Abstract
Mass extinctions occur frequently in natural history. While studies of animals that became extinct can be informative, it is the survivors that provide clues for mechanisms of adaptation when conditions are adverse. Here, we describe a survival pathway used by many species as a means for providing adequate fuel and water, while also providing protection from a decrease in oxygen availability. Fructose, whether supplied in the diet (primarily fruits and honey), or endogenously (via activation of the polyol pathway), preferentially shifts the organism towards the storing of fuel (fat, glycogen) that can be used to provide energy and water at a later date. Fructose causes sodium retention and raises blood pressure and likely helped survival in the setting of dehydration or salt deprivation. By shifting energy production from the mitochondria to glycolysis, fructose reduced oxygen demands to aid survival in situations where oxygen availability is low. The actions of fructose are driven in part by vasopressin and the generation of uric acid. Twice in history, mutations occurred during periods of mass extinction that enhanced the activity of fructose to generate fat, with the first being a mutation in vitamin C metabolism during the Cretaceous–Paleogene extinction (65 million years ago) and the second being a mutation in uricase that occurred during the Middle Miocene disruption (12–14 million years ago). Today, the excessive intake of fructose due to the availability of refined sugar and high-fructose corn syrup is driving ‘burden of life style’ diseases, including obesity, diabetes and high blood pressure.
Keywords: fructose, metabolic syndrome, metabolic water, uric acid, vasopressin
Introduction
During the last 450 million years, there have been at least five mass extinctions that have occurred due to a variety of causes, including changes in atmosphere gases, changing global temperatures, volcanic activity and an asteroid impact [1]. While often the focus is on those species that failed to survive, in many respects it is the survivors that deserve the most attention, for many of these animals have developed remarkable means of survival. Today, there are many examples of ‘extremophile’ species that can survive under remarkable situations, such as the Pompeii worm that can survive inferno (176°F) temperatures [2], or the occellated icefish that lives in the Antarctic seas in the absence of red blood cells [3], or the wood frog in northern Canada who freezes in winter, surviving because of the production of glycerol that acts as an antifreeze to allow slow circulation of blood in the freezing conditions [4].
One of the most important means for survival is to have sufficient food and water, as well as the necessary minerals, electrolytes and nutrients to maintain muscle mass and body functions. It is also important to be able to adapt in conditions where oxygen levels may decrease. One means for doing this is to store caches of food in one’s den, but there is always the danger that the cache could be stolen, or that the den itself may become unsafe if discovered by predators. Thus, the ideal means for assuring survival is for the body itself to aid in the storage of food, water and other critical needs.
There appears to be a common mechanism by which many animals survive, and that it involves a unique metabolic pathway mediated by fructose, a simple sugar present in fruit [5]. Fructose is also produced in the body under conditions of stress. In turn, the metabolism of fructose uniquely activates processes that stimulate survival, and it works through specific hormones (such as vasopressin) as well as metabolic products (uric acid) to mediate its effects. Here, we provide a brief description of this central pathway that appears to have a key role in the evolution of species (Fig. 1).
Fig. 1.
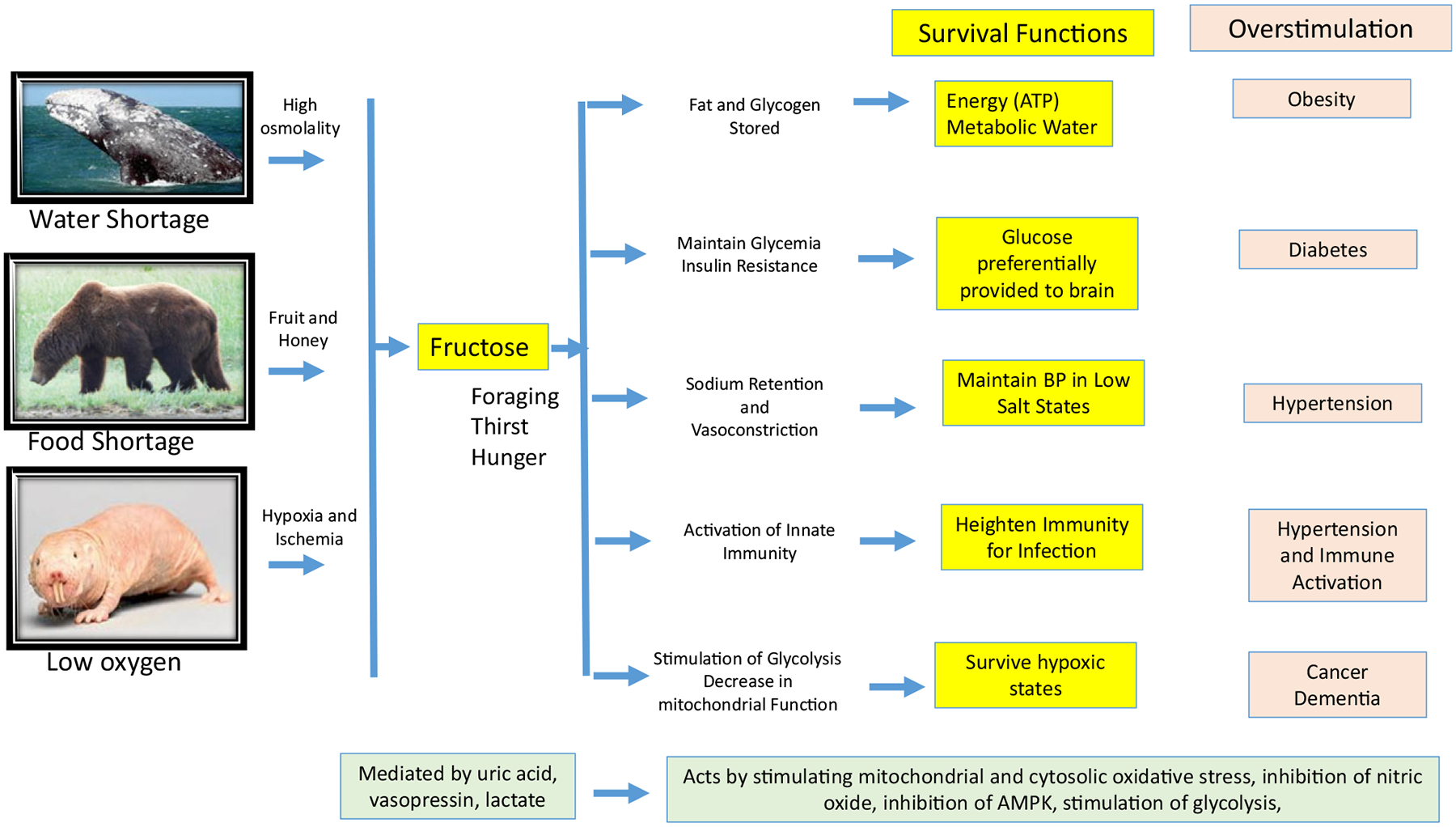
A Common Survival Pathway. The simple sugar, fructose, likely had a major role in evolution due to its unique metabolism that activates processes that prepares the animal for pending shortage in food, water or oxygen. Specifically, fructose shifts the energy provided in nutrients towards fuel storage (fat, glycogen) and away from energy (ATP) production by downregulating mitochondrial metabolism and the favouring of glycolysis. The fat and glycogen provide a source for energy and metabolic water when food and water are scarce. The switch towards glycolysis is associated with a reduction in energy demand and results in protection from hypoxic and ischaemic states. Gluconeogenesis and insulin resistance occur to raise serum glucose levels to provide fuel to the brain. Sodium is retained, and vasoconstrictors are stimulated to increase blood pressure, and innate immunity is also stimulated. Foraging develops, and thirst and hunger occur as a mechanism to increase weight, largely from the development of leptin resistance and hyperosmolality-driven thirst. These processes are mediated by endproducts of fructose metabolism that include vasopressin, lactate and uric acid. While providing a key survival advantage in conditions of scarce resources, excessive fructose can stimulate metabolic diseases, dementia and cancer.
