Abstract
INTRODUCTION
We investigated the effectiveness of a multidomain intervention to preserve cognitive function in older adults at risk for dementia in Germany in a cluster‐randomized trial.
METHODS
Individuals with a Cardiovascular Risk Factors, Aging, and Dementia (CAIDE) risk score ≥ 9 aged 60 to 77 years were recruited. After randomization of their general practitioner (GP), patients received a multidomain intervention (including optimization of nutrition and medication, and physical, social, and cognitive activity) or general health advice and GP treatment as usual over 24 months. Primary outcome was global cognitive performance (composite z score, based on domain‐specific neuropsychological tests).
RESULTS
Of 1030 participants at baseline, n = 819 completed the 24‐month follow‐up assessment. No differences regarding global cognitive performance (average marginal effect = 0.010, 95% confidence interval: –0.113, 0.133) were found between groups at follow‐up. Perceived restrictions in intervention conduct by the COVID‐19 pandemic did not impact intervention effectiveness.
DISCUSSION
The intervention did not improve global cognitive performance.
Highlights
Overall, no intervention effects on global cognitive performance were detected.
The multidomain intervention improved health‐related quality of life in the total sample.
In women, the multidomain intervention reduced depressive symptoms.
The intervention was completed during the COVID‐19 pandemic.
Keywords: cognitive function, dementia, lifestyle, prevention, randomized controlled trial
1. BACKGROUND
The global number of people living with dementia is rapidly increasing, owing in large part to population aging. While to date no cure is available, 1 the risk for cognitive decline and dementia is, in part, modifiable. Currently, there is evidence for 12 modifiable risk factors for dementia, that is, low levels of education, hearing loss, traumatic brain injury, arterial hypertension, obesity, excessive alcohol consumption, diabetes mellitus, depression, physical inactivity, smoking, social isolation, and exposure to air pollution. 2 Targeting these risk factors and providing person‐centered, cost‐effective, and sustainable means of reducing risk exposure have been highlighted as key components in the reduction of dementia cases by the World Health Organization's (WHO) global action plan on the public health response to dementia. 3
Growing numbers of randomized controlled trials (RCTs) have been launched to use evidence from observational studies regarding modifiable risk factors for interventions on cognitive performance. Owing to the multifactorial etiology of dementia, approaches targeting multiple risk factors simultaneously have been suggested as particularly promising in their potential to preserve cognitive function. 4 The pioneer study testing a multidomain lifestyle intervention in older adults at increased risk for dementia, the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) study, found beneficial intervention effects on global cognition, domain‐specific cognitive function, and physical functioning. 5 These promising results have resulted in the launch of the World Wide FINGERS (WW‐FINGERS) network as an effort to optimize the FINGER approach and adapt the intervention to different settings. 6 A recent review and meta‐analysis confirmed small beneficial effects of multidomain interventions on cognitive performance. 7 In Germany, respective approaches have been lacking so far.
The AgeWell.de‐study was designed to tailor the FINGER approach to older adults at risk for dementia in Germany. Expanding on the FINGER intervention, AgeWell.de included additional intervention components, targeting further modifiable risk factors for dementia, that is potential drug‐related problems (DRP) such as treatment with anticholinergics, 8 depressive symptoms, 2 , 9 and grief after loss experiences. 10 , 11
Our primary objective was to evaluate the effectiveness of the AgeWell.de multidomain intervention on changes in global cognitive performance. The secondary objective was the evaluation of intervention effectiveness on mortality, institutionalization/nursing home placement, activities of daily living (ADL) and instrumental activities of daily living (IADL), quality of life, health‐related quality of life, depressive symptoms, and social inclusion. We hypothesized that the multidomain intervention is superior to general practitioner (GP) treatment as usual (GPTAU) and health advice with regard to the preservation of cognitive functioning and secondary outcomes.
2. METHODS
2.1. Study design and participants
The study design and rationale 12 as well as baseline sample characteristics 13 have been described previously. AgeWell.de followed a pragmatic trial design to facilitate transfer to and implementation in real‐world‐settings; therefore, the study was embedded into the German primary care system. The primary care system holds a key role in the implementation of the federal law to strengthen health promotion and prevention in Germany (Gesetz zur Stärkung der Gesundheitsförderung und der Prävention (Präventionsgesetz—PrävG); therefore, GP practices (GPPs) were deemed a promising source of future implementation of the intervention in real‐world settings. Participants were recruited from consenting GPPs at five study sites in Germany (Leipzig, Greifswald, Kiel, Munich, Halle). All GPPs at the five study sites were eligible for participation, though an emphasis was put on established networks with specific GPPs. No further eligibility criteria were applied for selection of GPPs. Eligible patients were aged 60 to 77 years and had a Cardiovascular Risk Factors, Aging, and Dementia (CAIDE) risk score 14 of ≥ 9 points (range: 0–15 points), as assessed by their GP. Exclusion criteria were diagnosed or suspected dementia; terminal conditions or severe diseases which might interfere with safe conduct of the intervention; severe impairments of hearing, vision, or mobility; insufficient command of the German language; and concurrent participation in another intervention trial. The study was carried out in accordance with the principles of the Declaration of Helsinki in its revised version from 2000. The responsible ethics boards of the coordinating study center of AgeWell.de (Ethics Committee of the Medical Faculty of the University of Leipzig; ethical vote number: 369/17‐ek) and of all participating study sites approved the AgeWell.de‐study. Participants provided written informed consent to participate at their respective GPP. AgeWell.de is registered at the German Clinical Trials Register (DRKS; ID: DRKS00013555).
RESEARCH IN CONTEXT
Systematic review: PubMed and clinicaltrials.gov were searched for multidomain trials, that is, including ≥ 2 intervention components and assessing cognitive performance or risk for dementia as outcomes. While some trials reported beneficial intervention effects on cognitive performance on the total sample, others solely found beneficial effects on subgroups of participants or reported no beneficial intervention effects.
Interpretation: Overall results of our trial were negative. Beneficial effects in the total sample were detected for the secondary outcome health‐related quality of life. Post hoc analyses within the intervention group indicated positive effects of adherence to the intervention components nutrition and social activity on cognitive performance at follow‐up.
Future directions: Identifying optimal target groups for prevention of cognitive decline and defining intervention intensity required for achieving beneficial effects on cognitive performance remains a crucial challenge for future trials. More intensive interventions and implementing further measures to increase adherence might constitute a promising strategy for future studies.
2.2. Randomization and masking
Participating GPPs (clusters) were sequentially randomized to either the intervention (IG) or control group (CG), using computerized randomization lists. A block‐randomization algorithm with a targeted assignment ratio of 1:1 for GPPs and a block size of n = 6 was applied to achieve balanced sample sizes for the groups. GPPs were randomized after recruiting the first participants to avoid imbalances in recruited patients between clusters. The data management center at the Institute for General Practice of the Hannover Medical School handled all randomization procedures, with randomization lists concealed from investigators. GPs were blinded to their respective allocation in the recruitment phase. Although it was not possible to prevent patients from reporting their study experiences to their GP, group allocation was not actively revealed to participants.
2.3. Intervention procedures
Following the FINGER design, the AgeWell.de intervention included nutritional counseling, enhancement of physical and social activity, cognitive training, and the management of cardiovascular risk factors (overweight, smoking). AgeWell.de additionally assessed selected DRP particularly conducive to dementia (e.g., prescription of anticholinergic drugs, underuse of medicines for cardiovascular diseases or diabetes mellitus), depressive symptoms, and grief after loss experiences.
