Summary
Antimicrobial host defence peptides (HDP) are critical for the first line of defence against bacterial, viral, and fungal pathogens. Over the past decade we have become more aware that, in addition to their antimicrobial roles, they also possess the potent immunomodulatory capacity. This includes chemoattracting immune cells, activating dendritic cells and macrophages, and altering T-cell differentiation. Most examinations of their immunomodulatory roles have focused on tissues in which they are very abundant, such as the intestine and the inflamed skin. However, HDP have now been detected in the brain and the spinal cord during a number of conditions. We propose that their presence in the central nervous system (CNS) during homeostasis, infection, and neurodegenerative disease has the potential to contribute to immunosurveillance, alter host responses and skew developing immunity. Here, we review the evidence for HDP expression and function in the CNS in health and disease. We describe how a wide range of HDP are expressed in the CNS of humans, rodents, birds, and fish, suggesting a conserved role in protecting the brain from pathogens, with evidence of production by resident CNS cells. We highlight differences in methodology used and how this may have resulted in the immunomodulatory roles of HDP being overlooked. Finally, we discuss what HDP expression may mean for CNS immune responses.
Keywords: brain, central nervous system, host defence peptides, neurodegeneration, cathelicidin, defensins, dermcidin, hepcidin
Introduction
Antimicrobial host defence peptides (HDP) are a family of short peptides with diverse sequences, produced both constitutively and in response to bacterial, viral, and fungal infections. They are expressed in multiple tissues and fluids throughout the body, including serum, saliva, semen, sweat, lung, intestine, and skin [1–6]. Cells that are well documented to produce HDP include neutrophils, mast cells, macrophages, Paneth cells, and mucosal epithelium [7–10]. Features of abundant HDP are shown in Table 1.
Table 1:
Features of antimicrobial peptides expressed in the CNS. PDB—reference code for entry in the protein data bank. Created with BioRender.com.
| Peptide | Structure | Size (# residues, human form) | Charge |
|---|---|---|---|
| Cathelicidin |
 Human CAMP (LL-37) PDB: 2KSO Wang., 2008 |
37 | Cationic +6 |
| Beta defensin 1 |
 Human DEFB1 PDB: 1KJ5 Schibli et al., 2001 |
36 | Cationic +4 |
| Beta defensin 2 |
 Human DEFB4 PDB: 1FQQ Sawai et al., 2001 |
41 | Cationic +6 |
| Dermcidin |
 Human DCD PDB: 2KSG Jung et al., 2010 |
48 | Anionic −5 |
| Hepcidin |
 Human HAMP PDB: 2KEF Jordan et al., 2009 |
25 | Cationic +3 |
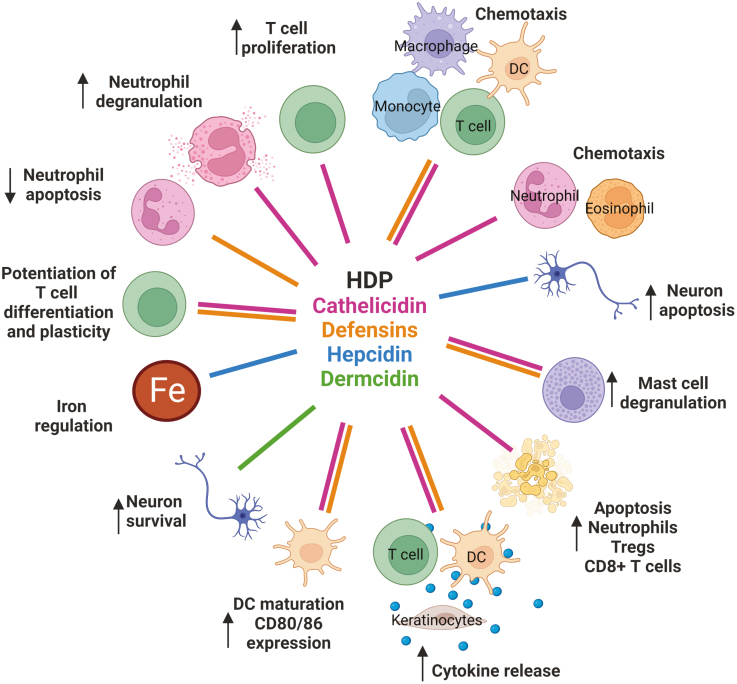
In addition to their antimicrobial capacities, HDP are potent immunomodulators. In particular, defensins, and cathelicidins can alter dendritic cell activation and differentiation [11–14], skew T-cell differentiation [13, 15], halt macrophage mRNA translation [16], and chemoattract immune cells including monocytes and T cells [17] (Fig. 1). As such, their production not only drives defence against infiltrating infection but also substantially alters immunity during infectious and inflammatory disease (HDP immunomodulatory capacities are summarized in Fig. 1).
Figure 1:
Known immunomodulatory effects of host defence peptides. Created with BioRender.com.
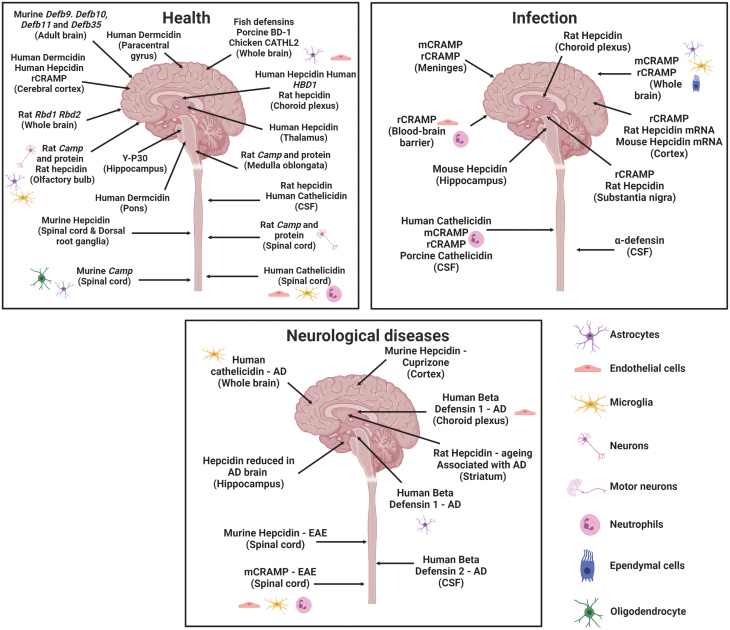
The expression of a variety of peptides has been widely described in many tissues, with a particular abundance in the intestine [18, 19], lung [20, 21], and skin [22–24]. However, one exception has been the central nervous system (CNS). The production of HDP by resident and infiltrating cells of the CNS, and their capacity to modulate immunity there, has not previously been reviewed, despite mounting evidence that HDP are indeed present at this site (Fig. 2 and Table 2). Here, we review the expression and discuss the potential functions of HDP throughout the nervous system of multiple species in health, infection, and neurodegenerative disease.
Figure 2:
The expression of host defence peptides across the central nervous system in health, infection and neurological disease. Created with BioRender.com.
Table 2:
| Peptide | Expression in the CNS | Cellular source | References |
|---|---|---|---|
| Cathelicidin | Whole brain, olfactory bulb, medulla oblongata, spinal cord, hippocampus, striatum, cerebellum, dorsolateral prefrontal cortex, anterior cingulate cortex, meninges, CSF | Microglia, astrocytes, motor neurons, Purkinje cells, olfactory bulb neurons, dorsolateral prefrontal cortex, anterior cingulate cortex, BBB endothleial cells, infiltrating neutrophils, meningeal cells, neuronal cell lines | Bals et al. (1998), Bergman et al. (2005), van Dijk et al. (2005), Bergman et al. (2006), Brandenburg et al. (2009), Brandenburg et al. (2008). Brandenburg et al. (2010), Lewis et al. (2014), Lee et al. (2015), Byfield et al. (2011), de Buhr et al. (2017), Postolache et al. (2020), Hassel et al. (2018) |
| Beta defensin 1 | Whole brain, choroid plexus, hippocampus, spinal cord | Neuronal cells, astrocytes | Huttner et al. (1997), Nakayama et al. (1999), Hiratsuka et al. (2001), Froy et al. (2007), Maxwell et al. (2003), Morrison et al. (2003), Zhang et al. (1998), Hao et al. (2001), Schluesener and Meyermann (1995), Williams et al. (2013), Fleming et al. (2006) |
| Beta defensin 2 | Whole brain (low levels) | Immortalized brain capillary endothelial cells, neuronal cells | Huttner et al. (1997); Hiratsuka et al. (2001), Froy et al. (2007), Maxwell et al. (2003), Morrison et al. (2003), Tiszlavicz et al. (2011), Hao et al. (2001), Soman et al. (2009) |
| Dermcidin | Pons, paracentral gyrus, locus ceruleus, nucleus raphe pontis, substantia nigra, lateral hypothalamic nuclei | Unknown | Porter et al. (2003) |
| Hepcidin | Whole brain, choroid plexus, cortex, thalamus, hippocampus, striatum, substantia nigra, choroid plexus, spinal cord, dorsal root ganglia | Astrocytes, epithelial cells of choroid plexus, neurons, immortalized mouse microglia | Hanninen et al. (2009),, Hanase et al. (2020), Raha-Chowdhury et al. (2015), Zechel et al. (2006), Pandur et al. (2019), Urrutia et al. (2013); Wang et al. (2008), Zarruk et al. (2015), and Varga et al. (2018) |
Cathelicidin
The short cationic peptide cathelicidin has multiple well-defined antimicrobial and immunomodulatory roles. Its expression has been described in many tissues such as the skin [25], intestine [6], airways [21], and reproductive system [26, 27]. Many cell types can produce cathelicidin; it is stored at a high concentration in neutrophil secondary granules and can also be produced by monocytes, macrophages, mast cells, adipocytes, and some T-cell subsets [10, 28–30]. It has direct and indirect anti-bacterial, anti-viral, and anti-fungal action [31–33], with direct killing observed against the respiratory syncytial virus [32], many bacterial species including Escherichia coli and Streptococcus pneumoniae (reviewed in [34]) and fungal species including Candida albicans [35]. As a consequence, mice lacking cathelicidin are more susceptible to a variety of infections [31, 36, 37].
