Abstract
INTRODUCTION
Proton pump inhibitors (PPIs) may increase dementia risk. However, it is currently unknown whether timing of exposure or age at dementia diagnosis influence the risk.
METHODS
We assessed associations between cumulative PPI use and dementia at different ages in a nationwide Danish cohort of 1,983,785 individuals aged 60 to 75 years between 2000 and 2018.
RESULTS
During follow‐up, there were 99,384 all‐cause dementia incidences. Incidence rate ratio (IRR) of dementia with PPI ever‐use compared with never‐use was 1.36 (95% CI, 1.29 to 1.43) for age 60 to 69 years at diagnosis, 1.12 (1.09 to 1.15) for 70 to 79 years, 1.06 (1.03 to 1.09) for 80 to 89 years, and 1.03 (0.91 to 1.17) for 90+ years. Longer treatment duration yielded increasing IRRs. For cases below 90 years, increased dementia rate was observed regardless of treatment initiation up to >15 years before diagnosis.
DISCUSSION
Regardless of timing of treatment initiation, PPI use was associated with increased dementia rate before age 90 years. Dementia rates increased with younger age at diagnosis.
Highlights
After following 1,983,785 individuals for a median of 10 years, 99,384 developed dementia
PPIs were used by 21.2% of cases and 18.9% of controls
PPI use was associated with increased dementia rate regardless of time of treatment onset
Magnitude of associations increased with younger age at diagnosis
PPI use was not associated with dementia occurring after age 90 years
Keywords: Alzheimer's disease, dementia, pharmacoepidemiology, proton pump inhibitor, risk factor
1. BACKGROUND
Worldwide, around 55 million people live with dementia, and with 10 million new cases annually, this number is expected to increase considerably due to an aging world population, making the disease and its consequences a major global public health challenge. 1 Recognizing potential modifiable risk factors for dementia is of high priority. 1
Proton pump inhibitors (PPIs) suppress acid production in the stomach and are used to treat gastric acid‐related conditions such as peptic ulcers and gastroesophageal reflux disease. Worldwide, the use of PPIs has increased rapidly over the last two decades, particularly among adults aged 40 years and above. 2 , 3 , 4 , 5 , 6 In Denmark, the total PPI use increased sixfold between 2000 and 2021. 7 Studies reported over‐prescription of PPIs in both primary and secondary care with around 50% of prescriptions not being accompanied with valid indications for treatment. 8 , 9 , 10
PPIs pass through the blood‐brain barrier 11 and their use has been associated with neurological adverse reactions such as migraine, peripheral neuropathies, and impairment of hearing, vision, and memory. 12 , 13 , 14 A recent study showed that PPIs potently and selectively inhibit the enzyme responsible for biosynthesis of the neurotransmitter acetylcholine (choline‐acetyltransferase), and thereby may inhibit neuronal signaling in the brain. 15
In mice, PPIs increased brain beta‐amyloid levels, 16 and thus may be involved in the pathological process of development of Alzheimer's disease (AD). 17 However, whether this applies to humans and leads to dementia is currently unknown.
Previous studies investigating the association between PPIs and risk of dementia reported conflicting findings with some studies reporting a positive association, 18 , 19 , 20 , 21 while other studies showed a negative or neutral association. 22 , 23 , 24 This could possibly be owing to the heterogeneity between data availability and study design. 25
Although not specifically designed to investigate risk differences according to age at dementia diagnosis, two previous studies observed greater risk estimates among the youngest cases of dementia compared to older cases. 18 , 20 However, most previous studies did not take this into account, 19 , 21 , 22 , 23 , 24 and thus, the association between PPI use and dementia risk in relation to age at dementia diagnosis remains largely unexplored.
RESEARCH IN CONTEXT
Systematic review: The existing literature on PPI use and risk of dementia is inconsistent with previous observational studies reporting conflicting findings. Most studies did not differentiate by age at diagnosis or consider the timing of exposure to PPI according to the diagnosis.
Interpretation: Our study based on highly valid real‐world data from Denmark found that PPI use, regardless of timing of treatment initiation, was associated with increased risk of all‐cause dementia before age 90 years. We consistently observed higher dementia rates with younger age at dementia diagnosis.
Future directions: Results from our data contribute with evidence highlighting age at dementia diagnosis to interact substantially with the association between PPI use and dementia risk. The increasing body of evidence related to potential serious adverse effects of PPIs call for scientific attention. Mechanistic studies are warranted to investigate the link between PPIs and cognition, and the interaction with age.
Furthermore, the majority of studies did not consider the timing of PPI use according to dementia diagnosis, and many did not apply latency windows between the exposure and outcome. Both factors are important when assessing a slowly progressing disease such as dementia in order to reduce reverse causation/protopathic bias. 26
In this nationwide population‐based study addressing previously understudied aspects of the association between PPI use and dementia risk, we aimed to assess the influence of age at dementia diagnosis, and timing of PPI use according to the diagnosis.
