Abstract
INTRODUCTION
Dementia is an emergent health priority for Indigenous peoples worldwide, yet little is known about disease drivers and protective factors.
METHODS
Database searches were conducted in March 2022 to identify original publications on risk, protective, genetic, neuroradiological, and biological factors related to dementia and cognitive impairment involving Indigenous peoples.
RESULTS
Modifiable risk factors featured across multiple studies include childhood adversity, hearing loss, low education attainment, unskilled work history, stroke, head injury, epilepsy, diabetes, hypertension, hyperlipidemia, depression, low BMI, poor mobility, and continence issues. Non‐modifiable risk factors included increasing age, sex, and genetic polymorphisms. Education, ex‐smoking, physical and social activity, and engagement with cultural or religious practices were highlighted as potential protective factors. There is a paucity of research on dementia biomarkers involving Indigenous peoples.
DISCUSSION
Greater understanding of modifiable factors and biomarkers of dementia can assist in strength‐based models to promote healthy ageing and cognition for Indigenous peoples.
Keywords: biomarkers, dementia, first nations, Indigenous, prevention
1. INTRODUCTION
Dementia is characterized by a progressive decline in cognition that translates into the loss of functional abilities and independence. It is a growing global health challenge with impacts on individuals, families, and systems of care. There are currently more than 50 million people living with dementia worldwide, with numbers estimated to exceed 152 million by 2050. 1 For Indigenous peoples, who comprise 6% of the world's population, dementia is an emerging health priority in light of increasing survivorship to old age. 2
The United Nations recognizes Indigenous peoples as those who, by self‐identification, have a “historical continuity with a given region prior to colonization and strong link to their lands” and maintain “distinct languages, cultures, beliefs, and knowledge systems” from the mainstream or dominant society. 3 Worldwide, Indigenous peoples often experience inequities in social and biological determinants of health as a legacy of colonization and corrosion of traditional ways of life. Adverse social determinants of disease such as intergeneration trauma, racial discrimination, cultural disconnection, land dispossession, and reduced access to health services and education lead to a greater burden of non‐communicable chronic diseases, including dementia. 2
There is currently no widely available and clinically effective disease‐modifying treatment for dementia and as such, modification of risk and protective factors are crucially important in strategies to promote healthy aging and prevent cognitive decline. The 2020 Lancet Commission report on dementia, prevention, intervention, and care suggests that, at a population level, up to 40% of dementia could be preventable by eliminating 12 risk factors. 4 It identified less education in early life, hypertension, hearing impairment, smoking, obesity, depression, physical inactivity, diabetes, social isolation, traumatic brain injury, excessive alcohol consumption, and air pollution as key risk factors in the development of cognitive problems. 4 These risk factors feature in the health and social profile of Indigenous peoples however, a greater understanding of the risk and protective factors unique to specific Indigenous groups and those shared across First Nation populations is important in developing more effective approaches to decrease the burden and sequalae of dementia. 2
The pathogenesis of dementia often begins decades before the onset of symptoms and changes in brain pathology detectable in midlife may offer insights into dementia risk in the future. Gray and white matter changes on magnetic resonance imaging (MRI) can support clinical diagnosis and assist in the differentiation of one type of dementia from another. Alzheimer's disease‐specific biomarkers such as amyloid beta and tau have been demonstrated using positron emission tomography (PET) imaging and in fluids (cerebrospinal fluid and plasma). 5 , 6 These disease‐specific biomarkers, alongside microvascular and inflammatory biomarkers involved in cellular signaling, immune responses, neuronal support, and apoptosis have also been studied in peripheral blood. 7 Advances in scientific understanding of genetic, neuroimaging, and biological markers augment understanding of dementia phenotype as a complex interaction between genetics, biology, and exposures across the life‐course. They may, in the future, have greater utility in clinical practice to identify those at higher risk of a neurodegenerative process, improve timely and accurate diagnosis, and monitor disease progression and effects of interventions. 8 Despite great strides in the field, the relationship between biomarkers and ethnoracial factors remains understudied. Large studies on cognition and aging such as the Australian Imaging Biomarkers and Lifestyle Study of Ageing (AIBL) and Alzheimer's disease neuroimaging initiative (ADNI) from North America, do not contain a broad representation of ethnoracial populations. 9 , 10
Our aim was to describe and evaluate the evidence base for risk, protective, and biomarkers associated with dementia and cognitive impairment in Indigenous peoples around the world. We also provide an update of prevalence and incidence of dementia and cognitive impairment in Indigenous populations. We build on existing systematic reviews on this subject in our synthesis of emerging research. 11 , 12 , 13 To our knowledge, this will be the first review of risk, protective and biomarkers of dementia and cognitive impairment involving Indigenous peoples.
RESEARCH IN CONTEXT
Systematic review: The authors reviewed the evidence base for risk, protective and biomarkers of dementia in Indigenous peoples worldwide. This is the first review of biomarkers of dementia and cognitive impairment involving Indigenous peoples.
Interpretation: Database searches yielded 1567 titles for screening, 132 articles for review and 39 were included in the systematic review. Prevalence rates of dementia varied widely from 0.6% to 37.2% with rates of MCI/CIND between 4.4% and 27.4% for those aged ≥60 years. There are many shared determinants of health and illness between Indigenous populations however, appreciation of unique population profiles can assist in the development of targeted strength‐based models to promote cognitive health and prevent impairment over the life‐course.
Future directions: Increased research is needed on modifiable protective factors and biomarkers of dementia involving Indigenous peoples.
2. METHODS
2.1. Search strategy
The protocol for this review was registered in PROSPERO (CRD42020207449). A literature search of Medline, Embase, CINAHL, Global Health, Emcare, and PsycINFO databases was conducted using the OVID platform on the 15th of March 2022. Search terms included all descriptors related to: Indigenous persons, dementia, cognitive impairment, prevalence, incidence, risk factor, protective factors, genetic markers, neuroimaging, and biomarkers. Indigenous population search terms were based on the Lancet‐Lowitja Institute Global Collaboration publication on the health of Indigenous and tribal peoples and checked against works in the same canon. 2 This review was limited to age‐related dementias such as Alzheimer's dementia (AD), vascular dementia (VaD), Lewy body dementia (LBD), and fronto‐temporal dementia (FTD), and does not address dementia secondary to primary neurological conditions (e.g., amyotrophic lateral sclerosis), retroviruses (e.g., human immunodeficiency virus), or other pathogens (e.g., syphilis). Cognitive impairment encompassed cognitive impairment not dementia (CIND) and mild cognitive impairment (MCI) diagnoses and pertained to those with evidence of cognitive decline from baseline without functional loss to meet a diagnosis of dementia. CIND and MCI were included in this review as they may represent an early stage of dementia with shared risk or protective factors. The full search strategy is detailed in Appendix SA. The review is reported according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA). 14
2.2. Study selection and data extraction strategy
References from the literature search underwent screening by two reviewers (H.N. and K.B.) based on predefined inclusion and exclusion criteria. We included original studies that focused on a recognized Indigenous population that comprised >10% of the study cohort. Indigenous peoples were chosen with reference to the UN statement and the Lancet‐Lowitja Institute Global Collaboration publication on the health of Indigenous and tribal peoples across all world continents, except Antarctica, with emphasis on self‐identification, connection to country, and acceptance within a cultural community. 2 , 3 Studies included dementia and/or cognitive impairment as outcome measures of interest and contained information on: prevalence, incidence, risk factors, protective factors, and/or biomarkers. Single case studies, dissertation abstracts, reviews and articles not available in English through publication or in translation were excluded. Risk factors are defined as identifiable events or conditions associated with an increased probability of disease. Protective factors are those associated with a decreased probability of disease. Studies involving genetic markers such as apolipoprotein E (APOE) and biological markers such as homocysteine were included when they were studied in relation to the outcomes of interest. Biomarkers were taken to be any molecule in the brain or biological fluids associated with a disease state that facilitates its diagnosis. Structural and functional neuroimaging markers that are important in diagnosis as a marker of disease states of interest were also included. These encompass descriptors of regional cerebral atrophy and white matter changes on MRI as well as fluorodeoxyglucose and amyloid radiotracer avidity using functional and molecular imaging modalities. Discrepancies were resolved through discussion with a third reviewer (D.L.) to achieve consensus.
2.3. Quality assessment
Systematic appraisal of included articles was performed using a checklist adapted from the National Institute of Health (NIH) Study Quality Assessment Tool for Observational Cohort and Cross‐Sectional Studies. 15 Engagement, governance, or co‐authorship with Indigenous people/communities/organizations and reporting of positive outcomes (strength‐based model) was included to assess the reporting of Indigenous health research in line with the CONSIDER Statement and Aboriginal and Torres Strait Islander quality appraisal tool. 16 , 17 Studies were assigned a quality rating of good, fair, or poor (Appendix SB). Discrepancies were resolved through discussion with the third reviewer (D.L.).
2.4. Data synthesis
The articles were observed to be highly heterogeneous and focused on different aspects of the research question. As such, pooling of the results in a meta‐analysis was not possible due to substantial variance in focus, methods, and outcome measures employed. The findings were therefore synthesized in descriptive form. Prevalence rates were not standardized for comparison between Indigenous populations due to the lack of an appropriate reference cohort. Indeed, using any one Indigenous group as a reference would assume the sameness of all Indigenous cohorts with one another, and using a non‐Indigenous cohort would omit differences between Indigenous and non‐Indigenous populations.
3. RESULTS
3.1. Study selection
Database searches identified 1567 articles after removal of duplications as summarized in the PRISMA flowchart (Figure 1). After screening of titles and abstracts, 132 articles underwent full‐text reviews and 39 met a priori inclusion criteria and were included in this systematic review.
FIGURE 1.
PRISMA flowchart.
3.2. Study characteristics
Of the 39 studies included in this systematic review, 26 studies reported on risk, protective or biomarkers of dementia or cognitive impairment involving an Indigenous population (Table 1). The majority of the included articles were cross‐sectional, case‐control, or cohort studies, and only two involved longitudinal follow‐ups. There were 18 studies on prevalence and 4 on incidence of dementia or MCI (Table 1). Indigenous populations studied included Aboriginal and Torres Strait Islander peoples of Australia, First Nations people of Canada, Choctaw and Cherokee Indians in the United States, Native Hawaiians, Chamorros of Guam, Melanau people of East Malaysia, Tibetans residing in the Qinghai‐Tibet plateau, the Otomi tribe native to Mexico, the Mamirauá and Amanã peoples of the Amazonian Basin in Brazil, and the Tsimane and Moseten tribespeople Indigenous to Bolivia. A Peruvian cohort with high Amerindian genetic ancestry was also included.
