Abstract
INTRODUCTION
We assessed TAR DNA‐binding protein 43 (TDP‐43) seeding activity and aggregates detection in olfactory mucosa of patients with frontotemporal lobar degeneration with TDP‐43‐immunoreactive pathology (FTLD‐TDP) by TDP‐43 seeding amplification assay (TDP43‐SAA) and immunocytochemical analysis.
METHODS
The TDP43‐SAA was optimized using frontal cortex samples from 16 post mortem cases with FTLD‐TDP, FTLD with tau inclusions, and controls. Subsequently, olfactory mucosa samples were collected from 17 patients with FTLD‐TDP, 15 healthy controls, and three patients carrying MAPT variants.
RESULTS
TDP43‐SAA discriminated with 100% accuracy post mortem cases presenting or lacking TDP‐43 neuropathology. TDP‐43 seeding activity was detectable in the olfactory mucosa, and 82.4% of patients with FTLD‐TDP tested positive, whereas 86.7% of controls tested negative (P < 0.001). Two out of three patients with MAPT mutations tested negative. In TDP43‐SAA positive samples, cytoplasmatic deposits of phosphorylated TDP‐43 in the olfactory neural cells were detected.
DISCUSSION
TDP‐43 aggregates can be detectable in olfactory mucosa, suggesting that TDP43‐SAA might be useful for identifying and monitoring FTLD‐TDP in living patients.
Keywords: frontotemporal dementia, frontotemporal lobar degeneration, olfactory mucosa, seeding, seeding amplification assays, TAR DNA‐binding protein 43
1. BACKGROUND
Frontotemporal dementia (FTD) is a progressive neurodegenerative disease characterized by a heterogeneous neuropathological and clinical presentation, and complex genotype–phenotype correlation. 1 , 2 , 3 , 4 , 5 Neuropathological prediction can only be made in monogenic disease where variants within granulin (GRN) or TAR DNA‐binding protein (TARDBP) or hexanucleotide repeat expansions in the chromosome 9 open reading frame 72 (C9orf72) genes lead to frontotemporal lobar degeneration (FTLD) with TDP‐43–immunoreactive pathology (FTLD‐TDP), while variants in the microtubule associated protein tau (MAPT) gene result in FTLD with tau inclusions (FTLD‐tau). 6 Additionally, frontotemporal dementia‐amyotrophic lateral sclerosis (FTD‐ALS) is associated with TDP‐43 neuropathology. 7 Currently, neither clinical presentation, cerebrospinal fluid (CSF) or blood biomarkers, nor imaging markers can predict the underlying neuropathological hallmarks at the single subject level in other patients with FTD. 8
Seeding amplification assay (SAA), which has demonstrated the ability to detect prions in various peripheral tissues, 9 has been adapted to detect TDP‐43 in the CSF of genetic FTD and ALS cases. 10 However, confirmatory studies are required to assess TDP43‐SAA accuracy.
Olfactory brushing, a non‐invasive procedure for sampling neurons of the olfactory mucosa, has proven useful for the in vivo diagnosis of human prion diseases, 11 , 12 and, more recently, of alpha‐synucleinopathies via the SAA. 13 , 14
Previous autopsy studies confirmed that aggregated TDP‐43 could be recovered in the olfactory bulb of patients with neurodegenerative disorders, such as Alzheimer's disease 15 or ALS. 16 Thus, the olfactory mucosa could represent a valuable source for testing TDP‐43 aggregation capacity, and it may represent an easily accessible tissue to study aberrantly misfolded or modified TDP‐43.
These considerations prompted the present study, which aims to evaluate whether TDP‐43 seeding and aggregates can be detected in patients with a diagnosis of FTD associated with FTLD‐TDP. 1 , 2 , 3 , 4 To achieve this, TDP43‐SAA was optimized in brain homogenates of FTLD‐TDP autopsy‐confirmed cases and then developed in olfactory mucosa samples of patients with FTD diagnosis associated with FTLD‐TDP and healthy controls, examining in parallel the presence of TDP‐43 aggregates by immunocytochemistry.
2. METHODS
2.1. Participants
In this cross‐sectional study, autopsy cases were obtained from the Brain Library of the Dementia Laboratory at Indiana University School of Medicine, Indianapolis, Indiana, USA while patients were recruited at the Universities of Brescia and Verona, Italy.
The autopsy series included frontal cortex tissues from 16 subjects: 10 diagnosed with FTLD‐TDP (5 carriers of GRN variants and 5 with C9orf72 repeat expansions) and 6 patients with non–TDP‐43 related diagnosis (3 with multiple system tauopathy with dementia [MSTD] and 3 control subjects lacking TDP‐43 pathology; 1 see Table S1 in supporting information for details). Several of these cases have been extensively characterized before. 17 , 18 , 19 Brain specimens from all the aforementioned cases were used to optimize the SAA.
The clinical series, recruited from February 2021 to June 2022, comprised 35 subjects: 17 patients with FTD diagnosis associated with FTLD‐TDP neuropathology (8 with GRN variants, 7 with C9orf72 expansions, 1 with TARDBP variant, and 1 with FTD‐ALS), 15 healthy controls (HC), and 3 patients with FTD diagnosis associated with FTLD‐tau, carrying MAPT variants. Demographic and clinical characteristics are reported in Table 1.
TABLE 1.
