 (0.84, 4.19) | 4.5 | 20.5 | 0.073 | |
| Visuospatial domain | – | – | – | – | |
| Figure copy | 2.07 (1.06, 4.03) | 14.3 | 31.5 | 0.064 | |
| Language Domain | – | – | – | – | |
| Naming | 6.17 (1.83, 20.79) | 6.9 | 17.4 | 0.460 | |
| MCI | 2.74 (1.42, 5.3) | 30.2 | 58.0 | 0.001 | |
Note: Hazard ratios with 95% confidence intervals are presented according to competing risks regression analysis adjusting for age, sex, education, depression, and study center. Hazard ratios for which the confidence intervals do not cross one (i.e., p < 0.05) are highlighted in bold. Values for the Visuospatial and Language domains are not shown since only one test was available for each domain.
Abbreviations: iRBD, isolated/idiopathic rapid eye movement sleep behavior disorder; MCI, mild cognitive impairment; TMT, Trail Making Test.
Impaired testing based on a z‐score cutoff of −1.5.
FIGURE 2.
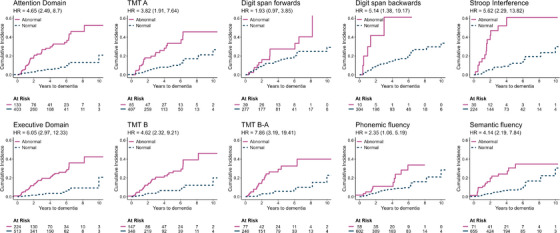
Cumulative incidence of dementia phenoconversion in iRBD stratified by impaired baseline testing (tests of attention and executive domains). Results are presented according to baseline neuropsychological test (impaired z‐score ≤ −1.5). Hazard ratios according to competitive risk regression are shown, adjusting for age, sex, education, depression, and study center, with 95% confidence intervals in parentheses. TMT, trail making test.
FIGURE 3.
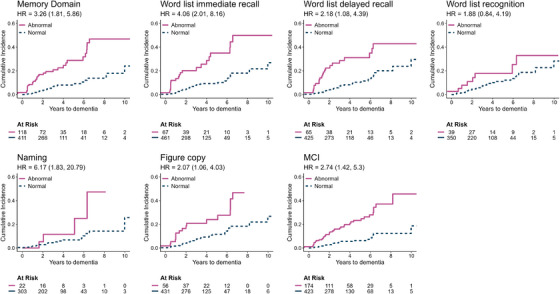
Cumulative incidence of dementia phenoconversion in iRBD stratified by impaired baseline testing (tests of memory, language, visuospatial domains, and MCI). Results are presented according to baseline neuropsychological test (impaired z‐score ≤ −1.5). Hazard ratios according to competitive risk regression are shown, adjusting for age, sex, education, depression, and study center, with 95% confidence intervals in parentheses. MCI, mild cognitive impairment.
4.4. Progression of neuropsychological tests over time
Participants were examined up to 10 years prior to phenoconversion or censoring (Figure S2A). The progression of neuropsychological testing is shown in Figure 4 and Table 3.
FIGURE 4.
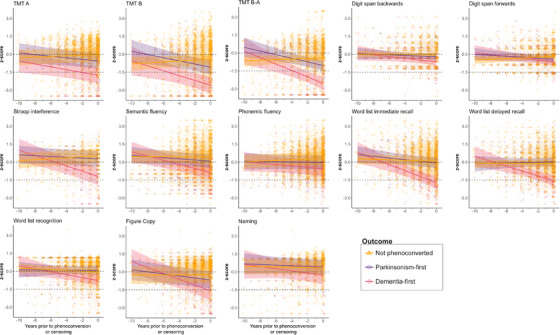
Annual progression of neuropsychological testing in iRBD. Neuropsychological testing and assessed up to 10 years prior to phenoconversion or censoring. Individual dots represent each participant; solid lines represent estimated progression by linear mixed‐effect modeling; shaded areas represent 95% confidence intervals; horizontal dotted lines indicate thresholds for reduced cognitive performance (z‐score < zero) and impaired performance (z‐score ≤ −1.5). MMSE, Mini‐Mental State Examination; MoCA, Montreal Cognitive Assessment; TMT, Trail Making Test.