2. METHODS
2.1. Study population and design
We identified a nationwide cohort of Danish residents aged 60 to 75 years in year 2000 or turning 60 years between 2000 and 2018. Exclusion criteria were registration of a dementia diagnosis (since 1977) or treatment with a dementia‐specific drug (since 1995) before study entry. To ensure sufficient information on previous medication use, we did not include individuals who immigrated to Denmark after 1995 (the initial year of prescription data availability). Included individuals were followed from January 1, 2000, or their 60th birthday, whichever came later, for up to 19 years until first onset of dementia, emigration, death, or December 31, 2018, whichever came first. The study population was established by linking the following nationwide Danish registers: (1) the National Patient Registry, 27 (2) the Danish Psychiatric Central Research Registry, 28 (3) the Danish National Prescription Registry, 29 and (4) the Danish Education Registry 30 (Table S1). All the registers included a pseudonymized unique personal identification number assigned to all residents in Denmark, permitting unambiguous linkage of data on the individual level. 27
Within the nationwide cohort, we nested a case‐control population comprising all cases of dementia that occurred during follow‐up, each matched with a set of control individuals sampled from the cohort. On the date of dementia incidence (index date), each dementia case was matched to five dementia‐free controls of the same sex and birth year (ie, age) through incidence density sampling. 31
2.2. Outcome
The primary outcome of interest was late‐onset all‐cause dementia (defined as dementia of any etiology from age 60 years). An individual was considered to have dementia either from the date of first dementia diagnosis or first redemption of a dementia‐specific drug (Table S1). In Denmark, dementia is typically diagnosed in hospital memory clinics, enabling identification of cases through diagnoses from the National Patient Registry, providing data on all diagnoses given in Danish hospitals since 1977. 27 The validity of dementia syndrome diagnoses in Danish hospital registers is high. 32 Prescriptions with dementia‐specific medication (for Alzheimer's disease, Lewy body dementia, and Parkinson's disease dementia) from the National Prescription Registry allow for identification of cases diagnosed and managed in primary care. 29 The age at dementia diagnosis/index date was grouped in 10‐year age groups (60 to 69 years; 70 to 79 years; 80 to 89 years; 90+ years).
2.3. Exposure
Information on PPI use (Anatomical Therapeutic Chemical Classification System [ATC] code A02BC) in the nested case‐control population was obtained from the Danish National Prescription Registry, holding complete data on all prescriptions redeemed from pharmacies in Denmark since January 1995. Prescriptions included the ATC‐code, date of purchase, package size, unit size, and number of purchased packages.
In Denmark, PPIs are primarily used through prescriptions (only around 2% of the total PPI use in the study period was bought over‐the‐counter 7 ).
Five‐year exposure lag time windows before index date (ie, omitting any prescriptions of PPI between the date of dementia diagnosis/matching and 5 years prior) was consistently applied to diminish reverse causation bias 26 (ie, reducing the confounding impact of potential changes in prescription patterns of PPI in the prodromal stages before dementia diagnosis).
A PPI user was defined as an individual who redeemed at least two prescriptions of PPI between 1995 and the beginning of the lag time window. Individuals only redeeming one prescription were categorized separately. PPI subtypes have different potencies and thus, different recommended daily dosages. Therefore, cumulative exposure to PPIs (whether continuous or discontinuous) was calculated as the total number of defined daily doses (DDDs) redeemed from prescriptions between January 1, 1995, until 5 years before the dementia incidence or matching. DDDs were calculated from prescription information on the quantity of purchased PPI (Table S2). Timing of treatment according to dementia diagnosis was computed as the time between the date of first PPI prescription and the index date.
2.4. Confounding variables
Age, sex, and highest attained educational level (low; medium; high) were considered demographic and socioeconomic confounders. Health‐related confounders included (1) the presence of cardiovascular disease (CVD) defined as a diagnosis of stroke, the presence of ischemic heart disease including acute myocardial infarction, or use of oral antithrombotic medication including anticoagulants; (2) diabetes mellitus type 1 or 2 defined from diagnoses or use of antidiabetics; (3) hypertension defined from diagnoses or use of antihypertensive drugs; and (4) dyslipidemia defined from diagnoses or use of statins. The health‐related confounders were included since they are acknowledged risk factors for dementia 33 , 34 as well as associated to use of PPI. 35 Variables were assessed at the beginning of the lag time window. Exact definitions of covariates are shown in Table S1.
2.5. Statistical analysis
We applied conditional logistic regression with categorical variables on the matched case‐control population and calculated adjusted incidence rate ratios (IRRs) with 95% confidence intervals (CIs) for the associations between PPI use and dementia according to age on index date. PPI never‐users constituted the reference group in all analyses. Associations between ever‐use of PPI (at least two prescriptions) as well as cumulative treatment duration (categorized as ≤3 months; >3 to 12 months; >12 to 36 months; >36 months) and all‐cause dementia were assessed. Additionally, association between timing of treatment initiation (ever‐use) according to index date and all‐cause dementia was assessed. The timing of PPI initiation was categorized as follows: (1) within 5 years of index; (2) >5 to 10 years before index; (3) >10 to 15 years before index; and (4) >15 years before index. For the PPI initiation subcategory of within 5 years of index, the 5‐year lag time window was not applied.