Demographic and clinical characteristics of FTD patients and controls.
| ID | Gene | Variant | Diagnosis | Age, years | Sex | Age at onset, years | CDR® plus NACC | SAA |
|---|---|---|---|---|---|---|---|---|
| BS1 | GRN | p.T272Sfs*10 | avPPA | 60 | F | 58 | 1 | + |
| BS2 | GRN | p.T272Sfs*10 | bvFTD | 65 | M | 64 | 0.5 | + |
| BS3 | GRN | p.T272Sfs*10 | bvFTD | 61 | F | 60 | 0.5 | + |
| BS4 | GRN | p.T272Sfs*10 | bvFTD | 51 | M | 51 | 0.5 | + |
| BS5 | GRN | p.T272Sfs*10 | PCA | 61 | F | 58 | 3 | + |
| BS6 | GRN | p.T272Sfs*10 | bvFTD | 50 | F | 47 | 2 | + |
| BS7 | GRN | p.T272Sfs*10 | bvFTD | 79 | F | 77 | 3 | + |
| BS8 | GRN | p.C246X | avPPA | 71 | M | 70 | 1 | + |
| BS9 | C9orf72 | expansion | avPPA | 57 | M | 56 | 1 | + |
| BS10 | C9orf72 | expansion | bvFTD | 58 | M | 53 | 3 | + |
| BS11 | C9orf72 | expansion | bvFTD | 51 | M | 49 | 1 | + |
| BS12 | C9orf72 | expansion | FTD‐ALS | 51 | F | 51 | 0.5 | – |
| BS13 | C9orf72 | expansion | bvFTD | 64 | M | 58 | 2 | – |
| BS14 | C9orf72 | expansion | bvFTD | 56 | F | 52 | 3 | + |
| BS15 | C9orf72 | expansion | FTD‐ALS | 56 | F | 56 | 0.5 | – |
| BS16 | TARDP | p.Asn267Ser | bvFTD | 55 | M | 54 | 1 | + |
| BS17 | – | – | FTD‐ALS | 55 | M | 51 | 3 | + |
| BS18 | MAPT | P301L | CBS | 55 | M | 51 | 2 | – |
| BS19 | MAPT | P301L | bvFTD | 53 | F | n.a. | 4 | – |
| BS20 | MAPT | IVS10+3 G > A | bvFTD | 35 | M | 33 | 0.5 | + |
| C1 | – | – | HC | 52 | F | – | – | – |
| C2 | – | – | HC | 56 | F | – | – | – |
| C3 | – | – | HC | 60 | M | – | – | – |
| C4 | – | – | HC | 57 | M | – | – | – |
| C5 | – | – | HC | 30 | M | – | – | – |
| C6 | – | – | HC | 24 | F | – | – | – |
| C7 | – | – | HC | 67 | F | – | – | – |
| C8 | – | – | HC | 71 | M | – | – | – |
| C9 | – | – | HC | 68 | F | – | – | – |
| C10 | – | – | HC | 72 | F | – | – | – |
| C11 | – | – | HC | 64 | F | – | – | – |
| C12 | – | – | HC | 54 | M | – | – | – |
| C13 | – | – | HC | 76 | M | – | – | + |
| C14 | – | – | HC | 60 | M | – | – | – |
| C15 | – | – | HC | 65 | F | – | – | + |
Abbreviations: +, positive; –, negative; avPPA, agrammatic variant of primary progressive aphasia; bvFTD, behavioral variant frontotemporal dementia; C9orf72, chromosome 9 open reading frame 72; CBS, corticobasal syndrome; CDR plus NACC, Clinical Dementia Rating staging instrument plus National Alzheimer's Coordinating Center behavior and language; FTD, frontotemporal dementia; F, female; FTD‐ALS, frontotemporal dementia‐amyotrophic lateral sclerosis; GRN, granulin; HC, healthy control; M, male; MAPT, microtubule‐associated protein tau; n.a., not available; PCA, posterior cortical atrophy; SAA, seeding amplification assay; TARDP, TAR DNA‐binding protein.
Each patient underwent an extensive clinical and neuropsychological assessment and magnetic resonance imaging scan. Genetic analyses were carried out according to standard protocols, as previously published. 20 Disease stage was assessed by the Clinical Dementia Rating (CDR) Dementia Staging Instrument plus National Alzheimer's Coordinating Center (NACC) behavior and language domains (CDR plus NACC).
HC underwent a brief standardized neuropsychological assessment (Mini‐Mental State Examination ≥27/30); psychiatric or other neurological illnesses were considered exclusion criteria.
Olfactory swabbing was performed on each subject to evaluate the TDP43‐SAA and TDP‐43 immunocytochemical pattern.
The study was conducted in accordance with the revised Declaration of Helsinki and Good Clinical Practice guidelines and was approved by the ethics committees of Verona (N28917) and Brescia (NP4541) hospitals.
The study was compliant with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline requirements.
2.2. Brain specimens collection and preparation
Post mortem frozen frontal lobe brain samples were homogenized (10% w/v) in lysis buffer (100 mM sodium chloride, 10 mM ethylenediaminetetraacetic acid [EDTA], 0.5% Nonidet P‐40, 0.5% sodium deoxycholate, 10 mM Tris, and pH 7.4). The brain homogenates were then clarified by centrifugation at 6000 g for 5 minutes at 4°C and the supernatants were collected, aliquoted, and stored at −80°C until SAA analysis. Supernatants were serially diluted in phosphate‐buffered saline (PBS) before SAA.
2.3. Olfactory mucosa sample collection and preparation
Nasal swab procedures were performed by otolaryngologists using FLOQBrushes (Copan Italia). The olfactory mucosa collection was carried out at the level of the agger nasi, as previously reported. 21 After this procedure, the swabs were placed in polypropylene tubes containing either saline or fixative (Diacyte, Diapath) solutions and then sealed. The tubes were then vortexed and centrifuged at 1369 g for 15 minutes. The resultant olfactory mucosa pellets in saline solution were frozen and kept at −80°C for SAA. Prior to SAA, olfactory mucosa samples were thawed, and a disposable inoculating loop (Fisherbrand) was used to transfer approximately 1 μL of the pellet into a tube containing 120 μL PBS. This tube was then sonicated at 120 W (Digital ultrasonic bath Mod.DU‐32, Argo Lab) until the pellet was fully dispersed. For immunocytochemical analysis, fixed cells were counted with Countess II FL Automated Cell Counters (Invitrogen) and diluted if necessary. The cell suspension was cytocentrifuged onto slides using a cytospin (CYTOSPIN IV, AHSI) and the slides were kept frozen until immunostaining was carried out. 14
RESEARCH IN CONTEXT
Systematic review: The authors reviewed the literature using traditional (e.g., PubMed) sources. The identification of peripheral markers for TAR DNA‐binding protein 43 (TDP‐43) in frontotemporal lobar degeneration (FTLD) remains challenging. Olfactory mucosa may represent a valuable source for testing TDP‐43 aggregation capacity.
