Abstract
The ability of several viroids to induce posttranscriptional gene silencing has been demonstrated; however, the structure recognized by the Dicer enzyme(s) responsible for the initiation of this mechanism remains a mystery. Here, we show that the hairpin known to be implicated in the replication of peach latent mosaic viroid has the ability to trigger the Dicer enzyme(s). This domain, which is composed of a succession of several small stems separated by symmetrical bulges, is reminiscent of the precursor micro-RNAs.
Posttranscriptional gene silencing (PTGS) is a process known to play an important role in antiviral defense in plants (12, 21). PTGS is triggered by the presence of double-stranded RNAs (dsRNA) that are then cleaved by an RNase called Dicer (7, 19). There are at least four distinct Dicer-like (DCL) genes encoding predicted DCL enzymes in the Arabidopsis sp. and rice genomes (17). This cleavage leads to the formation of single-stranded RNAs, 21 to 25 nucleotides (nt) in length, called small interfering RNAs (siRNA). The siRNA are incorporated into a multisubunit protein complex, the RNA-induced silencing complex, and act as a guide to direct this RNA degradation machinery to its target RNAs. Moreover, the siRNA can serve as primers for an RNA-dependent RNA polymerase (RdRP), thereby causing an amplification phenomenon.
Viroids are small (∼300-nt), single-stranded, circular RNA pathogens that infect higher plants (9). Based on the detection of siRNA of 21 to 25 nt, representing different regions of the viroid genome, species of the viroid families Pospiviroidae and Avsunviroidae have been shown to induce PTGS (6, 10, 12, 14, 15, 20). In order to determine how peach latent mosaic viroid (PLMVd), which belongs to the Avsunviroidae family (3), initiates PTGS, we adopted a model assay based on a wheat germ extract that contains DCL enzymes which convert dsRNA into siRNA (18). Initially, 32P-radiolabeled PLMVd transcripts were synthesized in vitro in the presence of 50 μCi of [α-32P]GTP (3,000 Ci/mmol) from the recombinant plasmid pPD1 that possesses two tandemly repeated PLMVd sequences (2). During transcription, RNAs of both polarities possessing hammerhead sequences were produced and self-cleaved efficiently, yielding 338-nt monomeric transcripts. Purified transcripts (0.5 pmol) of both plus and minus polarities were then heat denatured at 85°C and slowly cooled to room temperature in order to favor the formation of dsRNA (Fig. 1A). The resulting mixture was incubated with the wheat germ extract according to the manufacturer's recommendations (Promega) for 3 h at 25°C. The reaction was stopped by proteinase K treatment (15 min at 65°C) followed by phenol extraction. The nucleic acids were then ethanol precipitated and fractionated on 10% denaturing polyacrylamide gels containing 7 M urea. The dsRNA-induced Dicer activity resulted in the formation of 21- to 25-nt siRNA. This confirms the hypothesis that dsRNA complexes formed by intermolecular base pairing of viroid strands of both polarities can serve as substrates for DCL enzyme(s) (10, 12, 14, 15). However, the incubation of PLMVd transcripts of either plus or minus polarity, both of which are highly structured RNA species (Fig. 1B) (4, 16), alone also resulted in the detection of siRNA (Fig. 1E, lanes 3 to 4). The latter structures are much more likely to be found in the cellular environment than are intermolecular dsRNA and can also be substrates for DCL. The cleavage of both the plus and minus strands produced different siRNA profiles (i.e., the former gave 21- and 24-nt siRNA, while the latter yielded 21-, 23- and 24-nt siRNA), probably due to small structural differences between the RNA species that either generated different products or recruited a different DCL. As controls, a characteristic hairpin formed by a 27-bp stem was efficiently cleaved, while a delta ribozyme motif that does not include a stem longer than 7 bp was not (Fig. 1C to E).
FIG. 1.