All models included the above‐mentioned confounding variables as covariates. For educational level, <2% of the population had missing information, and thus were categorized separately as “unknown”. Information on all the remaining confounders was available for all included individuals. Data were analyzed and categorized using SAS software version 9.4 (SAS Institute) and R statistical software (R Core Team, 2020). 36
2.6. Sensitivity analyses
To test for the robustness of results, sensitivity analyses in three separate subpopulations were performed as follows: (1) association between PPI use and dementia in a subpopulation restricted to individuals with a Charlson Comorbidity Index 37 (CCI) score of zero (dementia and peptic ulcer disease were excluded from CCI definition) in order to test for associations among individuals expected to have a relatively low burden of chronic somatic comorbidity; (2) association between PPI use and dementia in a subpopulation restricted to individuals without stroke, since stroke could potentially be an intermediary on the causal pathway between PPI use and dementia 38 ; and (3) association between PPI use and dementia in a subpopulation restricted to individuals aged 60 to 65 years in 2000, where all included individuals had similar circumstances of exposure to PPI (in terms of age and calendar time) as well as prescription data availability used to measure the exposure.
An additional sensitivity analysis tested the association between each PPI subtype and dementia.
All main analyses were repeated with 2‐year lag time and without lag time instead of the standard 5‐year lag time window.
To test for potential competing risk of death among users of PPI, an analysis estimating the association between PPI use and mortality rate was performed (ever‐use vs never‐use).
3. RESULTS
The nationwide cohort included 1,983,785 eligible individuals followed for a total of 20.5 million person‐years with a median (first‐third quartile) follow‐up time of 10.3 years (5.1 to 15.6 years). During follow‐up, there were 99,384 (5.0%) incidences of all‐cause dementia. The establishment of the cohort and nested case‐control population is shown in Figure 1. Among all dementia cases, 55.4% were women, and the median age at time of diagnosis was 79 years (74 to 83 years) for women and 77 years (72 to 82 years) for men. Characteristics of cases and their matched controls are specified in Table 1.
FIGURE 1.
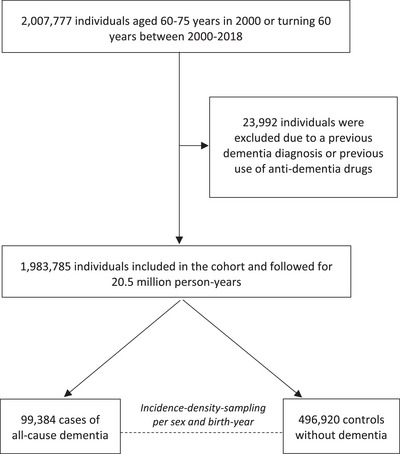
Flowchart of the establishment of the cohort and nested case‐control population. During follow‐up, 486,312 individuals were censored due to emigration (n = 11,970) or death (n = 474,342).
TABLE 1.
Characteristics of nested case‐control population.
| All‐cause dementia (n=99,384) | Controls (n=469,920) | |
|---|---|---|
| Sex: Female | 55,105 (55.4) | 275,525 (55.4) |
| Age at index (years, median) | 78 (73.0 to 82.6) | 78 (73.0 to 82.6) |
| Follow‐up (years, median) | 11.1 (7.0 to 14.8) | 18.2 (14.1 to 19.0) |
| Year of diagnosis (median) | 2012 (2008 to 2015) | – |
| Cardiovascular disease (CVD) | 43,006 (43.3) | 185,950 (37.4) |
| Stroke | 7333 (7.4) | 22,046 (4.4) |
| IHD/AMI | 17,819 (17.9) | 79,482 (16.0) |
| OAT/OAC | 39,082 (39.3) | 168,999 (34.0) |
| Diabetes | 10,482 (10.5) | 38,418 (7.7) |
| Hypertension | 61,763 (62.1) | 288,986 (58.2) |
| Dyslipidemia | 28,065 (28.2) | 126,799 (25.5) |
| Educational level | ||
| Low | 51,218 (51.5) | 246,521 (49.6) |
| Medium | 34,009 (34.2) | 172,612 (34.7) |
| High | 12,493 (12.6) | 69,039 (13.9) |
| Unknown | 1664 (1.7) | 8748 (1.8) |
| PPI user (2+ prescriptions) | 21,049 (21.2) | 93,751 (18.9) |
| Age at treatment initiation (years, median) | 68 (62 to 73) | 68 (63 to 73) |
| Duration of treatment (months, median) | 3.7 (0.9 to 21.2) | 3.6 (0.9 to 18.6) |
| One prescription only | 7350 (7.4) | 35,064 (7.1) |
Note. Values are shown either in ‘number of individuals (%)’ or ‘median (first‐third quartile)’. Shown numbers of PPI use include 5 years lag time window.
Abbreviations: AMI, acute myocardial infarction; CVD, cardiovascular disease; IHD, ischemic heart disease; OAT, oral antithrombotic medication; OAC, oral anticoagulants; PPI, proton pump inhibitor.
Users of PPIs (at least two prescriptions) comprised 21.2% of dementia cases and 18.9% of controls, while 7.4% of cases and 7.1% of controls redeemed one prescription only. The median age at first PPI prescription was 68 years for both cases and controls.
