Abstract
Background:
This study aimed to determine the reasons for conversion and elucidate the safety and efficacy of transition to tenofovir alafenamide/emtricitabine/bictegravir sodium (TAF/FTC/BIC) in highly active antiretroviral therapy (HAART)-experienced HIV-infected patients in real-world settings.
Methods:
We conducted a retrospective cohort study. The treatment conversion rationales, safety, and effectiveness in 1684 HIV-infected patients with previous HAART experience who switched to TAF/FTC/BIC were evaluated at Beijing Ditan Hospital from September 2021 to Auguest 2022.
Results:
Regimen simplification (990/1684, 58.79%) was the most common reason for switching, followed by osteoporosis or osteopenia (375/1684, 22.27%), liver dysfunction (231/1684, 13.72%), decline in tenofovir alafenamide/emtricitabine/elvitegravir/cobicistat (TAF/FTC/EVG/c) with food restriction (215/1684, 12.77%), virological failure (116/1684, 6.89%), and renal dysfunction (90/1684, 5.34%). In patients receiving non-nucleotide reverse transcriptase inhibitors (NNRTI)-containing regimens, lipid panel changes 1 year after switching indicated a difference of 3.27 ± 1.10 mmol/L vs. 3.40 ± 1.59 mmol/L in triglyceride (P = 0.014), 4.82 ± 0.74 mmol/L vs. 4.88 ± 0.72 mmol/L in total cholesterol (P = 0.038), 3.09 ± 0.70 mmol/L vs. 3.18 ± 0.66 mmol/L in low-density lipoprotein (P <0.001), and 0.99 ± 0.11 mmol/L vs. 0.95 ± 0.10 mmol/L in high-density lipoprotein (P <0.001). Conversely, among patients receiving booster-containing regimens, including TAF/FTC/EVG/c and lopinavir/ritonavir (LPV/r), lipid panel changes presented decreased trends. We also observed an improved trend in viral load suppression, and alanine transaminase (ALT), aspartate transaminase (AST), estimated glomerular filtration rate (eGFR), and serum creatinine levels after the transition (P <0.001).
Conclusion:
The transition to TAF/FTC/BIC demonstrated good treatment potency. Furthermore, this study elucidates the motivations behind the adoption of TAF/FTC/BIC in real-world scenarios, providing clinical evidence supporting the stable conversion to TAF/FTC/BIC for HAART-experienced patients.
Keywords: HIV; Antiretroviral therapy, highly active; Conversion; Viral load; Efficacy; Safety
Introduction
With the advent of the highly active antiretroviral therapy (HAART), the immune-suppression has been gradually recovering, leading to a dramatic reduction in human immunodeficiency virus (HIV) associated mortality and morbidity among the individuals with HIV. This has led to an extension of their lifespan, bringing them closer to the life expectancy of the general population.[1] Life-long HAART has been necessary, but adverse effects (AEs) and non-AIDS-defining events (NADEs) inevitably occur over time, impacting patient adherence and quality of life.[2] Previous studies have reported that AEs are the primary reasons for patients in clinical trials switching or discontinuing HAART.[3]
According to the guidelines for the diagnosis and treatment of HIV/acquired immune deficiency syndrome (AIDS) in China,[4] one of the preferred antiretroviral regimens comprises a combination of two nucleotide reverse transcriptase inhibitors (NRTIs), typically tenofovir disoproxil fumarate/lamivudine (TDF/3TC), along with a non-nucleotide reverse transcriptase inhibitor (NNRTI) such as efavirenz (EFV) or booster-containing protease inhibitor lopinavir/ritonavir (LPV/r). The main reasons for transitioning from HAART among the HIV-infected population in China are side effects, including lowered bone mineral density (BMD), rash, abnormal dreams, dizziness, headache, depression, drug-induced liver injuries (DILI), and metabolic changes.[4] This highlights the need for a well-tolerated, more potent, and simpler antiretroviral regimen among patients in China.
Tenofovir alafenamide/emtricitabine/bictegravir sodium (TAF/FTC/BIC, Biktarvy, GileadSciences, Inc. CA, USA) is a single-tablet co-formulated regimen offering a higher genetic barrier and reduced pill burden. Two pivotal phase 3 clinical trials, conducted as randomized, double-blind, multicenter, non-inferiority trials (NCT02607930[5] and NCT02607956[6]), have demonstrated that TAF/FTC/BIC is a safe, well-tolerated, and durable antiretroviral regimen, based on 144-week data. Another clinical trial (NCT02603120[7]) indicated that switching to TAF/FTC/BIC is non-inferior to the use of dolutegravir/abacavir/lamivudine (DTG/ABC/3TC); TAF/FTC/BIC displayed a notably lower incidence of treatment-associated AEs compared to dolutegravir.
In 2021, TAF/FTC/BIC was included in China’s National Reimbursement Drug List, making it accessible through resident medical insurance. A growing number of HAART-experienced patients gradually switched to TAF/FTC/BIC in the past year. However, there remains uncertainty regarding the reasons behind this transition and its impacts on patients. The objective of this study was to elucidate the reasons for conversion, as well as the safety, efficacy, and side effects of switching to TAF/FTC/BIC among HAART-experienced HIV-infected patients in real-world scenarios.
Methods
Ethical consideration
This retrospective study was conducted at Beijing Ditan Hospital, Capital Medical University, which is the referral hospital for HIV/AIDS cases in China. The Health Science Ethical Committee granted approval for the collection of clinical data in this anonymous study (No. DTEC-KY2021-022-03) based on an observational cohort and authorized exemption from informed consent.
