Abstract
The magnitude and durability of immune responses induced by replication-defective adenovirus serotype 5 (ADV5) vector-based vaccines were evaluated in the simian-human immunodeficiency virus/rhesus monkey model. A single inoculation of recombinant ADV5 vector constructs induced cellular and humoral immunity, but the rapid generation of neutralizing anti-Ad5 antibodies limited the immunity induced by repeated vector administration. The magnitude and durability of the immune responses elicited by these vaccines were greater when they were delivered as boosting immunogens in plasmid DNA-primed monkeys than when they were used as single-modality immunogens. Therefore, administration of ADV5-based vectors in DNA-primed subjects may be a preferred use of this vaccine modality for generating long-term immune protection.
Accumulating data suggest that an effective human immunodeficiency virus (HIV) vaccine should elicit potent and durable virus-specific cellular and humoral immune responses. Vaccine-elicited immune responses can contribute to ameliorating simian immunodeficiency virus (SIV) and simian-human immunodeficiency virus-induced disease in macaques (1, 2, 8, 9, 13, 15). However, the durability of the immune responses generated by vaccination may not be adequate to provide clinical protection over a prolonged period of time. In fact, we have recently shown that the immune responses in plasmid DNA-primed rhesus monkeys that were boosted with recombinant poxviruses decayed so rapidly following the boosting immunization that no incremental clinical protection was afforded by the delivery of the live recombinant vector (14). We sought to evaluate the durability of immune responses in rhesus monkeys generated with a plasmid DNA prime/recombinant adenovirus boost vaccine.
Serotype 5 human adenovirus made replication incompetent by mutation of the viral E1 and E3 genes (ADV5) has proven a safe and highly immunogenic vector in studies with laboratory animals and early-phase human clinical trials (4, 5, 17). These results have provided a rationale for advancing recombinant ADV5 into efficacy testing in human volunteers (11). However, only limited work has been done to determine the durability of the immunity that can be generated through immunization with these vaccine constructs. The present studies were performed in Indian-origin rhesus monkeys to evaluate the magnitude and persistence of the immune responses generated following recombinant ADV5 immunization.
ADV5 vaccine constructs were first evaluated for immunogenicity with or without prior immunization using plasmid DNA. DNA plasmids expressing codon-optimized HIV type 1 (HIV-1) and SIV immunogens were made synthetically, using a method that has been previously described (10). The full-length synthetic SIVmac239 gag-pol-nef gene encoding a fusion protein was cloned in the mammalian expression vector pVR1012 under the control of the cytomegalovirus immediate-early enhancer, promoter, and first intron. The pVR1012-HIV-1 89.6P Env plasmid expresses a modified form of the env gene (ΔCFI) with nucleotide deletions in the gp120 cleavage site, the gp41 fusion domain, and the spacing region between heptad repeats 1 and 2 (6, 12).
Recombinant E1/E3-deleted ADV5 constructs were generated by modification of a previously described method (17). The ADV5-89.6P Env constructs expressed gp140 rather than the gp145 protein expressed by the DNA constructs. Also, because ADV5 constructs expressing SIVmac239 Gag-Pol-Nef were unstable, an insert that expressed SIVmac239 Gag-Pol was used. ADV5 vectors were produced and amplified in 293 cells. Viruses were purified on a cesium chloride gradient and stored in phosphate-buffered saline with 15% glycerol at −20°C.
A PCR-based assay was used to select adult rhesus monkeys (Macaca mulatta) that expressed the Mamu-A*01 MHC class I allele (2). Two Mamu-A*01+ rhesus monkeys received priming immunizations with HIV-1 89.6P env and SIVmac239 gag-pol-nef genes expressed by the pVR1012 plasmid. Five milligrams of the env DNA vaccine and 5 mg of the gag-pol-nef DNA vaccine were administered intramuscularly as separate injections by using the needleless Biojector apparatus at weeks 0, 4, and 8. Two other Mamu-A*01+ rhesus monkeys were immunized intramuscularly with 1012 particles of ADV5-SIV gag-pol and 1012 particles of ADV5-HIV-1 89.6P env at weeks 0 and 8.
