Abstract
Background
Few longitudinal studies available characterize long COVID outcomes out to 24 months, especially in people with nonsevere acute coronavirus disease 2019 (COVID-19). This study sought to prospectively characterize incidence and duration of long COVID symptoms and their association with quality of life (QoL) from 1–24 months after mild-to-moderate COVID-19 using validated tools in a diverse cohort of unvaccinated people infected with SARS-CoV-2 in 2020.
Methods
At 1–3, 6, 12, 18, and 24 months post-COVID-19, 70 participants had orthostatic vital signs measured, provided blood, and completed surveys characterizing symptoms, QoL, and return to pre-COVID-19 health and activities using validated tools (FLU-PRO+, Fatigue Severity Scale, Insomnia Severity Index, General Practitioner Assessment of Cognition, Patient Health Questionnaire Depression 8-Item, Generalized Anxiety Disorder 7-Item, 36-Item Short-Form Health Survey, EuroQol EQ-5D-5L).
Results
During the study period, 33% of participants experienced long COVID (had not returned to pre-COVID-19 health status and reported at least 1 symptom >90 days postinfection); 8% had not returned to their pre-COVID-19 health status 24 months postinfection. Long COVID symptoms peaked 6 months post-COVID-19, frequently causing activity limitations. Having long COVID was significantly associated with decreased QoL in multiple domains. Frequencies of orthostatic hypotension and tachycardia reflected levels reported in the general population. Within-person weight increased significantly between months 1 and 6. Long COVID was associated with pre-COVID-19 obesity and hyperlipidemia, but not with high-sensitivity C-reactive protein levels 1–3 months postinfection.
Conclusions
Long COVID occurs in a significant proportion of unvaccinated people, even if the acute illness was not severe. Long COVID prevalence peaked 6–12 months post-COVID-19, and a small proportion of participants still reported not returning to their pre-COVID-19 health status 24 months post-COVID-19.
Keywords: COVID-19, long COVID, longitudinal cohort, quality of life, SARS-CoV-2
Thirty-three percent of unvaccinated adults with mild-to-moderate acute COVID-19 experienced long COVID symptoms; 8% still had not returned to their pre-COVID-19 health status 24 months postinfection. Long COVID symptoms peaked and quality of life nadired at 6–12 months postinfection.
Millions of people globally currently experience or have previously experienced one or more symptoms or sequelae of coronavirus disease 2019 (COVID-19), known as long COVID [1–4]. Symptoms most commonly associated with long COVID include somatic disturbances (eg, fatigue, insomnia, and pain), neurological or cognitive deficits, and persistent respiratory symptoms, though many other symptoms and sequelae are commonly reported [5, 6]. According to the United States (US) Census Household Pulse Survey results from June 2023, 15.7% of US adults have ever experienced long COVID and 6.0% experience it currently [7]. Notably, 81.3% of those currently experiencing long COVID report activity limitations [7].
Despite the large global burden, limited prospective symptom data exist on long COVID through 24 months from acute severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection [8]. Published reports of long COVID more than a year after infection largely represent retrospective or cross-sectional analyses, which may be limited in their ability to evaluate the longitudinal course of long COVID progression and resolution. Moreover, data are often limited to patients hospitalized during acute COVID-19 [9–18].
Here, we prospectively characterize the incidence and duration of long COVID symptoms and their association with quality of life (QoL), orthostatic vital signs, and a commonly measured marker of inflammation—C-reactive protein—at 1–3, 6, 12, 18, and 24 months after mild-to-moderate COVID-19 using validated tools in a cohort of unvaccinated people infected with pre-Alpha strains of SARS-CoV-2 in 2020.
METHODS
We enrolled a convenience sample of nonhospitalized adults with a positive SARS-CoV-2 polymerase chain reaction (PCR) test within the Johns Hopkins Health System between April and August 2020 [19]. Recruitment efforts were targeted toward patients aged >40 years, with those aged <40 years being called in random order thereafter. Most patients were consented within 48 hours of a positive test and were followed during acute infection, while a subset was enrolled within the first month after a positive test and began follow-up at month 1–3. One hundred ten participants completed a baseline assessment and submitted acute samples, of whom 51 participated in extended follow-up and are included here. Nineteen participants who joined the study at month 1–3 also consented to extended follow-up and are included in this analysis, resulting in a total sample size of 70. During follow-up, participants completed surveys; had height, weight, and orthostatic vital signs measured; and donated blood 1–3, 6, 12, 18, and 24 months post–acute COVID-19 illness onset. At enrollment, participants were asked about the current or past presence of 77 medical problems/diagnoses and open-ended questions about whether they had any other medical problems. Participants were enrolled for extended follow-up regardless of whether or not they reported symptoms at any time.
Patient Consent Statement
Informed oral consent was obtained from all participants. This study was approved by the Johns Hopkins University School of Medicine Institutional Review Board, who approved an oral consent process due to the risks of obtaining written consent from participants with acute COVID-19. Written consent forms were provided to participants.