Cathelicidin is also a powerful immunomodulator and understanding its impact is a burgeoning field of immunology. For example, it can mature dendritic cells and up-regulate their T cell priming capacities [11, 13, 14], chemoattract innate and adaptive immune cells, enhance T-cell survival and Th17 differentiation [15, 17, 38], and induce re-epithelialization and re-endothelialization following damage [39, 40]. It is therefore important to understand whether cathelicidin is expressed in the CNS, and whether its immunomodulatory roles are important at that site.
Health
During steady state, cathelicidin is expressed in the human CNS: in patients with conditions that do not clinically alter cerebrospinal fluid (CSF) composition (idiopathic cephalgia, ischialgia due to discopathy, and idiopathic facial nerve palsy) cathelicidin has been detected in the fluid (in the range 0.01–0.07μM) [41]. Interestingly, this is similar to the mean cathelicidin concentration in healthy plasma, which is 0.07 μM (n = 58, SD = 0.20) [7]. Dot blot hybridization demonstrated cathelicidin expression in whole healthy human brain homogenate to be, strikingly, at similar levels to the colon and the lung [21]. A more recent study that generated a single cell atlas of the human spinal cord showed that cathelicidin mRNA was expressed—albeit at low concentrations—in some astrocyte and oligodendrocyte populations [42]. Finally, recent work from our laboratory has shown that cathelicidin is expressed at the protein level in human post-mortem brain tissue from donors who did not die from neurological causes [43]. In these samples, cathelicidin was expressed by neutrophils, CD68+ microglia/macrophages, and endothelial cells, which to our knowledge is the first demonstration of these cells expressing cathelicidin in a healthy brain [43]. Thus, cathelicidin mRNA and protein are expressed during homeostasis in the human CNS.
Cathelicidin is also present in the central nervous systems of rodents, birds, and small mammals. mRNA encoding rat cathelicidin was detected in the olfactory bulb, medulla oblongata, and spinal cord of healthy brains [44]. In this study, rat cathelicidin was also detected in primary cell cultures of the hippocampus, striatum, cerebellum, and medulla oblongata. Furthermore, CMAP27, a chicken cathelicidin-like antimicrobial peptide, is expressed at the mRNA level in the brain [45]. This data suggests cathelicidin expression is conserved across different species in the steady-state CNS. In contrast, cathelicidin mRNA was not detected in healthy mouse brains using northern blot analysis [46], nor in our examinations of the healthy mouse spinal cord [43], making mice unusual in their lack of CNS cathelicidin expression.
Infection
As HDPs are critical in the innate immune response, it is likely they are important innate responders to infection in the CNS. Not surprisingly, cathelicidin levels are elevated in human CSF during infection. During active bacterial meningitis, cathelicidin is increased up to 0.02 μM in the CSF of patients, compared to 0.0025 μM in the healthy controls of this study [47]. Cathelicidin concentration positively correlates with CSF bacterial count [48] and with CSF white cell counts [49]. Similarly, CSF cathelicidin was elevated in children with tuberculous meningitis compared to healthy controls [50]. Moreover, in human CSF cathelicidin levels are increased in tuberculosis meningitis-positive HIV patients compared to tuberculosis meningitis-182 negative HIV patients [51].
Experiments to determine the cellular source of CNS cathelicidin have determined a surprisingly wide range of resident and infiltrating cells able to produce it. During Neisseria meningitidis infection, rat cathelicidin production increased in endothelial cells and infiltrating neutrophils in the meninges, as measured by immunohistochemical analysis [52]. It was also detected in the brains of rats 12, 22, and 39 h after Streptococcus pneumoniae infection [53] and can be produced by microglia and astrocytes following Pneumococcal meningitis infection [47]. Moreover, neutrophil extracellular traps coated with high concentrations of cathelicidin are released in the CSF following bacterial meningitis infection in rats [54] and Streptococcus suis infection in piglets [55]. In mice, cathelicidin is expressed in the meninges and brain parenchyma after pneumococcal infection and mice lacking cathelicidin have increased mortality following infection with Streptococcus pneumonia to induce meningitis [56]. Moreover, cathelicidin is expressed in the meninges and brain parenchyma of mice after pneumococcal infection.
Therefore, in many species cathelicidin is upregulated during CNS infection and can be produced by glial cells, endothelial cells, and infiltrating neutrophils. As cathelicidin has potent anti-bacterial and anti-viral activity, it is highly likely that cathelicidin will be involved in clearance of CNS invading pathogens. However, cathelicidin also has powerful immunomodulatory roles. In one study, it promoted signal transduction in glial cells leading to IL-6 production, in a manner dependent on ERK1/2, p38 MAPK, and NFκB [57]. Its role in glial cell function appears complex, with some evidence that it may play a regulatory role—for example cathelicidin-knockout glial cells have a pronounced pro-inflammatory response following meningitis infection [58]. Unpicking how regulation of immune responses is separate from cathelicidin’s direct anti-endotoxic and anti-inflammatory effects will take considerable work.
Neurodegeneration
HDP-secreting neutrophils migrate into the CNS during neuroinflammation. Neutrophil infiltration into the spinal cord and brain tissue has been observed in mouse models of Alzheimer’s disease (AD) [59, 60] and multiple sclerosis (MS) [61, 62]. Importantly, cathelicidin-positive neutrophils are present in the spinal cord in experimental autoimmune encephalomyelitis (EAE), a mouse model of MS. Depletion of neutrophils or cathelicidin attenuates the development of EAE [43, 60, 62–64] and in AD models this can improve cognitive decline [65].
In humans, neutrophils are present in the CSF and in active lesions of neuromyelitis optica patients [66]. Moreover, it has been shown that neutrophils release extracellular traps (NETs) in the brain parenchyma of AD patients [60]. MS peripheral blood neutrophils have increased activation markers and enhanced degranulation [67]. As cathelicidins, as well as other HDPs including defensins, are secreted during degranulation and are present on NETs, these studies suggest HDP could have a functional role during neutrophil effector mechanisms in these diseases.
Immunohistochemistry in post-mortem brains from patients with Alzheimer’s disease (AD) showed cathelicidin expression to be increased in microglia and astrocytes compared to healthy donor brain samples [68]. We have recently shown that cathelicidin is expressed in active lesions in the brains of patients with MS and in the spinal cords of mice undergoing the model of MS experimental autoimmune encephalomyelitis (EAE) [43]. In both cases, the majority of cathelicidin was released by neutrophils, but it was also seen expressed by microglia and by endothelial cells. Not only is cathelicidin expressed in the CNS but also it plays a key role in promoting damaging inflammation such that mice lacking the peptide are resistant to developing severe EAE. Therefore, during MS cathelicidin plays a role in potentiating harmful immune responses.
Defensins
Defensins are cationic peptides with a characteristic series of cysteine residues, which form an antiparallel β-sheet structure. There are two classes, α- and β-defensins [69], which have broad anti-microbial, anti-viral, and anti-fungal activity [70]—for example they have demonstrated activity against Staphylococci [71], herpes simplex virus [72], influenza virus [73], HIV [74], and Candida albicans [75].
α-defensins are stored in neutrophil primary granules at high concentrations and are released from Paneth cells in the intestine [69, 76]. They have been associated with CNS infections previously, being elevated in the CSF of children with bacterial meningitis (with a median of 23μg/ml in infected subjects and with no control subjects having detectable defensins in the CSF) [77]. Using LC–MS/MS analysis and ELISA techniques, α-defensin 1 was detected in the CSF of patients with West Nile neuroinvasive disease and non-WNV CNS infections [78]. It is expected that these CSF defensins are being released from neutrophils, and there are no published papers to our knowledge showing the expression of α-defensins by CNS-resident cells. To examine this further, we have carried out mining of published sequencing datasets of neurons, astrocytes, microglia, and oligodendrocytes. None of these datasets showed any expression of the alpha defensin genes Defa1, Defa2, Defa3, Defa4, Defa5, or Defa6. It is likely therefore that they are only being released by neutrophils, and also that the methods we use currently have led to the impact and quantity of this being underestimated (see note at end on Methodology). For the remainder of this review, we will focus on β-defensins.
β-defensins are not only associated with infection but also have significant immunomodulatory roles. Their production is increased in monocytes by LPS but also by IFN-γ and vitamin D [9, 79] indicating inflammation-related and not only infection-related mediators can switch on defensin expression.
Defensins have multiple roles in many immune cells. For example, human β-defensin 3 modulates TLR4 signalling [80], chemoattracts monocyte/macrophages [81], alters macrophage differentiation and increases their IL-4 production [82], and enhances dendritic cell responses to bacterial DNA in a TLR-9 dependent manner [12]. Mouse βD-14 switches CD4+ CD25− T cells into regulatory T cells inducing expression of FOXP3 and CTLA-4 [83]. β-defensins can also be anti-inflammatory, as hBD3 in the presence of LPS inhibits IL-6 and TNF-α accumulation in the human myelomonocytic cell line THP-1 and peripheral blood monocytes derived macrophages [84]. As a family of peptides, therefore, they have the varied immunomodulatory capacity that affects both innate and adaptive immune cells.
Health
In humans, early work analyzing the widespread expression of hBD-1 in the CNS showed it is not expressed in the mRNA isolated from the brain (although the specific region was not specified) [85]. Further, more specific, a study of frozen brain tissue of patients without CNS disorders showed that hBD-1 mRNA is expressed in the choroid plexus but not in the cerebral cortex, cerebellum, pia mater, or leptomeningeal vessels [86]. In this study, hBD-2 was not detected in any brain regions examined. The first study used northern blot analysis to assess hBD-1 expression, whilst the latter used RT-PCR analysis. This highlights the requirement for multiple experimental techniques to fully understand HDP expression within tissues; as detailed below (see ‘A note on methodology’), detection of HDP is sometimes difficult. Moreover, this data suggests in humans there is a regional expression of hBD-1 as well as differential expression of individual defensins. The choroid plexus serves as an interface between the CNS and the periphery, is a niche for resident immune cells, and has been shown to be the site of T-cell stimulation in the CNS [87–90]. As the choroid plexus was the only site in the brain that expressed hBD-2 in this study, it is possible that hBD-2 expression at this site is part of the immune surveillance of the CNS by immune cells. Further understanding of the function of human defensins in the CNS during steady-state may help elucidate these differences.