Study population
We conducted an observational cohort study, with eligibility criteria as follows: age >18 years, HAART experience, switching to TAF/FTC/BIC treatment, and undergoing follow-up for 1 year. Participants were excluded if they were HAART-naïve and initiated TAF/FTC/BIC or those who were HAART-experienced and switched to TAF/FTC/BIC for less than 1 year.
Study procedure
In accordance with the National Free Antiretroviral Treatment Program (NFATP),[8] free antiretroviral regimens comprising 2NRTIs + NNRTI or LPV/r were provided to the HIV-infected population. Additionally, TAF/FTC/BIC was incorporated into the National Reimbursement Drug List in 2021 and became available through resident medical insurance. Patients had the option to receive either free or medically insured antiretroviral regimens. By 2021, some patients receiving free HAART were transitioned to TAF/FTC/BIC. This study aimed to evaluate the reasons for switching, assess antiretroviral efficacy, gage tolerability, and examine changes in serum lipid profiles and BMD among patients on TAF/FTC/BIC after 1 year of follow-up.
The clinical data were abstracted and collected from electronic medical records encompassing demographic data, clinical characteristics, reason for treatment change, and laboratory tests, in which liver function, renal function, and serum lipid profile (triglyceride [TG], total cholesterol [TC], low-density lipoprotein [LDL], and high-density lipoprotein [HDL]) were monitored every 3–6 months. CD4 cell counts (Flow cytometry, Thermo Fisher Scientific, MA, USA), HIV viral load (VL) (real-time quantitative polymerase chain reaction), and BMD were assessed annually in accordance with antiretroviral therapy guidelines in China.[4]
Clinical definition
Dyslipidemia was diagnosed based on the following criteria: TG ≥2.3 mmol/L was defined as hypertriglyceridemia, TC >6.2 mmol/L was defined as hypercholesterolemia, LDL >3.12 mmol/L and HDL <1 mmol/L, as per the guidelines on the prevention and treatment of dyslipidemia in adults in China.[9]
Liver and renal functions were evaluated periodically in patients undergoing HAART, and liver dysfunction was evaluated based on established diagnostic criteria.[10] Renal dysfunction was evaluated based on the estimated glomerular filtration rate (eGFR) and was defined as eGFR <90 mL∙min–1∙1.73 m–2 and/or the presence of markers indicative of renal damage persisting for at least 3 months.
Virological success was defined as achieving a VL with lower target not detected (TND) while virological failure was defined as a VL above TND six months after receiving HAART.[4]
In HIV-infected patients receiving HAART, side effects on the central nervous system included abnormal dreams, nightmares, dizziness, headaches, and depression.
BMD was evaluated yearly in patients receiving HAART, using dual-energy X-ray absorptiometry (DXA). These evaluations included scans of the lumbar spine (L1–L4) and right and left hips, with osteopenia or osteoporosis defined as T/Z scores falling between –1.0 to –2.5 and less than –2.5, respectively.[11]
Assessment of efficacy and safety
Efficacy assessment was used to evaluate HIV VL changes 12 months after switching to TAF/FTC/BIC among HAART-experienced patients.
Safety assessments included the evaluation of changes in the lipid panel, including TG, TC, LDL, HDL, and BMD at the lumbar spine (L1–L4) and the right and left hips, also conducted 12 months after the switch to TAF/FTC/BIC in HAART-experienced patients. Liver and renal functions were assessed at the same 12-month post-transition mark.
Statistical analysis
Categorical variables were expressed as percentages, while continuous variables were presented as mean ± standard deviation or median (interquartile range [IQR]) due to their statistical distribution. Column charts were employed to visualize changes in the lipid panel and BMD one year after the transition to TAF/FTC/BIC. The change in the lipid panel and BMD was calculated based on the paired samples t-test, while the chi-squared test was used to evaluate the prevalence of hepatic and renal dysfunction 1 year after switching to TAF/FTC/BIC. All statistical analyses were performed using SPSS (version 22.0; SPSS Institute, Chicago IL, USA), with a significance level set as P <0.05 for all statistical tests.
Results
Baseline characteristics and previous HAART components
Starting in September 2021, we enrolled 1684 HIV-infected patients who previously received HAART for inclusion in this study. The median age of these patients was 37.8 years (IQR: 31.0–42.0 years). Additionally, the average duration of HAART for these individuals was 5.6 years (IQR: 3.0–8.0 years). Within this cohort, baseline CD4 cell counts averaged at 666.79 cells/μL (IQR: 462.00–829.25 cells/μL), and an HIV VL above TND was detected in 116 patients (6.89%).
Among the study patients, the baseline HAART components indicated TDF + 3TC as the most common backbone regimen (1439/1684, 85.45%), followed by zidovudine + lamivudine (AZT + 3TC) (26/1684, 1.54%), whereas EFV was the most common anchor drug (1396/1684, 82.90%), followed by LPV/r (31/1684, 1.84%), in accordance with the NFATP in China. A total of 215 patients (215/2164, 12.77%) also received TAF/FTC/EVG/c, another single-tablet co-formulated regimen. Table 1 details the baseline clinical characteristics, previous HAART components, and baseline laboratory tests, including the lipid panel and hepatic and renal functions.
Table 1.