TABLE 3.
Annual progression of neuropsychological testing in iRBD.
| Annual progression—z‐scores [95% CI] | Contrast p‐values | |||||
|---|---|---|---|---|---|---|
| Unconverted (A) | Parkinsonism‐first (B) | Dementia‐first (C) | A vs. B | A vs. C | B vs. C | |
| Attention | ||||||
| TMT A | 0.03 [−0.01, 0.06] | –0.07 [−0.13, 0.00] | –0.12 [−0.21, −0.03] | 0.030 | 0.006 | 0.588 |
| Digit span backward | –0.01 [−0.03, 0.02] | –0.03 [−0.08, 0.03] | –0.07 [−0.15, 0.00] | 0.751 | 0.183 | 0.554 |
| Digit span forward | 0.03 [0.00, 0.06] | –0.04 [−0.09, 0.01] | –0.07 [−0.14, 0.00] | 0.046 | 0.019 | 0.743 |
| Stroop interference | 0.04 [0.01, 0.07] | –0.03 [−0.09, 0.02] | –0.22 [−0.31, −0.13] | 0.057 | <0.001 | 0.002 |
| Executive | ||||||
| TMT B | 0.04 [−0.01, 0.09] | –0.13 [−0.23, −0.04] | –0.20 [−0.34, −0.07] | 0.004 | 0.002 | 0.660 |
| TMT B‐A | 0.03 [−0.02, 0.07] | –0.15 [−0.24, −0.06] | –0.27 [−0.4, −0.14] | 0.001 | <0.001 | 0.282 |
| Phonemic fluency | 0.03 [0.01, 0.05] | –0.01 [−0.06, 0.04] | –0.06 [−0.13, 0.01] | 0.225 | 0.033 | 0.488 |
| Semantic fluency | 0.00 [−0.03, 0.02] | –0.05 [−0.10, 0.01] | –0.17 [−0.25, −0.10] | 0.272 | <0.001 | 0.025 |
| Memory | ||||||
| Word list immediate recall | –0.02 [−0.06, 0.02] | –0.07 [−0.14, 0.00] | –0.25 [−0.35, −0.16] | 0.461 | <0.001 | 0.009 |
| Word list delayed recall | 0.00 [−0.04, 0.04] | 0.02 [−0.06, 0.09] | –0.20 [−0.3, −0.10] | 0.935 | <0.001 | 0.001 |
| Word list recognition | –0.03 [−0.07, 0.01] | –0.01 [−0.08, 0.07] | –0.12 [−0.23, −0.01] | 0.834 | 0.272 | 0.206 |
| Visuospatial | ||||||
| Figure copy | 0.00 [−0.03, 0.04] | –0.09 [−0.15, −0.02] | –0.25 [−0.34, −0.15] | 0.038 | <0.001 | 0.012 |
| Language | ||||||
| Naming | –0.01 [−0.05, 0.03] | –0.03 [−0.11, 0.05] | –0.09 [−0.23, 0.05] | 0.909 | 0.533 | 0.736 |
Note: The annual progression of neuropsychological tests of interest stratified by phenoconversion status are described as rate of decline in z‐score [95% confidence interval], with significant rates of change (i.e., rates of change different from zero) bolded. Contrast p‐values for detecting significant differences in rate between phenoconversion types are shown.
Dementia phenoconverters demonstrated a broad decline in neuropsychological testing in multiple cognitive domains over time. TMT B‐A and TMT B progressively declined to an impaired range (z‐score ≤ −1.5) at 4–6 years prior to dementia, and diverged from normal values (z‐score < zero) as early as 10 years prior to dementia (annual z‐score decline in TMT B‐A = −0.27; TMT B = −0.20 points per year). TMT A similarly diverged from normal values 10 years prior to the onset of dementia, although at a slower rate (annual z‐score decline = −0.12), with the average test scored as impaired only 2 years prior to phenoconversion. Stroop interference and Word list are immediate and delayed recalls testing diverged from normal values approximately 6–8 years prior to phenoconversion, and crossed into the impaired range within the year prior to phenoconversion (annual z‐score decline in Stroop interference = −0.22, Word list immediate recall = −0.25, Word list delayed recall = −0.20 points per year). Other tests, including Figure copy and Semantic fluency, reached or neared impaired values at the time of phenoconversion, at rates ranging between −0.17 and −0.25 points per year. Phonemic fluency and Naming did not change over time in dementia‐phenoconverters.
