Abstract
M141R is a myxoma virus gene that encodes a cell surface protein with significant amino acid similarity to the family of cellular CD200 (OX-2) proteins implicated in the regulation of myeloid lineage cell activation. The creation of an M141R deletion mutant myxoma virus strain (vMyx141KO) and its subsequent infection of European rabbits demonstrated that M141R is required for the full development of a lethal infection in vivo but is not required for efficient virus replication in susceptible cell lines in vitro. Minor secondary sites of infection were detected in the majority of rabbits infected with the M141R deletion mutant, demonstrating that the M141R protein is not required for the dissemination of virus within the host. When compared to wild-type myxoma virus-infected rabbits, vMyx141KO-infected rabbits showed higher activation levels of both monocytes/macrophages and lymphocytes in situ through assessments of inducible nitric oxide synthase-positive and CD25+ infiltrating cells in infected and lymphoid tissues. Purified peripheral blood mononuclear cells from vMyx141KO-infected rabbits demonstrated an increased ability to express gamma interferon upon activation by phorbol myristate acetate plus ionomycin compared to cells purified from wild-type myxoma virus-infected rabbits. We concluded that the M141R protein is a bona fide CD200-like immunomodulator protein which is required for the full pathogenesis of myxoma virus in the European rabbit and that its loss from the virus results in increased activation levels of macrophages in infected lesions and draining lymph nodes as well as an increased activation level of circulating T lymphocytes during infection. We propose a model whereby M141R transmits inhibitory signals to tissue macrophages, and possibly resident CD200R+ dendritic cells, that reduce their ability to antigenically prime lymphocytes and possibly provides anergic signals to T cells directly.
Natural selection not only drives the genetic evolution of prokaryotic and eukaryotic genomes but also sculpts the genomic variation of viruses (26, 27, 40). The ability of a given virus to successfully avoid effective detection and destruction by the host immune system depends on the combined interactions of multiple pathways, the consequence of which is to either minimize virus recognition by immune cells or to counteract any antiviral response that occurs when a virus infection is perceived by the host (53). Poxviruses are large DNA viruses which replicate solely in the cytoplasm of infected cells and encode many of the factors required for virus replication (34). The large size of the poxviral genome affords this virus family the luxury of encoding numerous factors for evading or counteracting both the innate and specific immune responses of the host (30, 47). Full genomic sequencing of many of the large poxvirus and herpesvirus genomes has provided us with a wealth of information regarding the genetic makeup of viruses and has also identified many new potential immunomodulatory genes (2, 30, 47).
Myxoma virus (MV) belongs to the Leporipoxvirus genus and is thus a member of the Chordopoxvirus subfamily (17). Like other family members, MV has a characteristic large, linear, double-stranded DNA genome with terminal inverted repeats and closed hairpin loop structures (33). Myxoma virus causes a mild, benign infection in its well-adapted North and South American leporid hosts (Sylvilagus californicus and Sylvilagus brasiliensis, respectively), but infection of the European rabbit results in fulminant myxomatosis, a mercurial, systemic, lethal disease resulting in approximately 100% mortality (18). The well-characterized pathogenesis of myxomatosis has provided an instructive model with which to dissect the host/pathogen relationship and also to understand the coevolution of hosts and poxvirus pathogens (33). MV encodes multiple proteins which are dispensable for virus replication in cultured cells and whose function is to protect the virus from the antiviral responses of the host immune system (8, 31). One such candidate immunomodulator is an MV-encoded homolog of CD200, previously designated vOX-2, which is encoded by the M141R open reading frame (8, 11).
CD200 (originally called OX-2) is a type 1 membrane glycoprotein and a member of the immunoglobulin superfamily that is expressed on a wide variety of cell types, including endothelial cells, B and T cells, and neurons (5, 10, 52). The CD200 molecule consists of two immunoglobulin (Ig) domains, one variable-like Ig domain at the N terminus and one constant-like Ig domain towards the C terminus (6). CD200 has a C-terminal transmembrane domain and a minute cytoplasmic domain that is not thought to be capable of independent signaling (7, 36). The function of the cellular CD200 protein has generated some controversy, in that both activating stimulatory and inhibitory (tolerogenic) immunoregulatory signals have been attributed to the expression of this molecule (9, 16, 20-23, 42). These apparently incongruent conclusions may be partly explained by the identification of two novel CD200 receptor-like family members, CD200RLa and CD200RLb, which have been shown to cooperate with DAP12, enabling these receptors to provide activating signals (55).
Mice lacking expression of the CD200 molecule have yielded some clues to the roles that CD200 and its receptor (CD200R) play in the immune system. CD200-deficient mice have more numerous and activated macrophage-lineage cells, including brain microglia, and three separate models of inflammation and injury were used to demonstrate the role that CD200 plays in the control of myeloid-lineage cell activation (28). Thus, CD200 has the capacity to limit the activation of myeloid-lineage cells in diverse tissues and to prevent the pathology associated with inappropriate activation of this cell type (7). The identification of the CD200R was also critical for the dissection of CD200's biology. Wright et al. (56) cloned the original CD200R and showed that its expression, unlike that of CD200, was restricted to cells of the myeloid lineage. The 67-amino-acid cytoplasmic domain of CD200R contains three conserved tyrosine residues, one of which is contained within an NPXY motif, thus suggesting a signaling capability (56). Most recently, it was shown that CD200 binding to CD200R induces tyrosine phosphorylation of CD200R, resulting in interactions with the adapter proteins Dok1 and Dok2 (57) and an inhibition of activation of extracellular signal-regulated kinase, Jun N-terminal protein kinase, and p38 mitogen-activated protein kinase. Cells that express CD200R include members that are implicated in antiviral responses, particularly monocytes/macrophages and dendritic cells, which are thought to be key for priming host T cells against antigens of the invading virus (7).
Thus, considering the role of CD200 as a negative regulator capable of delivering a locally restrictive signal to myeloid-lineage cells, it is not difficult to imagine why a virus would benefit from the expression of such a molecule on the surfaces of infected cells. The ability to ligate CD200R and deliver a tolerogenic signal to cells dedicated to the eradication of intracellular pathogens at the site of infection and in the lymph nodes, where the priming of virus-specific effector T cells takes place, would provide a significant advantage to a virus bent on evading the host immune response. In the current literature, CD200-like genes have been identified for some, but not all, members of the double-stranded DNA virus families of poxviruses, herpesviruses, and adenoviruses (13, 19, 37). The fact that selected members of these three rather different families of viruses encode CD200-like proteins highlights the probable value afforded by the expression of these molecules during infection. On the other hand, the fact that not all family members of each of these groups of viruses encode CD200-like genes underscores the observation that the protein product confers an evolutionary benefit rather than a necessary function. To date, viral CD200 (vCD200) function has only been examined for Kaposi's sarcoma-associated herpesvirus (KSHV; also called human herpesvirus 8 [HHV-8]), for which the K14 gene product has been shown to be a bona fide ligand of CD200R (13, 19). However, due to the lack of an appropriate animal model system, the role of K14, or any other vCD200, in infected animals remains unknown. The advantage of the poxvirus system is that individual genes can be deleted and the biological consequences can be evaluated both in vivo and in vitro (31).
M141R was first described as a potential CD200-like gene candidate following a complete genomic sequence analysis of the myxoma virus genome (11). The M141R protein possesses significant homology to the CD200 protein and thus made an attractive candidate for evaluation of a potential role as a vCD200 in the context of an infected animal host. This paper reports an initial characterization of the M141R protein's role in the micromanipulation of host immune responses, and we show that this protein possesses the CD200-like properties of reducing the activation levels of both macrophages and T cells in the infected host.
MATERIALS AND METHODS
Cells and viruses.
Wild-type myxoma virus (vMyx), which refers to strain Lausanne unless otherwise noted, vMyxgfp, an M141R deletion mutant (vMyx141KO), and an M141R revertant mutant virus (vMyx141rev) were passaged on baby green monkey kidney (BGMK) cells (obtained from S. Dales, University of Western Ontario) and rabbit kidney (RK13) cells (ATCC CCL-37) in Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum (FBS; Gibco BRL), 100 U of penicillin per ml, and 100 μg of streptomycin per ml. Rabbit CD4+ T lymphocytes (RL5) (National Institutes of Health AIDS Reagent Program) were maintained in RPMI 1640 (Gibco BRL) supplemented with 10% FBS, 100 U of penicillin per ml, and 100 μg of streptomycin per ml. Primary rabbit monocytes were cultured in RPMI supplemented with 20% FBS. Cells were infected at a multiplicity of infection (MOI) of 5.