Baseline clinical and laboratory characteristics and previous HAART components.
| Characteristics | Values (n = 1684) |
|---|---|
| Age (years) | 37.8 (31.0, 42.0) |
| Male | 1628 (96.67) |
| Time on HAART (years) | 5.6 (3.0, 8.0) |
| Baseline CD4 T cells (cells/μL) | 666.79 (462.00, 829.25) |
| Baseline VL | |
| TND | 1568 (93.11) |
| Above TND | 116 (6.89) |
| Baseline laboratory test | |
| TG (mmol/L) | 3.23 ± 1.17 |
| TC (mmol/L) | 4.84 ± 0.75 |
| LDL (mmol/L) | 3.14 ± 0.70 |
| HDL (mmol/L) | 0.99 ± 0.12 |
| ALT (U/L) | 34.70 ± 10.75 |
| AST (U/L) | 25.20 ± 8.52 |
| T-BIL (μmol/L) | 12.66 ± 2.99 |
| D-BIL (μmol/L) | 5.12 ± 2.24 |
| eGFR (mL · min–1 · 1.73 m–2) | 103.02 ± 9.16 |
| Cr (μmol/L) | 77.72 ± 10.34 |
| Previous ART components | |
| ABC/3TC/DTG | 3 (0.18) |
| TAF/FTC/EVG/c | 215 (12.77) |
| TDF + 3TC | 1439 (85.45) |
| AZT + 3TC | 26 (1.54) |
| ABC + 3TC | 1 (0.06) |
| LPV/r | 31 (1.84) |
| EFV | 1396 (82.90) |
| NVP | 10 (0.59) |
| RAL | 1 (0.06) |
| DTG | 28 (1.66) |
Data were shown as n (%), mean ± standard deviation, or median (interquartile range). 3TC: Lamivudine; ABC: Abacavir; ALT: Alanine transaminase; ART: antiretroviral therapy; AST: Aspartate transaminase; AZT: Zidovudine; Cr: Creatinine; D-BIL: Direct bilirubin; DTG: Dolutegravir; EFV: Efavirenz; eGFR: Estimated glomerular filtration rate; EVG/c: Elvitegravir/cobicistat; FTC: Emtricitabine; HAART: Highly active antiretroviral therapy; HDL: High-density lipoprotein; LDL: Low-density lipoprotein; LPV/r: Lopinavir/ritonavir; NVP: Nevirapine; RAL: Raltegravir; TAF: Tenofovir alafenamide; T-BIL: Total bilirubin; TC: Total cholesterol; TDF: Tenofovir disoproxil fumarate; TG: Triglyceride; TND: Target not detected; VL: Viral load.
The reasons for the switch to TAF/FTC/BIC among HAART-experienced patients
Among HAART-experienced patients who transitioned to TAF/FTC/BIC treatment, simplifying regimens (990/1684, 58.79%) were the primary motive for this switch, followed by osteoporosis or osteopenia (375/1684, 22.27%), liver dysfunction (231/1684, 13.72%), the inability to adhere to TAF/FTC/EVG/c with food restrictions (215/1684, 12.77%), virological failure (116/1684, 6.89%), renal dysfunction (90/1684, 5.34%), central nervous system side effects (47/1684, 2.79%), and dyslipidemia (30/1684, 1.78%) [Table 2]. It is worth noting that these reasons were not mutually exclusive.
Table 2.
The reasons for the switch to TAF/FTC/BIC among HAART-experienced patients.
| Reasons | Values (n = 1684) |
|---|---|
| Simplify regimens | 990 (58.79) |
| Osteoporosis or osteopenia | 375 (22.27) |
| Liver dysfunction | 231 (13.72) |
| Decline to taking ART with food | 215 (12.77) |
| Virological failure | 116 (6.89) |
| Renal dysfunction | 90 (5.34) |
| CNS side effects | 47 (2.79) |
| Dyslipidemia | 30 (1.78) |
Data were presented as n (%). These reasons were not mutually exclusive. ART: Antiretroviral therapy; CNS: Central nervous system; HAART: Highly active antiretroviral therapy; TAF/FTC/BIC: Tenofovir alafenamide/emtricitabine/bictegravir sodium.
The safety and efficacy after switching to TAF/FTC/BIC
Lipid changes
The study cohort was divided into three subcategories: patients receiving LPV/r-containing regimens, patients receiving TAF/FTC/EVG/c, and patients receiving 2NRTIs + NNRTIs at baseline. We examined lipid panel changes one year after switching within these three subcategories.
Among patients receiving 2NRTIs + NNRTI regimens at baseline, compared to baseline, there was a notable increase in lipid panel changes observed 12 months after transitioning to TAF/FTC/BIC: 3.27 ± 1.10 mmol/L vs. 3.40 ± 1.59 mmol/L in TG (t = 4.547, P = 0.014), 4.82 ± 0.74 mmol/L vs. 4.88 ± 0.72 mmol/L in TC (t = 18.715, P = 0.038), 3.09 ± 0.70 mmol/L vs. 3.18 ± 0.66 mmol/L in LDL (t = 18.733, P <0.001), and 0.99 ± 0.11 mmol/L vs. 0.95 ± 0.10 mmol/L in HDL (t = –17.830, P <0.001), respectively. These findings indicated a significant increase in serum lipid panel one year after the switch in this subcategory [Figure 1 and Supplementary Table 1, http://links.lww.com/CM9/B842].
Figure 1.