With respect to unconverted and parkinsonism‐first phenoconverted participants, a more modest decline in cognitive testing was observed up until the point of phenoconversion or censoring. In fact, certain tests (including Stroop interference, Digit span forward, and Phonemic fluency) subtly improved in unconverted participants over time, perhaps consistent with practice/learning effects. In parkinsonism‐first participants, a significant decline was seen in TMT B‐A (decline in z‐score = −0.15 points/year), TMT B (−0.13 points/year), TMT A (−0.07 points/year), and Figure copy (−0.09 points/year). When comparing between phenoconversion types, although overall mean scores on the TMT subtests were significantly lower in dementia‐first participants versus parkinsonism‐first participants, the rates of decline were not significantly different. By contrast, both mean overall scores and rates of decline were significantly worse in dementia‐first participants on Stroop interference, Figure copy, and Word list immediate and delayed recall. When comparing the progression of unconverted and parkinsonism‐first phenoconverters, only the trajectories of the TMT subscores, figure copy, and digit span forward were significantly different.
Given that neuropsychological testing methods and procedures differed among centers, we examined the progression of sub‐scores in the MoCA and MMSE, which , , were two standardized cognitive screening methods used by the majority of study centers. Total MoCA and MMSE raw scores crossed into impaired ranges (MoCA < 26; MMSE < 28) in dementia phenoconverters at 5–6 years prior to phenoconversion, while scores of parkinsonism‐first and unconverted participants remained stable, or in the case of MoCA scores in unconverted participants, subtly improved (Figure S3 and Table S3). Similar to the overall trends observed in detailed cognitive testing, the decline in total scores was driven primarily by dysfunction in subtests assessing attention, executive, visuospatial and memory functions, with general stability observed in language, naming, and orientation (data not shown).
MCI frequency in dementia participants progressively increased over time between approximately 25%–75% (Figure S2B). By contrast, MCI frequency in unconverted and parkinsonism participants fluctuated over time between approximately 25%–45%. Since almost one‐third of all iRBD participants had MCI at baseline, we conducted a secondary analysis of the data that included only those who did not have baseline MCI (Table S4). Overall longitudinal trends remained consistent between groups, although there was a general increase in the rates of decline in the dementia‐first group, which may reflect the removal of a floor effect in those with baseline MCI.
5. DISCUSSION
This multicentric study is the largest prospective longitudinal analysis of neuropsychological testing in iRBD. We confirmed numerous predictors of dementia, and demonstrate several important findings: (1) reduced baseline cognitive performance in iRBD is most evident on tests of attention and executive function; (2) impaired cognitive performance in multiple domains predicted dementia, with attention and executive dysfunction demonstrating the strongest predictive value; (3) cognitive changes in iRBD begin up to 10 years prior to phenoconversion, starting with a decline in executive function followed by attention, then memory and visuospatial dysfunction closer to time of phenoconversion; and (4) among specific tests, the TMT B and B‐A were particularly useful as both the most sensitive tests for early DLB with the highest amplitude of change over time and on longitudinal analysis, progression of episodic memory impairment best‐distinguished dementia‐first from parkinsonism‐first phenoconversion.
5.1. Cognitive performance in iRBD at baseline and longitudinally
Despite high inter‐center variability, reduced baseline performance was consistently observed in tests of attention and executive function. In dementia phenoconverters, TMT B and TMT B‐A were impaired at baseline, and reductions were observed in TMT A, Word list immediate recall, and Word list delayed recall. Moreover, impaired baseline testing in the attention or executive domains was highly predictive of the eventual development of DLB. Impaired Word list immediate recall was also highly predictive of dementia, which may relate to its sensitivity to attentional and executive capacity. 27
Overall, TMT B and B‐A were optimal tests for use in iRBD, as they were the most sensitive markers of early cognitive dysfunction (declining up to 10 years prior to dementia then becoming clearly impaired 5 years prior), and demonstrated a high degree of progression. Early decline in TMT sub‐scores was also observed in parkinsonism‐first phenoconverters, which suggests a similar degenerative process affecting processing speed/attention/executive function. Indeed, impaired scores on TMT A and B are frequently observed in early PD, even among those that remain dementia‐free at follow‐up 28 and impaired TMT B is a strong predictor of phenoconversion. 29 This makes the TMT subtests sensitive early markers of cognitive dysfunction in iRBD, with the advantages of wide international use and limited language dependency. 30 By contrast, longitudinal evaluation of verbal memory (Word list immediate and delayed recalls), and to a lesser extent Stroop interference, may be a method of differentiating between the two phenoconversion subtypes over time.