The physical activity component consisted of a standardized program including exercises for strength and flexibility/balance to be conducted at home twice a week. Respective exercises were demonstrated and instructed by the study nurses at participants’ homes, following the baseline interview. Individual goals for aerobic exercise on 3 to 5 days a week were scheduled with each participant. Further, participants received a pedometer and were instructed to document the number of steps walked daily.
Participants were provided with tablet computers equipped with the cognitive training program NeuroNation to enhance cognitive activity and were instructed to use the program three times per week for at least 15 minutes, freely choosing between exercises targeting different cognitive domains. The program provided exercises for verbal fluency, memory, visuospatial attention, executive function, mental arithmetic, reasoning, and information processing. All exercises were adaptive, that is, difficulty was adjusted for participants’ performance in the respective exercise in prior training sessions to increase motivation and avoid frustration on the side of participants.
The nutritional intervention component included advice based on the guidelines of the German Nutrition Society, targeting, for example, the consumption of five portions of fruit and vegetables a day, regular intake of fish, low consumption of salt and sugar, and sufficient hydration based on water and unsweetened beverages. 15
To optimize the participants’ medication, attending GPs provided information on participants’ diagnoses, lab values for creatinine and hemoglobin A1c, and prescribed medication, while participants provided information on their actual current medication during the baseline visit. Based on this information, an electronically supported evaluation was conducted to identify anticholinergic drugs, using an adapted list, based on Durán et al. 16 and Gray et al. and was adapted specifically for the German drug market. 8 In total, our list consisted of 85 anticholinergic drugs, including for example, first‐generation antihistamines, tricyclic antidepressants, and urinary tract spasmolytics (see Appendix S1 in supporting information for further details). Further, discrepancies between reported medication by GP and participant were recorded. After the evaluation, standardized recommendations on participants’ medication were sent to the attending GP by mail, including information on anticholinergic drugs, potentially missing medications for cardiovascular diseases or diabetes mellitus, potentially serious drug–drug interactions, potential contraindications because of renal impairment, and suggestions for modification of participants’ medication, if applicable (see Appendix S1 for further details).
Management of vascular risk factors was provided based on diagnoses and lifestyle risk factors (e.g., smoking, and overweight/obesity) reported by the attending GP and the participant. Participants received oral and written information on the respective risk factors and possible ways of reducing or eliminating risk factors, as applicable.
Social activity was enhanced by setting individual goals for social activities with the participant. In cases of prevalent depressive symptoms, participants were encouraged to contact their attending GP to receive adequate support. Participants experiencing symptoms of grief after bereavement were provided information on grief reactions, including addresses of self‐help groups, helplines, and other sources of support to be contacted when needed.
Trained study nurses instructed participants on how to conduct the intervention components during structured face‐to‐face visits at the participants’ homes after the baseline examination. This visit comprised instructions on how to conduct the individual intervention components and setting of individual goals with participants. All IG participants met the study nurse for face‐to‐face contacts at baseline; an interim session 12 months after baseline; and at trial completion, that is, 24 months after the baseline interview. Additionally, regular monitoring and booster sessions with the study nurse were held at 2, 4, 8, 16, and 20 months after baseline to monitor adherence to the intervention and maintain motivation via telephone. Figure 1 describes the process of intervention delivery and contacts with participants throughout the study.
FIGURE 1.
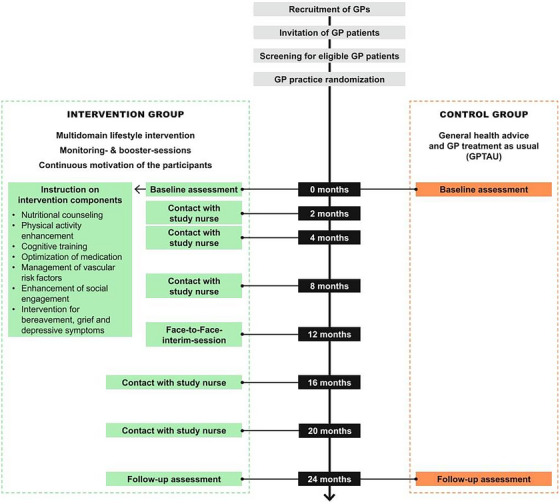
Intervention design and participant contacts throughout the AgeWell.de study. GP, general practitioner.
Study nurses assessed the degree to which participants were able to achieve their goals in the respective intervention domains (nutrition, physical, social, and cognitive activity) and current state of motivation for behavior change per domain, using structured interviews. Where applicable, study nurses offered information on group‐based activities (e.g., for physical activity) available at participants’ place of residence. The monitoring and booster sessions also served to identify potential barriers to behavior change and need for additional support from the study team. Interview guides for contacts with participants were based on motivational interviewing (MI) techniques, 17 aiming to resolve participants’ conflicting beliefs about and fostering motivation for behavior change. While MI is directive in nature and aims at eliciting behavior change, it emphasizes honoring participants’ autonomy, therefore allowing for different levels of difficulty of goals set by participants. All contacts with participants were documented using standardized data entry forms and recorded in the electronic database. Participants in the CG received GPTAU and written health advice on the intervention components at the baseline interview.
2.4. Diversity, equity, and inclusion
The design of the research project and the intervention was discussed with members of the community senior citizen board in Leipzig in the research planning phase, that is, before beginning of the study, to enhance inclusion of the target group. Inclusion and exclusion criteria were designed to keep barriers to participation at a minimum, allowing as many eligible GP patients as possible participation in the study. The multicentric design of AgeWell.de with five recruiting centers across Germany was deemed a feasible approach to recruit participants from a variety of both urban and rural areas. Subgroup analyses of intervention effects were prespecified to report stratified results for men and women.
2.5. Outcomes
Following the approach of FINGER, primary outcome was global cognitive performance at 24‐month follow‐up, as measured by a composite z score. The composite score was based on a subset of tests of the Consortium to Establish a Registry for Alzheimer's Disease (CERAD) 18 neuropsychological test battery, covering the cognitive domains attention (assessed using the Trail Making Test Part A [TMT‐A] 19 ), executive function (TMT Part B [TMT‐B] – TMT‐A 19 ), learning/memory (CERAD Wordlist Memory 18 ), language (CERAD Verbal Fluency Test “Animals” 18 ), and perceptual–motor skills (CERAD Constructional Practice 18 ). In addition, we assessed the domain social cognition using the Reading the Mind in the Eyes test (revised version). 20 The respective assessments were chosen to cover all cognitive domains required for a diagnosis of mild/major neurocognitive disorder (NCD) according to the Diagnostic and Statistical Manual of Mental Disorders Fifth Edition (DSM‐V) criteria. 21
Secondary outcomes included mortality (information obtained from attending GP or elected confidant), nursing home placement (information obtained from participant, GP, or elected confidant), ADL (assessed using the Barthel Index 22 ), and IADL (Amsterdam IADL Questionnaire 23 ), quality of life (assessed using the World Health Organization Quality of Life questionnaire [WHOQOL‐OLD] 24 ), health‐related quality of life (EuroQol 5D Visual Analogue Scale [EQ‐5D‐VAS] 25 ), depressive symptoms (Geriatric Depression Scale 26 ), and social inclusion (Lubben Social Network Scale [LSNS‐6] 27 ). Cost effectiveness of the intervention will be part of the health economic analysis that is pending.
Trial‐related adverse events (AEs) were documented throughout the intervention period regardless of causal relationship to the intervention.