TABLE 1.
Studies included in this systematic review.
| Study | Prevalence and/or incidence | Risk, protective, genetic, and/or biomarkers | Both | |
|---|---|---|---|---|
| Asia | ||||
| 1. | Pu'un et al., 2014 18 | ⬤ | ⬤ | ⬤ |
| 2. | Huang et al., 2016 19 | ⬤ | ⬤ | ⬤ |
| 3. | Chen et al., 2021 20 | ⬤ | ||
| Australia & Oceania | ||||
| 4. | Smith et al., 2008 21 | ⬤ | ||
| 5. | Smith et al., 2010 22 | ⬤ | ||
| 6. | Cotter et al., 2012 23 | ⬤ | ||
| 7. | Li et al., 2014 24 | ⬤ | ||
| 8. | Radford et al., 2015 25 | ⬤ | ||
| 9. | Russell et al., 2016 26 | ⬤ | ||
| 10. | LoGiudice et al., 2016 27 | ⬤ | ⬤ | ⬤ |
| 11. | Radford et al., 2017 28 | ⬤ | ||
| 12. | Radford et al., 2019 29 | ⬤ | ||
| 13. | Derrig et al., 2020 30 | ⬤ | ⬤ | ⬤ |
| 14. | Russell et al., 2021 31 | ⬤ | ||
| 15. | Ma'u et al., 2021 32 | ⬤ | ||
| 16. | Russell et al., 2022 33 | ⬤ | ||
| 17. | Thompson et al., 2022 34 | ⬤ | ||
| 18. | Lavrencic et al., 2022 35 | ⬤ | ⬤ | ⬤ |
| North America | ||||
| 19. | Hendrie et al., 1993 36 | ⬤ | ||
| 20. | Rosenberg et al., 1996 37 | ⬤ | ||
| 21. | Henderson et al., 2002 38 | ⬤ | ||
| 22. | Borenstein et al., 2007 39 | ⬤ | ||
| 23. | Sundar et al., 2007 40 | ⬤ | ||
| 24. | Galasko et al., 2007 41 | ⬤ | ⬤ | ⬤ |
| 25. | Jervis et al., 2007 42 | ⬤ | ||
| 26. | Weiner et al., 2008 43 | ⬤ | ||
| 27. | Weiner et al., 2009 44 | ⬤ | ||
| 28. | Weiner et al., 2011 45 | ⬤ | ||
| 29. | Jacklin et al., 2012 46 | ⬤ | ||
| 30. | MacDonald et al., 2015 47 | ⬤ | ||
| 31. | Mayeda et al., 2016 48 | ⬤ | ||
| 32. | Kirkpatrick et al., 2019 49 | ⬤ | ||
| 33. | Carty et al., 2020 50 | ⬤ | ⬤ | ⬤ |
| 34. | Smith et al., 2021 51 | ⬤ | ||
| 35. | Suchy‐Dicey et al., 2021 52 | ⬤ | ||
| South America | ||||
| 36. | Stryjer et al., 2011 53 | ⬤ | ||
| 37. | Brucki et al., 2014 54 | ⬤ | ||
| 38. | Marca Ysabel et al., 2021 55 | ⬤ | ||
| 39. | Gatz et al., 2022 56 | ⬤ | ⬤ | ⬤ |
Odds ratios (ORs) were the most frequent means of assessing the relationship between variables of interest and dementia or cognitive impairment. MacDonald et al. and Ma'u et al. reported on the Population Attributable Fractions (PAF) of modifiable risk factors and their contribution to dementia in an Indigenous Canadian and Maori cohort, respectively. 32 , 47 These PAF calculations utilize the strength of each risk factor, its prevalence in a population, and overlap within the same person to estimate the proportional reduction in dementia at a population level if exposure to the risk factor was eliminated. 57
3.3. Quality assessments
Quality assessments were undertaken on studies on risk, protective factors, and biomarkers (Table 2). Fifteen out of 26 studies were assessed to be of “Good” quality, 9 “Fair” quality, and 2 “Poor” quality based on the modified NIH tool. Engagement, governance, or co‐authorship with Indigenous people/communities/organizations was explicit in 17 studies and 8 studies modeled strength‐based or positive outcomes. A summary of quality assessments is provided in the Appendices (Appendix SC).
TABLE 2.
Descriptive summary of included studies.
| Author, year | Title | Study design | Indigenous population | Age; mean/median age (SD) | Outcomes of interest | Risk or protective factors | Genetic or biomarker | QA |
|---|---|---|---|---|---|---|---|---|
| Asia | ||||||||
| Pu'un et al., 2014 18 | Dementia among elderly Melanau: A community survey of an Indigenous people in East Malaysia | Cross‐sectional |
Melanau of Malaysia N = 344 |
≥60 years; median 70.4 (±6.7) | Dementia | Dementia was associated with age (OR 1.19 a , 95% CI 1.12–1.27), no education (OR 7.56 a 95%CI 1.70–33.57) and multiple cardiovascular illnesses (OR 3.76 a , 95% CI 1.25–11.28). | Fair | |
| Huang et al., 2016 19 | Lower prevalence of Alzheimer's disease among Tibetans: Association with religious and genetic factors | Cross‐sectional |
Tibetans living in the Qinghai‐Tibet plateau N = 3974 |
>60 years | AD | Age groups 75−79 (OR 4.04, 95% CI 1.27–12.80), 80−84 (OR 3.58, 95% CI 0.96–13.35), ≥85 (OR 35.73, 95% CI 9.64–132.42), eating beef (OR 6.17, 95% CI 2.46–15.49), and head trauma (OR 44.62, 95% CI 2.55–782.17) were associated with AD. Kowtow (0.11, 95% CI 0.027–0.44), turning prayer beads (0.20, 95% CI 0.084–0.47), and eating chicken (OR 0.05, 95% CI 0.005–0.52) were negatively associated with AD. | CLU genotypes AA+GA of rs2279590 were associated with AD (OR 4.48, 95% CI 1.07–18.79). Genotypes GG+GC of rs9331888 (OR 0.18, 95% CI 0.038–0.89) and kowtow (OR 0.20, 95% CI 0.046–0.89) were negatively associated with AD. There was no difference in the distribution of APOE alleles between those with and without dementia. | Good |
| Chen et al., 2020 20 | The association between neprilysin gene polymorphisms and Alzheimer's disease in Tibetan populations | Case‐ control |
Tibetans N = 212 |
≥50 years; Mean 68.4 years (±8.3) for AD cohort | AD | No significant difference in gender, age (matched), history of hypertension and T2DM between AD and control groups. | No significant correlation between six polymorphisms of NEP gene loci (rs9829757, rs1816558, rs6776185, rs3736187, rs701109, rs989692) and AD. Allele C of NEP gene locus (rs701109) and allele T of gene locus (rs3736187) were associated with males with AD (p < 0.05). APOE ε4 was not detected in either cases or control groups. | Fair |
| Australia & Oceania | ||||||||
| Smith et al., 2010 22 | Factors associated with dementia in Aboriginal Australians | Cross‐sectional |
Aboriginal Australians N = 363 |
≥ 45 years; mean 60.9 (±11.9) | Dementia | Older age, male gender (OR 3.1, 95% CI 1.4‐6.8), no formal education (OR 2.7, 95% CI 1.1‐6.7), current smoking (OR a 4.5, 95% CI 1.1‐18.6), previous stroke (OR a 17.9, 95% CI 5.9‐49.7), epilepsy (OR a 33.5, 95% CI 4.8‐232.3), head injury (OR a 4.0, 95% CI 1.7‐9.4), poor mobility (OR 13.4 a , 95% CI 4.1‐43.9), daytime incontinence (OR 116.8 a , 95% CI 21.9‐622.8), incontinence at night (OR 87.4 a , 95% CI 18.4‐415.7), any urinary problems including urinary tract infections, incontinence (OR 4.2 a , 95% CI 1.8‐10.2) and falls (OR 2.7 a , 95% CI 1.2‐6.1) was associated with dementia. | N/A | Good |
| LoGiudice et al., 2016 27 | Incidence and predictors of cognitive impairment and dementia in Aboriginal Australians: A follow‐up study of 5 years | Longitudinal cohort |
Aboriginal peoples in Australia N = 189 |
≥ 45 years Mean 65.4 (±10.3) | Dementia; MCI |
Factors associated with CIND or dementia were age (OR 1.12 a , 95% CI 1.06−1.17), poor mobility (OR 3.08 a , 95% CI 1.09, 8.72), head injury (OR 5.22 a , 95% CI 1.85−14.70), analgesic medication (OR 3.60 a , 95% CI 1.35−9.62), and BMI (OR 0.90 a , 95%CI 0.82−0.98). ***** Factors associated with incident MCI or dementia from normal were stroke (OR 9.54 a , 95% CI 2.39‐38.13), head injury (OR 3.74 a , 95% CI 1.14‐12.24), analgesic medication (OR13.48 a , 95% CI 3.16‐57.44), BMI (OR 0.90 a , 95% CI 0.81‐1.00) and higher SBP (OR 1.03 a , 95% CI 1.00‐1.06). |
N/A | Good |
| Radford et al., 2017 28 | Childhood stress and adversity is associated with late‐life dementia in Aboriginal Australians | Cross‐sectional |
Aboriginal and Torres Strait Islander people of Australia N = 296 |
≥60 years; median 66.6 (±6.3) | Dementia; AD |
Each standard deviation increase in CTQ scores were associated with all‐cause dementia (OR 1.70, 95% CI 1.14−2.54) and AD (OR 1.77, 95% CI 1.08−2.91). CTQ scores and dementia remained significant after controlling for depression and anxiety variables (OR 1.61 a , 95% CI 1.05−2.45). There were no significant associations between CTQ scores and smoking, alcohol abuse, diabetes, or cardiovascular risk factors. |
N/A | Good |
| Radford et al., 2019 29 | Factors associated with the high prevalence of dementia in older Aboriginal Australians | Cross‐sectional |
Aboriginal and Torres Strait Islander people of Australia N = 336 |
≥60 years; median 66.6 (±6.3) | Dementia; AD; MCI |
Factors associated with all‐cause dementia included age (OR 2.88 a , 95% CI 1.93−4.31), childhood trauma (OR 1.59 a , 95% CI 1.06−2.40), unskilled work (OR 2.41 a , 95% CI 1.12−5.19), stroke (OR 3.35 a , 95% CI 1.57−7.15), head injury with loss of consciousness (OR 0.58 a , 95% CI 1.20−5.56), epilepsy (OR 3.17 a , 95% CI 0.88−11.49), high risk current alcohol use (OR 3.23 a , 95% CI 1.17‐8.93), depression (OR 1.60 a , 95% CI 1.08‐2.37), low BMI (OR 3.44, 95% CI 1.38‐8.61), mobility impairment (OR 2.40, 95% CI 1.20‐4.80), ADL impairment (OR 3.98 a , |
N/A | Good |
|
95% CI 2.66‐5.96), incontinence (OR 5.56, 95% CI 2.63−11.76, hospitalization in the past year (OR 2.09 a , 95% CI 1.06‐4.14), residing in residential care (47.72, 95% CI 9.76−233.34), living alone (OR 2.13 a , 95% CI 1.03‐4.43) and loneliness (OR 3.86 a , 95% CI 1.70‐8.75. Mild physical activity (OR 0.34 a 95% CI 0.14‐0.79), moderate physical activity (OR 0.44 a 95% CI 0.19‐0.99 and social activities (OR 0.41 a 95% CI 0.27‐0.62) were associated with a lower odds of dementia. ***** Age (OR 3.31 a 95% 2.01‐5.46), childhood trauma (OR 1.90 a , 95% CI 1.15‐3.16) and stroke (OR 3.08, 95% CI 1.21‐7.88) were significant independent factors associated with probable or possible AD. Lifetime low risk alcohol consumption was associated with a lower likelihood of AD relative to abstinence (OR 0.22 a , 95% CI 0.05‐1.00). |
