Abstract
The transcription of the α1-acid glycoprotein gene is induced by inflammatory cytokines and glucocorticoids. C/EBPβ is a major transcription factor involved in the induction of the agp gene by some cytokines. In this report, we have identified a novel transcriptional intermediary factor, TIF1β, which could enhance the transcription of the agp gene by the glucocorticoid receptor (GR) and C/EBPβ. TIF1β belongs to a subgroup of RING (really interesting new gene) finger proteins that contain a RING finger preceding two B box-type fingers and a putative coiled-coil domain (RBCC domain). Immunoprecipitation experiments showed that the interaction between GR and TIF1β is ligand independent. The overexpression of the TIF1β gene enhances GR-regulated expression in a ligand- and glucocorticoid-responsive element (GRE)-dependent manner. TIF1β can also augment C/EBPβ-mediated activity on wild-type and GRE-mutated agp genes, but this augmentation is diminished when all three C/EBPβ-binding elements are mutated. Functional and biochemical characterizations indicated that the bZIP domain of C/EBPβ and the RBCC domain, plant homeodomain finger, and bromodomain of TIF1β are crucial for the interactions of these proteins. Taken together, these results suggest that TIF1β serves as a converging mediator of signal transduction pathways of glucocorticoids and some inflammatory cytokines.
The acute-phase reaction to inflammatory stimuli is accompanied by an increase in a variety of serum proteins, collectively named acute-phase proteins. The synthesis of these proteins is regulated by glucocorticoids and inflammatory cytokines, such as interleukin 1 (IL-1), IL-6, and tumor necrosis factor alpha (5–7, 62). C/EBPβ was initially identified as the key transcription factor involved in the regulation of the α1-acid glycoprotein (AGP) gene during the acute-phase response (termed AGP/EBP) (18). C/EBPβ was also shown to be involved in the regulation of a number of other genes, such as those for IL-6 and albumin (termed NF-IL-6, LAP, IL-6DBP, or CRP2) (2, 14, 20, 49, 61). In addition to C/EBPβ-binding motifs, a glucocorticoid-responsive element (GRE) also exists between −120 and −107 in the 5′-flanking region of the agp gene (8, 18). Previous reports showed that maximal induction of the agp gene by glucocorticoids also requires another C/EBPβ-binding element located downstream of GRE (34, 50, 60). The synergistic interaction between cytokines and glucocorticoids has been attributed to protein-protein interactions between C/EBPβ and the glucocorticoid receptor (GR) (45).
GR belongs to a family of nuclear receptors that function as ligand-dependent transcription factors (9, 48). Transcriptional activation of target genes by nuclear receptors is mediated by two activation regions, AF1, located in the N terminus, and AF2, located in the C terminus of the hormone-binding domain of the receptor. GR-mediated transcription is promoter dependent and cell specific (for a review, see reference 24).
Results from studies of transcriptional interference or squelching between AF1 and AF2 of steroid receptors suggested the existence of coactivators or transcriptional intermediary factors which interact specifically with the AF1 and AF2 domains (3, 39, 55). Recent studies have led to the identification of several proteins that interact with nuclear receptors in a ligand-dependent manner and play essential roles in mediating their transcriptional activities. These proteins include RIP140 (15), TIF1 (36), Trip1/SUG1 (38, 59), SRC-1/p160 (26, 31, 47), TIF2/Grip1 (29, 58), ARA70 (63), and CBP/p300 (16, 27, 31). Several of these factors showed markedly different affinities for various nuclear receptors (56, 59). CBP and p300 are large nuclear proteins and have been demonstrated to interact functionally with a number of sequence-specific transcriptional activators (for a review, see reference 30). Previous data indicated that competition for limiting amounts of CBP may account for many of the inhibitory actions of both GR and the retinoic acid receptor on AP1 activation (31).
Genes for two related TIF1 proteins, TIF1α and TIF1β, have been cloned and shown to be members of the RING (really interesting new gene) finger family (for reviews, see references 12 and 52). The RING finger motif can be defined simply as Cys3-His-Cys4, a new class of the zinc finger. At least 80 members of the RING finger family have been identified. Many members, including the tumor suppressor BRCA-1 (42), the oncogene product Mel18 (32), and the mediator of the tumor necrosis factor receptor, TRAF2 (51), have been implicated as being in control of cell growth, cell differentiation, and development. The functions of these RING fingers remain to be defined, although some reports have suggested that they are the interface for protein-protein interactions (4, 10).
To delineate the mechanisms of transcriptional regulation of the agp gene by C/EBPβ, we have initiated studies on proteins that interact with C/EBPβ by purifying them using a number of procedures, including anti-C/EBPβ antibody immunoaffinity chromatography (40, 41). In this report, we present results on the identification and characterization of the roles of TIF1β in the activation of the agp gene. These results indicate that the enhancement of GR or C/EBPβ activity by TIF1β occurs through direct protein-protein interactions.
MATERIALS AND METHODS
Plasmids and constructs.
The EST clone containing partial human TIF1β cDNA (from nucleotides 1882 to 2673) was obtained from Research Genetics. An 0.8-kb DNA fragment insert isolated from the plasmid was used as a probe for screening the day-16 mouse embryo cDNA library (Novagen). A cDNA clone with a 2.8-kb insert containing the complete open reading frame of TIF1β was obtained. Mammalian expression plasmids were constructed by cloning the following TIF1β fragments into cytomegalovirus (CMV) expression vector pcDNA3 (Invitrogen): the full-length EcoRI-HindIII fragment (pcDNA3-TIF1β), and EcoRI-SacI fragment (residues 1 to 563), an EcoRI-PvuII fragment (residues 1 to 372), and a fragment resulting from BamHI deletion (residues 80 to 383 deleted) of the full-length EcoRI-HindIII fragment. An SfiI-HindIII fragment (residues 14 to 834), a BamHI fragment (residues 80 to 383), a BamHI-SacI fragment (residues 383 to 563), and a BamHI-HindIII fragment (residues 383 to 834) were cloned into the pGEX-1 vector (Pharmacia) for the production of glutathione S-transferase (GST) fusion proteins. The full-length EcoRI fragment of C/EBPβ, an N-terminal NcoI fragment (amino acids 21 to 151 [C/EBPβ-N), or a C-terminal NcoI-HindIII fragment (amino acids 151 to 296 [C/EBPβ-C]) was ligated to the pRSET vector (Invitrogen) for recombinant protein production.