Fructose as a survival factor
Fructose is unique from all other nutrients as its metabolism results in an intracellular alarm signal that triggers the organism to go into a ‘safety mode’ [5]. Specifically, while fructose can be metabolized by hexokinase, the enzyme fructokinase C (also known as ketohexokinase, or KHK) is the primary enzyme that metabolizes fructose, generating fructose-1-phosphate so rapidly that ATP and intracellular phosphate levels fall (Fig. 2). The effect is dependent on the concentration of fructose and can result in significant reductions (20–60%) of intracellular ATP as well as GTP [6] in the organs where fructokinase C is expressed, which includes the liver, kidney, brain, pancreatic islets and adipose tissues [7, 8]. Fructokinase C can also be induced in tissues, such as the ischaemic heart [9]. The loss of intracellular phosphate activates the enzyme AMP deaminase, and this accelerates the production of inosine monophosphate (IMP) and uric acid [6]. The effect is further amplified by the inhibition by IMP of aldolase B, whose role is to metabolize the fructose-1-phosphate to eventually release the sequestered phosphate. The metabolism of fructose also drives production of vasopressin, in the supraoptic nucleus of the hypothalamus [10], and circulating levels of vasopressin, noted by the stable metabolite, copeptin, are also regulated in part by fructose [11, 12].
Fig. 2.
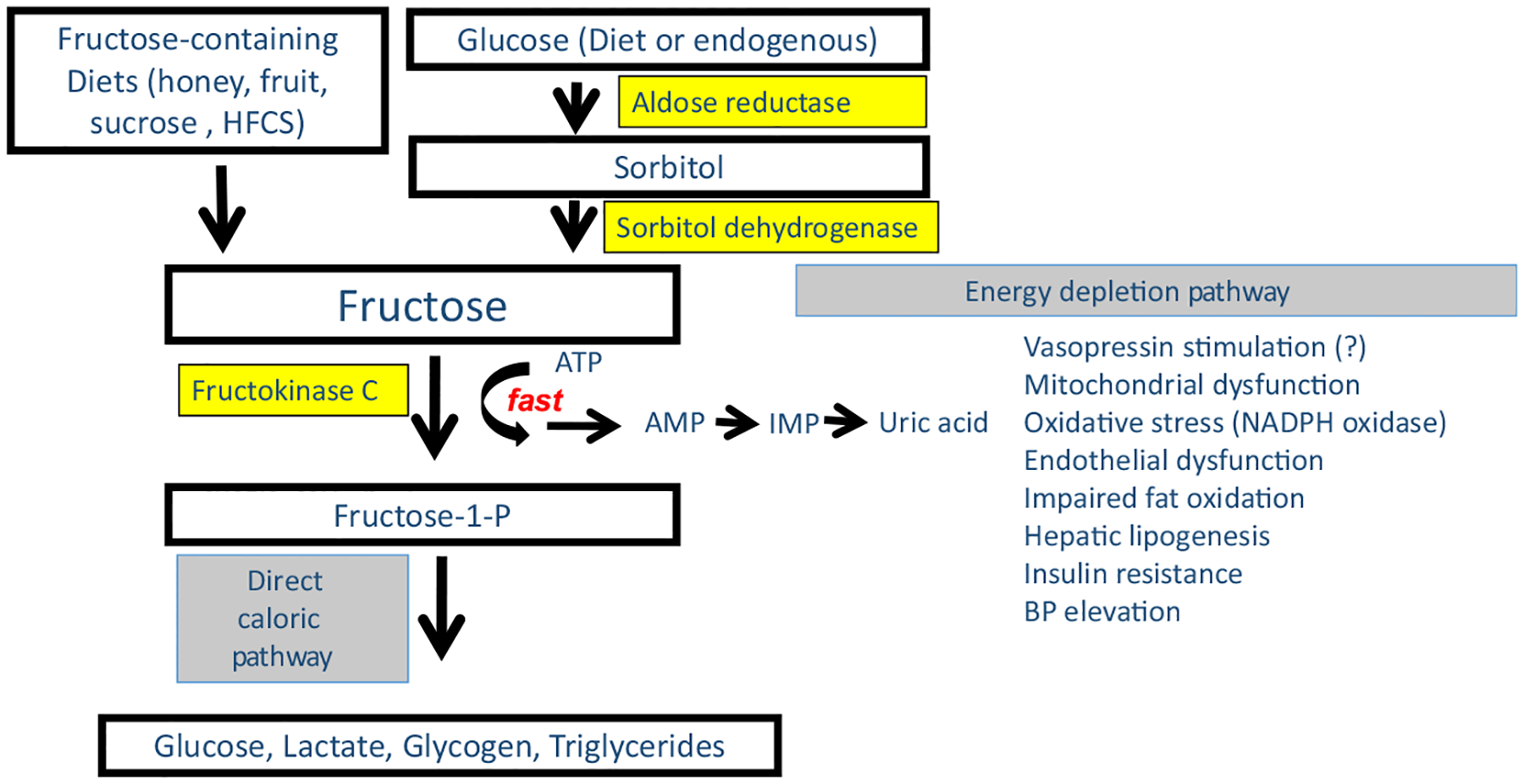
Biochemical Basis of Fructose Metabolism. Fructose can be obtained either from the diet or can be produced from glucose endogenously via the aldose reductase-sorbitol dehydrogenase (polyol) pathway. The unique feature of fructose metabolism is that it reduces the energy (ATP) and intracellular phosphate in the cell during its metabolism that sets off an ‘alarm’ signal that triggers the survival pathway. Specifically, fructose is metabolized by fructokinase C (KHK-C) in various tissues including the intestine, liver, kidney, islets, adipose tissue and brain, where it causes a rapid phosphorylation of fructose to fructose-1-phosphate. Fructose-1-phosphate is then metabolized further by aldolase B and other enzymes to lead to the production of glucose, lactate, glycogen and triglycerides. However, the initial phosphorylation of fructose by KHK-C results in a fall in intracellular phosphate that activates AMP deaminase-2 that triggers the degradation of AMP to IMP and eventually uric acid. Vasopressin is also stimulated by fructose metabolism, although it is not known if it is mediated by the energy depletion pathway. These processes lead to mitochondrial dysfunction and a shift from energy production to energy storage.