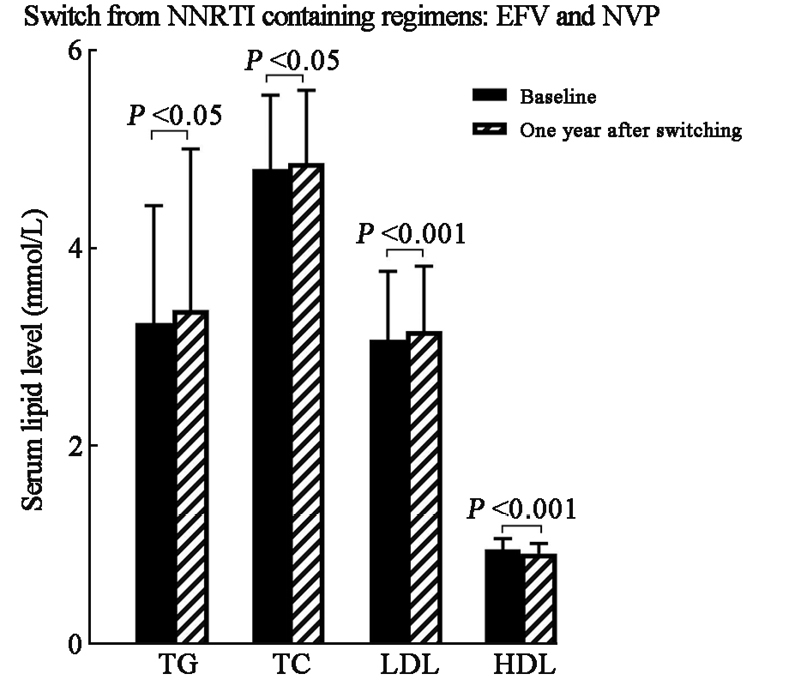
Serum lipid change 1 year after switching to TAF/FTC/BIC among subcategories receiving NNRTI-containing regimens at baseline. BIC: Bictegravir sodium; EFV: Efavirenz; FTC: Emtricitabine; HDL: High-density lipoprotein; LDL: Low-density lipoprotein; NNRTI: Non-nucleotide reverse transcriptase inhibitor; NVP: Nevirapine; TAF: Tenofovir alafenamide; TC: Total cholesterol; TG: Triglyceride.
Among patients receiving LPV/r-containing regimens at baseline, a comparison between baseline and the lipid panel measurements one year after the switch indicated the following changes: 3.02 ± 1.10 mmol/L vs. 2.99 ± 1.13 mmol/L in TG (t = –0.822, P = 0.807), 4.61 ± 1.03 mmol/L vs. 4.57 ± 1.18 mmol/L in TC (t = –0.476, P = 0.417), 2.85 ± 0.93 mmol/L vs. 2.80 ± 0.97 mmol/L in LDL (t = –0.597, P = 0.837), and 0.99 ± 0.19 mmol/L vs. 1.05 ± 0.17 mmol/L in HDL (t = 2.323, P = 0.142), respectively. These results suggested a decreased trend in serum lipid panel year after the switch from LPV/r-containing regimens though without statistical significance. [Figure 2 and Supplementary Table 1, http://links.lww.com/CM9/B842].
Figure 2.
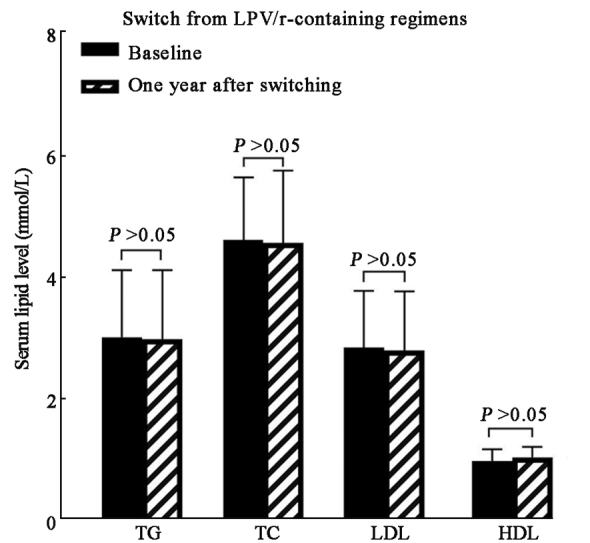
Serum lipid change 1 year after switching to TAF/FTC/BIC among subcategories receiving LPV/r-containing regimens at baseline. BIC: Bictegravir sodium; FTC: Emtricitabine; HDL: High-density lipoprotein; LDL: Low-density lipoprotein; LPV/r: Lopinavir/ritonavir; TAF: Tenofovir alafenamide; TC: Total cholesterol; TG: Triglyceride.
Among patients receiving TAF/FTC/EVG/c at baseline, lipid panel changes indicated the following results: 2.96 ± 1.01 mmol/L vs. 2.92 ± 0.93 mmol/L in TG (t = –3.598, P = 0.634), 4.95 ± 0.73 mmol/L vs. 4.82 ± 0.71 mmol/L in TC (t = –11.473, P = 0.032), 3.51 ± 0.53 mmol/L vs. 3.43 ± 0.47 mmol/L in LDL (t = –5.904, P = 0.081), and 1.03 ± 0.16 mmol/L vs. 1.07 ± 0.13 mmol/L in HDL (t = 6.003, P = 0.007), respectively, indicating that serum TC level decreased and HDL level increased significantly, while TG and LDL showed a decreasing trend one year after the switch within this subcategory [Figure 3 and Supplementary Table 1, http://links.lww.com/CM9/B842].