||||||||
| Derrig et al., 2020 30 | Mild cognitive impairment in Aboriginal Australians | Cross‐sectional |
Aboriginal and Torres Strait Islander peoples of Australia N = 287 |
≥60 years; Mean 65.9 years (±5.6) | aMCI; non‐aMCI | aMCI was associated with older age (OR 1.68 a , 95% CI 1.12‐2.53), head injury (OR 3.19 a , 95% CI 1.35‐7.56), symptoms of depression (OR 1.5 a 2, 95% CI: 1.04‐2.24), and lower blood pressure (OR 0.53 a , 95% CI: 0.33‐0.86). naMCI was associated with low education (OR = 4.46 a , 95% CI: 1.53 to 13.05), unskilled work history (OR a = 5.62, 95% CI: 2.07 to 13.90), higher body mass index (OR = 1.99 a , 95% CI: 1.30 to 3.04), and moderate to severe hearing loss (OR = 2.82 a , 95% CI: 1.06 to 7.55). | N/A | Good |
| Ma'u et al., 2021 32 | Differences in the potential for dementia prevention between major ethnic groups within one country: A cross sectional analysis of population attributable fraction of potentially modifiable risk factors in New Zealand | Cross‐sectional |
Māori people of New Zealand N = 1286 |
N/A | Dementia | The total weighted PAF for dementia was 51.4% for Maori people. PAF contributions for 12 risk factors were: obesity (7.3%), hearing loss (6.5%), education (5.6%), social isolation (5.1%), hypertension (5.0%), physical inactivity (5.0%), traumatic brain injury (3.5%), smoking (4.3%), depression (4.2%), diabetes (2.4%), air pollution (1.8%), alcohol (0.7%). | N/A | Good |
| Russell et al., 2022 33 | Factors associated with the increased risk of dementia found in the Torres Strait | Cross‐sectional |
Aboriginal and Torres Strait Islander peoples of Australia N = 274 |
≥45 years; Mean 65.1 years (±10.8) | Dementia | Age (OR 1.14 a , 95% CI 1.09‐1.20), chronic kidney disease (OR 2.77 a , 95% CI 1.11‐6.91), cerebrovascular disease (OR = 32.47, 95% CI 8.99‐117.31), higher ICIQ score (OR 1.23 a , 95% CI 1.01‐1.49) were associated with all‐cause dementia (p < 0.05). Lower education, poor mobility, hearing impairment, diabetes, dyslipidaemia and pain and falls screening test scores were associated with dementia in univariate analyses but effects were attenuated in multivariable models. | Good | |
| Thompson et al., 2022 34 | Using health check data to understand risks for dementia and cognitive impairment among Torres Strait Islander and Aboriginal peoples in Northern Queensland—A data linkage study | Cohort | Torres Strait Islander and Aboriginal population in Australia | Mean 48.9 (±10.8) years | Dementia; CIND |
Increasing age was significantly associated with CIND and Dementia. Education beyond primary school (secondary or further education (RR = 0.38, 95% CI 0.19−0.76) and moderate physical activity of ≥20 min for ≥5 days in the previous 7 days by self‐reports (RR = 0.26, 95% CI 0.13−0.52) were significant as possible protective factors associated with CIND and dementia. |
N/A | Good |
|
Albuminuria, with or without comorbid hypertension, was associated with later risk of CIND and Dementia but did not reach statistical significance, after adjusting for age. Other vascular risk measures including hypertension, diabetes, hyperglycaemia triglycerides, waist circumference, BMI showed inconclusive or had unexpected associations (lower waist circumference and lower triglycerides) with later CIND and dementia risk. |
||||||||
| Lavrencic et al., 2022 35 | Dementia incidence, APOE genotype, and risk factors for cognitive decline in aboriginal Australians: A longitudinal cohort study | Longitudinal cohort |
Aboriginal and Torres Strait Islander peoples in Australia N = 155 |
≥60 years; mean 65.7 (±5.7) | Dementia; MCI |
Risk factors associated with incident MCI or dementia include older age (OR 2.29 a , 95% CI 1.42‐3.70), male sex (OR 4.14 a , 95% CI 0.60‐10.70), unskilled work history (OR 5.09 a , 95% CI 1.95‐13.26), polypharmacy (OR 3.11 a , 95% CI 1.17‐8.28) and past smoking (OR a 0.24, 95% CI 0.08‐0.75). Hearing loss, lower education and vision problems were strong risk factors in bivariate models, but effects were attenuated in multivariable models. Protective factors include years of education and being an ex‐smoker (vs. never smoked). |
APOE ε4 allele frequency was 24%. Homozygous or heterozygous APOE ε4 was associated with incident MCI/dementia (OR 3.96, 95% CI 1.25‐12.50). | Good |
| North America | ||||||||
| Rosenberg et al., 1996 37 | Genetic factors for the development of Alzheimer disease in the Cherokee Indian | Case‐control | Cherokee Indians | >65 | AD | N/A | There is an inverse relationship between degree of genetic Cherokee Indian ancestry and representation of AD, independent of APOE ε4 allele status. This relationship diminished with increasing age. For a decrease of 10% in Cherokee ancestry, the odds of developing AD are estimated to be nine times greater at age 65 years but only 1.34 times greater at age 80 years. | Poor |
| Henderson et al., 2002 38 | Apolipoprotein E4 and tau allele frequencies among Choctaw Indians | Case‐control |
Choctaw Indians from the USA N = 821 |
mean 75 years for Dementia APOE cohort | Dementia | Lower prevalence of APOE ε4 in the Choctaw population compared to Caucasian comparators. Among those who identify > 1/2 Choctaw ancestry the ε4 allele frequency was 6%, about half that of Caucasians. In those with less than or equal to ½ Choctaw ancestry, there was evidence of an association between the APOE ε4 genotype and disease (p < 0.05) that was consistent with the degree of association seen in Caucasians. Low prevalence of the tau H2 genotype of 6% (1/5th of Caucasian population) but statistical significance limited by low numbers of individuals with dementia. | Poor | |
| Borenstein et al., 2007 39 | Cycad exposure and risk of dementia, MCI, and PDC in the Chamorro population of Guam | Cross‐sectional |
Chamorros on Guam N = 1984 |
≥65 years; Mean 79 years (±7.0) for GD cohort | GD; MCI |
Picking (OR 1.42 a , 95% CI 1.05‐1.91), processing (OR 1.57 a , 95% CI 1.19−2.06) and eating (OR 1.42 a , 95% CI 1.13‐1.79) fadang in young adulthood were associated with GD. Picking fadang OR 2.87 (95% CI 1.48‐5.56) and eating fadang as a young adult OR 2.18 (95% CI 1.34‐3.55) were associated with PDC. PARPs for GD for picking 0.13 (95% CI 0.03‐0.22), processing 0.12 (95% CI 0.03‐0.22), and eating fadang 0.22 (95% CI 0.07‐0.38). Associations among men were more consistently significant across all outcome groups compared with women in subanalyses by sex. Consumption of fruit bats or exposure to cycad used as a topical medicine were not significant for any of the outcomes. ***** Picking (OR 1.84 a , 95% CI 1.32‐2.57), processing (OR 1.83 a , 95% CI 1.34‐2.49), and eating (OR 1.84 a , 95% CI 1.39‐2.43) fadang in young adulthood were associated with MCI. PAR proportions for MCI for picking 0.26 (95% CI 0.12‐0.40), processing 0.23 (95% CI 0.09‐0.38), and eating fadang 0.28 (95% CI 0.07‐0.50). |
APOE ε4 cases varied minimally across case groups or controls and was not statistically significant. | Good |
| Sundar et al., 2007 40 | Two sites in the MAPT region confer genetic risk for Guam ALS/PDC and dementia | Case‐control |
Chamorros on Guam N = 600 |
Mean 74.9 (±7.2) years for GD cohort | GD | N/A | MAPT gene SNP 2 (OR 1.61, 95% CI 1.00−2.62), SNP 9 (OR 1.06, 95% CI 0.57−1.97) and SNP 6 and 9 (OR 3.02, 95% CI 1.10−8.25) were associated with GD. No significant evidence of an association was found between APOE and GD. | Fair |
| Galasko et al., 2007 41 | Prevalence of dementia in Chamorros on Guam: Relationship to age, gender, education, and APOE | Cross‐sectional |
Chamorros on Guam N = 2029 |
≥65 years; mean 73.8 (±6.0) | GD; MCI |
Age (OR 1.11, 95% CI 1.08–1.13) and low education (OR 0.87, 95% CI 0.84–0.90) were associated with dementia. Gender was not significantly associated with dementia or GD after adjusting for age and education. ***** MCI participants (77.4 ± 6.3 years) were older than cognitively normal participants (72.8 ± 5.2 years) with greater ratio of women to men. |
APOE ε4 frequency was 5.3% for those with all‐cause dementia compared to 4.0% for unaffected controls. APOE ε4 was not significantly associated with all‐cause dementia, GD, PDC or vascular dementia. | Good |
| Weiner et al., 2008 43 | Atherosclerosis risk factors in American Indians with Alzheimer disease: Preliminary findings | Case‐control |
American Indians from the United States N = 68 |
Median 78 (range 56‐91) years for AD cases | AD |
There is an association between history of hypertension and diabetes and AD in a small sample of American Indians. A high percentage of Choctaw cases and controls had a history of hypertension compared to white participants. History of diabetes was most prevalent in Indian ADs; less so in Indian controls and White participants with AD. Age, education, waist size, BMI, history of high cholesterol, history of myocardial infarction or history of stroke did not differ significantly between the three groups. |
Plasma homocysteine concentrations increased with age (p < 0.001) but was not significantly associated with degree of Indian heritage (p = 0.63); sex (p = 0.14) or AD (p = 0.14). | Fair |