Interpretation: TDP‐43 aggregates can be detectable in olfactory mucosa of patients with frontotemporal lobar degeneration with TDP‐43–immunoreactive pathology (FTLD‐TDP) by TDP‐43 seeding amplification assay (TDP43‐SAA). TDP43‐SAA–positive samples presented intracellular deposits of phosphorylated TDP‐43 in the cytoplasm of olfactory neural cells. These findings suggest that TDP43‐SAA might be a useful test for identifying and monitoring FTLD‐TDP in living patients.
Future directions: Future studies are aimed to assess accuracy of the assay in larger patient cohorts and analyze biological features and seeding kinetics of olfactory mucosa TDP‐43 aggregates.
2.4. TDP43‐SAA
The recombinant TDP‐43 substrate was obtained from the laboratory of Prion Biology at the Scuola Internazionale Superiore di Studi Avanzati (SISSA) in Trieste and prepared as previously described. 10
SAA reactions were performed in untreated 96‐well plates with a clear bottom (Nunc MicroWell 96‐Well Optical‐Bottom Plates, Thermo Scientific). Each well was preloaded with a single glass bead (3 mm in diameter, Sigma). The reaction mix (0.5 M guanidine hydrochloride [Gdn‐HCl], 25 mM Tris‐HCl, 5 mM EDTA, 10 μM Thioflavin [ThT]), and 0.00924 mg/mL [0.61 μM] or 0.01 mg/mL [0.67 μM] TDP‐43 of recombinant HuTDP‐43[263‐414] were used for olfactory mucosa and brain homogenate sample analyses, respectively. For brain homogenate‐seeded reactions, 2 μL of diluted brain homogenate was added to 198 μL of the reaction mix; for olfactory mucosa‐seeded reactions, 4 μL of diluted olfactory mucosa was added to 196 μL of the reaction mix. The plate was subsequently sealed with a plate sealer film (Nalgene Nunc International) and incubated at 40°C for 60 hours with intermittent cycles of 15 seconds of shaking (100 rpm double orbital) and 30 minutes of rest. ThT fluorescence measurements (450 ± 10 nm excitation and 480 ± 10 nm emission; bottom read) were taken every 30 minutes.
The criteria used for discriminating positive and negative SAA tests of brain homogenate and olfactory mucosa are akin to those previously described for prion SAA. 11 Briefly, all the data were normalized, and a ThT fluorescence threshold was calculated as the average fluorescence during the initial 10 hours of incubation plus 5 or 50 standard deviations (SD) for olfactory mucosa and brain homogenate samples, respectively. A sample was considered positive if at least two of the four replicate wells exceeded the calculated threshold. If only one of the quadruplicates crossed the threshold, the analysis was repeated. If the data were confirmed, the sample was considered an undetermined negative.
2.5. TDP‐43 immunocytochemical analyses
Nasal swabs were also processed for immunocytochemistry. Slides were incubated overnight at 4°C with primary antibodies: anti‐β3 tubulin (1:400; catalog number [cn] T2200; Sigma), anti‐β3 tubulin (1:400; cn 322600; Invitrogen), anti‐β4 tubulin (1:500; cn T7941; Sigma), anti‐CK18 (1:300; cn ab32118; Abcam), anti‐p62 (1:150; cn 610833; BD), anti‐PGP9.5 (1:600; cn GTX634797; Genetex), anti‐TARDBP (1:200; cn sc‐376311; Santa Cruz), anti‐TDP‐43 C‐term (1:200; cn T1580; Merck), anti‐TDP‐43 pS409/410 (1:800; cn TIP‐PTD‐P01; Cosmobio) 22 antibodies. The next day, slides were washed and incubated for 1 hour at room temperature with Alexa Fluor‐conjugated secondary antibodies (1:1000, Life Technologies). After washing, slides were incubated with DAPI (1:2000) for 5 minutes and mounted with DABCO (Sigma‐Aldrich). Images were acquired using an Axiolab fluorescence microscope (Zeiss).
2.6. Statistical analyses
SAA relative fluorescence responses were analyzed and plotted using Graphpad Prism 8.3 software. Endpoint‐dilution analysis was carried out and used to determine seeding doses as per methods of Spearman–Kärber as has been previously described. 23 The Fisher exact test was used to evaluate differences of TDP43‐SAA results between groups. P‐values < 0.05 were considered statistically significant. Statistical analyses were performed with SPSS 29.0 (IBM Corp.).
2.7. Data availability
Data can be made available upon reasonable request to the corresponding authors.
3. RESULTS
3.1. TDP43‐SAA optimization in autopsy‐confirmed cases
TDP43‐SAA was initially optimized using frontal cortex of FTLD‐TDP and non FTLD‐TDP cases. Seeding reactions were performed by testing various concentrations of the low complexity domain (LCD; aa 263‐414) of human recombinant TDP‐43. After a first round of optimization, we identified the optimal substrate concentration to discriminate between groups. When the reaction was performed using 0.01 mg/mL substrate, no increase in ThT fluorescence was observed in wells containing brain homogenates from control cases, which lacked TDP‐43 pathology; ThT fluorescence above a set threshold occurred instead within 30 hours in wells with brain homogenates originating from FTLD‐TDP cases (Figure 1A). More specifically, all 10 FTLD‐TDP cases exhibited seeding reactions at 30 hours, whereas 6 non FTLD‐TDP samples remained negative until 60 hours (Figure 1B and Table S1).
FIGURE 1.