Other plasmid constructs, such as pCMV-C/EBPβ, rat AGP (wild type [WT])-CAT, AGP (C mutant)-CAT, AGP (D mutant)-CAT, AGP (E mutant)-CAT, AGP (CDE mutant)-CAT, and AGP (GRE mutant)-CAT, were as described previously (40, 59). Briefly, the plasmids were obtained by ligation of the wild-type or mutant (see below) rat agp gene promoter sequence from −736 to +1 to the chloramphenicol acetyltransferase (CAT) reporter gene. The C, D, E, and GRE mutants correspond to serial 3-base substitutions at positions −74 to −72 (ACA to GTG), −96 to −94 (CAA to TGG), −106 to −104 (AGA to GAG), and −118 to −116 (ACA to GTG), respectively. pMMTV-CAT is the mouse mammary tumor virus long terminal repeat ligated to the CAT reporter gene. The mammalian cell expression vector (pRSV-hGR) and recombinant baculovirus containing GR were kindly provided by M.-J. Tsai of Baylor College of Medicine. pRSV-CREB and pCMV-PKAc were obtained from Susan Taylor.
Recombinant proteins and antibodies.
Human TIF1β (from nucleotide 1882 to 2673) was cloned into the pRSET vector and expressed in Escherichia coli BL21(DE3)(pLysS). This recombinant protein was purified on a nickel column and used for rabbit immunization. Monoclonal and polyclonal antibodies to C/EBPβ were as described previously (18). Anti-GR antibody was purchased from Santa Cruz Biotech.
Cell cultures, transient transfection, and CAT assay.
BHK, HeLa, and P388D1 cells were cultured in Iscove’s modified Dulbecco’s medium supplemented with 10% fetal calf serum. DNA transfection was performed by the calcium phosphate precipitation method. BHK cells were grown in 6- or 3.5-cm-diameter petri dishes to 30 to 40% confluence. The amounts of CAT reporter plasmid DNA and expression plasmid DNA used in each experiment are described in the figure legends. pcDNA3 plasmid DNA was used to adjust the total amount of DNA for each transfection to be equal. pCMV/SEAP (Tropix) (0.5 μg) was included in each transfection as an internal control for transfection efficiency. During the 24-h posttransfection period, the cells were placed in fresh medium and, in some experiments, induced with 1 μM dexamethasone (water soluble; Sigma). Cells were harvested 24 h later and extracted with 100 μl of 0.25 M Tris-HCl (pH 7.8). The acetylated forms of chloramphenicol were separated by thin-layer chromatography and quantified with an image analyzer (BAS 1000; Fuji). All transfection experiments were repeated two to four times.
Preparation of whole-cell extracts, immunoprecipitation, and Western blotting.
Whole-cell extracts from P388D1 cells were prepared by lysing the cells with buffer containing 25 mM HEPES (pH 7.6), 0.3 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5% Nonidet P-40 (NP-40), and 0.5 mM dithiothreitol (DTT). For immunoprecipitation analysis, 1 mg of whole-cell extracts was precleaned with preimmune serum and protein A-Sepharose in 0.5 ml of immunoprecipitation buffer (25 mM HEPES [pH 7.6], 0.25 M NaCl, 1 mM EDTA, 0.1% NP-40, 0.5 mM DTT, 6% glycerol) at 4°C for 2 h. The precleaned supernatants were incubated with 5 μg of anti-TIF1β or anti-C/EBPβ antibody and protein A-Sepharose in the presence or absence of 1 μM dexamethasone at 4°C for 90 min. After extensive washes, the protein complex was dissolved in sodium dodecyl sulfate (SDS) loading buffer and subjected to SDS-polyacrylamide gel electrophoresis (PAGE). The separated polypeptides were blotted onto a Hybond-C membrane (Amersham) and probed with anti-TIF1β, anti-C/EBPβ, or anti-GR antibody. The results were detected with an enhanced chemiluminescence kit (Amersham).
Protein-protein interaction assay.
Glutathione-Sepharose 8A beads (Pharmacia) were mixed with 3 μg of wild-type or deletion mutant recombinant GST-TIF1β fusion protein or GST only in 500 μl of phosphate-buffered saline containing 1% Triton X-100 on a rotary shaker for 20 min at room temperature. The beads were washed three times with phosphate-buffered saline, combined with 100 ng of recombinant full-length C/EBPβ, truncated C/EBPβ-N, or truncated C/EBPβ-C in a final volume of 500 μl of binding buffer (25 mM HEPES [pH 7.6], 0.25 M NaCl, 1 mM EDTA, 0.1% NP-40, 0.5 mM DTT, 6% glycerol), and incubated on a rotary shaker for 2 h at 4°C. The beads were washed three times with binding buffer, and the bound proteins were subjected to SDS-PAGE and Western blot analysis.
RESULTS
Isolation and characterization of cDNA clones for TIF1β.
The RING finger protein family consists of members found in animals, plants, and viruses, but the function of the RING finger domain remains to be defined. By comparison with sequences of RING finger domains similar to those of inhibitors of apoptosis or RING-1, a number of human EST clones carrying putative RING finger domains were identified. Sequence analysis revealed that one of these clones, containing an 0.8-kb insert, was highly homologous to a mouse protein, TIF1β (37). Rabbit antibodies were generated by use of a recombinant protein derived from the EST clone. In serendipitous Western blot experiments for identifying C/EBPβ-interacting proteins, we used a rabbit anti-TIF1β antibody as a control. Surprisingly, it reacted with a protein of ∼100 kDa that appeared in the eluent of the anti-C/EBPβ immunoaffinity column (data not shown). This observation prompted us to study the possible physical and functional interactions between TIF1β and C/EBPβ. Using the 0.8-kb DNA fragment as a probe to screen the mouse cDNA library, a 2.8-kb cDNA clone that could encode a protein of 834 amino acids was obtained.
TIF1β and TIF1α are strongly homologous in the N- and C-terminal regions. The N-terminal region is a RING finger preceding two B box-type fingers and a putative coiled-coil domain (RBCC motif) (36). The C-terminal region consists of a plant homeodomain (PHD) finger followed by a bromodomain (36).
TIF1β appears to be widely expressed, since Northern blotting reveals a major 3-kb TIF1β transcript in all human tissues (data not shown). The subcellular localization of TIF1β was then determined by indirect immunofluorescence, which showed granular staining of TIF1β only in the nucleoplasm and not in the nucleolus (data not shown).
TIF1β stimulates the C/EBPβ-mediated activation of the agp gene.
The physical interaction of C/EBPβ and TIF1β was examined by an immunoprecipitation assay. P388D1 is a mouse macrophage cell line which expresses C/EBPβ constitutively. Anti-C/EBPβ antibody can bring down TIF1β in P388D1 whole-cell extracts (Fig. 1A). Direct protein-protein interactions were studied by pull-down assays with GST-TIF1β (full length) and both full-length C/EBPβ and truncated forms of C/EBPβ, C/EBPβ-N and C/EBPβ-C (Fig. 1B). The results showed that the bZIP domain of C/EBPβ (i.e., C/EBPβ-C) is sufficient for its direct interaction with TIF1β.