Figure 2 reports adjusted IRRs of all‐cause dementia according to ever‐use and cumulative use of PPI stratified by age groups at index. Ever‐use of PPI was associated with an increased IRR of all‐cause dementia occurring before age 90 years ranging from IRR 1.36 (95% CI, 1.29 to 1.43) among cases 60 to 69 years old on index to IRR 1.06 (1.03 to 1.09) among cases 80 to 89 years old on index. Increasing treatment duration of PPI yielded increasing IRRs in a duration‐response manner. Overall, associations reduced consistently with older age at dementia diagnosis, and were neutral among those aged 90+ years at diagnosis.
FIGURE 2.
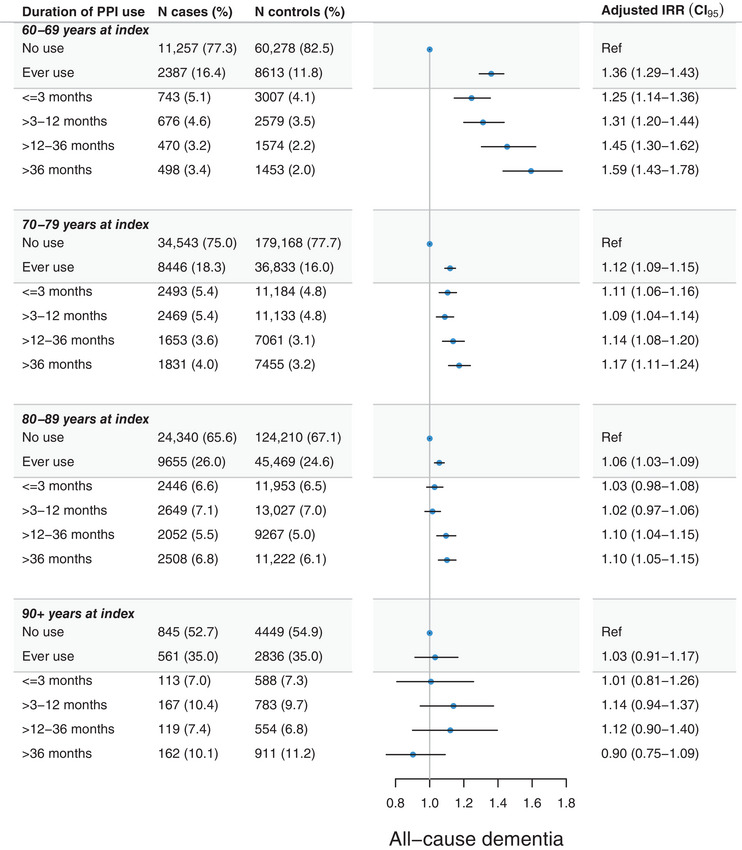
Adjusted incidence rate ratios (IRRs) and 95% confidence intervals (CIs) of the association between cumulative duration of proton pump inhibitor (PPI) use and all‐cause dementia. Adjustments were made for educational level, cardiovascular disease, diabetes, hypertension, and dyslipidemia, with a 5‐year lag time window applied. Estimates for “one prescription only” are not shown.
Regardless of timing of treatment initiation up to >15 years before index, ever‐use of PPI was associated with an increased rate of dementia occurring before age 90 years (Figure 3). No category of timing of treatment initiation was associated with dementia after age 89 years.
FIGURE 3.
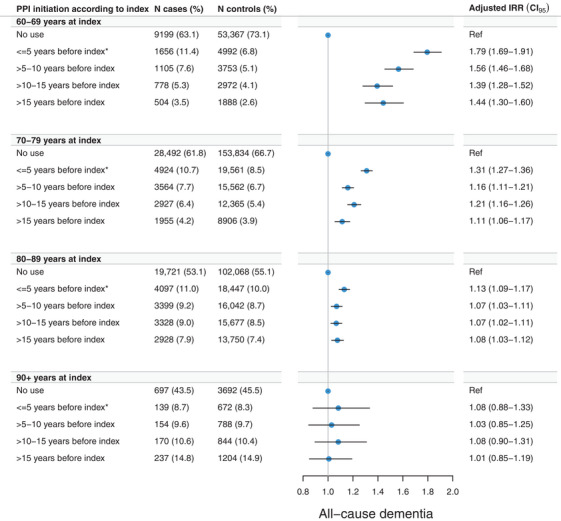
Adjusted incidence rate ratios (IRRs) and 95% confidence intervals (CIs) of the association between timing of initiation of proton pump inhibitor (PPI) use and all‐cause dementia. Adjustments were made for educational level, cardiovascular disease, diabetes, hypertension, and dyslipidemia. Asterisks (*) denote that for the category of “<= 5 years before index” there was no 5‐year lag time window applied, whereas a 5‐year lag time window was applied for all other categories. Estimates for “one prescription only” are not shown.
3.1. Sensitivity analyses
In a subpopulation of individuals with a CCI score of zero, estimates of PPI use and dementia generally reduced compared to those of the main population, but overall associations with dementia occurring before age 80 years remained stable (Figure S1). Results remained robust in sensitivity analyses assessing the association between PPI use and all‐cause dementia in (1) a subpopulation of individuals without stroke (Figure S2), as well as in (2) a subpopulation of individuals aged 60 to 65 years in year 2000 (Figure S3).
Ever‐use of each PPI subtype yielded comparable results with elevated rates of dementia before age 80 years (Figure S4).