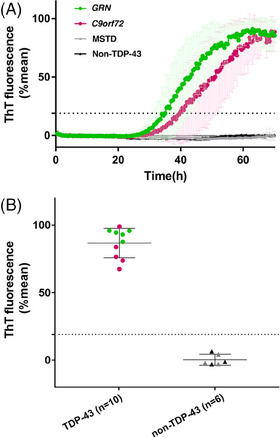
TDP‐43 seeding activity in frontal cortex of FTLD‐TDP and non–FTLD‐TDP. A, Curves of TDP43‐SAA from the frontal cortex of FTLD‐TDP (green line, GRN; purple line, C9orf72), MSTD (gray line), and non‐TDP‐43 (black line) cases. Curves derived from the average percentage of ThT fluorescence from four replicate reactions (normalized as described in the Methods section). Results are expressed as means of these averages (thick lines) and standard deviations (thin lines) as a function of SAA reaction time. B, Final average relative ThT fluorescence from four replicates readings obtained from frontal cortex of FTLD‐TDP (green dots, GRN; purple dots, C9orf72, i.e. TDP‐43) and non‐FTLD‐TDP (gray triangles, MSTD; black triangles, HC, i.e. non‐TDP‐43) at 60 hours. Bars represent the average ± standard deviation for each case. The dashed line marks the fluorescence threshold for positive results. C9orf72, chromosome 9 open reading frame 72 expansion; FTLD‐TDP, frontotemporal lobar degeneration with TDP‐43‐immunoreactive pathology; GRN, granulin variants; MSTD, multiple system tauopathy with dementia; SAA, seeding amplification assay; TDP‐43, TAR DNA‐binding protein–43‐immunoreactive pathology cases; ThT, thioflavin T
To evaluate the sensitivity of SAA under our optimized conditions, we performed SAA end‐point dilution analysis of representative frontal cortex sample originating from FTLD‐TDP and non FTLD‐TDP cases. Seeding reactions occurred with up to 10−3 brain tissue dilutions of FTLD‐TDP cases whereas no seeding activity was observed in the non FTLD‐TDP cases (see Figure S1 in supporting information). End‐point dilution analysis of brain‐tissue samples indicated that the concentrations of seeding doses resulting in 50% ThT‐positive replicate reactions (SD50) for these samples were 3 to 4 log10 SD50 per milligram of brain tissue.
3.2. TDP‐43 seeding detection by SAA in olfactory mucosa samples
After our optimization using autopsy‐verified brain homogenates we proceeded with testing olfactory mucosa. TDP‐43 seeding activity was detectable in olfactory mucosa samples of patients with FTD associated with FTLD‐TDP using SAA. The average percentage of ThT fluorescence readings from four replicate reactions, presented as a function of TDP43‐SAA reaction time, clearly increased in FTD cases associated with FTLD‐TDP (mean [SD] age, 58.9 [7.7]; female, 47.1%), compared to age‐matched HC (age, 58.4 [14.5]; female, 53.0%) and patients with MAPT variants (age, 47.7 [11.0]; female, 33.3%; Figure 2A).
FIGURE 2.
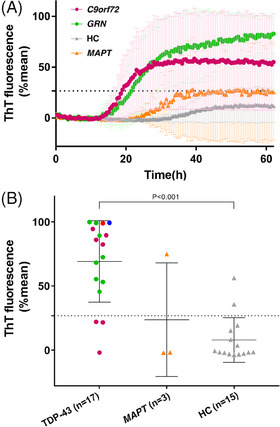
TDP‐43 seeding activity in olfactory mucosa of FTLD‐TDP and non–FTLD‐TDP cases. A, Curves of TDP43‐SAA from the olfactory mucosa of patients with FTLD‐TDP (green line = GRN; purple line = C9orf72), FTLD‐tau (orange line), and HC (gray line). Curves derived from the average percentage of ThT fluorescence from four replicate reactions (normalized as described in the methods section). Results are expressed as means of these averages (thick lines) and standard deviations (thin lines) as a function of SAA reaction time. B, Final average relative ThT fluorescence from four replicates readings obtained from the olfactory mucosa of FTLD‐TDP (green dots, GRN; purple dots, C9orf72; blue dot, TARDP; red dot, FTD‐ALS, i.e. TDP‐43), HC (gray triangles), and FTLD‐tau with MAPT variants (yellow triangles) at 60 hours. Bars represent the average ± standard deviation for each case. The dashed line marks the fluorescence threshold for positive results. C9orf72, chromosome 9 open reading frame 72 expansion; FTD‐ALS, frontotemporal dementia‐amyotrophic lateral sclerosis; FTLD‐tau; frontotemporal lobar degeneration with tau inclusions; FTLD‐TDP, frontotemporal lobar degeneration with TDP‐43‐immunoreactive pathology; GRN, granulin variants; HC, healthy controls; MAPT, microtubule associate protein tau variants; SAA, seeding amplification assay; TARDP, TAR DNA‐binding protein variants; TDP‐43, TAR DNA‐binding protein 43; ThT, thioflavin T
Olfactory mucosa samples from a total of 88.2% (14/17) of patients with FTLD‐TDP tested positive for the TDP43‐SAA, while 86.7% (13/15) of HC tested negative (P < 0.001). Olfactory mucosa samples from two out of three FTLD‐tau MAPT variant carriers tested negative (see Figure 2B and Table 1).
As shown in Figure 3, when examining FTD subgroups, all the GRN cases (including both p.T272Sfs*10 and p.C246X variants), the TARDP case, and the sporadic FTD‐ALS case tested positive for the TDP43‐SAA. However, three out of seven individuals with C9orf72 repeat expansions tested negative. Among these, two negative C9orf72 cases were the only subjects in this group at the prodromal disease stage (CDR plus NACC = 0.5), while the third presented with behavioral variant FTD and a CDR plus NACC score of 2. Regarding the MAPT variant carriers, the two FTLD‐tau patients with the P301L variant tested negative. In contrast, the patient with the IVS10+3 G > A variant tested positive. One of the two HC with a TDP43‐SAA positive test was 76 years old, and the possibility of a concomitant neurodegenerative disorder cannot be ruled out (see Table 1 for details).
FIGURE 3.