FIG. 1.
Protein-protein interactions between C/EBPβ and TIF1β. (A) P388D1 whole-cell extracts were immunoprecipitated with anti-C/EBPβ polyclonal antibody (C/EBPβ) or preimmune serum (PI). The precipitated proteins were subjected to SDS-PAGE followed by Western blotting with anti-C/EBPβ monoclonal or anti-TIF1β polyclonal antibodies. (B) Several recombinant C/EBPβ constructs were incubated with glutathione bead-immobilized GST-TIF1β (lanes 4 to 6) or GST (lanes 7 to 9). After extensive washes, the protein complexes were subjected to SDS-PAGE and immunoblotted with anti-C/EBPβ antibody. Lanes 1 to 3 represent direct loading of different recombinant C/EBPβ constructs. FL, N, and C represent full-length C/EBPβ, C/EBPβ-N, and C/EBPβ-C, which are described in Materials and Methods.
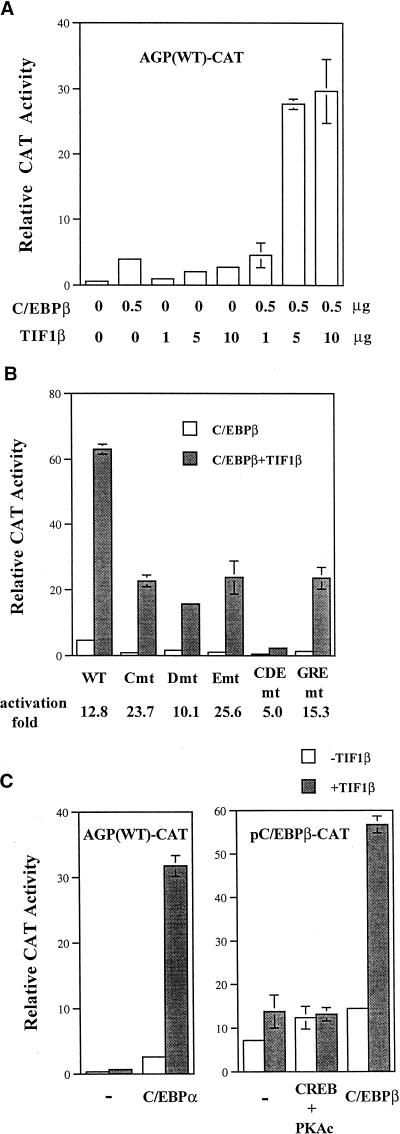
To characterize the functional role of TIF1β in the activation of the agp gene by C/EBPβ, we performed cotransfection experiments. TIF1β augmented the activation of the agp gene by C/EBPβ in a dose-dependent manner (Fig. 2A). To further elucidate the roles of C/EBPβ-binding motifs in the activation effect between C/EBPβ and TIF1β, we performed transfection assays with reporters containing mutated C/EBPβ-binding motifs (C, D, E, and CDE mutants). Compared to the results obtained with the wild-type reporter gene, there was stimulation of C/EBPβ activity by TIF1β with the C-, D-, or E-site-mutated reporter gene (13-fold for the wild type and 26-, 10-, and 24-fold for the C, D, and E mutants, respectively). Induction by these factors was dramatically reduced when a reporter containing mutations of all three C/EBPβ-binding sites (i.e., the CDE mutant) was tested (Fig. 2B). Transactivation of the GRE-mutated agp gene by C/EBPβ and TIF1β was comparable to that of the wild-type gene (Fig. 2B). Thus, the augmentation effect of TIF1β on the activation of the C/EBPβ gene is exclusively C/EBPβ-binding motif dependent, and GR is apparently not required.
FIG. 2.
Stimulation of C/EBPβ-mediated gene activation by TIF1β. (A) BHK cells were cotransfected with 2 μg of AGP (WT)-CAT, 0.5 μg of pCMV-C/EBPβ (C/EBPβ), and increasing amounts of pcDNA3-TIF1β (TIF1β) (1, 5, and 10 μg). (B) In 3.5-cm-diameter petri dishes, BHK cells were cotransfected with 0.5 μg of AGP (WT)-CAT, AGP (C mutant)-CAT, AGP (D mutant)-CAT, AGP (E mutant)-CAT, AGP (CDE mutant)-CAT, or AGP (GRE mutant)-CAT and 0.1 μg of pCMV-C/EBPβ with or without 2 μg of pcDNA3-TIF1β. The data represent the average activity of two independent duplicate experiments. The fold induction by TIF1β is indicated below the panel. mt, mutant. (C) (Left panel) In 3.5-cm-diameter petri dishes, BHK cells were cotransfected with 0.5 μg of AGP (WT)-CAT and 0.05 μg of pCMV-C/EBPα in the presence or absence of 2 μg of pcDNA3-TIF1β. (Right panel) BHK cells were transfected with 0.25 μg of pC/EBPβ-CAT, and 0.1 μg of pCMV-C/EBPβ or both 0.1 μg of pRSV-CREB and 0.1 μg of pCMV-PKAc in the absence or presence of 2 μg of pcDNA3-TIF1β. Error bars indicate standard deviations. −, pcDNA3 vector control.
To further assess the activation specificity of TIF1β and other factors, we conducted transient transfection assays with expression vectors for C/EBPα, CREB, and TIF1β. As shown in Fig. 2C, left panel, TIF1β also augmented the C/EBPα-mediated transactivation of the agp gene. The pC/EBPβ-CAT reporter contains the promoter from the c-ebpβ gene (nucleotides −390 to +82) (17). It has been reported that there are C/EBP- and CREB-responsive elements in the regulatory region of c-ebpβ (17, 44). TIF1β potentiated C/EBPβ activity but not CREB activity in the c-ebpβ gene promoter (Fig. 2C, right panel).
To define the regions of TIF1β that could interact with C/EBPβ physically and functionally, we constructed mammalian cell expression vectors and prepared recombinant GST fusion proteins of various deletion mutants of TIF1β (Fig. 3A and B, upper panels). Mutants of TIF1β with either the PHD finger and the bromodomain deleted (amino acids 1 to 563) or the RBCC domain deleted (amino acids 80 to 383 deleted) failed to potentiate the activation of the agp gene by C/EBPβ (Fig. 3A, lower panel). Physical interaction experiments with recombinant proteins derived from C/EBPβ and deletion mutants of TIF1β indicated that the RBCC domain of TIF1β was sufficient to interact with C/EBPβ (Fig. 3B, lower panel). The region of amino acids 383 to 563 seemed to interact weakly with C/EBPβ. Taken together, these results suggest that TIF1β interacts with C/EBPβ through the RBCC domain and enhances C/EBPβ transcriptional activity through the PHD finger and the bromodomain.
FIG. 3.
