Abstract
The epidemic of coronavirus disease 2019 (COVID-19) has broken the normal spread mode of respiratory viruses, namely, mainly spread in winter, resulting in over 230 million confirmed cases of COVID-19. Many studies have shown that severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) can affect the nervous system by varying degrees. In this review, we look at the acute neuropsychiatric impacts of COVID-19 patients, including acute ischemic stroke, encephalitis, acute necrotizing encephalopathy, dysosmia, and epilepsy, as well as the long-term neuropsychiatric sequelae of COVID-19 survivors: mental disorder and neurodegenerative diseases. In particular, this review discusses long-term changes in brain structure and function associated with COVID-19 infection. We believe that the traditional imaging sequences are important in the acute phase, while the nontraditional imaging sequences are more meaningful for the detection of long-term neuropsychiatric sequelae. These long-term follow-up changes in structure and function may also help us understand the causes of neuropsychiatric symptoms in COVID-19 survivors. Finally, we review previous studies and discuss some potential mechanisms of SARS-CoV-2 infection in the nervous system. Continuous focus on neuropsychiatric sequelae and a comprehensive understanding of the long-term impacts of the virus to the nervous system is significant for formulating effective sequelae prevention and management strategies, and may provide important clues for nervous system damage in future public health crises.
Keywords: COVID-19, SARS-CoV-2, nervous system, imaging sequences, pathogenesis, neuropsychiatric sequelae, brain function alteration
Introduction
The epidemic of coronavirus disease 2019 (COVID-19) has broken the normal spread mode of respiratory viruses: the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) spread not only in the winter of 2019 and 2020 but also in the summer of 2020 and 2021 (Wang et al., 2020a). According to the WHO Coronavirus (COVID-19) Dashboard (https://covid19.who.int/), there have been over 230 million confirmed cases of COVID-19 worldwide, as of 28 September 2021. Although >5.9 billion people have been vaccinated against the COVID-19, the epidemic has not been effectively controlled in many parts of the world.
The coronavirus was discovered by Fred Beaudette and Charles Hudson in 1937, and named by June Almeida in 1967. Because the spike glycoproteins on the virus particles under the electron microscope were like the crown, it was named “corona” in Arabic. So far, besides SARS-CoV-2, six coronaviruses that can infect humans have been discovered, including human coronaviruses (HCoV) OC43, 229E, NL63, and HKU1; severe acute respiratory syndrome coronavirus (SARS-CoV); and Middle-East respiratory syndrome coronavirus (MERS-CoV) (Drosten et al., 2003; Hamre and Procknow, 1966; Lau et al., 2006; Lia et al., 2004; McIntosh et al., 1967; Zaki et al., 2012; Zhou et al., 2020a). A detailed comparison of these seven coronaviruses is shown in Table 1.
Table 1:
Characteristic of seven coronaviruses.
| Characteristic | SARS-CoV-2 | MERS-CoV | HCoV- HKU1 | HCoV- NL63 | SARS-CoV | HCoV- OC43 | HCoV-229E |
|---|---|---|---|---|---|---|---|
| Occurrence time | December 2019 | September 2012 | January 2005 | April 2004 | April 2003 | 1967 | 1965 |
| Genus | β-CoVs, lineage B | β-CoVs, lineage C | β-CoVs, lineage A | α-CoVs | β-CoVs, lineage B | β-CoVs, lineage A | α-CoVs |
| Binding receptor | ACE2 | DPP4 (CD26) | __ | ACE2 | ACE2 | 9-O-acetylated sialic acid | Human aminopeptidase N (CD13) |
| Receptor distribution | Nervous, respiratory, cardiovascular, digestive, and urinary systems | Respiratory, digestive, and urogenital systems | Respiratory system | Digestive and urinary systems | Respiratory, digestive, and urinary systems | Respiratory and digestive systems | Respiratory and digestive systems |
| Severity of infection | Severe symptoms | Severe symptoms | Mild or moderate symptoms | Mild or moderate symptoms | Severe symptoms | Mild or moderate symptoms | Mild or moderate symptoms |
Coronaviruses can be divided into four genera: α, β, γ, and δ, Among them, β-CoVs can be divided into four independent lineages A, B, C, and D. ACE2: angiotensin-conversion enzyme 2. DPP4: dipeptidyl peptidase 4 (also known as CD26).
The four HCoV strains (229E, NL63, OC43, and HKU1) are common coronaviruses that usually cause mild or moderate upper respiratory tract infections. Symptoms mainly include cough, rhinorrhea, headache, sore throat, fever, etc., which are common in children (Gaunt et al., 2010; Kuypers et al., 2007; Lau et al., 2006; Zeng et al., 2018). However, MERS-CoV, SARS-CoV, and SARS-CoV-2 not only can cause severe symptoms of the respiratory system (Booth et al., 2003; Mackay and Arden, 2015; Ozma et al., 2020; Peiris et al., 2003), but can also cause damage to other multiple systems, such as the urinary and cardiovascular systems (Amsalem et al., 2021; Mackay and Arden, 2015; Shi et al., 2020; Su et al., 2020; Yin and Wunderink, 2018). In addition, as the first symptom and sequelae of COVID-19, neuropsychiatric symptoms have been attracted more and more attention (AboTaleb, 2020; Mao et al., 2020; Paterson et al., 2020; Varatharaj et al., 2020). The detail mechanisms of virus infection in the brain or neuropsychiatric symptoms in acute and chronic stages are still unclear. Therefore, timely and continuous study of the development of neuropsychiatric symptoms in patients with COVID-19 has important clinical significance for disease monitoring and treatment strategy.
In this review, we reviewed previous studies related to MERS-CoV, SARS-CoV, and COVID-19 case reports, as well as the acute neuropsychiatric symptoms of COVID-19 patients, and the long-term neuropsychiatric sequelae of COVID-19 survivors may be caused by changes in brain function. Then, we discussed several potential mechanisms of SARS-CoV-2 infection of brain.
Acute neuropsychiatric symptoms of COVID-19 patients
Imaging examinations, especially MRI, have advantages for nervous system diseases, but in the early stage of the epidemic, nervous system symptoms have not been attracted attention. In addition, strict isolation and preventive measures may limit many patients with mild neurological symptoms from receiving detailed imaging examination (Jain et al., 2020). Therefore, most of the symptoms in the acute stage of COVID-19 patients were reported by cases.
Acute ischemic stroke
Stroke refers to a group of diseases with acute cerebral vascular circulation disorder, and the typical clinical manifestations are limb hemiplegia, aphasia, mental symptoms, vertigo, ataxia, choking cough, and even to coma and death in severe cases (Sacco et al., 2013). Acute ischemic stroke is currently reported as the most common cerebral vascular disease in patients with COVID-19 (Wang et al., 2020b), and is also the most common neurological manifestation associated with COVID-19 found in neuroimaging (Jain et al., 2020). The imaging findings of acute ischemic stroke related to COVID-19 were the same as those of localized ischemic stroke caused by other diseases.
Ischemic stroke often occurs 2 weeks after COVID-19 patients show neurological symptoms (Ellul et al., 2020a; Zhou et al., 2020b). Large retrospective studies in several countries have reported cases of acute ischemic stroke with different severities (Benussi et al., 2020; Jain et al., 2020; Klok et al., 2020; Lodigiani et al., 2020; Mao et al., 2020; Varatharaj et al., 2020). Detailed sample size and incidence rate of acute ischemic stroke are shown in Table 2. Through the studies in these five countries, we found that there were significant differences in the incidence of cerebral vascular circulation disorder reported by various countries, which may be related to the following two reasons: on the one hand, these six studies were retrospective studies, and there were deviations in data selection. More prospective studies are needed to verify this result in the future. On the other hand, we found two studies with low incidence rate of acute ischemic stroke. They used prophylactic thromboprophylaxis for all hospitalized patients or 100% intensive care patients and 75% of the general wards (Jain et al., 2020; Lodigiani et al., 2020). This finding may be of great significance to the formulation of treatment plan for hospitalized COVID-19 patients to prevent the occurrence of acute cerebral vascular circulation disorder.