Dicer activity assays performed using a wheat germ extract and various RNA substrates. (A to D) Schematic representations of the secondary structures of the tested RNAs. (A) Duplex of PLMVd strands of plus and minus polarity. (B) Single PLMVd strand of either plus or minus polarity. The hammerhead cleavage sites are identified (hh), and the + and - symbols designate their polarities. (C) Model hairpin including a 27-bp stem. (D) Trans-acting delta ribozyme (the nucleotide sequences of the latter two RNA substrates can be retrieved elsewhere) (11, 13). (E) Autoradiogram of a polyacrylamide gel electrophoresis (PAGE) gel of Dicer activity assays performed using a wheat germ extract. Lanes 1 and 2 are duplexes of the PLMVd strands shown in panel A. The reaction conditions were identical except that the wheat germ extract was inactivated by heat treatment prior to the incubation for lane 2. Lanes 3 and 4 are PLMVd strands of either plus or minus polarity, respectively. Lanes 5 and 6 are the assays with the model hairpin (R31-27) and delta ribozyme (Rz-12), respectively. To the left of the gel, the positions of the RNA markers of 21 and 26 nt are indicated. XC indicates xylene cyanol. The bottom part (below the dashed line) has been overexposed in order to reveal the bands corresponding to the small products.
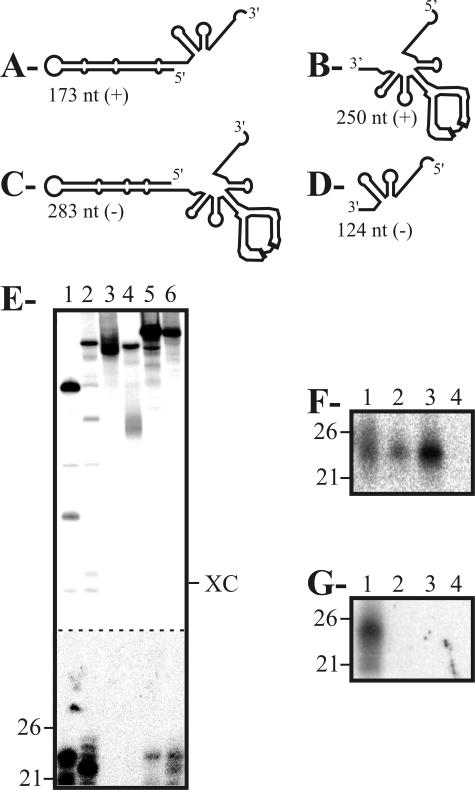
Subsequently, we investigated which region(s) of PLMVd was (were) a substrate for DCL(s). Transcripts containing two different regions of PLMVd for both polarities were synthesized from the pPD1 plasmid, and their ability to be cleaved by Dicer-like enzymes was assessed. Regardless of the polarity of the transcripts, only those regions including the long P11 hairpin, which is composed of a succession of small stems separated by symmetrical bulges, resulted in the production of siRNA (Fig. 2E). These observations were confirmed by Northern blot hybridization using probes directed against either the P11 hairpin or the right domain of PLMVd (Fig. 2F and G).
FIG. 2.
Dicer activity assays performed with various PLMVd-derived fragments. (A to D) Schematic representations of the plus-polarity (A, B) and minus-polarity (C, D) PLMVd fragments tested. The length, in nucleotides, is indicated for each fragment. (E) Autoradiogram of a PAGE gel of Dicer activity assays performed as described above. Lanes 1 to 6 are the 173-nt (lane 1), 283-nt (lane 2), 250-nt (lane 3), 124-nt (lane 4), and 338-nt fragments of plus and minus polarity (lanes 5 and 6, respectively). Adjacent to the gel, the positions of the RNA markers of 21 and 26 nt are indicated. XC indicates xylene cyanol. The bottom part (below the dashed line) has been overexposed in order to reveal the bands corresponding to the small products. (F, G) Autoradiograms of Northern blot hybridizations of cleavage assays performed in the presence of nonradioactive PLMVd RNA fragments of plus polarity. The conditions for hybridization have been described previously (12). The 5′-32P-oligonucleotides used as probes were complementary to a part of the P11 hairpin (5′-1GCCCACTGATGAGCCGCTGAAATGCGGCGAAACTTTTGAT40-3′) (F) and the right domain (5′-142CCGCTTGGTTCCCGAAGGAAAAGTCCCACCTTACCTCATTG182-3′) (G) of PLMVd. Lanes 1 to 4 are the duplexes of PLMVd strands of plus and minus polarity (lane 1), the 338-nt fragments (lane 2), and the 173-nt (lanes 3) and 250-nt (lane 4) fragments of plus polarity. Adjacent to the gel, the positions of the RNA markers of 21 and 26 nt are indicated.