The cellular immune responses elicited by these vaccines were assessed by tetramer staining and enzyme immunospot (ELISPOT) assays, using pooled peptides and 9-mer peptides representing the SIV Gag p11C, SIV Pol p68A, and HIV-1 Env p41A epitopes. Cytotoxic T lymphocytes (CTL) specific for the Mamu-A*01-restricted immunodominant SIV Gag p11C and subdominant SIV Pol p68A and HIV-1 Env p41A epitopes (7) were monitored in all four Mamu-A*01+ monkeys by tetramer staining of fresh peripheral blood mononuclear cells (PBMC). Tetramer staining of whole blood was done as described previously (2, 7, 8, 14).
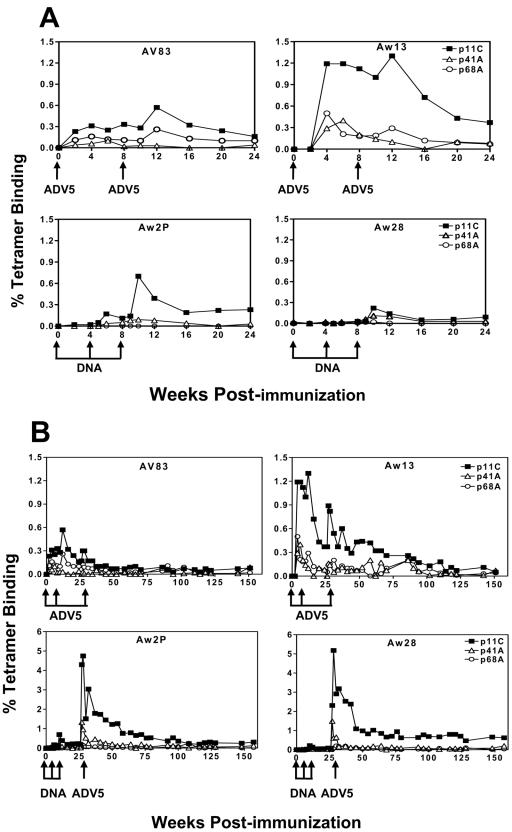
SIV Gag p11C/Mamu-A*01 tetramer-binding CD3+ CD8+ cells were detected in freshly isolated PBMC in the two ADV5 vaccine-immunized monkeys 2 weeks after the first immunization (Fig. 1A, top panel), reaching up to 1.1% circulating CD3+ CD8+ cells specific for Gag p11C in monkey Aw13. CD8+ T lymphocytes specific for the subdominant SIV Pol p68A and HIV-1 Env p41A epitopes were also detectable following one inoculation with these ADV5 vaccines. CD3+ CD8+ T cells that bound the dominant SIV Gag p11C/Mamu-A*01 tetrameric complex were detected in the monkeys that received plasmid DNA vaccinations 2 weeks after the third DNA immunization (Fig. 1A, bottom panel). CTL responses to the two subdominant epitopes were of very low magnitude in these monkeys.
FIG. 1.
Mamu-A*01-restricted CD8+ T-lymphocyte responses following ADV5 prime/ADV5 boost or DNA prime/ADV5 boost immunizations. (A) Two Mamu-A*01+ rhesus monkeys received intramuscular inoculations of 1012 particles of each of two ADV5 constructs, one expressing SIV Gag-Pol and the other expressing HIV-1 89.6P Env, on weeks 0 and 8. On weeks 0, 4, and 8, two other Mamu-A*01+ rhesus monkeys were inoculated with 5 mg of each of two plasmid DNA constructs, one expressing SIV Gag-Pol and the other expressing HIV-1 89.6P Env. (B) All four monkeys were boosted on week 26 with 1012 particles of each of the two ADV5 constructs. Vaccine-elicited CD8+ T-cell responses specific for the immunodominant SIV Gag p11C and subdominant HIV-1 Env p41A and SIV Pol p68A epitopes were measured by tetramer staining of freshly isolated PBMC. The percentages of CD3+ CD8+ T cells that bound the Mamu-A*01/peptide-tetramer complexes are shown.