In an analysis estimating competing risk of death, ever‐use of PPI was associated with an increased rate of death, adjusted IRR 2.24 (2.22 to 2.25).
Associations remained largely unchanged in sensitivity analyses with no lag time and with a 2‐year lag time instead of a 5‐year lag time.
4. DISCUSSION
In this real‐world population‐based case‐control study nested in a nationwide cohort of individuals aged 60 to 75 years in 2000 to 2018, PPI use was associated with an increased rate of all‐cause dementia before age 90 years. Associations displayed a duration‐response pattern and were largest among the youngest cases of dementia (age 60 to 69 years at diagnosis) and decreased with advancing age at diagnosis. Initiation of treatment with PPI up to >15 years prior to diagnosis was associated with an increased dementia rate. PPI use showed risk estimates close to unity for dementia occurring in age 80 to 89 years, and no association was observed for dementia occurring from age 90 years. Sensitivity analyses supported the overall findings.
In line with recent observational studies, we found an increased rate of dementia with PPI use. 18 , 19 , 20 , 21 Our findings of larger associations with dementia occurring in earlier ages are consistent with two previous studies that stratified by age at dementia diagnosis. 18 , 20 In contrast, a study based on national Finnish registers by Taipale et al. reported no clinically meaningful association between PPIs and AD. 23 In that study, individuals had a maximum of 17 years of available exposure history compared to 24 years in our study. Since only AD cases were included, potential increased risk of vascular cognitive decline cannot be ruled out.
We observed that the age at dementia diagnosis interacted significantly with the association of interest, that is, the magnitude of the effect of PPI use on dementia rate differed depending on this variable. Previous studies that reported no association between PPIs and dementia, including Taipale et al., did not stratify by age at dementia diagnosis. 22 , 23 Thus, potential risk differences according to age at diagnosis were not accounted for and may therefore explain the differences between study findings.
Our finding of higher risk estimates for PPI initiation closest to dementia diagnosis could represent an acceleration of dementia neuropathology related to PPI use, but these results are more likely influenced by reverse causation. Nevertheless, PPI initiation up to >15 years before dementia diagnosis consistently yielded increased dementia rates before age 90 years, potentially indicative of a long‐acting influence of PPIs on the brain in congruence with underlying neurobiological processes leading to dementia which expectedly progress gradually over decades. 39
A study by Friesen et al. based on registers from one Canadian province followed three cohorts with different age groups at study start and found highest risk estimates for dementia with PPI use in the youngest cohort (start age 46 to 55 years). 24 Although the authors did not stratify by age at dementia diagnosis, this aligns with our study results displaying higher dementia rates occurring at a younger age. Overall, Friesen et al. reported no consistently increased dementia risk with PPI use. However, while the highest cumulative dose category of PPI use in Friesen et al. was >180 DDDs, corresponding to about >6 months’ duration, we assessed longer duration intervals up to >36 months (>1095 DDDs). Considering our observation of the largest associations with long‐term PPI use, further stratification of cumulative PPI use in Friesen et al. may have yielded different results.
The observed association between cumulative PPI exposure and increased dementia rate may be explained by PPI‐related impacts on cognition, for example, through neurodegeneration and/or cerebrovascular damage. 15 , 16 , 40 One clinical trial found impairment in different cognitive domains among individuals randomized to short‐term PPI use. 13 PPIs can affect brain cells through various mechanisms, for example, by increasing beta‐amyloid levels, involved in the development of AD. 16 Long‐term exposure may additionally increase vascular dysfunction by impairing endothelial function, 40 thereby accelerating the progression of cerebrovascular damage potentially leading to vascular dementia. Listed side‐effects of PPI use include neurological, psychological, and psychiatric symptoms/conditions. 12 , 14 Although the mechanisms of these side effects have not been fully described, they suggest non‐negligible effects on the nervous system related to PPI use. Mechanistic studies are needed to investigate the potential link between PPI use and cognition. Furthermore, future studies should explore if the potential PPI‐associated risk of dementia varies across dementia subtypes.
The association between PPI use and dementia was unambiguously largest among the youngest cases of dementia, potentially suggestive of a critical window of exposure where midlife PPI use affects dementia risk to a larger degree compared to late‐life use. Further, the finding could signify a declining impact of individual risk factors with advancing age owing to lengthy ongoing neuropathological processes. Arguably, dementia occurring at different ages can be considered different diseases in terms of characteristics and risk factors. 41 Whether the finding is causal has yet to be determined.
Residual confounding factors could still have impacted the results, especially if unmeasured risk factors for dementia (eg, lifestyle‐related) were more prevalent in PPI users compared to non‐users, and if such potential differences between exposed and unexposed participants were more pronounced in the younger age groups compared to the older age groups.
The sensitivity analysis restricted to individuals with a CCI score of zero aimed at reducing confounding from somatic comorbidity. Though the associations to some extent decreased in magnitude, the overall findings remained stable, indicating that the observed association was not entirely explained by somatic comorbidity as defined in the CCI.