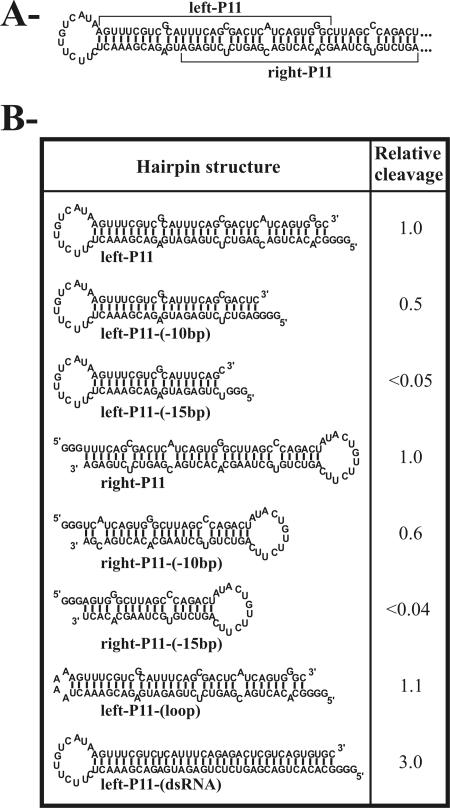
In order to identify the important structural features of the P11 stem that trigger the DCL enzyme(s), cleavage experiments were performed using substrates derived from the P11 hairpin. The left portion of the P11 hairpin substrate (left-P11) of 83 nt in size and the left-P11-(-10bp) (as identified in Fig. 3) of 63 nt had almost the same levels of cleavage. These substrates contain, respectively, 34 and 24 bp, if we consider that the bulged nucleotides adopt a stacked, non-Watson-Crick base-pairing structure. Conversely, removal of a further 5 bp resulted in an uncleavable substrate [left-P11-(-15bp)] of 53 nt. The latter substrate included only a 19-bp stem, which is smaller than the 21 bp required for Dicer activity (8). Similar results were obtained when using the right portion of the P11 stem, to which a loop allowing the two strands to connect was added [right-P11-(-10bp) and right-P11-(-15bp)]. The sequence composing the loop was also shown to be unimportant for cleavage to occur [i.e., left-P11-(loop)]. A substrate in which the bulged nucleotides from the upper strands were substituted by residues permitting the formation of Watson-Crick base pairs was cleaved significantly more efficiently than the initial imperfect stem [i.e., 3.0 for the left-P11-(dsRNA)], demonstrating DCL's preference for perfect dsRNA. Similar results were obtained when the experiments were repeated using transcripts derived from the P11 hairpin of minus polarity as well as changing the positions and identities of the bulged nucleotides (data not shown). Taken together, these data demonstrated that DCL enzymes prefer dsRNA of at least 21 bp in length, regardless of their sequence, but can tolerate the presence of bulged nucleotides.
FIG. 3.
Dicer activity assays performed with various transcripts derived from the P11 hairpin. (A) The sequence and secondary structure of the hairpin is illustrated. This is a variant sequence of the pPD1 insert sequence (see reference 2). Three guanosines were added at the 5′ end of each transcript to ensure efficient transcription from the oligonucleotides. (B) The Dicer assays were performed as described in the text. The cleavage levels were determined and are reported as relative values in comparison to a value of 1.0 that was arbitrarily assigned to the left-P11 substrate.
Previous studies indicated that accumulated siRNA in infected plants form a population of sequences representing the entire PLMVd genome (12, 14), whereas in wheat germ extract they appeared to be restricted to the sequences of the P11 hairpin. In plants, the siRNA can subsequently be used as a primer by an RdRP, thereby producing more dsRNA susceptible to the activity of DCL enzyme(s) (7, 19). Incubation of a full-length PLMVd RNA with a 5′-32P-radiolabeled RNA primer complementary to the left-terminal P11 loop produced RNA fragments of the predicted sizes, confirming the presence of the RdRP activity in the wheat germ extract (Fig. 4, lane 1). Similar results were obtained using other RNA primers complementary to various regions of the viroid and to different PLMVd fragments (e.g., Fig. 4, lane 2). Moreover, the production of dsRNA by RdRP from a nonradioactive PLMVd fragment and an RNA primer was monitored by the detection of siRNA by Northern blot hybridization after incubation in the wheat germ extract. Using a probe against the right domain of PLMVd, a faint tiny signal was observed in the presence of the RNA primer, while no signal was detected if PLMVd was incubated alone (data not shown). This shows that the amplification took place in the wheat germ extract, although inefficiently compared to that in the plant cell. The amplification is presumably at a higher level in plants than in wheat germ extracts because the enzymatic extract is exhausted after several minutes, while the phenomenon of accumulation in the living cell occurs for an extended period of time.