Figure 3.
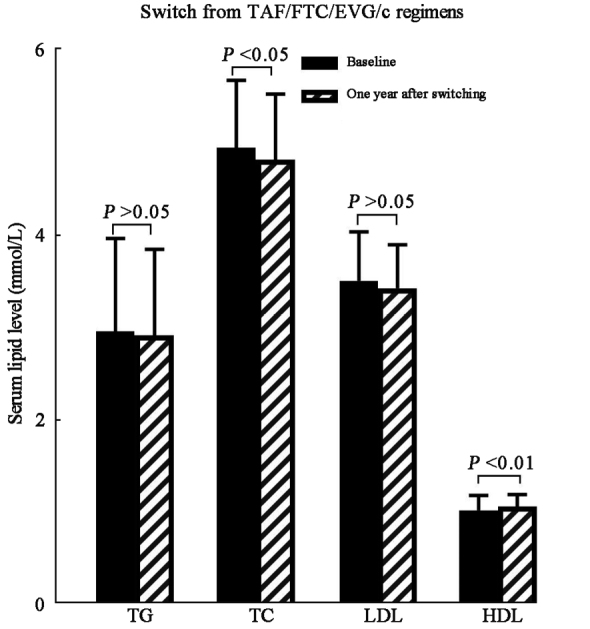
Serum lipid change 1 year after switching to TAF/FTC/BIC among subcategories receiving TAF/FTC/EVG/c at baseline. BIC: Bictegravir sodium; EVG/c: Elvitegravir/cobicistat; FTC: Emtricitabine; HDL: High-density lipoprotein; LDL: Low-density lipoprotein; TAF: Tenofovir alafenamide; TC: Total cholesterol; TG: Triglyceride.
Change of absolute value of BMD
BMD was evaluated at baseline and one year after switching to TAF/FTC/BIC, based on DXA scans of the lumbar spine (L1–L4) and the right and left hips. Although the absolute BMD value did not significantly improve, there was a trend of improvement in BMD of the lumbar spine (L1–L4) one year after transitioning to TAF/FTC/BIC treatment in these patients [Figure 4 and Supplementary Table 2, http://links.lww.com/CM9/B842].
Figure 4.
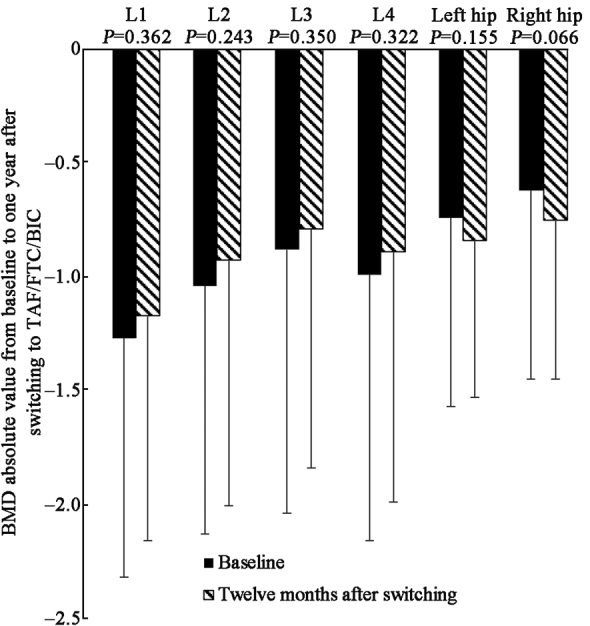
Change of BMD absolute value at baseline and 1 year after switching to TAF/FTC/BIC. BIC: Bictegravir sodium; BMD: Bone mineral density; FTC: Emtricitabine; TAF: Tenofovir alafenamide.
Evaluation of HIV VL
Among these HAART-experienced patients switching to TAF/FTC/BIC, which included 116 patients with a baseline VL above TND, ranged from 98 copies/mL to 95,421 copies/mL, resistance was detected among patients with VL >1000 copies/mL, and TAF/FTC/BIC was switched based on resistance results. The HIV VL was tested periodically to detect virological failure, and results indicated that the VL was suppressed below the threshold of detection after 12 months of switching, suggesting potent antiretroviral effects.
Hepatic and renal function
Hepatic and renal functions were evaluated at baseline and one year after the transition to TAF/FTC/BIC, and the results indicated that TAF/FTC/BIC improved transaminase ALT, AST, eGFR, and serum creatinine levels (P <0.001) [Supplementary Table 3, http://links.lww.com/CM9/B842], which indicated that prevalence of hepatic and renal dysfunction decreased after switching to TAF/FTC/BIC.
Discussion
The inclusion of TAF/FTC/BIC in the National Reimbursement Drug List in China represents a notable development. This study marks the evaluation of the reasons behind conversion to TAF/FTC/BIC treatment, along with an assessment of its safety and efficacy within a real-world observational cohort in China. Our findings indicate that this switch resulted in favorable outcomes regarding viral suppression, safety, and efficacy in a real-world setting.
In this study, we found that simplified regimens were the primary motivation for transitioning to TAF/FTC/BIC. Cao et al[12] reported that, despite substantial developments in the NFATP in China, maintaining adherence to optimal regimens remains a huge challenge. The NFATP-recommended HAART, consisting of 2NRTIs + NNRTI/PI/r, affected the adherence of patients due to excessive tablet burden. In contrast, TAF/FTC/BIC, a single-tablet co-formulated regimen, provided optimal regimens and improved compliance, which contributed to enhanced virological success among the HIV-infected population.