On week 26, all four monkeys were boosted by intramuscular inoculation with 1012 particles of each of the ADV5 vaccine constructs. The two monkeys that had received prior vaccinations with ADV5 had little or no increase in their epitope-specific CTL responses (Fig. 1B). In fact, the peak tetramer responses after the third inoculations were actually smaller in magnitude than those reached following the second ADV5 inoculations. On the other hand, the two monkeys that were primed with plasmid DNA vaccines demonstrated a dramatic expansion of their p11C-specific CD8+ T-cell responses following ADV5 vaccine boosting (Fig. 1B, bottom panel), with peak Gag p11C/Mamu-A*01 tetramer-binding responses of approximately 5%. The HIV-1 Env p41A/Mamu-A*01 tetramer-binding responses reached a peak following ADV5 inoculation of 1.3%. Responses in these monkeys to SIV Pol p68A were extremely low.
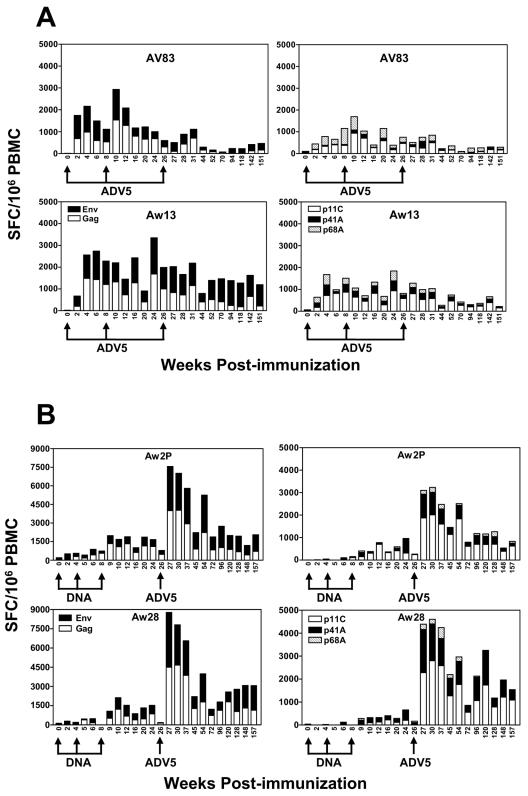
Gamma interferon (IFN-γ) ELISPOT assays were used to assess the vaccine-elicited cellular immune responses in these monkeys as described previously (12, 14). Consistent with the tetramer staining, the SIV Gag- and HIV-1 Env-specific PBMC IFN-γ ELISPOT responses elicited by the first ADV5 inoculations in monkeys AV83 and Aw13 were boosted only marginally by the second vaccination in week 8. The third injection of ADV5 vaccines in week 26 had no boosting effect (Fig. 2A, left panels). Similar patterns of PBMC ELISPOT responses were observed to 9-mer peptides representing the Gag p11C, Pol p68A, and Env p41A epitopes (Fig. 2A, right panels). The two monkeys receiving DNA prime immunizations had a dramatic expansion of their IFN-γ ELISPOT responses to SIV Gag and HIV-1 Env antigens following boosting with ADV5 vaccines, a fivefold increase in the total spot-forming cell (SFC) responses to both antigens (Fig. 2B, left panels). The ELISPOT responses to individual epitope peptides were similarly increased (Fig. 2B, right panels). Importantly, the vaccine-elicited cellular immune responses were remarkably durable, readily detected as late as 151 weeks following initial immunization of the monkeys.
FIG. 2.
Cellular immune responses to SIV Gag and HIV-1 Env following ADV5 prime/ADV5 boost or DNA prime/ADV5 boost immunizations. (A) Two Mamu-A*01+ monkeys were immunized with ADV vectors (see Fig. 1A). (B) Two Mamu-A*01+ rhesus monkeys were inoculated with the DNA/ADV vaccine (see Fig. 1B) and analyzed by interferon gamma ELISPOT responses in freshly isolated PBMC with in vitro exposure to peptide pools spanning the SIVmac251 Gag and HIV-1 89.6P Env proteins or 9-mer peptides representing the SIV Gag p11C, HIV-1 p41A, and SIV Pol p68A epitopes. Total SFC per 106 cells are shown. IFN-γ ELISPOT responses in medium control wells were consistently <15% of the response in wells containing peptide (data not shown).