Validated survey tools used are described and displayed in the Supplementary Methods, Supplementary Table 1, and the Supplementary References. Participants reported the presence and severity of 49 long COVID symptoms: 38 derived from the FLU-PRO+ and 11 additional symptoms (after the month 1–3 visit) based on results from a Long COVID Patient-Led Research Collaborative survey with input from the collaborative (Supplementary Table 2) [5, 20]. Participants were asked at each study visit if they had any other symptoms not already listed. Additionally, participants were asked, “Have you returned to your usual health today?” and “Have you returned to your usual activities today?” We defined long COVID as self-reported lack of return to usual pre-COVID-19 health status plus endorsement of at least 1 symptom (from the 49 queried or any other self-reported symptom) 90 or more days after acute COVID-19 illness onset. Six participants across 7 study visits reported not returning to their usual health but endorsed no symptoms and were classified as not having long COVID. Beginning at the month 6 visit, participants were asked if they had experienced any respiratory illness, been hospitalized, or received COVID-19 testing since their last visit. Participants also submitted nasal and oral swabs for reverse-transcription PCR testing for COVID-19 reinfection at each study visit.
The following additional validated tools were introduced in surveys after the month 1–3 visit. Participants reporting fatigue completed the Fatigue Severity Scale (FSS), and those endorsing sleep disturbances completed the Insomnia Severity Index (ISI). Depression and anxiety were assessed among all participants using the Patient Health Questionnaire Depression 8-Item (PHQ-8) and Generalized Anxiety Disorder 7-Item (GAD-7). Self-assessed cognition was evaluated among all participants using the General Practitioner Assessment of Cognition (GPCOG) informant interview. QoL was measured among all participants using the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36) and the EuroQol EQ-5D-5L visual analog scale. We used the RAND 36-Item Health Survey 1.0 scoring method to convert SF-36 questions into 8 QoL domains: physical functioning (PF), physical limitation of role fulfillment (PL), emotional limitation of role fulfillment (EL), energy/fatigue (EF), emotional well-being (EW), social functioning (SF), pain (PA), and general health (GH). PL and EL were consistently scored as perfect (100) by participants, so they are not shown or discussed here.
Orthostatic vital signs were recorded per Centers for Disease Control and Prevention guidelines, and we used standard definitions of orthostatic hypotension [21] and orthostatic tachycardia [22]. Complete blood counts with differential were run same-day at onsite clinical laboratories. High-sensitivity C-reactive protein (hs-CRP) (cobas, Roche, Switzerland), N-terminal-pro-b-type natriuretic peptide (NT-proBNP) (Dimension Vista, Siemens, Germany), and high-sensitivity cardiac troponin (hs-cTnI) (Atellica, Siemens, Germany) were quantified from frozen serum at the Johns Hopkins Hospital Clinical Laboratory (hs-CRP) and the Clinical Chemistry Research Laboratory at the University of Maryland Baltimore (NT-proBNP, hs-cTnI).
Survey data were collected and stored using the REDCap platform [23]. Analyses were performed using Stata version 16.0 software [24]. At each timepoint, participants missing data were excluded only from analyses requiring those missing data, but not from the overall cohort evaluation. The number of participants followed up at each timepoint are shown in Supplementary Table 3, and sample sizes for each analysis are provided in table headers and legends. The t test or rank-sum test was used to compare continuous variables between groups, and Pearson χ2 or Fisher exact test was used to compare categorical variables. Fisher exact test was used only where the number of participants reporting a given condition was <5. Paired t tests were used to evaluate within-person changes in vital signs and laboratory values over time.
RESULTS
Cohort Characteristics
Among the 70 participants, the median age was 55 years (interquartile range [IQR], 43–63 years) and 63% were female sex at birth. Fourteen percent identified as Hispanic of any race, 26% as non-Hispanic Black/African American, 53% as non-Hispanic White, and 7% as other race(s) (Table 1). Thirty-seven (53%) had body mass index (BMI) ≥30 kg/m2, 27 (39%) had hypertension, and 9 (13%) had preexisting diabetes. All participants were unvaccinated at the time of SARS-CoV-2 infection and the month 1–3 study visit, as COVID-19 vaccines were not available to the public at that time. Sixty-five (93%) received at least 1 dose of a COVID-19 vaccine during the 24-month follow-up period. Among the 51 participants who submitted acute COVID-19 symptom data, the median peak FLU-PRO score was 0.375 (IQR, 0.156–0.625). Further characterization of the acute course of infection in this cohort has been described previously [19]. Of the 70 participants in the study, 2 required hospitalization during the acute phase of COVID-19. Both hospitalized participants required supplemental oxygen during their hospital stay. Two participants enrolled in outpatient randomized controlled trials of convalescent plasma, 1 of whom was subsequently hospitalized and later received remdesivir and dexamethasone. Four participants (6%) tested newly positive for COVID-19 or self-reported having been reinfected during the follow-up period. One participant died of unrelated causes approximately 18 months into the follow-up period.
Table 1.