While fructose is found in the diet, which in the wild is principally from fruits and honey, another source of fructose is from production via the polyol pathway. Aldose reductase converts glucose to sorbitol which is then metabolized to fructose by sorbitol dehydrogenase (Fig. 2). In turn, aldose reductase can be stimulated by high glucose levels (such as in diabetes), by high-salt diets (which increases osmolality, a known stimulant of aldose reductase), heat, tissue hypoxia, oxidative stress and by fructose and uric acid [13–17]. In western societies, the main sources of fructose are from table sugar (sucrose) and the sweetener, high-fructose corn syrup (HFCS). Table 1 summarizes the main sources of exogenous and endogenous fructose.
Table 1.
Sources of dietary and endogenous fructose
| Dietary sources of fructose |
|---|
| Ripe fruit |
| Honey |
| Refined sugar (sucrose) (Sugarcane, Sugar Beets) |
| High-fructose corn syrup |
| Palm Sugar, Maple Sugar |
| Nectar |
| Endogenous sources (Aldose Reductase Dependent) |
| High-glycemic carbohydrates |
| Hyperglycaemia (Diabetes) |
| Hyperuricemia (or increases at the organ level) |
| Heat Stress |
| Oxidative stress |
| Hyperosmolality (from dehydration or high-salt diet) |
| Hypoxia |
| Ischaemia |
Fructose stimulates fat and glycogen storage
Fructose can increase weight and fat stores by several mechanisms [18]. First, ingesting fructose generates a poor satiety response as it does not directly stimulate insulin or leptin [19]. However, of greater significance is the observation that fructose metabolism drives the development of central leptin resistance, resulting in persistent hunger (and/or impaired satiety) which results in exaggerated food intake [20]. The mechanisms are not fully understood, but the generation of uric acid and the development of intracellular oxidative stress may play a role [21].
In addition, fructose drives the production of fatty liver, which is mediated both by an increase in hepatic lipogenesis and a reduction in fatty acid oxidation [22]. These effects result from intracellular and mitochondrial oxidative stress driven by NADPH oxidase, leading to a decrease in aconitase-2 and a rise in citrate that stimulates the lipogenesis pathway [23], and a reduction in enoyl CoA hydratase (a component of the fatty acid beta-oxidation pathway) [24]. Further, fructose metabolism leads to the accumulation of malonyl CoA and decreases the uptake of fatty acids into mitochondria by decreasing the activity of carnitine palmitoyl transferase 1a activity [22].
Fructose also stimulates gluconeogenesis and glycogen accumulation. Much of the fructose is metabolized to glucose and lactate, the latter which is also a gluconeogenic substrate. While fructose increases glucose production, much of the glucose is taken up by the liver via stimulation of glucokinase to make glucose-6-phosphate [25, 26] and then used to produce glycogen [27, 28].
The increase in fat and glycogen driven by fructose metabolism is much greater than that observed with glucose alone and is consistent with a relative reduction in mitochondrial energy (ATP) production with preferential storage of the energy as a fuel [22, 24]. As such, fructose metabolism is a primary nutrient used by many animals to gain fat mass. This has been shown to occur in long-distance migrating birds before their flights [29], in bears and other mammals preparing for hibernation [30], and also in certain types of fruit-eating fish such as the Pacu [31].
Fat and glycogen as a source of metabolic water
While fat and glycogen are used as fuel (ATP) sources during food shortage, they are also a major source of water in settings where water is less available, as fat generates about 1.1 g of water for every g of fat oxidized, while for glycogen the ratio is about 3–4 g of water per g of glycogen oxidation [32, 33]. Being able to produce water from stored fat and glycogen is critical for any animal that has a lack of water availability, but especially for mammals living in the desert and salt water oceans and for animals that do not drink during prolonged periods of hibernation or long-distance migration. Indeed, desert mammals, such as the jerboa, sand rat and camel, have some of the highest fat content among all mammals, only to be surpassed by hibernating animals such as the grizzly bear and marine mammals such as the whale. The high-fat content in these species provides a key source for water, especially for the marine mammals that cannot drink sea water (Fig. 2) [34]. While the foods these animals ingest provide some water, some studies suggest that 20–40 per cent of water in desert and ocean mammals may derive from fat [34, 35].
The mechanism for the fat accumulation in these mammals likely involves fructose. First, we have found that heat stress, dehydration or high-salt diets all induce hyperosmolality which activates the enzyme aldose reductase leading to the generation of fructose in tissues such as the kidney, liver and brain [10, 36–38]. These same processes of heat stress and dehydration are likely to be present in desert animals while dehydration and hyperosmolality are likely common in marine mammals in the hypertonic sea environment. The endogenously produced fructose then stimulates fat production which would provide a source of water.
As mentioned earlier, both dietary and endogenous fructose stimulate vasopressin production. For example, dehydration-induced hyperosmolality induces fructose generation in the hypothalamus where it mediates the release of vasopressin [10]. In turn, our group has found that vasopressin has a major role in driving obesity and fatty liver in fructose fed animals (unpublished data). Since desert mammals and marine mammals have some of the highest vasopressin levels known, it is very likely that the obesity in these animals is mediated by endogenous fructose generation and vasopressin.
Thus, one of the primary functions of fructose is to conserve water by stimulating vasopressin which reduces water loss via the kidney while also stimulating fat and glycogen production as a source of metabolic water [5]. Furthermore, there is some evidence that vasopressin may reduce nonsensible water losses by acting on vasopressin-2 (V2) receptors in the lung to reduce water loss through the lungs [39, 40] and through its temperature lowering (antipyretic) effects (mediated by the V1b vasopressin receptor) [41], and in frogs the ancestral vasopressin (vasotocin) may reduce water losses through the skin [42]. Thus, fructose may coordinate, through its action on vasopressin, a host of responses to help conserve water [5].
Interestingly, fructose ingestion may also increase thirst, which would act as another mechanism to stimulate a further increase in water content. When fructose is ingested, serum vasopressin is stimulated that will reduce urinary water losses by stimulating urinary concentration [36]. However, while water is retained, serum osmolality does not fall, but remains elevated (Fig. 3). The reason is that the water shifts from the extracellular to intracellular space, likely due to the rapid generation of glycogen. Since serum osmolality remains elevated, thirst continues to stimulate water intake.
Fig. 3.