| Weiner et al. 2009 44 | Brain MRI, apolipoprotein E genotype, and plasma homocysteine in American Indian Alzheimer disease patients and Indian controls | Case‐control |
American Indians from the United States N = 21 |
>60 years | AD |
No statistically significant differences between AD subjects and controls in age, gender, degree of Indian heritage, history of hypertension, diabetes, high cholesterol, waist circumference or BMI. Education was lower in the AD group but this was not statistically significant. |
More AD subjects had APOE ε4 alleles than controls (p = 0.043) but there was no significant relationship between the presence of an APOEε4 allele and Indian heritage in the AD group or in the combined AD and control groups. Median plasma homocysteine concentrations were higher in AD subjects but did not achieve statistical significance. There were no significant differences in neuroimaging findings between the two groups, but AD subjects had greater volume of WMH and greater WMHV/WBV ratio (median 1.63% vs. 0.65%) and a far greater range of WMHV. In those with AD, WBV correlated with BMI (p = 0.015) and age (p = 0.005). | Fair |
| Weiner et al., 2011 45 | The relationship of cardiovascular risk factors to Alzheimer disease in Choctaw Indians | Case‐control |
American Indians from the United States N = 78 |
Median 78 years (range 54‐91) years for AD cases | AD | There were no significant differences between Choctaws with and without AD in gender, number or types of cardiovascular risk factors (history of hypertension, diabetes, high cholesterol and myocardial infarction). Indian AD groups had a significantly higher percentage of affected first‐degree relatives than the Indian controls. | Choctaw groups with and without AD had lower proportion of one or more APOE ε4 alleles than White participants. There was a significant relationship between homocysteine concentration and onset age for American Indians with AD (p = 0.005). | Fair |
| MacDonald et al., 2015 47 | Implications of risk factors for Alzheimer's disease in Canada's Indigenous population | Cohort | Indigenous peoples of Canada (First Nation, Inuit, Metis) | N/A | Dementia | Physical inactivity (32.5%, 95% CI 10.1%−51.1%), low educational attainment (22.4%, 95% CI 14.6%−29.6%), smoking (19.4%, 95% CI 5.8%−32.9%), midlife obesity (16.8%, 95% CI 10.3‐23.6), midlife hypertension (14.2%, 95% CI 4.2%−25.1%) and diabetes mellitus (6.0%, 95% CI 2.6%−9.7%) contribute to dementia rates in the Indigenous population. The combined PAR for AD for all six modifiable risk factors was 79.6% among on‐reserve Indigenous, 74.9% among off‐reserve Indigenous, and 67.1% among non‐Indigenous peoples in Canada. | N/A | Good |
| Carty et al. 2020 50 | Risk factors for Alzheimer's disease and related Dementia diagnoses in American Indians | Cohort |
American Indians N = 3464 |
≥55 years | Dementia | Age (RR 1.12 a 95% CI 1.11‐1.14), hypertension (RR 2.75 a 95% CI 1.75‐4.31), depression (RR 2.49 a 95% CI 1.79‐3.46), hyperlipidaemia (RR 1.41 a 95% CI 1.08‐1.84) and diabetes (RR 2.07 a 95% CI 1.41‐3.03) were associated with increased risk of a dementia diagnosis. Female sex (RR 0.70 a 95% CI 0.52‐0.94), being married/having a life partner (RR 0.67 a 95% CI 0.48‐0.94) were associated with lower risk of a dementia diagnosis. | Fair | |
| Smith M et al., 2021 51 | Disparities in Alzheimer disease and mild cognitive impairment among Native Hawaiians and Pacific Islanders | Cohort |
Native Hawaiians & Pacific Islanders N = 87 |
N/A | AD; MCI |
The mean age of Native Hawaiians and Pacific Islanders at time of diagnosis with AD or MCI was 73.2 (±12.5) years. Mean age at diagnosis was lower in this group with greater percentage with a diagnosis <65 years compared to other racial groups (p = 0.02). There was a higher proportion of women than men with diagnosis of AD over MCI 67%, p = .028). Hypertension, hyperlipidaemia, and type II diabetes were found to be higher among the Native Hawaiians and Pacific Islanders (hypertension 74%, p = 0.012; hyperlipidemia 70%, p =0 .05; T2DM 28%, p = 0.002). There were no statistically significant differences in marital status, insurance and number of comorbidities between the different racial groups. |
Fair | |
| Suchy‐Dicey et al., 2021 52 | APOE genotype, hippocampus, and cognitive markers of Alzheimer's disease in American Indians: Data from the Strong Heart Study | Cohort |
American Indians N = 811 |
≥64 years Mean 73.1 (±6.0) | Cognitive impairment b | APOE ε4 carrier frequencies were 4/3 21.0%; ε4/4 0.7%; ε4/2 0.6% (total 22.3%). Non‐APOE ε4 carrier frequencies include ε3/3 72.5%; ε3/2 5.1%; ε2/2 0%. Sociodemographic and clinical features were similar between APOE ε4 carriers and non‐carriers apart from higher proportion with LDL and chronic kidney disease (not significant). MRI‐defined brain volumes (brain, hippocampal, intracranial volumes) and multidomain cognitive test scores were similar between APOE ε4 carriers and non‐carriers and not significantly associated with carrier status, after adjusting for sociodemographic and clinical conditions. | Good | |
| South America | ||||||||
| Marca Ysabel 2021 55 | Dissecting the role of Amerindian genetic ancestry and the APOE ε4 allele on Alzheimer disease in an admixed Peruvian population | Case‐control |
Peruvian N = 207 |
≥65 years | AD | N/A | Genetic ancestry surrounding the APOE is predominately Amerindian (60.6%). APOE ε4 allele frequency was 9.2% in cognitively impaired individuals versus 4.6% in those cognitively normal and significantly associated with increased risk of AD (OR 5.02 a , 95% CI 2.3−12.5) | Fair |
| Gatz et al., 2022 56 | Prevalence of dementia and mild cognitive impairment in Indigenous Bolivian forger‐horticulturalist | Cohort |
Indigenous Tsimane and Moseten people of Bolivia N = 623 |
≥60 years | Dementia; MCI |
Dementia cases were equal between women and men. ***** MCI was more prevalent among women compared to men (p = 0.02 for sex difference). ***** Cognitive impairment was associated with visuospatial impairments, parkinsonian symptoms and gait abnormalities. |
The number of APOE ε4 alleles did not distinguish between those with cognitive impairment and those without however, carrying two (vs. none) alleles was associated with significantly greater odds of cognitive impairment (Tsimane OR 10.7 95% CI 1.47, 78.6; Moseten OR 11.5 95% CI 1.28‐102.8). On CT, medial temporal atrophy (OR 8.89 95% CI 1.85‐42.9), internal carotid artery calcification morphology (OR 6.10 95% CI 1.23‐30.4), basal ganglia calcification (OR 5.27 95% CI 1.85‐∞), calcification of the lenticulostriate arteries (OR 4.77 95% CI 1.04‐22.0) and lenticulostriate arteries calcification density (OR 1.11 95% CI 1.01‐1.23) was associated with cognitive impairment for Tsimane but not for Moseten. Poorer cognition was associated with severity of intracranial vascular calcification, greater severity of cerebral atrophy, especially of white matter volume. | Good |
Abbreviations: AD, Alzheimer's dementia; aMCI, amnestic mild cognitive impairment; APOE4, apolipoprotein E allele; BMI, body mass index; CIND, cognitive impairment not dementia; CLU, clusterin; CT, computed tomography; CTQ, Childhood Trauma Questionnaire; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; Fhx dementia, family history of dementia; GD, Guam dementia; ICIQ, International Consultation on Incontinence Questionnaire; MAPT, microtubule‐associated protein tau; MCI, mild cognitive impairment; MRI, magnetic resonance imaging; NEP, neprilysin; OR, odds ratio; PAF, population attributable fractions; PAR, population attributable risk; PDC, parkinsonism dementia complex; PTSD, post‐traumatic stress disorder; QA, quality assessment; RICE, Retrospective Indigenous Childhood Enrichment Scale; RR, relative risk; SBP, systolic blood pressure; T2DM, type 2 diabetes mellitus; WHMH, white matter hyperintensities; WHV, white matter hyperintensities volume.
Adjusted/multivariate model.
Cognitive impairment was defined as deficits (1.5 SD below mean) in leaning and retention tasks in multidomain psychometric tests such as the California Verbal Learning Test, Weschler Adult Intelligence Scale, Controlled Oral Word Association, and Modified Mini Mental Status Examination.
3.4. Prevalence and incidence of dementia and MCI
Most studies included participants aged ≥60 (±5 years). For those studies with participants aged ≥45 years, prevalence rates for ≥60 years and age‐standardized prevalence rates are reported where available. Across different Indigenous populations, prevalence rates of dementia ranged from 0.6% to 37.2% and rates of MCI/CIND varied from 4.4% to 27.4%. Low rates of dementia were reported for Cree Indians (0.5%), Tibetans (1.3%), Tsimane (1.2%), and Moseten (0.6%) cohorts 19 , 36 , 56 and highest for Aboriginal and Torres Strait Islander peoples (up to 26.8% in those ≥65 years). 21 Dementia incidence reported for Aboriginal and Torres Strait Islander peoples ranged from 21.0 to 35.9 per 1000 person‐years for those aged ≥60 years. 22 , 25 Mayeda et al. 48 reported a dementia incidence of 22.2 per 1000 person‐years for American Indians ≥60 years (Table 3).
TABLE 3.