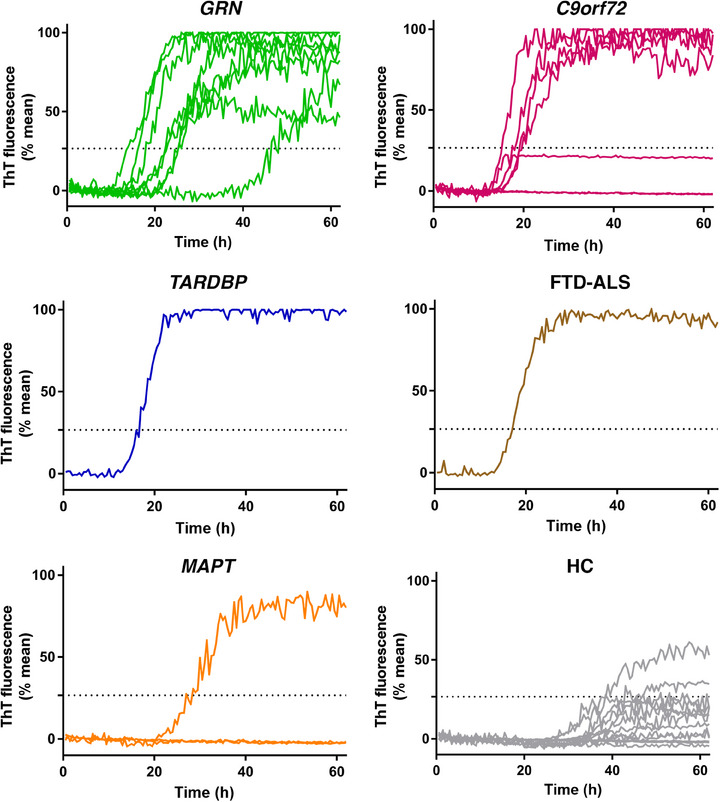
TDP‐43 seeding activity in olfactory mucosa in different subgroups. Representative curves of TDP43‐SAA from the olfactory mucosa, derived from the average percentage of ThT fluorescence from four replicate reactions (normalized as described in the Methods section) in each subject, grouped according to genetic trait or diagnosis. C9orf72, chromosome 9 open reading frame 72 expansion; FTD‐ALS, frontotemporal dementia‐amyotrophic lateral sclerosis; GRN, granulin variants; HC, healthy controls; MAPT, microtubule associate protein tau variants; SAA, seeding amplification assay; TARDP, TAR DNA‐binding protein variants; TDP‐43, TAR DNA‐binding protein 43; ThT, thioflavin T
3.3. Detection and characterization of TDP‐43 aggregates by immunocytochemical analysis in olfactory mucosa samples
Spurred by the positive results of TDP43‐SAA in olfactory mucosa, we conducted immunostaining analyses on olfactory swab samples. As shown in Figure 4, olfactory mucosa preparations from FTLD‐TDP patients showed rounded inclusions of phosphorylated TDP‐43 (pTDP‐43) within the cytoplasm or the apical section of ciliated cells, and infrequently in the nucleus. Additionally, cells positive for pTDP‐43 displayed ubiquitin‐binding protein p62 granular deposits in the cytoplasm or at the level of the cilia, which did not always colocalize with TDP‐43. We were unable to identify distinct patterns in the intracellular deposition of pTDP‐43 and p62 within FTLD‐TDP subgroups.
FIGURE 4.
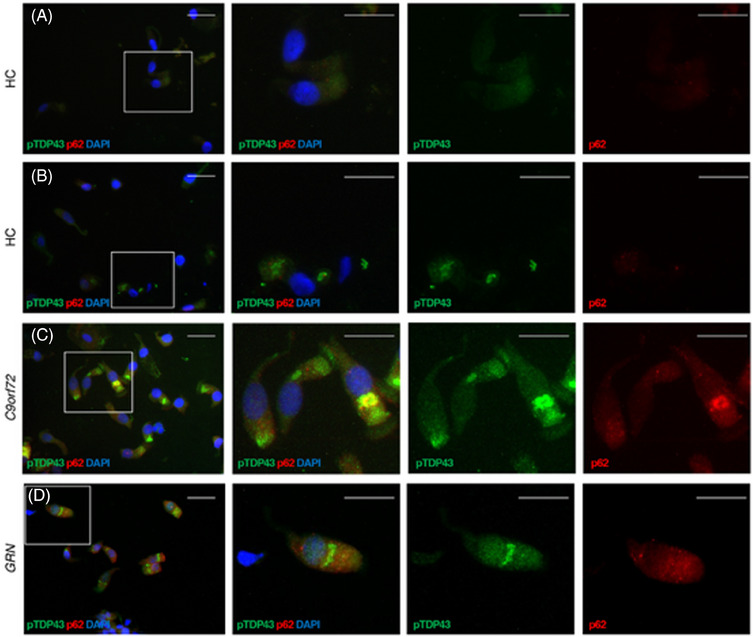
Distribution pattern of TDP‐43 aggregates. Cells obtained by olfactory brushing stained with phosphorylated TDP‐43 (pTDP43, green), p62 (red), and DAPI (blue). A and B, Two representative healthy controls (HC). C, One representative patient with C9orf72 repeat expansion (C9orf72). D, One representative patient with granulin variant (GRN). See Results section for details. Original 40X images (scale bar 25 μM) on the left, with insets on the three right columns. C9orf72, chromosome 9 open reading frame 72 expansion; TDP‐43, TAR DNA‐binding protein 43
We also found less intense cytoplasmic positivity for pTDP‐43 in three HC (see Figure 4), though these samples tested negative in the SAA. This suggests that pTDP‐43 deposits observed in the control samples were not aggregated into fibrillary structures.