Construction of recombinant myxoma viruses.
vMyxgfp has been described previously (29). The basis for the vMyx141KO virus was the plasmid myx1044, which was generated as previously described (11) and contains the entire M141R open reading frame plus both 5′ and 3′ flanking sequences. PCR amplification of the myx1044 template was used to generate the 5′ flanking region (FR) of the M141R fragment by use of the following primers: M141Rko/FLF (5′-AAAAGCTTCGGAAGAAGAGAGACGATG-3′) and M141Rko/FLR (5′-AAGGATCCTCTATTTTGCTCGCATCTTT-3′). M141Rko/FLF and M141Rko/FLR were designed to create HindIII and BamHI sites (underlined), respectively, at the 5′ and 3′ ends of the 5′ FR. The resulting PCR product, M141RkoFL, and the myx1044 plasmid were digested with HindIII and BamHI, which in the latter case removed the majority of the M141R coding sequence and left only the 3′ flanking region of the M141R gene. The digested myx1044 plasmid was then ligated to the PCR product containing the 5′ FR fragment, creating an internal BamHI site between the two flanking regions. The consequent plasmid, pBSM141RkoFL/FR, was digested with BamHI, and the ends were repaired by use of the Klenow fragment and T4 polymerase to enable the blunt-end insertion of selectable markers as follows. A plasmid containing the enhanced green fluorescent protein (EGFP) gene under the control of the poxvirus synthetic early/late promoter (12) and the Escherichia coli guanosine phosphoribosyltransferase (GPT) gene driven by the vaccinia virus 7.5K promoter (pT7 E/L EGFP-GPT) was digested with XbaI to release the EGFP/GPT cassette. This fragment's ends were blunted by use of the Klenow fragment and T4 polymerase and then ligated to the blunt ends of pBSM141RkoFL/FR, creating pBSM141RkoFL/gfp/gpt/FR, which was used for transfection into myxoma virus-infected cells. M141R deletion mutant myxoma virus growth was selected on the basis of mycophenolic acid resistance as previously described (35). The wild-type myxoma virus was used as the background strain to create both recombinant viruses, designated vMyxgfp and vMyx141KO. PCR and sequence analysis using the M141Rkodetect forward (5′-AAATACAACGACGGTAGCCG-3′) and reverse (5′-TACTGTCTCGTTTCCACCG-3′) primers confirmed the absence of an intact M141R open reading frame. In order to create the revertant strain vMyx141rev, in which the complete M141R open reading frame was restored, we cotransfected the vMyx141KO virus and the original myx1044 plasmid containing the intact M141R gene. Under these conditions, homologous recombination between the M141R flanking regions of the virus and the full M141R open reading frame on the plasmid occurred, and consequently the EGFP/GPT cassette was excised from the virus and replaced with the intact full-length M141R gene, creating a colorless vMyx141rev virus when assessed under fluorescent illumination. Multiple rounds of focus purification were completed, and PCR analysis of the M141R gene from the revertant virus genomic DNA was used to verify the purity of the revertant virus strain.
Single-step growth analysis in tissue culture.
BGMK, RK13, and RL5 cells (105) were infected with wild-type myxoma virus, vMyxgfp, or vMyx141KO at an MOI of 5 for 1 h. The inoculum was removed and the cells were washed three times with serum-free medium and then once with medium supplemented with serum. Viruses were harvested at 0, 4, 8, 12, 24, and 48 h postinfection (hpi). Virus titers were determined by serial dilution and infection of BGMK cells, followed by crystal violet staining of fixed monolayers in order to visualize foci (38). All growth analyses were performed in triplicate.
Generation of polyclonal anti-M141R antiserum.
The peptides M141RA and M141RB were chosen based on their predicted antigenic and hydrophilic indices. A rabbit polyclonal anti-M141R antiserum was generated by the use of keyhole limpet hemocyanin (KLH)-conjugated peptides as immunogens. The peptide M141RA [(C)TWKKNNETTI] corresponds to residues 51 to 60 and M141RB [(C)TRGRSTNRST] corresponds to residues 142 to 151 of the M141R amino acid sequence, with an amino-terminal cysteine included for coupling to KLH. Both peptides were conjugated to KLH by use of an Imject Immunogen EDC conjugation kit (Pierce) according to the manufacturer's instructions and were suspended in Freund's complete adjuvant (Sigma) for the first injection into New Zealand White rabbits. Four to five subsequent injections for boosting antibody production were carried out in Freund's incomplete adjuvant (Sigma). The antibody titer was assessed by Western blot analysis of vMyxgfp-infected BGMK cell lysates. A positive anti-M141R serum was obtained from the rabbit injected with peptide M141RB, and the antiserum was subsequently purified by use of a column for affinity purification which had been prepared with the M141RB peptide conjugated to bovine serum albumin by use of an Aminolink Plus immobilization kit (Pierce) according to the manufacturer's protocol.
M141R expression in infected cells.
Cells (106) were infected with vMyxgfp at an MOI of 10 for 1 h at 37°C. The inoculum was removed and the cells were washed once with serum-free medium (Gibco BRL). At various times, infected cells were collected and lysed directly in 50 μl of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) buffer, and samples were analyzed by 12% SDS-PAGE and transferred to an Optitran nitrocellulose filter (Schleicher & Schuell). The filter was then blocked overnight at 4°C in 5% skim milk-Tris-buffered saline (TBS)-Tween, incubated for 1 h at 4°C with a 1:500 dilution of the affinity-purified anti-M141R polyclonal antibody in 5% skim milk-TBS-Tween, washed three times with TBS-Tween, and incubated for 30 min with a 1:3,000 dilution of a donkey anti-rabbit-horseradish peroxidase (Amersham) antibody in 5% skim milk-TBS-Tween. The filter was then washed three times and bands were detected by the use of ECL Plus (Amersham).
Primary lymphocyte stimulation assays.
Diluted heparinized whole rabbit blood was layered over an equivalent volume of Lympholyte-Rabbit (Cedarlane) and centrifuged for 30 min at 1,500 × g at room temperature. Erythrocytes were lysed in ACK lysis buffer for 3 min and then immediately diluted in phosphate-buffered saline (PBS), and the remaining cells were collected by centrifugation. The mononuclear cell fraction was then counted, and cultures were set up at 106 cells per ml in RPMI 1640 (Gibco Life) supplemented with 20% fetal bovine serum, 2% penicillin-streptomycin for 6 h in the presence of 25 ng/ml phorbol myristate acetate (PMA) (Sigma) and 1 μM ionomycin (Sigma) at the manufacturer's recommended dilution of 4 μl per 6 ml of medium. Stimulated samples were further supplemented with Golgistop (BD Biosciences) for the final 4 h (37°C, 5% CO2) of incubation, washed, and then stained with antibodies for flow cytometric analyses.
Flow cytometry.
Purified rabbit peripheral blood mononuclear cells (PBMCs) were stained for surface expression of CD8 with mouse anti-rabbit CD8 (Spring Valley), washed, and then stained with goat anti-mouse-fluorescein isothiocyanate (FITC) (Jackson Immunoresearch). The cells were then washed, fixed, and permeabilized by use of a Cytofix/Cytoperm kit with Golgistop (BD Biosciences). The cells were then stained for intracellular gamma interferon (IFN-γ) expression with biotin-conjugated mouse anti-bovine IFN-γ (Serotec), washed, and stained with phycoerythrin (PE)-conjugated streptavidin (BD Biosciences). Finally, the cells were washed and resuspended in 2% paraformaldehyde (Merck, Darmstadt, Germany) in phosphate-buffered saline. Live gated events were assessed for anti-CD8-FITC and anti-IFN-γ-PE reactivity on a BD LSR II flow cytometer. Batch fluorescence-activated cell sorting (FACS) analysis was carried out by the use of FlowJo software (Tree Star Inc).
Infection of rabbits with recombinant and wild-type myxoma viruses.
Specific-pathogen-free female New Zealand White rabbits (Oryctolagus cuniculus) were housed in level II biocontainment facilities per Health Canada's Laboratory Safety Guidelines, 2nd ed., and the Canadian Food Inspection Agency guidelines in Containment Standards for Veterinary Facilities. All protocols and procedures were done in accord with the regulations of the Animals for Research Act (Ontario) and the guidelines and policy statements of the Canadian Council on Animal Care. Each rabbit was inoculated intradermally with 1,000 PFU of virus per site, and injections were performed on both hindlimbs. The rabbits were monitored twice daily by research and veterinary staff for symptoms of myxomatosis, including but not limited to the size, number, and quality of primary and secondary lesions, orthopnea, mouth breathing, cyanosis, decreased or absent food/water intake, fecal output, and dehydration (18, 33). Moribund animals were immediately sacrificed by intravenous injection with euthanyl administered following anesthesia. For a comparative pathogenesis study, three rabbits were inoculated with the vMyx141KO virus, three were inoculated with wild-type myxoma virus (strain Lausanne), and four were inoculated with vMyxgfp. Disease progress was followed for 28 days or until euthanasia was necessary. For a histological study, six rabbits each were inoculated with vMyx141KO or vMyxgfp. Two animals per group were sacrificed on days 3 and 7 postinfection. Primary and secondary lesions, spleens, and inguinal and popliteal lymph nodes were harvested, and paired samples of each were either snap-frozen in OCT medium for later cryopreservation or placed in neutral buffered formalin.
Histology and immunohistochemistry.
Immunohistochemistry was performed on both frozen and formalin-fixed tissue sections. Rabbits were sacrificed and selected tissues were fixed in 10% formalin, while paired samples of each tissue were snap-frozen in OCT compound (Tissue Tek; Miles Diagnostics). Formalin-fixed tissues were embedded in paraffin, cut into 5-μm-thick sections, and stained with hematoxylin and eosin. Frozen sections were incubated with anti-rabbit CD11b (for monocytes, macrophages, and neutrophils; Spring Valley Labs) or anti-murine inducible nitric oxide synthase (iNOS) (BD Transduction Laboratories). Slides were then processed with a Vectastain ABC kit according to the manufacturer's instructions (Vector Laboratories). The reaction was then developed with 3,3′-diaminobenzidine tetrahydrochloride and analyzed by light microscopy. For anti-CD25 (BD Biosciences) immunohistochemistry, an anti-mouse IgG conjugated to Alexa Fluor 594 tyramide signal amplification kit was used to visualize the signal (Molecular Probes). Images were taken under a Zeiss confocal microscope with a 40× water immersion lens. Nonfluorescent immunohistochemistry (hematoxylin and eosin and anti-iNOS staining) images were taken under a Nikon OPTIPHOT microscope.
Confocal fluorescence analysis.
For confocal analyses, 105 BGMK cells were grown on 1-mm-wide glass coverslips and allowed to adhere for 4 h. Each of the virus strains (vMyxgfp or vMyx141KO) was then used to infect the cells at an MOI of 1 for 1 h at 37°C, followed by the addition of Dulbecco's modified Eagle medium. At 48 hpi, the cells were washed three times in PBS and fixed in 4% paraformaldehyde for 1 h at room temperature (RT). The fixed cells were washed three times in PBS, blocked by incubation in 4% FBS in PBS for 1 h at RT, washed three times in PBS, and then incubated overnight at 4°C with a 1:100 dilution of anti-M141R antiserum or preimmune serum in 4% FBS in PBS. Cells were washed three times in PBS and then incubated with a 1:500 dilution of the secondary antibody, the Cy5-conjugated mouse anti-rabbit IgG heavy plus light chains (Jackson Immunoresearch), in 4% FBS in PBS for 1 h at RT. The coverslips were then washed three times in PBS and mounted onto slides for confocal microscopic analysis using a Zeiss LSM 510 META NLO microscope with C-Apo 40×/1.2-numerical aperture (NA) and C-Apo 63×/1.2-NA (water immersion) lenses.
