Abstract
Giant cell arteritis (GCA) may manifest with aggressive intracranial stenosis resistant to medical therapy, and patients may develop refractory neurologic deficits and cerebral infarcts, making GCA a life-threatening condition.
We report the case of a 68-year-old woman recently diagnosed with GCA, medicated with prednisolone 60 mg daily. Two weeks later, the patient was admitted to our Stroke Unit after a sudden episode of global aphasia. Magnetic resonance angiography showed two recent ischaemic lesions, besides an erythrocyte sedimentation rate of 17 mm/hour. A cerebral angiography revealed bilateral stenosis and dilation in the petrous, cavernous and supraclinoid segments of internal carotid arteries (ICA). The patient was started on intravenous methylprednisolone pulses (250 mg daily for five days). Computed tomography (CT) angiography and Doppler ultrasound showed severe vascular disease affecting multiple territories, without significant intracranial involvement. The hypothesis of GCA with extracranial vasculitic involvement was considered as the aetiology of ischaemic cerebral infarctions in multiple territories and, given the severity of the disease, it was decided to add tocilizumab. Despite this, the patient evolved with significant worsening neurological deficits and a CT scan confirmed the presence of new vascular events. Endovascular treatment (EVT) with balloon angioplasty was conducted on both ICAs, with improved calibre and downstream filling. After that, the patient presented sustained clinical improvement, without recurrence of any ischaemic events at the one-year follow-up.
This clinical case stands out for the importance of EVT as an effective therapy in patients with medically refractory GCA with symptomatic intracranial stenosis, improving their prognosis.
LEARNING POINTS
Giant cell arteritis may manifest with aggressive and symptomatic intracranial arterial stenoses.
Endovascular treatment is an effective intervention to prevent ischaemic complications in intracranial giant cell arteritis.
Keywords: Giant cell arteritis, intracranial stenosis, ischaemic stroke, vascular imaging, endovascular treatment
CASE DESCRIPTION
We present the case of a 68-year-old Caucasian woman with a previous history of dyslipidemia, who was in her usual state of health until she started feeling persistent pain in shoulders and hips, morning stiffness, and shoulder and pelvic girdle weakness. She also reported anorexia, weight loss accounting for 10% of her body weight, and night sweats, without fever.
One month later, she reported bilateral temporoparietal headache, jaw claudication and scalp hyperaesthesia, later associated with vertigo, dizziness, and a decline in visual acuity (without diplopia or amaurosis fugax).
Blood tests showed increased acute phase reactants (erythrocyte sedimentation rate (ESR) of 76 mm/h and C-reactive protein (CRP) of 13 mg/dl). Cranioencephalic (CE) magnetic resonance (MR) imaging showed sequelae of cortical microinfarction of the left postero inferior cerebellar artery, stenosis of the V4 segment of the left vertebral artery (VA) and severe bilateral stenosis of the petrous and cavernous segments of internal carotid arteries (ICA). Doppler ultrasound revealed parietal thickening and lack of compressibility in the temporal arteries, compatible with the halo sign and hypoechoic wall thickening of the axillary arteries.
The diagnoses of giant cell arteritis (GCA) and polymyalgia rheumatica (PMR) were made, and the patient was medicated with acetylsalicylic acid (ASA) 100 mg once daily, and prednisolone (PDN) 60 mg (1 mg per kilogram) once daily.
After two weeks of corticotherapy, the patient was clinically and analytically improved (a decrease of ESR to 19 mm/h and CRP to 0.01 mg/dl).
Nevertheless, the patient presented a sudden episode of global aphasia, lasting 15 minutes. She was observed in an Internal Medicine consultation, reporting dizziness, blurred vision, and gait imbalance, and presenting left central facial paresis (CFP). MR angiography revealed two recent ischaemic lesions located in the left occipital and right temporal regions.
Consequently, she was admitted to our Stroke Unit, presenting left CFP and tongue deviation to the right. In the subsequent days, the patient exhibited subtle horizontal-rotatory nystagmus, exhaustible in levoversion, slight pronation of the left upper limb in the extended arms test and left dysmetria (National Institutes of Health Stroke Scale - NIHSS - 2). Blood analysis revealed an ESR of 17 mm/hour, without other relevant alterations.