2.6. Sample sizes
Following the approach of FINGER, we assumed a mean decrease of −0.21 points (standard deviation [SD]: 0.5) in the composite z score of global cognitive performance in the CG, compared to the IG, over the 24‐month intervention period. This strategy was based on previous findings from a study of participants with mild Alzheimer's disease (AD), which indicated a linear decline in global cognitive function over time and an expected −0.42 point decline over a 24‐month intervention period in the CG, compared to the IG. 28 However, because AgeWell.de, just as did FINGER, included participants at increased risk for dementia (not people already having mild AD), we expected a decline in global cognitive performance in the CG that was about half as large (−0.21 points) over 24 months, which was included in the sample size calculation as a reference effect size. To detect a 50% difference in change in cognitive performance between groups, a sample size of n = 475 individuals per group was deemed sufficient, applying two‐sided t tests with an alpha level of 0.05 indicating significance and 90% power. Additionally, assuming a cluster size of ≈ n = 6 patients per GPP, an estimated intra‐cluster correlation (ICC) of 0.02 and a dropout rate ≤ 10% in each group, the total sample size was set at n = 1152 individuals (n = 576 per group).
2.7. Statistical analyses
We computed z scores for all domain‐specific cognitive tests at baseline and at 24‐month follow‐up, standardized to the baseline mean and SD, with higher scores indicating better performance. To evaluate global cognitive performance (primary endpoint), a composite z score was calculated by averaging z scores of the single domain‐specific tests, given that a minimum of three out of the six domain‐specific tests had been scored, to achieve the best possible balance between outcome validity and minimizing risk of selection bias when excluding observations with missing data.
For secondary outcomes, Amsterdam IADL scores were zero‐skewness log‐transformed to approximate a normal distribution. Scores for EQ‐5D VAS, WHOQOL‐OLD, Amsterdam IADL, and LSNS were standardized to z scores as described above. Because the distribution of ADL scores was highly skewed to the left, the Barthel Index was dichotomized (100 points vs. < 100 points) to enhance interpretability. Due to insufficient cases regarding nursing home placement (n = 1) and mortality (n = 11) during the trial, no effects could be analyzed for these outcomes.
Main analysis followed the intention‐to‐treat (ITT) principle, including all participants who attended both the baseline and the 24‐month follow‐up assessment. We generated an index variable, indicating missingness of information in any variable at baseline (value = 0 if no information was missing, value = 1 if any information was missing). Inspection of incomplete data revealed no indication of systematic item missingness; therefore, missing values were assumed to be missing at random (MAR). Missing values were replaced by multiple imputation by chained equations, following recommendations for handling of missing data in RCTs. 29 , 30 Pooled estimates of 50 imputed datasets were used in all analyses. For comparison, the main analysis of unimputed data is shown in Appendix S1 (Table S1 in supporting information).
We applied generalized linear regression models (GLM) to estimate mean group differences between IG and CG in the primary and all secondary outcomes at 24‐month follow‐up. To account for non‐normal distributions, we used GLM with negative binomial distribution errors for depressive symptoms, and GLM with logit link and binomial distribution errors for ADL. All other outcomes were assumed approximately normally distributed and estimated with identity link and Gaussian distribution errors. All models included an indicator of treatment group and were adjusted for age at baseline, sex, education, and the baseline value of the respective outcome to counteract possible baseline imbalances and regression to the mean. 31 , 32 Education was assessed using the Comparative Analysis of Social Mobility in Industrial Nations (CASMIN) scale, taking into account general and vocational education and providing a certificate‐based assessment of education with three levels (low, intermediate, high). 33 Treatment effects are reported as average marginal effects (AME), that is, the sample average of the estimated differences in the outcome scores between the treatment groups at 24‐month follow‐up (IG vs. CG), with an alpha level of 0.05 (two‐tailed) indicating significance.
Additionally, we assessed prespecified subgroup differences in intervention effectiveness by sex and age. For that, we included an interaction term between treatment group and sex (age) in the models for all outcomes and evaluated whether the estimated mean group difference at 24‐month follow‐up was modified by these two demographics.
Supplementing the main analyses, we estimated intervention effectiveness using data only from IG participants adherent to the protocol. Study nurses assessed intervention adherence on seven occasions (six monitoring and booster sessions, one face‐to‐face interim assessment) in the IG, asking to what extent participants were able to reach their goals in the domains nutrition, physical activity, cognitive activity, and social activity (response options: not at all [0]–absolutely [4]). Adherence to all intervention components was summed across all points in time, leading to a score ranging from 0 to 28. The subset of IG participants scoring ≥ 12 points contributed to this sensitivity analysis, providing a suitable trade‐off between measuring goal achievement and available participant data across occasions.
Adherence to the individual intervention components nutrition, physical, social, and cognitive activity within the intervention group was further evaluated by calculating mean scores for goal achievement in the four components, assessed across all contacts with participants (range: 0–4, higher scores indicating better adherence). The impact of adherence to the respective intervention components on the primary outcome was subsequently analyzed within the intervention group only.
To provide further information on the cognitive status of participants, we assessed mild cognitive impairment (MCI) 34 and mild or major neurocognitive disorder according to DSM‐V 21 at baseline and 24‐month follow‐up, using established criteria. Interaction terms between MCI status and treatment group were calculated to assess possible effects of baseline MCI status on treatment effects for the primary outcome.
To assess possible effects of the COVID‐19 pandemic and respective governmental measures (e.g., restrictions of social contacts, closure of cultural and recreational facilities) on domains relevant to our intervention, paper‐based questionnaires were sent out to all participants in April and October 2020. 35 Participants reported perceived restrictions in the domains healthy nutrition, physical activity, cognitive activity, and social activity on a 5‐point scale (response options: not restricted, slightly restricted, moderately restricted, restricted, very restricted/not possible to pursue the respective action). No further instructions or examples for respective activities were given on how to answer the questions on perceived restrictions. Responses were dichotomized, with the response options “moderately restricted,” “restricted,” “very restricted/not possible to pursue the respective action” indicating perceived restrictions, while “not restricted” and “slightly restricted” indicated no perceived restrictions. We applied interaction terms between perceived restrictions in the respective intervention domains and group membership to assess possible effects of restrictions on treatment effects for the primary outcome.
All analyses were conducted using Stata/SE 16.0 (StataCorp). All models were run using cluster‐robust standard errors to account for intra‐cluster correlation of participants in GPPs.
3. RESULTS
Between June 2018 and October 2019, n = 123 participating GPPs recruited eligible participants across the five recruiting study sites, with n = 64 and n = 59 GPPs randomized to the IG and CG, respectively. Practices recruited n = 1176 patients who consented to participate. After excluding patients who did not fulfill the inclusion criteria or did not complete the baseline examination, the baseline sample comprised n = 1030 participants (IG/CG: n = 487/543). Of these, n = 819 (79.5%) completed the 24‐month follow‐up assessment between July 2020 and January 2022 and were included in the ITT analyses (IC/CG: n = 378/441). These participants were recruited by n = 117 GPPs (IG/CG: 59/58), with numbers of recruited patients per practice ranging from 1 to 15 in the IG and 1 to 25 in the CG. GPPs recruited an average of n = 6 patients in both the IG and CG, with no evidence of differences in numbers of recruited patients per cluster detected between IG and CG practices (P = 0.4405). Dropout rates amounted to n = 22.4% (n = 109) in the IG and 18.8% (n = 102) in the CG. Due to delays in follow‐up assessments caused by the COVID‐19 pandemic and respective restrictions regarding personal contact, the mean duration between baseline and follow‐up interviews was M = 25.6 months (SD: 2.8). Flow of GPPs and participants is described in Figure 2.