We proceeded to characterize the olfactory mucosa cells positive for pTDP‐43 (see Figure S2 in supporting information). In earlier studies, we established that olfactory neurons and supporting cells can be distinguished based on their unique morphology and their positivity for β3 tubulin. 14 , 21 In our cases, numerous cells tested positive for β3 tubulin, yet pTDP‐43 deposits were primarily seen in ciliated neural cells in olfactory mucosa samples obtained from patients but not from HC (Figure S2). These cells likely represent glia‐like sustentacular cells, as they do not exhibit the typical thin, elongated shape of olfactory neurons. 24
To further characterize the neuronal population, we labeled olfactory mucosa samples with a marker for immature neurons, PGP9.5 25 (see Figure S2). A small number of neuron‐shaped cells showed PGP9.5 positivity at the plasma membrane, representing metaplastic olfactory neurons, but these cells did not exhibit pTDP‐43 positive inclusions.
Finally, to assess respiratory cell contamination, cell preparations were immunostained with anti‐β4 and anti‐β3 tubulin. Though most cells tested positive for β3 tubulin, rare globose cells with respiratory cell morphology expressed β4 tubulin in the cilia (see Figure S2).
These findings suggest that olfactory mucosa swabs are mainly composed by differentiated olfactory neural cells, including supporting cells and olfactory sensory neurons, with a minimal contamination from respiratory cells.
4. DISCUSSION
Despite recent advances in biomarker research, no fluid biomarkers or imaging tracers have yet demonstrated reliable utility for the diagnosis of FTLD‐TDP in patients with FTD during life. 20 , 26 Consequently, clinical trials for disease‐modifying agents have largely focused on genetically defined disease, often excluding most patients with FTD with sporadic disease from experimental treatments. 27
This study demonstrated that (1) TDP‐43 seeding activity can be detected in the olfactory mucosa, (2) TDP‐43 seeding activity shows a strong association with FTLD‐TDP cases, and (C) pTDP‐43 aggregates are specifically present in the cytoplasm of neural cells of the olfactory mucosa mirroring the characteristics of FTLD‐TDP neuropathology. 28
We set up TDP43‐SAA in brain tissues of a well‐characterized autopsy cohort, demonstrating 100% accuracy in differentiating cases with FTLD‐TDP and non–FTLD‐TDP pathology. This assay was applied to the study of olfactory mucosa of patients with a FTD diagnosis associated with FTLD‐TDP and HC. The TDP43‐SAA correctly identified 88.2% of patients with FTLD‐TDP. Interestingly, all three negative results were observed among patients with C9orf72 repeat expansion. However, these findings argue that lack of TDP‐43 seeding activity may also depend on the clinical stage and the subtype of neuronal inclusions (i.e., type of FTLD‐TDP). 29 , 30 Notably, the two samples of olfactory mucosa obtained from the C9orf72 repeat expansion subjects who were in the prodromal stage did not show pTDP‐43 deposits by immunocytochemical analysis.
The analysis of olfactory mucosa also demonstrated a good ability (86.7%) to correctly identify clinically evaluated HC. However, at this stage we cannot rule out the presence TDP‐43 co‐pathology associated with aging in the two HC resulting positive at TDP‐SAA.
Finally, we cannot draw definitive conclusions on the positive testing in a patient with FTLD‐tau with MAPT IVS10+3 variant, but intronic variants in the MAPT gene (i.e., IVS10+16) have been previously found to be associated with TDP‐43 co‐pathology and TDP‐43 inclusions. 31
Comparably to other proteins causative of neurodegenerative dementias, 32 , 33 TDP‐43 contains an amyloid‐like region, which likely makes it prone to aggregation via a prion‐like seeding mechanism, and this process might relate to disease pathogenesis and progression. 19 Previous studies have already explored the potential utility of testing TDP‐43 seeding activity in CSF 10 or serum TDP‐43 levels to identify FTLD‐TDP cases, 34 showing promising results.
More recently, plasma GFAP/neurofilament light chain ratio has been proposed as a potential marker to differentiate FTLD‐TDP and FTLD‐tau, 27 but validation at the single‐subject level is yet to be performed.
Our study shows that olfactory swabbing is a non‐invasive, easy‐to‐perform, and repeatable procedure to assess TDP‐43 seeding activity in olfactory mucosa of living individuals and to explore the biological features of TDP‐43 aggregates in olfactory neuroepithelium of subjects with FTD diagnosis associated with FTLD‐TDP pathology. In this view, peculiar characteristics of olfactory neurons are undergoing constant self‐renewal within a 2‐ to 3‐month timespan, making these cells suitable for research related to nerve cell degeneration and protein misfolding. 14
Therefore, we have demonstrated that deposits of pTDP‐43 are present in the cytoplasm of cells of neuronal origin, and mostly absent in the nuclei, mimicking TDP‐43 deposits in the brain tissue. 28 In particular, pTDP‐43 aggregates were mainly detected in glial‐like sustentacular cells but less in olfactory neurons. We speculated that this distinct involvement might be related to TDP‐43 cell tropism for aggregation or to a different half‐life of these neural cells.
Accordingly, the olfactory mucosa could be viewed as a dynamic peripheral neural platform exhibiting continuous replication and it provides an ideal setting for studying TDP‐43 pathological processes with different approaches to further understand pathogenetic mechanisms of disease.
As observed in a few olfactory mucosa samples of HC, cytoplasmic positivity for pTDP‐43 was not associated with TDP43‐SAA, indicating that TDP43 deposits were not aggregates in fibrillary structures. In this respect, it should be considered that recent evidence has hypothesized a new aspect of looking at TDP‐43 phosphorylation, not as a driver of TDP‐43 pathology but rather as a protective cellular response against TDP‐43 aggregation. 35
We acknowledge that our findings entail certain limitations. First, this is a pilot study conducted on a relatively small sample of well‐characterized FTD patients with monogenic disease. Larger cohorts will be required to confirm the accuracy of the TDP43‐SAA and validate these findings in clinical phenotypes with predictable pathology. Additionally, neuropathologic analysis of such patients would assess the type of FTLD‐TDP present, presence of co‐pathology, and atypical findings, which will lead to better interpret the data. Second, it is essential to point out that the lack of a group of patients with non‐monogenic FTD and other causative FTD‐related mutations may limit the generalizations of our results to all FTD forms associated with FTLD‐TDP pathology. Third, because TDP‐43 proteinopathy can be a common co‐pathology in various neurodegenerative diseases and aging‐related pathologies (i.e., limbic‐predominant age‐related TDP‐43), 36 we cannot definitively determine whether the occurrence of TDP‐43 pathology could be responsible for SAA positivity.