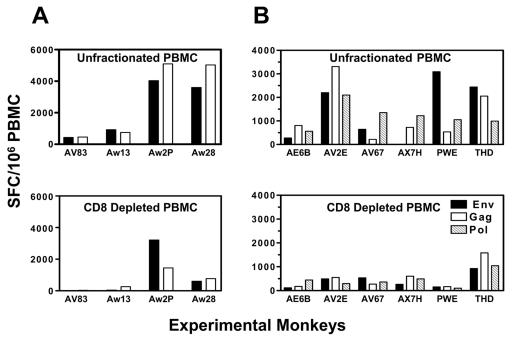
To assess T-lymphocyte subsets responsive to these vaccine regimens, unfractionated and CD8+ T-lymphocyte-depleted PBMC from the four monkeys were assessed in ELISPOT assays. Another cohort of six monkeys that received DNA immunizations on weeks 0, 4, and 8 and an ADV5 boost immunization on week 26 were also evaluated. Freshly isolated PBMC from these two cohorts of immunized monkeys were incubated with phycoerythrin-labeled anti-CD8 antibody and then with antiphycoerythrin-labeled magnetic beads (Miltenyi Biotech). The labeled PBMC were then sorted using a Miltenyi AutoMACS cell sorter to deplete CD8+ T lymphocytes. IFN-γ ELISPOT responses were measured in unfractionated and CD8+ T-lymphocyte-depleted PBMC from these monkeys 2 weeks following the final ADV5 immunizations. Cellular immune responses elicited by DNA prime/ADV5 boost vaccination were mediated by both CD4+ and CD8+ T lymphocytes, whereas inoculations with ADV5/ADV5 vaccines elicited predominantly CD8+ T-lymphocyte responses (Fig. 3A). Similar patterns were observed in another six animals receiving DNA prime/ADV5 boost (Fig. 3B) (12).
FIG. 3.
Cellular immunity elicited by DNA prime and ADV5 boost immunizations is mediated by both CD4+ and CD8+ T lymphocytes. Freshly isolated PBMC from two cohorts of immunized monkeys (A and B) were depleted of CD8+ T lymphocytes. IFN-γ ELISPOT responses were measured in unfractionated as well as CD8+ T-cell-depleted PBMC of the monkeys 2 weeks following the final ADV5 immunizations.
The humoral immune responses were assessed by measuring the titer of anti-HIV gp140 antibody in the plasma of the monkeys during the course of the immunizations using a lectin capture enzyme-linked immunosorbent assay method as described previously (18). Consistent with the pattern seen in cellular immune responses, the ADV5 vaccine boost of the plasmid DNA-primed monkeys increased antibody titers by 2 logs. The two monkeys vaccinated with ADV5 vectors generated modest anti-HIV-1 gp140 enzyme-linked immunosorbent assay titers, but little boosting was observed with subsequent ADV5 immunizations (data not shown).
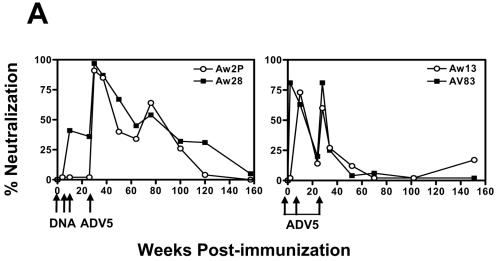
Plasma samples were also assessed for antibody neutralization of HIV-1 89.6 using an 89.6 Env-pseudotyped viral vector and TZM-bl target cells. The single round viral vector was generated by cotransfection of 293 T cells with the HIV-1 SG3.1 env molecular clone (a gift from Beatrice Hahn, University of Alabama) and the PSVIIIenv plasmid encoding the HIV-1 89.6 Env protein (a gift from Dana Gazbuda, Dana-Farber Cancer Institute). Viral supernatant was collected 48 hours after transfection, clarified by centrifugation and filtration with a 0.45-μm filter, and stored at −80°C. To assay for virus neutralization, the pseudotyped virus was mixed with heat-inactivated monkey plasma at a final dilution in plasma of 1:5. Target cells were added 30 minutes later. The target cells were TZM-bl cells (NIH AIDS Research and Reference Reagent Program). These cells are a HeLa cell clone that was engineered to express CD4 and CCR5. They also contain an integrated reporter gene for firefly luciferase. Luciferase expression is induced in trans by viral Tat protein, and the amount of luminescence detected is directly proportional to the number of infectious virus particles added to the target cells. ADV5 vaccine boost of the plasmid DNA-primed monkeys elicited antibodies that produced greater than 95% virus neutralization (Fig. 4A). These responses declined slowly and could still be detected up to 70 weeks following immunization. In the monkeys immunized with ADV5 vector only, neutralizing titers could be detected following the second inoculation and a small but detectable boost was apparent after the third immunization. These responses were less potent and durable than those induced by DNA/ADV5 immunization (Fig. 4A).