Functional and biochemical characterization of C/EBPβ-interacting domains of TIF1β. (A) (Upper panel) Schematic representation of several TIF1β expression vectors; numbers denote amino acid positions. (Lower panel) Transient transfection assays. BHK cells (in 3.5-cm-diameter petri dishes) were transfected with 0.5 μg of AGP (WT)-CAT, 0.1 μg of pCMV-C/EBPβ, and 2 μg of pcDNA3-TIF1β (full length [fl] or from amino acid 1 to 563 or 1 to 372, or full length but with amino acids 80 to 383 deleted). (B) (Upper panel) Schematic representation of several GST-TIF1β fusion proteins. (Lower panel) Protein pull-down assay. Glutathione bead-immobilized recombinant GST-TIF1β (fl or from amino acid 80 to 383, 383 to 563, or 383 to 834) incubated with full-length recombinant C/EBPβ (100 ng). After extensive washes, the protein complex was analyzed by immunoblotting with anti-C/EBPβ antibody. −, pcDNA3 vector control.
TIF1β enhances the transcriptional activity of GR.
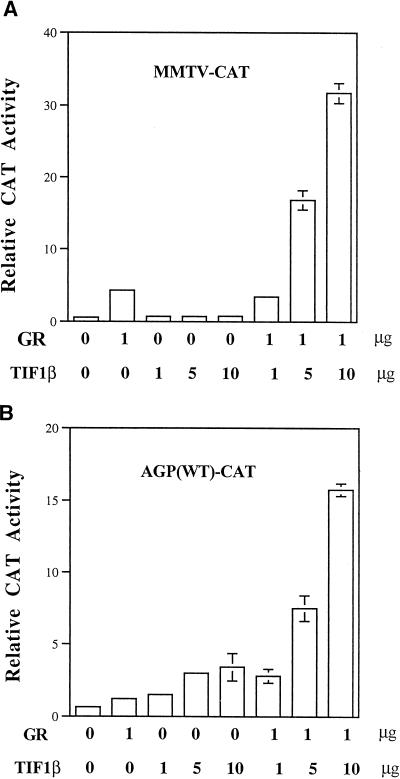
TIF1α was identified as a protein that interacts directly with the ligand-binding domains of several nuclear receptors in a ligand- and AF2-dependent manner both in vivo and in vitro. It was suggested that TIF1α mediates the transcriptional activation of the target gene by the AF2 domain of nuclear receptors (36). TIF1α and TIF1β share highly conserved domains. To further investigate the role of TIF1β in nuclear receptor-mediated transactivation of target gene expression, we performed transient transfection assays. As shown in Fig. 4, in the presence of exogenous GR and dexamethasone, TIF1β stimulated the transcription of mouse mammary tumor virus and the agp promoter in a dose-dependent manner. TIF1β did not augment the transcriptional activity of GR in the absence of dexamethasone (data not shown).
FIG. 4.
TIF1β potentiates GR-activated gene expression. (A) HeLa cells were transiently transfected with 2 μg of pMMTV-CAT reporter plasmid, 1 μg of pRSV-hGR (GR), and increasing amounts of pcDNA3-TIF1β (TIF1β) (1, 5, and 10 μg). Each assay was done in the presence of 1 μM dexamethasone. (B) BHK cells were transiently transfected with 2 μg of AGP (WT)-CAT reporter plasmid. Other plasmids and conditions of treatment are as described for panel A. Relative CAT activity normalized with an internal control represents an average of two independent duplicate experiments. Error bars indicate standard deviations.
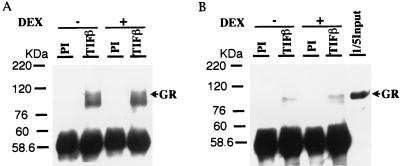
To determine the molecular basis of target gene activation by TIF1β and GR, we conducted an analysis of the interaction between TIF1β and GR by an immunoprecipitation assay. Polyclonal antibody to TIF1β but not preimmune serum immunoprecipitated GR from P388D1 whole-cell extracts in the absence or presence of dexamethasone (Fig. 5A; the anti-GR antibody detected two isoforms, 95 and 90 kDa). This result indicates that TIF1β and GR coexist in a complex. Direct protein-protein interactions were examined by an immunoprecipitation assay with GST-TIF1β fusion protein and recombinant GR. TIF1β interacted with GR in the absence or presence of dexamethasone (Fig. 5B).
FIG. 5.
Protein-protein interactions between GR and TIF1β. (A) Immunoprecipitation of GR by anti-TIF1β antibody. P388D1 whole-cell extracts were immunoprecipitated with anti-TIF1β or control (PI) antibody in the presence (+) or absence (−) of 1 μM dexamethasone (DEX) and subjected to SDS-PAGE and Western blotting with anti-GR polyclonal antibody. (B) Recombinant GST-TIF1β was incubated with recombinant GR in the presence (+) or absence (−) of 1 μM dexamethasone and then immunoprecipitated with anti-TIF1β antibody or preimmune serum. After extensive washes, the protein complex was analyzed by Western blotting with anti-GR antibody. Recombinant GR used for the interaction assay (1/5 input) was included as a control.
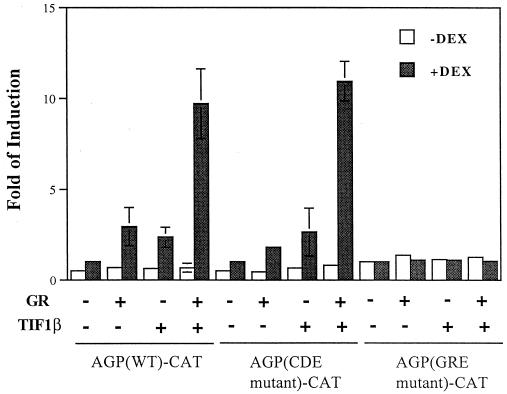
Previous studies indicated that maximal induction of the agp gene by glucocorticoid requires the downstream C/EBPβ-binding sequences (34, 50, 60). To further study the TIF1β-mediated GR induction of the agp gene, we performed transfection assays using agp promoters containing mutated C/EBPβ-binding sites or mutated GRE. As indicated by the previous results, the activation of the agp gene by TIF1β and GR was observed only in the presence of dexamethasone (Fig. 6). Reporters containing mutated C/EBPβ-binding elements (namely, C, D, and E mutants) remained responsive to TIF1β and GR. In contrast, the reporter containing mutated GRE was unresponsive to TIF1β and GR (Fig. 6). The activation of the agp gene by TIF1β alone in the presence of dexamethasone was likely due to the effect of endogenous GR. These results show that the transcriptional activation of the agp gene by TIF1β and GR is dependent on GRE but independent of C/EBPβ-binding motifs.
FIG. 6.