FIG. 4.

Autoradiogram of a 10% PAGE gel of primer extension by the RdRP in wheat germ extract. Lane 1 is the extension of primer P11 (5′-GGG6UUUGAUGAUAUGAGUUUCGUC324-3′) on the 338-nt PLMVd template. Lane 2 is the extension of primer P10 (5′-GGG290ACUCAUCUUCCAGAAUCACU270-3′) on the 250-nt PLMVd fragments. Lanes 3 and 4 are controls with DNase digestion and without template, respectively. To the left of the gel, the positions of the RNA markers are indicated. XC indicates xylene cyanol.
In summary, this study identifies the P11 hairpin of PLMVd as the domain recognized by the DCL enzyme(s), thereby initiating PTGS. It seems unlikely that the P11 hairpin evolved specifically to be a Dicer substrate, because the viroid evades PTGS-mediated destruction by replicating in the chloroplast (3, 12). The selective pressure on the P11 sequence and structure came primarily from its implication in the mechanism of replication. It seems most likely that PTGS induction occurs during the cytoplasmic phase of the viroid's life cycle (e.g., as it moves from cell to cell). On the other hand, the structure of P11 recalls that of the pre-micro-RNAs (pre-miRNA), which are bulged hairpins of variable sizes, sometimes with branched structures, that are cleaved by DCL1 to give miRNA (1, 5, 17). These miRNA subsequently interact with specific mRNAs in order to regulate their translation. It seems reasonable to suggest that this mechanism might be involved in the pathogenesis of this viroid (20); however, physical evidence remains to be reported. In contrast to the DCL2 and DCL3 that have been associated with cytoplasmic siRNA biogenesis, DCL1 was demonstrated to be a nuclear enzyme (17, 21, 22). However, this localization is incompatible with the hypothesis that PLMVd might take in the miRNA biogenesis pathway. Clearly, additional experimentation should reveal many other intimate details related to the viroid's PTGS mechanism.
Acknowledgments
This work was supported by a grant from the Canadian Institute of Health Research (CIHR) to J.-P.P. The RNA group is supported by a grant from the CIHR.
REFERENCES
- 1.Bartel, D. 2004. MicroRNAs: genomics, biogenesis, mechanism and function. Cell 116:281-297. [DOI] [PubMed] [Google Scholar]
- 2.Beaudry, D., F. Bussière, F. Lareau, C. Lessard, and J. P. Perreault. 1995. The RNA of both polarities of the peach latent mosaic viroid self-cleaves in vitro solely by single hammerhead structures. Nucleic Acids Res. 23:745-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bussière, F., J. Lehoux, D. A. Thompson, L. J. Skrzeczkowski, and J. P. Perreault. 1999. Subcellular localization and rolling circle replication of peach latent mosaic viroid: hallmarks of group A viroids. J. Virol. 73:6353-6360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bussière, F., J. Ouellet, F. Côté, D. Lévesque, and J. P. Perreault. 2000. Mapping in solution shows the peach latent mosaic viroid to possess a new pseudoknot in a complex, branched secondary structure. J. Virol. 74:2647-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carmell, M. A., and G. J. Hannon. 2004. RNase III enzymes and the initiation of gene silencing. Nat. Struct. Mol. Biol. 11:214-218. [DOI] [PubMed] [Google Scholar]
- 6.Denti, M. A., A. Boutla, M. Tsagris, and M. Tabler. 2004. Short interfering RNAs specific for potato spindler tuber viroid are found in the cytoplasm but not in the nucleus. Plant J. 37:762-769. [DOI] [PubMed] [Google Scholar]
- 7.Dillin, A. 2003. The specifics of small interfering RNA specificity. Proc. Natl. Acad. Sci. USA 100:6289-6291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elbashir, S. M., W. Lendeckel, and T. Tuschl. 2001. RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev. 15:188-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flores, R., J. W. Randles, M. Bar-Joseph, and T. O. Diener. 2000. Viroids, p. 1009-1024. In M. H. V. Van Regenmortel, C. M. Fauquet, D. H. L. Bishop, E. B. Carsten, M. K. Estes, S. M. Lemon, J. Maniloff, M. A. Mayo, D. J. McGeoch, C. R. Pringle, and R. B. Wickner (ed.), Virus taxonomy. Seventh Report of the International Committee on the Taxonomy of Viruses. Academic Press, San Diego, Calif.