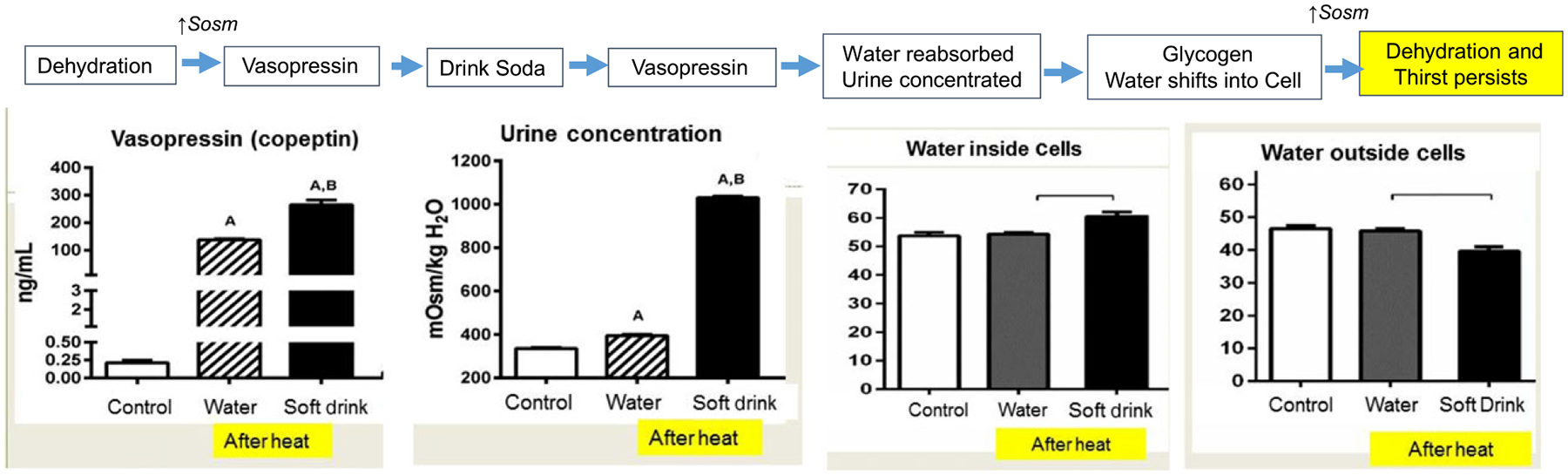
Fructose Stimulates Thirst by Shifting Water into the Cell. The administration of fructose (such as in a soft drink) following heat stress is associated with remarkable stimulation of vasopressin (noted by serum copeptin) compared to intake of water. The increased vasopressin is associated with an amplification in urinary concentration compared to water hydration. However, serum osmolality fails to decrease because the water retained through the action of vasopressin shifts into the cell, resulting in a persistently contracted extracellular volume with increased intracellular volume (measured by bioimpedance). This is likely because glycogen is being rapidly made, and glycogen incorporates water into its lattice structure [33, 95]. The original study excluding the bioimpedance data is from Ref [36].
Fructose induced insulin resistance as a mechanism for providing fuel to the brain
Fructose induces insulin resistance in animals [43], and the mechanism is due in part to enhanced gluconeogenesis mediated by fructokinase-dependent activation of the AMP deaminase pathway with the generation of uric acid [44]. This specific pathway results in inhibition of AMP activated-protein kinase by malonyl CoA and uric acid, and activates AMP deaminase, due to low intracellular phosphate levels, that keeps this process persistent [22, 44]. In addition, fructose metabolism results in oxidative stress, mitochondrial dysfunction and lipid accumulation in the liver affecting insulin sensitivity in this tissue [45]. While these changes result in higher levels of insulin in the circulation, over time fructose may cause low-grade islet damage leading to a fall in insulin secretion [46].
There is a survival benefit to becoming insulin resistant, for during starvation glucose as a fuel may be limited and hypoglycaemia could develop. However, the insulin resistance will result in decreased uptake of glucose into insulin-dependent tissues such as skeletal muscle, which can rely on fatty acids, resulting in maintaining or even increasing serum glucose. This is critical for brain function, as the brain prefers glucose as its major fuel source, and will continue utilizing glucose as uptake of glucose into the brain does not require insulin.
Fructose maintains blood pressure by retaining sodium and effects of uric acid
Fructose ingestion has been shown to increase blood pressure acutely in humans (as opposed to glucose) [47]. The mechanism likely involves stimulation of salt reabsorption in the kidney [48], increasing serum vasopressin levels [11] and the production of uric acid [49, 50]. Indeed, the effects of uric acid to raise blood pressure are mediated by the induction of oxidative stress and decreasing the biological activity of endothelial nitric oxide [51, 52].
There is some evidence that endogenously produced fructose may regulate blood pressure. A high serum osmolality, such as occur in dehydration or high-salt intake, has been found to stimulate endogenous fructose pathway through the activation of aldose reductase. In this regard, blood pressure rise can be prevented in hyperosmolar animals lacking fructokinase [15]. High-salt intake also interacts with dietary fructose to raise blood pressure [53].
While there is increasing evidence that salt intake may acutely regulate blood pressure via osmolality and potentially endogenous fructose production, the long-term elevation of blood pressure appears to be mediated by intrarenal inflammation that results in persistent renal vasoconstriction that impairs sodium excretion [54]. Of note, there is also some evidence that high blood pressure mediated by uric acid might transition to this mechanism over time [55].
Fructose as an activator of the innate immune response
Fructose has been shown to activate immune responses in nonimmune cells, such as by inducing leucocyte adhesion molecules (intercellular adhesion molecule-1, ICAM-1), in vascular endothelial cells and by stimulating chemokine secretion (monocyte chemoattractant protein-1, MCP-1) and inflammasome, and oxidative stress in tubular cells [56–58]. In the setting of heat stress, hydration with fructose also preferentially increases the expression of inflammasomes and interleukin-1 beta (IL-1β) compared to water hydration alone [59]. Fructose has also been reported to activate immune cells in vitro, resulting in increased IL-1β and IL-6 secretion from dendritic cells and interferon-γ from T cells in association with a shift towards glycolysis [60].
One of the mechanisms by which fructose may activate the immune system is via the production of advanced glycation endproducts [60], but there is also increasing evidence that uric acid, a metabolite generated during fructose metabolism, may also have a critical role. While crystalline uric acid is a well-known activator of the immune system, leading to stimulation of inflammasome-dependent IL-1β secretion [61], soluble and/or microcrystalline uric acid can also can activate dendritic cells [62] and T cells [63, 64] and monocytes [65, 66], as well as stimulate inflammasome generation and NF-κB activation in nonimmune cells [57, 67, 68].
Fructose as a mechanism for enhancing survival under hypoxic conditions
One of the key effects of fructose is to enhance glycolysis and reduce mitochondrial metabolism [22, 24]. This effect might be amplified by the high lactate generation induced by fructose that can further reduce mitochondrial function [69]. The increase in glycolysis with reduced mitochondrial function allows for less oxygen utilization and has been shown to be beneficial under conditions of hypoxia. Indeed, hypoxia is a stimulus for endogenous fructose production as aldose reductase is activated by hypoxia through a HIF-1 alpha dependent pathway. Some animals, such as the naked mole rat, will start producing fructose when it is in hypoxic burrows and uses the fructose to help maintain survival [70]. Fructose is also used by some animals in utero, including whales and other cetaceans where it has concentrations similar to glucose in other species [71]. While the function of fructose is not specifically known, we speculate that it likely helps as a means to both survive under low oxygen conditions during diving but also to stimulate fat as a means for generating metabolic water when they are born.