GRE-dependent and C/EBPβ-binding-element-independent augmentation of the activation of AGP-CAT by GR and TIF1β. AGP (WT)-CAT, AGP (CDE mutant)-CAT, or AGP (GRE mutant)-CAT was used as a reporter (see Materials and Methods). In 3.5-cm-diameter petri dishes, BHK cells were cotransfected with one reporter plasmid (1 μg) and 0.5 μg of pRSV-hGR (GR), 5 μg of pcDNA3-TIF1β (TIF1β), or both in the presence or absence of 1 μM dexamethasone (DEX). The fold induction for each experiment is shown. The values are the averages of at least two independent experiments. Error bars indicate standard deviations.
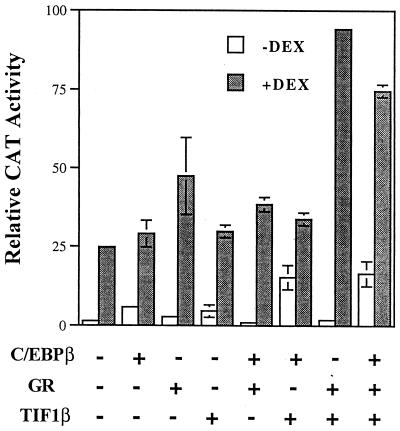
We further examined the effect of TIF1β on the agp gene by cotransfecting pRSV-GR and pCMV-C/EBPβ simultaneously. As shown in Fig. 7, the net effect of the transactivation of the agp gene by cotransfection of pCMV-C/EBPβ, pRSV-GR, and pCMV-TIF1β seemed to be the result of pRSV-GR plus pCMV-TIF1β. Taken together, these results suggest that there is no additive or synergistic activation of the agp gene by the overexpression of GR, TIF1β, and C/EBPβ under these experimental conditions.
FIG. 7.
Activation of the agp gene by C/EBPβ, GR, and TIF1β. BHK cells (grown in 6-cm petri dishes) were transfected with 2 μg of AGP (WT)-CAT and 0.5 μg of pCMV-C/EBPβ (C/EBPβ), 1 μg of pRSV-hGR (GR), or 5 μg of pcDNA3-TIF1β (TIF1β) in various combinations in the absence or presence of 1 μM dexamethasone (DEX). Normalized relative CAT activity represents an average of two independent experiments. Error bars indicate standard deviations.
DISCUSSION
TIF1β was originally identified as a protein that interacts directly with two chromosomal proteins, mHP1α and mMOD1 (37). In this report, TIF1β was identified as a coactivator for C/EBPβ and GR in the activation of the agp gene. The specificity of TIF1β for GR or C/EBPβ was demonstrated by transfection assays with wild-type or mutant reporter plasmids containing GRE and/or C/EBPβ-binding elements. Direct protein-protein interactions between TIF1β and GR or C/EBPβ were apparently responsible for the activation of the agp gene by these proteins. These results suggest that TIF1β may act as an integrator or coactivator for both glucocorticoid and cytokine signaling pathways leading to the activation of C/EBPβ. The identification of TIF1β as a coactivator provides further clues about the mechanisms of transactivation by GR and C/EBPβ and the regulation of their target genes, such as acute-phase response genes.
The competition for a common coactivator, CBP, by AP-1 and the nuclear receptor provides an example of how genes that contain either an AP-1- or a nuclear receptor-binding site could be regulated (31). However, a more complex pattern of regulation was observed for genes containing composite response elements. One GRE and three C/EBPβ-binding sites are located in the upstream regulatory region of the agp gene. The regulation of this gene by interactions between TIF1β, GR, and C/EBPβ seems to be complex and depends on the steady-state levels of these factors. When pRSV-GR, pCMV-C/EBPβ, and pCMV-TIF1β were cotransfected into cells, no apparent changes in the transactivation of the agp gene were seen compared to those seen with pRSV-GR and pCMV-TIF1β (Fig. 7). In fact, the slight decrease in activation observed could have been attributed to competition between C/EBPβ and GR for TIF1β.
Results from the deletion analysis indicated that the RBCC domain and the PHD domain-bromodomain are essential for the function of TIF1β. The RBCC domain is the C/EBPβ-interacting domain. Although the actual functional significance of these domains is unknown, it is currently assumed that they are involved in protein-protein interactions. The RBCC motif has been found in the N-terminal part of several putative transcriptional factors, ribonucleoproteins, and proto-oncogene products, including PML, RFP, RPT-1, SS-A/Ro, XNF7, and PWA33 (for a review, see reference 21). Three RBCC domain-containing proteins, PML, RFP, and TIF1α, have been identified in the context of fusion oncoproteins resulting from chromosomal translocations. The RBCC domain is fused to truncated products of other genes (23, 36, 54). Mutations in the RBCC domain of PML prevent PML nuclear body formation (11). Many PHD finger-containing proteins have been implicated in interactions between chromosomal proteins (1). These include products of the Drosophila genes trithorax and polycomblike. It is interesting to note that bromodomains are found in the adaptor proteins p300, CBP, and GCN5, as well as in SWI/SNF2 (13, 30, 35). These proteins reside in large multiprotein complexes. Thus, the overall structure of TIF1β implies that it activates gene transcription by taking part in the formation of multiprotein complexes.
A TIF1β-related protein, TIF1α, was found to interact with several nuclear hormone receptors. TIF1α contains a nuclear receptor-binding motif, LXXLL (28, 37). However, there is no motif resembling LXXLL in the TIF1β sequence. Inhibition of RXRα activity was observed as a result of ectopic expression of TIF1α in the transient transfection assays. The RBCC domain-containing protein PML exerts a very powerful enhancing effect on the transactivating properties of several steroid hormone receptors (25). It is likely that a specific functional interaction exists between coactivators and nuclear receptors. The activation effect of TIF1α or TIF1β on GR- or RXRα-induced gene expression, respectively, remains to be studied.
In addition to GR and C/EBPβ, TIF1β has been reported to interact with the transcriptional silencing domain of the Drosophila Kruppel-related KRAB proteins and to serve as a corepressor (22, 33, 43). The mechanism of repression by TIF1β remains elusive. To test the possibility that TIF1β is a general mediator of various transcriptional factors, we performed cotransfection assays with mammalian cell expression vectors for CREB and the protein kinase A catalytic subunit in the presence of the pC/EBPβ-CAT reporter. TIF1β could not enhance CREB activity (Fig. 2C). Another member of the C/EBP family, C/EBPα, was also tested, and the results showed that TIF1β could augment the C/EBPα-activated expression of AGP (WT)-CAT (Fig. 2C). Thus, in addition to C/EBPβ, the transcriptional activity of another member of the C/EBP family may be modulated by TIF1β.