β-defensins are expressed in the brain of a wide variety of species. Porcine BD-1 is expressed in the brain of 4–5-week-old pigs [91]. Studies using RT-PCR show that Rbd1 and Rbd2 are expressed at low levels in the rat brain [92, 93] and the bovine β-defensin is expressed in the meninges and choroid plexus of healthy adult cows [94]. Likewise, Defb9, Defb10, Defb11, and Defb35 are expressed in the adult mouse brain and Defb10, Defb11, and Defb35 are expressed in the neonate [95, 96]. In health, the levels of the beta-defensin homologue, gcdefb1, in Chinese grass Carp showed the highest expression in the brain compared to other tissues [97]. In other healthy fish such as mandarin [98] and orange-spotted grouper [99], β-defensin transcripts are present but are expressed at low levels in the brain. The duck β-defensin-2 homologue is also expressed at low levels in the brain in healthy ducklings [100]. In addition, members of the β-defensin family are differentially expressed; in rainbow trout, for example, four novel members of the family were identified but only omBD-3 was expressed at low levels in the brain [101], and in the blunt snout bream maBD-2 was expressed in the brain, but not maBD-2 [102].
Thus, defensins are expressed widely and are conserved across different species. It is likely that the expression of defensins during a steady state plays a role in immune surveillance and may have important functions in regulating immune responsiveness of resident CNS cells. However, whether the expression of different defensins, and their expression in different brain regions possess different functions is unclear and warrants further investigation.
Infection
Surprisingly, there are very few studies investigating the expression of β-defensins in the CNS following infection. One study demonstrated the expression of hBD-2 mRNA and protein by immortalized human brain capillary endothelial cells after Chlamydophila pneumoniae infection [103]. Another study demonstrated that stimulating astrocytes cell cultures with LPS, IL-1β, or TNF-α—to model infection—stimulated the production of hBD1 and hBD2 mRNA and protein, whilst meningeal fibroblasts and microglia were only able to express hBD1 mRNA [104]. These results suggest a possible role for hBD2 in early immune responses of the brain.
Neurodegeneration
It has been suggested that β-defensins could play a role in the neuroimmune function and during neurodegeneration [105]. Williams and colleagues hypothesize that conditions such as hyperglycaemia and increased insulin resistance, which are present in many neurological conditions, may alter defensin expression; for example, high glucose induces hBD2 and hBD3 mRNA expression from primary epithelial cells in vitro. In addition, they suggest that abnormal expression of β-defensins could contribute to loss of AMP-induced regulation of dendritic cells and chronic inflammation.
Similarly, levels of hBD-2 were significantly elevated in the sera and in the CSF of patients with AD compared to age-matched controls [106]. In addition, hBD-1, but not hBD-2 or h-BD-3, is present within hippocampal astrocytes as well as in neurons and the choroid plexus and is increased in patients with AD [107].
Dermcidin
Dermcidin is a non-classical HDP that shares no homology with other known antimicrobial peptides [3]. It is secreted constitutively by eccrine sweat glands at a concentration of ~1–10 µg/ml, and is transported to the epidermal surface [3]. Dermcidin is proteolytically cleaved into an active form which is 47 amino acids in length [3] and, unlike other positively charged HDPs, its charge is −5 [3]. It has potent antimicrobial activity, contributing to the immune defence of the skin [108]. Broad spectrum activity against several different pathogens has been described such as Staphylococcus epidermidis, Pseudomonas aeruginosa, Pseudomonas putida, methicillin-resistant S. aureus, Listeria monocytogenes, and Salmonella typhimurium [109–111]. This peptide was originally identified in humans [3], and interestingly dermcidin has no homologue in rodents or other mammals except for primates [108].
Health
Dermcidin is expressed in the uninfected brain; northern blot analysis showed dermcidin expression specifically in the pons of healthy adult and foetal human brains, with low expression also noted in the paracentral gyrus of the cerebral cortex [112].
An AP-Dermcidin fusion protein showed strong binding to neurons in the locus ceruleus, nucleus raphe pontis, substantia nigra, and the lateral hypothalamic nuclei and weak binding to almost all neurons in the healthy human adult brain [112]. The authors suggest that dermcidin could be acting as a survival factor for neurons that have increased sensitivity to reactive oxygen species [112]. Similarly, Y-P30 (the first 30 amino acids of the dermcidin precursor protein [108]), has been detected in neonatal rats and human foetal brains in the neocortex and hippocampus. Under oxidative stress conditions, Y-P30 has been shown to promote the survival of retinoblastoma cells, hepatocellular carcinoma cell line HuH7, and the prostate cancer cell line PC-3M [113, 114].
Infection
Dermcidin has broad antimicrobial activity against many bacteria including Staphylococcus, Listeria and Salmonella species, but to our knowledge its activity during CNS infection has not been investigated. High dermcidin production is exclusive to the pons of the healthy human brain [112]. As the pons has an active relationship with the periphery [115], expression of dermcidin in this brain region could be an important mechanism for innate defence against infection. However, it remains to be clarified whether dermcidin is expressed in the non-inflamed brain at the protein level. Alternatively, it is possible that dermcidin has more important neuro-modulatory and neurological maintenance functions, some of which are yet to be discovered.
Neurodegeneration
In serum samples from AD patients, dermcidin expression was increased and it was suggested as a potential biomarker for disease [116], but other studies are so far not available.
As discussed above, the dermcidin precursor protein Y-P30 can promote neuronal survival [112–114]; however, this has not been investigated under neurodegenerative conditions. Other research has demonstrated that Y-P30 also promotes neurite outgrowth from thalamic and cerebellar neurons and is neuroprotective following optic nerve damage [113, 114, 117–119]. Therefore, as Y-P30 can be neuroprotective during injury, perhaps if the dermcidin expression is dysregulated this could lead to abnormal neuronal maintenance and potential neurodegeneration. However, testing this hypothesis will require significant further study.
Hepcidin
Hepcidin is a cysteine-rich cationic peptide produced in the liver, which has multiple immunomodulatory and antimicrobial activities including against Candida albicans, Aspergillus fumigatus, Escherichia coli, Staphylococcus aureus, Staphylococcus epidermidis, and group B Streptococcus [120]. Mutations in HAMP have been identified in patients suffering from hereditary hemochromatosis [121]. This role appears to be unrelated to its action as an antimicrobial peptide, as these patients do not have increased susceptibility to infections, but to its immunomodulatory action. Hepcidin also has many non-immunomodulatory functions throughout the brain, specifically its role with regard to iron homeostasis which has been reviewed extensively elsewhere [122, 123].
Health
Hepcidin mRNA is present in the uninfected human brain with relatively high expression found in the cortex, cerebellum, thalamus, medulla oblongata, and hippocampus, with the highest expression in the cortex and thalamus [124]. Hepcidin has also been detected within granular structures of astrocytes and in the epithelial cells of the choroid plexus [125].
It is also widely distributed in healthy mouse and rat brains and spinal cord. In mice, hepcidin-1 and hepcidin-2 are present in the CNS and immunohistochemistry of hepcidin-1 was observed in many regions such as the olfactory bulb, cortex, hippocampus, amygdala, thalamus, hypothalamus, mesencephalon, cerebellum, pons, spinal cord, as well as in dorsal root ganglia of the peripheral nervous system [126]. The same study showed using immunohistochemistry that hepcidin-1 was expressed by neurons and glia cells in the adult mouse CNS [126]. The authors failed to show a similar distribution of hepcidin by in situ hybridization which they suggest is because the mRNA signal is below the detection limit [126]. As hepcidin is an important iron regulator in the periphery, the authors suggest it is possible the same is occurring in the CNS.
In rats, Raha-Chowdhury showed by RT-PCR that hepcidin mRNA was expressed at low levels throughout the brain, while in situ hybridization showed hepcidin mRNA was restricted to the endothelium of blood vessels and the choroid plexus [127]. Hepcidin protein was expressed in the sub-ventricular zone, cortex, and the CSF, and associated with the epithelial cells of the choroid plexus, endothelial cells, pericytes, and astrocytes. The authors suggested that due to their observation that hepcidin is expressed in all layers of the BBB blood vessel walls and pericytes, peripheral hepcidin could also be crossing the intact BBB into the CNS [127].
Infection
There is increasing evidence that hepcidin acts as an antimicrobial agent in the CNS. Intravenous LPS injection in rats significantly increases hepcidin mRNA and protein expression in the cortex and the substantia nigra but not in the striatum or hippocampus [128, 129]. Similarly, peripheral administration of LPS in mice increases hepcidin gene expression in the choroid plexus [130]. It is not clear whether the regional specificity of hepcidin upregulation is physiologically functional, or if it is due to differences in the sensitivity of methods used, as other studies have failed to detect hepcidin mRNA in the cortex [127]. This LPS-induced expression was mediated through the IL-6/STAT3 pathway in the mouse cortex and hippocampus [131]; in IL-6 KO mice, hepcidin mRNA levels in these regions are significantly reduced [131]. Another study showed that this same pathway also occurs in the choroid plexus during ageing in rats [132].
Interestingly, there may be cell-specific regulation of LPS-mediated expression of hepcidin in the brain. Hepcidin mRNA is expressed at a much lower level in neurons than in astrocytes, and treatment with IL-6 in IL-6 KO astrocytes and neurons resulted in higher increased expression in astrocytes compared to neurons [133]. There is also evidence for cell-cell communication in this pathway, with LPS increasing hepcidin expression in neurons only when in culture with microglia, indicating microglia were the source of IL-6 (131). Thus, bacterial agents directly activate inflammatory signalling pathways which lead to the production of hepcidin in CNS resident cells—this is likely part of the CNS immune response.
Hepcidin is an important modulator of iron homeostasis and acts as a regulator of cellular iron release by binding to ferroportin 1 [134]. Its production in immortalized mouse microglia cells increases after stimulation with the inflammatory mediator CX3CL1, typically expressed on neurons [135]; it is also produced by astrocytes and microglia in response to LPS, TNF, and IL-6 [136]. This emphasizes how HDPs are able to support communication between CNS cells and demonstrates the relationship between inflammation and iron metabolism in the CNS.
