Abstract
Background
Until now, only a few cases of Valsalva-induced barotraumas (pneumothorax, pneumomediastinum and subcutaneous emphysema) have been described, and none of them among COVID-19 patients.
Case description
A man in his 50s was admitted for SARS-CoV-2-related acute respiratory distress syndrome (ARDS). Initial evolution was favourable with non-invasive ventilatory support, high-flow oxygen nasal cannula and the best supportive drugs available at the time. During the Valsalva manoeuvre while defecating, the patient reported sudden chest pain and showed a new acute hypoxemic respiratory failure due to a pneumothorax. It led to multiple complications (pulmonary embolism, haemoptysis, and cardiac arrest), and despite the best supportive care, led to the patient’s death.
Discussion
The Valsalva manoeuvre can be an overlooked cause of pneumothorax in patients with COVID-19. Predisposition to barotrauma in COVID-19 patients could be explained by several factors, including the extensive use of non-invasive and invasive ventilation during the pandemic, and the histological changes observed in the lungs of those infected with COVID-19.
Conclusion
We report the first description of a Valsalva-induced barotrauma in a COVID-19 infection. We emphasise the importance of treating constipation particularly in severe COVID-19 cases, to prevent complications such as barotrauma.
LEARNING POINTS
Pneumothorax is a common complication of severe COVID-19 infection, but Valsalva manoeuvre-induced pneumothorax in COVID-19 patients has never been reported previously.
Particular care should be taken to prevent and treat constipation in hospitalised patients as it may cause a wide range of complications, including barotraumatism.
The extensive use of non-invasive and invasive ventilation may play a role in barotrauma, but causal association has not been proven.
Keywords: COVID-19, Valsalva, pneumothorax
INTRODUCTION
Several publications have reported various types of barotrauma during the COVID-19 pandemic: pneumothorax, pneumomediastinum and subcutaneous emphysema, mostly in patients receiving invasive and non-invasive ventilation.
We describe the case of a patient hospitalised with SARS-CoV-2 pneumonia, complicated by an acute respiratory distress syndrome (ARDS). The patient developed a pneumothorax, caused by a Valsalva manoeuvre during strained defecation, leading to multiple complications and eventually death.
CASE DESCRIPTION
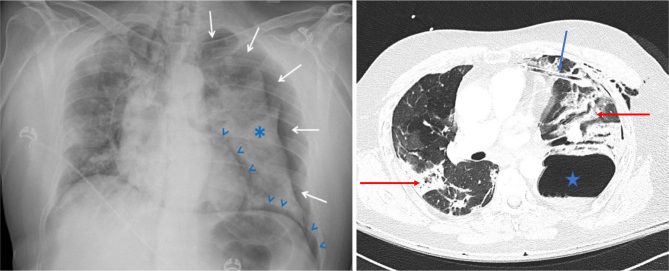
A non-smoking male in his 50s was admitted for COVID-19-related ARDS, attributed to the Delta variant, and managed with non-invasive ventilation, intermittent high-flow oxygen therapy and pharmacological interventions including dexamethasone, tocilizumab and casirivimab/imdevimab. Initial CT imaging revealed 25%–50% lung parenchyma involvement without pulmonary embolism. Oxygen requirements diminished progressively, transitioning to nasal cannula on day 8 amid reports of constipation. On day 14, straining defecation precipitated acute respiratory failure and chest pain, with imaging disclosing a 4 cm left pneumothorax and pneumomediastinum (Fig. 1).
Figure 1.
Left panel: Four centimetres left-sided pneumothorax (white arrows) with associated left-sided lung atelectasis (asterisk) and pneumomediastinum (blue arrowheads) that appeared on day 14. Discrete right-sided mediastinal shift. Right panel: CT scan on day 15 showing a large left basal pneumatocele with an undrained fluid component (blue star). A chest tube was in the antero-medial position (blue arrow). There is densification of pulmonary infiltrate as previously described (red arrows).
Subsequent ICU transfer and chest tube insertion initially ameliorated respiratory failure (Fig. 1, left panel). However, complications such as pulmonary embolism, haemoptysis and worsening hypoxemic respiratory failure ensued, necessitating the use of invasive mechanical ventilation (Fig. 2). Cardiac arrest on day 22 (Fig. 3) culminated in unsuccessful resuscitation and the patient’s death.