Nevertheless, based on our real‐world data consistently showing the highest PPI‐associated dementia risk among the youngest cases of dementia, we have identified age at diagnosis as an important effect‐modifier. This influence should be investigated in future studies, especially as to whether it translates to a critical window of exposure which could shape the basis for future guidelines and interventions. In the context of PPIs being among the most prescribed drugs worldwide and with high prevalence of inappropriate use, further elucidation of long‐term safety in relation to dementia as well as interventions promoting appropriate use is an important public health matter. 2 , 3 , 4 , 5 , 6 , 8 , 9 , 10
Strengths of the study include the large nationwide study population, long follow‐up period, highly valid dementia diagnoses, 32 extensive details on cumulative exposure to PPI based on redeemed prescriptions, and information on confounding factors. These strengths were important to reliably investigate the research question of interest.
This study had limitations. First, data on prescriptions before 1995, over‐the‐counter use, and in‐hospital intravenous use of PPI throughout the study period were not available, leading to underestimating PPI use in the cohort and possible misclassification of PPI users as non‐users, resulting in possible bias toward the null. However, PPI use before 1995 as well as over‐the‐counter use and intravenous use was relatively limited; thus, we do not consider this limitation to have impacted the interpretation or generalizability of our findings. 7 Our study period and age‐restrictions were chosen to enhance detectability of exposure among cohort members. Additionally, PPI users had a higher mortality rate compared to non‐users. This competing risk of death further diluted the association between PPI use and dementia. However, since our main finding was increased risk, we do not expect the competing death risk to have impacted the overall interpretation.
Second, we could not differentiate between vascular dementia and neurodegenerative etiologies of dementia due to low validity of non‐AD subtype diagnoses. 32 Considering the previously reported positive association between PPI use and risk of stroke, 38 the observed association with all‐cause dementia could be reflecting the association with stroke. However, the sensitivity analysis restricted to individuals without stroke supported the main results, making it improbable that our findings are entirely explained by stroke as an intermediary on the causal pathway.
Third, dementia is underdiagnosed in the general population, but we do not expect potential under‐registered dementia diagnoses to be differentially distributed among exposed and non‐exposed, and thus should not impact the external validity of our findings. This study was based on national Danish registers and is therefore generalizable to populations with similar demographics and healthcare. Nonetheless, increasing and inappropriate PPI use has been reported in both Western and Eastern countries with rates comparable to those of the Danish population, making Denmark a relevant research setting for the aim of our study. 2 , 3 , 4 , 5 , 6 , 8 , 9 , 10
Finally, we cannot exclude residual confounding by indication particularly among long‐term PPI users. Although labelled indications for PPIs are not known to be associated with dementia, these individuals may have a higher prevalence of unmeasured factors related to dementia risk such as obesity and smoking. 42 Danish healthcare registers do not hold information on lifestyle factors and therefore these could not be taken directly into account. 43 Nonetheless, we adjusted for important consequences related to unhealthy lifestyle, for example, hypertension, dyslipidemia, diabetes, and CVD. Although we included several potential confounders and greatly limited reverse causation with 5‐year lag windows, biases from residual confounding cannot be ruled out. Still, the results remained robust in sensitivity analyses aiming at reducing potential confounding.
In conclusion, exposure to PPIs was found to be associated with an increased rate of all‐cause dementia occurring before 90 years of age regardless of time of treatment initiation according to the diagnosis. Longer cumulative duration of PPI use yielded higher risk estimates. Further studies are warranted to determine if these findings represent a causal effect of PPIs on dementia risk, and to explore risk differences according to age at dementia diagnosis and dementia subtypes.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest. Author disclosures are available in the Supporting Information.
CONSENT STATEMENT
Studies based on national Danish registers do not require patient consent or ethical approval under Danish legislation. The Danish Health Data Board and the Danish Data Protection Agency approved this study (ID No. P‐2021‐250).
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
The Danish Dementia Research Centre is supported by the Danish Ministry of Health (grant number 1604063), which was not involved in the design of the study, data management/analysis, interpretation of data, or writing/reviewing the manuscript.