That attention and executive dysfunction were the earliest signs of cognitive decline in iRBD may not be surprising, giving that these domains are frequently impaired at the time of PD diagnosis, 31 , 32 with pathological correlates in the fronto‐striatal network. 8 , 33 Our results indicate that this dysfunction occurs earlier/more accelerated for certain tests (e.g., TMT) and in a different pattern in others (e.g., Stroop) in those with DLB versus PD. By contrast, visuospatial and episodic memory impairments, reflective of posterior cortical degeneration, are highly sensitive in detecting the transition to PD with dementia. 34 Similarly, in this study, longitudinal changes in verbal episodic memory best distinguished between types of phenoconversion. Our observations support the “dual syndrome hypothesis” in which two partially overlapping cognitive syndromes occur in PD with dementia: (1) fronto‐striatal deficits common in PD; and (2) a posterior cortical syndrome that results in symptoms of DLB. 35
On other tests of attention (Stroop interference), memory (Word list immediate and delayed recalls), and visuospatial function (Figure copy) scores of dementia phenoconverters deviated from normal 6–8 years prior to phenoconversion and reached or neared impaired values much closer to time of phenoconversion. While visuospatial dysfunction is a prominent feature of DLB, 36 , 37 it was not a strong baseline predictor of dementia. This is likely due to similar degrees of baseline prevalence in both dementia‐first and parkinsonism‐first phenoconverters, and the fact that visuospatial testing declined in both, although at different rates. Finally, some tests improved in those that remained unconverted, as observed in longitudinal testing in normal aging. 38 , 39 This could indicate a learning effect in the cognitively well that becomes lost as cognition becomes increasingly impaired; alternatively, this could reflect factors that influence testing, such as anxiety or depression (although these tend to be stable in iRBD 40 , 41 ).
The neuropsychological profiles in this study are in keeping with previous single‐center iRBD studies, 4 , 5 , 13 , 14 and retrospective studies examining pre‐diagnostic PD without selecting for iRBD. 37 , 41 We demonstrate here that the onset of cognitive decline occurs earlier than previously reported and emerges in a temporal pattern, in which executive and attentional domains are first affected, followed by memory and/or visuospatial domains. Cognitive screening in combination with neuroimaging or fluid biomarkers could improve model prediction and identify subjects for targeted preventative trials early in the neurodegenerative process when they are more likely to be beneficial. This is important if the pathomechanisms that drive neurodegeneration are substantially different between PD and DLB. 6 , 7
5.2. MCI in iRBD
MCI in iRBD demonstrates selective neuroimaging and neurophysiological abnormalities, 8 , 10 , 12 , 42 is a risk factor for phenoconversion, 1 , 5 , 12 , 13 and may identify a subset of iRBD participants with a malignant phenotype. 2 In this study, however, the presence of baseline MCI conferred comparatively moderate dementia risk (HR = 2.7), which reflects its high overall frequency in iRBD. Additionally, in contrast to an increasing frequency in dementia phenoconverters, MCI frequency fluctuated in parkinsonism‐first and unconverted participants over time, similar to fluctuations in cognitive ability observed in PD patients with MCI and patients with MCI that later develop Alzheimer's disease. 43 , 44 Although fluctuations may have been resultant from learning effects in the unconverted, no learning effect was seen in parkinsonism‐first participants. Therefore, although MCI may confer a higher risk of phenoconversion and may reflect a malignant iRBD subtype, a sizeable number of iRBD participants with MCI do not imminently develop dementia or phenoconvert. By contrast, impaired executive and attentional dysfunction specifically appear to be more sensitive markers of eventual dementia.