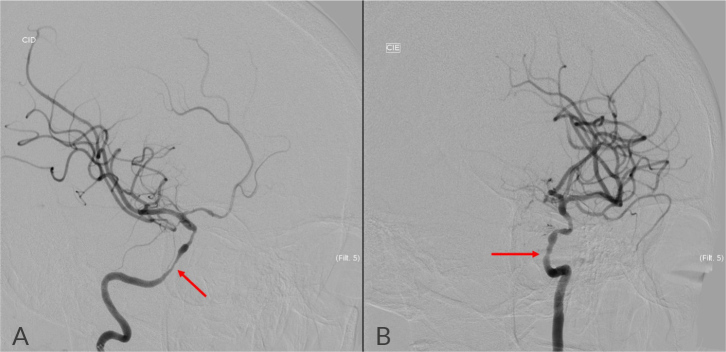
Cerebral angiography (Fig. 1) demonstrated bilateral stenosis and dilation in the petrous, cavernous and supraclinoid segments of ICA, with stenosis of 70% on the right and 50% on the left. The VA exhibited irregularities and thin calibre from the origin, particularly on the right, with posterior circulation sustained through anastomoses of the VA, left costocervical artery and left occipital artery, and with V4 dominance on the left. There was no evidence of atheromatous plaque.
Figure 1.
Cerebral angiography showed bilateral stenosis and dilation in the petrous, cavernous and supraclinoid segments of ICA, with stenosis of approximately 70% on the right (A) and 50% on the left (B).
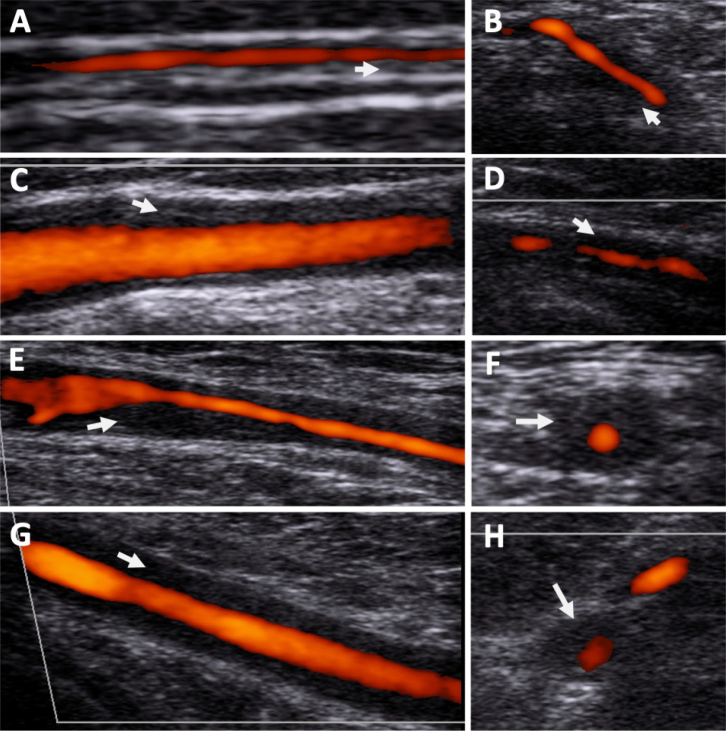
Vascular Doppler ultrasound (Fig. 2) was repeated at our hospital showing a bilateral temporal halo sign as well as bilateral axillary and subclavian diffuse parietal hypoechoic wall thickening. There was significant stenosis observed in the right axillary and left subclavian arteries and left VA wall thickening and bilateral common carotid artery hypoechoic wall thickening.
Figure 2.
Vascular ultrasound showing evidence of hypoechoic vessel wall thickening compatible with large vessel Vasculitis. (A) Right temporal artery parietal branch; (B) Left temporal artery common; (C) Right common carotid artery; (D) Left vertebral artery; (E) Right axillary artery; (F) Right axillary artery transverse plane; (G) Left subclavian artery; (H) Left subclavian artery transverse plane. Arrows show thickened vessel wall.