Table 2:
Summary of studies on COVID-19 patients with acute ischemic stroke.
| Studies | Countries | Recruitment time | Sample size | Morbidity |
|---|---|---|---|---|
| (Mao et al., 2020) | China | 16 January 2020 to 19 February 2020 | 214 | 5 (5.7%) |
| (Jain et al., 2020) | New York | 1 March 2020 to 13 April 2020 | 3218 | 35 (1.1%) |
| (Varatharaj et al., 2020) | UK | 2 April 2020 to 26 April 2020 | 153 | 57 (45.6%) |
| (Lodigiani et al., 2020) | Italian | 13 February 2020 to 10 April 2020 | 388 | 9 (2.3%) |
| (Benussi et al., 2020) | Italian | 21 February 2020 to 5 April 2020 | 173 | 43 (76.7%) |
| (Klok et al., 2020) | Netherlands | 7 March 2020 to 5 April 2020 | 184 | 3 (1.6%) |
Moreover, we also summarized six (21 COVID-19 patients) detailed case reports of acute ischemic stroke (Avula et al., 2020; Basi et al., 2020; Beyrouti et al., 2020; Diaz-Segarra et al., 2020; Oxley et al., 2020; Zhai et al., 2020), and found that the common symptoms were disorientation, hemiplegia, and dysarthria. In addition, young and elderly patients with COVID-19 may suffer from acute ischemic stroke, but most of them are older. At present, it is reported that the minimum age of onset of COVID-19 related acute ischemic stroke is 33 years old (Oxley et al., 2020), and most of them are over 60 years old.
Many potential causes of COVID-19 related acute ischemic stroke have been suggested, but the exact pathogenesis is unclear (Bhatia and Srivastava, 2020). Some scholars believe that the occurrence of acute cerebral vascular circulation disorders are not related to SARS-CoV-2 infection, because most of these patients have underlying diseases, including diabetes, hypertension, and cardiovascular diseases, which are known high risk factors for cerebrovascular diseases (Li et al., 2020a), so the time of cerebrovascular diseases during the infection of SARS-CoV-2 may be just an accident (Spence et al., 2020). However, we agree with most scholars that SARS-CoV-2 combined with vascular endothelial cells induces hypercoagulability, thereby activating the contact and complement system to cause neurological damage (Spence et al., 2020; Yaghi et al., 2020). There are two aspects of evidence to support this conjecture: first, acute ischemic stroke also occurs in some young patients without basic diseases (Oxley et al., 2020); second, in the cases with D-dimer level examination, the index increased significantly (Diaz-Segarra et al., 2020), and several studies have shown that high levels of D-dimer predict disease severity and hospitalized COVID-19 patients’ mortality (Li et al., 2020a; Yao et al., 2020; Yu et al., 2020; Zhang et al., 2020).
Encephalitis
Encephalitis is an inflammatory process of the central nervous system, and is divided into three categories according to infection and the time of symptoms, including primary encephalitis, para-infection or postinfectious encephalitis, and autoimmune or paraneoplastic encephalitis (Achar and Ghosh, 2020). It is difficult to distinguish between primary encephalitis and postinfectious encephalitis caused by SARS-CoV-2. These two types of encephalitis were distinguished only by detecting cerebrospinal fluid (CSF) reverse transcriptase–polymerase chain reaction (RT–PCR), intrathecal synthesis of SARS-CoV-2 specific antibody, or by biopsy to detect whether there is SARS-CoV-2 antigen or RNA in brain tissue (Koralnik and Tyler, 2020). If any of these three tests is positive, it supports the diagnosis of primary encephalitis, inflammation caused by direct virus infection. The distinction between the first two categories and the third category also depends on laboratory examination. Autoimmune or paraneoplastic encephalitis can be excluded when CSF pleocytosis and protein elevation are detected (Ellul and Solomon, 2018). In addition, although electroencephalogram and imaging examinations (CT and MRI) may not distinguish the encephalitis, but they can show the damage of inflammation caused for various reasons to the nervous system.
So far, the symptoms of encephalitis in patients with COVID-19 were mostly reported by cases. We reviewed these case reports and divided them into the following three categories according to whether they were positive in laboratory and imaging examination: (i) CSF SARS-CoV-2 RT–PCR was positive. This was the first reported case of encephalitis related to the COVID-19 in the world: after the 24-year-old male patient was admitted to the hospital, SARS-CoV-2 RNA was not detected in nasopharyngeal swabs, nor IgM antibodies in serum samples, but SARS-CoV-2 RNA was detected in CSF (Moriguchi et al., 2020). Another 40-year-old female patient was hospitalized with encephalitis and found that nasopharyngeal swabs and CSF SARS-CoV-2 RT–PCR were positive (Huang et al., 2020b). The SARS-CoV strain was isolated from a previous brain tissue specimen of SARS patients with obvious central nervous system symptoms (Xu et al., 2005), which also indirectly proves that the virus can directly invade the nervous system. (ii) No SARS-CoV-2 RNA was found in the CSF, but there was polycythemia, elevated lymphocytes, or elevated protein (Bernard-Valnet et al., 2020; Duong et al., 2020; Pilotto et al., 2020). However, some scholars believe that although SARS-CoV-2 RNA was not detected in CSF, it may be related to the relatively short virus residence or low virus load in the nervous system, or lack of effective detection methods (Duong et al., 2020; Ye et al., 2020). (iii) There were obvious imaging changes, but all laboratory examinations of CSF were normal. Most of the imaging findings were normal on CT, but abnormal brain parenchymal morphology and signal could be seen on MRI, and fluid-attenuated inversion recovery (FLAIR) and diffusion weighted imaging (DWI) could better display the lesions (Dogan et al., 2020; Khoo et al., 2020; Wong et al., 2020; Ye et al., 2020; Zambreanu et al., 2020; Zuhorn et al., 2021).
The mechanism of COVID-19-associated encephalitis is still unclear. According to these case reports, although there are cases that support the direct invasion of the SARS-CoV-2 into the brain tissue (the virus is found in CSF or brain tissue biopsy), most support the overreaction of nervous system immune cells mainly mediated by autoimmunity/antibodies. Further research is needed to support or refute this hypothesis, which is of significance to the clinical development of a reasonable treatment plan.
Acute necrotizing encephalopathy (ANE)
Encephalopathy refers to the clinical manifestation of pathology affecting brain function under the action of various factors of extracranial or intracranial origin. Encephalopathy and psychosis are two different mental states. The typical characteristics are disorientation, short-term memory impairment, and inattention in the abnormal awakening state (Perugula and Lippmann, 2016). ANE is a rare para-infectious encephalopathy infected by influenza and other viruses. It mainly occurs in children with influenza, and the typical imaging features are multiple symmetrical edema and necrosis areas in the central nervous system (Kansagra and Gallentine, 2011; Krett et al., 2020; Poyiadji et al., 2020). However, the reported ANE-related SARS-CoV-2 infections were more common in adults.
The first case of ANE was reported in Radiology. A 58-year-old female confirmed with COVID-19 had not only cough and fever, but also mental state changes. Since lumbar puncture was an invasive examination, the patient did not test for SARS-CoV-2 in CSF. But the changes of CT and MRI were consistent with the typical imaging findings of ANE (Poyiadji et al., 2020). From the subsequent case reports, it was found that the common mental state changes of ANE related to the COVID-19 were slow response to commands, poor memory, disorientation, and obvious language difficulties, and SARS-CoV-2 was not detected in the cases tested for CSF (Al Mazrouei et al., 2020; Farhadian et al., 2020; Kihira et al., 2020; Krett et al., 2020; Umapathi et al., 2020).
According to previous studies, ANE caused by SARS-CoV-2 infection was related to intracranial cytokine storm, destroying the blood–brain barrier (BBB), but no virus directly invaded the nervous system (Rossi, 2008). The pathogenesis section next describes how cell storms damage the nervous system. Studies have found that cytokine analysis of CSF showed increased levels of interleukin-1 (IL-1), interleukin-6 (IL-6), interferon gamma (IFN-γ), and tumor necrosis factor alpha (TNF-α), showing that the proinflammatory cytokines were dysregulated. It indirectly proved that cytokine storm was involved in the pathogenesis of ANE (Farhadian et al., 2020; Krett et al., 2020). However, it is necessary to further study the pathogenesis of COVID-19 related ANE.