Demographic Characteristics of Participants Who Provided Consent for Extended Follow-up (N = 70)
| Characteristic | No. (%) |
|---|---|
| Demographic characteristics | |
| Age, y, median (IQR) | 55 (43–63) |
| BMIa, kg/m2, median (IQR) | 30 (25–35) |
| Sex (recorded at birth) | |
| Female | 44 (62.9) |
| Male | 26 (37.1) |
| Race/ethnicity | |
| Hispanic | 10 (14.3) |
| Non-Hispanic Black/African American | 18 (25.7) |
| Non-Hispanic Other | 5 (7.1) |
| Non-Hispanic White | 37 (52.9) |
| Baseline comorbidities | |
| Overweight (BMI ≥25 kg/m2)a | 54 (77.1) |
| Obesity (BMI ≥30 kg/m2)a | 37 (52.9) |
| Hypertension | 27 (38.6) |
| Hyperlipidemia | 15 (21.4) |
| Depression | 13 (18.6) |
| Asthma/COPD/emphysema | 11 (15.7) |
| Anxiety | 10 (14.3) |
| Diabetes (type 2) | 9 (12.9) |
| Taking insulinb | 4 (5.7) |
| Past or current cancer diagnosis | 7 (10.0) |
| Immunocompromisedc | 9 (12.9) |
| Immunosuppressing medicationd | 7 (10.0) |
| Solid organ transplant recipient | 2 (2.9) |
| Autoimmune disorder | 3 (4.3) |
| Living with HIVe | 2 (2.9) |
| Chronic kidney diseasef | 2 (2.9) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; HIV, human immunodeficiency virus; IQR, interquartile range.
aBaseline BMI was missing for 3 participants.
bFour people with type 2 diabetes took insulin, 4 did not, and 1 did not answer.
cSee Supplementary Methods for definition of immunocompromised status.
dImmunosuppressing medications included prednisone or other steroid at any dosage (n = 5), tacrolimus (n = 2), hydroxychloroquine (n = 1), and mycophenolate mofetil/mycophenolic acid (n = 1).
eBoth participants with HIV reported taking antiretroviral therapy.
fChronic kidney disease stages included stage 2 (n = 1) and stage 4 (n = 1).
Long COVID
At the month 6 and 12 study visits, 24% of participants who completed the survey at that time point had long COVID. At month 18, 15% of respondents had long COVID and at month 24, 6% had long COVID (Table 2). Twenty-four (33%) participants ever had long COVID during the 24-month follow-up period. Long COVID was associated with pre-COVID-19 obesity and hyperlipidemia but not any other comorbidity or demographic characteristic (Supplementary Table 4). Long COVID status was not associated with acute hospitalization, COVID-19 treatment, or reinfection, although these outcomes were rare in our cohort.
Table 2.
Quality of Life, Mental Health, and Cognition by Long COVID Status at Each Timepointa
| Tool | Month 6 (n = 33) | Month 12a (n = 42) | Month 18a (n = 33) | Month 24 (n = 51) | ||||
|---|---|---|---|---|---|---|---|---|
| Long COVID (n = 8) | Long COVID (n = 10) | Long COVID (n = 5) | Long COVID (n = 3) | |||||
| Diff. in Meansb | P Valuec | Diff. in Meansb | P Valuec | Diff. in Meansb | P Valuec | Diff. in Meansb | P Valuec | |
| SF-36 quality of life domaind | ||||||||
| PF | 12.3 | .067 | 14.7 | .027* | 1.0 | .821 | 15.8 | .157 |
| EF | 25.5 | .007** | 26.3 | .003** | −0.2 | .825 | 29.7 | .037* |
| EW | 10.2 | .106 | 10.1 | .059 | 6.2 | .142 | −6.2 | .791 |
| SF | 5.6 | .353 | 14.2 | .062 | −4.7 | .454 | 16.6 | .198 |
| PA | 3.3 | .508 | −0.27 | .716 | −15.4 | .085 | 13.2 | .453 |
| GH | 11.0 | .396 | 12.0 | .049* | 7.5 | .619 | 9.1 | .495 |
| EQ-5D-5Le | 4.0 | .199 | 14.6 | .013* | 10.8 | .045* | 10.8 | .128 |
| GAD-7e | — | — | −1.66 | .600 | −2.40 | .230 | −2.56 | .174 |
| PHQ-8e | — | — | −2.92 | .209 | −0.39 | .791 | −7.69 | .003** |
| GPCOGe | 0.65 | .143 | 0.28 | .562 | 0.07 | .978 | 0.77 | .403 |
Abbreviations: COVID, coronavirus disease 2019; EQ-5D-5L, EuroQol Quality of Life visual analog scale (general health question only); GAD-7, Generalized Anxiety Disorder 7-Item; GPCOG, General Practitioner Assessment of Cognition (self-report section only); PHQ-8, Patient Health Questionnaire 8-Item Depression Scale.
aOne participant at month 12 and 3 participants at month 18 were missing quality of life data and were excluded.
bDifference between mean scores among those who had long COVID and those who did not (without long COVID – with long COVID). Positive values indicate higher scores among those without long COVID.
cStatistical significance was determined using rank-sum tests: *P < .05, **P < .01; — indicates insufficient data.
dSF-36 quality of life domains: EF, Energy/Fatigue; EW, Emotional Well-being; GH, General Health; PA, Pain; PF, Physical Functioning; SF, Social Functioning.
eQuality of life and cognition were not evaluated at the month 1–3 timepoint. GAD-7 and PHQ-8 scores were not evaluated at the month 1–3 or month 6 timepoint.