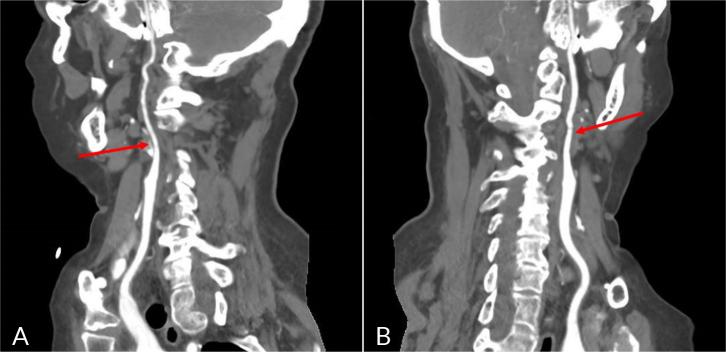
A repeated CE CT angiography (Fig. 3) showed multiple irregularities in the calibre of the intracranial segments of the ICA. It identified subocclusive stenosis in the ophthalmic segments of both carotid arteries and in the laceration of the right ICA, also stenosis greater than 50% in the cavernous segment of the left ICA. An occlusion of the V2 segment of the left VA was found, in addition to stenosis greater than 50% in the V3 segment.
Figure 3.
Cranioencephalic CT angiography showed multiple irregularities in the calibre of the intracranial segments of the ICA, identifying sub occlusive stenosis in the ophthalmic segments of both carotid arteries (A, right ICA; B, left ICA).
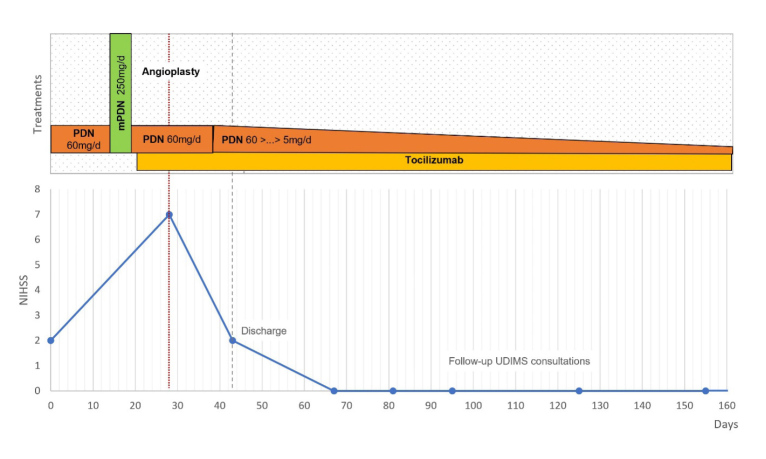
The diagnosis of GCA with large vessel cranial and extracranial involvement was reinforced, with extra and intracranial artery stenosis as the aetiology of ischaemic cerebral infarctions in multiple places. After multidisciplinary consulting, the patient was started on endovenous methylprednisolone pulses (250 mg daily for five days) followed by PDN 60 mg/day with taper, and it was decided to add tocilizumab (first administration endovenous 8 mg/kg, followed by subcutaneous 162 mg weekly) (Fig. 4).
Figure 4.
The treatment course of the case along with the evolution of the patient’s NIHSS; the date of admission was set as the zero day. Abbreviations: mPDN, methylprednisolone, PDN, prednisolone.