Among the HAART-experienced patients who transitioned to TAF/FTC/BIC, a group consisting of 116 patients with a VL above TND at baseline, the evaluation of VL indicated virological success one year after the switch. TAF/FTC/BIC incorporates an integrase strand transfer inhibitor (INSTI) with a high genetic barrier, a characteristic that facilitates rapid HAART initiation and contributes to effective virological suppression.[13] In this study, the 116 patients with VL above TND received 2NRTIs + NNRTI at baseline switched to TAF/FTC/BIC treatment. This new regimen, characterized by a high genetic barrier, led to the complete suppression of HIV VL,[14] indicating the efficacy of TAF/FTC/BIC in suppressing HIV viral replication in the real world.
In this study, we found that, among the subcategories receiving NNRTI-containing regimens at baseline, particularly TDF + 3TC + EFV, there was a notable increase in serum lipid panel parameters, including TG, TC, and LDL, one year after switching to TAF/FTC/BIC, while HDL decreased significantly in vivo. Previous studies have indicated that switching from TDF to TAF in antiretroviral treatment regimens affects the lipid profile.[15] According to these studies, 38% of patients receiving TDF-containing regimens presented with serum LDL levels above cardiovascular targets. This prevalence increased to 60% after the transition to TAF-containing regimens. Plum et al[16] also conducted a retrospective study, confirming that switching from TDF- to TAF-based regimens results in a significant increase in serum TG, TC, and HDL levels. Their calculations indicated that cardiovascular risk increased after switching from TDF- to TAF-based regimens due to dyslipidemia. A multicenter cohort study indicated that,[17] after an 18-month follow-up, switching from TDF- to TAF-containing regimens led to increases in adjusted mean TC, HDL, LDL, and TG levels in vivo, indicating that switching to TAF among patients receiving TDF-containing regimens worsened serum lipid panels.
The slight elevation in lipoproteins observed after switching from NNRTI or dolutegravir/raltegravir was mainly due to the withdrawal of tenofovir, a drug known for its lipid-lowering effect.
Previous studies indicated that antiretroviral regimens, including backbone drugs, switched from TDF/FTC to TAF/FTC with different anchor drugs including NNRTI and INSTIs substantially increased serum TG, TC, and LDL levels.[15] This finding is consistent with our conclusion, and indicates that TAF/FTC/BIC worsens serum lipid panels and increases the risk of cardiovascular disorders. Therefore, it is advisable for HIV-infected patients receiving TAF/FTC/BIC to undergo periodic screening of their serum lipid panels and other cardiovascular risk factors.
Among the patients receiving LPV/r-containing regimens at baseline, we found a decreasing trend in the serum lipid panel one year after the switch, while among the patients receiving TAF/FTC/EVG/c, serum TC levels decreased and HDL level increased significantly, while TG and LDL levels also exhibited a trend of reductions. These two regimens included booster ritonavir and cobicistat, which resulted in increased serum lipid levels in vivo. Gatell et al[18] reported that switching from a ritonavir-boosted protease inhibitor to a dolutegravir-based regimen in patients with high cardiovascular risk resulted in improved fasting lipid levels. However, Daar et al[19] found that switching to fixed-dose TAF/FTC/BIC from boosted protease inhibitor-based regimens did not result in lipid changes, indicating that further investigation is warranted to assess lipid changes in patients switching from booster-containing regimens to TAF/FTC/BIC.
Although the TDF-containing regimen is recommended as the first-line treatment according to the guidelines for antiretroviral therapy in China, the bone-related complications, such as osteopenia and osteoporosis, are currently the focus of attention. Among HAART-naïve patients, the initiation of a TDF-containing regimen was consistently associated with a reduction in bone density.[20] A meta-analysis and systematic review indicated that,[21] in comparison with TDF, the TAF-containing regimen presented a lower loss of BMD in the treatment of both HAART-naïve and HAART-experienced patients. Specifically, the TAF-containing regimen resulted in reduced loss of BMD in the spine and hip among HAART-naive patients, while the group that switched from the TDF- to the TAF-containing regimen showed increases in hip and spine BMD by 1.47% and 1.56%, respectively. Kanda et al[22] also indicated that within the HIV-infected population, spine BMD significantly increased, while hip BMD showed an increasing trend after switching from a TDF- to a TAF-containing regimen at the 1-year follow-up. The protective mechanism of TAF on bone density remains unclear, but previous research has indicated that TAF functions as an alanine ester prodrug of tenofovir (TFV), resulting in higher intracellular drug concentrations within HIV-infected cells (as determined by pharmacokinetic studies) and lower serum TFV concentration than those found with TDF.[23,24] The reduced TFV serum concentration decreased uptake by osteoblasts in vivo, ultimately mitigating BMD loss.[25] In this study, we found that switching to TAF/BIC/BIC results in an increased trend in spine BMD at the 1-year follow-up, indicating that the use of DXA is advisable for screening osteopenia or osteoporosis in patients receiving HAART, especially for those on TDF-contained regimens. Furthermore, TAF/FTC/BIC may be a viable alternative for individuals within the HIV-infected population who are experiencing a reduction in bone density.