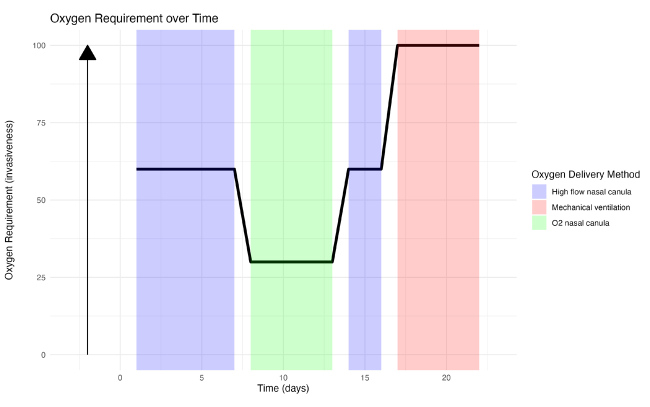
Figure 2.
Evolution of the oxygen need according to time since hospitalisation (X axis) and oxygen requirement (Y axis). The maximal invasiveness of the oxygen delivery corresponds to mechanical ventilation.
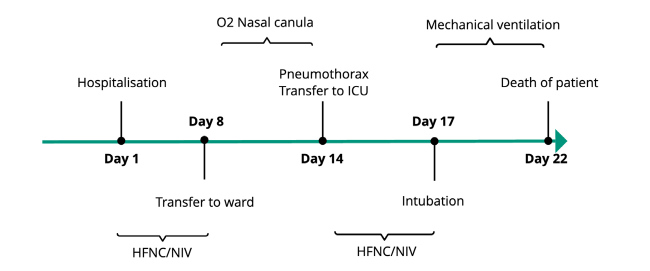
Figure 3.
Timeline of the patient’s evolution since admission in relation to the type of oxygen delivered and setting of care. The pneumothorax happened on day 14, while the patient was oxygenated via a nasal cannula.
Abbreviation: NIV, non-invasive ventilation; HFNC, high-flow nasal canula; ICU, intensive care unit.
DISCUSSION
We describe, to the best of our knowledge, the first case of pneumothorax induced by a Valsalva manoeuvre in a COVID-19 infection. Our patient suffered from acute chest pain and respiratory distress during sustained defecating exertion which strongly suggests that the pneumothorax was induced by the Valsalva manoeuvre.
In the literature, the Valsalva manoeuvre has been associated with the hyperacute onset of barotraumas, mostly pneumomediastinum and less often with pneumothorax. Valsalva-induced barotrauma could be the result of the large pressure gradient being generated against a closed glottis. The rise of intra-alveolar pressure provokes a rupture of the alveolar wall, leading the air to propagate to the mediastinum through the perivascular and bronchovascular sheaths. This phenomenon was first described in 1944 and is known as the Macklin effect[1].
Many cases have been reported: in the settings of labour, during drug inhalation, while playing a musical instrument, blowing balloons or endoscopic investigation (without iatrogenic perforation)[2–5]. Some of the cases were associated with known risk factors of spontaneous barotraumas such as tobacco use, inhaled drugs, or underlying lung disease (asthma, COPD, interstitial lung disease). Most of these cases were treated conservatively and none were fatal.
Among COVID-19 patients, a recent matched case-control study comparing 24 pneumothorax to 48 control patients admitted to a critical care unit investigated risk factors associated with pneumothorax development. Interestingly, as previously described in spontaneous pneumothorax, a higher BMI seems to be a protective factor in COVID-19-associated pneumothorax. A prolonged time from symptoms onset to intubation has also been associated with an increased risk of pneumothorax[6].
A recent case report described the occurrence of pneumothorax following a severe COVID-19 infection in a previously healthy patient, who was transiently mechanically ventilated. Subpleural bullae could be observed at the time of the pneumothorax, although it was not present at admission. Of note, the patient was coughing when the pneumothorax occurred, pointing to a possible role of raised intra-alveolar pressure similar to a Valsalva effect[7]. Lung lesions secondary to the infection and the mechanical ventilation are other possible contributing factors.
Thus, the Valsalva manoeuvre, due to any reasons (cough or constipation for example in our case) can be an overlooked cause of pneumothorax in patients with COVID-19. Constipation is prevalent in hospitalised patients, especially in the intensive care unit, and COVID-19 patients are particularly prone to constipation due to prolonged bed rest, opioid use, or dehydration.
Since the beginning of the pandemic, some data have suggested a higher prevalence of barotrauma among COVID-19 infected patients than in ARDS of other aetiologies. Nevertheless, the existence of a specific COVID-19 ARDS sub-phenotype is still controversial[8], and studies comparing the barotrauma incidence in COVID-19 ARDS and non-COVID-19 ARDS are still lacking.
One factor that could explain the predisposition to develop barotrauma is the extensive use of non-invasive and invasive ventilation during the pandemic in severe respiratory forms of COVID-19. Barotraumas are frequent in mechanically ventilated patients suffering from ARDS. Some authors have postulated that patient self-induced lung injury, a concept describing lung injuries due to patient’s respiratory effort and harmful patient-ventilator interaction, could favour barotrauma in spontaneously breathing patients with non-invasive ventilation[9].