Finally, hepcidin release from astrocytes has been shown to induce neuronal apoptosis. Astrocyte-specific hepcidin knockdown mice had decreased levels of cleaved caspase 3 in neurons (a marker of increased apoptosis). The authors showed that the lack of hepcidin production by astrocytes protects neurons from inflammation-stimulated apoptosis by reducing neuronal iron concentration [137]. It is possible that hepcidin production in the CNS can induce apoptosis of other cells such as resident or infiltrating immune cells, which acts as an important immunomodulatory function.
Neurodegeneration
It is well known that iron accumulation is a hallmark of neurodegenerative disease [138–140] and the implications of iron pathophysiology in neurological diseases have been reviewed extensively elsewhere [141–143]. Abnormal iron levels in the brain are involved in the formation of free radicals, which have been associated with oxidative damage and neuronal death [141]. Hepcidin regulates iron accumulation in microglia and astrocytes in diseases including AD, sporadic amyotrophic lateral sclerosis, Parkinson’s disease, and Sanfilippo syndrome [144–147]. Hepcidin levels increase with age in the rat in the cortex, striatum, hippocampus, and substantia nigra [148] and this was associated with increased pathological hallmarks of AD. However, a study showed that hepcidin was significantly reduced in post-mortem hippocampal lysates from patients with AD compared to healthy controls [149]. It is currently unclear why hepcidin expression is decreased in patients with AD [141, 149]. A reduction in hepcidin would lead to increased brain iron content which could partially explain the increased iron expression in the brains of patients with AD [141]. A recent study showed that overexpression of hepcidin in astrocytes of APP/PS1 mice significantly improved cognitive decline and partially reduced Aβ plaque formation in the cortex. They showed that overexpression of hepcidin reduced iron content in neurons which reduced iron accumulation-induced oxidative stress and cortex neuronal death [150]. Therefore, perhaps a similar mechanism is occurring in the human AD brain. Moreover, expression of the HAMP gene is upregulated in the spinal cord during the mouse model of multiple sclerosis, experimental autoimmune encephalomyelitis (EAE) [151], and in the cortex during the cuprizone model of demyelination [152]. Therefore, it is likely that dysregulated hepcidin leads to aberrant iron accumulation in multiple neurological diseases.
A note on Amyloid-β
Interestingly, a review by Gosztyla and colleagues collated evidence indicating a relationship between Aβ peptides in AD and their proposed function as HDP [153]. Aβ peptides are considered the driving force leading to the development of AD; however, drugs targeting Aβ have not been successful in terms of reducing cognitive decline [154]. Moreover, one of the common adverse reactions of anti-Aβ treatment is increased incidence of infections, suggesting a link between Aβ and the fight against infection [153, 155, 156]. Specifically, Aβ has been suggested to function as an antimicrobial HDP. This was first proposed by Robinson and Bishop in the bioflocculant hypothesis, which stated that Aβ deposited by glial cells forms a web that surrounds neurons and protects them from pathogens [157]. They reference a study [158] showing intracerebroventricular injection of LPS promoted Aβ deposition in transgenic mice that overexpress mutated human APP, and suggest this provides evidence that Aβ can bind pathogens [157]. Since this was proposed, multiple studies have demonstrated evidence in support of the bioflocculant hypothesis. For example, Aβ can inhibit viral replication of influenza [159] and has shown to have antimicrobial activity against many microorganisms [153, 160] such as Candida albicans, Streptococcus pneumoniae, and Pseudomonas aeruginosa, and Aβ exhibited higher potency than cathelicidin for some pathogens [161]. Aβ oligomers bind AD-associated herpes simplex virus [162], and prevent the virus from entering cells [163] suggesting a protective role for Aβ in CNS innate immunity [164]. Intriguingly, amyloid fibrils are present on NETs [165] suggesting amyloid fibrils may be an important mediator of innate immunity. Finally, Aβ can be expressed by immortalized microglial cells following LPS exposure [166], demonstrating that it could be released from activated immune cells within the CNS in response to infection.
A note on methodology
The central nervous system (CNS) was long considered an immune privileged site [167], separated from peripheral cells by the blood brain barrier. However, we now understand that immune cells do cross this barrier [168, 169] and patrol the brain and spinal cord during homeostasis, infection, and neurological disease. Indeed, granulocytes, including neutrophils, are present in the naïve mouse brain [170], comprising the third-largest tissue-resident leukocyte population in health [171]. In particular, neutrophils were noted in the dura mater, pia mater, and ependyma [171]. As neutrophils are one of the main cellular sources of HDP, storing cathelicidin and defensins in particular in abundance, neutrophil infiltration into the CNS is likely to contribute to HDP expression at this site.
HDP-secreting neutrophils also migrate into the CNS during neuroinflammation. Neutrophil infiltration into the spinal cord and brain tissue has been observed in mouse models of Alzheimer’s disease (AD) [59, 60] and multiple sclerosis (MS) [61, 62]. Importantly, cathelicidin-positive neutrophils are present in the spinal cord in EAE. Depletion of neutrophils or cathelicidin attenuates the development of EAE [43, 60, 62–64] and in AD models this can improve cognitive decline [65]. In humans, neutrophils are present in the CSF and in active lesions of neuromyelitis optica patients [66]. In other human neurological diseases, alterations to neutrophil populations have been noted.
This raises the possibility that if HDP are stored in infiltrating and/or resident neutrophils, or other granular cells, mRNA assays may not detect expression as the HDP is not actively being transcribed. Therefore, further studies are required which utilize a variety of complementary techniques to elucidate under what conditions HDP are expressed and by which cell types in the CNS. This is particularly true for understanding the role of neutrophil-derived and NET-coated HDP in the nervous system; we know neutrophils are present but unpicking their roles is technologically challenging.
In many of the papers described in this review, the cellular source of the HDP was not identified. In cells other than neutrophils, published single-cell RNA sequencing data from CNS studies can be exploited to pinpoint the expression of HDP. Likewise, as reagents improve, the cellular source of HDP in species other than humans and mice can be determined through co-localization with specific cell markers.
Finally, there are limitations in the availability of effective antibodies for detection the of HDP. For example, few reliable anti-HDP flow cytometry or immunofluorescence antibodies exist. Thus, the development of better reagents is essential to generate a complete map of HDP expression across the CNS across health, infection, and disease.
Concluding remarks
Host defence peptides are not limited to mucosal sites or active infections; instead, we have shown that they are expressed in the central nervous system of a wide range of species. This broad expression—during infection and also sterile inflammation and neurodegeneration—suggests the peptides have multiple roles. We propose HDP have important functions not only as the first line of defence against pathogens but also as important immunomodulators. As the field develops and we understand the immunomodulatory roles of HDP in more detail, it is likely we will understand nervous system HDP to have roles we have not so far considered.
Contributor Information
Katie J Smith, Centre for Inflammation Research, University of Edinburgh, 47 Little France Crescent, EH16 4TJ, Edinburgh, UK.
Emily Gwyer Findlay, Centre for Inflammation Research, University of Edinburgh, 47 Little France Crescent, EH16 4TJ, Edinburgh, UK.
Conflict of Interest
The authors declare no conflicts of interest.
Author Contributions
KJS and EGF both contributed to the conception and design of this article, to the writing of the original draft, and to editing and review.
Funding
This work was funded by a Royal Society Dorothy Hodgkin Fellowship (DH150175) and a Royal Society Fellows’ Enhancement award (RGF/EA/180049), both to EGF.