FIGURE 2.
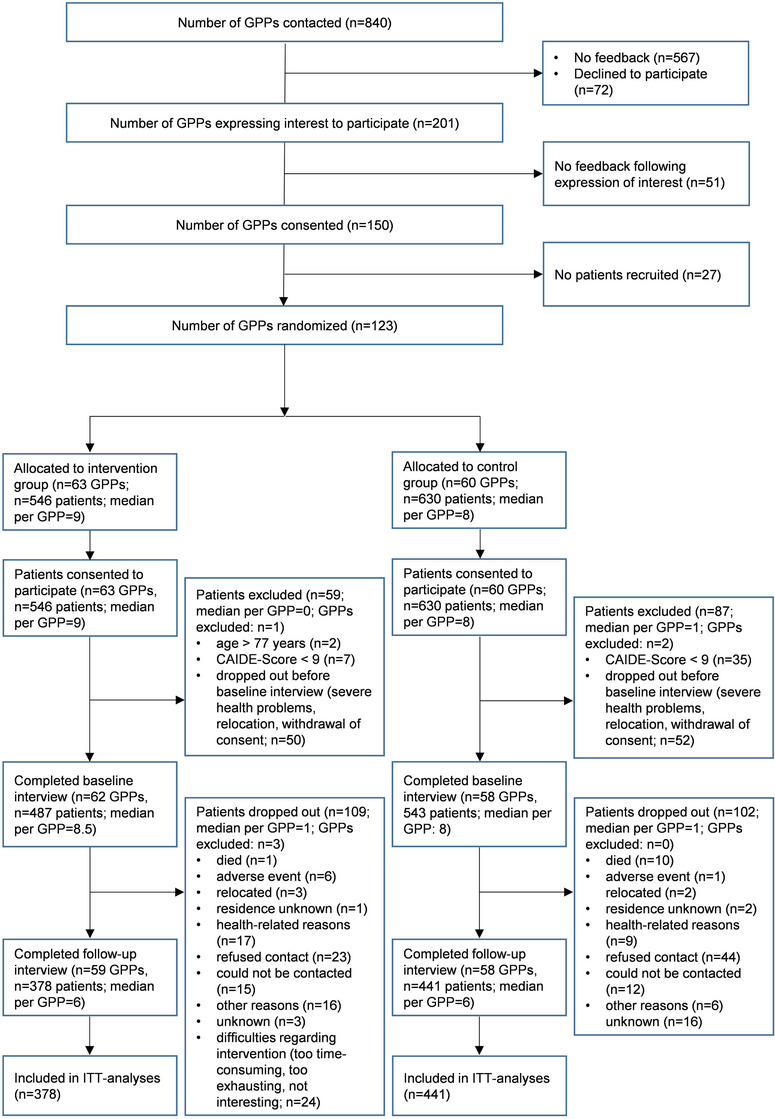
Participant flow in AgeWell.de throughout trial and analyses. CAIDE, Cardiovascular Risk Factors, Aging and Dementia; GPP, general practitioner practice; ITT, intention to treat.
3.1. Baseline characteristics
Characteristics of participants at baseline are reported in Table 1. Baseline values for components of the primary outcome, that is, domain‐specific cognitive tests, are described in Appendix S1 (Table S2 in supporting information). As described earlier, 13 IG and CG participants did not differ regarding values of primary or secondary outcomes and covariates. Participants who dropped out during the intervention did not differ from those who completed the 24‐month follow‐up assessment in terms of age (P = 0.443), sex (P = 0.353), or group allocation (P = 0.153). Those who dropped out before the 24‐month follow‐up assessment more often had low levels of education than participants who completed the 24‐month follow‐up (P < 0.001).
TABLE 1.
Baseline characteristics of AgeWell.de study participants.
| Intervention (n = 378) | Control (n = 441) | |||
|---|---|---|---|---|
| Variables | Raw values | z scores a | Raw values | z scores a |
| Sociodemographic characteristics | ||||
| Age in years | 69.1 (4.9) | – | 69.0 (4.9) | – |
| Female sex, n (%) | 199 (52.7) | – | 234 (53.1) | – |
| Education, n (%) | ||||
| Low | 83 (18.8) | – | 98 (25.9) | – |
| Intermediate | 238 (54.0) | – | 196 (51.9) | – |
| High | 120 (27.2) | – | 84 (22.2) | – |
| Primary outcome | ||||
| Cognitive performance (composite score) | −0.009 (0.568) | −0.004 (0.974) | −0.005 (0.594) | 0.003 (1.021) |
| Secondary outcomes | ||||
| ADL | 0.963 (0.189) | – | 0.961 (0.193) | – |
| IADL | 67.26 (3.20) | –0.054 (0.948) | 67.31 (3.39) | 0.046 (1.039) |
| Quality of life | 74.51 (10.57) | 0.066 (0.961) | 73.12 (10.93) | –0.058 (1.025) |
| Health‐related quality of life | 78.27 (14.93) | 0.090 (0.964) | 75.63 (16.02) | –0.078 (1.024) |
| Depressive symptoms | 1.484 (1.859) | – | 1.493 (1.849) | – |
| Social inclusion | 17.78 (5.47) | 0.056 (0.979) | 17.20 (5.68) | –0.047 (1.012) |
Notes: Data are mean (SD), except when indicated otherwise.
Abbreviations: ADL, activities of daily living (dichotomized score); IADL, instrumental activities of daily living; SD, standard deviation.
z scores were standardized to baseline mean and SD, with higher values indicating better performance.
3.2. Intervention effects
3.2.1. Primary outcome
Overall, ITT analyses revealed no significant intervention effect on global cognitive performance (composite score; AME = 0.010, 95% confidence interval [CI]: −0.113, 0.133). The estimated mean change in the composite score at 24‐month follow‐up was 0.063 (standard error [SE]: 0.041) in the intervention group and 0.053 (SE: 0.048) in the CG. This corresponds to a 15.7% difference in change in global cognitive performance at 24‐month follow‐up between the groups (see Figure 3).
FIGURE 3.
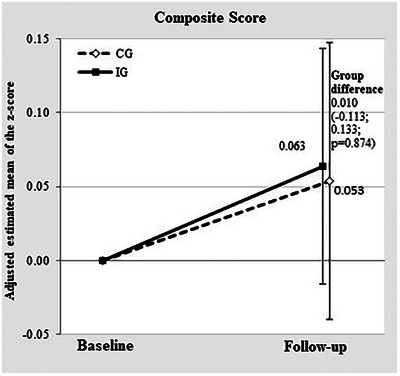
Mean change in global cognitive performance (primary outcome) from baseline to 24‐month follow‐up in IG and CG. CG, control group; IG, intervention group.
3.2.2. Secondary outcomes
The intervention had no significant effect on the secondary outcomes quality of life (AME = 0.003, 95% CI: −0.108, 0.113), depressive symptoms (AME = −0.246, 95% CI: −0.529, 0.038), social inclusion (AME = 0.049, 95% CI: −0.091, 0.188), ADL (AME = 0.012, 95% CI: −0.014, 0.037), and IADL (AME = −0.038, 95% CI: −0.266, 0.191). However, the intervention improved health‐related quality of life in the IG (AME = 0.194, 95% CI: 0.064, 0.324). AMEs for the primary and all secondary outcomes at 24‐month follow‐up are displayed in Table 2.