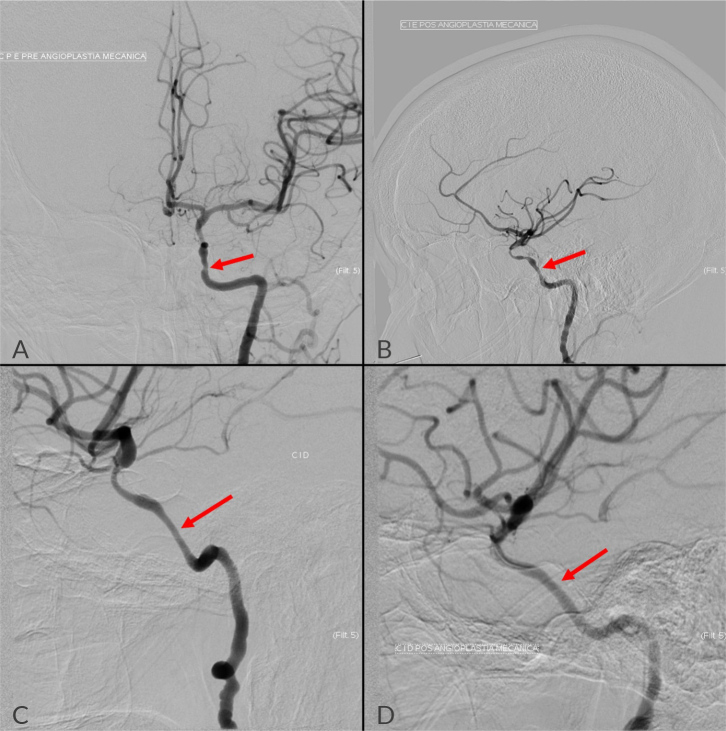
Despite complete normalisation of acute phase reactants and resolution of inflammatory symptoms such as PMR, the patient did not show neurologic improvement. A few days later, the patient reported left temporal pain and presented with significant worsening neurological deficits. She was drowsy, failing to answer questions and perform tasks, with decreased strength and sensitivity in the left upper limb, and maintaining the nystagmus, left CFP and left dysmetria (NIHSS 7). A CE CT scan confirmed the presence of a recent right occipital cortico-subcortical ischaemic lesion in the territory of the right posterior cerebral artery. Given the evidence of disease progression with new vascular events, and following consultation with Neuroradiology, a bilateral balloon angioplasty (Fig. 5) was conducted on both ICA, with improved calibre and downstream filling, and without immediate complications. After endovascular treatment (EVT), the patient showed sustained clinical improvement, without recurrence of ischaemic events (NIHSS 2).
Figure 5.
Pretreatment angiography showed the previously described changes – stenosis of 70% on the right ICA (A) and 50% on the left (C). After endovascular treatment, it was noted an improved blood flow in both ICA, more pronounced on the right side (B, right ICA; D, left ICA).
To complement the study, a fluorodeoxyglucose (FDG)-positron emission tomography (PET) scan was also performed, showing large vessel vasculitis affecting predominantly the thoracic aorta, subclavian, axillary, carotid and iliofemoral arteries.
The patient was later discharged under ASA 100 mg once daily, rosuvastatin/ezetimibe 10/10 mg once daily, PDN 30 mg once daily on tapering and tocilizumab 162 mg once weekly.
During one-year follow-up consultations, the patient continued to show good neurologic progress (NIHSS 0). Follow-up vascular ultrasound performed at 3 and 12 months found complete resolution of temporal artery halo signs, as well as an improvement in axillary-subclavian artery wall thickening and stenosis. The patient continued corticoid tapering allowing a dose reduction to a minimum of PDN 2.5 mg once daily, while maintaining tocilizumab.
DISCUSSION
GCA is the most common systemic vasculitis, predominantly affecting large- and medium-sized arteries in people aged over 50 years, with a prevalence 2–3 times higher in women[1,2]. Recently, the clinical spectrum of GCA has been expanding to include both cranial and extracranial arteries[1,3]. Initially considered uncommon, large vessel involvement is currently acknowledged in up to 75% of patients with GCA: subclavian and axillary arteries (42.5% – 70%), aorta (50% – 65%), cervical carotids (35% – 40%) and VA (10%)[4–6]. The recent increasing use of high-sensitivity MR vessel-wall imaging also showed up to 40% intracranial arterial involvement[7].
While temporal artery biopsy has traditionally represented the gold standard diagnostic tool for GCA, the recognised complications and high false negative rate have resulted in increased utilisation of imaging investigations in GCA diagnosis[8]. Recently, vascular ultrasound has gained popularity, facilitating a quick, cost-effective and non-invasive GCA diagnosis, being also useful in disease monitoring, as in most patients wall thickening improves throughout treatment[1,3]. MR angiography can also be performed for GCA diagnosis and, together with FDG-PET and CT angiography, plays a key role in detecting inflammation and damage in extracranial vessels[1,8].
Ischaemic stroke is a rare but well-described complication in GCA, occurring in 2% – 7% of patients, commonly resulting from vertebrobasilar system involvement[1,9–12]. In patients experiencing severe intracranial manifestations of GCA, mortality rates may reach up to 58% despite intensive medical therapy[9].