Pourhadi N, Janbek J, Jensen‐Dahm C, Gasse C, Laursen TM, Waldemar G. Proton pump inhibitors and dementia: A nationwide population‐based study. Alzheimer's Dement. 2024;20:837–845. 10.1002/alz.13477
REFERENCES
- 1. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396:413‐446. doi: 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lassalle M, Le Tri T, Bardou M, et al. Use of proton pump inhibitors in adults in France: a nationwide drug utilization study. Eur J Clin Pharmacol. 2020;76:449‐457. doi: 10.1007/s00228-019-02810-1 [DOI] [PubMed] [Google Scholar]
- 3. Mishuk AU, Chen L, Gaillard P, Westrick S, Hansen RA, Qian J. National trends in prescription proton pump inhibitor use and expenditure in the United States in 2002‐2017. J Am Pharm Assoc. 2021;61:87‐94. doi: 10.1016/j.japh.2020.09.015. e7. [DOI] [PubMed] [Google Scholar]
- 4. Ying J, Li LC, Wu CY, Yu ZW, Kan LD. The status of proton pump inhibitor use: a prescription survey of 45 hospitals in China. Rev Española Enfermedades Dig. 2019;111. doi: 10.17235/reed.2019.6155/2019 [DOI] [PubMed] [Google Scholar]
- 5. Pottegård A, Broe A, Hallas J, de Muckadell OBS, Lassen AT, Lødrup AB. Use of proton‐pump inhibitors among adults: a Danish nationwide drug utilization study. Therap Adv Gastroenterol. 2016;9:671‐678. doi: 10.1177/1756283x16650156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Othman F, Card TR, Crooks CJ. Proton pump inhibitor prescribing patterns in the UK: a primary care database study. Pharmacoepidemiol Drug Saf. 2016;25:1079‐1087. doi: 10.1002/pds.4043 [DOI] [PubMed] [Google Scholar]
- 7. Schmidt M, Hallas J, Laursen M, Friis S. Data Resource Profile: danish online drug use statistics (MEDSTAT). Int J Epidemiol. 2016;45:1401‐1402g. doi: 10.1093/ije/dyw116 [DOI] [PubMed] [Google Scholar]
- 8. Haastrup P, Paulsen MS, Zwisler JE, et al. Rapidly increasing prescribing of proton pump inhibitors in primary care despite interventions: a nationwide observational study. Eur J Gen Pract. 2014;20:290‐293. doi: 10.3109/13814788.2014.905535 [DOI] [PubMed] [Google Scholar]
- 9. Pasina L, Nobili A, Tettamanti M, et al. Prevalence and appropriateness of drug prescriptions for peptic ulcer and gastro‐esophageal reflux disease in a cohort of hospitalized elderly. Eur J Intern Med. 2011;22:205‐210. doi: 10.1016/j.ejim.2010.11.009 [DOI] [PubMed] [Google Scholar]
- 10. Liu Y, Zhu X, Li R, Zhang J, Zhang F. Proton pump inhibitor utilisation and potentially inappropriate prescribing analysis: insights from a single‐centred retrospective study. BMJ Open. 2020;10:e040473. doi: 10.1136/bmjopen-2020-040473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cheng F, Ho Y, Hung L, Chen C, Tsai T. Determination and pharmacokinetic profile of omeprazole in rat blood, brain and bile by microdialysis and high‐performance liquid chromatography. J Chromatogr A. 2002;949:35‐42. doi: 10.1016/S0021-9673(01)01225-0 [DOI] [PubMed] [Google Scholar]
- 12. Makunts T, Alpatty S, Lee KC, Atayee RS, Abagyan R. Proton‐pump inhibitor use is associated with a broad spectrum of neurological adverse events including impaired hearing, vision, and memory. Sci Rep. 2019;9:17280. doi: 10.1038/s41598-019-53622-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Akter S, Hassan MR, Shahriar M, Akter N, Abbas MG, Bhuiyan MA. Cognitive impact after short‐term exposure to different proton pump inhibitors: assessment using CANTAB software. Alzheimers Res Ther. 2015;7:79. doi: 10.1186/s13195-015-0164-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Y, Wintzell V, Ludvigsson JF, Svanström H, Pasternak B. Proton pump inhibitor use and risk of depression and anxiety in children: nationwide cohort study. Clin Transl Sci. 2022;15:1112‐1122. doi: 10.1111/cts.13225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kumar R, Kumar A, Nordberg A, Långström B, Darreh‐Shori T. Proton pump inhibitors act with unprecedented potencies as inhibitors of the acetylcholine biosynthesizing enzyme—A plausible missing link for their association with incidence of dementia. Dement. 2020;16:1031‐1042. doi: 10.1002/alz.12113. Alzheimer's. [DOI] [PubMed] [Google Scholar]
- 16. Badiola N, Alcalde V, Pujol A, et al. The proton‐pump inhibitor lansoprazole enhances amyloid beta production. PLoS One. 2013;8:e58837. doi: 10.1371/journal.pone.0058837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Paroni G, Bisceglia P, Seripa D. Understanding the amyloid hypothesis in Alzheimer's disease. J Alzheimer's Dis. 2019;68:493‐510. doi: 10.3233/JAD-180802 [DOI] [PubMed] [Google Scholar]
- 18. Choi HG, Kim J‐H, Kim JH, et al. Associations between proton pump inhibitors and Alzheimer's disease: a nested case–control study using a Korean nationwide health screening cohort. Alzheimers Res Ther. 2022;14:91. doi: 10.1186/s13195-022-01032-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang P, Li Z, Chen P, et al. Regular proton pump inhibitor use and incident dementia: population‐based cohort study. BMC Med. 2022;20:271. doi: 10.1186/s12916-022-02478-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gomm W, von Holt K, Thomé F, et al. Association of proton pump inhibitors with risk of dementia. JAMA Neurol. 2016;73:410. doi: 10.1001/jamaneurol.2015.4791 [DOI] [PubMed] [Google Scholar]