How could TIF1β function as a coactivator? TIF1β seems to be a bifunctional protein involved in the remodeling of the chromatin template in both the repression and the activation of transcription (37). Thus, the interaction between GR and TIF1β or between C/EBPβ and TIF1β may promote the conversion of a transcriptionally inactive heterochromatin-like structure to an active euchromatin-like open structure by triggering the release of HP1 and MOD1 (37). We previously identified a nucleolar phosphoprotein, Nopp140, that functions as a mediator between C/EBPβ and the general transcription factor TIFIIB (40). In light of the analogous features shared by the interactions between C/EBPβ and Nopp140 or TIF1β, we also tested the physical interaction between TIF1β and TFIIB. Our results did not offer conclusive evidence on any physical interaction between TIF1β and TFIIB (data not shown). Thus, the mechanism of activation of the agp gene by C/EBPβ and TIF1β is different from that of C/EBPβ and Nopp140.
The present results revealed a direct protein-protein interaction between TIF1β and GR and showed that this interaction is ligand independent. However, the functional interaction between GR and TIF1β is ligand dependent. It is speculated that TIF1β may participate in the formation of a coactivator complex to activate target gene expression. CBP has been demonstrated to interact with other nuclear receptor coactivators (SRC-1/ACTR/pCIP family) to form a functional complex and to result in a synergistic response to the nuclear receptors (19, 31, 57). These coactivators have been identified as histone acetyltransferases that remodel chromatin structure to facilitate transcriptional activation (19, 46, 53). Further experiments to identify other TIF1β-interacting proteins may yield important mechanistic insights.
ACKNOWLEDGMENTS
This research was supported by grants NSC86-2311-B001-089 and NSC88-2311-B001-114 (to C.-J.C.) and NSC86-2311-B001-094-Y (to S.-C.L.) from the National Science Council.
We thank Susan Taylor, Sophia Tasi, and Ming-Jer Tasi for plasmids and Bertrand Chin-Ming Tan for critical reading of the manuscript.
REFERENCES
- 1.Aasland R, Gilson T J, Stewart A F. The PHD finger: implications for chromatin-mediated transcriptional regulation. Trends Biochem Sci. 1995;20:56–59. doi: 10.1016/s0968-0004(00)88957-4. [DOI] [PubMed] [Google Scholar]
- 2.Akira S, Isshiki H, Sugita T, Tanabe O, Kinoshita S, Nishio Y, Nakajima T, Hirano T, Kishimoto T. A nuclear factor for IL-6 expression (NF-IL6) is a member of a C/EBP family. EMBO J. 1990;9:1897–1906. doi: 10.1002/j.1460-2075.1990.tb08316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barettino D, Vianco-Ruiz M D M, Stunnenberg H G. Characterization of the ligand-dependent transactivation domain of thyroid hormone receptor. EMBO J. 1994;13:3039–3049. doi: 10.1002/j.1460-2075.1994.tb06603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barlow P N, Luisi B, Milner A, Elliott M, Everett R. Structure of the C3HC4 domain by 1H-nuclear magnetic resonance spectroscopy. A new structural class of zinc-finger. J Mol Biol. 1994;237:201–211. doi: 10.1006/jmbi.1994.1222. [DOI] [PubMed] [Google Scholar]
- 5.Baumann H, Firestone G L, Burgess T L, Gross K W, Yamamoto K R, Held W A. Dexamethasone regulation of α1-acid glycoprotein and other acute phase reactants in rat liver and hepatoma cells. J Biol Chem. 1983;258:563–570. [PubMed] [Google Scholar]
- 6.Baumann H, Gauldie J. Regulation of hepatic acute phase plasma protein genes by hepatocyte stimulating factors and other mediators of inflammation. Mol Biol Med. 1990;7:147–159. [PubMed] [Google Scholar]
- 7.Baumann H, Jahreis G P, Morella K K. Interaction of cytokine- and glucocorticoid-response elements of acute-phase plasma protein genes. J Biol Chem. 1990;265:22275–22281. [PubMed] [Google Scholar]
- 8.Baumann H, Maquat L E. Localization of DNA sequences involved in dexamethasone-dependent expression of the rat α1-acid glycoprotein gene. Mol Cell Biol. 1986;6:2551–2561. doi: 10.1128/mcb.6.7.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beato M, Herrlich P, Schutz G. Steroid hormone receptors: many actors in search of a plot. Cell. 1995;83:851–857. doi: 10.1016/0092-8674(95)90201-5. [DOI] [PubMed] [Google Scholar]
- 10.Borden K L B, Boddy M N, Lally J, O’Reilly N J, Martin S, Howe K, Solomon E, Freemont P S. The solution structure of the RING finger domain from the acute promyelocytic leukemia proto-oncoprotein PML. EMBO J. 1995;14:1532–1541. doi: 10.1002/j.1460-2075.1995.tb07139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borden K L B, Lally J M, Martin S R, O’Reilly N J, Solomon E, Freemont P S. In vivo and in vitro characterization of the B1 and B2 zinc-binding domains from the acute promyelocytic leukemia protooncoprotein PML. Proc Natl Acad Sci USA. 1996;93:1601–1606. doi: 10.1073/pnas.93.4.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borden K L B, Freemont P S. The RING finger domain: a recent example of a sequence-structure family. Curr Opin Struct Biol. 1996;6:395–401. doi: 10.1016/s0959-440x(96)80060-1. [DOI] [PubMed] [Google Scholar]
- 13.Brownell J E, Zhou J, Ranalli T, Kobayashi R, Edmondson D G, Roth S Y, Allis C D. Tetrahymena histone acetyltransferase A: a homology to yeast Gcn5p linking histone acetylation to gene activation. Cell. 1996;84:843–851. doi: 10.1016/s0092-8674(00)81063-6. [DOI] [PubMed] [Google Scholar]
- 14.Cao Z, Umek R M, McKnight S L. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991;5:1538–1552. doi: 10.1101/gad.5.9.1538. [DOI] [PubMed] [Google Scholar]
- 15.Cavailles V, Dauvois S, L’Horset F, Lopez G, Hoare S, Kushner P J, Parker M G. Nuclear factor RIP140 modulates transcriptional activation by the estrogen receptor. EMBO J. 1995;14:3741–3751. doi: 10.1002/j.1460-2075.1995.tb00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chakravarti D, LaMorte V J, Nelson M C, Nakajima T, Schulman I G, Juguilon H, Montminy M, Evans R M. Role of CBP/p300 in nuclear receptor signalling. Nature. 1996;383:99–103. doi: 10.1038/383099a0. [DOI] [PubMed] [Google Scholar]