TABLE 2.
Between‐group differences in primary and secondary outcomes at 24‐month follow‐up.
| Outcome | AME | 95% CI | P |
|---|---|---|---|
| Primary outcome | |||
| Cognitive performance (composite score) | 0.010 | –0.113; 0.133 | 0.874 |
| Secondary outcomes | |||
| ADL | 0.012 | –0.014; 0.037 | 0.374 |
| IADL | –0.038 | –0.266; 0.191 | 0.746 |
| Quality of life | 0.003 | –0.108; 0.113 | 0.964 |
| Health‐related quality of life | 0.194 | 0.064; 0.324 | 0.003 |
| Depressive symptoms | –0.246 | –0.529; 0.038 | 0.090 |
| Social inclusion | 0.049 | –0.091; 0.188 | 0.495 |
Notes: All models adjusted for age, sex, education, and baseline score. Generalized linear regression models (GLMs) were used to estimate mean group differences (IG vs. CG) in scores at 24‐month follow‐up. All outcomes are z scores (higher values indicate better performance), standardized to baseline mean and SD, except depressive symptoms (original scores) and ADL (dichotomized score). Significant effects in bold type.
Abbreviations: ADL, activities of daily living; AME, average marginal effect; CI, confidence interval; IADL, instrumental activities of daily living; SD, standard deviation.
3.2.3. Sensitivity analyses
Analyses of adjusted between‐group differences at 24‐month follow‐up in components of the composite score revealed beneficial intervention effects on the domain social cognition only (AME = 0.132, 95% CI: 0.004, 0.261). No further intervention benefits were detected for the other components of the composite score (Appendix S1, Table S3 in supporting information). Supplementing the main analyses, ITT analyses using unimputed data were conducted, revealing highly comparable results with beneficial intervention effects seen only on health‐related quality of life (AME = 0.198, 95% CI: 0.069, 0.328; Appendix S1, Table S1). Further, ITT analyses without adjustment for covariates provided results highly comparable to the main analyses (AMEhealth‐related quality of life = 0.264, 95% CI: 0.115, 0.413; Appendix S1, Table S4 in supporting information). Finally, analyses including only IG participants adherent to the intervention (n = 730) also resembled results of the main analyses (AMEhealth‐related quality of life = 0.208, 95% CI: 0.064, 0.352; Appendix S1, Table S5 in supporting information).
Prespecified subgroup analyses for age and sex revealed three subgroup differences in treatment effects at 24‐month follow‐up. First, adjusting for covariates, continuous age modified the association of group membership on health‐related quality of life at 24‐month follow‐up (βgroup*age = −0.050, 95% CI: −0.075, −0.025, P < 0.001), such that scores in IG participants were higher than scores in CG participants at age ≤ 70. Stratified analyses by age group (60–69 years vs. 70–77 years) revealed beneficial intervention effects among younger (β = 0.405, 95% CI: 0.243, 0.568), but not among older participants (β = −0.045, 95% CI: −0.235, 0.145). Second, we found a significant group x sex interaction on quality of life, such that the intervention benefit was larger for women than for men (βgroup*sex = −0.225, 95% CI: −0.432, −0.018, P = 0.033). However, no intervention effect on quality of life was detected for women (β = 0.110, 95% CI: −0.051, 0.271) or men (β = −0.120, 95% CI: −0.261, 0.020) when stratifying analyses by sex. Finally, we detected sex‐specific intervention effects on depressive symptoms (βgroup*sex = 0.347, 95% CI: 0.021, 0.674, P = 0.037). The intervention reduced depressive symptoms in women (β = −0.328, 95% CI: −0.537, −0.119), but not in men (β = 0.011, 95% CI: −0.266, 0.289), according to analyses stratified by sex.
Data on MCI status and mild NCD at baseline and 24‐month follow‐up were available for 787/819 and 772/819 participants, respectively. Overall, 8.57% (n = 70) of participants revealed signs of MCI at baseline, with no differences between groups (IG/CG: n = 31/39, χ2 = 0.12, P = 0.728). Regarding mild NCD, 10.5% of participants fulfilled criteria for mild NCD at baseline, again, with no differences between groups (IG/CG: n = 45/40, χ2 = 0.88, P = 0.643). No participants with signs of major NCD were detected at baseline or 24‐month follow‐up. Controlling for age, sex, and education, MCI at baseline had no effect on global cognitive performance at follow‐up (β = −0.037; 95% CI: −0.239; 0.164), and no interaction of MCI status with group allocation was detected (βgroup*MCI‐status = −0.57; 95% CI: −0.400; 0.286).
Regarding potential influences of the cluster design of the AgeWell.de study on our findings regarding the primary endpoint, we found no evidence for an influence of the number of patients recruited in individual GPPs on overall cognitive performance at follow‐up (β = −0.002, 95% CI: −0.024, 0.020). Adjusting for age, sex, education, and baseline cognitive performance, the number of patients recruited per GPP did not change the intervention effect on global cognitive performance (β = 0.002, 95% CI: −0.030, 0.034).
To assess potential contamination of the CG, we further assessed changes in lifestyle concerning physical, social, and cognitive activity as well as nutrition in the CG. Interaction terms (group x time) revealed no evidence for group‐specific changes in physical activity (F = 0.913, P = 0.340), social activity (F = 1.175, P = 0.279), cognitive activity (F = 0.122, P = 0.727), or nutrition (F = 3.315, P = 0.069) between baseline and follow‐up.
Mean adherence to the intervention components (range for each intervention component: 0–4 points, higher values indicating better adherence) within the IG were as follows: nutrition: M = 2.80, SD = 0.71; cognitive activity: M = 2.90, SD = 0.78; physical activity: M = 2.66, SD = 0.82; social activity: M = 2.81, SD = 0.76. Better adherence to the intervention components nutrition (β = 0.137, 95% CI: 0.037, 0.237) and social activity (β = 0.142, 95% CI: 0.036, 0.248) was linked to better global cognitive performance at 24‐month follow‐up within the IG. Adherence to the intervention components cognitive activity (β = 0.061, 95% CI: −0.062, 0.185) and physical activity (β = 0.081, 95% CI: −0.015, 0.178) was not linked to better global cognitive performance within the IG.
3.2.4. Effect of COVID‐19 on intervention effects
A total of n = 707 questionnaires with information on COVID‐19–related restrictions regarding intervention conduct were available for analyses. Results are displayed in Table 3. Perceived restrictions regarding nutrition due to the pandemic were more frequently reported by IG than by CG participants (9.0% vs. 3.7%; P = 0.004).
TABLE 3.
Participants reporting perceived restrictions in intervention components of AgeWell.de.
| N | Intervention component | Intervention group, % (n) | Control group, % (n) | P |
|---|---|---|---|---|
| 707 | Nutrition | 9.0 (30) | 3.7 (14) | 0.004 |
| 705 | Physical activity | 55.2 (182) | 51.5 (193) | 0.328 |
| 700 | Cognitive activity | 14.4 (47) | 11.5 (43) | 0.262 |
| 698 | Social activity | 65.9 (216) | 61.2 (226) | 0.192 |
Note: Percentages of participants reporting restrictions in the domains healthy nutrition, physical activity, cognitive activity, and social activity (responses: moderately restricted, restricted, or very restricted/not possible to pursue the respective action).