We confirmed a high baseline frequency of 30% MCI in iRBD by Level I PD‐MCI criteria. Previous single‐center studies using Level II PD‐MCI criteria showed a similar frequency of between 30% and 55%. 5 , 12 , 13 However, MCI in dementia‐first participants specifically was lower than previously reported, 5 which likely reflects the reduced sensitivity of Level I PD‐MCI criteria. 45
5.3. Strengths and limitations
Strengths include a relatively large and international iRBD population, prospective follow‐up at up to 10 years prior to phenoconversion, neuropsychological testing in multiple domains, and large numbers of phenoconverters, allowing more precise estimates of the cognitive trajectories of early DLB. However, several limitations should be noted. First, each center used its own testing protocol and not every center performed every test. Therefore, a pragmatic approach to data harmonization was required. To mitigate variation, the harmonization procedures used normatives based upon the specific test and localized to country, if possible, while analyses incorporated covariates to account for age, sex, education, depression, and study center. Nevertheless, the high degree of inter‐center variability highlights the need for establishing study protocols for testing in iRBD. The source of variability likely arises from differences in the specific test, administration, environmental, cultural, and sociodemographic factors. 46 Notably, high variability was also observed in a large multicenter study of non‐demented PD subjects despite the use of normatives and stricter pooling criteria. 47 Second, we used a broad indicator of depression (namely, clinical diagnosis, screening questionnaire, or the use of antidepressants). As antidepressants have many indications besides depression, this may have overestimated its frequency, although excluding depression as a covariate had a minor impact on the results (Table S5). Third, we could not assess the impact of other medications as this information was not systematically collected. Fourth, since motor dysfunction is strongly correlated with progression and phenoconversion, 1 , 41 we did not include standardized motor scores (i.e., MDS‐UPDRS‐III) in the statistical models, since the absence of collinearity is a fundamental assumption of linear modeling. As such, subclinical motor dysfunction may have influenced cognitive tests with motor demands or timed components, such as the TMT, although notably, the TMT B‐A subscore is insensitive to motor dysfunction as the motor components for TMT B and TMT A are identical. Fifth, we did not incorporate imaging, fluid, or tissue biomarkers as complementary measures of disease progression. Neuroimaging, in particular has demonstrated patterns of degeneration that correlate with cognitive dysfunction and progression in iRBD, 9 , 10 and in combination with clinical markers may predict the timing of phenoconversion to overt disease. 12 Such patterns may explain the sequence of cognitive decline on specific tests and cognitive domains we observed. Moreover, since dementia‐first phenoconverters were older, the degree to which other degenerative processes (e.g., other proteinopathies or vascular disease) contributed toward cognitive dysfunction is unclear. Finally, although this study involved six countries and participants in a range of ages and levels of education, the majority were Caucasian and/or from countries of high socioeconomic status, which may limit generalizability.
To conclude, we analyzed the evolution of neuropsychological testing in prodromal synucleinopathy. We demonstrate that the pathological processes that underly cognitive dysfunction in iRBD begin very early in the disease course and follow a domain‐specific pattern of decline in prodromal DLB.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest. Author disclosures are available in the supporting information.
CONSENT STATEMENT
All human participants provided informed consent.
Supporting information
Supplementary information
ACKNOWLEDGMENTS
S.J. was supported by an Edmond J. Safra Fellowship in Movement Disorders from the Michael J. Fox Foundation. The Oxford Discovery cohort (lead M.T. Hu) is supported by Parkinson's UK and the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC), UK. K.Y. Jung received research fund supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP) (2017M3C7A1029688, 2017R1A2B2012280) and NRF‐2022R1H1A2092329. D.A. was partially supported by a grant from the Italian Ministry of Health to IRCCS Ospedale. Policlinico San Martino (Fondi per la Ricerca Corrente, and Italian Neuroscience network (RIN)). J.Y. Lee received the National Research Foundation of Korea (NRF) grant funded by the Ministry of Science and ICT (MSIT) (NRF‐ 2022R1A2C4001834). J.F.G. was funded by a grant from the Canadian Institutes of Health Research (CIHR) and he holds a Canada Research Chair in Cognitive Decline in Pathological Aging. R.B.P. was funded by a grant from the CIHR.