- 17.Chang C J, Shen B J, Lee S C. Autoregulated induction of the acute-phase response transcription factor gene, agp/ebp. DNA Cell Biol. 1995;14:529–537. doi: 10.1089/dna.1995.14.529. [DOI] [PubMed] [Google Scholar]
- 18.Chang C J, Chen T T, Lei H Y, Chen D S, Lee S C. Molecular cloning of a transcription factor, AGP/EBP, that belongs to members of the C/EBP family. Mol Cell Biol. 1990;10:6642–6653. doi: 10.1128/mcb.10.12.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen H, Lin R J, Schiltz R L, Chakravarti D, Nash A, Nagy L, Privalsky M L, Nakatani Y, Evans R M. Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300. Cell. 1997;90:569–580. doi: 10.1016/s0092-8674(00)80516-4. [DOI] [PubMed] [Google Scholar]
- 20.Descombes P, Chojkier M, Lichtsteiner S, Falvey E, Schibler U. LAP, a novel member of the C/EBP gene family, encodes a liver-enriched transcription activator protein. Genes Dev. 1990;4:1541–1551. doi: 10.1101/gad.4.9.1541. [DOI] [PubMed] [Google Scholar]
- 21.Freemont P S. A novel zinc finger coiled-coil domain in a family of nuclear proteins. Trends Biochem Sci. 1992;17:344–345. doi: 10.1016/0968-0004(92)90308-v. [DOI] [PubMed] [Google Scholar]
- 22.Friedman J R, Fredericks W J, Jensen D E, Speicher D W, Huang X-P, Neilson E G, Rauscher F J., III KAP-1, a novel corepressor for the highly conserved KRAB repression domain. Genes Dev. 1996;10:2067–2078. doi: 10.1101/gad.10.16.2067. [DOI] [PubMed] [Google Scholar]
- 23.Goddard A D, Borrow J, Freemont P S, Solomon E. Characterization of a zinc finger gene disrupted by the t(15;17) in acute promyelocytic leukemia. Science. 1991;254:1371–1374. doi: 10.1126/science.1720570. [DOI] [PubMed] [Google Scholar]
- 24.Gronemeyer H. Transcription activation by oestrogen and progesterone receptors. Annu Rev Genet. 1991;25:89–123. doi: 10.1146/annurev.ge.25.120191.000513. [DOI] [PubMed] [Google Scholar]
- 25.Guiochon-Mantel A, Savouret J F, Quignon F, Delabre K, Milgrom E, The H D. Effect of PML and PML-RAR on the transactivation properties and subcellular distribution of steroid hormone receptors. Mol Endocrinol. 1995;9:1791–1803. doi: 10.1210/mend.9.12.8614415. [DOI] [PubMed] [Google Scholar]
- 26.Halachmi S, Marden E, Martin G, MacKay H, Abbondanza C, Brown M. Estrogen receptor-associated proteins: possible mediators of hormone-induced transcription. Science. 1994;264:1455–1458. doi: 10.1126/science.8197458. [DOI] [PubMed] [Google Scholar]
- 27.Hanstein B, Eckner R, DiRenzo J, Halachmi S, Liu H, Searcy B, Kurokawa R, Brown M. p300 is a component of an estrogen receptor coactivator complex. Proc Natl Acad Sci USA. 1996;93:11540–11545. doi: 10.1073/pnas.93.21.11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heery D M, Kalkhoven E, Hoare S, Parker M G. A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature. 1997;387:733–736. doi: 10.1038/42750. [DOI] [PubMed] [Google Scholar]
- 29.Hong H, Kohli K, Trivedi A, Johnson D L, Stallcup M R. GRIP1, a novel mouse protein that serves as a transcriptional coactivator in yeast for the hormone binding domains of steroid receptors. Proc Natl Acad Sci USA. 1996;93:4948–4952. doi: 10.1073/pnas.93.10.4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janknecht R, Hunter T. Transcriptional control: versatile molecular glue. Curr Biol. 1996;6:951–954. doi: 10.1016/s0960-9822(02)00636-x. [DOI] [PubMed] [Google Scholar]
- 31.Kamei Y, Xu L H, Heinzel T, Torchia J, Kurokawa R, Gloss B, Lin S-C, Heyman R, Rose D, Glass C, Rosenfeld M. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell. 1996;85:403–414. doi: 10.1016/s0092-8674(00)81118-6. [DOI] [PubMed] [Google Scholar]
- 32.Kanno M, Hasegawa M, Ishida A, Isono K, Taniguchi M. Mel-18, a polycomb group-related mammalian gene, encodes a transcriptional negative regulator with tumor suppressive activity. EMBO J. 1995;14:101–107. doi: 10.1002/j.1460-2075.1995.tb00254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim S-S, Chen Y-M, O’Leary E, Witzgall R, Vidal M, Bonventre J V. A novel member of the RING finger family, KRIP-1, associates with the KRAB-A transcriptional repressor domain of zinc finger proteins. Proc Natl Acad Sci USA. 1996;93:15299–15304. doi: 10.1073/pnas.93.26.15299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klein E S, DiLorenzo D, Posseckert G, Beato M, Ringold G M. Sequences downstream of the glucocorticoid regulatory element mediate cycloheximide inhibition of steroid induced expression from the rat α1-acid glycoprotein promoter: evidence for a labile transcription factor. Mol Endocrinol. 1988;2:1343–1351. doi: 10.1210/mend-2-12-1343. [DOI] [PubMed] [Google Scholar]
- 35.Laurent B C, Treich I, Carlson M. The yeast SNF2/SWI2 protein has DNA-stimulated ATPase activity required for transcriptional activation. Genes Dev. 1993;7:583–591. doi: 10.1101/gad.7.4.583. [DOI] [PubMed] [Google Scholar]
- 36.Le Douarin B, Zechel C, Garnier J-M, Lutz Y, Tora L, Pierrat B, Heery D, Gronemeyer H, Chambon P, Losson R. The N-terminal part of TIF1, a putative mediator of the ligand-dependent activation function (AF-2) of nuclear receptors, is fused to B-raf in the oncogenic protein T18. EMBO J. 1995;14:2020–2033. doi: 10.1002/j.1460-2075.1995.tb07194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le Douarin B, Nielsen A L, Garnier J-M, Ichinose H, Jeanmougin F, Losson R, Chambon P. A possible involvement of TIF-1α and TIF-1β in the epigenetic control of transcription by nuclear receptors. EMBO J. 1996;15:6701–6715. [PMC free article] [PubMed] [Google Scholar]
- 38.Lee J W, Ryan F, Swaffield J C, Johnston S A, Moore D A. Interaction of thyroid-hormone receptor with a conserved transcriptional mediator. Nature. 1995;374:91–94. doi: 10.1038/374091a0. [DOI] [PubMed] [Google Scholar]
- 39.Meyer M-E, Gronemeyer H, Turcotte B, Bocquel M-T, Tasset D, Chambon P. Steroid hormone receptors compete for factors that mediate their enhancer function. Cell. 1989;57:433–442. doi: 10.1016/0092-8674(89)90918-5. [DOI] [PubMed] [Google Scholar]