In addition to adverse bone events, the initiation of a TDF-containing regimen was associated with renal dysfunction, which occurred in 1% of patients. Compared with TDF, TAF exhibited a 90% reduction in serum TFV concentration, leading to lower nephrotoxicity in patients receiving the TAF-containing regimen. A meta-analysis and systematic review indicated superior safety profiles for TAF compared to TDF in pooled data from 26 clinical studies spanning 12,500 person-years of follow-up.[26] Surial et al[17] reported that, among HIV-infected patients from the Swiss HIV Cohort Study who transitioned from TDF to a TAF-containing regimen, the regimen switch led to improvements in eGFR and proteinuria in this cohort. These findings are consistent with our conclusion, indicating that periodic screening of renal function is necessary for patients on HAART, especially among those on TDF-containing regimens, and that TAF/FTC/BIC is a viable option for the HIV-infected population.
This study had several limitations. Firstly, it was retrospective rather than prospective, and potential bias was present in this study. Secondly, the study included only 1684 HIV-infected patients who switched to TAF/FTC/BIC, and it was conducted as a single-center study. The results should be further evaluated in different medical centers. Thirdly, the conclusions drawn in this study were based on clinical data from a 1-year switch to TAF/FTC/BIC, which necessitates further evaluation through long-term observations.
In this study, we found that TAF/FTC/BIC presented strong virological efficacy. It leads to an increase in serum lipid panel for patients using NNRTI-containing regimens, a decrease trend in lipid panel for those using booster-containing regimens, an improved spine BMD trend, and protective effects on hepatic and renal functions one year after switching to this treatment. Additionally, we have investigated the rationale behind the transition to TAF/FTC/BIC treatment in real-world clinical settings, providing valuable clinical evidence supporting the stable transition to TAF/FTC/BIC among HAART-experienced patients in China.
Funding
This work was supported by the Beijing Municipal Administration of Hospitals’ Ascent Plan (No. DFL20191802), Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (No. ZYLX202126), and Capital’s Funds for Health Improvement and Research (No. 2020-2-2174).
Conflicts of interest
None.
Supplementary Material
Footnotes
How to cite this article: Xiao J, Gao GJ, Ding Y, Li JL, Gao CY, Xu QH, Wu L, Liang HY, Ni L, Wang F, Duan YJ, Yang D, Zhao HX. Reasons, safety and efficacy analysis for conversion of HAART-experienced patients to TAF/FTC/BIC. Chin Med J 2023;136:2931–2937. doi: 10.1097/CM9.0000000000002939
References
- 1.Zhang F Dou Z Ma Y Zhao Y Liu Z Bulterys M, et al. Five-year outcomes of the China National Free Antiretroviral Treatment Program. Ann Intern Med 2009;151:241–251. doi: 10.7326/0003-4819-151-4-200908180-00006. [DOI] [PubMed] [Google Scholar]
- 2.Crowell TA Ganesan A Berry SA Deiss RG Agan BK Okulicz JF, et al. Hospitalizations among HIV controllers and persons with medically controlled HIV in the U.S. Military HIV Natural History Study. J Int AIDS Soc 2016;19:20524. doi: 10.7448/IAS.19.1.20524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arribas JR DeJesus E van Lunzen J Zurawski C Doroana M Towner W, et al. Simplification to single-tablet regimen of elvitegravir, cobicistat, emtricitabine, tenofovir DF from multi-tablet ritonavir-boosted protease inhibitor plus coformulated emtricitabine and tenofovir DF regimens: Week 96 results of STRATEGY-PI. HIV Clin Trials 2017;18:118–125. doi: 10.1080/15284336.2017.1330440. [DOI] [PubMed] [Google Scholar]
- 4.AIDS and Hepatitis C Professional Group, Society of Infectious Diseases, Chinese Medical Association, Chinese Center for Disease Control and Prevention . Chinese guidelines for diagnosis and treatment of HIV/AIDS (in Chinese). Chin J AIDS STD 2021;27:1182–1201. doi: 10.13419/j.cnki.aids.2021.11.02 [Google Scholar]
- 5.Gallant J Lazzarin A Mills A Orkin C Podzamczer D Tebas P, et al. Bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection (GS-US-380-1489): A double-blind, multicentre, phase 3, randomised controlled non-inferiority trial. Lancet 2017;390:2063–2072. doi: 10.1016/S0140-6736(17)32299-7. [DOI] [PubMed] [Google Scholar]
- 6.Orkin C DeJesus E Sax PE Arribas JR Gupta SK Martorell C, et al. Fixed-dose combination bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir-containing regimens for initial treatment of HIV-1 infection: Week 144 results from two randomised, double-blind, multicentre, phase 3, non-inferiority trials. Lancet HIV 2020;7:e389–e400. doi: 10.1016/S2352-3018(20)30099-0. [DOI] [PubMed] [Google Scholar]
- 7.Wohl D Clarke A Maggiolo F Garner W Laouri M Martin H, et al. Patient-reported symptoms over 48 weeks among participants in randomized, double-blind, phase III non-inferiority trials of adults with HIV on co-formulated bictegravir, emtricitabine, and tenofovir alafenamide versus co-formulated abacavir, dolutegravir, and lamivudine. Patient 2018;11:561–573. doi: 10.1007/s40271-018-0322-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao DC Wen Y Ma Y Zhao Y Zhang Y Wu YS, et al. Expansion of China’s free antiretroviral treatment program. Chin Med J 2012;125:3514–3521. [PubMed] [Google Scholar]