FIG. 4.
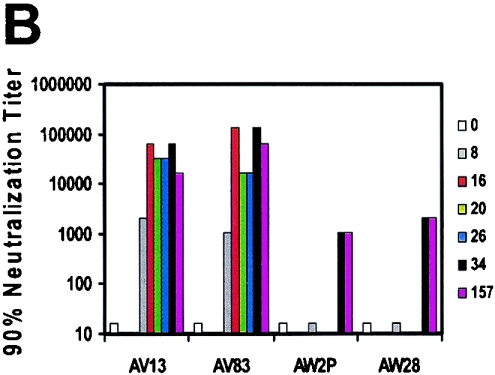
Humoral immune responses elicited by DNA prime and ADV5 boost immunizations. (A) Neutralizing antibodies against HIV-1 89.6 were measured during the course of immunizations. The percent neutralization value for each plasma sample is based on a comparison to the corresponding preimmune plasma for each monkey. All plasma were heat inactivated and diluted 1:5. (B) ADV5-specific neutralizing antibody responses are generated following a single inoculation of an ADV5 vaccine construct. Ad5-specific neutralizing antibody responses were assessed using a luciferase-based virus neutralization assay. Ninety-percent-neutralization titers were defined as the maximum serum dilution that neutralized 90% of luciferase activity.
To evaluate the mechanism accounting for failed ADV5 boosting, we determined the magnitude of antivector immunity elicited in the monkeys receiving these vaccines. ADV5-specific neutralizing antibody responses were assessed in serum of these immunized monkeys by using a luciferase-based virus neutralization assay (3, 16). As shown in Fig. 4B, Ad5-specific neutralizing-antibody responses were generated in the monkeys following a single ADV5 vaccination. Following the second recombinant Ad5 inoculation, there was a four- to fivefold increase in titer, with no decrement in this titer, even 133 weeks following the last boost. The animals that received a single ADV5 immunization also had no decrease in the Ad5 neutralizing-antibody titer.
In this report, the durability of ADV and DNA/ADV vaccine responses has been assessed. The findings suggest that ADV5 vaccines may be more useful as components of heterologous prime/boost regimens than as single-modality immunogens. The magnitudes of the total HIV- and SIV-specific PBMC SFC responses and the PBMC CTL epitope-specific tetramer and SFC responses were greater in the monkeys receiving plasmid DNA/ADV5 immunizations than in monkeys receiving ADV5-alone immunizations. Moreover, the ADV5 alone-immunized monkeys developed CD8+ T-lymphocyte-biased responses. The plasmid DNA/ADV5-immunized monkeys developed both CD4+ and CD8+ T-lymphocyte responses. Finally, the present data suggest that multiple immunizations with the same ADV5 vectors are likely to prove of limited utility, since durable, high-titer anti-ADV5 antibody responses are generated following even a single ADV5 administration that will neutralize the immunogenicity of subsequent inoculated ADV5 vaccine constructs.
It should be noted that there are some inconsistencies between the findings reported in the present study and results recently published by others. In a nonhuman primate study done by Casimiro et al., immunizations with a DNA prime/ADV5 boost and with an ADV5 prime/ADV5 boost regimen were comparably immunogenic (5). Importantly, the highest dose of ADV5 given to monkeys in that study was 1011 particles. In the present study, 1012 particles of ADV5 were used to boost the animals, and the responses were of a greater magnitude in the monkeys that were immunized with DNA prime/ADV5 boost. The differences in the results in these two studies may be attributable to the different doses of recombinant adenovirus vectors used for immunization. Further, the antigen-specific T-cell responses in the DNA-primed/ADV5-boosted animals in the present study were of lower magnitudes than those described in a previous study by Shiver et al. (15). In that earlier study, the plasmid DNA vaccines were administered with the adjuvant CRL1005. The use of the adjuvant may have contributed to the large magnitude responses seen following recombinant adenovirus vector boost (5).