Although glucocorticoids remain the primary treatment for GCA, tocilizumab, an interleukin-6 inhibitor, has emerged as a crucial adjunctive therapeutic option, ensuring effective disease control allowing lower cumulative corticosteroid doses and fewer disease relapses[1,3,13].
Infrequently, GCA may manifest with aggressive intracranial arterial stenoses that prove resistant to medical therapy, and patients may develop refractory neurologic deficits and cerebral infarcts, making GCA a potentially life-threatening condition[9].
EVT, such as percutaneous transluminal angioplasty (PTA) with or without stenting or calcium channel blocker infusion, stands as a potential intervention to prevent ischaemic complications in intracranial GCA, despite lack of evidence about its safety and efficacy in these patients[9]. PTA seems safe and effective in peripheral vessels, though its long-term durability remains uncertain[14]. The occurrence of restenosis is estimated at 33% following a single-session treatment and 18.4% after multiple interventions[15]. Furthermore, there is a notable risk of spontaneous dissection in this population, attributed to vessel-wall inflammation, with an estimated incidence of 25%[16].
Considering the uncertain efficacy and substantial potential risks, EVT for intracranial GCA is rarely described in the literature[9]. To our knowledge, there are other 15 patients described in the literature where intracranial EVT has been performed, comprising a total of 20 EVT procedures (Table 1). When including our patient and accounting for the complications reported, the prevalence of restenosis was 6/19 procedures (32%) and that of dissection was 2/19 procedures (11%) (Table 1). Despite the low numbers, this seems inferior to the figures presented in PTA in peripheral arteries. Of note, all patients underwent intracranial EVT while on a high dose of corticosteroids and 11/16 patients (69%) had an additional immunosuppressant drug prior to the procedure (Table 1).
Table 1.
Intracranial endovascular treatment in patients with giant cell arteritis with intracranial vasculitis stenosis. Data shows a systematic literature review including the patient described in this work.
| Reference | Age (years) | Sex | Number of treatments | Technique | Vessels | Stenosis grade | Previous corticosteroid treatment and adjuvant immunosuppressant | Complications | Worst NIHSS/mRS* | Post treatment NIHSS/mRS* | Clinical follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Current article | 68 | F | 1 | Endovascular intracranial angioplasty | Bilateral ICA | 70% in right ICA and 50% in left ICA | PDN 60 mg, mPDN (500 mg IV) 5 days, tocilizumab | None | NIHSS 7 | NIHSS 0 | 12 months |
| Takatsuki et al. 2023[17] | 68 | F | 2 | Endovascular intracranial angioplasty | Left MCA × 2, left ICA × 2 | 84% in left MCA | mPDN (500 mg IV) 3 days and then PDN 60 mg and MTX (unknown dose) | Restenosis. Balloon angioplasty was repeated. | NIHSS 8 | NIHSS 4 | 8 months |
| Simonsen et al. 2020[2] | 65 | F | 1 | Endovascular intracranial angioplasty | Left ICA | Unknown | mPDN (1 g IV) 3 days, then PDN (100 mg with taper) and tocilizumab. CPM and then MTX |
Restenosis of the vessel at the initial treatment, only treated with immunosuppressant. | Unknown | mRS 2 | 90 days |
| 64 | F | 2 | Endovascular intracranial angioplasty | Left ICA × 3, right ICA × 2 | Unknown | mPDN (1 g IV) 3 days, then PDN (100 mg with taper). CPM (15 mg/kg, every 3 weeks) | Small dissection. Restenosis. Balloon angioplasty was repeated. |
Unknown | mRS 3 | 90 days | |