Another postulated explanation is the alveolar damage secondary to infection and inflammation. These changes in pulmonary parenchyma could weaken the alveoli membrane and thus lead to alveolar rupture, provoking air leaks and barotrauma formation as explained by the Macklin effect[10]. Similar pathological evolutions have been previously observed in a variety of viral pneumonia, including Middle East respiratory syndrome (MERS) coronavirus and SARS-CoV-1.
Corticosteroids have been recommended as first-line therapy in hypoxemic COVID-19 infected patients since the RECOVERY study was published, and they have been extensively prescribed since then. High-dose corticoid therapy (the so-called Meduri protocol) has been used for years in ARDS of any aetiologies without evidence of increasing incidence of barotrauma. Further studies evaluating the possible causal role of drugs in barotrauma and COVID-19 are lacking.
CONCLUSION
Our case describes a barotrauma induced by a Valsalva manoeuvre in a COVID-19 patient, an association not previously described in the literature. It stands out due to the absence of known risk factors for spontaneous barotrauma, the lack of previous invasive ventilation and the catastrophic consequences for a patient whose clinical course was initially favourable.
Emphasising constipation prevention and treatment is crucial to mitigate this complication in severe COVID-19 cases, where barotrauma is common and could significantly exacerbate hypoxemia, prolong hospital stays and increase mortality. Although numerous risk factors may contribute to these complications, definitive data that evaluate the causal relationships between COVID-19, barotrauma and ventilation methods are still limited. This gap highlights the need for further research into the exact causes and prevention strategies.
APPENDIX
REFERENCES
- Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn MR, Watson RL, Thetford JT, Wong JI, Kamangar N. High incidence of barotrauma in patients with severe coronavirus disease 2019. J Intensive Care Med. 2021;36:646–654. doi: 10.1177/0885066621989959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama H, Uchida K, Aoyama K, Pechlivanoglou P, Englesakis M, Yamada Y, et al. Assessment of therapeutic interventions and lung protective ventilation in patients with moderate to severe acute respiratory distress syndrome: a systematic review and network meta-analysis. JAMA Netw Open. 2019;2:e198116. doi: 10.1001/jamanetworkopen.2019.8116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belletti A, Todaro G, Valsecchi G, Losiggio R, Palumbo D, Landoni G, et al. Barotrauma in coronavirus disease 2019 patients undergoing invasive mechanical ventilation: a systematic literature review. Crit Care Med. 2021 doi: 10.1097/CCM.0000000000005283. published ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattarello S, Camporota L, Gattinoni L. COVID-19 pneumonia: therapeutic implications of its atypical features. Anaesth Crit Care Pain Med. 2023;42:101182. doi: 10.1016/j.accpm.2022.101182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz MJ, van Meenen DM, Bos LD. COVID-19-related acute respiratory distress syndrome: lessons learned during the pandemic. Lancet Respir Med. 2022;10:1108–1110. doi: 10.1016/S2213-2600(22)00401-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal M, Hussain A, Majeed Z, Elfaki H. Pneumomediastinum in a cannabis smoker precipitated by vigorous sexual intercourse. BMJ Case Rep. 2021;14:e244804. doi: 10.1136/bcr-2021-244804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay T, Bellomo R, Rechnitzer T, See E, Ali Abdelhamid Y, Deane AM. Constipation, diarrhea, and prophylactic laxative bowel regimens in the critically ill: a systematic review and meta-analysis. J Crit Care. 2019;52:242–250. doi: 10.1016/j.jcrc.2019.01.004. [DOI] [PubMed] [Google Scholar]
- Diaz R, Heller D. StatPearls [Internet] Treasure Island (FL): StatPearls Publishing; 2022. [accessed 27 November 2022]. Barotrauma and mechanical ventilation. Available at: http://www.ncbi.nlm.nih.gov/books/NBK545226/ [PubMed] [Google Scholar]
- Acute Respiratory Distress Syndrome Network. Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- Elabbadi A, Urbina T, Berti E, Contou D, Plantefève G, Soulier Q, et al. Spontaneous pneumomediastinum: a surrogate of P-SILI in critically ill COVID-19 patients. Crit Care. 2022;26:350. doi: 10.1186/s13054-022-04228-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menter T, Haslbauer JD, Nienhold R, Savic S, Hopfer H, Deigendesch N, et al. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;77:198–209. doi: 10.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo HH, Sweeney RT, Regula D, Leung AN. Best cases from the AFIP: fatal 2009 influenza A (H1N1) infection, complicated by acute respiratory distress syndrome and pulmonary interstitial emphysema. RadioGraphics. 2010;30:327–333. doi: 10.1148/rg.302095213. [DOI] [PubMed] [Google Scholar]