Joza S, Hu MT, Jung K‐Y. et al. Prodromal dementia with Lewy bodies in REM sleep behavior disorder: A multicenter study. Alzheimer's Dement. 2024;20:91–102. 10.1002/alz.13386
REFERENCES
- 1. Postuma RB, Iranzo A, Hu M, et al. Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: a multicentre study. Brain. 2019;142:744‐759. doi: 10.1093/BRAIN/AWZ030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fereshtehnejad SM, Zeighami Y, Dagher A, Postuma RB. Clinical criteria for subtyping Parkinson's disease: biomarkers and longitudinal progression. Brain. 2017;140:1959‐1976. doi: 10.1093/BRAIN/AWX118 [DOI] [PubMed] [Google Scholar]
- 3. Arnaldi D, Chincarini A, Hu MT, et al. Dopaminergic imaging and clinical predictors for phenoconversion of REM sleep behaviour disorder. Brain. 2021;144:278‐287. doi: 10.1093/BRAIN/AWAA365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marchand DG, Postuma RB, Escudier F, et al. How does dementia with Lewy bodies start? Prodromal cognitive changes in REM sleep behavior disorder. Ann Neurol. 2018;83:1016‐1026. doi: 10.1002/ANA.25239 [DOI] [PubMed] [Google Scholar]
- 5. Genier Marchand D, Montplaisir J, Postuma RB, Rahayel S, Gagnon JF. Detecting the cognitive prodrome of dementia with Lewy bodies: a prospective study of REM sleep behavior disorder. Sleep. 2017;40:1‐11. doi: 10.1093/sleep/zsw014 [DOI] [PubMed] [Google Scholar]
- 6. Foffani G, Obeso JA. A cortical pathogenic theory of Parkinson's disease. Neuron. 2018;99:1116‐1128. doi: 10.1016/J.NEURON.2018.07.028 [DOI] [PubMed] [Google Scholar]
- 7. Adler CH, Beach TG. Neuropathological basis of nonmotor manifestations of Parkinson's disease. Mov Disord. 2016;31:1114‐1119. doi: 10.1002/MDS.26605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rahayel S, Postuma RB, Montplaisir J, et al. Cortical and subcortical gray matter bases of cognitive deficits in REM sleep behavior disorder. Neurology. 2018;90:e1759‐e1770. doi: 10.1212/WNL.0000000000005523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rahayel S, Tremblay C, Vo A, et al. Brain atrophy in prodromal synucleinopathy is shaped by structural connectivity and gene expression. Brain. 2022;145:3162‐3178. doi: 10.1093/BRAIN/AWAC187 [DOI] [PubMed] [Google Scholar]
- 10. Rahayel S, Postuma RB, Montplaisir J, et al. A prodromal brain‐clinical pattern of cognition in synucleinopathies. Ann Neurol. 2021;89:341‐357. doi: 10.1002/ANA.25962 [DOI] [PubMed] [Google Scholar]
- 11. Berg D, Borghammer P, Fereshtehnejad SM, et al. Prodromal Parkinson disease subtypes — key to understanding heterogeneity. Nat Rev Neurol. 2021;17:349‐361. doi: 10.1038/s41582-021-00486-9 [DOI] [PubMed] [Google Scholar]
- 12. Arnaldi D, Chincarini A, De Carli F, et al. The fate of patients with REM sleep behavior disorder and mild cognitive impairment. Sleep Med. 2021;79:205‐210. doi: 10.1016/j.sleep.2020.02.011 [DOI] [PubMed] [Google Scholar]
- 13. Terzaghi M, Toscano G, Casoni F, et al. Assessment of cognitive profile as a prodromal marker of the evolution of rapid eye movement sleep behavior disorder. Sleep. 2019;42:1‐8. doi: 10.1093/SLEEP/ZSZ103 [DOI] [PubMed] [Google Scholar]
- 14. Youn S, Kim T, Yoon IY, et al. Progression of cognitive impairments in idiopathic REM sleep behaviour disorder. Journal of Neurology, Neurosurgery & Psychiatry. 2016;87:890‐896. doi: 10.1136/JNNP-2015-311437 [DOI] [PubMed] [Google Scholar]
- 15. Yoo D, Lee J‐Y, Kim YK, et al. Mild cognitive impairment and abnormal brain metabolic expression in idiopathic REM sleep behavior disorder. Parkinsonism Relat Disord. 2021;90:1‐7. doi: 10.1016/j.parkreldis.2021.07.022 [DOI] [PubMed] [Google Scholar]