- 40.Miau L H, Chang C J, Tsai W H, Lee S C. Identification and characterization of a nucleolar phosphoprotein, Nopp140, as a transcription factor. Mol Cell Biol. 1997;17:230–239. doi: 10.1128/mcb.17.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miau L H, Chang C J, Shen B J, Tsai W H, Lee S C. Identification of heterogeneous nuclear ribonucleoprotein K (hnRNP K) as a repressor of C/EBPβ-mediated gene activation. J Biol Chem. 1998;273:10784–10791. doi: 10.1074/jbc.273.17.10784. [DOI] [PubMed] [Google Scholar]
- 42.Miki Y, Swensen J, Shattuck-Eidens D, Futreal P A, Harshman K, Tavtigian S, Liu Q, Cochran C, Bennett L M, Ding W, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- 43.Moosmann K, Georgiev O, Le Douarin B, Bourquin J-P, Schaffer W. Transcriptional repression by RING finger protein TIF1β that interacts with the KRAB repressor domain of KOX1. Nucleic Acids Res. 1996;24:4859–4867. doi: 10.1093/nar/24.24.4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Niehof M, Manns M P, Trautwein C. CREB controls LAP/C/EBPβ transcription. Mol Cell Biol. 1997;17:3600–3613. doi: 10.1128/mcb.17.7.3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nishio Y, Isshiki H, Kishimoto T, Akira S. A nuclear factor for interleukin-6 expression (NF-IL6) and the glucocorticoid receptor synergistically activate transcription of the rat α1-acid glycoprotein gene via direct protein-protein interaction. Mol Cell Biol. 1993;13:1854–1862. doi: 10.1128/mcb.13.3.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ogryzko V V, Schiltz R L, Russanva V, Howard B H, Nakatani Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell. 1996;89:373–380. doi: 10.1016/s0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- 47.Onate S A, Tsai S Y, Tsai M-J, O’Malley B W. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science. 1995;270:1354–1357. doi: 10.1126/science.270.5240.1354. [DOI] [PubMed] [Google Scholar]
- 48.Parker M G. Steroid and related receptors. Curr Opin Cell Biol. 1993;5:499–504. doi: 10.1016/0955-0674(93)90016-j. [DOI] [PubMed] [Google Scholar]
- 49.Poli V, Mancini F P, Cortese R. IL-6DBP, a nuclear protein involved in interleukin-6 signal transduction, defines a new family of leucine zipper proteins related to C/EBP. Cell. 1990;63:643–653. doi: 10.1016/0092-8674(90)90459-r. [DOI] [PubMed] [Google Scholar]
- 50.Ratajczak T, Williams P M, DiLorenzo D, Ringold G M. Multiple elements within the glucocorticoid regulatory unit of the rat α1-acid glycoprotein gene are recognition sites of C/EBP. J Biol Chem. 1992;267:11111–11119. [PubMed] [Google Scholar]
- 51.Rothe M, Pan M-G, Henzel W J, Ayres T M, Goeddel A V. The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell. 1995;83:1243–1252. doi: 10.1016/0092-8674(95)90149-3. [DOI] [PubMed] [Google Scholar]
- 52.Saurin A J, Borden K L B, Boddy M N, Freemont P S. Does this have a familiar RING? Trends Biochem Sci. 1996;21:208–214. [PubMed] [Google Scholar]
- 53.Spencer T E, Jenster G, Burcin M M, Allis C D, Zhou J, Mizzen C A, McKenna N J, Onate S A, Tsai S Y, Tsai M-J, O’Malley B W. Steroid receptor coactivator-1 is a histone acetyltransferase. Nature. 1997;389:194–198. doi: 10.1038/38304. [DOI] [PubMed] [Google Scholar]
- 54.Takahashi M, Inaguma Y, Hiai H, Hirose F. Developmentally regulated expression of a human “finger”-containing gene encoded by the 5′ half of the ret transforming gene. Mol Cell Biol. 1988;8:1853–1856. doi: 10.1128/mcb.8.4.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tasset D, Tora L, Formental C, Scheer E, Chambon P. Distinct classes of transcriptional activating domains function by different mechanisms. Cell. 1990;62:1177–1187. doi: 10.1016/0092-8674(90)90394-t. [DOI] [PubMed] [Google Scholar]
- 56.Thenot S, Henriquet C, Rochefort H, Cavailles V. Differential interaction of nuclear receptors with the putative human transcriptional coactivator hTIF1. J Biol Chem. 1997;272:12062–12068. doi: 10.1074/jbc.272.18.12062. [DOI] [PubMed] [Google Scholar]
- 57.Torchia J, Rose D W, Inostroza J, Kamei Y, Westin S, Glass C K, Rosenfeld M G. The transcriptional co-activator pCIP binds CBP and mediates nuclear-receptor function. Nature. 1997;387:677–684. doi: 10.1038/42652. [DOI] [PubMed] [Google Scholar]
- 58.Voegel J J, Heine M J S, Zechel C, Chambon P, Gronemeyer H. TIF2, a 160 KDa transcriptional mediator for the ligand-dependent activation function AF-2 of nuclear receptors. EMBO J. 1996;15:3667–3675. [PMC free article] [PubMed] [Google Scholar]
- 59.vom Baur E, Zechel C, Heery D, Heine M J S, Garnier J M, Vivat V, Le Douarin B, Gronemeyer H, Chambon P, Losson R. Differential ligand-dependent interactions between the AF-2 activating domain of nuclear receptors and the putative transcriptional intermediary factors mSUG1 and TIF1. EMBO J. 1996;15:110–124. [PMC free article] [PubMed] [Google Scholar]
- 60.Williams P, Ratajczak T, Lee S C, Ringold G M. AGP/EBP (LAP) expressed in rat hepatoma cells interacts with multiple promoter sites and is necessary for maximal glucocorticoid induction of the rat α1-acid glycoprotein gene. Mol Cell Biol. 1991;11:4959–4965. doi: 10.1128/mcb.11.10.4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Williams S C, Cantwell C A, Johnson P F. A family of C/EBP-related proteins capable of forming covalently linked leucine zipper dimers in vitro. Genes Dev. 1991;5:1553–1567. doi: 10.1101/gad.5.9.1553. [DOI] [PubMed] [Google Scholar]
- 62.Won K-A, Baumann H. The cytokine response element of the rat α1-acid glycoprotein gene is a complex of several interacting regulatory sequences. Mol Cell Biol. 1990;10:3965–3978. doi: 10.1128/mcb.10.8.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yeh S, Chang C. Cloning and characterisation of a specific coactivator, ARA70, for the androgen receptor in human prostate cells. Proc Natl Acad Sci USA. 1996;93:5517–5521. doi: 10.1073/pnas.93.11.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]