| 72 | F | 2 | Endovascular intracranial angioplasty | Left ICA × 2, right ICA × 1, left VA × 1 | Unknown | mPDN (1g IV) 3 days, then PDN (100 mg with taper). CPM 10 mg/Kg for 6 treatments and then MTX | Restenosis. Balloon angioplasty was repeated. | Unknown | mRS 1 | 90 days | |
| 71 | F | 2 | Endovascular intracranial angioplasty | Left ICA × 2, right ICA × 2, left vertebral × 1 | Unknown | mPDN (1 g IV) 3 days, then PDN (100 mg with taper). CPM (10 mg/Kg) for 6 treatments and then MTX | Dissection of vertebral artery. Restenosis. Balloon angioplasty was repeated. |
Unknown | mRS 4 | 90 days | |
| Lago et al. 2020[18] | 72 | F | 1 | Endovascular intracranial angioplasty | Left ICA × 1 | Unknown | PDN (60 mg/day with taper) | None | Unknown | mRS 2 | 16 months |
| 73 | F | 1 | Endovascular intracranial angioplasty | Left VA | Unknown | PDN (unknown dose) | None | Unknown | mRS 3 | 17 months | |
| Togo et al. 2018[19] | 80 | F | 1 | Endovascular intracranial angioplasty | Right VA | Unknown | mPDN (1 g IV) 3 days, then PDN 45 mg. CPM (670 mg | None | Unknown | mRS 3 | 27 months |
| Guerrero et al. 2015[20] | 59 | F | 1 | Endovascular intracranial angioplasty and stent placement | Left ICA | 90% | mPDN (1 g IV) 3 days, then PDN (1 mg/Kg daily). MTX (7.5 mg weekly) | None | Unknown | mRS 2 | 32 months |
| Neutel et al. 2014[21] | 65 | M | 1 | Endovascular intracranial angioplasty | Left ICA | Unknown | PDN (1 mg/Kg/day). MTX (15 mg weekly) | None | Unknown | Unknown | 1 year |
| Dementovych et al. 2012[22] | Unknown | Unknown | 1 | Endovascular intracranial angioplasty and stent placement | Right vertebral artery at the V3 and V4 junction | Better than 80% | PDN (50 mg od) and AZA (50 mg od) | None | NIHSS 13 | NIHSS 3 | 30 days |
| Chausson et al. 2010[23] | 78 | F | 1 | Endovascular intracranial angioplasty and stent placement | Bilateral ICA | Better than 90% | mPDN (120 mg IV) | None | Unknown | Unknown | 7 days |
| Steiger et al. 2018[24] | 77 | F | 1 | Endovascular intracranial angioplasty and stent placement | Right VA | High-dose corticosteroids. Interleukin-6 receptor blocker therapy | None | Unknown | mRS 1 | 60 days | |
| Espígol-Frigolé et al. 2009[25] | Unknown | Unknown | 1 | Endovascular intracranial angioplasty | Bilateral ICA Bilateral VA |
Unknow | High-dose corticosteroids | Unknown | Unknown | Unknown | 18 months |
| Unknown | Unknown | 1 | Endovascular intracranial angioplasty | Bilateral ICA | Unknown | High-dose corticosteroids | Unknown | Unknown | Unknown | 18 months |
Abbreviations:
AZA, azathioprine; CPM, cyclophosphamide; F, female; ICA, internal carotid artery; IV, intravenous; MCA, middle cerebral artery; mPDN, methylprednisolone; mRS, Modified Rankin Scale; MTX, methotrexate; NIHSS, National Institutes of Health Stroke Scale; PDN, prednisolone; VA, vertebral artery.
mRS based on the mRS reported in the study or inferred from clinical description.
Although it seems reasonable to postpone intravascular procedures of vasculitic arteries until disease activity is stabilised, so that the occurrence of complications may be reduced, the occurrence of neurologic manifestations often warrants urgent treatment.
Therefore, we believe that in cases of intracranial stenoses refractory to medical therapy, EVT is a possible and effective treatment.
Acknowledgements
The authors express their gratitude to Dr Catarina Perry da Câmara, Neuroradiologist at Centro Hospitalar Universitário de Lisboa Central, for her significant contribution in performing diagnostic and therapeutic angioplasty procedures, which proved essential for clinical improvement of the patient.
Footnotes
Conflicts of Interests: The Authors declare that there are no competing interests.
Patient Consent: Written informed consent was obtained from the patient.