References
- 1.Sørensen OE, Gram L, Johnsen AH, Andersson E, Bangsbøll S, Tjabringa GS, et al. Processing of seminal plasma hCAP-18 to ALL-38 by gastricsin: a novel mechanism of generating antimicrobial peptides in vagina. J Biol Chem 2003, 278, 28540–6. doi: 10.1074/jbc.M301608200. [DOI] [PubMed] [Google Scholar]
- 2.Mathews M, Jia HP, Guthmiller JM, Losh G, Graham S, Johnson GK, et al. Production of beta-defensin antimicrobial peptides by the oral mucosa and salivary glands. Infect Immun 1999, 67, 2740–5. doi: 10.1128/IAI.67.6.2740-2745.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schittek B, Hipfel R, Sauer B, Bauer J, Kalbacher H, Stevanovic S, et al. Dermcidin: a novel human antibiotic peptide secreted by sweat glands. Nat Immunol 2001, 2, 1133–7. [DOI] [PubMed] [Google Scholar]
- 4.Murakami M, Ohtake T, Dorschner RA, Gallo RL.. Cathelicidin antimicrobial peptides are expressed in salivary glands and saliva. J Dent Res 2002, 81, 845–50. [DOI] [PubMed] [Google Scholar]
- 5.Bals R, Wang X, Wu Z, Freeman T, Bafna V, Zasloff M, et al. Human beta-defensin 2 is a salt-sensitive peptide antibiotic expressed in human lung. J Clin Invest 1998, 102, 874–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hase K, Eckmann L, Leopard JD, Varki N, Kagnoff MF.. Cell differentiation is a key determinant of cathelicidin LL-37/human cationic antimicrobial protein 18 expression by human colon epithelium. Infect Immun 2002, 70, 953–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sørensen O, Cowland JB, Askaa J, Borregaard N.. An ELISA for hCAP-18, the cathelicidin present in human neutrophils and plasma. J Immunol Methods 1997, 206, 53–9. [DOI] [PubMed] [Google Scholar]
- 8.Di Nardo A, Vitiello A, Gallo RL.. Cutting edge: mast cell antimicrobial activity is mediated by expression of cathelicidin antimicrobial peptide. J Immunol 2003, 170, 2274–8. [DOI] [PubMed] [Google Scholar]
- 9.Duits LA, Ravensbergen B, Rademaker M, Hiemstra PS, Nibbering PH.. Expression of beta-defensin 1 and 2 mRNA by human monocytes, macrophages and dendritic cells. Immunology 2002, 106, 517–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dudal S, Turriere C, Bessoles S, Fontes P, Sanchez F, Liautard J, et al. Release of LL-37 by Activated Human Vγ9Vδ2 T Cells: a microbicidal weapon against <em>Brucella suis</em>. J Immunol 2006, 177, 5533. [DOI] [PubMed] [Google Scholar]
- 11.Davidson DJ, Currie AJ, Reid GSD, Bowdish DME, MacDonald KL, Ma RC, et al. The cationic antimicrobial peptide LL-37 modulates dendritic cell differentiation and dendritic cell-induced T-cell polarization. J Immunol 2004, 172, 1146. [DOI] [PubMed] [Google Scholar]
- 12.McGlasson SL, Semple F, MacPherson H, Gray M, Davidson DJ, Dorin JR.. Human β-defensin 3 increases the TLR9-dependent response to bacterial DNA. Eur J Immunol 2017, 47, 658–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Findlay EG, Currie AJ, Zhang A, Ovciarikova J, Young L, Stevens H, et al. Exposure to the antimicrobial peptide LL-37 produces dendritic cells optimized for immunotherapy. OncoImmunology 2019, 8, 1608106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minns D, Smith KJ, Findlay EG.. Orchestration of adaptive T-cell responses by neutrophil granule contents. Mediators Inflamm 2019, 2019, 8968943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minns D, Smith KJ, Alessandrini V, Hardisty G, Melrose L, Jackson-Jones L, et al. The neutrophil antimicrobial peptide cathelicidin promotes Th17 differentiation. Nat Commun 2021, 12, 1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brook M, Tomlinson GH, Miles K, Smith RW, Rossi AG, Hiemstra PS, et al. Neutrophil-derived alpha defensins control inflammation by inhibiting macrophage mRNA translation. Proc Natl Acad Sci USA 2016, 113, 4350–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang D, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, et al. LL-37, the neutrophil granule–and epithelial cell–derived cathelicidin, utilizes formyl peptide receptor–like 1 (Fprl1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T-cells. J Exp Med 2000, 192, 1069–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antoni L, Nuding S, Weller D, Gersemann M, Ott G, Wehkamp J, et al. Human colonic mucus is a reservoir for antimicrobial peptides. J Crohn’s Colitis 2013, 7, e652–e64. [DOI] [PubMed] [Google Scholar]
- 19.Dupont A, Heinbockel L, Brandenburg K, Hornef MW.. Antimicrobial peptides and the enteric mucus layer act in concert to protect the intestinal mucosa. Gut Microbes 2014, 5, 761–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agerberth B, Grunewald J, Castaños-velez E, Olsson B, Jornvall H, Wigzell H, et al. Antibacterial components in bronchoalveolar lavage fluid from healthy individuals and sarcoidosis patients. Am J Respir Crit Care Med 1999, 160, 283–90. [DOI] [PubMed] [Google Scholar]
- 21.Bals R, Wang X, Zasloff M, Wilson JM.. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc Natl Acad Sci USA 1998, 95, 9541–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dongsheng L, Jiawen L, Yiqun D, Xiaoyong Z.. Expression of LL-37, human beta defensin-2, and CCR6 mRNA in patients with psoriasis vulgaris. J Huazhong Univ Sci Technol [Med Sci] 2004, 24, 404–6. [DOI] [PubMed] [Google Scholar]
- 23.Frohm M, Gunne H, Bergman AC, Agerberth B, Bergman T, Boman A, et al. Biochemical and antibacterial analysis of human wound and blister fluid. Eur J Biochem 1996, 237, 86–92. [DOI] [PubMed] [Google Scholar]
- 24.Yamasaki K, Gallo RL.. Antimicrobial peptides in human skin disease. Eur J Dermatol 2008, 18, 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braff MH, Di Nardo A, Gallo RL.. Keratinocytes store the antimicrobial peptide cathelicidin in lamellar bodies. J Investig Dermatol 2005, 124, 394–400. [DOI] [PubMed] [Google Scholar]
- 26.Valore EV, Park CH, Igreti SL, Ganz T.. Antimicrobial components of vaginal fluid. Am J Obstet Gynecol 2002, 187, 561–8. [DOI] [PubMed] [Google Scholar]
- 27.Malm J, Sørensen O, Persson T, Frohm-Nilsson M, Johansson B, Bjartell A, et al. The human cationic antimicrobial protein (hCAP-18) is expressed in the epithelium of human epididymis, is present in seminal plasma at high concentrations, and is attached to spermatozoa. Infect Immun 2000, 68, 4297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cruse G, Fernandes VE, de Salort J, Pankhania D, Marinas MS, Brewin H, et al. Human lung mast cells mediate pneumococcal cell death in response to activation by pneumolysin. J Immunol 2010, 184, 7108–15. [DOI] [PubMed] [Google Scholar]
- 29.Agerberth B, Charo J, Werr J, Olsson B, Idali F, Lindbom L, et al. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood 2000, 96, 3086–93. [PubMed] [Google Scholar]
- 30.Zhang LJ, Guerrero-Juarez CF, Hata T, Bapat SP, Ramos R, Plikus MV, et al. Innate immunity. Dermal adipocytes protect against invasive Staphylococcus aureus skin infection. Science 2015, 347, 67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner RA, et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature 2001, 414, 454–7. [DOI] [PubMed] [Google Scholar]
- 32.Currie SM, Findlay EG, McHugh BJ, Mackellar A, Man T, Macmillan D, et al. The human cathelicidin LL-37 has antiviral activity against respiratory syncytial virus. PLoS One 2013, 8, e73659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.López-García B, Lee PH, Yamasaki K, Gallo RL.. Anti-fungal activity of cathelicidins and their potential role in Candida albicans skin infection. J Invest Dermatol 2005, 125, 108–15. [DOI] [PubMed] [Google Scholar]
- 34.Vandamme D, Landuyt B, Luyten W, Schoofs L.. A comprehensive summary of LL-37, the factotum human cathelicidin peptide. Cell Immunol 2012, 280, 22–35. [DOI] [PubMed] [Google Scholar]
- 35.Ordonez SR, Veldhuizen EJA, van Eijk M, Haagsman HP.. Role of soluble innate effector molecules in pulmonary defense against fungal pathogens. Front Microbiol 2017, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chromek M, Arvidsson I, Karpman D.. The antimicrobial peptide cathelicidin protects mice from Escherichia coli O157:H7-mediated disease. PLoS One 2012, 7, e46476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chromek M, Slamová Z, Bergman P, Kovács L, Podracká L, Ehrén I, et al. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat Med 2006, 12, 636–41. [DOI] [PubMed] [Google Scholar]
- 38.Kurosaka K, Chen Q, Yarovinsky F, Oppenheim JJ, Yang D.. Mouse cathelin-related antimicrobial peptide chemoattracts leukocytes using formyl peptide receptor-like 1/mouse formyl peptide receptor-like 2 as the receptor and acts as an immune adjuvant. J Immunol 2005, 174, 6257–65. [DOI] [PubMed] [Google Scholar]
- 39.Carretero M, Escámez MJ, García M, Duarte B, Holguín A, Retamosa L, et al. In vitro and in vivo wound healing-promoting activities of human cathelicidin LL-37. J Investig Dermatol 2008, 128, 223–36. [DOI] [PubMed] [Google Scholar]
- 40.Soehnlein O, Wantha S, Simsekyilmaz S, Döring Y, Megens RTA, Mause SF, et al. Neutrophil-derived cathelicidin protects from neointimal hyperplasia. Sci Transl Med 2011, 3, 103ra98–ra98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Byfield FJ, Wen Q, Leszczynska K, Kulakowska A, Namiot Z, Janmey PA, et al. Cathelicidin LL-37 peptide regulates endothelial cell stiffness and endothelial barrier permeability. Am J Physiol Cell Physiol 2011, 300, C105–C12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yadav A, Matson KJE, Li L, Hua I, Gaur P, Alkaslasi MR, et al. The Human Motoneuron Expression Signature is Defined by ALS-Related Genes. bioRxiv 2022, 2022.03.25.485808. [Google Scholar]