Statistical and sequence analysis.
All experiments were performed at least in triplicate. Values are expressed as means ± standard deviations for individual samples. The myxoma virus M141R sequence was previously submitted to GenBank and assigned accession number NC-001132 (11). The M141R sequence was analyzed by the use of MacVector and DSGENE (Accelrys) and with NCBI BLAST (3).
RESULTS
Myxoma virus M141R resembles CD200.
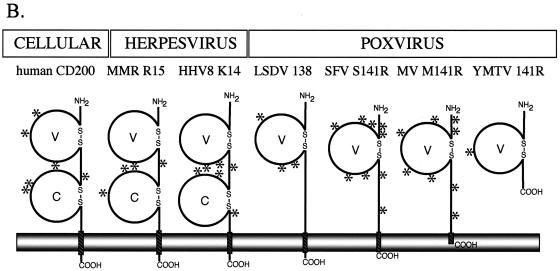
The M141R open reading frame first came to our attention during the sequencing of the myxoma virus genome (11). The putative amino acid sequence suggested that M141R possesses an N-terminal signal sequence and a single C-terminal transmembrane domain. However, even more interesting was the prediction that M141R contains a variable-like Ig domain that appears to be conserved with that of human CD200 (Fig. 1A). An alignment of the M141R sequence with those of human CD200 and a viral homolog (K14) from HHV-8 indicated 20% sequence identity and 40% amino acid similarity (Fig. 1A). Three genera of the poxviruses appear to encode homologs of CD200, including members of the leporipox, yatapox, and capripox viruses. Based on the predicted structure of human CD200, we propose that M141R folds into a similar structure but contains only a single Ig domain (Fig. 1B).
FIG. 1.
(A) ClustalW alignment of human CD200, myxoma virus M141R, and HHV-8 K14. Amino acid identities are shown as black boxes, and similarities are shown as shaded boxes. The bold line above the CD200 sequence denotes the V (variable)-like Ig domain. Arrowheads denote the two conserved cysteines that define the Ig domain boundary; asterisks identify Ig superfamily consensus residues, including those conserved in V-like Ig domains. (B) Predicted structural features of human CD200 and selected putative viral homologues. Macaca mulatta rhadinovirus (MMR) and Human herpesvirus 8 (HHV-8), both belonging to the Gammaherpesvirinae subfamily, are predicted to encode CD200-like proteins. The Chordopoxvirinae subfamily members Lumpy skin disease virus of the genus Capripoxvirus (LSDV), leporipoxviruses Shope fibroma virus (SFV) and Myxoma virus (MV), and yatapoxviruses Yaba-like disease virus (not illustrated) and Yaba-monkey tumor virus (YMTV) encode CD200-like proteins. Predicted variable (V)- and constant (C)-like Ig domains, formed by disulfide bonds (S-S), and transmembrane (hatched rectangles) domains are illustrated, as are the locations of potential N-glycosylation (*) sites. All of the proteins except for YMTV 141R are predicted to be type I membrane glycoproteins.
M141R is expressed as a 21-kDa surface protein.
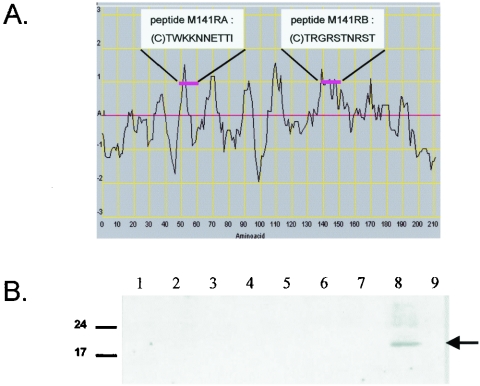
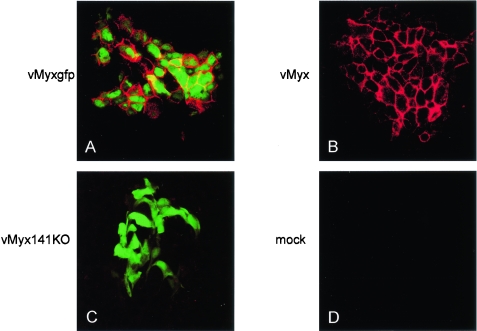
To help characterize M141R's function and cellular localization, we designed two peptides against predicted antigenic regions within M141R and used these peptides to raise polyclonal antibodies in a rabbit (Fig. 2A). Tests of the rabbit serum indicated that antibodies against peptide M141RB were generated. To further study the basic biology of M141R, we collected cells infected with either vMyxgfp or vMyx141KO (described below) at various times postinfection and probed them with anti-M141R. M141R was expressed by 12 h postinfection in vMyxgfp-infected but not vMyx141KO-infected BGMK cells (Fig. 2B). This was later than expected given M141R's predicted early promoter (11) but may have reflected the limits of detection of our antibody, a relatively low level of expression, or a combination of both factors. The predicted signal sequence and transmembrane domain suggest that M141R might be anchored to the cell surface. We were unable to detect any M141R in concentrated supernatants from infected cells, confirming that M141R is not secreted into the extracellular environment (data not shown). The observed molecular mass of the M141R protein in cell lysates fell within the range of 17 to 24 kDa, which is consistent with a predicted cleaved mature peptide of ∼21 kDa. To test whether we could localize M141R to the surfaces of infected cells as predicted by the amino acid sequence, we infected BGMK cells with wild-type myxoma virus, vMyxgfp, or vMyx141KO and stained nonpermeabilized cells with anti-M141R. We were able to detect M141R at the surfaces of nonpermeabilized vMyxgfp- and wild type (vMyx)-infected BGMK cells (Fig. 3A and B). However, no M141R expression was detected at the surfaces of cells infected with vMyx141KO (Fig. 3C), and no nonspecific staining was detected in uninfected cells (Fig. 3D).
FIG. 2.
(A) Peptides tested for production of anti-M141R polyclonal antibodies. The peptides M141RA and M141RB were chosen based on their antigenic indices, locations within the predicted secondary structure of the M141R protein (i.e., not within the cleavable signal sequence or transmembrane domain), and facility of synthesis. A positive anti-M141R serum was obtained from the rabbit injected with peptide M141RB and was subsequently affinity purified. (B) Time course of M141R protein expression. Infected or mock-infected BGMK cells were harvested at 0, 2, 4, and 12 h postinfection (hpi) into SDS loading buffer and analyzed by SDS-PAGE, and then the M141R protein was detected by Western blotting with the anti-M141R serum. Dashes indicate the positions of the molecular mass markers. Lanes: 1, mock infection at 12 hpi; 2, vMyxgfp infection at 0 hpi; 3, vMyx141KO infection at 0 hpi; 4, vMyxgfp infection at 2 hpi; 5, vMyx141KO infection at 2 hpi; 6, vMyxgfp infection at 4 hpi; 7, vMyx141KO infection at 4 hpi; 8, vMyxgfp infection at 12 hpi; 9, vMyx141KO infection at 12 hpi.
FIG. 3.
Confocal immunofluorescence analysis demonstrating the cell surface localization of M141R expressed during myxoma virus infection of BGMK cells. (A) vMyxgfp-infected cells; (B) vMyx (wild-type myxoma virus)-infected cells (does not express gfp); (C) vMyx141KO-infected cells; (expresses gfp); (D) mock-infected cells. All cells were stained with the anti-M141R antibody at a 1/500 dilution (red).
M141R is nonessential in vitro.
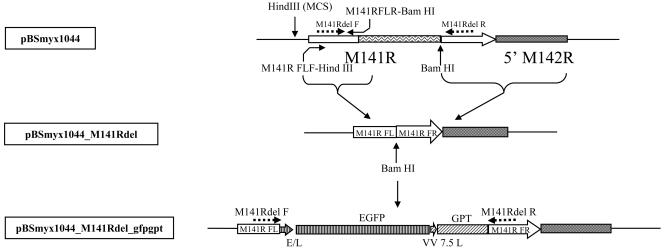
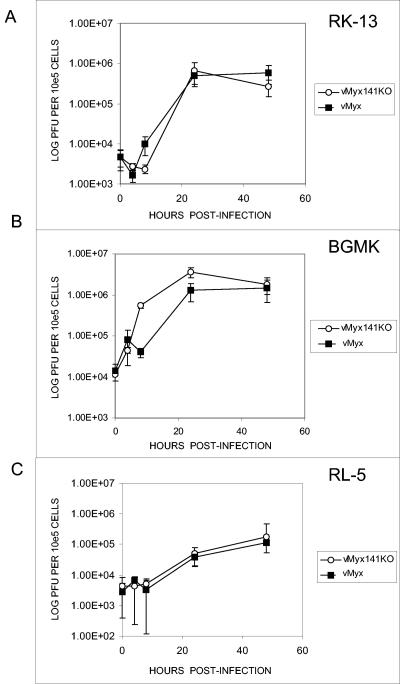
An important tool for studying the role of M141R during virus infections is the production of viruses in which M141R has been disrupted or deleted. To this end, we deleted most of the coding region of M141R and replaced it with an EGFP/GPT cassette (Fig. 4). The knockout virus was prepared and the construct purity was confirmed by PCR amplification of the M141R locus (data not shown). Our hypothesis was that M141R would have an immunomodulatory function during myxoma virus infections of rabbits. To examine if M141R plays a role in the in vitro replication of myxoma virus in susceptible cell lines, we investigated the growth kinetics of the vMyx141KO and wild-type virus strains. The ability of vMyx141KO to grow in vitro was assessed by conventional single-step growth analyses using cultured rabbit RK-13 fibroblasts, the rabbit CD4+ T-lymphocyte cell line RL-5, and primate BGMK cells. No differences in growth or replication kinetics were observed for vMyx141KO compared to the wild-type control virus for the cell lines tested (Fig. 5), confirming that M141R is dispensable for infection and replication in vitro.