Despite these limitations, our study has demonstrated that TDP‐43 seeding activity and aggregates can be detected in the olfactory mucosa of patients with FTD associated with FTLD‐TDP pathology, suggesting that the TDP43‐SAA could serve as a useful tool for FTLD‐TDP identification and monitoring.
CONFLICT OF INTEREST STATEMENT
AB was partially supported by Fondazione Cariplo (grant n° 2021‐1516), and by the Fondation pour la Recherche sur Alzheimer. BB was partially supported by ADDF, unrelated to the present work; received personal fees from Alector, UCB, AviadiBio, Lilly, and Wave Life Sciences; and is listed as an inventor on a pending patent on the use of non‐invasive brain stimulation to increase cognitive functions in patients with neurodegenerative disorders. BG was partially supported by a R01‐AG075802 grant. MB, EB, CS, LS, AC, SC, EB, FG, MI, AS, CF, MF, KLN, LC, HJG, MPC, MP, AP, SM, GL, and GZ report no disclosures. This work was supported by project funding from Target ALS Foundation. Author disclosures are available in the supporting information.
CONSENT STATEMENT
The study was conducted in accordance with the revised Declaration of Helsinki and Good Clinical Practice guidelines and was approved by the ethics committees of Verona (N28917) and Brescia (NP4541) hospitals. Informed consent was obtained from all subjects included in the study.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
The authors wish to thank Drs Antonella Alberici and Ilenia Libri for support, Dr. Masato Hasegawa for providing pTDP‐43 antibody, Dr. Brad Glazier for critical reading of the manuscript, and patients and their families for participating in this study.
Fontana E, Bongianni M, Benussi A, et al. Detection of TDP‐43 seeding activity in the olfactory mucosa from patients with frontotemporal dementia. Alzheimer's Dement. 2024;20:1156–1165. 10.1002/alz.13541
Elena Fontana and Matilde Bongianni contributed equally to the present work.
Barbara Borroni and Gianluigi Zanusso contributed equally to the present work.
Contributor Information
Barbara Borroni, Email: bborroni@inwind.it.
Gianluigi Zanusso, Email: gianluigi.zanusso@univr.it.
REFERENCES
- 1. Cairns NJ, Bigio EH, Mackenzie IRA, et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 2007;114(1):5‐22. http://link.springer.com/10.1007/s00401‐007‐0237‐2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mackenzie IR, Neumann M. Reappraisal of TDP‐43 pathology in FTLD‐U subtypes. Acta Neuropathol. 2017;134(1):79‐96. doi: 10.1007/s00401-017-1716-8 [DOI] [PubMed] [Google Scholar]
- 3. Gorno‐Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006‐1014. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=21325651&retmode=ref&cmd=prlinks [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134(Pt 9):2456‐2477. doi: 10.1093/brain/awr179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Logroscino G, Piccininni M, Graff C, et al. Incidence of syndromes associated with frontotemporal lobar degeneration in 9 European countries. JAMA Neurol. 2023;80(3):279. doi: 10.1001/jamaneurol.2022.5128. Published online January 30, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moore KM, Nicholas J, Grossman M, et al. Age at symptom onset and death and disease duration in genetic frontotemporal dementia: an international retrospective cohort study. Lancet Neurol. 2020;19(2):145‐156. doi: 10.1016/S1474-4422(19)30394-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mackenzie IRA, Rademakers R. The role of transactive response DNA‐binding protein‐43 in amyotrophic lateral sclerosis and frontotemporal dementia. Curr Opin Neurol. 2008;21(6):693‐700. doi: 10.1097/WCO.0b013e3283168d1d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Borroni B, Padovani A. Dementia: a new algorithm for molecular diagnostics in FTLD. Nat Rev Neurol. 2013;9(5):241‐242. 10.1038/nrneurol.2013.72 [DOI] [PubMed] [Google Scholar]
- 9. Bongianni M, Orrù C, Groveman BR, et al. Diagnosis of human prion disease using real‐time quaking‐induced conversion testing of olfactory mucosa and cerebrospinal fluid samples. JAMA Neurol. 2017;74(2):155. http://archneur.jamanetwork.com/article.aspx?doi. doi: 10.1001/jamaneurol.2016.4614 [DOI] [PubMed] [Google Scholar]
- 10. Scialò C, Tran TH, Salzano G, et al. TDP‐43 real‐time quaking induced conversion reaction optimization and detection of seeding activity in CSF of amyotrophic lateral sclerosis and frontotemporal dementia patients. Brain Commun. 2020;2(2):fcaa142. doi: 10.1093/braincomms/fcaa142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Orrú CD, Bongianni M, Tonoli G, et al. A test for Creutzfeldt–Jakob disease using nasal brushings. N Engl J Med. 2014;371(6):519‐529. http://www.nejm.org/doi/abs/10.1056/NEJMoa1315200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hermann P, Appleby B, Brandel JP, et al. Biomarkers and diagnostic guidelines for sporadic Creutzfeldt‐Jakob disease. Lancet Neurol. 2021;20(3):235‐246. doi: 10.1016/S1474-4422(20)30477-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stefani A, Iranzo A, Holzknecht E, et al. Alpha‐synuclein seeds in olfactory mucosa of patients with isolated REM sleep behaviour disorder. Brain. 2021;144(4):1118‐1126. doi: 10.1093/brain/awab005 [DOI] [PubMed] [Google Scholar]