Further, we assessed possible effects of perceived restrictions in the respective intervention domains on the treatment effect on global cognitive performance. Adjusting for covariates, we found no evidence for influences of perceived restrictions in the domains nutrition (βgroup*perceived restrictions = −0.542, 95% CI: −1.264, 0.180, P = 0.139), physical activity (βgroup*perceived restrictions = −0.076, 95% CI: −0.291, 0.140, P = 0.487), social activity (βgroup*perceived restrictions = −0.043, 95% CI: −0.282, 0.196, P = 0.720), or cognitive activity (βgroup*perceived restrictions = −0.092, 95% CI: −0.442, 0.258, P = 0.603) on between‐group differences in cognitive performance at 24‐month follow‐up.
3.3. Adverse events
Throughout the intervention period, 163 AEs occurred, with 30 (18.4%) AEs considered serious adverse events (SAEs; Table 4). One AE with probable causal link to the intervention was recorded when a participant's medication was adjusted (dose reduction of extended‐release oxycodone due to presumed mild anticholinergic effects) following the suggestions of the AgeWell.de study personnel on DRP, leading to slight symptoms of withdrawal. Burden for the participant was considered mild and the AE could be solved during the course of the intervention (careful dose titration leading to a still reduced total daily dose compared to the dose before intervention). No SAEs suspected to be related to study participation were reported.
TABLE 4.
Adverse events during the Agewell.de intervention.
| Type of AE | Total (n = 1.030) | Intervention group (n = 487) | Control group (n = 543) | Causal relation to intervention a |
|---|---|---|---|---|
| Total | 163 | 152 | 11 | 1 |
| Loss/grief | 28 | 25 | 3 | 0 |
| Depressive symptoms | 21 | 21 | 0 | 0 |
| Physical injury | 44 | 43 | 1 | 0 |
| Hospitalization | 56 | 49 | 7 | 0 |
| Personal burden | 5 | 5 | 0 | 0 |
| Other | 24 | 24 | 0 | 1 |
| Thereof: SAEs | ||||
| Total | 30 | 25 | 5 | 0 |
| Life‐threatening condition | 2 | 2 | 0 | 0 |
| Hospitalization/prolongation thereof | 26 | 25 | 5 | 0 |
| Irreversible harm / disability | 5 | 5 | 0 | 0 |
Abbreviations: AE, adverse event; SAE, serious adverse event.
AEs considered probably or definitely causally related to study participation; in each case of recorded AE/SAE, more than one option could apply, leading to differences between total n of AEs/SAEs and individual causes for AE/SAE; AEs labeled “other” included, for example, diseases not fitting the category “physical injury” or burden due to sickness of a spouse or relative.
4. DISCUSSION
We investigated the effectiveness of the AgeWell.de multidomain intervention, comprising enhancement of physical, cognitive, and social activity; optimized nutrition and medication; management of cardiovascular risk factors; and, if necessary, an intervention targeting grief and depressive symptoms in a sample of older adults at increased risk for dementia in Germany. We hypothesized that the multidomain intervention would be superior to general health advice and GPTAU regarding preserved cognitive performance and several secondary outcomes after a 24‐month intervention period.
Other than the FINGER multidomain intervention, which revealed overall beneficial effects on cognitive performance, 5 the AgeWell.de intervention revealed no intervention effect on the primary outcome global cognitive performance. Regarding secondary outcomes, the intervention improved health‐related quality of life in the overall sample and reduced depressive symptoms in women. Evidence on sex differences in effectiveness of treatment of depressive symptoms is mixed, with certain studies reporting greater benefits for men, while others reported stronger effects on women or no sex differences. 36 Possibly, the intervention component targeting depressive symptoms (referral to GP and other professional sources of help) might have appealed more to women in our study. Previous studies suggest higher rates of help seeking for depressive symptoms and a larger variety of coping strategies in women. 37 However, baseline values of depressive symptoms in our study sample were rather low.
As described earlier, 13 participants in AgeWell.de had a mean Montreal Cognitive Assessment score of 24.5 points at baseline, indicating that a substantial proportion had cognitive performance levels slightly below corresponding age‐ and education‐specific norms. However, we assessed MCI and mild/major NCD using established criteria and investigated possible influences on the treatment effect. While MCI at baseline was linked to worse performance in the primary outcome in general, we found no effect of MCI status on the treatment effect, suggesting that lack of effectiveness is unlikely to be explained by baseline cognitive performance.
While FINGER offered several guided activities for IG participants (e.g., supervised training, meetings with other study participants 5 ), our intervention was performed independently by participants. Accounting for a pragmatic trial design, this was deemed a feasible approach to be readily implemented in real‐world settings. Despite regular telephone contact with participants to offer support and monitor adherence, this might have led to a slightly lower intensity than in FINGER, possibly contributing to the non‐significant findings. Post hoc analyses in IG participants indicated that better adherence to the intervention components nutrition and social activity was linked to improvement in cognitive performance at 24‐month follow‐up, implying that more intense interventions and better adherence might increase effectiveness of multidomain interventions on cognitive performance. In the FINGER and Healthy Ageing Through Internet Counselling in the Elderly trials, better adherence to the intervention predicted greater benefits on primary outcomes. 38 , 39
The CG received GPTAU and advice on the intervention components. Due to high standards of care in the German primary care setting, parts of the intervention, for example, management of cardiovascular conditions, might have also been applied in the CG. Additionally, the assessment process and information provided on lifestyle and brain health might have raised awareness among the CG, possibly stimulating beneficial lifestyle changes. Therefore, our results might constitute rather conservative estimates. However, we assessed group‐specific changes in the intervention components, with no evidence for positive lifestyle changes in the CG, indicating a bias in the results due to the design involving an active CG is unlikely.
Targeting at‐risk individuals, for example, based on dementia risk scores, has been suggested as a feasible approach for primary prevention of dementia. 5 , 6 Participants in AgeWell.de had on average higher dementia risk scores than participants in FINGER, as we exclusively enrolled patients with a CAIDE score ≥ 9 points (FINGER: ≥ 6 points). However, this might have resulted in a study population too severely impaired to benefit from the intervention, as higher baseline CAIDE scores have been linked to decreased cognitive function in longitudinal studies. 40
The COVID‐19 pandemic imposed severe challenges on intervention conduct and extended the duration between baseline and 24‐month follow‐up interview for a substantial proportion of participants. We took great effort to keep participants in the study, maintain motivation, and also assessed perceived impact of the pandemic and its restrictions on conduct of individual intervention components (physical, social, and cognitive activity, nutrition) by sending out questionnaires in April and October 2020. 35 Intervention group participants more often reported restrictions regarding the intervention component “nutrition” than participants in the CG, possibly due to COVID‐19–related restrictions interfering with participants’ goals regarding nutrition. While most studies on nutrition habits in older adults during the pandemic reported no changes in a recent systematic review, 41 some studies detected unfavorable changes, for example, increased overall food consumption 42 or higher intake of foods high in calories. 43 As we did not assess specific dietary changes during the pandemic, we cannot fully explain the between‐group differences detected for restrictions on healthy nutrition in our study. We found no evidence for a group‐specific impact of pandemic‐related restrictions, reported by participants, on cognitive performance at 24‐month follow‐up. However, these analyses used self‐reported data and might, therefore, provide biased estimates. The actual impact of restrictions in social contacts, physical activity, and so forth, due to the pandemic and respective legislative measures to curb spreading of the virus might have had a stronger impact than captured by our data. Therefore, we cannot fully rule out possible effects of COVID‐19 on our findings.