FIG. 4.
Construction of M141R deletion mutant virus strain vMyx141KO. The pBSmyx1044 plasmid containing the entire M141R gene was used as a template to amplify the left flanking region (M141RFL) of the M141R gene, using the primers M141RFLF-HindIII and M141RFLR-BamHI. pBSmyx1044 was digested with HindIII and BamHI to excise all but the right flanking region of M141R (FR). The M141RFL PCR product was also digested with HindIII/BamHI and then ligated into digested pBSmyx1044 to form a plasmid consisting of only the flanking regions of M141R, joined by a BamHI site, creating pBSmyx1044_M141Rdel. A cassette with the EGFP and GPT genes under the control of early/late and VV7.5L promoters, respectively, was cloned into the BamHI site, creating the pBSmyx1044_M141Rdel_gfpgpt plasmid. This plasmid was subsequently transfected into vMyx-infected cells to generate an M141R deletion mutant virus expressing EGFP (vMyx141KO) via homologous recombination.
FIG. 5.
Single-step growth analysis of vMyx141KO and vMyx wild-type control myxoma viruses on the RK13 (A), BGMK (B), and RL-5 (C) cell lines. The cell lines were infected with the viruses and harvested at the indicated time points, and the titers of infectious virus present at each time point were determined by subsequent infections of BGMK cells. The same analysis was carried out on all three cell lines with the vMyxgfp virus. All growth analyses were performed in triplicate.
M141R is a virulence factor in infected rabbits.
To examine the virulence properties of M141R, we infected New Zealand White rabbits with a control myxoma virus (vMyxgfp) or vMyx141KO and monitored the progression of myxomatosis in the animals. Table 1 outlines the gross pathological observations made on days 0, 4, 7, and 9 postinfection. All of the vMyx141KO-infected animals made a full recovery following a moderately delayed course of myxomatosis and suffered only comparatively mild symptoms such as a reduced size and severity of primary lesions, restricted spread, and the establishment of secondary lesions (Table 1). To ensure that the reduced virulence of the vMyx141KO strain was strictly due to the deletion of the M141R open reading frame and not to inadvertent mutations introduced elsewhere in the viral genome, we created a revertant virus, termed vMyx141rev, via transfection with a plasmid containing the intact M141R gene and its insertion back into the vMyx141KO virus by homologous recombination. Revertant viruses were selected by a loss of EGFP expression and confirmed for M141R restoration. The fully pathogenic profile of the revertant strain, vMyx141rev, was indistinguishable from that of vMyxgfp, confirming that M141R is a biologically significant virulence factor of myxoma virus.
TABLE 1.
Pathogenicity of vMyx141KO mutant myxoma virus in European rabbits
| dpi | Exptl procedure or symptoms of infection
|
|
|---|---|---|
| Wild-type control virus (vMyxgfp)a | M141R deletion mutant virus (vMyx141KO) | |
| 0 | Inoculation of four rabbits with 1,000 PFU vMyxgfp per site on each hind limb | Inoculation of three rabbits with 1,000 PFU vMyx141KO virus per site on each hind limb |
| 3 | Primary lesions at inoculation sites (1.4 to 2.0 cm) were pink and raised, conjunctivitis on one rabbit | Primary lesions at inoculation sites (0.9 to 1.3 cm), very slightly raised |
| 7 | Primary lesions were raised approximately 1 to 2 cm and were necrotic, with 6 to 14+ satellites (0.7 to 2.5 cm), thickened ears with 2 to 15+ coalescing lesions at base (0.5 to 4.0 cm), multiple lesions on nose (2.2 cm), 1 to 6 eye lesions (0.5 to 1.0 cm), copious exudate from eyes, 1 to 2 genital lesions, anxious behavior | Primary sites begin to cavitate, zero to four satellites appear (0.3 to 0.7 cm), one to three ear lesions (0.7 to 2.7 cm), inflamed eyelids, genital lesion on one rabbit (0.5 cm) |
| 9 | Primary lesions were extremely protuberant and severely necrotic (open wound), copious exudate from primary lesions, eight or completely coalescing thick purple ear lesions, multiple lesions on nose/lips, eyes swollen shut, one or multiple genital lesions (2.1 cm), very edematous head, rough fur, abnormal posture/activity, decreased food and water intake, decreased fecal output, dehydration, abnormal respiration, all rabbits scored as moribund and were euthanized | Well-formed primary lesion scabs zero to three satellites (0.6 to 1.0 cm), zero to two ear lesions (0.9 to 1.0 cm), one eye lesion each on two rabbits (0.4 cm), genital lesion on one rabbit (1.0 cm) |
| 21 | Not applicable | Primary lesions consisting of small, dry scabs, few scars remaining from secondary lesions, all rabbits immune to challenge with 2,000 PFU strain Lausanne per rabbit |
The symptoms listed were indistinguishable from those observed with vMyx141rev-infected rabbits.
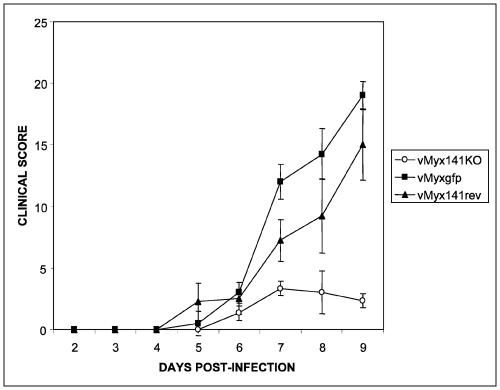
In addition to detailed observations of the clinical symptoms associated with myxomatosis, rabbits were evaluated daily for a defined list of diagnostic characteristics, including food and water intake, fecal output, hydration level, respiration stress, quality of primary lesions, quality and quantity of satellite and secondary lesions on the nose, eyes, ears, and genitals, posture, activity, and attitude (Fig. 6). Each diagnostic characteristic was evaluated numerically, and clinical scores were used to assist the animal care staff in monitoring the progression of myxomatosis. Certain symptoms, such as severe orthopnea, mouth breathing, cyanosis, and lack of food and/or water intake for >24 h, required that the rabbits be euthanized for ethical reasons. The clinical scores for both vMyx141KO- and vMyxgfp-infected rabbits suggested that we cannot differentiate between animals infected with either virus up to day 6 postinjection. After this critical point, the diagnostic scores for the rabbits infected with vMyxgfp rapidly increased while there was only a modest increase in the scores for the vMyx141KO-infected group (Fig. 6). By day 8 postinjection, the vMyx141KO-infected group exhibited a trend toward lower clinical scores and recovery, while the vMyxgfp-infected group's scores continued to rise until euthanasia was required. Recovered rabbits were rechallenged by infection with wild-type myxoma virus but were completely protected from myxomatosis. Based on the results of this study, we conclude that the M141R protein plays a critical role in the pathogenesis of myxoma virus and thereby fits the definition of a classic poxvirus virulence factor.
FIG. 6.
M141RKO pathogenesis study results. Three rabbits were infected with the vMyx141KO virus, three were infected with the vMyx141rev virus, and four were infected with the vMyxgfp control virus. The rabbits were each infected with 2,000 PFU intradermally, with 1,000 PFU injected into each hind leg. Rabbits were monitored daily for the clinical symptoms associated with myxomatosis. The clinical score was derived from well-defined values as described in the text. Specific symptoms such as severe orthopnea, mouth breathing, cyanosis, and no food/water intake for >24 h or a threshold score of 18 required the rabbits to be euthanized for ethical reasons. Although we only included data on the graph to day 9 postinfection, scores for animals infected with vMyx141KO continued to decrease until there was a complete recovery.
M141R does not alter macrophage recruitment and trafficking.
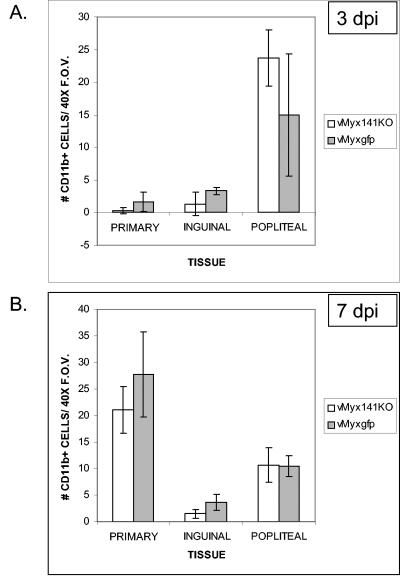
To examine the role of M141R in blocking the immune response during infection, we set out to compare the numbers of myeloid-lineage cells in several locations, including the site of injection and selected lymph nodes, of animals infected with either vMyx141KO or vMyxgfp. Our detection marker for macrophages/monocytes was rabbit CD11b. CD11b is a β2-integrin that is expressed on natural killer cells, monocytes, macrophages, and activated neutrophils. However, the monoclonal antibody used for our experiments specifically recognizes the rabbit CR3 complement receptor (48, 54) and predominantly reacts with rabbit macrophages when used for immunohistochemical applications. On day 3 postinfection, low levels of CD11b+ cells were observed at the primary site of infection and in the inguinal lymph node tissue, while larger numbers were seen in the popliteal lymph nodes regardless of the virus used (Fig. 7). By day 7 postinfection, larger numbers of CD11b+ cells were observed at the primary site of infection, with a reduction in the numbers of positively staining cells in the popliteal lymph nodes. Again, there was no significant difference in the total numbers of CD11b+ cells detected between the vMyx141KO- and vMyxgfp-infected animal groups. Therefore, we concluded that the presence of M141R does not interfere with macrophage recruitment or trafficking.