- 21. Ahn N, Nolde M, Günter A, et al. Emulating a target trial of proton pump inhibitors and dementia risk using claims data. Eur J Neurol. 2022;29:1335‐1343. doi: 10.1111/ene.15284 [DOI] [PubMed] [Google Scholar]
- 22. Cooksey R, Kennedy J, Dennis MS, et al. Proton pump inhibitors and dementia risk: evidence from a cohort study using linked routinely collected national health data in Wales. UK PLoS One. 2020;15:e0237676. doi: 10.1371/journal.pone.0237676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Taipale H, Tolppanen A‐M, Tiihonen M, Tanskanen A, Tiihonen J, Hartikainen S. No association between proton pump inhibitor use and risk of Alzheimer's disease. Am J Gastroenterol. 2017;112:1802‐1808. doi: 10.1038/ajg.2017.196 [DOI] [PubMed] [Google Scholar]
- 24. Friesen KJ, Falk J, Chateau D, Kuo IF, Bugden S. Signal and noise: proton pump inhibitors and the risk of dementia? Clin Pharmacol Ther. 2022. doi: 10.1002/cpt.2767 [DOI] [PubMed] [Google Scholar]
- 25. Ahn N, Nolde M, Krause E, et al. Do proton pump inhibitors increase the risk of dementia? A systematic review, meta‐analysis and bias analysis. Br J Clin Pharmacol. 2023;89:602‐616. doi: 10.1111/bcp.15583 [DOI] [PubMed] [Google Scholar]
- 26. Delgado‐Rodriguez M. Bias. J Epidemiol Community Heal. 2004;58:635‐641. doi: 10.1136/jech.2003.008466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schmidt M, Schmidt SAJ, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015:449. doi: 10.2147/CLEP.S91125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mors O, Perto GP, Mortensen PB. The Danish Psychiatric Central Research Register. Scand J Public Health. 2011;39:54‐57. doi: 10.1177/1403494810395825 [DOI] [PubMed] [Google Scholar]
- 29. Wallach Kildemoes H, Toft Sørensen H, Hallas J. The Danish National Prescription Registry. Scand J Public Health. 2011;39:38‐41. doi: 10.1177/1403494810394717 [DOI] [PubMed] [Google Scholar]
- 30. Jensen VM, Rasmussen AW. Danish education registers. Scand J Public Health. 2011;39:91‐94. doi: 10.1177/1403494810394715 [DOI] [PubMed] [Google Scholar]
- 31. Etminan M, Pharmacoepidemiology II. The Nested case‐control study–a novel approach in pharmacoepidemiologic research. Pharmacotherapy. 2004;24:1105‐1109. doi: 10.1592/phco.24.13.1105.38083 [DOI] [PubMed] [Google Scholar]
- 32. Phung TKT, Andersen BB, Høgh P, Kessing LV, Mortensen PB, Waldemar G. Validity of dementia diagnoses in the danish hospital registers. Dement Geriatr Cogn Disord. 2007;24:220‐228. doi: 10.1159/000107084 [DOI] [PubMed] [Google Scholar]
- 33. Jørgensen K, Nielsen TR, Nielsen A, Waldemar G. Potential for prevention of dementia in Denmark. Alzheimer's Dement 2023. doi: 10.1002/alz.13030 [DOI] [PubMed] [Google Scholar]
- 34. Zhang YR, Xu W, Zhang W, et al. Modifiable risk factors for incident dementia and cognitive impairment: an umbrella review of evidence. J Affect Disord. 2022;314:160‐167. doi: 10.1016/j.jad.2022.07.008 [DOI] [PubMed] [Google Scholar]
- 35. Teramura‐Grönblad M, Hosia‐Randell H, Muurinen S, Pitkala K. Use of proton‐pump inhibitors and their associated risks among frail elderly nursing home residents. Scand J Prim Health Care. 2010;28:154‐159. doi: 10.3109/02813432.2010.493315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Core Tea. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2020. https://www.R‐project.orgn.d [Google Scholar]
- 37. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373‐383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 38. Yang M, He Q, Gao F, Nirantharakumar K, et al. Regular use of proton‐pump inhibitors and risk of stroke: a population‐based cohort study and meta‐analysis of randomized‐controlled trials. BMC Med. 2021;19:316. doi: 10.1186/s12916-021-02180-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Karr JE, Graham RB, Hofer SM, Muniz‐Terrera G. When does cognitive decline begin? A systematic review of change point studies on accelerated decline in cognitive and neurological outcomes preceding mild cognitive impairment, dementia, and death. Psychol Aging. 2018;33:195‐218. doi: 10.1037/pag0000236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nolde M, Bahls M, Friedrich N, et al. Association of proton pump inhibitor use with endothelial function and metabolites of the nitric oxide pathway: a cross‐sectional study. Pharmacother J Hum Pharmacol Drug Ther. 2021;41:198‐204. doi: 10.1002/phar.2504 [DOI] [PubMed] [Google Scholar]
- 41. Wong JFW, Kirk A, Perlett L, Karunanayake C, Morgan D, O'Connell ME. Characteristics of young‐onset and late‐onset dementia patients at a remote memory clinic. Can J Neurol Sci /J Can Des Sci Neurol. 2020;47:320‐327. doi: 10.1017/cjn.2020.8 [DOI] [PubMed] [Google Scholar]
- 42. Hvid‐Jensen F, Pedersen L, Nielsen R, et al. Lifestyle factors among proton pump inhibitor users and nonusers: a cross‐sectional study in a population‐based setting. Clin Epidemiol. 2013:493. doi: 10.2147/CLEP.S49354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jensen HAR, Davidsen M, Møller SR, et al. Danskernes sundhed – Den Nationale Sundhedsprofl 2021. Danish Heal Auth—Sundhedsstyrelsen 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information