REFERENCES
- 1.Farina N, Tomelleri A, Campochiaro C, Dagna L. Giant cell arteritis: Update on clinical manifestations, diagnosis, and management. Eur J Intern Med. 2023;107:17–26. doi: 10.1016/j.ejim.2022.10.025. [DOI] [PubMed] [Google Scholar]
- 2.Simonsen NCZ, Speiser L, Hansen IT, Jayne D, von Weitzel-Mudersbach P. Endovascular Treatment of Intracerebral Giant Cell Arteritis. Front Neurol. 2020;11:287. doi: 10.3389/fneur.2020.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Serôdio JF, Trindade M, Favas C, Alves JD. Extra-Cranial Involvement in Giant Cell Arteritis. In: Chaudhry AI, editor. Giant-Cell Arteritis [Internet] IntechOpen; 2022. [Google Scholar]
- 4.Prieto-González S, Arguis P, García-Martínez A, Espígol-Frigolé G, Tavera-Bahillo I, Butjosa M, et al. Large vessel involvement in biopsy-proven giant cell arteritis: prospective study in 40 newly diagnosed patients using CT angiography. Ann Rheum Dis. 2012;71:1170–1176. doi: 10.1136/annrheumdis-2011-200865. [DOI] [PubMed] [Google Scholar]
- 5.Blockmans D, de Ceuninck L, Vanderschueren S, Knockaert D, Mortlemans L, Bobbaers H. Repetitive 18F-fluorodeoxyglucose positron emission tomography in giant cell arteritis: a prospective study of 35 patients. Arthritis Rheum. 2006;55:131–137. doi: 10.1002/art.21699. [DOI] [PubMed] [Google Scholar]
- 6.Bull Haaversen AC, Brekke LK, Kermani TA, Molberg O, Diamantopoulos AP. Extended ultrasound examination identifies more large vessel involvement in patients with giant cell arteritis. Rheumatology (Oxford) 2023;62:1887–1894. doi: 10.1093/rheumatology/keac478. [DOI] [PubMed] [Google Scholar]
- 7.Siemonsen S, Brekenfeld C, Holst B, Kaufmann-Buehler A-K, Fiehler J, Bley TA. 3T MRI reveals extra- and intracranial involvement in giant cell arteritis. AJNR Am J Neuroradiol. 2015;36:91–97. doi: 10.3174/ajnr.A4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong J, Chan S, Shetty A. A Case of Giant Cell Arteritis Presenting As Catastrophic Posterior Circulation Stroke: A Diagnostic Dilemma. Cureus. 2022;14(8):e27961. doi: 10.7759/cureus.27961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caton MT, Jr, Mark IT, Narsinh KH, Baker A, Cooke DL, Hetts SW, et al. Endovascular Therapy for Intracranial Giant Cell Arteritis: Systematic Review, Technical Considerations and the Effect of Intra-arterial Calcium Channel Blockers. Clin Neuroradiol. 2022;32:1045–1056. doi: 10.1007/s00062-022-01171-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez-Gay MA, Vazquez-Rodriguez TR, Gomez-Acebo I, Pego-Reigosa R, Lopez-Diaz MJ, Vazquez-Triñanes MC, et al. Strokes at time of disease diagnosis in a series of 287 patients with biopsy-proven giant cell arteritis. Medicine (Baltimore) 2009;88:227–235. doi: 10.1097/MD.0b013e3181af4518. [DOI] [PubMed] [Google Scholar]
- 11.Salvarani C, Della Bella C, Cimino L, Macchioni P, Formisano D, Bajocchi G, et al. Risk factors for severe cranial ischaemic events in an Italian population-based cohort of patients with giant cell arteritis. Rheumatology (Oxford) 2009;48:250–253. doi: 10.1093/rheumatology/ken465. [DOI] [PubMed] [Google Scholar]
- 12.Soriano A, Muratore F, Pipitone N, Boiardi L, Cimino L, Salvarani C. Visual loss and other cranial ischaemic complications in giant cell arteritis. Nat Rev Rheumatol. 2017;13:476–484. doi: 10.1038/nrrheum.2017.98. [DOI] [PubMed] [Google Scholar]
- 13.Stone JH, Tuckwell K, Dimonaco S, Klearman M, Aringer M, Blockmans D, et al. Trial of Tocilizumab in Giant-Cell Arteritis. N Engl J Med. 2017;377:317–328. doi: 10.1056/NEJMoa1613849. [DOI] [PubMed] [Google Scholar]