Dysosmia
Dysosmia is one of the most common symptoms in patients with COVID-19 in the acute stage, which is characterized by decreased or loss of olfaction after virus infection. However, at the beginning of the disease outbreak, we did not know enough about this symptom. Subsequent studies found dysosmia may not only be the first symptom before respiratory symptoms (Lechien et al., 2020), but also occur in some asymptomatic infected people (Gane et al., 2020). The incidence rate of dysosmia reported by various countries was different. A study of 114 COVID-19 patients in France found that 47% of the patients had a dysosmia. Anosmia appeared 4.4 (±1.9) days after infection. The average time of anosmia was 8.9 (± 6.3) days, and 98% of patients recovered within 28 days (Klopfenstein et al., 2020). A multicenter study in Belgium found that 357 (85.6%) patients had infection related dysosmia, and 72.6% recovered olfactory function within 8 days after the rehabilitation of the COVID-19 (Lechien et al., 2020). However, 11 (5.1%) of 214 confirmed hospitalized patients in Wuhan developed dysosmia, and the authors said that this may be because of incomplete evaluation, which may make this incidence inaccurate (Mao et al., 2020). The mechanism of olfactory disorder needs further research.
Epilepsy
Epilepsy is a disease with complex etiology and it is defined as a kind of neurological disorder caused by excessive discharge of brain neurons from the perspective of physiological mechanism (Scharfman, 2007; Sirven, 2015; Stafstrom and Carmant, 2015). Combined with literature reports, we found that epilepsy in patients with COVID-19 can be divided into two types: first, there is no history of epilepsy and family history. Epileptic symptoms can occur after virus infection, which can be accompanied by cerebrovascular diseases (Sora and Viroj, 2020; Karimi et al., 2020; Lu et al., 2020; Lyons et al., 2020). Second, the patient has a well-controlled history of epilepsy and relapses after infection (Anand et al., 2020). Because no virus traces were found in CSF examinations (Anand et al., 2020; Lyons et al., 2020), the mechanism of epilepsy mainly focuses on two aspects: one is the excessive excitation of neurons induced by cytokine storm, leading to seizures (Libbey and Fujinami, 2011; Singhi, 2011), and the other is the drug side-effects caused by the use of antiviral drugs in hospitalized patients (Ying et al., 2021).
The long-term neuropsychiatric sequelae of COVID-19 survivors
It has been nearly 2 years since the outbreak of the COVID-19. With the continuous increase in the number of infections, the sequelae after the recovery of COVID-19 have been widely reported, but more attention has been paid to the symptoms of the respiratory system and cardiovascular system (Arnold et al., 2021; Blanco et al., 2021; Trinkmann et al., 2021; Wu et al., 2021; Xiong et al., 2021; Yan et al., 2021). According to a previous report, there was still a high incidence rate of neuropsychiatric symptoms in 31 to 50 months after SARS-CoV infection (Lam et al., 2009). Therefore, we believe that the world may face a large number of survivors of COVID-19 neuropsychiatric sequelae. Timely understanding of possible symptoms and possible pathogenesis has important socio-economic value in the prevention and treatment of neuropsychiatric sequelae.
Mental disorder
The outbreak of COVID-19 has had a long-term mental health impact on patients, doctors, and even ordinary people without SARS-CoV-2 infection, including anxiety, depression, post-traumatic stress disorder, and insomnia (Lai et al., 2020; Li et al., 2020c; Troyer et al., 2020). Suicide by emergency doctors also occurred in New York, and some scholars even worry that the COVID-19 pandemic may trigger a wave of suicide (Thakur and Jain, 2020).
At present, there are some differences in the frequency of mental diseases in COVID-19 survivors. Mazza et al (Mazza et al., 2021) conducted a prospective study of mental disorder in survivors of the COVID-19. They included 226 COVID-19 survivors and assessed their mental status 1 and 3 months after discharge. The study found that compared with 1 month after discharge, 3 months after discharge the post-traumatic stress disorder, anxiety, and insomnia symptoms of the survivors of the COVID-19 decreased over time, while the depressive symptoms persisted. However, several large cohort studies followed up for 6 months found that the neuropsychiatric sequelae of COVID-19 survivors were mainly sleep difficulties, anxiety, and depression (Al-Aly et al., 2021; Huang et al., 2021a; Taquet et al., 2021a). A recent article published in The Lancet shows that the incidence of anxiety or depression at 12 months is slightly higher than that at 6 months, and the proportion of anxiety or depression in women is twice that in men, and age is positively correlated with anxiety or depression (Huang et al., 2021b). We think that the reason for this difference may be related to the number of participants included in the study and the length of follow-up.
The pathogenesis of mental disorder in survivors of COVID-19 is unclear whether it is due to direct virus infection or the continuous effect of immune response caused by a cytokine storm (Al-Aly et al., 2021; Rogers et al., 2020). The previously mentioned 3-month follow-up study found that there was a significant positive correlation between systemic immune-inflammation index (SII = platelets × neutrophils/lymphocytes) and depressive symptoms, which may support the role of immune response in the occurrence of mental disorder. The impact of the social environment may not be ignored, such as the reduction of social activities in the recovery stage after discharge or unemployment caused by incomplete recovery of the body.
Neurodegenerative diseases
Neurodegenerative diseases are a kind of chronic progressive diseases characterized by protein deposition, neuroinflammation, and progressive loss of neurons and axons, mainly including Alzheimer's disease (AD), Parkinson's disease (PD), and multiple sclerosis (MS). There are many causes of neurodegenerative diseases, including neuroinflammation, oxidative stress, aging, and so on (Beltrán-Castillo et al., 2018; Singh et al., 2019; Tejera et al., 2019). Whether the SARS-CoV-2 infection will cause neurodegenerative diseases or accelerate the premature occurrence of neurodegenerative diseases still needs a longer follow-up. Many studies have shown that the neuroinflammatory response is an indispensable key process for the progress of most neurodegenerative diseases (Baik et al., 2016; Tejera et al., 2019).
AD is one of neurodegenerative diseases caused by neuroinflammatory response, synaptic pruning, and neuron loss (Zhang and Jiang, 2015), and its typical clinical manifestation is cognitive impairment. In view of the various damage that SARS-CoV-2 can cause to the nervous system in the acute phase, its long-term effect on cognitive function is also worthy of attention (Calderón-Garcidueñas et al., 2020; Chen et al., 2021). A study on the two-way association between COVID-19 and neuropsychiatric diseases showed that the incidence of first diagnosis of AD in people over 65 years old was 1.6% (95% CI 1.2–2.1) in 14 to 90 days after the diagnosis of COVID-19 (Taquet et al., 2021b). This study provided preliminary evidence for the link between viral infection and AD. In addition, another study found that the mortality of COVID-19 patients with AD (62.2%) was much higher than COVID-19 patients without AD (26.2%) (Bianchetti et al., 2020). So far, the mechanism of AD caused by SARS-CoV-2 infection is still unclear. Some scholars believe that the virus can interact with glutamate and GABAergic may stimulate the excitotoxic reaction caused by the binding of ACE2 receptor in neurons, resulting in brain tissue injury (Guo et al., 2020). In addition, viruses invading the nervous system can also widely infiltrate the whole brain through the trans-synaptic transfer, which may be the reason for neurodegenerative changes several months and years after acute infection (Li et al., 2020b; Li et al., 2012).
PD is a neurodegenerative disease involving the basal ganglia, which is often characterized by motor retardation, static tremor or rigidity. Different from the AD described previously, the mortality of COVID-19 patients with PD (19.7%) was only slightly higher than COVID-19 patients without PD (12.8%) (Fasano et al., 2020b), and there was no significant difference in the probability of SARS-CoV-2 infection between patients with PD and the general population (Fasano et al., 2020a). Previous studies have shown that patients with PD can show motor and cognitive dysfunction, which may be related to the common pathogenesis of AD and PD (Bezdicek et al., 2019; Das et al., 2019; Van Bulck et al., 2019). Ponsen et al. (Ponsen et al., 2004) found that anosmia was the early clinical symptoms of PD, while previous studies said that anosmia was often the first symptoms of SARS-CoV-2 infection (Lechien et al., 2020). This may be indirect evidence for the interaction between PD and COVID-19 infection. The wide expression of ACE2 receptor in the brain may provide a molecular basis for later neurodegenerative changes (Lukiw et al., 2020). Unfortunately, there is no effective clinical evidence to prove these potential mechanisms.