While the shunting to glycolysis provides some protection from hypoxia, lactate and uric acid, which are other products of fructose metabolism, also aid survival. For example, both substances have been reported to improve oxygen affinity for haemocyanin, the haemoglobin equivalent in crustaceans, that may aid improving oxygen delivery brackish water in which oxygen levels are low [72].
Other survival pathways
The fructose survival pathway likely was an important method for increasing fat stores in preparation for times when food or water shortage was likely. However, almost all species try to maintain some fat stores at all times, and in humans, this approximates to about 25% in women and 20% in men [73]. Women tend to carry more fat, likely as a protective mechanism to assure sufficient fat stores to sustain a successful pregnancy.
A consequence of activating the fructose survival pathway, however, is the induction of oxidative stress which is utilized to shift from an energy production to an energy storage state [23]. In turn, chronic oxidative stress has been postulated to have a role in ageing and cancer [74]. Thus, there is likely a fine balance between activating the survival pathway to provide critical needs and super stimulation of the pathway where it may cause deleterious consequences.
Caloric restriction has been reported to increase lifespan [75]. The reduction of oxidative stress associated with storing fat would predict this. Nevertheless, while it might increase survival in a laboratory setting in which food is provided daily, in the wild the potential for periods of food or water deprivation could act to increase mortality in the setting of absent or low-fat stores.
Because fructose has the downside of inducing oxidative stress, some animals that use fructose for survival upregulate antioxidant pathways as a protective mechanism to counter the effects of chronic fructose-dependent or independent oxidative stress in animals [76]. One such system is upregulation of the transcription fact, nuclear factor erythroid 2-related 2 (NRF2) which has been observed in hibernating bats, deep-sea diving seals and naked mole rats [76]. The antioxidant effects may block some aspects of fructose metabolism (such as fat storage) while allowing the benefits of other systems (such as generation of lactate and uric acid that may aid survival in hypoxic settings).
Evolution and survival during major extinctions
As mentioned, mass extinctions have occurred intermittently during the history of life, and adaptation is often critical. One of the major mechanisms of adaptation has been through genetic changes driven by evolution, and more recently, the power of epigenetic modifications has also been appreciated. For humans, there appear to have been two major mutations that occurred during extinctions that appear to involve the fructose pathway, and likely acted by enhancing our fat stores.
Around 65 million years ago, an asteroid fragment (the Chicxulub meteorite) crashed in the Yucatan, causing a massive impact that spewed dust into the air, covering much of the globe and driving temperatures down 7°C [77]. The world became like a nuclear winter, and more than 75 per cent of all life became extinct, including the nonavian dinosaurs (known as the Cretaceous–Paleogene extinction) [78]. Early mammals existed during this period, including the earliest primates [79], and one group of primates (the dry nosed haplorrhines) acquired a mutation in vitamin C synthesis (L-gulonolactone oxidase) that rapidly took over the whole family, suggesting a survival benefit [80].
While vitamin C has many functions, one action is to block the effects of fructose to stimulate fat synthesis, likely by blocking the mitochondrial oxidative stress mediated by fructose [23, 81]. Our group has found that fructose induced metabolic syndrome in vitamin C deficient mice can be blocked dose dependently by increasing doses of vitamin C (unpublished data).
This may lead to the interesting question of why vitamin C is present in fruits, given the observation that many species use fruits as a source of fructose to gain fat in preparation for long-distance migration or hibernation. However, the vitamin C content is highest early in the season, and as a fruit ripens, it sweetens (by increasing its fructose content) while its vitamin C content falls [82]. Thus, when animals ingest fruit in the fall, prior to winter, the fruit is maximally sweet with the lowest vitamin C content.
The other major extinction was the ‘Middle Miocene Disruption’ that occurred around 12–14 million years ago in Europe, and led to the extinction of many mammals including the apes that were living in this region [83]. During this time, there was a period of global cooling with a fall in temperatures of approximately 5°C, that resulted in a reduction in fruit, which was primary food for the ancestral apes. In particular, the loss of the fig tree, which can fruit all year long, resulted in periods of starvation during the cooler months [83]. The middle Miocene witnessed the death knell of the uricase gene for the entire ape lineage [84, 85]. Uricase is the enzyme that degrades uric acid, and the uricase gene had been slowly losing its activity via the accumulation of deleterious amino acid replacements during the preceding Oligocene along with its transporter URAT1 [86], but the complete pseudogenization of uricase during the mid-Miocene led to the ultimate loss of enzymatic activity [85]. One of the consequences of the loss of uricase in early hominoids was a greater uric acid response to fructose, which in turn is associated with greater stimulation of fat and glucose production from the same dose of fructose [44, 85]. Thus, the uricase mutation has been hypothesized to act as a survival factor for the European apes during this period of global cooling and food shortage [87]. Indeed, it appears that apes bearing these mutations subsequently migrated to Africa where they became the ancestors of humans and the African great apes, and to southeastern Asia where they became the ancestors of the orangutan [88, 89].
A survival pathway gone wrong: the role in obesity today
The geneticist, James Neel, proposed more than 50 years ago that mutations acquired during periods of starvation in our past might have a role in the epidemic of diabetes that is occurring today [90]. Our data support this ‘thrifty gene hypothesis’ and suggest that mutations affecting the fructose survival pathway may have enhanced the ability of ancestral humans to survive when food sources were limited, but that today it may increase our risk for obesity and diabetes. Indeed, we have proposed that the primary culprit is the dramatic increase in fructose intake from the introduction of refined sugar and HFCS, but diets high in salt, umami and alcohol (representing foods that rapidly generate uric acid) are also contributing. Interestingly, fructose is likely not only involved in driving obesity and diabetes, but also hypertension, and cardiovascular disease. Furthermore, the ability to stimulate aerobic glycolysis (Warburg effect) likely explains the increasing association of fructose as a fuel for various cancers.
Climate change and survival of life on the planet
While fructose is a survival nutrient, as is vasopressin a survival hormone, the behaviour of these molecules may change as the world around us changes. Global warming will place new stresses on both humans and animals in the wild. Given that heat stress can activate fructose-vasopressin pathways, we suggest that this may be important in driving the obesity and diabetes epidemic. Indeed, others have already reported an association between global warming and obesity [91]. Furthermore, heat stress and global warming have been linked with epidemics of chronic kidney disease in hot regions in Central America, Mexico, and India [92], and experimental studies suggest that they may be mediated by activation of the fructose-vasopressin pathways in the kidneys [37, 38].