- 14.Chatterjee S, Flamm SD, Tan CD, Rodriguez ER. Clinical diagnosis and management of large vessel vasculitis: giant cell arteritis. Curr Cardiol Rep. 2014;16:498. doi: 10.1007/s11886-014-0498-z. [DOI] [PubMed] [Google Scholar]
- 15.Both M, Aries PM, Müller-Hülsbeck S, Jahnke T, Schäfer PJ, Gross WL, et al. Balloon angioplasty of arteries of the upper extremities in patients with extracranial giant-cell arteritis. Ann Rheum Dis. 2006;65:1124–1130. doi: 10.1136/ard.2005.048470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nuenninghoff DM, Hunder GG, Christianson TJH, McClelland RL, Matteson EL. Incidence and predictors of large-artery complication (aortic aneurysm, aortic dissection, and/or large-artery stenosis) in patients with giant cell arteritis: a population-based study over 50 years. Arthritis Rheum. 2003;48:3522–3531. doi: 10.1002/art.11353. [DOI] [PubMed] [Google Scholar]
- 17.Takatsuki K, Kojima Y, Ikeuchi Y, Kitayama J, Tanaka A, Inoue Y. Intracranial vascular stenosis in giant cell arteritis successfully treated by two balloon angioplasty procedures. Mod Rheumatol Case Rep. 2022;7:166–171. doi: 10.1093/mrcr/rxac080. [DOI] [PubMed] [Google Scholar]
- 18.Lago A, Tembl JI, Fortea G, Morales L, Nieves C, Campins M, et al. Stroke and temporal arteritis: A study of 6 cases. Neurologia (Engl Ed) 2020;35:75–81. doi: 10.1016/j.nrl.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Togo M, Kono T, Hoshi T, Imamura H, Todo K, Adachi H, et al. Successful endovascular therapy for multiple intracranial arterial stenosis associated with medically intractable giant cell arteritis. J Neurol Sci. 2018;384:104–106. doi: 10.1016/j.jns.2017.11.027. [DOI] [PubMed] [Google Scholar]
- 20.Guerrero AM, Sierra-Hidalgo F, Calleja P, Navia P, Campollo J, Díaz-Guzmán J. Intracranial internal carotid artery angioplasty and stenting in giant cell arteritis. J Neuroimaging. 2015;25:307–309. doi: 10.1111/jon.12116. [DOI] [PubMed] [Google Scholar]
- 21.Neutel D, Biscoito L, Campos J, e Melo TP, Albuquerque L. Giant cell arteritis with symptomatic intracranial stenosis and endovascular treatment. Neurol Sci. 2014;35:609–610. doi: 10.1007/s10072-013-1596-1. [DOI] [PubMed] [Google Scholar]
- 22.Dementovych N, Mishra R, Shah QA. Angioplasty and stent placement for complete occlusion of the vertebral artery secondary to giant cell arteritis. J Neurointerv Surg. 2012;4:110–113. doi: 10.1136/jnis.2011.004689. [DOI] [PubMed] [Google Scholar]
- 23.Chausson N, Olindo S, Signaté A, Cohen-Ténoudji P, Aveillan M, Saint-Vil M, et al. Angioplastie carotidienne bilatérale chez une patiente avec infarctus cérébral sur maladie de Horton [Bilateral intracerebral angioplasty in a patient with stroke caused by giant cell arteritis] Rev Neurol (Paris) 2010;166:328–332. doi: 10.1016/j.neurol.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 24.Steiger R, Walchhofer L-M, Rietzler A, Mair KJ, Knoflach M, Glodny B, et al. Cerebral Phosphorus Magnetic Resonance Spectroscopy in a Patient with Giant Cell Arteritis and Endovascular Therapy. Case Rep Radiol. 2018;2018:7806395. doi: 10.1155/2018/7806395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Espígol-Frigolé G, Gomez-Choco M, Obach V, Sanroman L, Prieto-González S, Argelich R, et al. Percutaneous transluminal angioplasty of internal carotid and vertebral arteries in giant cell arteritis: report of 2 cases. APMIS. 2009;117(suppl):104–105. [Google Scholar]