- 43.Smith KJ, Minns D, McHugh BJ, Holloway RK, O’Connor R, Williams A, et al. The antimicrobial peptide cathelicidin is critical for the development of Th17 responses in severe inflammatory disease. bioRxiv 2022, 2022.01.27.477976. [Google Scholar]
- 44.Bergman P, Termen S, Johansson L, Nystrom L, Arenas E, Jonsson AB, et al. The antimicrobial peptide rCRAMP is present in the central nervous system of the rat. J Neurochem 2005, 93, 1132–40. [DOI] [PubMed] [Google Scholar]
- 45.van Dijk A, Veldhuizen EJ, van Asten AJ, Haagsman HP.. CMAP27, a novel chicken cathelicidin-like antimicrobial protein. Vet Immunol Immunopathol 2005, 106, 321–7. [DOI] [PubMed] [Google Scholar]
- 46.Gallo RL, Kim KJ, Bernfield M, Kozak CA, Zanetti M, Merluzzi L, et al. Identification of CRAMP, a cathelin-related antimicrobial peptide expressed in the embryonic and adult mouse. J Biol Chem 1997, 272, 13088–93. [DOI] [PubMed] [Google Scholar]
- 47.Brandenburg LO, Varoga D, Nicolaeva N, Leib SL, Wilms H, Podschun R, et al. Role of glial cells in the functional expression of LL-37/rat cathelin-related antimicrobial peptide in meningitis. J Neuropathol Exp Neurol 2008, 67, 1041–54. [DOI] [PubMed] [Google Scholar]
- 48.Savonius O, Helve O, Roine I, Andersson S, Saukkoriipi A, González Mata A, et al. Cerebrospinal fluid cathelicidin correlates with the bacterial load and outcomes in childhood bacterial meningitis. Pediatr Infect Dis J 2018, 37. [DOI] [PubMed] [Google Scholar]
- 49.Savonius O, Helve O, Roine I, Andersson S, Fernández J, Peltola H, et al. Swiftly decreasing cerebrospinal fluid cathelicidin concentration predicts improved outcome in childhood bacterial meningitis. J Clin Microbiol 2016, 54, 1648–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Visser DH, Solomons RS, Ronacher K, van Well GT, Heymans MW, Walzl G, et al. Host immune response to tuberculous meningitis. Clin Infect Dis 2015, 60, 177–87. [DOI] [PubMed] [Google Scholar]
- 51.Seipone ID, Singh R, Patel VB, Singh A, Gordon ML, Muema DM, et al. Tuberculous meningitis is associated with higher cerebrospinal HIV-1 viral loads compared to other HIV-1-associated meningitides. PLoS One 2018, 13, e0192060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bergman P, Johansson L, Wan H, Jones A, Gallo RL, Gudmundsson GH, et al. Induction of the antimicrobial peptide CRAMP in the blood-brain barrier and meninges after meningococcal infection. Infect Immun 2006, 74, 6982–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brandenburg LO, Varoga D, Nicolaeva N, Leib SL, Podschun R, Wruck CJ, et al. Expression and regulation of antimicrobial peptide rCRAMP after bacterial infection in primary rat meningeal cells. J Neuroimmunol 2009, 217, 55–64. [DOI] [PubMed] [Google Scholar]
- 54.Mohanty T, Fisher J, Bakochi A, Neumann A, Cardoso JFP, Karlsson CAQ, et al. Neutrophil extracellular traps in the central nervous system hinder bacterial clearance during pneumococcal meningitis. Nat Commun 2019, 10, 1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Buhr N, Reuner F, Neumann A, Stump-Guthier C, Tenenbaum T, Schroten H, et al. Neutrophil extracellular trap formation in the Streptococcus suis-infected cerebrospinal fluid compartment. Cell Microbiol 2017, 19. [DOI] [PubMed] [Google Scholar]
- 56.Merres J, Höss J, Albrecht L-J, Kress E, Soehnlein O, Jansen S, et al. Role of the cathelicidin-related antimicrobial peptide in inflammation and mortality in a mouse model of bacterial meningitis. J Innate Immun 2014, 6, 205–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brandenburg LO, Jansen S, Wruck CJ, Lucius R, Pufe T.. Antimicrobial peptide rCRAMP induced glial cell activation through P2Y receptor signalling pathways. Mol Immunol 2010, 47, 1905–13. [DOI] [PubMed] [Google Scholar]
- 58.Kress E, Merres J, Albrecht LJ, Hammerschmidt S, Pufe T, Tauber SC, et al. CRAMP deficiency leads to a pro-inflammatory phenotype and impaired phagocytosis after exposure to bacterial meningitis pathogens. Cell Commun Signal 2017, 15, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baik SH, Cha M-Y, Hyun Y-M, Cho H, Hamza B, Kim DK, et al. Migration of neutrophils targeting amyloid plaques in Alzheimer’s disease mouse model. Neurobiol Aging 2014, 35, 1286–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zenaro E, Pietronigro E, Della Bianca V, Piacentino G, Marongiu L, Budui S, et al. Neutrophils promote Alzheimer’s disease-like pathology and cognitive decline via LFA-1 integrin. Nat Med 2015, 21, 880–6. [DOI] [PubMed] [Google Scholar]
- 61.Rumble JM, Huber AK, Krishnamoorthy G, Srinivasan A, Giles DA, Zhang X, et al. Neutrophil-related factors as biomarkers in EAE and MS. J Exp Med 2015, 212, 23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Steinbach K, Piedavent M, Bauer S, Neumann JT, Friese MA.. Neutrophils amplify autoimmune central nervous system infiltrates by maturing local APCs. J Immunol 2013, 4531–9. [DOI] [PubMed] [Google Scholar]
- 63.Määttä JA, Sjöholm UR, Nygårdas PT, Salmi AA, Hinkkanen AE.. Neutrophils secreting tumor necrosis factor alpha infiltrate the central nervous system of BALB/c mice with experimental autoimmune encephalomyelitis. J Neuroimmunol 1998, 90, 162–75. [DOI] [PubMed] [Google Scholar]
- 64.Carlson T, Kroenke M, Rao P, Lane TE, Segal B.. The Th17-ELR+ CXC chemokine pathway is essential for the development of central nervous system autoimmune disease. J Exp Med 2008, 205, 811–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cruz Hernández JC, Bracko O, Kersbergen CJ, Muse V, Haft-Javaherian M, Berg M, et al. Neutrophil adhesion in brain capillaries reduces cortical blood flow and impairs memory function in Alzheimer’s disease mouse models. Nat Neurosci 2019, 22, 413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG.. The spectrum of neuromyelitis optica. Lancet Neurol 2007, 6, 805–15. [DOI] [PubMed] [Google Scholar]
- 67.Naegele M, Tillack K, Reinhardt S, Schippling S, Martin R, Sospedra M.. Neutrophils in multiple sclerosis are characterized by a primed phenotype. J Neuroimmunol 2012, 242, 60–71. [DOI] [PubMed] [Google Scholar]
- 68.Lee M, Shi X, Barron AE, McGeer E, McGeer PL.. Human antimicrobial peptide LL-37 induces glial-mediated neuroinflammation. Biochem Pharmacol 2015, 94, 130–41. [DOI] [PubMed] [Google Scholar]
- 69.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol 2003, 3, 710–20. [DOI] [PubMed] [Google Scholar]
- 70.Mookherjee N, Anderson MA, Haagsman HP, Davidson DJ.. Antimicrobial host defence peptides: functions and clinical potential. Nat Rev Drug Discovery 2020, 19, 311–32. [DOI] [PubMed] [Google Scholar]
- 71.Sass V, Schneider T, Wilmes M, Körner C, Tossi A, Novikova N, et al. Human β-defensin 3 inhibits cell wall biosynthesis in Staphylococci. Infect Immun 2010, 78, 2793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Daher KA, Selsted ME, Lehrer R.. Direct inactivation of viruses by human granulocyte defensins. J Virol 1986, 60, 1068–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Holthausen DJ, Lee SH, Kumar VT, Bouvier NM, Krammer F, Ellebedy AH, et al. An amphibian host defense peptide is virucidal for human H1 hemagglutinin-bearing influenza viruses. Immunity 2017, 46, 587–95. [DOI] [PubMed] [Google Scholar]
- 74.Sun X, Yau VK, Briggs BJ, Whittaker GR.. Role of clathrin-mediated endocytosis during vesicular stomatitis virus entry into host cells. Virology 2005, 338, 53–60. [DOI] [PubMed] [Google Scholar]
- 75.Järvå M, Phan TK, Lay FT, Caria S, Kvansakul M, Hulett MD.. Human β-defensin 2 kills Candida albicans through phosphatidylinositol 4,5-bisphosphate-mediated membrane permeabilization. Sci Adv 2018, 4, eaat0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jones DE, Bevins CL.. Paneth cells of the human small intestine express an antimicrobial peptide gene. J Biol Chem 1992, 267, 23216–25. [PubMed] [Google Scholar]
- 77.Maffei FA, Heine RP, Whalen MJ, Mortimer LF, Carcillo JA.. Levels of antimicrobial molecules defensin and lactoferrin are elevated in the cerebrospinal fluid of children with meningitis. Pediatrics 1999, 103, 987–92. [DOI] [PubMed] [Google Scholar]
- 78.Fraisier C, Papa A, Granjeaud S, Hintzen R, Martina B, Camoin L, et al. Cerebrospinal fluid biomarker candidates associated with human WNV neuroinvasive disease. PLoS One 2014, 9, e93637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Edfeldt K, Liu PT, Chun R, Fabri M, Schenk M, Wheelwright M, et al. T-cell cytokines differentially control human monocyte antimicrobial responses by regulating vitamin D metabolism. Proc Natl Acad Sci USA 2010, 107, 22593–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Biragyn A, Ruffini PA, Leifer CA, Klyushnenkova E, Shakhov A, Chertov O, et al. Toll-like receptor 4-dependent activation of dendritic cells by β-defensin 2. Science 2002, 298, 1025–9. [DOI] [PubMed] [Google Scholar]
- 81.Röhrl J, Yang D, Oppenheim JJ, Hehlgans T.. Human beta-defensin 2 and 3 and their mouse orthologs induce chemotaxis through interaction with CCR2. J Immunol (Baltimore, Md: 1950) 2010, 184, 6688–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Candela ME, Allsop DJP, Carter RN, Semple F, Kilanowski F, Webb S, et al. Classical macrophage polarisation is limited by human β-defensin-3 via an autocrine IL-4 dependent process. bioRxiv 2021, doi: 10.1101/2021.05.06.442606. [DOI] [Google Scholar]
- 83.Navid F, Boniotto M, Walker C, Ahrens K, Proksch E, Sparwasser T, et al. Induction of regulatory T cells by a murine β-defensin. J Immunol 2012, 188, 735–43. [DOI] [PubMed] [Google Scholar]