- 9.Chinese Medical Association . The guideline for prevention and treatment of hyperlipidemia in Chinese adults (in Chinese). Chin J Cardiovasc Dis 2007;35:390–409. doi: 10.3760/j.issn:0253-3758.2007.05.003 [Google Scholar]
- 10.Aithal GP Watkins PB Andrade RJ Larrey D Molokhia M Takikawa H, et al. Case definition and phenotype standardization in drug-induced liver injury. Clin Pharmacol Ther 2011;89:806–815. doi: 10.1038/clpt.2011.58. [DOI] [PubMed] [Google Scholar]
- 11.Zeng YQ Xiao J Li CL Wang Y Zhang L Pang XL, et al. Prevalence and risk factors for bone mineral density changes in antiretroviral therapy-naive human immunodeficiency virus-infected adults: A Chinese cohort study. Chin Med J 2020;133:2940–2946. doi: 10.1097/CM9.0000000000001317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao W, Hsieh E, Li T. Optimizing treatment for adults with HIV/AIDS in China: Successes over two decades and remaining challenges. Curr HIV/AIDS Rep 2020;17:26–34. doi: 10.1007/s11904-019-00478-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michienzi SM, Barrios M, Badowski ME. Evidence regarding rapid initiation of antiretroviral therapy in patients living with HIV. Curr Infect Dis Rep 2021;23:7. doi: 10.1007/s11908-021-00750-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffmann C, Rockstroh JK. HIVBOOK2010. Medizin Fokus Verlag; 2010. Medizin Fokus Verlag, Hamburg. [Google Scholar]
- 15.Gazzola L Tagliaferri G De Bona A Mondatore D Borsino C Bini T, et al. Dyslipidaemia after switch to tenofovir alafenamide (TAF)-based cART regimens in a cohort of HIV-positive patients: What clinical relevance? HIV Med 2021;22:140–145. doi: 10.1111/hiv.12984. [DOI] [PubMed] [Google Scholar]
- 16.Plum PE Maes N Sauvage AS Frippiat F Meuris C Uurlings F, et al. Impact of switch from tenofovir disoproxil fumarate-based regimens to tenofovir alafenamide-based regimens on lipid profile, weight gain and cardiovascular risk score in people living with HIV. BMC Infect Dis 2021;21:910. doi: 10.1186/s12879-021-06479-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Surial B Mugglin C Calmy A Cavassini M Günthard HF Stöckle M, et al. Weight and metabolic changes after switching from tenofovir disoproxil fumarate to tenofovir alafenamide in people living with HIV: A cohort study. Ann Intern Med 2021;174:758–767. doi: 10.7326/M20-4853. [DOI] [PubMed] [Google Scholar]
- 18.Gatell JM Assoumou L Moyle G Waters L Johnson M Domingo P, et al. Switching from a ritonavir-boosted protease inhibitor to a dolutegravir-based regimen for maintenance of HIV viral suppression in patients with high cardiovascular risk. AIDS 2017;31:2503–2514. doi: 10.1097/QAD.0000000000001675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daar ES DeJesus E Ruane P Crofoot G Oguchi G Creticos C, et al. Efficacy and safety of switching to fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide from boosted protease inhibitor-based regimens in virologically suppressed adults with HIV-1: 48 week results of a randomised, open-label, multicentre, phase 3, non-inferiority trial. Lancet HIV 2018;5:e347–e356. doi: 10.1016/S2352-3018(18)30091-2. [DOI] [PubMed] [Google Scholar]
- 20.Stellbrink HJ Orkin C Arribas JR Compston J Gerstoft J Van Wijngaerden E, et al. Comparison of changes in bone density and turnover with abacavir-lamivudine versus tenofovir-emtricitabine in HIV-infected adults: 48-week results from the ASSERT study. Clin Infect Dis 2010;51:963–972. doi: 10.1086/656417. [DOI] [PubMed] [Google Scholar]
- 21.Wang H, Lu X, Yang X, Xu N. The efficacy and safety of tenofovir alafenamide versus tenofovir disoproxil fumarate in antiretroviral regimens for HIV-1 therapy: Meta-analysis. Medicine (Baltimore) 2016;95:e5146. doi: 10.1097/MD.0000000000005146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanda N Okamoto K Okumura H Mieno M Sakashita K Sasahara T, et al. Outcomes associated with treatment change from tenofovir disoproxil fumarate to tenofovir alafenamide in HIV-1-infected patients: A real-world study in Japan. HIV Med 2021;22:457–466. doi: 10.1111/hiv.13061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ray AS, Fordyce MW, Hitchcock MJ. Tenofovir alafenamide: A novel prodrug of tenofovir for the treatment of human immunodeficiency virus. Antiviral Res 2016;125:63–70. doi: 10.1016/j.antiviral.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 24.Lee WA He GX Eisenberg E Cihlar T Swaminathan S Mulato A, et al. Selective intracellular activation of a novel prodrug of the human immunodeficiency virus reverse transcriptase inhibitor tenofovir leads to preferential distribution and accumulation in lymphatic tissue. Antimicrob Agents Chemother 2005;49:1898–1906. doi: 10.1128/AAC.49.5.1898-1906.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moran CA, Weitzmann MN, Ofotokun I. Bone loss in HIV infection. Curr Treat Options Infect Dis 2017;9:52–67. doi: 10.1007/s40506-017-0109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta SK Post FA Arribas JR Eron JJ Jr. Wohl DA Clarke AE, et al. Renal safety of tenofovir alafenamide vs. tenofovir disoproxil fumarate: A pooled analysis of 26 clinical trials. AIDS 2019;33:1455–1465. doi: 10.1097/QAD.0000000000002223. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