- 16. Joza S, Hu MT, Jung K‐Y, et al. Progression of clinical markers in prodromal Parkinson's disease and dementia with Lewy bodies: a multicentre study. Brain. 2023;139:16‐17. doi: 10.1093/brain/awad072 [DOI] [PubMed] [Google Scholar]
- 17. American Academy of Sleep Medicine , ed. International Classification of Sleep Disorders. 3rd ed. American Acad. of Sleep Medicine; 2014. [Google Scholar]
- 18. Postuma RB, Adler CH, Dugger BN, et al. REM sleep behavior disorder and neuropathology in Parkinson's disease. Mov Disord. 2015;30:1413‐1417. doi: 10.1002/MDS.26347 [DOI] [PubMed] [Google Scholar]
- 19. American Psychiatric Association, American Psychiatric Association , eds. Diagnostic and Statistical Manual of Mental Disorders: DSM‐5. 5th ed. Washington, D.C: American Psychiatric Association; 2013. [Google Scholar]
- 20. Litvan I, Goldman JG, Tröster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society Task Force guidelines. Mov Disord. 2012;27:349‐356. doi: 10.1002/MDS.24893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Balduzzi S, Rücker G, Schwarzer G. How to perform a meta‐analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22:153‐160. doi: 10.1136/EBMENTAL-2019-300117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Scrucca L, Santucci A, Aversa F. Regression modeling of competing risk using R: an in depth guide for clinicians. Bone Marrow Transplant. 2010;94:1388‐1395. doi: 10.1038/bmt.2009.359 [DOI] [PubMed] [Google Scholar]
- 23. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Statist Assoc. 1999;94:496‐509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 24. Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest package: tests in linear mixed effects models. Journal of Statistical Software. 2017;82:1‐26. doi: 10.18637/JSS.V082.I13 [DOI] [Google Scholar]
- 25. Lenth RV. Emmeans: estimated marginal means, aka least‐squares means. 2022. Accessed February 14, 2023. https://CRAN.R‐project.org/package=emmeans
- 26. Fox J, Weisberg S. Visualizing fit and lack of fit in complex regression models with predictor effect plots and partial residuals. Journal of Statistical Software. 2018;87:1‐27. doi: 10.18637/JSS.V087.I09 [DOI] [Google Scholar]
- 27. Saury J‐M, Emanuelson I. Neuropsychological assessment of hippocampal integrity. Applied Neuropsychology: Adult. 2017;24:140‐151. doi: 10.1080/23279095.2015.1113536 [DOI] [PubMed] [Google Scholar]
- 28. De Roy J, Postuma RB, Brillon‐Corbeil M, et al. Detecting the cognitive prodrome of dementia in Parkinson's disease. Journal of Parkinson's Disease. 2020;10:1033‐1046. doi: 10.3233/JPD-191857 [DOI] [PubMed] [Google Scholar]
- 29. Arnaldi D, Mattioli P, Famà F, et al. Stratification tools for disease‐modifying trials in prodromal synucleinopathy. Mov Disord. 2022;37:52‐61. doi: 10.1002/mds.28785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Strauss E, Sherman EMS, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. Oxford University Press; 2006. [Google Scholar]
- 31. Aarsland D, Bronnick K, Williams‐Gray C, et al. Mild cognitive impairment in Parkinson disease: a multicenter pooled analysis. Neurology. 2010;75:1062. doi: 10.1212/WNL.0B013E3181F39D0E [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Williams‐Gray CH, Foltynie T, Brayne CEG, Robbins TW, Barker RA. Evolution of cognitive dysfunction in an incident Parkinson's disease cohort. Brain. 2007;130:1787‐1798. doi: 10.1093/brain/awm111 [DOI] [PubMed] [Google Scholar]
- 33. Amboni M, Tessitore A, Esposito F, et al. Resting‐state functional connectivity associated with mild cognitive impairment in Parkinson's disease. J Neurol. 2015;262:425‐434. doi: 10.1007/s00415-014-7591-5 [DOI] [PubMed] [Google Scholar]