Knowledge of the fructose survival pathway, however, may become important as we try to both adapt to the changing world and also try to help animals in the wild cope with changing climates and food and water availability. Thus, studies investigating these pathways in different species and in different environments can provide new insights into the physiology of survival. Biomimicry, or the study of the adaptation of species to different types of stress, may provide key insights that may aid the survival of humans and other life on our planet [30, 93, 94].
Footnotes
Conflict of interest
RJJ, MAL, DRT, CR-J and LGL all have equity in a startup company, Colorado Research Partners, LLC, that is developing inhibitors of fructose metabolism. There are no other conflicts.
References
- 1.Hull P Life in the aftermath of mass extinctions. Curr Biol 2015; 25: R941–52. [DOI] [PubMed] [Google Scholar]
- 2.Ravaux J, Hamel G, Zbinden M et al. Thermal limit for metazoan life in question: in vivo heat tolerance of the Pompeii worm. PLoS ONE 2013; 8: e64074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sidell BD, Vayda ME, Small DJ et al. Variable expression of myoglobin among the hemoglobinless Antarctic icefishes. Proc Natl Acad Sci USA 1997; 94: 3420–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larson DJ, Middle L, Vu H, et al. Wood frog adaptations to overwintering in Alaska: new limits to freezing tolerance. J Exp Biol 2014; 217: 2193–200. [DOI] [PubMed] [Google Scholar]
- 5.Johnson RJ, Stenvinkel P, Jensen T et al. Metabolic and kidney diseases in the setting of climate change, water shortage, and survival factors. J Am Soc Nephrol 2016; 27: 2247–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van den Berghe G, Bronfman M, Vanneste R, Hers HG. The mechanism of adenosine triphosphate depletion in the liver after a load of fructose. A kinetic study of liver adenylate deaminase. Biochem J 1977; 162: 601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diggle CP, Shires M, Leitch D et al. Ketohexokinase: expression and localization of the principal fructose-metabolizing enzyme. J Histochem Cytochem 2009; 57: 763–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oppelt SA, Zhang W, Tolan DR. Specific regions of the brain are capable of fructose metabolism. Brain Res 2017; 1657: 312–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mirtschink P, Krishnan J, Grimm F et al. HIF-driven SF3B1 induces KHK-C to enforce fructolysis and heart disease. Nature 2015; 522: 444–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song Z, Roncal-Jimenez CA, Lanaspa-Garcia MA et al. Role of fructose and fructokinase in acute dehydration-induced vasopressin gene expression and secretion in mice. J Neurophysiol 2017; 117: 646–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolf JP, Nguyen NU, Dumoulin G, Berthelay S. Influence of hypertonic monosaccharide infusions on the release of plasma arginine vasopressin in normal humans. Horm Metab Res 1992; 24: 379–83. [DOI] [PubMed] [Google Scholar]
- 12.Chapman CL, Johnson BD, Sackett JR, Parker MD, Schlader ZJ. Soft drink consumption during and following exercise in the heat elevates biomarkers of acute kidney injury. Am J Physiol Regul Integr Comp Physiol 2019; 316: R189–R98. [DOI] [PubMed] [Google Scholar]
- 13.Hwang YC, Kaneko M, Bakr S et al. Central role for aldose reductase pathway in myocardial ischemic injury. FASEB J 2004; 18: 1192–9. [DOI] [PubMed] [Google Scholar]
- 14.Lanaspa MA, Ishimoto T, Li N et al. Endogenous fructose production and metabolism in the liver contributes to the development of metabolic syndrome. Nat Commun 2013; 4: 2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lanaspa MA, Kuwabara M, Andres-Hernando A et al. High salt intake causes leptin resistance and obesity in mice by stimulating endogenous fructose production and metabolism. Proc Natl Acad Sci USA 2018; 115: 3138–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanchez-Lozada LG, Andres-Hernando A, Garcia-Arroyo FE et al. Uric acid activates aldose reductase and the polyol pathway for endogenous fructose and fat production causing development of fatty liver in rats. J Biol Chem 2019; 294: 4272–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-Arroyo FE, Gonzaga G, Munoz-Jimenez I et al. Antioxidant supplements as a novel mean for blocking recurrent heat stress-induced kidney damage following rehydration with fructose-containing beverages. Free Radic Biol Med 2019; 141: 182–91. [DOI] [PubMed] [Google Scholar]
- 18.Ishimoto T, Lanaspa MA, Le MT et al. Opposing effects of fructokinase C and A isoforms on fructose-induced metabolic syndrome in mice. Proc Natl Acad Sci USA 2012; 109: 4320–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teff KL, Elliott SS, Tschop M et al. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J Clin Endocrinol Metab 2004; 89: 2963–72. [DOI] [PubMed] [Google Scholar]
- 20.Shapiro A, Mu W, Roncal C, Cheng KY, Johnson RJ, Scarpace PJ. Fructose-induced leptin resistance exacerbates weight gain in response to subsequent high-fat feeding. Am J Physiol Regul Integr Comp Physiol 2008; 295: R1370–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lanaspa MA, Tapia E, Soto V, Sautin Y, Sanchez-Lozada LG. Uric acid and fructose: potential biological mechanisms. Semin Nephrol 2011; 31: 426–32. [DOI] [PubMed] [Google Scholar]
- 22.Softic S, Meyer JG, Wang G-X et al. Dietary sugars alter hepatic fatty acid oxidation via transcriptional and post-translational modifications of mitochondrial proteins. Cell Metab 2019; 30: 735–53.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lanaspa MA, Sanchez-Lozada LG, Choi YJ et al. Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: potential role in fructose-dependent and - independent fatty liver. J Biol Chem 2012; 287: 40732–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lanaspa MA, Cicerchi C, Garcia G et al. Counteracting roles of AMP deaminase and AMP kinase in the development of fatty liver. PLoS ONE 2012; 7: e48801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lanaspa MA, Andres-Hernando A, Orlicky DJ et al. Ketohexokinase C blockade ameliorates fructose-induced metabolic dysfunction in fructose-sensitive mice. J Clin Invest 2018; 128: 2226–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Schaftingen E, Vandercammen A, Detheux M, Davies DR. The regulatory protein of liver glucokinase. Adv Enzyme Regul 1992; 32: 133–48. [DOI] [PubMed] [Google Scholar]