- Das KM, Lee EY, Al Jawder SE, Enani MA, Singh R, Skakni L, et al. Acute Middle East respiratory syndrome coronavirus: temporal lung changes observed on the chest radiographs of 55 patients. Am J Roentgenol. 2015;205:W267–274. doi: 10.2214/AJR.15.14445. [DOI] [PubMed] [Google Scholar]
- Filice GA. SARS, pneumothorax, and our response to epidemics. Chest. 2004;125:1982–1984. doi: 10.1378/chest.125.6.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zheng X, Tong Q, Li W, Wang B, Sutter K, et al. Overlapping and discrete aspects of the pathology and pathogenesis of the emerging human pathogenic coronaviruses SARS-CoV, MERS-CoV, and 2019-nCoV. J Med Virol. 2020;92:491–494. doi: 10.1002/jmv.25709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes KT, Beasley MB. Pulmonary manifestations of acute lung injury: more than just diffuse alveolar damage. Arch Pathol Lab Med. 2017;141:916–922. doi: 10.5858/arpa.2016-0342-RA. [DOI] [PubMed] [Google Scholar]
- Agarwal A, Hunt B, Stegemann M, Rochwerg B, Lamontagne F, Siemieniuk RA, et al. A living WHO guideline on drugs for Covid-19. BMJ. 2020:m3379. doi: 10.1136/bmj.m3379. [DOI] [PubMed] [Google Scholar]
- The RECOVERY Collaborative Group. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar J, Ferrando C, Martínez D, Ambrós A, Muñoz T, Soler JA, et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8:267–276. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- Meduri GU, Golden E, Freire AX, Taylor E, Zaman M, Carson SJ, et al. Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial. Chest. 2007;131:954–963. doi: 10.1378/chest.06-2100. [DOI] [PubMed] [Google Scholar]
Footnotes
Conflicts of Interests: The Authors declare that there are no competing interests.
Patient Consent: Written informed consent for publication of this case report and accompanying images was not obtained from the patient or the relatives after all possible attempts were made.
REFERENCES
- 1.Murayama S, Gibo S. Spontaneous pneumomediastinum and Macklin effect: overview and appearance on computed tomography. World J Radiol. 2014;6:850–854. doi: 10.4329/wjr.v6.i11.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.La Verde M, Palmisano A, Iavarone I, Ronsini C, Labriola D, Cianci S, et al. A rare complication during vaginal delivery, Hamman’s syndrome: a case report and systematic review of case reports. Int J Environ Res Public Health. 2022;19:4618. doi: 10.3390/ijerph19084618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mégarbane B, Chevillard L. The large spectrum of pulmonary complications following illicit drug use: features and mechanisms. Chem Biol Interact. 2013;206:444–451. doi: 10.1016/j.cbi.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 4.Dejene S, Ahmed F, Jack K, Anthony A. Pneumothorax, music and balloons: a case series. Ann Thorac Med. 2013;8:176–178. doi: 10.4103/1817-1737.114283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tseng W-C, Yeh C-C, Jao S-W, Wu Z-F, Lin S-L. Bilateral tension pneumothorax during colonoscopy in a patient with chronic obstructive pulmonary disease: a case report. J Clin Anesth. 2016;34:432–435. doi: 10.1016/j.jclinane.2016.05.030. [DOI] [PubMed] [Google Scholar]
- 6.Lee SJ, Kim J, Lee KH, Lee JA, Kim CH, Lee SH, et al. Risk factors of pneumothorax and pneumomediastinum in COVID-19: a matched case–control study. BMC Infect Dis. 2023;23:137. doi: 10.1186/s12879-023-08104-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yagyu K, Miki Y, Nakagawa H, Shoji S, Shirano M, Amo K. Life-threatening pneumothorax after release from isolation with COVID-19 pneumonia. Radiol Case Rep. 2023;18:903–906. doi: 10.1016/j.radcr.2022.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajdev K, Spanel AJ, McMillan S, Lahan S, Boer B, Birge J, et al. Pulmonary barotrauma in COVID-19 patients with ARDS on invasive and non-invasive positive pressure ventilation. J Intensive Care Med. 2021;36:1013–1017. doi: 10.1177/08850666211019719. [DOI] [PubMed] [Google Scholar]
- 9.Carteaux G, Parfait M, Combet M, Haudebourg A-F, Tuffet S, Mekontso Dessap A. Patient-self inflicted lung injury: a practical review. J Clin Med. 2021;10:2738. doi: 10.3390/jcm10122738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manna S, Maron SZ, Cedillo MA, Voutsinas N, Toussie D, Finkelstein M, et al. Spontaneous subcutaneous emphysema and pneumomediastinum in non-intubated patients with COVID-19. Clin Imaging. 2020;67:207–213. doi: 10.1016/j.clinimag.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]