MS is an autoimmune disease, and its pathogenesis is related to neuronal degeneration caused by focal demyelination and inflammation. Since MS patients need to take immunosuppressive drugs, unlike AD or PD, we are no longer concerned with whether MS is a long-term neuropsychiatric sequelae of patients affected by COVID-19, but whether the probability of the patient being infected with SARS-CoV-2 will be increased because of suffering from MS. The current research results on this issue are not uniform. The literature showed that the probability of COVID-19 pneumonia in patients with MS was about 2.5 times higher than that in the general population (Crescenzo et al., 2020), but more studies showed that there was no difference between these two groups (Fan et al., 2020; Louapre et al., 2020). This contradictory result requires more long-term studies to explore whether there is a relationship between SARS-CoV-2 infection and MS.
Changes of brain structure and functional imaging in long-term follow-up of COVID-19 survivors
Brain structural changes in COVID-19 survivors at a 3-month follow-up
In a recent study, 51 COVID-19 survivors were followed up for 3 months to study the changes of brain structural. They divided the 51 participants into a mild group (MG, N = 19) and a severe group (SG, N = 32) based on the severity of their symptoms during the hospitalization period, and respectively compared the images collected by these two groups with the images of 31 healthy controls (HCs). The study showed that the conventional axial T2-FLAIR scans of all COVID-19 survivors showed no abnormalities, but the analysis of structural 3D T1-weighted images (T1WI) and high-resolution diffusion tension images (DTI) found that compared with HC group, the white matter tracts of MG had a few changed, and there was no significant change in gray matter. However, the reduction in cortical thickness and white matter microstructure changes in the SG were more significant than those in the MG, mainly in the frontal lobe and limbic system. These results provide evidence for the nervous system damage of COVID-19 survivors (Qin et al., 2021).
Brain function changes in COVID-19 survivors at a 1-year follow-up
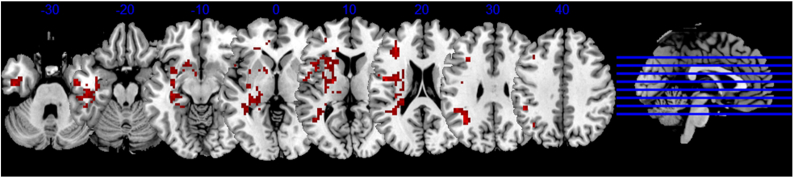
To explore the long-term brain function changes of COVID-19 survivors. Our team recruited 22 COVID-19 rehabilitation patients who were followed up for 1 year and 29 HCs. By comparing the fMRI and clinical data of the two groups, we found that compared with the HC group, the amplitude low-frequency fluctuation (ALFF) value of COVID-19 rehabilitation patients was significantly higher in multiple brain regions, including left anterior central gyrus, hippocampus, caudate, and putamen (Fig. 1). In addition, the increased ALFF value of the left caudate was significantly positively correlated with the score of the ascension insomnia scale, and the ALFF value of the left anterior central gyrus was also positively correlated with the neutrophil count during hospitalization. Our study provides imaging evidence for the long-term neuropsychiatric sequelae of COVID-19 rehabilitation patients, and analyzing the relationship between imaging changes and inflammatory markers may help to reveal the neurobiological mechanism of COVID-19 related neuropsychiatric sequelae (Du et al., 2022).
Figure 1:
Long-term brain function changes in COVID-19 survivors. The red area indicates the brain area with increased amplitude low-frequency fluctuation value in the COVID-19 rehabilitation patients followed up for 1 year compared with the normal control.
Potential mechanism of SARS-CoV-2 infection in the nervous system
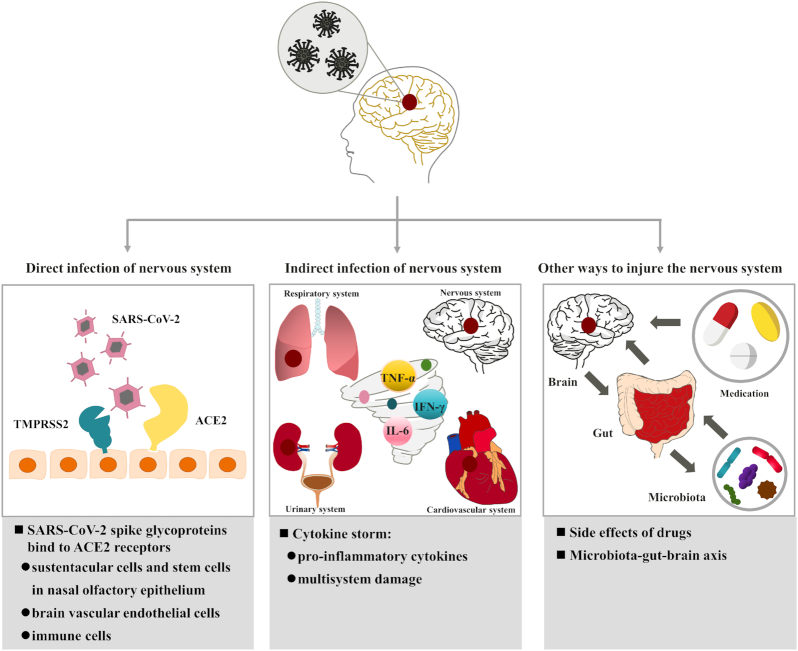
With the reports of acute and long-term nervous system symptoms in patients with COVID-19 patients and survivors, some studies have speculated that the symptoms are related to SARS-CoV-2 infection of the nervous system (Achar and Ghosh, 2020; Ellul et al., 2020a; Niazkar et al., 2020; Troyer et al., 2020; Wang et al., 2020a). In addition, Zhou et al. (Zhou et al., 2020a) found that the genetic sequence of the SARS-CoV-2 was 79.6% similar to that of the SARS-CoV, so it may provide indirect evidence to support the symptoms of brain damage caused by SARS-CoV-2 infection. Although the exact pathogenesis of SARS-CoV-2 infection of the nervous system remains unclear, previous studies have suggested some potential mechanisms of SARS-CoV-2 infection in the nervous system (Fig. 2).
Figure 2:
Potential mechanism of SARS-CoV-2 infection in nervous system. TMPRSS2: transmembrane serine protease 2. TNF-α: tumor necrosis factor alpha. IFN-γ: interferon gamma and IL-6: interleukin-6.
Direct infection of the nervous system
The spike glycoproteins on the surface of SARS-CoV-2 play a vital role in the virus invading the nervous system. Compared to SARS-CoV, the spike glycoproteins on SARS-CoV-2 have a higher affinity for the angiotensin-conversion enzyme 2 (ACE2) receptors (Shang et al., 2020; Wang et al., 2020c). However, ACE2 receptors exist not only in cardiomyocyte, gastrointestinal epithelial cells, kidney, bladder, and urinary epithelial cells (Zou et al., 2020), but also widely in nasal epithelial cells, vascular endothelial cells, brain neurons, astrocytes, and oligodendrocytes (Barrantes, 2020). Previous studies have found that the receptor binding domain of virus was on the S1 subunit of the homotrimeric spike glycoproteins expressed by the SARS-CoV, which provides a basis for the binding of the virus to ACE2 receptor on the cell membrane (Shang et al., 2020; Wang et al., 2020c; Zhu et al., 2013). After they interact, the viral spike glycoproteins are hydrolyzed and cleaved by transmembrane serine protease 2 (TMPRSS2) and lysosomal protease cathepsins (Shang et al., 2020). The virus completes the most critical step in crossing the cell membrane (Wang et al., 2020c). Similar to the SARS-CoV transmembrane process, SARS-CoV-2 first recognizes cells with low levels of TMPRSS2 through the preactivation of furin (Shang et al., 2020), and then also uses TMPRSS2 to cleave and trigger spike glycoproteins (Hoffmann et al., 2020). Finally, the SARS-CoV-2 RNA enters healthy cells to replicate. The process by which the virus enters the cell remains to be verified.