Return to Activities
When asked if they had returned to their usual pre-COVID-19 activities, 93% reported that they had at month 1–3, as had 94% at month 6, 80% at month 12, 87% at month 18, and 96% at month 24 (Table 3). Between 11% and 44% of participants reported that their symptoms interfered with completing their daily activities across all timepoints, peaking at month 6 (Table 3). Eleven participants (16%) reported not having returned to their usual activities after reporting a return to usual activities at an earlier timepoint, showcasing long COVID's waxing-waning nature. At each study event, a higher proportion of participants reporting having returned to their pre-COVID-19 activities than to their pre-COVID-19 health status, indicating that some participants returned to work or other activities before feeling fully recovered.
Table 3.
Prevalence of Long COVID, Return to Health, Return to Activities, and Activity Limitations at Each Timepoint
| Status | Month 1–3 (n = 56a) | Month 6 (n = 33b) |
Month 12 (n = 45c) | Month 18 (n = 37d) | Month 24 (n = 52e) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | (%) | No. | (%) | No. | (%) | No. | (%) | No. | (%) | |
| Long COVID | NA | NA | 8 | (24.2) | 10 | (22.2) | 5 | (13.5) | 3 | (5.8) |
| Not returned to health | 10 | (17.9) | 9 | (27.3) | 11 | (24.4) | 7 | (18.9) | 4 | (7.7) |
| Not returned to activities | 4 | (7.1) | 2 | (6.1) | 7 | (15.6) | 5 | (13.5) | 1 | (1.9) |
| Symptoms interfering with activities | 6 | (10.7) | 11 | (33.3) | 7 | (15.6) | 4 | (10.8) | 9 | (17.3) |
| A little bit | 5 | (8.9) | 7 | (21.2) | 5 | (11.1) | 2 | (5.4) | 7 | (13.5) |
| Somewhat | 0 | (0) | 4 | (12.1) | 1 | (2.2) | 1 | (2.7) | 1 | (1.9) |
| Quite a bit | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) | 1 | (1.9) |
| Very much | 1 | (1.8) | 0 | (0) | 1 | (2.2) | 1 | (2.7) | 0 | (0) |
| Prefer not to answer | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) | 2 | (3.8) |
Percentages reflect the proportion of the total number listed at the top of each column for whom each characteristic is true.
Abbreviation: NA, not applicable.
aAt month 1–3, 1 participant was missing return to activities and activity limitation data.
bAt month 6, 8 participants were missing activity limitation data.
cAt month 12, 2 participants were missing return to health status, 3 participants were missing activity limitation data, and 1 participant could not have long COVID status determined.
dAt month 18, 1 participant was missing return to health status and could not have long COVID status determined, and 6 participants were missing activity limitation data.
eAt month 24, 1 participant was missing return to health status and long COVID status could not be determined, and 5 participants were missing activity limitation data.
Biomarkers, Orthostatic Vital Signs, and Weight
Body mass index and orthostatic vital signs were recorded at each study visit, and blood samples were obtained for biomarker analysis. Rates of orthostatic hypotension and/or orthostatic tachycardia, hs-CRP, NT-proBNP, hs-cTnI, and select values from complete blood counts with differential are shown in Supplementary Table 5. No laboratory values at any time point were associated with ever having long COVID. Rates of orthostatic hypotension and tachycardia were consistent with recent population prevalence estimates, suggesting no increased rates of orthostatic tachycardia or orthostatic hypotension in this cohort [21, 22]. For participants who completed multiple timepoints, we evaluated within-person changes in weight and laboratory values over time. Within-person weight increased over time and was significantly different between months 1–3 and 6 (n = 31, mean difference = 0.48 kg/m2, P = .024) and between months 1–3 and 24 (n = 36, mean difference = 0.58 kg/m2, P = .006). Weight gain remained significant after stratification among those without long COVID, but not among those with long COVID. Those with long COVID had a higher median BMI at baseline and throughout follow-up than those without long COVID, were more likely to be overweight or obese, and experienced less total weight gain. When comparing month 1–3 to month 6, 11 participants with long COVID had a median BMI increase from 32.65 to 32.80 kg/m2 (P = .724), and 20 participants without long COVID had a median BMI increase from 30.71 to 31.52 kg/m2 (P = .014). Between months 1–3 and month 24, 13 participants with long COVID had a median BMI increase from 33.44 to 33.98 kg/m2 (P = .359), while 23 participants without long COVID had a median BMI increase from 28.65 to 29.93 kg/m2 (P = .007). No other significant within-person changes in clinical or laboratory values were observed.