- 27.Hengist A, Koumanov F, Gonzalez JT. Fructose and metabolic health: governed by hepatic glycogen status? J Physiol 2019; 597: 3573–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ciudad CJ, Carabaza A, Guinovart JJ. Glycogen synthesis from glucose and fructose in hepatocytes from diabetic rats. Arch Biochem Biophys 1988; 267: 437–47. [DOI] [PubMed] [Google Scholar]
- 29.Bairlein F How to get fat: nutritional mechanisms of seasonal fat accumulation in migratory songbirds. Naturwissenschaften 2002; 89: 1–10. [DOI] [PubMed] [Google Scholar]
- 30.Stenvinkel P, Jani AH, Johnson RJ. Hibernating bears (Ursidae): metabolic magicians of definite interest for the nephrologist. Kidney Int 2013; 83: 207–12. [DOI] [PubMed] [Google Scholar]
- 31.Junk WJ. Temporary fat storage, an adaptation of some fish species to the waterlevel fluctuations and related environmental changes of the Amazon river. Amazoniana 1985; 9: 315–51. [Google Scholar]
- 32.Meerman R, Brown AJ. When somebody loses weight, where does the fat go? BMJ 2014; 349: g7257. [DOI] [PubMed] [Google Scholar]
- 33.Olsson KE, Saltin B. Variation in total body water with muscle glycogen changes in man. Acta Physiol Scand 1970; 80: 11–8. [DOI] [PubMed] [Google Scholar]
- 34.Ortiz RM. Osmoregulation in marine mammals. J Exp Biol 2001; 204: 1831–44. [DOI] [PubMed] [Google Scholar]
- 35.Williams JB, Ostrowski S, Bedin E, Ismail K. Seasonal variation in energy expenditure, water flux and food consumption of Arabian oryx Oryx leucoryx. J Exp Biol 2001; 204: 2301–11. [DOI] [PubMed] [Google Scholar]
- 36.Garcia-Arroyo FE, Cristobal M, Arellano-Buendia AS et al. Rehydration with soft drink-like beverages exacerbates dehydration and worsens dehydration-associated renal injury. Am J Physiol Regul Integr Comp Physiol 2016; 311: R57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garcia-Arroyo FE, Tapia E, Blas-Marron MG et al. Vasopressin mediates the renal damage induced by limited fructose rehydration in recurrently dehydrated rats. Int J Biol Sci 2017; 13: 961–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roncal Jimenez CA, Ishimoto T, Lanaspa MA et al. Fructokinase activity mediates dehydration-induced renal injury. Kidney Int 2014; 86: 294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guetta J, Klorin G, Tal R et al. Vasopressin-2 receptor antagonist attenuates the ability of the lungs to clear edema in an experimental model. Am J Respir Cell Mol Biol 2012; 47: 583–8. [DOI] [PubMed] [Google Scholar]
- 40.Fay MJ, Du J, Yu X, North WG. Evidence for expression of vasopressin V2 receptor mRNA in human lung. Peptides 1996; 17: 477–81. [DOI] [PubMed] [Google Scholar]
- 41.Daikoku R, Kunitake T, Kato K, Tanoue A, Tsujimoto G, Kannan H. Body water balance and body temperature in vasopressin V1b receptor knockout mice. Auton Neurosci 2007; 136: 58–62. [DOI] [PubMed] [Google Scholar]
- 42.Jorgensen CB. 200 years of amphibian water economy: from Robert Townson to the present. Biol Rev Camb Philos Soc 1997; 72: 153–237. [DOI] [PubMed] [Google Scholar]
- 43.Bremer AA, Stanhope KL, Graham JL, et al. Fructose-fed rhesus monkeys: a nonhuman primate model of insulin resistance, metabolic syndrome, and type 2 diabetes. Clin Transl Sci 2011; 4: 243–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cicerchi C, Li N, Kratzer J et al. Uric acid-dependent inhibition of AMP kinase induces hepatic glucose production in diabetes and starvation: evolutionary implications of the uricase loss in hominids. FASEB J 2014; 28: 3339–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Madlala HP, Maarman GJ, Ojuka E. Uric acid and transforming growth factor in fructose-induced production of reactive oxygen species in skeletal muscle. Nutr Rev 2016; 74: 259–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roncal-Jimenez CA, Lanaspa MA, Rivard CJ et al. Sucrose induces fatty liver and pancreatic inflammation in male breeder rats independent of excess energy intake. Metabolism 2011; 60: 1259–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown CM, Dulloo AG, Yepuri G, Montani JP. Fructose ingestion acutely elevates blood pressure in healthy young humans. Am J Physiol Regul Integr Comp Physiol 2008; 294: R730–7. [DOI] [PubMed] [Google Scholar]
- 48.Cabral PD, Hong NJ, Hye Khan MA, et al. Fructose stimulates Na/H exchange activity and sensitizes the proximal tubule to angiotensin II. Hypertension 2014; 63: e68–73. [DOI] [PubMed] [Google Scholar]
- 49.Nakagawa T, Hu H, Zharikov S et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Renal Physiol 2006; 290: F625–31. [DOI] [PubMed] [Google Scholar]
- 50.Perez-Pozo SE, Schold J, Nakagawa T, Sanchez-Lozada LG, Johnson RJ, Lillo JL. Excessive fructose intake induces the features of metabolic syndrome in healthy adult men: role of uric acid in the hypertensive response. Int J Obes (Lond) 2010; 34: 454–61. [DOI] [PubMed] [Google Scholar]
- 51.Sanchez-Lozada LG, Soto V, Tapia E et al. Role of oxidative stress in the renal abnormalities induced by experimental hyperuricemia. Am J Physiol Renal Physiol 2008; 295: F1134–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sanchez-Lozada LG, Tapia E, Lopez-Molina R et al. Effects of acute and chronic L-arginine treatment in experimental hyperuricemia. Am J Physiol Renal Physiol 2007; 292: F1238–44. [DOI] [PubMed] [Google Scholar]
- 53.Hayasaki T, Ishimoto T, Doke T et al. Fructose Increases the Activity of Sodium Hydrogen Exchanger in Renal Proximal Tubules that is Dependent on Ketohexokinase. J Nutr Biochem 2019; 71: 54–62. in press. [DOI] [PubMed] [Google Scholar]
- 54.Rodriguez-Iturbe B, Pons H, Johnson RJ. Role of the immune system in hypertension. Physiol Rev 2017; 97: 1127–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Watanabe S, Kang DH, Feng L et al. Uric acid, hominoid evolution, and the pathogenesis of salt-sensitivity. Hypertension 2002; 40: 355–60. [DOI] [PubMed] [Google Scholar]
- 56.Glushakova O, Kosugi T, Roncal C et al. Fructose induces the inflammatory molecule ICAM-1 in endothelial cells. J Am Soc Nephrol 2008; 19: 1712–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hu QH, Zhang X, Pan Y, Li YC, Kong LD. Allopurinol, quercetin and rutin ameliorate renal NLRP3 inflammasome activation and lipid accumulation in fructose-fed rats. Biochem Pharmacol 2012; 84: 113–25. [DOI] [PubMed] [Google Scholar]