The intervention was feasible and safe, with only one mild AE causally linked to study participation. Differences in absolute numbers of AEs between groups are explained by trial design. AEs were documented upon each contact with participants, with regular telephone contacts in the IG, while CG participants had no regular contact with study personnel between baseline and 24‐month follow‐up assessment. Therefore, AEs in the CG were only assessed during the 24‐month follow‐up interview.
Our study has several limitations. First, we did not reach the aspired number of participants. A higher number of GPPs than initially planned were recruited (actual number: 123; planned: 96), and extensive efforts were made to support GPs in recruitment of participants. However, possibly due to time constraints on the side of GPs or lack of eligible patients willing to participate, our study did not reach the aspired number of participants. Second, more participants than initially expected dropped out during the intervention (IG: 22.4%, CG: 18.8%). Third, only selected DRP with a presumed close relationship to dementia were addressed. Thus, we cannot exclude that a full systematic assessment of medications considered potentially inadequate in elderly patients would have had more beneficial effects. Further, we did not assess whether blinding of GPs and participants was successful. Although group allocation was not actively revealed to GPs or patients, we cannot rule out that participants gained awareness on group assignments. Therefore, we were unable to assess potential impact of awareness of group allocation on treatment effects. Due to a skewed distribution of numbers of participants recruited per GPP, with many GPPs recruiting only one patient, reporting of results per cluster was not feasible, possibly limiting generalizability of our findings. Goals for behavior change in the intervention components were set individually with participants. Owing to the principles of MI, study nurses respected participants’ autonomy when setting goals; however, this may have introduced bias due to variations in number and difficulty of goals set by participants. More objective measures of goal achievement might enhance predictability of intervention effectiveness in future studies. Finally, due to the very low numbers of deaths and only one case of institutionalization during the intervention period, we were not able to assess intervention effects on the respective secondary outcomes. Low rates of mortality may be partially due to study exclusion criteria, excluding patients with conditions deemed fatal by the GP. Recent meta‐analyses, however, reported no effects of multidomain interventions on mortality. 7
AgeWell.de is the first study completing an adapted FINGER intervention in another setting, providing evidence that the multidomain intervention is feasible and safe to conduct by older adults at increased risk for dementia. Our intervention included the assessment of DRP with anticholinergic effects; personalized recommendations for adjustments of medication; and, where necessary, support for grief and depressive symptoms, addressing further known risk factors for dementia. Last, we completed our trial during the COVID‐19 pandemic, taking extensive efforts to maintain motivation and keep participants in the study. To the best of our knowledge, our study is the first multidomain RCT targeting cognitive performance in older adults providing data on an intervention conducted during the COVID‐19 pandemic, which might improve knowledge on conduct and evaluation of an extensive intervention during challenging conditions.
5. CONCLUSION
We found no beneficial effects of a multidomain intervention on cognitive performance among a high‐risk sample of older adults in Germany. However, the intervention benefitted health‐related quality of life in the total sample and improved depressive symptoms in women. Post‐hoc analyses indicate the need for higher‐intensity interventions and more ambitious goals in multidomain trials targeting dementia risk reduction. Future studies might investigate the effectiveness of approaches tailored to the needs of different subgroups of older adults, for example, based on lifestyle or (cardiovascular) risk profile to maximize intervention effectiveness.
CONFLICT OF INTEREST STATEMENT
Walter E. Haefeli reports grants from BAYOOCARE GmbH, Daiichi Sankyo GmbH, and Bayer AG, and speaker fees from Bristol Myers‐Squibb GmbH & Co. KGaA and Daiichi Sankyo GmbH. Christian Brettschneider reports payments for lectures on public health in the MBA Program “Health Care Management” from Hamburg University and travel grants from University Zürich. Laura Lunden reports grants from Hevert‐Arzneimittel GmbH & Co. KG. All other authors declare that they have no competing interests. The authors have no declarations of interest to disclose in supporting information.
CONSENT STATEMENT
Participants provided written informed consent to participate at their respective GP practice.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
Members of the AgeWell.de study group: Principal Investigator and Co‐Principal Investigators: Steffi G. Riedel‐Heller (PI), Wolfgang Hoffmann, Jochen Gensichen, Walter E. Haefeli, Hanna Kaduszkiewicz, Hans‐Helmut König, Thomas Frese, David Czock, Jochen René Thyrian, Birgitt Wiese; Franziska Berg, Andrea Bischhoff, Christian Brettschneider; Mandy Claus, Juliane Döhring, Alexander Eßer, Corinna Gräble, Caroline Jung‐Sievers, Kerstin Klauer‐Tiedtke, Kerstin Krebs‐Hein, Sebastian Lange, Paula Liegert, Dagmar Lochmann, Tobias Luck, Melanie Luppa, Silke Mamone, Andreas Meid, Michael Metzner, Lydia Neubert, Anke Oey, Susanne Röhr, Franziska‐Antonia Zora Samos, Karin Schumacher, Hanna Seidling, Theresa Terstegen, Sandy Thieme, Lars Wamsiedler, Tanja Wehran, Marina Weißenborn, Flora Wendel, Ines Winkler, Isabel Zöllinger, Andrea Zülke, Ina Zwingmann. Further, the authors want to thank all participating GPs and study participants of the AgeWell.de trial for their cooperation. AgeWell.de is funded by the German Federal Ministry for Education and Research (BMBF; reference numbers: 01GL1704A, 01GL1704B, 01GL1704C, 01GL1704D, 01GL1704E, 01GL1704F). The BMBF had no role in the design of this study and had no role during its execution, analyses, interpretation of the data, writing of the present paper, or decision to submit results.
Open access funding enabled and organized by Projekt DEAL.
Zülke AE, Pabst A, Luppa M, et al. A multidomain intervention against cognitive decline in an at‐risk‐population in Germany: Results from the cluster‐randomized AgeWell.de trial. Alzheimer's Dement. 2024;20:615–628. 10.1002/alz.13486
Andrea E. Sulked and Alexander Pabst share first authorship.
TRIAL REGISTRATION: German Clinical Trials Register (DRKS; ID: DRKS00013555)
REFERENCES
- 1. Yiannopoulou KG, Papageorgiou SG. Current and future treatments in Alzheimer disease: an update. J Cent Nerv Syst Dis. 2020;12:1179573520907397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet commission. Lancet North Am Ed. 2020;396(10248):413‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization . Global action plan on the public health response to dementia 2017‐2025. 92415134 2017.