Therefore, the SARS-CoV-2 may not only complete the cross synaptic transfer of the virus between neurons and neurons and between neurons and satellite cells directly through exocytosis, endocytosis, and vesicle transport (Li et al., 2012), but it may also invade the nervous system through the following three ways in which SARS-CoV-2 spike glycoproteins bind to ACE2 receptors in different cells: (i) SARS-CoV-2 may bind to ACE2 receptors of sustentacular cells (Bilinska et al., 2020) and stem cells (Brann et al., 2020) in the nasal olfactory epithelium, then transport to the next level neurons through reverse axons (Desforges et al., 2019), and finally infect the nervous system. SARS-CoV (McCray et al., 2007), MERS-CoV (Jacomy and Talbot, 2003; Li et al., 2016), and HCoV-OCR43 (Li et al., 2016) have also been reported to invade the brain through olfactory neurons, which indirectly provides evidence for SARS-CoV-2 to enter the brain through the olfactory pathway. (ii) SARS-CoV-2 may bind to ACE2 receptors in vascular endothelial cells (Pezzini and Padovani, 2020), which form the main component of the BBB). BBB refers to the barrier between plasma and brain cells formed by brain capillary wall and glial cells, as well as the barrier between plasma and CSF formed by the choroid plexus (Abbott, 2002; Abbott et al., 2006). The BBB can effectively prevent harmful molecules and ions from entering brain tissue from the blood (Abbott, 2002; Abbott et al., 2006; Daneman and Prat, 2015; Pardridge, 2005). Electron microscopic images showed that SARS-CoV-2 entered vascular endothelial cells through endocytosis and exocytosis to achieve intercellular transfer of the virus (Paniz-Mondolfi et al., 2020). Moreover, SARS-CoV-2 does not replicate in the process of cross endothelial cell transfer, but replicates in the cytoplasm when it reaches its neurons, astrocytes, and oligodendrocytes (Baig et al., 2020). (iii) SARS-CoV-2 may bind to ACE2 receptors in immune cells (Desforges et al., 2014). SARS-CoV-2 may use lymphocytes, granulocytes, and monocytes involved in immune responses to pass through the BBB. Recently, a new hypothesis has been proposed that when the body is infected by the SARS-CoV, macrophages not only play a defensive role, but also use the “Trojan horse” mechanism to help the virus enter the nervous system (Abassi et al., 2020). In other words, after the immune response caused by virus infection, macrophages carry the virus through the BBB and specifically anchor in the brain parenchyma. ACE2 receptors on the surface of sustentacular cells and stem cells in nasal olfactory epithelium, vascular endothelial cells of the BBB, and immune cells provide binding sites for the SARS-CoV-2 to directly invade the nervous system.
Indirect infection of the nervous system
Cytokine storm refers to an uncontrolled excessive inflammatory reaction, which starts locally and spreads further in the body through systemic circulation, which may be the mechanism by which the virus indirectly infects the nervous system (Jose and Manuel, 2020). When the human immune system cannot produce an appropriate adaptive immune response to SARS-CoV-2, the persistence of the virus and the extension and amplification of the inherent immune mechanism lead to the dysfunction of immune response, resulting in the high inflammatory state of cytokine storm (Pelaia et al., 2020). Cytokine storm has also been observed in patients infected with SARS-CoV and MERS-CoV (Mahallawi et al., 2018; Wong et al., 2004). Like SARS-CoV and MERS-CoV, patients infected with SARS-CoV-2 may have elevated levels of proinflammatory cytokines, such as TNF-α, IFN-γ, and IL-6 (Han et al., 2020; Huang et al., 2020a). In particular, there are only elevated levels of antiinflammatory cytokines in COVID-9 patients, including IL-2, IL-4, and IL-10 (Benameur et al., 2020). This may be because IL-6 can down regulate intraendothelial adhesion and tight junction proteins and increase the paracellular permeability of microvascular endothelial cells, which may affect the integrity of BBB (Rochfort et al., 2014). Therefore, the cytokine storm caused by SARS-CoV-2 may damage the nervous system from two aspects. On the one hand, it increases the microvascular permeability of the nervous system, so that the virus can enter the brain through the BBB (Daneman and Prat, 2015; Pardridge, 2005). A cytokine storm can also activate the coagulation system and lead to the formation of cerebral microthrombosis (Benameur et al., 2020; Ehrlich et al., 1998). Therefore, SARS-CoV-2 may indirectly damage the nervous system through the cytokine storm.
Other ways to injure the nervous system
Besides the mechanisms mentioned previously, the side-effects of therapeutic drugs and the microbiota–gut–brain axis may also be the potential mechanisms causing neurological symptoms, but they need to be further investigated by further research.
Severe patients and patients in the acute phase of COVID-19 were often treated with corticosteroids (Liu et al., 2020; Mehta et al., 2020; Mo et al., 2020; Wan et al., 2020), and a number of studies have shown that 35% of patients treated with corticosteroids have internal neuropsychiatric symptoms such as cognitive and sleep disorders, mania, and depression (Brown and Chandler, 2001; Warrington and Bostwick, 2006), which indicates that corticosteroid treatment may mediate nervous system damage in patients with COVID-19 (Troyer et al., 2020).
The concept of the microbiota–gut–brain axis was proposed in 2009 (Rhee et al., 2009). The gut microbiota may affect the nervous system through the microbiota–gut–brain axis in regulating anxiety, emotion, cognition, and pain (Cryan and Dinan, 2012). An animal experiment showed that mice infected with Trichuris muris showed obvious anxiety-like behaviors, and cognitive dysfunction-like behaviors were observed 30 days after the symptoms of the infection subsided (Bercik et al., 2010). Therefore, based on previous studies, we suspect that changes in the gut microbiota of patients with COVID-19 may cause neuropsychiatric symptoms through regulating the microbiota–gut–brain axis.
Summary and prospect
With the increasing number of COVID-19 cases worldwide, more and more evidence has showed that neurological symptoms are related to viral infections, but the exact pathogenesis is still unclear. We believe that imaging examinations have advantages in showing acute damage to the nervous system and long-term neuropsychiatric sequelae. In the acute phase of virus infection, conventional sequences such as T2-FLAIR and DWI can suggest that patients with COVID-19 are complicated with acute ischemic stroke or encephalitis when other tests are negative. However, nontraditional MRI sequences, especially 3D T1WI, DTI, and fMRI, may be more meaningful to show long-term changes in brain structure and function. These long-term follow-up changes in structure and function may also help us understand the causes of neuropsychiatric symptoms in COVID-19 survivors. The underlying mechanisms associated with SARS-CoV-2 infection are diverse and unclear. We believe that the research on the mechanism of infection should not only study the way of invading the nervous system, but should also pay attention to the underlying diseases that may increase the susceptibility of this group of people. This may require more research to explore the pathogenesis of such comorbidities.
Our present paper, therefore, mainly focused on the damage to the nervous system, the acute and long-term neuropsychiatric sequelae, and the potential mechanism of SARS-CoV-2 infection. In light of the continuous attention to neuropsychiatric sequelae and current understanding about the long-term damage of the virus to the nervous system, formulating effective sequelae prediction and prevention is of great significance, and thus can provide important clues for the nervous system damage of future public health crises.
ACKNOWLEDGEMENTS
The study was supported by National Natural Science Foundation of China (82102157), Hunan Provincial Natural Science Foundation of China (2021JJ40895), the Science and Technology Innovation Program of Hunan Province (2020SK53423), and the Clinical Research Center For Medical Imaging In Hunan Province (2020SK4001).
Contributor Information
Yanyao Du, Department of Radiology, Second Xiangya Hospital of Central South University, Changsha 410011, Hunan Province, China.
Wei Zhao, Department of Radiology, Second Xiangya Hospital of Central South University, Changsha 410011, Hunan Province, China; Clinical Research Center for Medical Imaging in Hunan Province, Changsha 410011, Hunan, China.
Lei Du, Department of Psychiatry and Behavioral Neuroscience, University of Cincinnati College of Medicine, Cincinnati 45255, OH, USA.
Jun Liu, Department of Radiology, Second Xiangya Hospital of Central South University, Changsha 410011, Hunan Province, China; Clinical Research Center for Medical Imaging in Hunan Province, Changsha 410011, Hunan, China; Department of Radiology Quality Control Center, Hunan Province, Changsha 410011, Hunan, China.
Author contributions
Y.Y.D., W.Z., L.D. and J.L. discussed the structure of the article and they all contributed to the article. Y.Y.D. wrote the first draft. W.Z., L.D. and J.L. modified the manuscript. All authors provided input on the final version of the manuscript. All authors provided input to the final version for publication.
Conflicts of interest statement
The authors declare that they have no competing interests.