Symptoms
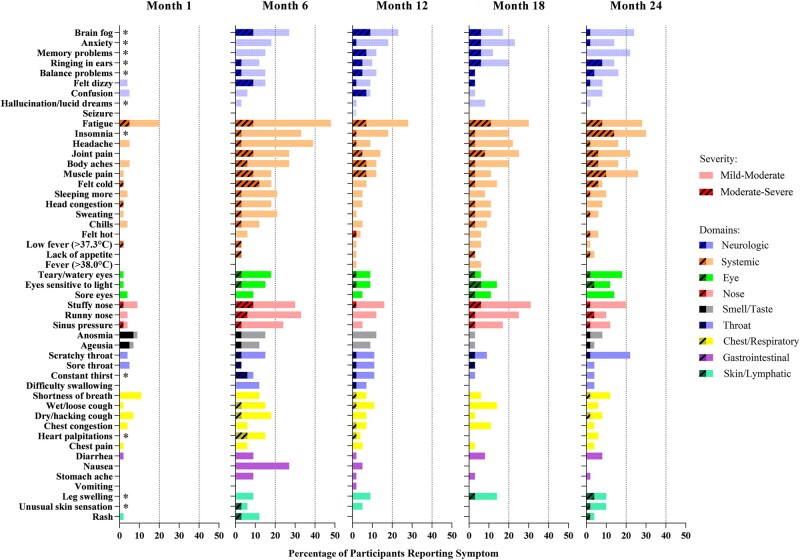
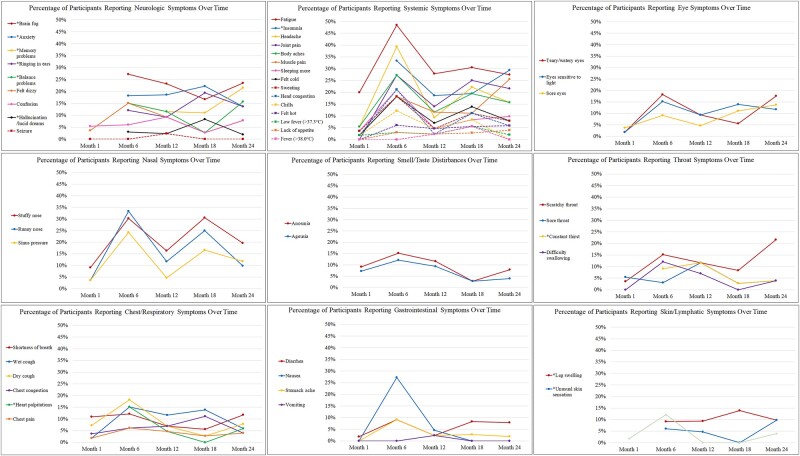
Overall, participants reported significantly fewer symptoms at month 1–3 than at later timepoints (comparing the 38 FLU-PRO+ symptoms captured at all timepoints). Participants reported the greatest number of symptoms at the month 6 timepoint, with gradual decreases thereafter. The most commonly reported symptoms across timepoints were systemic and neurological: fatigue, sleep disturbances, anxiety, problems with concentration or brain fog, memory problems, balance problems, tinnitus, muscle and joint pain, and body aches (Figure 1, Supplementary Table 2). Upper respiratory symptoms were common, but appeared to follow a seasonal pattern with higher frequency of report in colder months. Gastrointestinal symptoms, anosmia, and ageusia decreased consistently over time (Figure 2).
Figure 1.
Percentage of participants reporting symptoms by symptom domain at each timepoint. Fifty-six participants completed a survey at the month 1–3 timepoint, 33 completed a survey at month 6, 46 completed a survey at month 12, 37 completed a survey at month 18, and 52 completed a survey at month 24. *Beginning with the month 6 timepoint, 11 additional long COVID symptoms were added [5].
Figure 2.
Percentage of participants reporting symptoms over time, by symptom domain. *Beginning with the month 6 timepoint, 11 additional long COVID symptoms were added [5].
Fatigue and Sleep Disturbances
Fatigue was the most common symptom across all timepoints, with at least 20% of participants reporting it at each timepoint (Supplementary Table 2). Fatigue Severity Scale 9 (FSS-9) and ISI scores were calculated for those who reported fatigue or sleep disturbances, respectively (Supplementary Table 6). Postexertional fatigue was common, being reported by 17%–50% of participants across timepoints (Supplementary Table 6). Both insomnia and sleeping more than usual were highly reported across all timepoints (Supplementary Table 2). Between 5% and 11% of participants reported both insomnia and sleeping more than usual on the same survey, indicating multifaceted dysregulation of the sleep-wake cycle.
Self-reported Cognitive Impairment
Cognitive deficits—including brain fog or problems with concentration, memory problems, and confusion—represented a large fraction of neurologic symptoms reported (Supplementary Table 2). Five percent of participants endorsed any of the 3 cognitive symptoms at month 1–3, increasing to 30% at month 6, 24% at month 12, 19% at month 18, and 29% at month 24. The proportions of participants indicating impairment on the self-assessment portion of the GPCOG were stable across timepoints, with a trend toward more impairment at month 6 (Supplementary Table 6).
Quality of Life: SF-36
Having long COVID was significantly associated with decreased QoL in the domains of physical functioning at month 12; energy/fatigue at months 6, 12, and 24; and general health at months 12 and 18 (Table 2, Supplementary Figure 1). Similar associations between QoL and return to health status were observed regardless of symptom endorsement. In the overall cohort, median domain scores were consistent or improved slightly across timepoints, and within-person changes over time were not significant (Supplementary Table 6). QoL was significantly worse in 5 domains (physical functioning, energy/fatigue, social functioning, pain, and general health) in people with ≥3 symptoms vs those with <3 symptoms at month 6, when participants tended to report more symptoms overall (Supplementary Figure 2).