Prevalence and incidence.
| Indigenous population sample size | Age; mean/median age (SD) | Outcome: | |||
|---|---|---|---|---|---|
| Study | Indigenous population | Prevalence | Incidence | ||
| Asia | |||||
| Pu'un et al., 2014 18 † | Melanau of East Malaysia | 344 | ≥60 years; mean 70.4 (±6.7) |
Dementia 10.5%; Any cognitive impairment 27.3% |
|
| Huang et al., 2016 19 † | Tibetans from the Qinghai‐Tibet plateau | 3974 | ≥60 years | Dementia 1.3% (95% CI 0.9−1.7) | |
| Australia & Oceania | |||||
| Smith et al., 2008 21 | Aboriginal and Torres Strait Islander peoples Kimberley region of WA, Australia | 363 | ≥45 years; mean 60.7 (±11.9) |
Dementia 12.4% (95% CI 9.0‐15.8); 26.8% (95% CI 18.8‐34.8) ≥65 years CIND of 8.0%; 13.4% ≥65 years |
|
| Cotter et al., 2012 23 | Aboriginal and Torres Strait Islander peoples, Australia | 1668 (multiple data sources) | ≥45 years | Dementia 0.4–6.3 per 1000 persons | |
| Li et al., 2014 24 | Aboriginal and Torres Strait Islander peoples from the Northern Territory, Australia | 11,646 (multiple data sources) | ≥45 years; median 72 | Dementia 3.7%; 6.5% (95% CI 5.8‐6.8)* | Dementia 27.3 cases per 1000 person‐years (95% CI 22.8‐31.8)* |
| Radford et al., 2015 25 | Aboriginal and Torres Strait Islander peoples from New South Wales, Australia | 336 | ≥60 years; median 66.6 (±6.3) |
Dementia 13.4% (95% CI 9.6‐17.2); 21.0% (95% CI 12.8‐29.2)* MCI 17.7% (95% CI 3.4‐21.9) |
|
| Russell et al., 2016 26 | Torres Strait Islanders from the Torres Strait, Australia | 20 | ≥ 45 years; mean 65.8 (±9.5) |
Dementia 5.0% CIND 20.0% |
|
| LoGiudice et al., 2016 27 † | Aboriginal and Torres Strait Islander peoples Kimberley region of WA, Australia | 189 | ≥45 years; mean 65.4 (±10.3) |
Dementia: 7.3 (95% CI 3.7‐14.5) per 1000 person‐years; 21.0 (95% CI 10.5‐42.1) per 1000 person years for ≥60 years MCI or dementia 28.5 (95% CI 20.0‐40.5) per 1000 person‐years; 52.6 (95% CI 33.9, 81.5) per 1000 person‐years for ≥60 years |
|
| Derrig et al., 2020 30 † | Aboriginal and Torres Strait Islander peoples from New South Wales, Australia | 287 | ≥60 years; mean 65.9 (±5.6) | MCI 18.4% | |
| Russell et al., 2021 31 | Torres Strait Islanders from the Torres Strait, Australia | 274 | ≥45 years; mean 65.1 (±10.8) |
Dementia 14.2% CIND 21.9% |
|
| Lavrencic et al., 2022 35 † | Aboriginal and Torres Strait Islander peoples from New South Wales, Australia | 155 | ≥60 years; mean 65.7 (±5.7) |
Dementia: 35.9 per 1000 person‐years (95% CI 18.3‐53.6)* MCI and dementia: 75.2 (95% CI 50.6‐99.8)* |
|
| North America | |||||
| Hendrie et al., 1993 36 | The Cree of Manitoba, Canada | 192 | ≥65 years |
Dementia 4.2% AD 0.5% |
|
| Galasko et al., 2007 41 † | Chamorros of Guam, United States | 1984 | ≥65 years; mean 73.8 (±6.0) |
Dementia 12.2% (95% CI 11.7‐12.9) GD/AD 8.8% (95% CI 8.3‐9.4) MCI 4.4% |
|
| Jervis et al., 2007 | American Indians, United States | 140 |
≥60 years; mean 69.8 (±6.4) |
Dementia 14.6% based on the mini‐Mental State Examination; 37.2% based on the Mattis Dementia Rating Scale CIND 27.4% for MDRS; 13.4% for >65 |
|
| Jacklin et al., 2012 46 | First Nations people of Alberta, Canada | 129 774 | N/A | Dementia 7.5(95% CI 6.6‐8.5) per 1000* | |
| Mayeda et al., 2016 48 | American Indians and Alaska Natives, United States | 4543 | ≥60 years; mean 73.4 | Dementia 22.2 (95% CI 20.9–23.5) per 1000 person | |
| Kirkpatrick et al., 2019 49 | American Indians, United States | 52 |
Mean 64 (±7.1) |
44.2% (95% CI 30‐50) with cognitive impairment based on the Montreal Cognitive Assessment 15 participants underwent further evaluation: non‐amnestic MCI (27%); vascular MCI (33%); vascular dementia (13%) |
|
| Carty et al. 2020 50 † | American Indians, United States | 3464 | ≥55 years, median 64 | Dementia 5.7%; 6.6%* | |
| South America | |||||
| Stryjer et al., 2011 53 | Otomis tribe from Queretaro, Mexico | 65 | ≥65 years; mean 73.3 (±7.7) | Dementia 12.3% based on the diagnostic and statistical manual of mental disorders‐fourth edition (DSM‐IV); 7.7% based on Brookdale test and 6.1% with both | |
| Brucki et al., 2014 54 | Mamirauá and Amanã peoples of the Amazonian Basin, Brazil | 163 | ≥50 years; mean 62.3 (±9.2) |
Dementia 4.9%; 12.3 ≥65 years CIND 6.1%; 7.7% for ≥65 years |
|
| Gatz et al., 2022 56 † | Indigenous peoples of Bolivia | 623 | ≥60 years |
Dementia in Tsimane 1.2% (95% CI 0.4‐2.7) ; 2.7% (95% CI 0.1‐5.4)*; in Moseten 0.6% (95% CI 0.0‐3.2) ; 0.9%* (95% CI 0.0‐2.6)* MCI in Tsimane 7.7% (95% CI 5.2‐10.3)*; in Moseten 9.8% (95% CI: 4.9, 14.6)* |
|
Abbreviations: AD, Alzheimer's dementia; CI, confidence interval; CIND, cognitive impairment not dementia; GD, Guam dementia; MCI, mild cognitive impairment; N/A, not applicable; SD, standard deviation.
*Age‐adjusted values.
†Articles included in Table 2.
3.5. Risk and protective factors
Modifiable risk factors featured across multiple (>1) studies that demonstrated a statistically significant association include childhood adversity, hearing loss, low education attainment, unskilled work history, stroke, head injury, epilepsy, diabetes, hypertension, hyperlipidemia, depression, low BMI, poor mobility, and continence issues by self‐reports or using the International Consultation on Incontinence Questionnaire score. Non‐modifiable risk factors across different studies were increasing age, sex, and genetic polymorphisms, including APOE ɛ4. Age was reported to be statistically significant in 11 studies, and sex differences were reported in 7 studies. Gene associations with dementia or MCI were highlighted in 13 studies (Table 4).
TABLE 4.
Compilation of studied risk factors with measures of association.
| Studied variable of interest | Pu'un, 2014 18 | Huang, 2016 19 | Chen, 2020 20 | Smith, 2010 22 | LoGiudice, 2016 27 | Radford 2017 28 | Radford, 2019 29 | Derrig, 2020 30 | Russell, 2022 33 | Thompson 2022 34 | Lavrencic, 2022 35 | Rosenberg, 1996 37 | Henderson, 2002 38 | Bronstein, 2007 39 | Sundar 2007 40 | Galasko, 2007 41 | Weiner, 2008 43 | Weiner, 2009 44 | Weiner, 2011 45 | Carty et al., 2020 50 | Smith et al., 2020 51 | Suchy‐Dicey et al., 2022 52 | Marca Ysabel 2021 55 | Gatz, 2022 56 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | Asia | Australia & Oceania | North America | South America | ||||||||||||||||||||
| NON‐MODIFIABLE | ||||||||||||||||||||||||
| Age | * | |||||||||||||||||||||||
| Sex | M | M | M | M | F | F | * F | |||||||||||||||||
| Family history of dementia | ||||||||||||||||||||||||
| Ethnicity | ||||||||||||||||||||||||
| APOE4 | NS | NS | NS | NS | NS | NS | ||||||||||||||||||
| Tau | NS | |||||||||||||||||||||||
| NEP | M | |||||||||||||||||||||||
| CLU | ||||||||||||||||||||||||
| MAPT | ||||||||||||||||||||||||
| Homocysteine | NS | NS | ||||||||||||||||||||||
| POTENTIALLY MODIFIABLE | ||||||||||||||||||||||||
| Childhood trauma/adversity | ||||||||||||||||||||||||
| Religious practices | ||||||||||||||||||||||||
| Education | * | |||||||||||||||||||||||
| Unskilled work history | * | |||||||||||||||||||||||
| Social activities | ||||||||||||||||||||||||
| Physical activity | ||||||||||||||||||||||||
| Hospitalisation in past year | ||||||||||||||||||||||||
| Married/life partner | ||||||||||||||||||||||||
| Living alone | ||||||||||||||||||||||||
| Living in residential care | ||||||||||||||||||||||||
| Loneliness | ||||||||||||||||||||||||
| Diet: beef | ||||||||||||||||||||||||
| Diet: chicken | ||||||||||||||||||||||||
| Exposure to cyad‐derived products | ||||||||||||||||||||||||
| Smoking | ** | |||||||||||||||||||||||
| Alcohol | ||||||||||||||||||||||||
| Stroke | ||||||||||||||||||||||||
| Head injury | ||||||||||||||||||||||||
| Epilepsy | ||||||||||||||||||||||||
| Cardiovascular disease | ||||||||||||||||||||||||
| Renal disease/Albuminuria | ||||||||||||||||||||||||
| Obesity/high BMI | * | |||||||||||||||||||||||
| low BMI | ||||||||||||||||||||||||
| Hyperlipidaemia | ||||||||||||||||||||||||
| Depression/PTSD | * | |||||||||||||||||||||||
| Diabetes/HbA1c | ||||||||||||||||||||||||
| Hypertension/high BP | ||||||||||||||||||||||||
| Low BP | * | |||||||||||||||||||||||
| Hearing loss | * | |||||||||||||||||||||||
| Vision impairment | ||||||||||||||||||||||||
| Analgesics | ||||||||||||||||||||||||
| Polypharmacy | ||||||||||||||||||||||||
| Mobility impairment | ||||||||||||||||||||||||
| Falls | ||||||||||||||||||||||||
| Urinary incontinence | ||||||||||||||||||||||||
| ADL impairment | ||||||||||||||||||||||||
Note: Blue indicates a statistically significant negative association. Green indicates a statistically significant positive association. NS indicates not statistically significant.
Abbreviations: ADL, activities of daily living; APOE4, apolipoprotein E allele; BMI, body mass index; CLU, clusterin; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; F, female; HbA1c, hemoglobin A1C; M, male; MAPT, microtubule‐associated protein tau; NEP, neprilysin; PTSD, post‐traumatic stress disorder; SBP, systolic blood pressure.
Mild cognitive impairment/cognitive impairment not dementia only.
Ex‐smoker compared to never smoked.