References
- Abassi Z, Knaney Y, Karram Tet al. (2020) The lung macrophage in SARS-CoV-2 infection: a friend or a foe?. Front Immunol. 11:1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott NJ, Rönnbäck L, Hansson E (2006) Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 7:41–53. [DOI] [PubMed] [Google Scholar]
- Abbott NJ (2002) Astrocyte-endothelial interactions and blood-brain barrier permeability. J Anat. 200:629–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AboTaleb HA (2020) Neurological complications in COVID-19 patients and its implications for associated mortality. Curr Neurovasc Res. 17:522–30. [DOI] [PubMed] [Google Scholar]
- Achar A, Ghosh C (2020) COVID-19-associated neurological disorders: the potential route of CNS invasion and blood-brain relevance. Cells. 9:2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Mazrouei SS, Saeed GA, Al Helali AAet al. (2020) COVID-19-associated encephalopathy: neurological manifestation of COVID-19. Radiol Case Rep. 15:1646–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Aly Z, Xie Y, Bowe B (2021) High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 594:259–64. [DOI] [PubMed] [Google Scholar]
- Amsalem D, Dixon LB, Neria Y (2021) The Coronavirus Disease 2019 (COVID-19) outbreak and mental health: current risks and recommended actions. JAMA Psychiatry. 78:9–10. [DOI] [PubMed] [Google Scholar]
- Anand P, Al-Faraj A, Sader Eet al. (2020) Seizure as the presenting symptom of COVID-19: a retrospective case series. Epilepsy Behav. 112:107335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold DT, Hamilton FW, Milne Aet al. (2021) Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: results from a prospective UK cohort. Thorax. 76:399–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avula A, Nalleballe K, Narula Net al. (2020) COVID-19 presenting as stroke. Brain Behav Immun. 87:115–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig AM, Khaleeq A, Ali Uet al. (2020) Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 11:995–8. [DOI] [PubMed] [Google Scholar]
- Baik SH, Kang S, Son SMet al. (2016) Microglia contributes to plaque growth by cell death due to uptake of amyloid β in the brain of Alzheimer's disease mouse model. Glia. 64:2274–90. [DOI] [PubMed] [Google Scholar]
- Barrantes FJ (2020) Central nervous system targets and routes for SARS-CoV-2: current views and new hypotheses. ACS Chem Neurosci. 11:2793–803. [DOI] [PubMed] [Google Scholar]
- Basi S, Hamdan M, Punekar S (2020) Clinical course of a 66-year-old man with an acute ischaemic stroke in the setting of a COVID-19 infection. BMJ Case Rep. 13:e235920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltrán-Castillo S, Eugenín J, von Bernhardi R (2018) Impact of aging in microglia-mediated D-serine balance in the CNS. Mediators Inflamm. 2018:7219732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benameur K, Agarwal A, Auld SCet al. (2020) Encephalopathy and encephalitis associated with cerebrospinal fluid cytokine alterations and Coronavirus Disease, Atlanta, Georgia, USA, 2020. Emerg Infect Dis. 26:2016–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benussi A, Pilotto A, Premi Eet al. (2020) Clinical characteristics and outcomes of inpatients with neurologic disease and COVID-19 in Brescia, Lombardy, Italy. Neurology. 95:e910–20. [DOI] [PubMed] [Google Scholar]
- Bercik P, Verdu EF, Foster JAet al. (2010) Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology. 139:2102–12. e1. [DOI] [PubMed] [Google Scholar]
- Bernard-Valnet R, Pizzarotti B, Anichini Aet al. (2020) Two patients with acute meningoencephalitis concomitant with SARS-CoV-2 infection. Eur J Neurol. 27:e43–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyrouti R, Adams ME, Benjamin Let al. (2020) Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry. 91:889–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezdicek O, Ballarini T, Buschke Het al. (2019) Memory impairment in Parkinson's disease: the retrieval versus associative deficit hypothesis revisited and reconciled. Neuropsychology. 33:391–405. [DOI] [PubMed] [Google Scholar]
- Bhatia R, Srivastava MVP (2020) COVID-19 and stroke: incidental, triggered or causative. Annals Indian Acad Neurol. 23:318–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchetti A, Rozzini R, Guerini Fet al. (2020) Clinical presentation of COVID19 in dementia patients. J Nutr Health Aging. 24:560–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilinska K, Jakubowska P, Von Bartheld CSet al. (2020) Expression of the SARS-CoV-2 entry proteins, ACE2 and TMPRSS2, in cells of the olfactory epithelium: identification of cell types and trends with age. ACS Chem Neurosci. 11:1555–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco JR, Cobos-Ceballos MJ, Navarro Fet al. (2021) Pulmonary long-term consequences of COVID-19 infections after hospital discharge. Clin Microbiol Infect. 27:892–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth CM, Matukas LM, Tomlinson GAet al. (2003) Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 289:2801–9. [DOI] [PubMed] [Google Scholar]
- Brann DH, Tsukahara T, Weinreb Cet al. (2020) Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci Adv. 6:eabc5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown ES, Chandler PA (2001) Mood and cognitive changes during systemic corticosteroid therapy. Prim Care Companion J Clin Psychiatry. 3:17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Torres-Jardón R, Franco-Liraet al. (2020) Environmental nanoparticles, SARS-CoV-2 brain involvement, and potential acceleration of Alzheimer's and Parkinson's diseases in young urbanites exposed to air pollution. J Alzheimers Dis. 78:479–503. [DOI] [PubMed] [Google Scholar]
- Chen X, Laurent S, Onur OAet al. (2021) A systematic review of neurological symptoms and complications of COVID-19. J Neurol. 268:392–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crescenzo F, Marastoni D, Bovo Cet al. (2020) Frequency and severity of COVID-19 in multiple sclerosis: a short single-site report from northern Italy. Mult Scler Relat Disord. 44:102372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Dinan TG (2012) Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 13:701–12. [DOI] [PubMed] [Google Scholar]
- Daneman R, Prat A (2015) The blood–brain barrier. Cold Spring Harb Perspect Biol. 7:32–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das T, Hwang JJ, Poston KL (2019) Episodic recognition memory and the hippocampus in Parkinson's disease: a review. Cortex. 113:191–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desforges M, Le Coupanec A, Brison Eet al. (2014) Neuroinvasive and neurotropic human respiratory coronaviruses: potential neurovirulent agents in humans. Adv Exp Med Biol. 807:75–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desforges M, Le Coupanec A, Dubeau Pet al. (2019) Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system?. Viruses. 12:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Segarra N, Edmond A, Kunac Aet al. (2020) COVID-19 ischemic strokes as an emerging rehabilitation population: a case series. Am J Phys Med Rehabil. 99:876–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogan L, Kaya D, Sarikaya Tet al. (2020) Plasmapheresis treatment in COVID-19-related autoimmune meningoencephalitis: case series. Brain Behav Immun. 87:155–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosten C, Günther S, Preiser Wet al. (2003) Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 348:1967–76. [DOI] [PubMed] [Google Scholar]
- Du YY, Zhao W, Zhou XLet al. (2022) Amplitude of low-frequency fluctuation changes in COVID-19 patients: a resting-state fMRI study at a one-year follow-up. Neural Regen Res. 17:1576–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong L, Xu P, Liu A (2020) Meningoencephalitis without respiratory failure in a young female patient with COVID-19 infection in Downtown Los Angeles, early April 2020. Brain Behav Immun. 87:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich LC, Hu S, Sheng WSet al. (1998) Cytokine regulation of human microglial cell IL-8 production. J Immunol. 160:1944–8. [PubMed] [Google Scholar]
- Ellul M, Solomon T (2018) Acute encephalitis - diagnosis and management. Clin Med (Lond). 18:155–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellul MA, Benjamin L, Singh Bet al. (2020a) Neurological associations of COVID-19. Lancet Neurol. 19:767–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan M, Qiu W, Bu Bet al. (2020) Risk of COVID-19 infection in MS and neuromyelitis optica spectrum disorders. Neurol Neuroimmunol Neuroinflamm. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhadian S, Glick LR, Vogels CBFet al. (2020) Acute encephalopathy with elevated CSF inflammatory markers as the initial presentation of COVID-19. Res Sq. rs.3.rs–28583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano A, Cereda E, Barichella Met al. (2020a) COVID-19 in Parkinson's disease patients living in Lombardy, Italy. Mov Disord. 35:1089–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano A, Elia AE, Dallocchio Cet al. (2020b) Predictors of COVID-19 outcome in Parkinson's disease. Parkinsonism Relat Disord. 78:134–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gane SB, Kelly C, Hopkins C (2020) Isolated sudden onset anosmia in COVID-19 infection. A novel syndrome?. Rhinology. 58:299–301. [DOI] [PubMed] [Google Scholar]
- Gaunt ER, Hardie A, Claas ECet al. (2010) Epidemiology and clinical presentations of the four human coronaviruses 229E, HKU1, NL63, and OC43 detected over 3 years using a novel multiplex real-time PCR method. J Clin Microbiol. 48:2940–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo T, Zhang D, Zeng Yet al. (2020) Molecular and cellular mechanisms underlying the pathogenesis of Alzheimer's disease. Mol Neurodegener. 15:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamre D, Procknow JJ (1966) A new virus isolated from the human respiratory tract. Proc Soc Exp Biol Med. 121:190–3. [DOI] [PubMed] [Google Scholar]