- 84.Semple F, Webb S, Li HN, Patel HB, Perretti M, Jackson IJ, et al. Human beta-defensin 3 has immunosuppressive activity in vitro and in vivo. Eur J Immunol 2010, 40, 1073–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhao C, Wang I, Lehrer RI.. Widespread expression of beta-defensin hBD-1 in human secretory glands and epithelial cells. FEBS Lett 1996, 396, 319–22. [DOI] [PubMed] [Google Scholar]
- 86.Nakayama K, Okamura N, Arai H, Sekizawa K, Sasaki H.. Expression of human beta-defensin-1 in the choroid plexus. Ann Neurol 1999, 45, 685. [DOI] [PubMed] [Google Scholar]
- 87.Strominger I, Elyahu Y, Berner O, Reckhow J, Mittal K, Nemirovsky A, et al. The choroid plexus functions as a niche for T-cell stimulation within the central nervous system. Front Immunol 2018, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dendrou CA, Fugger L, Friese MA.. Immunopathology of Multiple Sclerosis. Nature Publishing Group; 2015. pp. 545–58. [DOI] [PubMed] [Google Scholar]
- 89.Ransohoff RM, Engelhardt B.. The Anatomical and Cellular Basis of Immune Surveillance in the Central Nervous System. Nature Publishing Group; 2012. p. 623–35. [DOI] [PubMed] [Google Scholar]
- 90.Baruch K, Schwartz M.. CNS-specific T cells shape brain function via the choroid plexus. Brain Behav Immun 2013, 34, 11–6. [DOI] [PubMed] [Google Scholar]
- 91.Zhang G, Wu H, Shi J, Ganz T, Ross CR, Blecha F.. Molecular cloning and tissue expression of porcine β-defensin-1. FEBS Lett 1998, 424, 37–40. [DOI] [PubMed] [Google Scholar]
- 92.Hiratsuka T, Nakazato M, Date Y, Mukae H, Matsukura S.. Nucleotide sequence and expression of rat beta-defensin-1: its significance in diabetic rodent models. Nephron 2001, 88, 65–70. [DOI] [PubMed] [Google Scholar]
- 93.Froy O, Hananel A, Chapnik N, Madar Z.. Differential effect of insulin treatment on decreased levels of beta-defensins and Toll-like receptors in diabetic rats. Mol Immunol 2007, 44, 796–802. [DOI] [PubMed] [Google Scholar]
- 94.Stolzenberg ED, Anderson GM, Ackermann MR, Whitlock RH, Zasloff M.. Epithelial antibiotic induced in states of disease. Proc Natl Acad Sci USA 1997, 94, 8686–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Morrison GM, Semple CA, Kilanowski FM, Hill RE, Dorin JR.. Signal sequence conservation and mature peptide divergence within subgroups of the murine beta-defensin gene family. Mol Biol Evol 2003, 20, 460–70. [DOI] [PubMed] [Google Scholar]
- 96.Maxwell AI, Morrison GM, Dorin JR.. Rapid sequence divergence in mammalian beta-defensins by adaptive evolution. Mol Immunol 2003, 40, 413–21. [DOI] [PubMed] [Google Scholar]
- 97.Yang K, Hou B, Ren F, Zhou H, Zhao T.. Characterization of grass carp (Ctenopharyngodon idella) beta-defensin 1: implications for its role in inflammation control. Biosci Biotechnol Biochem 2018, 1, 87–94. [DOI] [PubMed] [Google Scholar]
- 98.Wang G, Li J, Zou P, Xie H, Huang B, Nie P, et al. Expression pattern, promoter activity and bactericidal property of β-defensin from the mandarin fish Siniperca chuatsi. Fish Shellfish Immunol 2012, 33, 522–31. [DOI] [PubMed] [Google Scholar]
- 99.Guo M, Wei J, Huang X, Huang Y, Qin Q.. Antiviral effects of β-defensin derived from orange-spotted grouper (Epinephelus coioides). Fish Shellfish Immunol 2012, 32, 828–38. [DOI] [PubMed] [Google Scholar]
- 100.Soman SS, Arathy DS, Sreekumar E.. Discovery of Anas platyrhynchos avian beta-defensin 2 (Apl_AvBD2) with antibacterial and chemotactic functions. Mol Immunol 2009, 46, 2029–38. [DOI] [PubMed] [Google Scholar]
- 101.Casadei E, Wang T, Zou J, González Vecino JL, Wadsworth S, Secombes CJ.. Characterization of three novel beta-defensin antimicrobial peptides in rainbow trout (Oncorhynchus mykiss). Mol Immunol 2009, 46, 3358–66. [DOI] [PubMed] [Google Scholar]
- 102.Liang T, Wang DD, Zhang GR, Wei KJ, Wang WM, Zou GW.. Molecular cloning and expression analysis of two β-defensin genes in the blunt snout bream (Megalobrama amblycephala). Comp Biochem Physiol B Biochem Mol Biol 2013, 166, 91–8. [DOI] [PubMed] [Google Scholar]
- 103.Tiszlavicz Z, Endrész V, Németh B, Megyeri K, Orosz L, Seprényi G, et al. Inducible expression of human β-defensin 2 by Chlamydophila pneumoniae in brain capillary endothelial cells. Innate Immun 2011, 17, 463–9. [DOI] [PubMed] [Google Scholar]
- 104.Hao HN, Zhao J, Lotoczky G, Grever WE, Lyman WD.. Induction of human beta-defensin-2 expression in human astrocytes by lipopolysaccharide and cytokines. J Neurochem 2001, 77, 1027–35. [DOI] [PubMed] [Google Scholar]
- 105.Williams WM, Castellani RJ, Weinberg A, Perry G, Smith MA.. Do β-defensins and other antimicrobial peptides play a role in neuroimmune function and neurodegeneration? Sci World J 2012, 2012, 905785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Szekeres M, Ivitz E, Datki Z, Kálmán J, Pákáski M, Várhelyi ZP, et al. Relevance of defensin β-2 and α defensins (HNP1-3) in Alzheimer’s disease. Psychiatry Res 2016, 239, 342–5. [DOI] [PubMed] [Google Scholar]
- 107.Williams WM, Torres S, Siedlak SL, Castellani RJ, Perry G, Smith MA, et al. Antimicrobial peptide β-defensin-1 expression is upregulated in Alzheimer’s brain. J Neuroinflammation 2013, 10, 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schittek B. The multiple facets of dermcidin in cell survival and host defense. J Innate Immun 2012, 4, 349–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lai YP, Peng YF, Zuo Y, Li J, Huang J, Wang LF, et al. Functional and structural characterization of recombinant dermcidin-1L, a human antimicrobial peptide. Biochem Biophys Res Commun 2005, 328, 243–50. [DOI] [PubMed] [Google Scholar]
- 110.Cipáková I, Gasperík J, Hostinová E.. Expression and purification of human antimicrobial peptide, dermcidin, in Escherichia coli. Protein Expr Purif 2006, 45, 269–74. [DOI] [PubMed] [Google Scholar]
- 111.Vuong C, Voyich JM, Fischer ER, Braughton KR, Whitney AR, DeLeo FR, et al. Polysaccharide intercellular adhesin (PIA) protects Staphylococcus epidermidis against major components of the human innate immune system. Cell Microbiol 2004, 6, 269–75. [DOI] [PubMed] [Google Scholar]
- 112.Porter D, Weremowicz S, Chin K, Seth P, Keshaviah A, Lahti-Domenici J, et al. A neural survival factor is a candidate oncogene in breast cancer. Proc Natl Acad Sci USA 2003, 100, 10931–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cunningham TJ, Hodge L, Speicher D, Reim D, Tyler-Polsz C, Levitt P, et al. Identification of a survival-promoting peptide in medium conditioned by oxidatively stressed cell lines of nervous system origin. J Neurosci 1998, 18, 7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cunningham TJ, Jing H, Akerblom I, Morgan R, Fisher TS, Neveu M.. Identification of the Human cDNA for new survival/evasion peptide (DSEP): studies in vitro and in vivo of overexpression by neural cells. Exp Neurol 2002, 177, 32–9. [DOI] [PubMed] [Google Scholar]
- 115.Ángeles Fernández-Gil M, Palacios-Bote R, Leo-Barahona M, Mora-Encinas JP.. Anatomy of the brainstem: a gaze into the stem of life. Semin Ultrasound CT MRI 2010, 31, 196–219. [DOI] [PubMed] [Google Scholar]
- 116.Kang S, Jeong H, Baek JH, Lee SJ, Han SH, Cho HJ, et al. PiB-PET imaging-based serum proteome profiles predict mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis 2016, 53, 1563–76. [DOI] [PubMed] [Google Scholar]
- 117.Macharadze T, Landgraf P, Pape HC, Wahle P, Kreutz MR.. Y-P30 confers neuroprotection after optic nerve crush in adult rats. Neuroreport 2011, 22, 544–7. [DOI] [PubMed] [Google Scholar]
- 118.Cunningham TJ, Jing H, Wang Y, Hodge L.. Calreticulin binding and other biological activities of survival peptide Y-P30 including effects of systemic treatment of rats. Exp Neurol 2000, 163, 457–68. [DOI] [PubMed] [Google Scholar]
- 119.Landgraf P, Wahle P, Pape H-C, Gundelfinger ED, Kreutz MR.. The survival-promoting peptide Y-P30 enhances binding of pleiotrophin to syndecan-2 and -3 and supports its neuritogenic activity. J Biol Chem 2008, 283, 25036–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Park CH, Valore EV, Waring AJ, Ganz T.. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem 2001, 276, 7806–10. [DOI] [PubMed] [Google Scholar]
- 121.Roetto A, Papanikolaou G, Politou M, Alberti F, Girelli D, Christakis J, et al. Mutant antimicrobial peptide hepcidin is associated with severe juvenile hemochromatosis. Nat Genet 2003, 33, 21–2. [DOI] [PubMed] [Google Scholar]
- 122.Vela D. Hepcidin, an emerging and important player in brain iron homeostasis. J Transl Med 2018, 16, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang SM, Fu LJ, Duan XL, Crooks DR, Yu P, Qian ZM, et al. Role of hepcidin in murine brain iron metabolism. Cell Mol Life Sci 2010, 67, 123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hänninen MM, Haapasalo J, Haapasalo H, Fleming RE, Britton RS, Bacon BR, et al. Expression of iron-related genes in human brain and brain tumors. BMC Neurosci 2009, 10, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yanase K, Uemura N, Chiba Y, Murakami R, Fujihara R, Matsumoto K, et al. Immunoreactivities for hepcidin, ferroportin, and hephaestin in astrocytes and choroid plexus epithelium of human brains. Neuropathology 2020, 40, 75–83. [DOI] [PubMed] [Google Scholar]
- 126.Zechel S, Huber-Wittmer K, von Bohlen und Halbach O.. Distribution of the iron-regulating protein hepcidin in the murine central nervous system. J Neurosci Res 2006, 84, 790–800. [DOI] [PubMed] [Google Scholar]
- 127.Raha-Chowdhury R, Raha AA, Forostyak S, Zhao JW, Stott SR, Bomford A.. Expression and cellular localization of hepcidin mRNA and protein in normal rat brain. BMC Neurosci 2015, 16, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Qian ZM, He X, Liang T, Wu KC, Yan YC, Lu LN, et al. Lipopolysaccharides upregulate hepcidin in neuron via microglia and the IL-6/STAT3 signaling pathway. Mol Neurobiol 2014, 50, 811–20. [DOI] [PubMed] [Google Scholar]