Potential protective factors or those negatively associated with the occurrence of dementia or MCI reported to be statistically significant in at least one study include: higher education attainment, ex‐smoking (compared to never smoked or current smoker), Kowtow, turning prayer beads, physical activity by self‐reports, social activities, being married or having a life partner, and eating chicken (Table 4). Low BMI was reported to be associated with a greater odds of dementia and cognitive impairment in two studies 27 , 29 but was associated with a lower odds of MCI in one study 30 involving Aboriginal and Torres Strait Islander peoples. Seven out of nine studies that highlighted protective factors were assessed to be of good quality, 19 , 22 , 28 , 30 , 34 , 35 , 41 and two were of fair quality 18 , 50 (Table 2).
PAF were reported in three studies. 32 , 39 , 47 MacDonald et al. produced a combined PAF for AD of 79.6% and 74.9% among on‐reserve and off‐reserve Indigenous peoples in Canada, respectively. The relative contributions of six modifiable risk factors were physical inactivity (32.5%), low education attainment (22.4%), smoking (19.4%), midlife obesity (16.8%) midlife hypertension (14.2%), and diabetes mellitus (6.0%). 47 Ma'u et al. illustrated a PAF of 51.4% using 12 risk factors including obesity (7.3%), hearing loss (6.5%), education (5.6%), social isolation (5.1%), hypertension (5.0%), physical inactivity (5.0%), traumatic brain injury (3.5%), smoking (4.3%), depression (4.2%), diabetes (2.4%), air pollution (1.8%), and alcohol (0.7%) for Māori people of New Zealand. 32 Exposure to fadang in the unique population of Chamorros on Guam was estimated to contribute between 12% and 23% to outcomes of Guam dementia and MCI, after adjusting for age, sex, and education. 39
3.6. Genetic markers
Apolipoprotein E allele4 (APOE ε4) was the most studied genetic marker and featured in 12 studies. Half of these studies found no association between the distribution of APOE alleles between participants with and without dementia. 19 , 39 , 40 , 41 , 45 , 52 APOE ɛ4 allele frequency was reported in a study of Aboriginal and Torres Strait Islander peoples (24%), Chamorros (5.3% in dementia vs. 4.0% in cognitively normal group), American Indians (22.3%), and Peruvians with American Indian ancestry (9.2% in cognitively impaired vs. 4.6% in cognitively normal group). 35 , 41 , 52 , 55 Greater odds of MCI and dementia associated with being a homozygous or heterozygous carrier of the APOE ɛ4 allele (OR 3.96, 95% 1.25‐12.50) was described in one longitudinal cohort of Aboriginal and Torres Strait Islander peoples. 35 Marca‐Ysabel et al. found APOE ɛ4 to be associated with increased risk of AD (OR 5.02, 95% CI 2.3‐12.5). 55 Among the Indigenous Tsimane and Moseten peoples, the number of APOE ɛ4 alleles did not differ between those with cognitive impairment and those without; however, carrying two APOE ɛ4 alleles was associated with greater odds of cognitive impairment. 56 A couple of studies looked at the relationship between APOE ɛ4, degree of American Indian ancestry and dementia. Henderson et al. found that the prevalence of APOE ɛ4 in the Choctaw population was half that of Caucasian comparators. For those with less than 50% Choctaw ancestry, there was evidence of an association between the APOE ɛ4 genotype and disease. 38 However, Rosenberg reported an inverse relationship between the genetic degree of Cherokee ancestry and AD, independent of APOE ɛ4 status. 37
Other genes studied include clusterin (CLU), neprilysin (NEP), microtubule‐associated protein tau (MAPT) and Tau. 19 , 20 , 40 Huang et al. reported that CLU genotypes AA and GA of rs2279590 were associated with a 4‐fold increase in the odds of AD while CLU genotypes GG and GC of rs9331888 were negatively associated with AD in a sub‐sample of 39 Tibetans with AD and 56 without. 19 Chen et al. reported two different NEP SNPs (rs701109 and rs3736187 mutation) that may add to the risk of AD among male Tibetans rather than the whole population. 20 Sundar et al. reported MAPT SNPs 6, 7, and 9, homozygous genotypes (A/A, A/A, and C/C, respectively) contribute to susceptibility to Guam neurologic disorders. 40 Those with SNP6 and SNP9 AC/AC diplotype had a three‐fold increased risk for Guam dementia and a 4‐fold increased risk for Parkinsonism dementia complex compared to those with other diplotypes, after adjusting for SNP2. SNP2 allele carriers had a 1.6‐fold increased risk of Guam dementia and a 2‐fold increased risk of Parkinsonism dementia complex, after adjusting for SNPs 6 and 9. 40
3.7. Other biological and neuroradiological markers
Plasma homocysteine was a biological marker found to be higher in a small group of American Indians with AD compared to controls but did not reach statistical significance. 43 , 44
There were only three studies that reported on neuroimaging markers related to dementia and MCI, one using CT imaging and two using MRI. 44 , 52 , 56 There were no PET imaging or cerebrospinal fluid biomarker studies. In the Bolivian cohort, poorer cognition was associated with severity of intracranial vascular calcifications, greater medial temporal atrophy, and reduced white matter volume on CT brain scans of Tsimane participants. 56 In a small case‐control study of 21 participants by Weiner et al., AD participants demonstrated greater volume of white matter hyperintensity as a proportion of whole brain volumes on MRI, but this did not reach statistical significance. 44 Suchy‐Dicey et al. described MRI‐defined brain, hippocampal, and intracranial volumes against cognitive test scores in a larger cohort of 811 American Indians but found no significant association between neuroradiological parameters between APOE ɛ4 carriers and non‐carriers after adjusting for sociodemographic and clinical conditions. 52
4. DISCUSSION
4.1. Availability of evidence across Indigenous groups
Indigenous peoples live in every corner of the globe with the largest populations expected to reside in populous China, India, and Siberian regions of Russia. 58 However, most of the researched Indigenous groups included in this systematic review are from Western countries with a paucity of published scientific literature from these regions (Figure 2). Compared to most world regions where age‐standardized prevalence of dementia in those 60 and older lie between 5% and 7%, there was greater variance in the reported prevalence rates within and between Indigenous populations. 59 Prevalence rates were lowest for Cree Indians, Tibetans, Tsimane, and Moseten (0.5%–1.3%) and highest among Aboriginal and Torres Strait Islander peoples (>20%) in those ≥60 years. 19 , 21 , 25 , 36 , 56 Age‐adjusted dementia rates for American Indians ranged from 6.6% to 26.8% between studies, compared to the estimated prevalence of 10.8% in the general United States population. 60 This may reflect the heterogeneity of study methodologies. For instance, the study by Cotter et al. 23 based on databases reliant on routinely collected data reported no difference in dementia rates between Aboriginal and Torres Strait Islander peoples and non‐Indigenous Australians, which was not congruent with cohort studies based on expert clinical diagnoses of dementia. 21 , 25 , 31 The latter consistently report rates of dementia three to five times greater among different cohorts of Aboriginal and Torres Strait Islander peoples compared to non‐Indigenous Australians and are more likely to reflect true prevalence of disease. 21 , 25 , 31
FIGURE 2.
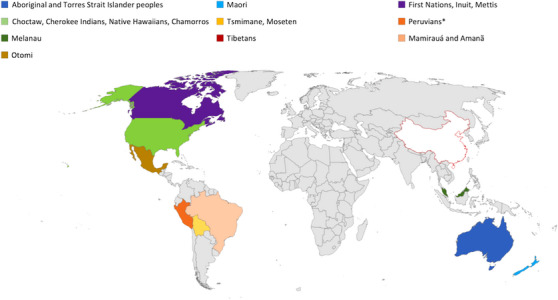
Geographical representation of Indigenous peoples included in this systematic review.
4.2. Evidence of risk and protective factors of dementia or cognitive impairment
Despite the diversity of Indigenous peoples worldwide, there are many similarities in the determinants of health and illness. Study of risk factors predominate the literature and emphasize medical factors such as head injury, epilepsy, diabetes, hypertension, and stroke. However, protective factors associated with dementia or MCI are less well understood. Poor mobility, falls, and continence issues found to be associated with dementia in cross‐sectional studies likely reflect syndromes related to a dementia diagnosis rather than its precipitant. This may have a bearing on the association between BMI and dementia across different studies. This relationship is proposed to be J‐shaped, whereby high BMI and obesity in middle age may increase the risk of dementia but low BMI is more likely to accompany cognitive impairment or dementia in the older population. 61
Risk and protective factors associated with dementia for Indigenous peoples are congruent with the evidence available for non‐Indigenous populations. 4 , 57 However, the significance of social determinants of health across the life‐course (childhood adversity, education attainment, skilled work history) and engagement with cultural or religious practices (Kowtow, turning prayer beads) were highlighted in this review of Indigenous peoples. As Radford et al. proposed, cultural connection and support from extended family may help mitigate experiences of trauma and adversity in childhood. 28 Male sex was associated with a greater risk of dementia in certain Indigenous cohorts, 22 , 35 , 39 , 41 , 46 in contrast to most non‐Indigenous populations where dementia is more common in women. 62 , 63 Higher rates of cluster risk factors such as stroke, head injury, and cardiac disease among men compared to women have been described in studies involving Aboriginal and Torres Strait Islander peoples. 22 , 29 , 35 Sex differences in exposure to other lifestyle and social risk factors such as heavy alcohol use, history of police custody and incarceration, and low education may also contribute to the higher rates of dementia and cognitive impairment among men in these communities. Among the Chamorros, there was slightly higher prevalence of parkinsonism‐dementia complex in men, but sex was not significantly associated with Guam dementia after adjusting for age and education. 39 , 41 Studies involving First Nations people of North America included in this review were equivocal and contained insufficient data on rates of risk factors to offer plausible explanations on sex differences. More work is required to delineate the differential risk factors for dementia for Indigenous men and women and to ascertain whether adjustments for education, cardiometabolic disease, and competing risk of death modifies these sex differences. 64
PAF calculations utilize the strength of each risk factor, its prevalence in a population, and overlap within the same person. Combined PAF for AD for First Nations people of Canada (79.6% for on‐reserve and 74.9% for off‐reserve) and Māori (51.4%) were considerably higher than global estimates (40%) and suggests that the preventable burden of dementia may be greater for certain Indigenous communities. 4 , 32 , 47 , 57 This is in keeping with the breadth of studies demonstrating higher prevalence of health and psychosocial factors associated with dementia in different Indigenous, compared to non‐Indigenous, populations. 2
4.3. Genetic factors associated with dementia or cognitive impairment
APOE ɛ4 is the most well recognized genetic risk‐factor for AD. The association of APOE allele with AD risk differs across ethnoracial groups with stronger effects observed in East Asian (ε3/ε4 OR: 3.1‐5.6; ε4/ε4 OR: 11.8‐33.1) and non‐Hispanic White populations (ε3/ε4 OR: 3.2; ε4/ε4 OR: 14.9) compared to African Americans and Hispanic peoples (ε3/ε4 OR:1.1‐2.2; ε4/ε4 OR: 2.2–5.7). 65 Most studies of APOE ɛ4 involving Indigenous peoples were small and may be poorly powered to discern an association. Where an association was reported, APOE ɛ4 carriers had increased odds of dementia or cognitive impairment comparable to other high‐risk ethnic groups, except among American Indians where the degree of Choctaw or Cherokee ancestry may modify this effect. The genotype ɛ2/ɛ3 that has been demonstrated to be protective across ethnic groups has not demonstrated in studies involving Indigenous peoples. 65
Genome‐wide association studies involving Indigenous peoples are limited and more work is required to elucidate genetic polymorphisms of significance to individual populations and across different cohorts. There was a notable absence of studies on AD susceptibility genes such as amyloid precursor protein, presenilin, presenilin 2, phosphatidylinositol‐binding clathrin assembly protein, or inflammatory markers (e.g., C‐reactive protein, interleukin‐6) that have been the subject of dementia research in non‐Indigenous populations. 66
4.4. Neuroradiological and biomarkers for dementia and cognitive impairment
This systematic review yielded few studies involving biological or imaging markers of dementia or MCI. Many of these studies were small scale, case‐control studies with limited statistical power. 44 , 56 In the broader literature, a study of 786 American Indian participants from the Strong Heart study (not included in this review) found that general cognitive functioning correlated with hippocampal volumes while processing speed was associated with brain volumes as well as cerebral vascular burden (infarcts and white matter disease). 67 In studies comparing ethnoracially diverse groups, cognitive decline was most associated with global gray matter atrophy in African American participants, volume of white matter hyperintensities in Hispanic participants, while regional temporal atrophy had greater relevance for White American participants. 68 This suggests t differences in the importance of neuroimaging markers as a reflection of the heterogenous brain changes driving cognitive change and dementia between ethnoracial groups. Larger studies on neuroimaging markers of dementia and MCI involving Indigenous peoples are needed to greater understand the brain changes relevant to these populations.