- 10.Itaya, A., A. Folimonov, Y. Matsuda, R. S. Nelson, and B. Ding. 2001. Potato spindle tuber viroid as inducer of RNA silencing in infected tomato. Mol. Plant-Microbe Interact. 14:1332-1334. [DOI] [PubMed] [Google Scholar]
- 11.Lamontagne, B., and S. Abou Elela. 2004. Evaluation of the RNA determinants for bacterial and yeast RNase III binding and cleavage. J. Biol. Chem. 279:2231-2241. [DOI] [PubMed] [Google Scholar]
- 12.Landry, P., D. Thompson, and J. P. Perreault. 2004. The role of viroids in gene silencing: the model case of Peach latent mosaic viroid. Can. J. Plant Pathol. 26:1-8. [Google Scholar]
- 13.Lévesque, D., S. Choufani, and J. P. Perreault. 2002. Delta ribozyme benefits from a good stability in vitro that becomes outstanding in vivo. RNA 8:464-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez de Alba, A. E., R. Flores, and C. Hernandez. 2002. Two chloroplastic viroids induce the accumulation of small RNAs associated with posttranscriptional gene silencing. J. Virol. 76:13094-13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papaefthimiou, I., A. J. Hamilton, M. A. Denti, D. C. Baulcome, M. Tsagris, and M. Tabler. 2001. Replicating potato spindle tuber viroid RNA is accompanied by short RNA fragments that are characteristic of post-transcriptional gene silencing. Nucleic Acids Res. 29:2395-2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pelchat, M., D. Lévesque, J. Ouellet, S. Laurendeau, S. Lévesque, J. Lehoux, D. A. Thompson, K. C. Eastwell, L. J. Skrzeczkowski, and J. P. Perreault. 2000. Sequencing of peach latent mosaic viroid variants from nine North American peach cultivars shows that this RNA folds into a complex secondary structure. Virology 271:37-45. [DOI] [PubMed] [Google Scholar]
- 17.Susi, P., M. Hohkuri, T. Wahlroos, and N. J. Kilby. 2004. Characteristics of RNA silencing in plants: similarities and differences across kingdoms. Plant Mol. Biol. 54:157-174. [DOI] [PubMed] [Google Scholar]
- 18.Tang, G., B. J. Reinhart, D. P. Bartel, and P. D. Zamore. 2003. A biochemical framework for RNA silencing in plants. Genes Dev. 17:49-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voinnet, O. 2003. RNA silencing bridging the gaps in wheat extracts. Trends Plant Sci. 8:307-309. [DOI] [PubMed] [Google Scholar]
- 20.Wang, M. B., X. Y. Bian, L. M. Wu, L. X. Liu, N. A. Smith, D. Isenegger, R. M. Wu, C. Masuta, V. B. Vance, J. M. Watson, A. Rzaian, E. S. Dennis, and P. M. Waterhouse. 2004. On the role of RNA silencing in the pathogenicity and evolution of viroids and viral satellites. Proc. Natl. Acad. Sci. USA 101:3275-3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waterhouse, P. M., M.-B. Wang, and T. Lough. 2001. Gene silencing as an adaptive defense against viruses. Nature 411:834-842. [DOI] [PubMed] [Google Scholar]
- 22.Xie, Z., L. K. Johansen, A. M. Gustafson, K. D. Kasschau, A. D. Lellis, D. Zilberman, S. E. Jacobsen, and J. C. Carrington. 2004. Genetic and functional diversification of small RNA pathways in plants. PLoS Biol. 2:E104. [Online.] [DOI] [PMC free article] [PubMed] [Google Scholar]