FIG. 7.
Quantification of CD11b+ cells in tissues from infected rabbits. The graphs show the average numbers of CD11b+ cells per 40× field of view present in the primary lesions and inguinal and popliteal lymph nodes of vMyx141KO- and vMyxgfp-infected rabbits at 3 days (A) and 7 days (B) postinfection (dpi). Tissues were harvested and snap-frozen at 3 and 7 days postinfection and then stained with an anti-CD11b monoclonal antibody as outlined in Materials and Methods. The data shown are average numbers of positively staining cells for five randomly selected 40× fields of view (FOV) per tissue per rabbit, with two rabbits per group (for each virus and day postinfection).
M141R downregulates macrophage activation.
The observation that similar numbers of macrophages migrated to the site of infection and into the draining lymph nodes regardless of the virus type led us to investigate whether M141R might act to regulate macrophage activation rather than recruitment. Primary lesions, spleens, and popliteal and inguinal lymph nodes from vMyx141KO- and vMyxgfp-infected rabbits were harvested at days 3 and 7 postinfection. The tissues were stained with an anti-iNOS monoclonal antibody and the numbers of iNOS-expressing cells present in each of the tissues of vMyx141KO- and vMyxgfp-infected animals were determined.
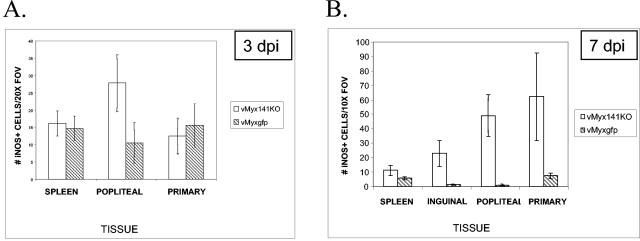
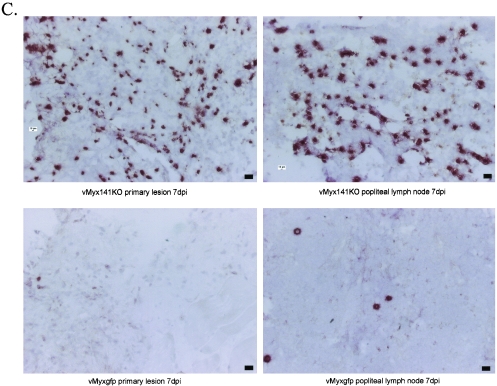
No significant differences in the numbers of iNOS+ cells were seen for the primary lesions or spleens early during infection. However, by day 3 the popliteal lymph nodes of vMyx141KO-infected rabbits displayed almost three times more iNOS+ cells than tissues from vMyxgfp-infected rabbits (Fig. 8A). By day 7, significantly more iNOS+ cells were detected in the primary lesions, spleens, and inguinal and popliteal lymph nodes of vMyx141KO-infected rabbits than in tissues from vMyxgfp-infected rabbits (Fig. 8B). The spleen sections of vMyxgfp-infected animals exhibited an average of 6 iNOS+ cells/field of view (FOV) while those of the vMyx141KO-infected rabbits exhibited nearly twice as many (11 iNOS+ cells/FOV) (Fig. 8B). Even more dramatic were the differences seen for the lymph nodes and the primary sites of infection by day 7 (Fig. 8C). An average of 62 iNOS+ cells/FOV were observed in sections of the primary sites of infection for vMyx141KO-infected rabbits versus an average of 7 iNOS+ cells/FOV at the same location for vMyxgfp-infected rabbits. An average of 49 iNOS+ cells/FOV were detected in the popliteal lymph nodes of vMyx141KO-infected rabbits versus an average of 1 iNOS+ cell/FOV in the lymph nodes of vMyxgfp-infected rabbits (Fig. 8C). We concluded that the loss of M141R from myxoma virus resulted in the activation and recruitment of significantly more iNOS+ cells to critical immune organs and sites of infection.
FIG. 8.
Quantification of iNOS+ cells in tissues from infected rabbits. (A) Numbers of iNOS-expressing cells present in the spleens, popliteal lymph nodes, and primary lesions of vMyx141KO- and vMyxgfp-infected rabbits at 3 days postinfection (dpi). (B) Numbers of iNOS-expressing cells present in the spleens, inguinal lymph nodes, popliteal lymph nodes, and primary lesions of vMyx141KO- and vMyxgfp-infected rabbits at 7 days postinfection. Tissues were harvested and snap-frozen on day 7 postinfection and then were stained with an anti-iNOS monoclonal antibody as outlined in Materials and Methods. The data shown are average numbers of positively staining cells for five randomly selected 10× fields of view (FOV) per tissue per rabbit, with two rabbits per group (for each virus and day postinfection). (C) Primary lesion and popliteal lymph node tissue sections from vMyx141KO- and vMyxgfp-infected rabbits. Tissues were harvested and snap-frozen on day 7 postinfection and then were stained with an anti-iNOS monoclonal antibody as outlined in Materials and Methods. Magnification, ×15. Bar, 10 μm.
M141R reduces T-cell activation levels in lymph nodes.
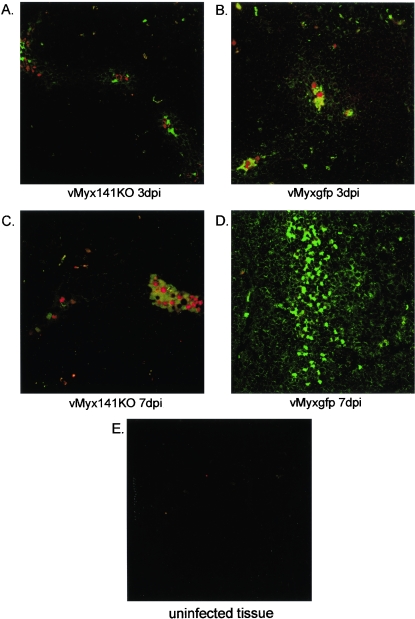
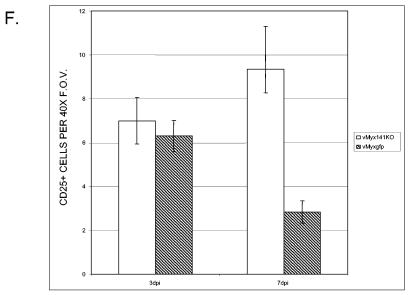
Since there was an apparent difference in the numbers of activated inflammatory cells in the tissues of vMyx141KO- versus vMyxgfp-infected rabbits, we sought to determine whether or not this would have implications on the level of T-cell activation in the regional lymph nodes. To detect T-cell activation, we used an anti-rabbit CD25 monoclonal antibody that recognizes the high-affinity chain of the interleukin-2 (IL-2) receptor. Lymph node tissues were harvested at 3 (Fig. 9A and B) and 7 (Fig. 9C and D) days postinfection and then stained with the anti-rabbit CD25 monoclonal antibody. In order to establish that the activated CD25+ lymphocytes were in fact localized within close proximity to virally infected cells, we employed confocal microscopy to visualize both CD25 (AlexaFluor594, red) expression and virus (EGFP, green) infection (Fig. 9A to E). Lymph node tissue sections from vMyx141KO (Fig. 9A and C)- and vMyxgfp (Fig. 9B and D)-infected and uninfected (Fig. 9E) rabbits revealed that CD25+ cells were indeed consistently and specifically located adjacent to or within the regions of infected tissue. In contrast, areas in which no virus was detected were also generally free of CD25+ T cells (Fig. 9E). CD25+ cells stained in the lymph nodes of vMyx141KO- and vMyxgfp-infected rabbits on 3 and 7 days postinfection were enumerated and plotted as average numbers of positively staining cells per five randomly selected 40× FOV per tissue per rabbit (Fig. 9F). Similar numbers of CD25+ cells were present in the lymph nodes of vMyx141KO- and vMyxgfp-infected rabbits on day 3 postinfection. However, by day 7 postinfection, an increase in CD25+ cell numbers was observed for vMyx141KO-infected lymph nodes, with a concomitant decrease in these cell numbers in vMyxgfp-infected lymph nodes.
FIG. 9.
Quantification of CD25+ cells in tissues from infected rabbits. Lymph node tissue sections from vMyx141KO (A and C)- and vMyxgfp (B and D)-infected rabbits were analyzed for both CD25 (red) expression and virus (green) infection and colocalization. Tissues were harvested and snap-frozen on days 3 (A and B) and 7 (C and D) postinfection and then were stained with an anti-rabbit CD25 monoclonal antibody as outlined in Materials and Methods. (E) Uninfected lymph node section. (F) Numbers of CD25+ cells present in lymph nodes of vMyx141KO- and vMyxgfp-infected rabbits at 3 and 7 days postinfection. The data shown are average numbers of positively staining cells for five randomly selected 40× fields of view (FOV) per tissue per rabbit, with two rabbits per group (for each virus and day postinfection).
M141R inhibits activation of peripheral T cells from myxoma virus-infected rabbits.