4.5. Indigenous perspectives in dementia research
The dementia prevention discourse is largely framed by a Western biomedical model with emphasis on risk factors and pathology. 69 Though this model allows for greater recognition of those at risk of cognitive decline and dementia, it often de‐emphasizes the cultural, historic, and psychosocial influences that are crucial in comprehensive understanding of possible determinants and correlates of health for Indigenous peoples. This biomedical model also reinforces a deficits approach that can negatively impact on the empowerment of people to strive for healthy ageing. Alternatively, a greater focus on proactive factors across the life‐course may be more conducive to a strengths‐based approach and the promotion of cognitive health and ageing for Indigenous peoples and their communities. There is increasing awareness of the Ethics around research involving Indigenous peoples evident in approaches to study design and governance of research teams. Some research groups are explicit in their engagement of Indigenous researchers and stakeholders to ensure that research involving Indigenous people serves to strengthen capacity, improve health outcomes and progress interests valued by Indigenous peoples and communities. 70
4.6. Future directions
Increased research is needed on modifiable protective factors of dementia involving Indigenous peoples to develop targeted strength‐based models that promote cognitive health and prevent impairment over the life‐course. The absence of studies on disease‐specific biomarkers of dementia or cognitive impairment involving an Indigenous cohort in this review highlights a significant research gap that is out of keeping with worldwide efforts to validate dementia biomarkers and to see to their incorporation into diagnostic and classifications schemes in research and clinical settings. 71 , 72 Greater efforts to engage Indigenous peoples in biomarker studies and clinical trials in culturally safe ways are needed to harness the potential to translate research to improve dementia diagnosis and care in the future. Research should be conducted with explicit partnership with Indigenous peoples and communities.
5. CONCLUSIONS
There are many shared determinants of health and illness across Indigenous people, but risk profiles may differ between populations. Greater understanding of modifiable risk and protective factors, backed by validated disease‐specific biomarkers, would be useful in the development of a strengths‐based model to promote cognitive health over the life‐course. This research will facilitate early recognition of people at risk, improve timely diagnosis and care for Indigenous peoples, their families, and communities.
AUTHOR CONTRIBUTIONS
Huong X. T. Nguyen, Dina LoGiudice, Bridgette J. McNamara, and Rosie Watson planned this systematic review. Huong X. T. Nguyen and Catherine Voutier were responsible for database searches. Huong X. T. Nguyen and Kate Bradley screened search results, reviewed full texts, extracted information, and completed quality assessments for included articles. Dina LoGiudice and Bridgette J. McNamara assisted to achieve consensus. Roslyn Malay provided feedback on the cultural appropriateness of the review. Huong X. T. Nguyen drafted the manuscript that was reviewed by all co‐authors prior to submission.
CONFLICT OF INTEREST STATEMENT
The authors report no disclosures or conflicts of interest relevant to the manuscript. Author disclosures are available in the supporting information.
CONSENT STATEMENT
Participant consent was not necessary for this research.
Supporting information
Supporting information
Supporting information
ACKNOWLEDGMENTS
We are grateful for the assistance of Mrs Catherine Voutier, senior clinical and research librarian in Melbourne Health, in database searches. This project is supported by the National Health and Medical Research Council (NHMRC) Centre for Research Excellence (#2006797): On Track (Teaching, Research, and Community Knowledges) promoting brain health with older Aboriginal and Torres Strait islander people. Huong X. T. Nguyen is supported by a NHMRC postgraduate scholarship. The funder had no role in the design, execution, analyses or interpretation, or publication of the study.
Nguyen HXT, Bradley K, McNamara BJ, Watson R, Malay R, LoGiudice D. Risk, protective, and biomarkers of dementia in Indigenous peoples: A systematic review. Alzheimer's Dement. 2024;20:563–592. 10.1002/alz.13458
REFERENCES
- 1. Guerchet M, Prince M, Prina M. Numbers of people with dementia worldwide: an update to the estimates in the World Alzheimer Report 2015. 2020.
- 2. Anderson I, Robson B, Connolly M, et al. Indigenous and tribal peoples' health (The Lancet–Lowitja Institute Global Collaboration): a population study. Lancet North Am Ed. 2016;388(10040):131‐157. [DOI] [PubMed] [Google Scholar]
- 3. United Nations permanent forum on indigenous issues: indigenous people, indigenous voice. 2007. Accessed 25/08/2022. https://www.un.org/esa/socdev/unpfii/documents/5session_factsheet1.pdf
- 4. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet North Am Ed. 2020;396(10248):413‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blennow K, Zetterberg H. Biomarkers for Alzheimer's disease: current status and prospects for the future. J Intern Med. 2018;284(6):643‐663. [DOI] [PubMed] [Google Scholar]
- 6. Ashton NJ, Schöll M, Heurling K, et al. Update on biomarkers for amyloid pathology in Alzheimer's disease. Biomark Med. 2018;12(7):799‐812. [DOI] [PubMed] [Google Scholar]
- 7. Ray S, Britschgi M, Herbert C, et al. Classification and prediction of clinical Alzheimer's diagnosis based on plasma signaling proteins. Nat Med. 2007;13(11):1359‐1362. [DOI] [PubMed] [Google Scholar]
- 8. Lange C, Suppa P, Pietrzyk U, et al. Prediction of Alzheimer's dementia in patients with amnestic mild cognitive impairment in clinical routine: incremental value of biomarkers of neurodegeneration and brain amyloidosis added stepwise to cognitive status. J Alzheimers Dis. 2018;61(1):373‐388. [DOI] [PubMed] [Google Scholar]
- 9. Ellis KA, Bush AI, Darby D, et al. The Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging: methodology and baseline characteristics of 1112 individuals recruited for a longitudinal study of Alzheimer's disease. Int Psychogeriatr. 2009;21(4):672‐687. [DOI] [PubMed] [Google Scholar]
- 10. Mueller SG, Weiner MW, Thal LJ, et al. Ways toward an early diagnosis in Alzheimer's disease: the Alzheimer's Disease Neuroimaging Initiative (ADNI). Alzheimers Dement. 2005;1(1):55‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Warren LA, Shi Q, Young K, et al. Prevalence and incidence of dementia among indigenous populations: a systematic review. Int Psychogeriatr. 2015;27(12):1959‐1970. [DOI] [PubMed] [Google Scholar]
- 12. De Souza‐Talarico JN, De Carvalho AP, Brucki SMD, et al. Dementia and cognitive impairment prevalence and associated factors in indigenous populations: a systematic review. Alzheimer Dis Assoc Disord. 2016;30(3):281‐287. [DOI] [PubMed] [Google Scholar]
- 13. Walker JD, Spiro G, Loewen K, et al. Alzheimer's disease and related dementia in indigenous populations: a systematic review of risk factors. J Alzheimer's Dis. 2020;78:1439‐1451. [DOI] [PubMed] [Google Scholar]
- 14. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264‐269. [DOI] [PubMed] [Google Scholar]
- 15. National Heart, Lung, and Blood Institute . Study Quality Assessment Tools. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
- 16. Huria T, Palmer SC, Pitama S, et al. Consolidated criteria for strengthening reporting of health research involving indigenous peoples: the CONSIDER statement. BMC Med Res Method. 2019;19(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harfield S, Pearson O, Morey K, et al. Assessing the quality of health research from an indigenous perspective: the aboriginal and Torres Strait Islander quality appraisal tool. BMC Med Res Method. 2020;20(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pu'un BI, Othman Z, Drahman I. Dementia among elderly Melanau: a community survey of an indigenous people in east Malaysia. Int Medical J. 2014;21(5):468‐471. [Google Scholar]
- 19. Huang F, Shang Y, Luo Y, et al. Lower prevalence of Alzheimer's disease among Tibetans: association with religious and genetic factors. J Alzheimer's Dis. 2016;50(3):659‐667. [DOI] [PubMed] [Google Scholar]
- 20. Chen S, Mima D, Jin H, et al. The association between Neprilysin gene polymorphisms and Alzheimer's disease in Tibetan population. Brain Behav. 2021;11(3):e02002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Smith K, Flicker L, Lautenschlager NT, et al. High prevalence of dementia and cognitive impairment in Indigenous Australians. Neurology. 2008;71(19):1470‐1473. [DOI] [PubMed] [Google Scholar]
- 22. Smith K, Flicker L, Dwyer A, et al. Factors associated with dementia in aboriginal Australians. Aust N Z J Psychiatry. 2010;44(10):888‐893. [DOI] [PubMed] [Google Scholar]
- 23. Cotter PR, Condon JR, Barnes T, et al. Do Indigenous Australians age prematurely? The implications of life expectancy and health conditions of older indigenous people for health and aged care policy. Aust Health Rev. 2012;36(1):68‐74. [DOI] [PubMed] [Google Scholar]
- 24. Li SQ, Guthridge SL, Eswara Aratchige P, et al. Dementia prevalence and incidence among the indigenous and non‐indigenous populations of the Northern Territory. Med J Aust. 2014;200(8):465‐469. [DOI] [PubMed] [Google Scholar]
- 25. Radford K, Mack HA, Draper B, et al. Prevalence of dementia in urban and regional Aboriginal Australians. Alzheimers Dement. 2015;11(3):271‐279. [DOI] [PubMed] [Google Scholar]