- 129.Wang Q, Du F, Qian ZM, Ge XH, Zhu L, Yung WH, et al. Lipopolysaccharide induces a significant increase in expression of iron regulatory hormone hepcidin in the cortex and substantia nigra in rat brain. Endocrinology 2008, 149, 3920–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Marques F, Falcao AM, Sousa JC, Coppola G, Geschwind D, Sousa N, et al. Altered iron metabolism is part of the choroid plexus response to peripheral inflammation. Endocrinology 2009, 150, 2822–8. [DOI] [PubMed] [Google Scholar]
- 131.Zhang FL, Hou HM, Yin ZN, Chang L, Li FM, Chen YJ, et al. Impairment of hepcidin upregulation by lipopolysaccharide in the interleukin-6 knockout mouse brain. Front Mol Neurosci 2017, 10, 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liu CB, Wang R, Dong MW, Gao XR, Yu F.. Expression of hepcidin at the choroid plexus in normal aging rats is associated with IL-6/Stat3 signaling pathway. Sheng Li Xue Bao 2014, 66, 639–46. [PubMed] [Google Scholar]
- 133.Ma J, Zhang FL, Zhou G, Bao YX, Shen Y, Qian ZM.. Different characteristics of hepcidin expression in IL-6+/+ and IL-6−/− neurons and astrocytes treated with lipopolysaccharides. Neurochem Res 2018, 43, 1624–30. [DOI] [PubMed] [Google Scholar]
- 134.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004, 306, 2090–3. [DOI] [PubMed] [Google Scholar]
- 135.Pandur E, Tamási K, Pap R, Varga E, Miseta A, Sipos K.. Fractalkine induces hepcidin expression of BV-2 microglia and causes iron accumulation in SH-SY5Y Cells. Cell Mol Neurobiol 2019, 39, 985–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Urrutia P, Aguirre P, Esparza A, Tapia V, Mena NP, Arredondo M, et al. Inflammation alters the expression of DMT1, FPN1 and hepcidin, and it causes iron accumulation in central nervous system cells. J Neurochem 2013, 126, 541–9. [DOI] [PubMed] [Google Scholar]
- 137.You LH, Yan CZ, Zheng BJ, Ci YZ, Chang SY, Yu P, et al. Astrocyte hepcidin is a key factor in LPS-induced neuronal apoptosis. Cell Death Dis 2017, 8, e2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ndayisaba A, Kaindlstorfer C, Wenning GK.. Iron in neurodegeneration—cause or consequence? Front Neurosci 2019, 13, 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lane DJR, Ayton S, Bush AI.. Iron and Alzheimer’s disease: an update on emerging mechanisms. J Alzheimers Dis 2018, 64, S379–s95. [DOI] [PubMed] [Google Scholar]
- 140.Filippi M, Rocca MA, Barkhof F, Brück W, Chen JT, Comi G, et al. Association between pathological and MRI findings in multiple sclerosis. Lancet Neurol 2012, 11, 349–60. [DOI] [PubMed] [Google Scholar]
- 141.Qian ZM, Ke Y.. Hepcidin and its therapeutic potential in neurodegenerative disorders. Med Res Rev 2020, 40, 633–53. [DOI] [PubMed] [Google Scholar]
- 142.Crichton RR, Dexter DT, Ward RJ.. Brain iron metabolism and its perturbation in neurological diseases. J Neural Transm (Vienna) 2011, 118, 301–14. [DOI] [PubMed] [Google Scholar]
- 143.Wang T, Xu SF, Fan YG, Li LB, Guo C.. Iron pathophysiology in Alzheimer’s Diseases. Adv Exp Med Biol 2019, 1173, 67–104. [DOI] [PubMed] [Google Scholar]
- 144.Shin JA, Kim YA, Kim HW, Kim HS, Lee KE, Kang JL, et al. Iron released from reactive microglia by noggin improves myelin repair in the ischemic brain. Neuropharmacology 2018, 133, 202–15. [DOI] [PubMed] [Google Scholar]
- 145.Cui J, Guo X, Li Q, Song N, Xie J.. Hepcidin-to-ferritin ratio is decreased in astrocytes with extracellular alpha-synuclein and iron exposure. Front Cell Neurosci 2020, 14, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Puy V, Darwiche W, Trudel S, Gomila C, Lony C, Puy L, et al. Predominant role of microglia in brain iron retention in Sanfilippo syndrome, a pediatric neurodegenerative disease. Glia 2018, 66, 1709–23. [DOI] [PubMed] [Google Scholar]
- 147.Niida-Kawaguchi M, Kakita A, Noguchi N, Kazama M, Masui K, Kato Y, et al. Soluble iron accumulation induces microglial glutamate release in the spinal cord of sporadic amyotrophic lateral sclerosis. Neuropathology 2020, 40, 152–66. [DOI] [PubMed] [Google Scholar]
- 148.Lu LN, Qian ZM, Wu KC, Yung WH, Ke Y.. Expression of iron transporters and pathological hallmarks of Parkinson’s and Alzheimer’s Diseases in the brain of young, adult, and aged rats. Mol Neurobiol 2017, 54, 5213–24. [DOI] [PubMed] [Google Scholar]
- 149.Raha AA, Vaishnav RA, Friedland RP, Bomford A, Raha-Chowdhury R.. The systemic iron-regulatory proteins hepcidin and ferroportin are reduced in the brain in Alzheimer’s disease. Acta Neuropathol Commun 2013, 1, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Xu Y, Zhang Y, Zhang JH, Han K, Zhang X, Bai X, et al. Astrocyte hepcidin ameliorates neuronal loss through attenuating brain iron deposition and oxidative stress in APP/PS1 mice. Free Radic Biol Med 2020, 158, 84–95. [DOI] [PubMed] [Google Scholar]
- 151.Zarruk JG, Berard JL, Passos dos Santos R, Kroner A, Lee J, Arosio P, et al. Expression of iron homeostasis proteins in the spinal cord in experimental autoimmune encephalomyelitis and their implications for iron accumulation. Neurobiol Dis 2015, 81, 93–107. [DOI] [PubMed] [Google Scholar]
- 152.Varga E, Pandur E, Abrahám H, Horváth A, Ács P, Komoly S, et al. Cuprizone Administration alters the iron metabolism in the mouse model of multiple sclerosis. Cell Mol Neurobiol 2018, 38, 1081–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gosztyla ML, Brothers HM, Robinson SR.. Alzheimer’s amyloid-β is an antimicrobial peptide: a review of the rvidence. J Alzheimers Dis 2018, 62, 1495–506. [DOI] [PubMed] [Google Scholar]
- 154.Mehta D, Jackson R, Paul G, Shi J, Sabbagh M.. Why do trials for Alzheimer’s disease drugs keep failing? A discontinued drug perspective for 2010-2015. Expert Opin Investig Drugs 2017, 26, 735–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gilman S, Koller M, Black RS, Jenkins L, Griffith SG, Fox NC, et al. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology 2005, 64, 1553–62. [DOI] [PubMed] [Google Scholar]
- 156.Orgogozo JM, Gilman S, Dartigues JF, Laurent B, Puel M, Kirby LC, et al. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology 2003, 61, 46–54. [DOI] [PubMed] [Google Scholar]
- 157.Bishop GM, Robinson SR.. The amyloid hypothesis: let sleeping dogmas lie?. Neurobiol Aging 2002, 23, 1101–5. [DOI] [PubMed] [Google Scholar]
- 158.Qiao X, Cummins DJ, Paul SM.. Neuroinflammation-induced acceleration of amyloid deposition in the APPV717F transgenic mouse. Eur J Neurosci 2001, 14, 474–82. [DOI] [PubMed] [Google Scholar]
- 159.White MR, Kandel R, Tripathi S, Condon D, Qi L, Taubenberger J, et al. Alzheimer’s associated β-amyloid protein inhibits influenza A virus and modulates viral interactions with phagocytes. PLoS One 2014, 9, e101364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Spitzer P, Condic M, Herrmann M, Oberstein TJ, Scharin-Mehlmann M, Gilbert DF, et al. Amyloidogenic amyloid-β-peptide variants induce microbial agglutination and exert antimicrobial activity. Sci Rep 2016, 6, 32228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Soscia SJ, Kirby JE, Washicosky KJ, Tucker SM, Ingelsson M, Hyman B, et al. The Alzheimer’s disease-associated amyloid β-protein is an antimicrobial peptide. PLoS One 2010, 5, e9505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Eimer WA, Vijaya Kumar DK, Navalpur Shanmugam NK, Rodriguez AS, Mitchell T, Washicosky KJ, et al. Alzheimer’s disease-associated β-amyloid is rapidly seeded by herpesviridae to protect against brain infection. Neuron 2018, 99, 56–63.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bourgade K, Garneau H, Giroux G, Le Page AY, Bocti C, Dupuis G, et al. β-Amyloid peptides display protective activity against the human Alzheimer’s disease-associated herpes simplex virus-1. Biogerontology 2015, 16, 85–98. [DOI] [PubMed] [Google Scholar]
- 164.Kumar DKV, Choi SH, Washicosky KJ, Eimer WA, Tucker S, Ghofrani J, et al. Amyloid-beta; peptide protects against microbial infection in mouse and worm models of Alzheimer’s disease. Sci Transl Med 2016, 8, 340ra72–ra72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Pulze L, Bassani B, Gini E, D’Antona P, Grimaldi A, Luini A, et al. NET amyloidogenic backbone in human activated neutrophils. Clin Exp Immunol 2016, 183, 469–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bitting L, Naidu A, Cordell B, Murphy GM Jr. Beta-amyloid peptide secretion by a microglial cell line is induced by beta-amyloid-(25-35) and lipopolysaccharide. J Biol Chem 1996, 271, 16084–9. [DOI] [PubMed] [Google Scholar]
- 167.Galea I, Bechmann I, Perry VH.. What is immune privilege (not)? Trends Immunol 2007, 28, 12–8. [DOI] [PubMed] [Google Scholar]
- 168.Perry VH, Anthony DC, Bolton SJ, Brown HC.. The blood-brain barrier and the inflammatory response. Mol Med Today 1997, 3, 335–41. [DOI] [PubMed] [Google Scholar]
- 169.Daneman R, Prat A.. The blood-brain barrier. Cold Spring Harb Perspect Biol 2015, 7, a0204–12-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Korin B, Ben-Shaanan TL, Schiller M, Dubovik T, Azulay-Debby H, Boshnak NT, et al. High-dimensional, single-cell characterization of the brain’s immune compartment. Nat Neurosci 2017, 20, 1300–9. [DOI] [PubMed] [Google Scholar]
- 171.Mrdjen D, Pavlovic A, Hartmann FJ, Schreiner B, Utz SG, Leung BP, et al. High-dimensional single-cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity 2018, 48, 380–95.e6. [DOI] [PubMed] [Google Scholar]