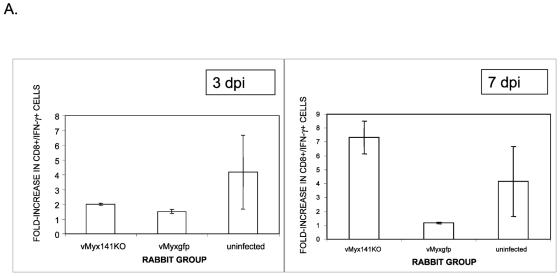
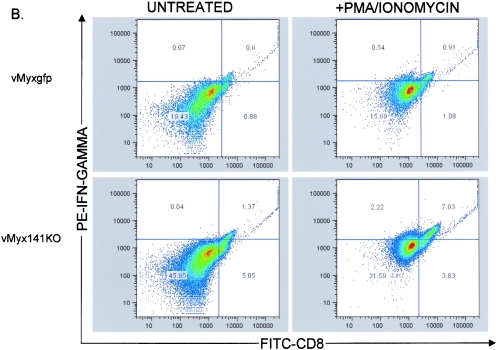
In an effort to determine if M141R could be partially responsible for producing a lymphoproliferation defect in myxoma virus-infected rabbits, we used the potent T-cell-activating PMA/ionomycin combination to stimulate peripheral lymphocytes collected from rabbits infected with either vMyxgfp or vMyx141KO to assess their ability to respond to in vitro stimulation. Groups of three rabbits were either left uninfected or infected with the vMyx141KO or vMyxgfp virus (2,000 PFU per rabbit). At 3 and 7 days postinfection (dpi), the animals were sacrificed, the PBMC fraction was purified, and the nonadherent fraction was resuspended in medium containing Golgistop to allow for the intracellular detection of IFN-γ. Two hours later, the experimental samples were stimulated with PMA plus ionomycin for 4 h. The activated cells were then stained for the surface expression of CD8, washed, permeabilized, and stained for the intracellular expression of IFN-γ. Following stimulation with PMA/ionomycin, there was a larger increase in the percentage of doubly positive lymphocytes from vMyx141KO-infected rabbits than that seen for lymphocytes from vMyxgfp-infected rabbits at 7 dpi compared to 3 dpi (Fig. 10A). Representative CD8 and IFN-γ antibody-staining profiles of purified PBMCs isolated from a single vMyx141KO infection and a single vMyxgfp-infected rabbit were stimulated with both PMA and ionomycin or left untreated and measured by flow cytometry. There was a twofold difference in the numbers of doubly positive (CD8+ IFN-γ+) cells in unstimulated PBMCs between the two infected animals (0.6% for vMyxgfp, 1.37% for vMyx141KO) (Fig. 10B). However, there was a sevenfold difference in the numbers of doubly positive cells between wild type- and vMyx141KO-infected rabbit PBMCs (0.91% for vMyxgfp, 7.03% for vMyx141KO) (Fig. 10B) following stimulation. This experiment was performed in triplicate with consistent results (Fig. 10A). We concluded that the expression of M141R by myxoma virus causes a global suppression of the ability of circulating rabbit T cells to respond to T-cell antigen-independent activation.
FIG. 10.
Activation of CD8+ lymphocytes from infected rabbits. (A) Increases in CD8+ IFN-γ+ cells upon stimulation of PBMCs isolated from rabbits at 3 (left) and 7 (right) days postinfection (dpi). PBMCs isolated from vMyx141KO-, vMyxgfp-, and mock-infected rabbits were cultured for 6 h in the presence of 25 ng/ml PMA plus 1 μM ionomycin and with Brefeldin A (BFA) for the final 4 h of the incubation. PBMCs were stained for the surface expression of CD8, fixed, permeabilized, and then stained for intracellular IFN-γ expression. Live gated events were assessed for anti-CD8-FITC and anti-IFN-γ-PE reactivity on a BD LSR II flow cytometer. Batch FACS analysis was then carried out with FlowJo software. (B) Representative dot plots of live gated PBMCs isolated from rabbits at 7 days postinfection from vMyxgfp- and vMyx141KO-infected rabbits. PBMCs were cultured for 6 h in the presence of 25 ng/ml PMA plus 1 μM ionomycin and with BFA for the final 4 h of incubation. PBMCs were stained for the surface expression of CD8, fixed, permeabilized, and stained for intracellular IFN-γ expression. Quadrant numbers represent percent total events assessed for anti-CD8-FITC and anti-IFN-γ-PE reactivity on a BD LSR II flow cytometer. Batch FACS analysis was then carried out with FlowJo software.
DISCUSSION
This paper constitutes the first characterization of a poxvirus-encoded homolog of CD200 (OX-2). To date, only one other virus-encoded CD200-like protein, the K14 protein of KSHV (HHV-8), has been investigated, but the precise function of the K14 protein has been controversial. The first report of its role by Chung et al. (13) showed that the purified form of K14, as well as its ectopic expression on B cells, induced the production of inflammatory cytokines such as IL-1, IL-6, MCP-1, and tumor necrosis factor (TNF) from myeloid-lineage cells. In contrast, Foster-Cuevas et al. (19) found that the expression of K14 on transfected CHO cells inhibited the production of TNF from macrophages. While our work suggests that the myxoma virus CD200-like protein exerts profound inhibition of macrophage activity in infected animals, our data cannot resolve the conflicting results with respect to the function of K14. Our findings are based on an in vivo model of poxvirus infection and pathogenesis, while the characterization of KSHV K14's function was carried out solely by ectopic gene expression experiments and not in the context of virus infection. In addition, myxoma virus and HHV-8 induce dramatically different pathological profiles. For example, myxomatosis is highly infectious and causes an acute lethal and disseminated infection in immunocompetent Oryctolagus rabbit hosts (17, 18, 33), while KSHV has been shown to be a necessary etiological agent causing a rare disease, Kaposi's sarcoma, which predominantly affects only immunocompromised persons and is rarely the cause of death (14, 15). On the other hand, the more benign nature of infection of myxoma virus in the Sylvilagus rabbit of South America is somewhat more similar to the HHV-8-induced skin lesions of immunocompromised humans. At this point, we cannot conclude whether the two different virus families would capture the same cellular gene and then exploit it for the same anti-immune purpose, but we can say that our data are fully congruent with a role for the M141R vCD200 as an inhibitor of the activation of myeloid cells.
When compared to the cellular CD200 amino acid (aa) sequence, M141R exhibits the most conservation of aa identity and similarity in the V-like Ig domain (Fig. 1). This is important in that it is through the N-terminal immunoglobulin domain that CD200 interacts with its cognate receptor, CD200R (25). Based on the known immunoregulatory function of cellular CD200, the genomic location of M141R, and its absence from many other poxvirus genomes, one would not predict that M141R would be obligatory for replication in vitro. Indeed, deletion of the M141R gene had no effect on the ability of the vMyx141KO virus to replicate in any of the cultured cell lines tested. M141R was predicted to possess a transmembrane domain at the C terminus, and we observed that it localized to the cell membrane in cells infected with the wild-type myxoma virus. This finding is consistent with M141R's putative CD200-like function, as it would require exposure to the extracellular milieu for the localized delivery of restrictive signals to local myeloid-lineage cells. The attenuated pathogenesis of vMyx141KO is also consistent with the prediction that a loss of M141R would result in a loss of the ability to counteract the cellular immune response.
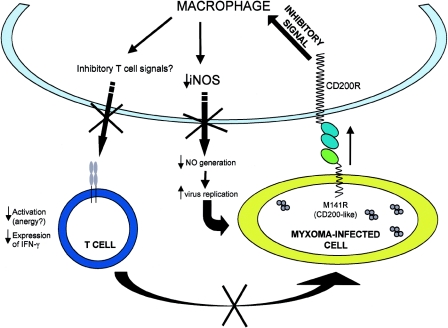
No differences in the overall numbers of infiltrating CD11b+ cells were observed in a comparison of primary site and lymph node tissues of wild type- and vMyx141KO-infected rabbits, but this was consistent with our expectations for the predicted behavior of a bona fide viral CD200. CD11b may not be a sensitive indicator of macrophage activation in the rabbit, and our results indicate that no overall differences in CD11b+ macrophage recruitment levels result from the presence or absence of the M141R protein during myxoma virus infection. Based on the functional characterization of both the cellular and herpesvirus CD200 proteins, we reasoned that a defect in the subsequent activation of myeloid-lineage cells, rather than a reduction in their recruitment, would result from exposure to the M141R protein. We therefore focused on a more explicit and generic indicator of macrophage activation, namely, expression of the inducible form of nitric oxide synthase (iNOS). Using iNOS induction, we observed significant differences in the numbers of activated macrophages with the capacity to generate high levels of the virotoxic compound NO when we compared wild type- and vMyx141KO-infected rabbits. Thus, a robust upregulation of iNOS expression occurred specifically in tissues of rabbits infected with the myxoma virus lacking M141R, in contrast to the M141R-expressing parental virus. This is consistent with our proposed model, with M141R inducing CD200-like inhibitory signals to tissue macrophages, and possibly resident dendritic cells, via CD200R (Fig. 11).
FIG. 11.
Potential interactions between myeloid-lineage cells and myxoma virus-infected cells. Note that the myxoma virus vCD200 (M141R) exerts its inhibitory effects by signaling CD200R on myeloid-lineage cells such as macrophages (or CD200R+ dendritic cells) that encounter myxoma virus-infected cells.
Recently, Banerjee and Dick (4) demonstrated that blocking the CD200-CD200R axis resulted in increased iNOS expression in the retinal neuron as well as an exacerbation of tissue damage in an experimental autoimmune uveoretinitis model in Lewis rats. Significantly, the overall numbers of macrophages were not altered by the CD200-CD200R blockade, but rather, only the numbers of iNOS-expressing macrophages increased. With our myxoma virus-infected rabbit model, we noted that the absence of M141R expression resulted in increased numbers of iNOS-expressing cells at both the site of infection and local lymph nodes compared to parental virus infection, but there were no changes in overall macrophage infiltration.
Nitric oxide has well-documented roles in the regulation of various physiological events, such as nerve impulse transmission, vascular regulation, and apoptosis, in addition to counteracting pathogens (1, 24, 43). The importance of iNOS and NO expression during virus infection in particular has been investigated, and a number of groups have shown that NO has significant antiviral activity in several DNA and RNA virus models. For example, NO has a demonstrated antiviral effect against viral hemorrhagic septicemia virus, as this fish rhabdovirus's replication was abrogated in the presence of NO donors (50, 51). Sanders et al. (44, 45) demonstrated that nitric oxide is a potent dose-dependent inhibitor of rhinovirus-induced proinflammatory cytokine (granulocyte-macrophage colony-stimulating factor) production. This same group has provided additional evidence that human rhinovirus infections enhance the level of NOS2 expression occurring in epithelial cells, suggesting that the induction of NO is an important factor for clearing this viral infection (46).