The breadth of the T-cell immune responses elicited using vaccine regimens that include ADV5 was significant. These immunizations generated CTL responses to both dominant and nondominant epitopes. Further, the vaccine-elicited T cells recognized multiple viral genes. Such breadth should be advantageous, since T-cell immune responses specific for a diversity of viral epitopes should diminish the likelihood that an infecting HIV will be able to escape from immune control by generating only a limited number of nucleotide mutations.
Importantly, the immune responses elicited by ADV5 alone and by plasmid DNA/ADV5 vaccination were remarkably durable. The half-life of the vaccine-elicited neutralizing antibodies to HIV was prolonged nearly threefold, and the vaccine-induced cellular immune responses remained readily detectable in PBMC of the monkeys more than 150 weeks following their initial immunization. Since these long-term immune responses most certainly reflect a persistence of memory immune cell populations, there is every reason to suppose that the tested vaccine vectors should confer some degree of immunity for a prolonged period of time following their administration. These vaccine vectors therefore represent viable modalities for moving forward into advanced-phase clinical testing.
Acknowledgments
We acknowledge Shawn Sumida and Jaap Goudsmit for assistance and reagent.
REFERENCES
- 1.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O′Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. I. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. 292:69-74. [DOI] [PubMed]
- 2.Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T.-M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M. E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, M. G. Lewis, E. A. Emini, J. W. Shiver, and N. L. Letvin. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290:486-492. [DOI] [PubMed] [Google Scholar]
- 3.Barouch, D. H., M. G. Pau, J. H. Custers, W. Koudstaal, S. Kostense, M. J. Havenga, D. M. Truitt, S. M. Sumida, M. G. Kishko, J. C. Arthur, B. Korioth-Schmitz, M. H. Newberg, D. A. Gorgone, M. A. Lifton, D. L. Panicali, G. J. Nabel, N. L. Letvin, and J. Goudsmit. 2004. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J. Immunol. 172:6290-6297. [DOI] [PubMed] [Google Scholar]
- 4.Barouch, D. H., P. F. McKay, S. M. Sumida, S. Santra, S. S. Jackson, D. A. Gorgone, M. A. Lifton, B. K. Chakrabarti, L. Xu, G. J. Nabel, and N. L. Letvin. 2003. Plasmid chemokines and colony-stimulating factors enhance the immunogenicity of DNA priming-viral vector boosting HIV-1 vaccines. J. Virol. 77:8729-8735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casimiro, D. R., L. Chen, T. M. Fu, R. K. Evans, M. J. Caulfield, M. E. Davies, A. Tang, M. Chen, L. Huang, V. Harris, D. C. Freed, K. A. Wilson, S. Dubey, D. M. Zhu, D. Nawrocki, H. Mach, R. Troutman, L. Isopi, D. Williams, W. Hurni, Z. Xu, J. G. Smith, S. Wang, X. Liu, L. Guan, R. Long, W. Trigona, G. J. Heidecker, H. C. Perry, N. Persaud, T. J. Toner, Q. Su, X. Liang, R. Youil, M. Chastain, A. J. Bett, D. B. Volkin, E. A. Emini, and J. W. Shiver. 2003. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J. Virol. 77:6305-6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chakrabarti, B. K., W. P. Kong, B. Y. Wu, Z. Y. Yang, J. Friborg, X. Ling, S. R. King, D. C. Montefiori, and G. J. Nabel. 2002. Modifications of the human immunodeficiency virus envelope glycoprotein enhance immunogenicity for genetic immunization. J. Virol. 76:5357-5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Egan, M. A., M. J. Kuroda, G. Voss, J. E. Schmitz, W. A. Charini, C. I. Lord, M. A. Forman, and N. L. Letvin. 