- Han H, Ma Q, Li Cet al. (2020) Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect. 9:1123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M, Kleine-Weber H, Schroeder Set al. (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 181:271–80 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Huang L, Wang Yet al. (2021a) 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 397:220–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Wang Y, Li Xet al. (2020a) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Yao Q, Gu Xet al. (2021b) 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet. 398:747–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YH, Jiang D, Huang JT (2020b) SARS-CoV-2 detected in cerebrospinal fluid by PCR in a case of COVID-19 encephalitis. Brain Behav Immun. 87:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacomy H, Talbot PJ (2003) Vacuolating encephalitis in mice infected by human coronavirus OC43. Virology. 315:20–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain R, Young M, Dogra Set al. (2020) COVID-19 related neuroimaging findings: a signal of thromboembolic complications and a strong prognostic marker of poor patient outcome. J Neurol Sci. 414:116923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jose RJ, Manuel A (2020) COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. 8:e46–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kansagra SM, Gallentine WB (2011) Cytokine storm of acute necrotizing encephalopathy. Pediatr Neurol. 45:400–2. [DOI] [PubMed] [Google Scholar]
- Karimi N, Razavi SA, Rouhani N (2020) Frequent convulsive seizures in an adult patient with COVID-19: a case report. Iran Red Crescent Med J. 22:e102828. [Google Scholar]
- Khoo A, McLoughlin B, Cheema Set al. (2020) Postinfectious brainstem encephalitis associated with SARS-CoV-2. J Neurol Neurosurg Psychiatry. 91:1013–4. [DOI] [PubMed] [Google Scholar]
- Kihira S, Delman BN, Belani Pet al. (2020) Imaging features of acute encephalopathy in patients with COVID-19: a case series. Am J Neuroradiol. 41:1804–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klok FA, Kruip M, van der Meer NJMet al. (2020) Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 191:145–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopfenstein T, Kadiane-Oussou NJ, Toko Let al. (2020) Features of anosmia in COVID-19. Med Mal Infect. 50:436–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koralnik IJ, Tyler KL (2020) COVID-19: a global threat to the nervous system. Ann Neurol. 88:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krett JD, Jewett GAE, Elton-Lacasse Cet al. (2020) Hemorrhagic encephalopathy associated with COVID-19. J Neuroimmunol. 346:577326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuypers J, Martin ET, Heugel Jet al. (2007) Clinical disease in children associated with newly described coronavirus subtypes. Pediatrics. 119:e70–6. [DOI] [PubMed] [Google Scholar]
- Lai J, Ma S, Wang Yet al. (2020) Factors associated with mental health outcomes among health care workers exposed to Coronavirus Disease 2019. JAMA Netw Open. 3:e203976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam MH, Wing YK, Yu MWet al. (2009) Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: long-term follow-up. Arch Intern Med. 169:2142–7. [DOI] [PubMed] [Google Scholar]
- Lau S, Woo P, Yip Cet al. (2006) Coronavirus HKU1 and other coronavirus infections in Hong Kong. J Clin Microbiol. 44:2063–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechien JR, Chiesa-Estomba CM, De Siati DRet al. (2020) Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 277:2251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Wohlford-Lenane C, Perlman Set al. (2016) Middle East Respiratory Syndrome coronavirus causes multiple organ damage and lethal disease in mice transgenic for human dipeptidyl peptidase 4. J Infect Dis. 213:712–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Li M, Wang Met al. (2020a) Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke Vasc Neurol. 5:279–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YC, Bai WZ, Hashikawa T (2020b) The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 92:552–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YC, Bai WZ, Hirano Net al. (2012) Coronavirus infection of rat dorsal root ganglia: ultrastructural characterization of viral replication, transfer, and the early response of satellite cells. Virus Res. 163:628–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Ge J, Yang Met al. (2020c) Vicarious traumatization in the general public, members, and non-members of medical teams aiding in COVID-19 control. Brain Behav Immun. 88:916–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lia V, Pyrc K, Jebbink MFet al. (2004) Identification of a new human coronavirus. Nat Med. 10:368–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libbey JE, Fujinami RS (2011) Neurotropic viral infections leading to epilepsy: focus on Theiler's murine encephalomyelitis virus. Future Virol. 6:1339–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Fang YY, Deng Yet al. (2020) Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J. 133:1025–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodigiani C, Iapichino G, Carenzo Let al. (2020) Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 191:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louapre C, Collongues N, Stankoff Bet al. (2020) Clinical characteristics and outcomes in patients with Coronavirus Disease 2019 and multiple sclerosis. JAMA Neurol. 77:1079–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Xiong W, Liu Det al. (2020) New onset acute symptomatic seizure and risk factors in coronavirus disease 2019: a retrospective multicenter study. Epilepsia. 61:e49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukiw WJ, Pogue A, Hill JM (2020) SARS-CoV-2 infectivity and neurological targets in the brain. Cell Mol Neurobiol. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons S, O'Kelly B, Woods Set al. (2020) Seizure with CSF lymphocytosis as a presenting feature of COVID-19 in an otherwise healthy young man. Seizure. 80:113–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay IM, Arden KE (2015) Middle East respiratory syndrome: an emerging coronavirus infection tracked by the crowd. Virus Res. 202:60–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahallawi WH, Khabour OF, Zhang Qet al. (2018) MERS-CoV infection in humans is associated with a pro-inflammatory Th1 and Th17 cytokine profile. Cytokine. 104:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L, Jin H, Wang M, Hu Yet al. (2020) Neurologic manifestations of hospitalized patients with Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 77:683–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza MG, Palladini M, De Lorenzo Ret al. (2021) Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: effect of inflammatory biomarkers at three-month follow-up. Brain Behav Immun. 94:138–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCray PB Jr., Pewe L, Wohlford-Lenane Cet al. (2007) Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. J Virol. 81:813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh K, Dees JH, Becker WBet al. (1967) Recovery in tracheal organ cultures of novel viruses from patients with respiratory disease. Proc Natl Acad Sci USA. 57:933–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P, McAuley DF, Brown Met al. (2020) COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet North Am Ed. 395:1033–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo P, Xing Y, Xiao Yet al. (2020) Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin Infect Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi T, Harii N, Goto Jet al. (2020) A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 94:55–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niazkar HR, Zibaee B, Nasimi Aet al. (2020) The neurological manifestations of COVID-19: a review article. Neurol Sci. 41:1667–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxley TJ, Mocco J, Majidi Set al. (2020) Large-vessel stroke as a presenting feature of COVID-19 in the young. N Engl J Med. 382:e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozma MA, Maroufi P, Khodadadi Eet al. (2020) Clinical manifestation, diagnosis, prevention and control of SARS-CoV-2 (COVID-19) during the outbreak period. Infez Med. 28:153–65. [PubMed] [Google Scholar]
- Paniz-Mondolfi A, Bryce C, Grimes Zet al. (2020) Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). J Med Virol. 92:699–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge WM (2005) The blood-brain barrier: bottleneck in brain drug development. NeuroRx. 2:3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson RW, Brown RL, Benjamin Let al. (2020) The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 143:3104–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris JS, Chu CM, Cheng VCet al. (2003) Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 361:1767–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelaia C, Tinello C, Vatrella Aet al. (2020) Lung under attack by COVID-19-induced cytokine storm: pathogenic mechanisms and therapeutic implications. Ther Adv Respir Dis. 14:1753466620933508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perugula ML, Lippmann S (2016) Encephalopathy or psychosis?. Innov Clin Neurosci. 13:41–2. [PMC free article] [PubMed] [Google Scholar]