- 34. Hobson P, Meara J. Mild cognitive impairment in Parkinson's disease and its progression onto dementia: a 16‐year outcome evaluation of the Denbighshire cohort. Int J Geriatr Psychiatry. 2015;30:1048‐1055. doi: 10.1002/gps.4261 [DOI] [PubMed] [Google Scholar]
- 35. Kehagia AA, Barker RA, Robbins TW. Cognitive impairment in Parkinson's disease: the dual syndrome hypothesis. Neurodegenerative Diseases. 2012;11:79‐92. doi: 10.1159/000341998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cagnin A, Bussè C, Jelcic N, Gnoato F, Mitolo M, Caffarra P. High specificity of MMSE pentagon scoring for diagnosis of prodromal dementia with Lewy bodies. Parkinsonism Relat Disord. 2015;21:303‐305. doi: 10.1016/j.parkreldis.2014.12.007 [DOI] [PubMed] [Google Scholar]
- 37. Cagnin A, Bussè C, Gardini S, et al. Clinical and cognitive phenotype of mild cognitive impairment evolving to dementia with Lewy bodies. Dementia and Geriatric Cognitive Disorders Extra. 2015;5:442‐449. doi: 10.1159/000441184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cooley SA, Heaps JM, Bolzenius JD, et al. Longitudinal change in performance on the Montreal Cognitive Assessment in older adults. Clin Neuropsychol. 2015;29:824. doi: 10.1080/13854046.2015.1087596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Goldberg TE, Harvey PD, Wesnes KA, Snyder PJ, Schneider LS. Practice effects due to serial cognitive assessment: implications for preclinical Alzheimer's disease randomized controlled trials. Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring. 2015;1:103‐111. doi: 10.1016/J.DADM.2014.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Darweesh SKL, Verlinden VJA, Stricker BH, Hofman A, Koudstaal PJ, Ikram MA. Trajectories of prediagnostic functioning in Parkinson's disease. Brain. 2017;140:429‐441. doi: 10.1093/BRAIN/AWW291 [DOI] [PubMed] [Google Scholar]
- 41. Fereshtehnejad SM, Yao C, Pelletier A, Montplaisir JY, Gagnon JF, Postuma RB. Evolution of prodromal Parkinson's disease and dementia with Lewy bodies: a prospective study. Brain. 2019;142:2051‐2067. doi: 10.1093/BRAIN/AWZ111 [DOI] [PubMed] [Google Scholar]
- 42. Vendette M, Montplaisir J, Gosselin N, et al. Brain perfusion anomalies in rapid eye movement sleep behavior disorder with mild cognitive impairment. Mov Disord. 2012;27:1255‐1261. doi: 10.1002/MDS.25034 [DOI] [PubMed] [Google Scholar]
- 43. Lawson RA, Yarnall AJ, Duncan GW, et al. Stability of mild cognitive impairment in newly diagnosed Parkinson's disease. Journal of Neurology, Neurosurgery & Psychiatry. 2017;88:648‐652. doi: 10.1136/JNNP-2016-315099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Malek‐Ahmadi M. Reversion from mild cognitive impairment to normal cognition: a meta‐analysis. Alzheimer Dis Assoc Disord. 2016;30:324‐330. doi: 10.1097/WAD.0000000000000145 [DOI] [PubMed] [Google Scholar]
- 45. Hoogland J, Boel JA, de Bie RMA, et al. Risk of Parkinson's disease dementia related to level I MDS PD‐MCI. Mov Disord. 2019;34:430‐435. doi: 10.1002/MDS.27617 [DOI] [PubMed] [Google Scholar]
- 46. Rindermann H, Becker D, Coyle TR. Survey of expert opinion on intelligence: causes of international differences in cognitive ability tests. Frontiers in Psychology. 2016;7:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hoogland J, van Wanrooij LL, Boel JA, et al. Detecting mild cognitive deficits in Parkinson's disease: comparison of neuropsychological tests. Mov Disord. 2018;33:1750‐1759. doi: 10.1002/MDS.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information