1999. Use of major histocompatibility complex class I/peptide/β2M tetramers to quantitate CD8+ cytotoxic T lymphocytes specific for dominant and nondominant viral epitopes in simian-human immunodeficiency virus-infected rhesus monkeys. J. Virol. 73:5466-5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egan, M. A., W. A. Charini, M. J. Kuroda, J. E. Schmitz, P. Racz, K. Tenner-Racz, K. Manson, M. Wyand, M. A. Lifton, C. E. Nickerson, T.-M. Fu, J. W. Shiver, and N. L. Letvin. 2000. Simian immunodeficiency virus (SIV) gag DNA-vaccinated rhesus monkeys develop secondary cytotoxic T-lymphocyte responses and control viral replication after pathogenic SIV infection. J. Virol. 74:7485-7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirsch, V. M., T. R. Fuerst, G. Sutter, M. W. Carroll, L. C. Yang, S. Goldstein, M. Piatak, Jr., W. R. Elkins, W. G. Alvord, D. C. Montefiori, B. Moss, and J. D. Lifson. 1996. Patterns of viral replication correlate with outcome in simian immunodeficiency virus (SIV)-infected macaques: effect of prior immunization with a trivalent SIV vaccine in modified vaccinia virus Ankara. J. Virol. 70:3741-3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang, Y., W. P. Kong, and G. J. Nabel. 2001. Human immunodeficiency virus type 1-specific immunity after genetic immunization is enhanced by modification of Gag and Pol expression. J. Virol. 75:4947-4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Letvin, N. L., D. H. Barouch, and D. C. Montefiori. 2002. Prospects for vaccine protection against HIV-1 infection and AIDS. Annu. Rev. Immunol. 20:73-99. [DOI] [PubMed] [Google Scholar]
- 12.Letvin, N. L., Y. Huang, B. K. Chakrabarti, L. Xu, M. S. Seaman, K. Beaudry, B. K. Schmitz, F. Yu, D. Rohne, K. L. Martin, A. Miura, W. Kong, Z. Yang, R. S. Gelman, O. G. Golubeva, D. C. Montefiori, J. R. Mascola, and G. J. Nabel. 2004. Heterologous envelope immunogens contribute to AIDS vaccine protection in rhesus monkeys. J. Virol. 78:7490-7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rose, N. F., P. A. Marx, A. Luckay, D. F. Nixon, W. J. Moretto, S. M. Donahoe, D. Montefiori, A. Roberts, L. Buonocore, and J. K. Rose. 2001. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell 106:539-549. [DOI] [PubMed] [Google Scholar]
- 14.Santra, S., D. H. Barouch, B. K. Schmitz, C. I. Lord, G. R. Krivulka, F. Yu, M. H. Beddall, D. A. Gorgone, M. A. Lifton, A. Miura, V. Philippon, K. Manson, P. D. Markham, J. Parrish, M. J. Kuroda, J. E. Schmitz, R. S. Gelman, J. W. Shiver, D. C. Montefiori, D. Panicali, and N. L. Letvin. 2004. Recombinant poxvirus boosting of DNA-primed rhesus monkeys augments peak but not memory T lymphocyte responses. Proc. Natl. Acad. Sci. USA 101:11088-11093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shiver, J. W., T. M. Fu, L. Chen, D. R. Casimiro, M. E. Davies, R. K. Evans, Z. Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415:331-335. [DOI] [PubMed] [Google Scholar]
- 16.Sprangers, M. C., W. Lakhai, W. Koudstaal, M. Verhoeven, B. F. Koel, R. Vogels, J. Goudsmit, M. J. Havenga, and S. Kostense. 2003. Quantifying adenovirus-neutralizing antibodies by luciferase transgene detection: addressing preexisting immunity to vaccine and gene therapy vectors. J. Clin. Microbiol. 41:5046-5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sullivan, N. J., A. Sanchez, P. E. Rollin, Z. Y. Yang, and G. J. Nabel. 2000. Development of a preventive vaccine for Ebola virus infection in primates. Nature 408:605-609. [DOI] [PubMed] [Google Scholar]
- 18.Yang, Z. Y., B. K. Chakrabarti, L. Xu, B. Welcher, W.-P. Kong, K. Leung, A. Panet, J. R. Mascola, and G. J. Nabel. 2004. Selective modification of variable loops alters tropism and enhances immunogenicity of human immunodeficiency virus type 1 envelope. J. Virol. 78:4029-4036. [DOI] [PMC free article] [PubMed] [Google Scholar]