- 26. Russell S, Strivens E, LoGiudice D, et al. Ageing on Hammond Island: is there cause for concern in the Torres Strait? Aust J Rural Health. 2016;24(5):342‐343. [DOI] [PubMed] [Google Scholar]
- 27. Lo Giudice D, Smith K, Fenner S, et al. Incidence and predictors of cognitive impairment and dementia in Aboriginal Australians: a follow‐up study of 5 years. Alzheimers Dement. 2016;12(3):252‐261. [DOI] [PubMed] [Google Scholar]
- 28. Radford K, Delbaere K, Draper B, et al. Childhood stress and adversity is associated with late‐life dementia in Aboriginal Australians. Am J Geriatr Psychiatry. 2017;25(10):1097‐1106. [DOI] [PubMed] [Google Scholar]
- 29. Radford K, Lavrencic LM, Delbaere K, et al. Factors associated with the high prevalence of dementia in older Aboriginal Australians. J Alzheimer's Dis. 2019;70(s1):S75‐S85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Derrig H, Lavrencic LM, Broe GA, et al. Mild cognitive impairment in Aboriginal Australians. Alzheimer's Dement Transl Res Clin. 2020;6(1):e12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Russell SG, Quigley R, Thompson F, et al. Prevalence of dementia in the Torres Strait. Australas J Ageing. 2021;40(2):e125‐e132. [DOI] [PubMed] [Google Scholar]
- 32. Ma'u E, Cullum S, Cheung G, et al. Differences in the potential for dementia prevention between major ethnic groups within one country: a cross sectional analysis of population attributable fraction of potentially modifiable risk factors in New Zealand. Lancet Reg Health West Pac. 2021;13:100191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Russell SG, Quigley R, Thompson F, et al. Factors associated with the increased risk of dementia found in the Torres Strait. Australas J Ageing. 2022;41(1):88‐96. [DOI] [PubMed] [Google Scholar]
- 34. Thompson F, Harriss LR, Russell S, et al. Using health check data to investigate cognitive function in aboriginal and Torres Strait Islanders living with diabetes in the Torres Strait. Endocrinol Diabetes Metab J. 2022;5(1):e00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lavrencic LM, Delbaere K, Broe GA, et al. Dementia incidence, APOE genotype, and risk factors for cognitive decline in Aboriginal Australians: a longitudinal cohort study. Neurology. 2022;98(11):e1124‐e1136. [DOI] [PubMed] [Google Scholar]
- 36. Hendrie HC, Hall KS, Pillay N, et al. Alzheimer's disease is rare in Cree. Int Psychogeriatr. 1993;5(1):5‐14. [DOI] [PubMed] [Google Scholar]
- 37. Rosenberg RN, Richter RW, Risser RC, et al. Genetic factors for the development of Alzheimer disease in the Cherokee Indian. Arch Neurol. 1996;53(10):997‐1000. [DOI] [PubMed] [Google Scholar]
- 38. Henderson JN, Crook R, Crook J, et al. Apolipoprotein E4 and tau allele frequencies among Choctaw Indians. Neurosci Lett. 2002;324(1):77‐79. [DOI] [PubMed] [Google Scholar]
- 39. Borenstein AR, Mortimer JA, Schofield E, et al. Cycad exposure and risk of dementia, MCI, and PDC in the Chamorro population of Guam. Neurology. 2007;68(21):1764‐1771. [DOI] [PubMed] [Google Scholar]
- 40. Sundar PD, Yu C‐E, Sieh W, et al. Two sites in the MAPT region confer genetic risk for Guam ALS/PDC and dementia. Hum Mol Genet. 2007;16(3):295‐306. [DOI] [PubMed] [Google Scholar]
- 41. Galasko D, Salmon D, Gamst A, et al. Prevalence of dementia in Chamorros on Guam: relationship to age, gender, education, and APOE. Neurology. 2007;68(21):1772‐1781. [DOI] [PubMed] [Google Scholar]
- 42. Jervis LL, Beals J, Fickenscher A, et al. Performance on the mini‐mental state examination and Mattis Dementia Rating Scale among older American Indians. J Neuropsychiatry Clin Neurosci. 2007;19(2):173‐178. [DOI] [PubMed] [Google Scholar]
- 43. Weiner MF, Rosenberg RN, Womack KB, et al. Atherosclerosis risk factors in American Indians with Alzheimer disease: preliminary findings. Alzheimer Dis Assoc Disord. 2008;22(3):245‐248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Weiner MF, de la Plata CM, Fields BAJ, et al. Brain MRI, apoliprotein E genotype, and plasma homocysteine in American Indian Alzheimer disease patients and Indian controls. Curr Alzheimer Res. 2009;6(1):52‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Weiner MF, Hynan LS, Rossetti H, et al. The relationship of cardiovascular risk factors to Alzheimer disease in Choctaw Indians. Am J Geriatr Psychiatry. 2011;19(5):423‐429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jacklin KM, Walker JD, Shawande M. The emergence of dementia as a health concern among First Nations populations in Alberta, Canada. Can J Public Health. 2012;104(1):e39‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. MacDonald JP, Barnes DE, Middleton LE. Implications of risk factors for Alzheimer's disease in Canada's indigenous population. Can Geriatr J. 2015;18(3):152‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mayeda ER, Glymour MM, Quesenberry CP, et al. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement. 2016;12(3):216‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kirkpatrick AC, Stoner JA, Donna‐Ferreira F, et al. High rates of undiagnosed vascular cognitive impairment among American Indian veterans. GeroScience. 2019;41(1):69‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Carty CL, Noonan C, Muller C, et al. Risk factors for Alzheimer's disease and related dementia diagnoses in American Indians. Ethn Dis Fall. 2020;30(4):671‐680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Smith M, Van N, Roberts A, et al. Disparities in Alzheimer disease and mild cognitive impairment among native Hawaiians and Pacific Islanders. Cogn Behav Neurol. 2021;34(3):200‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Suchy‐Dicey AM, Nelson LA, Su Y, et al. APOE genotype, brain imaging, and neuropsychological testing markers of Alzheimer's disease: data from the Strong Heart Study: epidemiology: dementia and risk in underrepresented populations. Alzheimer's & Dementia. 2020;16:e044951. [Google Scholar]
- 53. Stryjer R, Spivak B, Shiloh R, et al. Prevalence of dementia in an elderly illiterate native Mexican population of indigenous origin. J Public Health Epidemiol. 2011;3(11):556‐559. [Google Scholar]
- 54. Brucki SM, Nitrini R, Brucki SMD, et al. Subjective memory impairment in a rural population with low education in the Amazon rainforest: an exploratory study. Int Psychogeriatr. 2009;21(1):164‐171. [DOI] [PubMed] [Google Scholar]
- 55. Marca‐Ysabel MV, Rajabli F, Cornejo‐Olivas M, et al. Dissecting the role of amerindian genetic ancestry and the ApoE T4 allele on Alzheimer disease in an admixed Peruvian population. Neurobiol Aging. 2021;101:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gatz M, Mack WJ, Chui HC, et al. Prevalence of dementia and mild cognitive impairment in indigenous Bolivian forager‐horticulturalists. Alzheimers Dement. 2023;19(1):44‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673‐2734. [DOI] [PubMed] [Google Scholar]
- 58. Coates K, Coates KS. A global history of indigenous peoples. Springer; 2004. [Google Scholar]
- 59. Prince M, Bryce R, Albanese E, et al. The global prevalence of dementia: a systematic review and meta‐analysis. Alzheimers Dement. 2013;9(1):63‐75. e2. [DOI] [PubMed] [Google Scholar]
- 60. 2023 Alzheimer's disease facts and figures. Alzheimers Dement. 2023;19(4):1598‐1695. [DOI] [PubMed] [Google Scholar]
- 61. Li J, Joshi P, Ang TFA, et al. Mid‐ to late‐life body mass index and dementia risk: 38 years of follow‐up of the Framingham Study. Am J Epidemiol. 2021;190(12):2503‐2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mielke MM, Aggarwal NT, Vila‐Castelar C, et al. Consideration of sex and gender in Alzheimer's disease and related disorders from a global perspective. Alzheimers Dement. 2022;18(12):2707‐2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Eratne D, Loi SM, Farrand S, et al. Alzheimer's disease: clinical update on epidemiology, pathophysiology and diagnosis. Australas Psychiatry. 2018;26(4):347‐357. [DOI] [PubMed] [Google Scholar]
- 64. Lim U, Wang S, Park SY, et al. Risk of Alzheimer's disease and related dementia by sex and race/ethnicity: the Multiethnic Cohort Study. Alzheimers Dement. 2022;18(9):1625‐1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: a meta‐analysis. JAMA. 1997;278(16):1349‐1356. [PubMed] [Google Scholar]
- 66. Loy CT, Schofield PR, Turner AM, et al. Genetics of dementia. Lancet. 2014;383(9919):828‐840. [DOI] [PubMed] [Google Scholar]
- 67. Suchy‐Dicey A, Shibata D, Cholerton B, et al. Cognitive correlates of MRI‐defined cerebral vascular injury and atrophy in elderly American Indians: the Strong Heart Study. J Int Neuropsychol Soc. 2020;26(3):263‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gavett BE, Fletcher E, Harvey D, et al. Ethnoracial differences in brain structure change and cognitive change. Neuropsychology. 2018;32(5):529‐540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hulko W, Camille E, Antifeau E, et al. Views of first nation elders on memory loss and memory care in later life. J Cross Cult Gerontol. 2010;25(4):317‐342. [DOI] [PubMed] [Google Scholar]
- 70. Kinchin I, Mccalman J, Bainbridge R, et al. Does indigenous health research have impact? A systematic review of reviews. Int J Equity Health. 2017;16(1):1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):263‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Jack CR Jr, Bennett DA, Blennow K, et al. A/T/N: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016;87(5):539‐547. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Supporting information