The antiviral activity of NO has also been demonstrated in poxvirus infections. Karupiah et al. (32) showed that the ability of IFN-γ to limit both ectromelia and vaccinia virus replication corresponds to the production of NO, since macrophages treated with NOS inhibitors allowed a complete recovery of virus replication. The iNOS antiviral activity was evident when (i) iNOS cDNA was transfected into or (ii) NO-generating compounds were added to cells that did not express the enzyme, and the result in each case was a suppression of virus replication. The same group also carried out in vivo experiments by treating mice with an NOS inhibitor, followed by ectromelia virus infection (32). Instead of the normal course of ectromelia infection in mice, the disease in treated mice was classified as fulminant mousepox, significantly changing the outcome of infection and illuminating the significance of NO in determining success in the control of poxvirus infection. There is also evidence that NO can be used therapeutically to alter the course of poxvirus infection in humans. A clinical trial of acidified nitrite (also an NO donor) was carried out to evaluate its efficacy in treating molluscum contagiosum (a skin disease caused by infection with the molluscum contagiosum poxvirus) (39). Perhaps the most compelling evidence for the inhibition of poxvirus virulence by NO was provided by experiments in which mice were infected with a vaccinia virus encoding the iNOS protein, after which the inhibition of virus replication was evident at 24 h postinfection (41). We postulate that one consequence of M141R expression is a decreased ability of myeloid-lineage cells to locally express the inducible form of NOS, thereby strictly limiting the localized generation of virotoxic free radicals (Fig. 11).
The increased numbers of both activated macrophages and activated T cells within the tissues of rabbits infected with the vMyx141KO virus compared to those infected with vMyxgfp suggest that the M141R protein plays a role in dampening the activation of these cells during infection. It was previously reported that a close relative of myxoma virus (termed malignant rabbit fibroma virus) is capable of inhibiting the in vitro proliferative responses of rabbit lymphocytes to nonspecific T lymphocyte mitogens, resulting in a profound defect in the function of the host's immune responses (49). In this study, we sought to determine if the M141R protein could be at least partially responsible for producing a lymphoproliferation defect in myxoma virus-infected rabbits.
The results shown in Fig. 10A demonstrate a larger increase in the percentage of CD8+ IFN-γ+ T lymphocytes isolated from vMyx141KO-infected rabbits following stimulation than that seen for lymphocytes from vMyxgfp-infected rabbits. We concluded that expression of the M141R protein during myxoma virus infection of rabbits results in a generalized deficit in the ability of CD8+ lymphocytes to elaborate an IFN-γ response to nonspecific T-cell activators. This defect was more pronounced at day 7 postinfection than at the earlier 3-dpi time point. We also observed global effects of M141R expression during myxoma virus infection over the entire PBMC population. The defect in the ability of the majority of CD8+ T cells to produce IFN-γ was not necessarily expected given that (i) only a minority of the CD8+ lymphocytes would be predicted to respond to viral antigens and (ii) not all of the cells in this subset would have been directly exposed to the M141R protein at the surfaces of infected cells. Thus, the decreased production of IFN-γ by CD8+ T cells in rabbits infected with the M141R-expressing virus (wild type) was likely the result of either indirect interactions with compromised antigen-presenting cells (macrophages or dendritic cells) or a direct induction of T-cell anergy. Future experiments will hopefully provide clues to the mechanism of this M141R-induced defect in T-cell activation.
These results correlate well with our in vivo finding of reduced numbers of CD25+, activated T cells in the lymph nodes of wild-type myxoma virus-infected rabbits at day 7 postinfection. The priming and activation of virus-specific T cells would presumably take place in the draining lymph nodes of infected rabbits, and if T cells had been rendered less able to respond to activating signals by the presence of the M141R protein, one would predict that smaller numbers of activated T cells would be generated.
In conclusion, we have characterized the M141R protein as a potent immunomodulatory CD200-like virulence factor expressed by myxoma virus. M141R expression clearly provides a selective advantage to myxoma virus which is likely related to the ability of M141R to decrease the activation levels of myeloid-lineage cells. Consequently, this limits the expression of iNOS and thereby alters the local generation of NO as well as the ability to directly or indirectly provide stimulatory signals to CD8+ T lymphocytes. Our finding that M141R expression results in decreased numbers of both activated myeloid and T cells is entirely consistent with our hypothesis that M141R possesses CD200-like inhibitory properties. In addition, our study complements studies of vCD200 from KSHV (K14) (13, 19) showing the in vivo consequences of viral CD200-like protein expression during the course of a virus infection. Further experiments to determine if the M141R protein has other similar characteristics, such as binding to the rabbit CD200R and regulating the expression of TNF by macrophages, would provide additional insight into whether the two virus CD200-like genes are functionally analogous.
.
Acknowledgments
C.M.C. held a CIHR studentship and G.M. holds a Canada Research Chair in Molecular Virology. This work was funded by an operating grant from the CIHR.
We thank D. Kelvin for hosting some of this research and D. Hall for help with manuscript preparation.
REFERENCES
- 1.Aktan, F. 2004. iNOS-mediated nitric oxide production and its regulation. Life Sci. 75:639-653. [DOI] [PubMed] [Google Scholar]
- 2.Alcami, A., and U. H. Koszinowski. 2000. Viral mechanisms of immune evasion. Immunol. Today 21:447-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 4.Banerjee, D., and A. D. Dick. 2004. Blocking CD200-CD200 receptor axis augments NOS-2 expression and aggravates experimental autoimmune uveoretinitis in Lewis rats. Ocul. Immunol. Inflamm. 12:115-125. [DOI] [PubMed] [Google Scholar]
- 5.Barclay, A. N. 1981. Different reticular elements in rat lymphoid tissue identified by localization of Ia, Thy-1 and MRC OX 2 antigens. Immunology 44:727-736. [PMC free article] [PubMed] [Google Scholar]
- 6.Barclay, A. N., M. J. Clark, and G. W. McCaughan. 1986. Neuronal/lymphoid membrane glycoprotein MRC OX-2 is a member of the immunoglobulin superfamily with a light-chain-like structure. Biochem. Soc. Symp. 51:149-157. [PubMed] [Google Scholar]
- 7.Barclay, A. N., G. J. Wright, G. Brooke, and M. H. Brown. 2002. CD200 and membrane protein interactions in the control of myeloid cells. Trends Immunol. 23:285-290. [DOI] [PubMed] [Google Scholar]
- 8.Barrett, J., J.-X. Cao, S. Hota-Mitchell, and G. McFadden. 2001. Immunomodulatory proteins of myxoma virus. Semin. Immunol. 13:73-84. [DOI] [PubMed] [Google Scholar]
- 9.Borriello, F., J. Lederer, S. Scott, and A. H. Sharpe. 1997. MRC OX-2 defines a novel T-cell costimulatory pathway. J. Immunol. 158:4548-4554. [PubMed] [Google Scholar]
- 10.Bukovsky, A., J. Presl, J. Zidovsky, and P. Mancal. 1983. The localization of Thy-1.1, MRC OX 2 and Ia antigens in the rat ovary and fallopian tube. Immunology 48:587-596. [PMC free article] [PubMed] [Google Scholar]
- 11.Cameron, C., S. Hota-Mitchell, L. Chen, J. Barrett, J.-X. Cao, C. Macaulay, D. Willer, D. Evans, and G. McFadden. 1999. The complete DNA sequence of myxoma virus. Virology 264:298-318. [DOI] [PubMed] [Google Scholar]
- 12.Chakrabarti, S., J. R. Sisler, and B. Moss. 1997. Compact, synthetic, vaccinia virus early/late promoter for protein expression. BioTechniques 23:1094-1097. [DOI] [PubMed] [Google Scholar]
- 13.Chung, Y. H., R. E. Means, J. K. Choi, B. S. Lee, and J. U. Jung. 2002. Kaposi's sarcoma-associated herpesvirus OX2 glycoprotein activates myeloid-lineage cells to induce inflammatory cytokine production. J. Virol. 76:4688-4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Paoli, P. 2004. Human herpesvirus 8: an update. Microbes Infect. 6:328-335. [DOI] [PubMed] [Google Scholar]
- 15.Dourmishev, L. A., A. L. Dourmishev, D. Palmeri, R. A. Schwarts, and D. M. Lukac. 2003. Molecular genetics of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) epidemiology and pathogenesis. Microbiol. Mol. Biol. Rev. 67:175-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fallarino, F., C. Asselin-Paturel, C. Vacca, R. Bianchi, S. Gizzi, M. C. Fioretti, G. Trinchieri, U. Grohmann, and P. Puccetti. 2004. Murine plasmacytoid dendritic cells initiate the immunosuppressive pathway of tryptophan catabolism in response to CD200 receptor engagement. J. Immunol. 173:3748-3754. [DOI] [PubMed] [Google Scholar]
- 17.Fenner, F. 1979. Portraits of viruses: the poxviruses. Intervirology 11:137-157. [DOI] [PubMed] [Google Scholar]
- 18.Fenner, F., and F. N. Ratcliffe. 1965. Myxomatosis. Cambridge University Press, Cambridge, United Kingdom.