- 14. Brozzetti L, Sacchetto L, Cecchini MP, et al. Neurodegeneration‐associated proteins in human olfactory neurons collected by nasal brushing. Front Neurosci. 2020;14(March):1‐12. doi: 10.3389/fnins.2020.00145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Josephs KA, Dickson DW. TDP‐43 in the olfactory bulb in Alzheimer's disease. Neuropathol Appl Neurobiol. 2016;42(4):390‐393. doi: 10.1111/nan.12309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Takeda T, Uchihara T, Ohashi T, Seilhean D, Duyckaerts C, Uchiyama S, TDP‐43 Pathology Progression Along the Olfactory Pathway as a Possible Substrate for Olfactory Impairment in Amyotrophic Lateral Sclerosis.; 2015. http://links.lww.com/NEN/A733 [DOI] [PubMed]
- 17. Cracco L, Doud EH, Hallinan GI, et al. Distinguishing post‐translational modifications in dominantly inherited frontotemporal dementias: fTLD‐TDP Type A (GRN) vs Type B (C9orf72). Neuropathol Appl Neurobiol. 2022;48(6). doi: 10.1111/nan.12836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shi Y, Zhang W, Yang Y, et al. Structure‐based classification of tauopathies. Nature. 2021;598(7880):359‐363. doi: 10.1038/s41586-021-03911-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arseni D, Chen R, Murzin AG, et al. TDP‐43 forms amyloid filaments with a distinct fold in type A FTLD‐TDP. Nature. 2023;620(7975):898‐903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Borroni B, Benussi A, Archetti S, et al. Csf p‐tau181/tau ratio as biomarker for TDP pathology in frontotemporal dementia. Amyotroph Lateral Scler Frontotemporal Degener. 2015;16(1‐2):86‐91. doi: 10.3109/21678421.2014.971812 [DOI] [PubMed] [Google Scholar]
- 21. Bongianni M, Catalan M, Perra D, et al. Olfactory swab sampling optimization for α‐synuclein aggregate detection in patients with Parkinson's disease. Transl Neurodegener. 2022;11(1). doi: 10.1186/s40035-022-00311-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hasegawa M, Arai T, Nonaka T, et al. Phosphorylated TDP‐43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Ann Neurol. 2008;64(1):60‐70. doi: 10.1002/ana.21425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wilham JM, Orrú CD, Bessen RA, et al. Rapid end‐point quantitation of prion seeding activity with sensitivity comparable to bioassays. PLoS Pathog. 2010;6(12):e1001217. doi: 10.1371/journal.ppat.1001217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Choi R, Goldstein BJ. Olfactory epithelium: cells, clinical disorders, and insights from an adult stem cell niche. Laryngoscope Investig Otolaryngol. 2018;3(1):35‐42. doi: 10.1002/lio2.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Holbrook EH, Wu E, Curry WT, Lin DT, Schwob JE. Immunohistochemical characterization of human olfactory tissue. Laryngoscope. 2011;121(8):1687‐1701. doi: 10.1002/lary.21856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hu WT, Watts K, Grossman M, et al. Reduced CSF p‐Tau181 to Tau ratio is a biomarker for FTLD‐TDP. Neurology. 2013;81(22):1945‐1952. http://www.neurology.org/cgi/doi/10.1212/01.wnl.0000436625.63650.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cousins KAQ, Shaw LM, Chen‐Plotkin A, et al. Distinguishing frontotemporal lobar degeneration tau from TDP‐43 using plasma biomarkers. JAMA Neurol. 2022;79(11):1155. doi: 10.1001/jamaneurol.2022.3265. Published online 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Neumann M, Sampathu DM, Kwong LK, et al. Ubiquitinated TDP‐43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science (1979). 2006;314(5796):130‐133. doi: 10.1126/science.1134108 [DOI] [PubMed] [Google Scholar]
- 29. Neumann M, Lee EB, Mackenzie IR. Frontotemporal lobar degeneration TDP‐43‐immunoreactive pathological subtypes: clinical and mechanistic significance. Adv Exp Med Biol. 2021;1281:201‐217. doi: 10.1007/978-3-030-51140-1_13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mackenzie IRA, Frick P, Neumann M. The neuropathology associated with repeat expansions in the C9ORF72 gene. Acta Neuropathol. 2013;127(3):347‐357. http://link.springer.com/10.1007/s00401‐013‐1232‐4 [DOI] [PubMed] [Google Scholar]
- 31. Forrest SL, Shepherd CE, McCann H, Kwok JB, Halliday GM, Kril JJ. Globular glial tauopathy with a mutation in MAPT and unusual TDP‐43 proteinopathy in a patient with behavioural‐variant frontotemporal dementia. Acta Neuropathol. 2021;141(5):791‐794. doi: 10.1007/s00401-021-02297-0 [DOI] [PubMed] [Google Scholar]
- 32. Goedert M, Eisenberg DS, Crowther RA. Propagation of tau aggregates and neurodegeneration. Annu Rev Neurosci. 2017;40:189‐210. [DOI] [PubMed] [Google Scholar]
- 33. Zhu J, Pittman S, Dhavale D, et al. VCP suppresses proteopathic seeding in neurons. Mol Neurodegener. 2022;17(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Katisko K, Huber N, Kokkola T, et al. Serum total TDP‐43 levels are decreased in frontotemporal dementia patients with C9orf72 repeat expansion or concomitant motoneuron disease phenotype. Alzheimers Res Ther. 2022;14(1). doi: 10.1186/s13195-022-01091-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gruijs da Silva LA, Simonetti F, Hutten S, et al. Disease‐linked TDP‐43 hyperphosphorylation suppresses TDP‐43 condensation and aggregation. EMBO J. 2022;41(8):e108443. doi: 10.15252/embj.2021108443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nelson PT, Brayne C, Flanagan ME, et al. Frequency of LATE neuropathologic change across the spectrum of Alzheimer's disease neuropathology: combined data from 13 community‐based or population‐based autopsy cohorts. Acta Neuropathol. 2022;144(1):27‐44. doi: 10.1007/s00401-022-02444-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Data Availability Statement
Data can be made available upon reasonable request to the corresponding authors.