Depression and Anxiety
Participants with diagnosed anxiety or depression disorders prior to SARS-CoV-2 infection were not significantly more likely to report not having returned to pre-COVID-19 health status or activities or to be classified as having long COVID at any timepoint (Supplementary Table 7). Baseline anxiety and depression were not associated with ever having long COVID throughout the study (Supplementary Table 4). However, participants with diagnosed pre-COVID-19 anxiety or depression were more likely to report decreased QoL in all domains across multiple timepoints (Supplementary Table 7).
DISCUSSION
Here, we present a longitudinal prospective cohort study of unvaccinated people with mild-to-moderate acute COVID-19 followed from diagnosis through 24 months after initial infection with biosampling and measurement of BMI and orthostatic vital signs. We find that one-third of participants had long COVID during the study period, defined as self-reported lack of return to pre-COVID-19 health status and at least 1 symptom ≥90 days post–acute COVID-19 illness onset. Having long COVID was associated with decreased QoL in the domains of physical functioning, energy/fatigue, and general health. Risk factors for long COVID in this cohort included pre-COVID-19 obesity and hyperlipidemia. We report a high prevalence of somatic and neurologic symptoms, which remained common up to 24 months in our cohort. Symptoms peaked about 6 months postinfection, at which point reporting a higher number of symptoms was significantly associated with worse QoL in 5 domains (physical functioning, energy/fatigue, social functioning, pain, and general health). Although hs-CRP—a widely available inflammatory marker—is associated with severity of acute COVID-19, we find that it is not associated with concurrent or future risk of long COVID [25]. Although long COVID was found in a significant proportion of this cohort, we did not find greater than usual rates of orthostatic tachycardia or orthostatic hypotension in our cohort. This suggests that post-COVID-19 postural orthostatic tachycardia syndrome is not as frequent a finding as post-COVID-19 fatigue or other symptoms and sequelae. We additionally found that weight increased significantly in the post-COVID-19 period in this cohort. Whether this is reflective of a general population-wide trend toward weight gain during the COVID-19 pandemic [26] or not is difficult to discern without a COVID-19–negative control cohort.
We believe this is among the first comprehensive longitudinal cohorts with 24-month follow-up coupled with biosampling and objective weight and orthostatic vital sign measurement in nonsevere COVID-19. One large study from Switzerland assessed symptoms using electronic questionnaires in unvaccinated infected people compared to uninfected people also found the highest prevalence of long COVID at 6 months postinfection and also observed that a significant minority of people with mild-to-moderate acute COVID still had long COVID at 24 months postinfection [8]. A study from China evaluated people hospitalized with COVID-19 in early 2020 up to 24 months, finding that 55% had at least 1 symptom or sequela and that long COVID symptoms were associated with lower health-related QoL and decreased exercise capacity [13]. In a recent Spanish cross-sectional study of hospitalized and nonhospitalized COVID-19 survivors infected in March–April 2020 and surveyed at 24 months postinfection, 68% of participants reported at least 1 long COVID symptom [27]. Our finding that approximately one-third of our participants ever had long COVID during 24 months’ follow-up is consistent with the US Census Household Pulse Survey, which reported in June 2023 that 28.8% of US adults who ever had COVID-19 experienced long COVID [7], and with Chen and colleagues' long COVID pooled prevalence estimate of 34% among ambulatory patients [1]. We report that 6% of participants still had long COVID at 24 months’ follow-up, which if true in the general population, means that millions of people globally who acquired SARS-CoV-2 infection in 2020 may still be experiencing long COVID 2 years or more after infection. Other studies have reported associations of long COVID with obesity [28–30] and hyperlipidemia [31], which we observe as well. We did not observe a relationship between sex assigned at birth and long COVID, as described in other studies [1, 28, 29, 32], which may be due to sample size.
As others have observed [1, 5, 9, 10, 12–14, 27, 28, 31, 33], fatigue was the most frequently reported symptom in our study. FSS-9 scores among those endorsing fatigue or sleep disturbances in our cohort were similar to those with known fatigue-related disorders such as multiple sclerosis and sleep-wake disorders [34], and postexertional fatigue was common. The majority of those endorsing sleep disturbances in our cohort also screened positively for insomnia on the ISI. This suggests that long COVID may manifest in significant dysregulation of energy and sleep-wake functioning up to 2 years postinfection. We also found persistence of self-reported neurological and cognitive deficits, consistent with other studies [1, 10, 12–15, 27, 31, 35].
Our data extend to 24 months previously reported long COVID symptoms and their associations with QoL at earlier time points [13, 32, 33, 35–39]. Another prospective longitudinal study found that those with long COVID had decreased QoL in the SF-36 domains of physical functioning, energy/fatigue, social functioning, pain, and general health [36]—consistent with our findings—and the physical and mental health component scores of the SF-36 v.2.0 were significantly reduced in a long COVID case-control study [32]. Decreased QoL using the EuroQol visual analog scale has also been documented among adults up to 24 months postinfection [13, 33].