- 19.Foster-Cuevas, M., G. J. Wright, M. J. Puklavec, M. H. Brown, and A. N. Barclay. 2004. Human herpesvirus 8 K14 protein mimics CD200 in down-regulating macrophage activation through CD200 receptor. J. Virol. 78:7667-7676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorczynski, L., Z. Chen, J. Hu, Y. Kai, J. Lei, V. Ramakrishna, and R. M. Gorczynski. 1999. Evidence that an OX-2-positive cell can inhibit the stimulation of type 1 cytokine production by bone marrow-derived B7-1 (and B7-2)-positive dendritic cells. J. Immunol. 162:774-781. [PubMed] [Google Scholar]
- 21.Gorczynski, R., J. Bransom, M. Cattral, X. Huang, J. Lei, W. Min, Y. Wan, and J. Gauldie. 2001. Dendritic cells expressing TGFbeta/IL-10, and CHO cells with OX-2, increase graft survival. Transplant. Proc. 33:1565-1566. [DOI] [PubMed] [Google Scholar]
- 22.Gorczynski, R. M., M. S. Cattral, Z. Chen, J. Hu, J. Lei, W. P. Min, G. Yu, and J. Ni. 1999. An immunoadhesin incorporating the molecule OX-2 is a potent immunosuppressant that prolongs allo- and xenograft survival. J. Immunol. 163:1654-1660. [PubMed] [Google Scholar]
- 23.Gorczynski, R. M., Z. Chen, X. M. Fu, and H. Zeng. 1998. Increased expression of the novel molecule OX-2 is involved in prolongation of murine renal allograft survival. Transplantation 65:1106-1114. [DOI] [PubMed] [Google Scholar]
- 24.Guzik, T. J., R. Korbut, and T. Adamek-Guzik. 2003. Nitric oxide and superoxide in inflammation and immune regulation. J. Physiol. Pharmacol. 54:469-487. [PubMed] [Google Scholar]
- 25.Hatherley, D., and A. N. Barclay. 2004. The CD200 and CD200 receptor cell surface proteins interact through their N-terminal immunoglobulin-like domains. Eur. J. Immunol. 34:1688-1694. [DOI] [PubMed] [Google Scholar]
- 26.Hedrick, S. M. 2004. The acquired immune system: a vantage from beneath. Immunity 21:607-615. [DOI] [PubMed] [Google Scholar]
- 27.Hilleman, M. R. 2004. Strategies and mechanisms for host and pathogen survival in acute and persistent viral infections. Proc. Natl. Acad. Sci. USA 101:14560-14566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoek, R. M., S. R. Ruuls, C. A. Murphy, G. J. Wright, R. Goddard, S. M. Zurawski, B. Blom, M. E. Homola, W. J. Streit, M. H. Brown, A. N. Barclay, and J. D. Sedgwick. 2000. Down-regulation of the macrophage lineage through interaction with OX2 (CD200). Science 290:1768-1771. [DOI] [PubMed] [Google Scholar]
- 29.Johnston, J. B., J. W. Barrett, W. Chang, C. S. Chung, W. Zeng, J. Masters, M. Mann, F. Wang, J. Cao, and G. McFadden. 2003. Role of the serine-threonine kinase PAK-1 in myxoma virus replication. J. Virol. 77:5877-5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnston, J. B., and G. McFadden. 2003. Poxvirus immunomodulatory strategies: current perspectives. J. Virol. 77:6093-6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnston, J. B., and G. McFadden. 2004. Technical knockout: understanding poxvirus pathogenesis by selectively deleting viral immunomodulatory genes. Cell. Microbiol. 9:695-705. [DOI] [PubMed] [Google Scholar]
- 32.Karupiah, G., Q. W. Xie, R. M. Buller, C. Nathan, C. Duarte, and J. D. MacMicking. 1993. Inhibition of viral replication by interferon-gamma-induced nitric oxide synthase. Science 261:1445-1448. [DOI] [PubMed] [Google Scholar]
- 33.Kerr, P., and G. McFadden. 2002. Immune responses to myxoma virus. Viral Immunol. 15:229-246. [DOI] [PubMed] [Google Scholar]
- 34.Moss, B. 2001. Poxviridae: the viruses and their replication, p. 2849-2883. In D. M. Knipe et al. (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 35.Mossman, K., C. Upton, and G. McFadden. 1995. The myxoma virus-soluble interferon-γ receptor homolog, M-T7, inhibits interferon-γ in a species-specific manner. J. Biol. Chem. 270:3031-3038. [DOI] [PubMed] [Google Scholar]
- 36.Nathan, C., and W. A. Muller. 2001. Putting the brakes on innate immunity: a regulatory role of CD200. Nat. Immunol. 2:17-19. [DOI] [PubMed] [Google Scholar]
- 37.Nicholas, J. 2003. Human herpesvirus-8-encoded signalling ligands and receptors. J. Biomed. Sci. 10:475-489. [DOI] [PubMed] [Google Scholar]
- 38.Opgenorth, A., K. Graham, N. Nation, D. Strayer, and G. McFadden. 1992. Deletion analysis of two tandemly arranged virulence genes in myxoma virus, M11L and myxoma growth factor. J. Virol. 66:4720-4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ormerod, A. D., M. I. White, S. A. Shah, and N. Benjamin. 1999. Molluscum contagiosum effectively treated with a topical acidified nitrite, nitric oxide liberating cream. Br. J. Dermatol. 141:1051-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rappuoli, R. 2004. From Pasteur to genomics: progress and challenges in infectious diseases. Nat. Med. 10:1177-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rolph, M. S., W. B. Cowden, C. J. Medveczky, and I. A. Ramshaw. 1996. A recombinant vaccinia virus encoding inducible nitric oxide synthase is attenuated in vivo. J. Virol. 70:7678-7685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosenblum, M. D., E. B. Olasz, K. B. Yancey, J. E. Woodiff, Z. Lazarova, K. A. Gerber, and R. L. Truitt. 2004. Expression of CD200 on epithelial cells of the murine hair follicle: a role in tissue-specific immune tolerance? J. Investig. Dermatol. 123:880-887. [DOI] [PubMed] [Google Scholar]
- 43.Sanders, S. P. 1999. Asthma, viruses, and nitric oxide. Proc. Soc. Exp. Biol. Med. 220:123-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanders, S. P., J. Kim, K. R. Connolly, J. D. Porter, E. S. Siekierski, and D. Proud. 2001. Nitric oxide inhibits rhinovirus-induced granulocyte macrophage colony-stimulating factor production in bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 24:317-325. [DOI] [PubMed] [Google Scholar]
- 45.Sanders, S. P., E. S. Siekierski, J. D. Porter, S. M. Richards, and D. Proud. 1998. Nitric oxide inhibits rhinovirus-induced cytokine production and viral replication in a human respiratory epithelial cell line. J. Virol. 72:934-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanders, S. P., E. S. Siekierski, S. M. Richards, J. D. Porter, F. Imani, and D. Proud. 2001. Rhinovirus infection induces expression of type 2 nitric oxide synthase in human respiratory epithelial cells in vitro and in vivo. J. Allergy Clin. Immunol. 107:235-243. [DOI] [PubMed] [Google Scholar]
- 47.Seet, B. T., J. B. Johnston, C. R. Brunetti, J. W. Barrett, H. Everett, C. Cameron, J. Sypula, S. H. Nazarian, A. Lucas, and G. McFadden. 2003. Poxviruses and immune evasion. Annu. Rev. Immunol. 21:377-423. [DOI] [PubMed] [Google Scholar]
- 48.Smet, E. G., W. De Smet, L. Brys, and P. C. De Baetselier. 1986. Mab. 198: a monoclonal antibody recognizing the complement type 3 receptor (CR3) in the rabbit. Immunology 59:419. [PMC free article] [PubMed] [Google Scholar]
- 49.Strayer, D. S. 1989. Poxviruses, p. 173-192. In S. Specter, M. Bendinelli, and H. Friedman (ed.), Virus-induced immunosuppression. Plenum Press, New York, N.Y.
- 50.Tafalla, C., A. Figueras, and B. Novoa. 1999. Role of nitric oxide on the replication of viral haemorrhagic septicemia virus (VHSV), a fish rhabdovirus. Vet. Immunol. Immunopathol. 72:249-256. [DOI] [PubMed] [Google Scholar]
- 51.Tafalla, C. A., A. Figueras, and B. Novoa. 2001. Viral hemorrhagic septicemia virus alters turbot Scophthalmus maximus macrophage nitric oxide production. Dis. Aquat. Organ. 47:101-107. [DOI] [PubMed] [Google Scholar]
- 52.Webb, M., and A. N. Barclay. 1984. Localisation of the MRC OX-2 glycoprotein on the surfaces of neurones. J. Neurochem. 43:1061-1067. [DOI] [PubMed] [Google Scholar]
- 53.Welsh, R. M., L. K. Selin, and E. Szomolanyi-Tsuda. 2004. Immunological memory to viral infections. Annu. Rev. Immunol. 22:711-743. [DOI] [PubMed] [Google Scholar]
- 54.Wilkinson, J. M., G. McDonald, S. Smith, J. Galea-Lauri, J. Lewthwaite, B. Henderson, and P. A. Revell. 1993. Immunohistochemical identification of leucocyte populations in normal tissue and inflamed synovium of the rabbit. J. Pathol. 170:315-320. [DOI] [PubMed] [Google Scholar]
- 55.Wright, G. J., H. Cherwinski, M. Foster-Cuevas, G. Brooke, M. J. Puklavec, M. Bigler, Y. Song, M. Jenmalm, D. Gorman, T. McClanahan, M. R. Liu, M. H. Brown, J. D. Sedgwick, J. H. Phillips, and A. N. Barclay. 2003. Characterization of the CD200 receptor family in mice and humans and their interactions with CD200. J. Immunol. 171:3034-3046. [DOI] [PubMed] [Google Scholar]
- 56.Wright, G. J., M. J. Puklavec, A. C. Willis, R. M. Hoek, J. D. Sedgwick, M. H. Brown, and A. N. Barclay. 2000. Lymphoid/neuronal cell surface OX2 glycoprotein recognizes a novel receptor on macrophages implicated in the control of their function. Immunity 13:233-242. [DOI] [PubMed] [Google Scholar]
- 57.Zhang, S., H. Cherwinski, J. D. Sedgwick, and J. H. Philips. 2004. Molecular mechanisms of CD200 inhibition of mast cell activation. J. Immunol. 173:6786-6793. [DOI] [PubMed] [Google Scholar]