- 4. World Health Organization . Risk reduction of cognitive decline and dementia: WHO guidelines. 92415505 2019. [PubMed]
- 5. Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at‐risk elderly people (FINGER): a randomised controlled trial. Lancet North Am Ed. 2015;385(9984):2255‐2263. [DOI] [PubMed] [Google Scholar]
- 6. Rosenberg A, Mangialasche F, Ngandu T, Solomon A, Kivipelto M. Multidomain interventions to prevent cognitive impairment, Alzheimer's disease, and dementia: from finger to world‐wide fingERS. J Prev Alzheimers Dis. 2020;7(1):29‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hafdi M, Hoevenaar‐Blom MP, Richard E. Multi‐domain interventions for the prevention of dementia and cognitive decline. Cochrane Database Syst Rev. 2021;11:CD013572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gray SL, Anderson ML, Dublin S, et al. Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study. JAMA Intern Med. 2015;175(3):401‐407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Diniz BS, Butters MA, Albert SM, Dew MA, Reynolds CF. Late‐life depression and risk of vascular dementia and Alzheimer's disease: systematic review and meta‐analysis of community‐based cohort studies. Br J Psychiatry. 2013;202(5):329‐335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Corruble E, Falissard B, Gorwood P. DSM bereavement exclusion for major depression and objective cognitive impairment. J Affect Disord. 2011;130(1‐2):113‐117. [DOI] [PubMed] [Google Scholar]
- 11. Rosnick CB, Small BJ, Burton AM. The effect of spousal bereavement on cognitive functioning in a sample of older adults. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2010;17(3):257‐269. [DOI] [PubMed] [Google Scholar]
- 12. Zülke A, Luck T, Pabst A, et al. AgeWell.de—study protocol of a pragmatic multi‐center cluster‐randomized controlled prevention trial against cognitive decline in older primary care patients. BMC geriatrics. 2019;19(1):203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Röhr S, Zülke A, Luppa M, et al. Recruitment and baseline characteristics of participants in the AgeWell.de study‐a pragmatic cluster‐randomized controlled lifestyle trial against cognitive decline. Int J Environ Res Public Health. 2021;18(2):408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kivipelto M, Ngandu T, Laatikainen T, Winblad B, Soininen H, Tuomilehto J. Risk score for the prediction of dementia risk in 20 years among middle aged people: a longitudinal, population‐based study. Lancet Neurol. 2006;5(9):735‐741. [DOI] [PubMed] [Google Scholar]
- 15. Deutsche Gesellschaft für Ernährung e.V . 10 guidelines of the German Nutrition Society (DGE) on a wholesome diet. [September 13, 2022]; Available from: https://www.dge.de/fileadmin/public/doc/fm/10‐guidelines‐for‐a‐wholesome‐diet.pdf
- 16. Durán CE, Azermai M, Vander Stichele RH. Systematic review of anticholinergic risk scales in older adults. Eur J Clin Pharmacol. 2013;69:1485‐1496. [DOI] [PubMed] [Google Scholar]
- 17. Rollnick S, Miller WR. What is motivational interviewing? Behav Cogn Psychother. 1995;23(4):325‐334. [DOI] [PubMed] [Google Scholar]
- 18. Morris JC, Heyman A, Mohs RC, et al. The consortium to establish a registry for Alzheimer's disease (CERAD): i. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39(9):1159‐1165. [DOI] [PubMed] [Google Scholar]
- 19. Reitan RM. Trail Making Test: Manual for administration and scoring. Reitan Neuropsychology Laboratory; 1986. [Google Scholar]
- 20. Baron‐Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The “reading the mind in the eyes” test revised version: a study with normal adults, and adults with Asperger syndrome or high‐functioning autism. J Child Psychol Psychiatry Allied Discip. 2001;42(2):241‐251. [PubMed] [Google Scholar]
- 21. American Psychiatric Association . Diagnostic and statistical manual of mental disorders: DSM‐5. Volume 5: American psychiatric association; 2013. [Google Scholar]
- 22. Mahoney FI, Barthel DW. A simple index of independence useful in scoring improvement in the rehabilitation of the chronically ill. Md Med J. 1965;6:493‐507. [Google Scholar]
- 23. Am Sikkes S, Knol DL, Pijnenburg YAL, De Lange‐de Klerk ES, Uitdehaag BMJ, Scheltens P. Validation of the Amsterdam IADL Questionnaire©, a new tool to measure instrumental activities of daily living in dementia. Neuroepidemiology. 2013;41(1):35‐41. [DOI] [PubMed] [Google Scholar]
- 24. Winkler I, Matschinger H, Angermeyer MC. Der WHOQOL‐OLD. PPmP‐Psychotherapie·Psychosomatik·Medizinische Psychologie. 2006;56(02):63‐69. [DOI] [PubMed] [Google Scholar]
- 25. EuroQol Group . EuroQol‐a new facility for the measurement of health‐related quality of life. Health Policy. 1990;16(3):199‐208. [DOI] [PubMed] [Google Scholar]
- 26. Gauggel S, Birkner B. Validity and reliability of a German version of the geriatric depression scale (GDS). Zeitschrift fur Klinische Psychologie‐Forschung und Praxis. 1999;28(1):18‐27. [Google Scholar]
- 27. Lubben J, Blozik E, Gillmann G, et al. Performance of an abbreviated version of the Lubben social network scale among three European community‐dwelling older adult populations. Gerontologist. 2006;46(4):503‐513. [DOI] [PubMed] [Google Scholar]
- 28. Harrison J, Minassian SL, Jenkins L, Black RS, Koller M, Grundman M. A neuropsychological test battery for use in Alzheimer disease clinical trials. Arch Neurol. 2007;64(9):1323‐1329. [DOI] [PubMed] [Google Scholar]
- 29. Sterne JAC, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van Buuren S. Flexible imputation of missing data. CRC press; 2018. [Google Scholar]
- 31. Twisk J, Bosman L, Hoekstra T, Rijnhart J, Welten M, Heymans M. Different ways to estimate treatment effects in randomised controlled trials. Contemp Clin Trials Commun. 2018;10:80‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kahan BC, Jairath V, Doré CJ, Morris TP. The risks and rewards of covariate adjustment in randomized trials: an assessment of 12 outcomes from 8 studies. Trials. 2014;15(1):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brauns H, Scherer S, Steinmann S. The CASMIN educational classification in international comparative research: in: advances in cross‐national comparison. Springer; 2003:221‐244. [Google Scholar]
- 34. Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183‐194. [DOI] [PubMed] [Google Scholar]
- 35. Röhr S, Arai H, Mangialasche F, et al. Impact of the COVID‐19 pandemic on statistical design and analysis plans for multidomain intervention clinical trials: experience from world‐wide fingers. Alzheimers Dement. 2021;7(1):e12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Parker G, Blanch B, Crawford J. Does gender influence response to differing psychotherapies by those with unipolar depression? J Affect Disord. 2011;130(1‐2):17‐20. [DOI] [PubMed] [Google Scholar]
- 37. Howerton A, van Gundy K. Sex differences in coping styles and implications for depressed mood. Int J Stress Manag. 2009;16(4):333‐350. [Google Scholar]
- 38. Ngandu T, Lehtisalo J, Korkki S, et al. The effect of adherence on cognition in a multidomain lifestyle intervention (FINGER). Alzheimers Dement. 2022;18(7):1325‐1334. [DOI] [PubMed] [Google Scholar]
- 39. Richard E, van Moll Charante EP, Hoevenaar‐Blom MP, et al. Healthy ageing through internet counselling in the elderly (HATICE): a multinational, randomised controlled trial. Lancet Digit Health. 2019;1(8):e424‐e434. [DOI] [PubMed] [Google Scholar]
- 40. Reijmer YD, van den Berg E, van Sonsbeek S, et al. Dementia risk score predicts cognitive impairment after a period of 15 years in a nondemented population. Dement Geriatr Cogn Disord. 2011;31(2):152‐157. [DOI] [PubMed] [Google Scholar]
- 41. Larson EA, Karlen SB‐L, Magkos F. The effect of covid‐19‐related lockdowns on diet and physical activity in older adults: a systematic review. Aging Dis. 2021;12(8):1935‐1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Alhusseini N, Alqahtani A. COVID‐19 pandemic's impact on eating habits in Saudi Arabia. J Public Health Res. 2020;9(3):1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cicero AFG, Fogacci F, Giovannini M, et al. COVID‐19‐Related Quarantine effect on dietary habits in a northern italian rural population: data from the brisighella heart study. Nutrients. 2021;13(2):309. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information