Importantly, we found that pre-COVID-19 diagnoses of anxiety and depression were not associated with long COVID, although those with these diagnoses at baseline did experience consistently worse QoL than those without. There is disagreement in the literature regarding the impact of psychiatric conditions on long COVID risk, with some finding positive associations [29, 40] and others none [12].
Our cohort is diverse across age, sex, race, and ethnicity and did not select for long COVID, making it largely representative of the general population who experienced mild-to-moderate COVID-19 in 2020. Furthermore, we use validated tools to measure symptoms and QoL as well as objective clinical measurements and biomarkers, allowing us to compare our cohort to others and the general population. Limitations of this study include the cohort's relatively small size, the proportion of participants who missed survey timepoints, and attrition over the follow-up period, which may lead to sampling and attrition biases. More research is needed to characterize and address the lasting morbidity associated with long COVID and its biomarkers, especially in the face of new variants and a mostly vaccinated population.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Zoe O Demko, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Tong Yu, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Sarika K Mullapudi, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
M Gabriela Varela Heslin, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Chamia A Dorsey, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Christine B Payton, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Jeffrey A Tornheim, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Paul W Blair, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA; Austere Environments Consortium for Enhanced Sepsis Outcomes, Henry M. Jackson Foundation for the Advancement of Military Medicine, Bethesda, Maryland, USA.
Shruti H Mehta, Department of Epidemiology, Johns Hopkins University Bloomberg School of Public Health, Baltimore, Maryland, USA.
David L Thomas, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Yukari C Manabe, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Annukka A R Antar, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Notes
Acknowledgments. We would like to acknowledge Sidney Saint-Hilaire, Zihan Yang, Justin Chan, Mira Prabhu, and Tanique Bennett from the OutSMART Study Team; the Outpatient Clinical Characterization Protocol for Severe Infectious Diseases (Outpatient CCPSEI) Study Team; and the Johns Hopkins Institute for Clinical and Translational Research for their support of this work. We thank Erin Michos for discussions pertaining to cardiac markers.
Financial support. This work was supported by the Henry M. Jackson Foundation for the Advancement of Military Medicine (1007957 to P. W. B. and Y. C. M.); the Sherrilyn and Ken Fisher Center for Environmental Infectious Diseases Discovery Program (to P. W. B. and Y. C. M.); and the National Institute of Allergy and Infectious Diseases (award numbers K08AI143391 to A. A. R. A. and K23AI135102 to J. A. T.).
References
- 1. Chen C, Haupert SR, Zimmermann L, Shi X, Fritsche LG, Mukherjee B. Global prevalence of post COVID-19 condition or long COVID: a meta-analysis and systematic review. J Infect Dis 2022; 226:1593–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Callard F, Perego E. How and why patients made long Covid. Soc Sci Med 2021; 268:113426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol 2023; 21:133–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rahmati M, Udeh R, Yon DK, et al. A systematic review and meta-analysis of long-term sequelae of COVID-19 2-year after SARS-CoV-2 infection: a call to action for neurological, physical, and psychological sciences. J Med Virol 2023; 95:e28852. [DOI] [PubMed] [Google Scholar]
- 5. Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021; 38:101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thaweethai T, Jolley SE, Karlson EW, et al. Development of a definition of postacute sequelae of SARS-CoV-2 infection. JAMA 2023; 329:1934–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. National Center for Health Statistics . Long COVID: Household Pulse Survey. Atlanta, GA: Centers for Disease Control and Prevention; 2023.
- 8. Ballouz T, Menges D, Anagnostopoulos A, et al. Recovery and symptom trajectories up to two years after SARS-CoV-2 infection: population based, longitudinal cohort study. BMJ 2023; 381:e074425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ballering AV, van Zon SKR, Olde Hartman TC, Rosmalen JGM; Lifelines Corona Research Initiative. Persistence of somatic symptoms after COVID-19 in the Netherlands: an observational cohort study. Lancet 2022; 400:452–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Donnachie E, Hapfelmeier A, Linde K, et al. Incidence of post-COVID syndrome and associated symptoms in outpatient care in Bavaria, Germany: a retrospective cohort study using routinely collected claims data. BMJ Open 2022; 12:e064979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fernández-de-las-Peñas C, Martín-Guerrero JD, Cancela-Cilleruelo I, Moro-López-Menchero P, Rodríguez-Jiménez J, Pellicer-Valero OJ. Exploring the trajectory recovery curve of the number of post-COVID symptoms: the LONG-COVID-EXP-CM Multicenter Study. Int J Infect Dis 2022; 117:201–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gutierrez-Martinez L, Karten J, Kritzer MD, et al. Post-acute sequelae of SARS-CoV-2 infection: a descriptive clinical study. J Neuropsychiatry Clin Neurosci 2022; 34:393–405. [DOI] [PubMed] [Google Scholar]