- 58.Cirillo P, Gersch MS, Mu W et al. Ketohexokinase-dependent metabolism of fructose induces proinflammatory mediators in proximal tubular cells. J Am Soc Nephrol 2009; 20: 545–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Milagres T, Garcia-Arroyo FE, Lanaspa MA et al. Rehydration with fructose worsens dehydration-induced renal damage. BMC Nephrol 2018; 19: 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jaiswal N, Agrawal S, Agrawal A. High fructose-induced metabolic changes enhance inflammation in human dendritic cells. Clin Exp Immunol 2019; 197: 237–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Conforti-Andreoni C, Spreafico R, Qian HL, et al. Uric acid-driven Th17 differentiation requires inflammasome-derived IL-1 and IL-18. J Immunol 2011; 187: 5842–50. [DOI] [PubMed] [Google Scholar]
- 62.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature 2003; 425: 516–21. [DOI] [PubMed] [Google Scholar]
- 63.Shi Y, Galusha SA, Rock KL. Cutting edge: elimination of an endogenous adjuvant reduces the activation of CD8 T lymphocytes to transplanted cells and in an autoimmune diabetes model. J Immunol 2006; 176: 3905–8. [DOI] [PubMed] [Google Scholar]
- 64.Webb R, Jeffries M, Sawalha AH. Uric acid directly promotes human T-cell activation. Am J Med Sci 2009; 337: 23–7. [DOI] [PubMed] [Google Scholar]
- 65.Crisan TO, Cleophas MCP, Novakovic B et al. Uric acid priming in human monocytes is driven by the AKT-PRAS40 autophagy pathway. Proc Natl Acad Sci USA 2017; 114: 5485–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Crisan TO, Cleophas MC, Oosting M et al. Soluble uric acid primes TLR-induced proinflammatory cytokine production by human primary cells via inhibition of IL-1Ra. Ann Rheum Dis 2016; 75: 755–62. [DOI] [PubMed] [Google Scholar]
- 67.Xiao J, Zhang XL, Fu C, et al. Soluble uric acid increases NALP3 inflammasome and interleukin-1beta expression in human primary renal proximal tubule epithelial cells through the Toll-like receptor 4-mediated pathway. Int J Mol Med 2015; 35: 1347–54. [DOI] [PubMed] [Google Scholar]
- 68.Kanellis J, Watanabe S, Li JH et al. Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension 2003; 41: 1287–93. [DOI] [PubMed] [Google Scholar]
- 69.San-Millan I, Brooks GA. Reexamining cancer metabolism: lactate production for carcinogenesis could be the purpose and explanation of the Warburg Effect. Carcinogenesis 2017; 38: 119–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Park TJ, Reznick J, Peterson BL et al. Fructose-driven glycolysis supports anoxia resistance in the naked mole-rat. Science 2017; 356: 307–11. [DOI] [PubMed] [Google Scholar]
- 71.Goodwin RFW. Division of the common mammals into two groups according to the concentration of fructose in the blood of the foetus. J Physiol 1956; 132: 146–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Truchot J-P, Lallier FH. Modulation of the oxygen-carrying function of hemocyanin in crustaceans. Physiology 1992; 7: 49–52. [Google Scholar]
- 73.Frisch RE. Critical fatness hypothesis. Am J Physiol 1997; 273: E231–2. [DOI] [PubMed] [Google Scholar]
- 74.Harman D Aging: a theory based on free radical and radiation chemistry. J Gerontol 1956; 11: 298–300. [DOI] [PubMed] [Google Scholar]
- 75.Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev 2005; 126: 913–22. [DOI] [PubMed] [Google Scholar]
- 76.Stenvinkel P, Meyer CJ, Block GA, Chertow GM, Shiels PG. Understanding the role of the cytoprotective transcription factor NRF2 - Lessons from evolution, the animal kingdom and rare progeroid syndromes. Nephrol Dial Transplant 2019. 10.1093/ndt/gfz120 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schulte P, Alegret L, Arenillas I et al. The Chicxulub asteroid impact and mass extinction at the Cretaceous-Paleogene boundary. Science 2010; 327: 1214–8. [DOI] [PubMed] [Google Scholar]
- 78.Vellekoop J, Sluijs A, Smit J, et al. Rapid short-term cooling following the Chicxulub impact at the Cretaceous-Paleogene boundary. Proc Natl Acad Sci USA 2014; 111: 7537–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pozzi L, Hodgson JA, Burrell AS, Sterner KN, Raaum RL, Disotell TR. Primate phylogenetic relationships and divergence dates inferred from complete mitochondrial genomes. Mol Phylogenet Evol 2014; 75: 165–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Drouin G, Godin JR, Page B. The genetics of vitamin C loss in vertebrates. Curr Genomics 2011; 12: 371–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kc S, Carcamo JM, Golde DW. Vitamin C enters mitochondria via facilitative glucose transporter 1 (Glut1) and confers mitochondrial protection against oxidative injury. FASEB J 2005; 19: 1657–67. [DOI] [PubMed] [Google Scholar]
- 82.Nagy S Vitamin C contents of citrus fruit and their products: a review. J Agric Food Chem 1980; 28: 8–18. [DOI] [PubMed] [Google Scholar]
- 83.Andrews P An Apes’s view of human evolution. Cambridge: Cambridge University Press, 2015. [Google Scholar]
- 84.Oda M, Satta Y, Takenaka O, Takahata N. Loss of urate oxidase activity in hominoids and its evolutionary implications. Mol Biol Evol 2002; 19: 640–53. [DOI] [PubMed] [Google Scholar]
- 85.Kratzer JT, Lanaspa MA, Murphy MN et al. Evolutionary history and metabolic insights of ancient mammalian uricases. Proc Natl Acad Sci USA 2014; 111: 3763–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tan PK, Farrar JE, Gaucher EA, Miner JN. Coevolution of URAT1 and uricase during primate evolution: implications for serum urate homeostasis and gout. Mol Biol Evol 2016; 33: 2193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Johnson RJ, Andrews P. Fructose, uricase, and the back-to-Africa hypothesis. Evol Anthropol 2010; 19; 250–7. [Google Scholar]
- 88.Andrews P, Kelley J. Middle Miocene dispersals of apes. Folia Primatol (Basel) 2007; 78: 328–43. [DOI] [PubMed] [Google Scholar]
- 89.Begun DR. Middle Miocene hominoid origins. Science 2000; 287: 2375. [PubMed] [Google Scholar]
- 90.Neel JV. Diabetes mellitus: a “thrifty” genotype rendered detrimental by “progress”? Am J Hum Genet 1962; 14: 353–62. [PMC free article] [PubMed] [Google Scholar]
- 91.An R, Ji M, Zhang S. Global warming and obesity: a systematic review. Obes Rev 2018; 19: 150–63. [DOI] [PubMed] [Google Scholar]
- 92.Glaser J, Lemery J, Rajagopalan B et al. Climate change and the emergent epidemic of CKD from heat stress in rural communities: the case for heat stress nephropathy. Clin J Am Soc Nephrol 2016; 11: 1472–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Benyus J Biomimicry: innovation inspired by nature. New York, NY: William Morrow & Company, Inc, 1997. [Google Scholar]
- 94.Natterson-Horowitz B, Bowers K. ZOOBIQUITY: what animals can teach us about health and the science of healing. New York, NY: A.A. Knopf, 2012. [Google Scholar]
- 95.Fenn WO, Haege LF. The deposition of glycogen and water in the livers of cats. J Biol Chem 1940; 136: 87–101. [Google Scholar]