- Pezzini A, Padovani A (2020) Lifting the mask on neurological manifestations of COVID-19. Nat Rev Neurol. 16:636–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilotto A, Odolini S, Masciocchi Set al. (2020) Steroid-responsive encephalitis in Coronavirus Disease 2019. Ann Neurol. 88:423–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponsen MM, Stoffers D, Booij Jet al. (2004) Idiopathic hyposmia as a preclinical sign of Parkinson's disease. Ann Neurol. 56:173–81. [DOI] [PubMed] [Google Scholar]
- Poyiadji N, Shahin G, Noujaim Det al. (2020) COVID-19-associated Acute Hemorrhagic Necrotizing Encephalopathy: Imaging Features. Radiology. 296:E119–e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Wu J, Chen Tet al. (2021) Long-term microstructure and cerebral blood flow changes in patients recovered from COVID-19 without neurological manifestations. J Clin Invest. 131:e147329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SH, Pothoulakis C, Mayer EA (2009) Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol. 6:306–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochfort KD, Collins LE, Murphy RPet al. (2014) Downregulation of blood-brain barrier phenotype by proinflammatory cytokines involves NADPH oxidase-dependent ROS generation: consequences for interendothelial adherens and tight junctions. PLoS ONE. 9:e101815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JP, Chesney E, Oliver Det al. (2020) Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 7:611–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi A (2008) Imaging of acute disseminated encephalomyelitis. Neuroimaging Clin N Am. 18:149–61. [DOI] [PubMed] [Google Scholar]
- Sacco RL, Kasner SE, Broderick JPet al. (2013) An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 44:2064–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE (2007) The neurobiology of epilepsy. Curr Neurol Neurosci Rep. 7:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang J, Wan Y, Luo Cet al. (2020) Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci USA. 117:11727–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S, Qin M, Cai Yet al. (2020) Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur Heart J. 41:2070–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Kukreti R, Saso Let al. (2019) Oxidative stress: a key modulator in neurodegenerative diseases. Molecules. 1583, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhi P (2011) Infectious causes of seizures and epilepsy in the developing world. Dev Med Child Neurol. 53:600–9. [DOI] [PubMed] [Google Scholar]
- Sirven JI. (2015) Epilepsy: a spectrum disorder. Cold Spring Harb Perspect Med. 5:a022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sora Y, Viroj W (2020) COVID-19 and epilepsy. Annals Indian Acad Neurol. 23. DOI: 10.4103/aian.AIAN_254_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence JD, de Freitas GR, Pettigrew LCet al. (2020) Mechanisms of stroke in COVID-19. Cerebrovasc Dis. 49:451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafstrom CE, Carmant L (2015) Seizures and epilepsy: an overview for neuroscientists. Cold Spring Harb Perspect Med. 5:a022426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H, Yang M, Wan Cet al. (2020) Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 98:219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taquet M, Geddes JR, Husain Met al. (2021a) 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 8:416–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taquet M, Luciano S, Geddes JRet al. (2021b) Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatry. 8:130–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejera D, Mercan D, Sanchez-Caro JMet al. (2019) Systemic inflammation impairs microglial Aβ clearance through NLRP3 inflammasome. EMBO J. 38:e101064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur V, Jain A (2020) COVID 2019-suicides: a global psychological pandemic. Brain Behav Immun. 88:952–3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Trinkmann F, Müller M, Reif Aet al. (2021) Residual symptoms and lower lung function in patients recovering from SARS-CoV-2 infection. Eur Respir J. 57:2003002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyer EA, Kohn JN, Hong S (2020) Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun. 87:34–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umapathi T, Quek WMJ, Yen JMet al. (2020) Encephalopathy in COVID-19 patients; viral, parainfectious, or both?. eNeurologicalSci. 21:100275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bulck M, Sierra-Magro A, Alarcon-Gil Jet al. (2019) Novel approaches for the treatment of Alzheimer's and Parkinson's Disease. Int J Mol Sci. 719, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varatharaj A, Thomas N, Ellul MAet al. (2020) Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 7:875–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan S, Xiang Y, Fang Wet al. (2020) Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol. 92:797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Kream RM, Stefano GB (2020a) Long-term respiratory and neurological sequelae of COVID-19. Med Sci Monit. 26:e928996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, He W, Yu Xet al. (2020b) Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Infect. 80:639–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Zhang Y, Wu Let al. (2020c) Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell. 181:894–904. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington TP, Bostwick JM (2006) Psychiatric adverse effects of corticosteroids. Mayo Clin Proc. 81:1361–7. [DOI] [PubMed] [Google Scholar]
- Wong CK, Lam CW, Wu AKet al. (2004) Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 136:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong PF, Craik S, Newman Pet al. (2020) Lessons of the month 1: a case of rhombencephalitis as a rare complication of acute COVID-19 infection. Clin Med (Lond). 20:293–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Liu X, Zhou Yet al. (2021) 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study. Lancet Respir Med. 9:747–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Q, Xu M, Li Jet al. (2021) Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin Microbiol Infect. 27:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Zhong S, Liu Jet al. (2005) Detection of severe acute respiratory syndrome coronavirus in the brain: potential role of the chemokine mig in pathogenesis. Clin Infect Dis. 41:1089–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaghi S, Ishida K, Torres Jet al. (2020) SARS-CoV-2 and stroke in a New York healthcare system. Stroke. 51:2002–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, Huang H, Wang Cet al. (2021) Follow-up study of pulmonary function among COVID-19 survivors 1 year after recovery. J Infect. 83:381–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Cao J, Wang Qet al. (2020) D-dimer as a biomarker for disease severity and mortality in COVID-19 patients: a case control study. J Intensive Care. 8:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye M, Ren Y, Lv T (2020) Encephalitis as a clinical manifestation of COVID-19. Brain Behav Immun. 88:945–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Wunderink RG (2018) MERS, SARS and other coronaviruses as causes of pneumonia. Respirology. 23:130–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying W, Qian Y, Kun Z (2021) Drugs supply and pharmaceutical care management practices at a designated hospital during the COVID-19 epidemic. Res Social Adm Pharm. 17:1978–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HH, Qin C, Chen Met al. (2020) D-dimer level is associated with the severity of COVID-19. Thromb Res. 195:219–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki AM, van Boheemen S, Bestebroer TMet al. (2012) Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 367:1814–20. [DOI] [PubMed] [Google Scholar]
- Zambreanu L, Lightbody S, Bhandari Met al. (2020) A case of limbic encephalitis associated with asymptomatic COVID-19 infection. J Neurol Neurosurg Psychiatry. 91:1229–30. [DOI] [PubMed] [Google Scholar]
- Zeng ZQ, Chen DH, Tan WPet al. (2018) Epidemiology and clinical characteristics of human coronaviruses OC43, 229E, NL63, and HKU1: a study of hospitalized children with acute respiratory tract infection in Guangzhou, China. Eur J Clin Microbiol Infect Dis. 37:363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai P, Ding Y, Li Y (2020) The impact of COVID-19 on ischemic stroke. Diagn Pathol. 15:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Jiang L (2015) Neuroinflammation in Alzheimer's disease. Neuropsychiatr Dis Treat. 14:243–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Yan X, Fan Qet al. (2020) D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost. 18:1324–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Yang XL, Wang XGet al. (2020a) A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 579:270–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Li W, Wang Det al. (2020b) Clinical time course of COVID-19, its neurological manifestation and some thoughts on its management. Stroke Vasc Neurol. 5:177–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Liu Q, Du Let al. (2013) Receptor-binding domain as a target for developing SARS vaccines. J Thorac Dis. 5 Suppl 2:S142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X, Chen K, Zou Jet al. (2020) Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 14:185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuhorn F, Omaimen H, Ruprecht Bet al. (2021) Parainfectious encephalitis in COVID-19: “The Claustrum Sign”. J Neurol. 268:2031–4. [DOI] [PMC free article] [PubMed] [Google Scholar]