- 13. Huang L, Li X, Gu X, et al. Health outcomes in people 2 years after surviving hospitalisation with COVID-19: a longitudinal cohort study. Lancet Respir Med 2022; 10:863–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li D, Liao X, Liu Z, et al. Healthy outcomes of patients with COVID-19 two years after the infection: a prospective cohort study. Emerg Microbes Infect 2022; 11:1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Taquet M, Sillett R, Zhu L, et al. Neurological and psychiatric risk trajectories after SARS-CoV-2 infection: an analysis of 2-year retrospective cohort studies including 1 284 437 patients. Lancet Psychiatry 2022; 9:815–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Musheyev B, Boparai MS, Kimura R, et al. Longitudinal medical subspecialty follow-up of critically and non-critically ill hospitalized COVID-19 survivors up to 24 months after discharge. Intern Emerg Med 2023; 18:477–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang H, Li X, Huang L, et al. Lung-function trajectories in COVID-19 survivors after discharge: a two-year longitudinal cohort study. EClinicalMedicine 2022; 54:101668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fong SW, Goh YS, Torres-Ruesta A, et al. Prolonged inflammation in patients hospitalized for coronavirus disease 2019 (COVID-19) resolves 2 years after infection. J Med Virol 2023; 95:e28774. [DOI] [PubMed] [Google Scholar]
- 19. Blair PW, Brown DM, Jang M, et al. The clinical course of COVID-19 in the outpatient setting: a prospective cohort study. Open Forum Infect Dis 2021; 8:ofab007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Richard SA, Epsi NJ, Pollett S, et al. Performance of the inFLUenza Patient-Reported Outcome Plus (FLU-PRO Plus) instrument in patients with coronavirus disease 2019. Open Forum Infect Dis 2021; 8:ofab517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim MJ, FFarrell J. Orthostatic hypotension: a practical approach. Am Fam Physician 2022; 105:39–49. [PubMed] [Google Scholar]
- 22. Sebastian SA, Co EL, Panthangi V, et al. Postural orthostatic tachycardia syndrome (POTS): an update for clinical practice. Curr Probl Cardiol 2022; 47:101384. [DOI] [PubMed] [Google Scholar]
- 23. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. StataCorp . Stata statistical software: release 16. College Station, TX: StataCorp, LLC; 2019. [Google Scholar]
- 25. Cihakova D, Streiff MB, Menez SP, et al. High-value laboratory testing for hospitalized COVID-19 patients: a review. Future Virol 2021; 16:691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Restrepo BJ. Obesity prevalence among U.S. adults during the COVID-19 pandemic. Am J Prev Med 2022; 63:102–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fernández-de-las-Peñas C, Rodríguez-Jiménez J, Cancela-Cilleruelo I, et al. Post-COVID-19 symptoms 2 years after SARS-CoV-2 infection among hospitalized vs nonhospitalized patients. JAMA Netw Open 2022; 5:e2242106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Debski M, Tsampasian V, Haney S, et al. Post-COVID-19 syndrome risk factors and further use of health services in East England. PLoS Glob Public Health 2022; 2:e0001188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thompson EJ, Williams DM, Walker AJ, et al. Long COVID burden and risk factors in 10 UK longitudinal studies and electronic health records. Nat Commun 2022; 13:3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vimercati L, De Maria L, Quarato M, et al. Association between long COVID and overweight/obesity. J Clin Med 2021; 10:4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lapa J, Rosa D, Mendes JPL, Deusdará R, Romero GAS. Prevalence and associated factors of post-COVID-19 syndrome in a Brazilian cohort after 3 and 6 months of hospital discharge. Int J Environ Res Public Health 2023; 20:848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sneller MC, Liang CJ, Marques AR, et al. A longitudinal study of COVID-19 sequelae and immunity: baseline findings. Ann Intern Med 2022; 175:969–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Peluso MJ, Kelly JD, Lu S, et al. Persistence, magnitude, and patterns of postacute symptoms and quality of life following onset of SARS-CoV-2 infection: cohort description and approaches for measurement. Open Forum Infect Dis 2022; 9:ofab640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Valko PO, Bassetti CL, Bloch KE, Held U, Baumann CR. Validation of the fatigue severity scale in a Swiss cohort. Sleep 2008; 31:1601–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ali ST, Kang AK, Patel TR, et al. Evolution of neurologic symptoms in non-hospitalized COVID-19 “long haulers.” Ann Clin Transl Neurol 2022; 9:950–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McFann K, Baxter BA, LaVergne SM, et al. Quality of life (QoL) is reduced in those with severe COVID-19 disease, post-acute sequelae of COVID-19, and hospitalization in United States adults from northern Colorado. Int J Environ Res Public Health 2021; 18:11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Noujaim PJ, Jolly D, Coutureau C, Kanagaratnam L. Fatigue and quality-of-life in the year following SARS-Cov2 infection. BMC Infect Dis 2022; 22:541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. O’Brien K, Townsend L, Dowds J, et al. 1-year quality of life and health-outcomes in patients hospitalised with COVID-19: a longitudinal cohort study. Respir Res 2022; 23:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tabacof L, Tosto-Mancuso J, Wood J, et al. Post-acute COVID-19 syndrome negatively impacts physical function, cognitive function, health-related quality of life, and participation. Am J Phys Med Rehabil 2022; 101:48–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hirschtick JL, Titus AR, Slocum E, et al. Population-based estimates of post-acute sequelae of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (PASC) prevalence and characteristics. Clin Infect Dis 2021; 73:2055–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.