Abstract
Polarity formation in mammalian preimplantation embryos has long been a subject of controversy. Mammalian embryos are highly regulative, which has led to the conclusion that polarity specification does not exist until the blastocyst stage; however, some recent reports have now suggested polarity predetermination in the egg. Our recent time-lapse recordings have demonstrated that the first cleavage plane is not predetermined in the mouse egg. Here we show that, in contrast to previous claims, two-cell blastomeres do not differ and their precise future contribution to the inner cell mass and/or the trophectoderm cannot be anticipated. Thus, all evidence so far strongly suggests the absence of predetermined axes in the mouse egg. We observe that the ellipsoidal zona pellucida exerts mechanical pressure and space constraints as the coalescing multiple cavities are restricted to one end of the long axis of the blastocyst. We propose that these mechanical cues, in conjunction with the epithelial seal in the outer cell layer, lead to specification of the embryonic–abembryonic axis, thus establishing first polarity in the mouse embryo.
Keywords: Mouse preimplantation embryo, egg, embryonic axis, polarity, blastocoel, blastocyst
Various studies on experimentally manipulated embryos, including blastomere isolation (Tarkowski 1959; Tarkowski and Wroblewska 1967; Rossant 1976) and chimera formation (Tarkowski 1961), have demonstrated the highly regulative capacity of mammalian preimplantation embryos. This led to the long-held assumption that the polarity of most mammalian embryos remains undetermined until the blastocyst stage. However, recent studies (Gardner 1997, 2001; Piotrowska et al. 2001; Fujimori et al. 2003) have proposed that the embryonic–abembryonic (Em–Ab) axis of the mouse blastocyst arises perpendicular to the first cleavage plane. The second polar body (2pb) was used as a stationary marker for the “animal pole (A-pole)” during preimplantation development, and the first cleavage plane was considered to be meridional with only “occasional” exceptions (Gardner 1997, 2001), which led to the conclusion that polarity of the mouse embryo is predetermined in the egg (Gardner 1997, 2001; Piotrowska et al. 2001), as is the case for most nonmammalian species. Nonmeridional first cleavage (not passing through the 2pb) (Gardner and Davies 2003a) and movement of the 2pb toward the first cleavage furrow (Gray et al. 2004) were also observed, although the significance of these observations was not fully recognized. It has also been reported that the sperm entry position (SEP) determines the embryonic axis (Piotrowska and Zernicka-Goetz 2001); in this case, the first cleavage plane is defined as passing through both the 2pb and SEP. Moreover, it has been suggested that the blastomere in the two-cell embryo that inherits the SEP will divide earlier than the other and contribute preferentially to the inner cell mass (ICM) lineage (Piotrowska and Zernicka-Goetz 2001), although other authors have disputed these claims (Davies and Gardner 2002). Our recent time-lapse recordings (Hiiragi and Solter 2004) showed that, in contrast to previous assumptions, the first cleavage is not meridional and does not coincide with the “animal–vegetal (A–V)” axis in ∼50% of the embryos; moreover, the 2pb moves toward the first cleavage plane. We concluded that the first cleavage plane is not predetermined in the mouse egg, and that an “A–V axis” is a concept that can be discarded (see Terminology in Materials and Methods). We also presented a novel model for specification of the first cleavage plane (Hiiragi and Solter 2004), which is defined as the plane of apposition of the two pronuclei that have moved to the center of the mouse egg just before the pronuclear membranes are dissolved in M phase. Two recent reports re-examined this issue, and one of them confirmed our analysis using tubulin-GFP visualization of the mitotic spindle at second cleavage (Louvet-Vallee et al. 2005). The other (Plusa et al. 2005) repeated the statements previously made by this same group (Piotrowska and Zernicka-Goetz 2001), asserting that first cleavage passes close to the 2pb and the SEP. As in all previous publications, this group discounts the movement of the 2pb and of a marker for SEP toward the cleavage furrow and how this would influence the interpretation of their data.
Morphologically, the mouse blastocyst possesses an obvious asymmetry, with the ICM at one end of the long axis of the ellipsoidal embryo and the blastocoel surrounded by the trophectoderm (TE) at the other. Thus, in the light of recent controversial data (Alarcon and Marikawa 2003; Chroscicka et al. 2004), the intriguing question remains as to whether this asymmetry in the embryonic axis, the Em–Ab axis, is anticipated at an earlier point in development, as originally suggested (Gardner 1997, 2001; Piotrowska and Zernicka-Goetz 2001; Piotrowska et al. 2001; Fujimori et al. 2003; Gardner and Davies 2003a; Gray et al. 2004). In addition, the reported role of SEP in specifying the first cleavage plane or the future embryonic axis should also be revisited in the light of our findings (Hiiragi and Solter 2004) and results of previous manipulation experiments (Tarkowski 1959, 1961; Tarkowski and Wroblewska 1967; Rossant 1976), which argue against such a role. Here we have readdressed these issues using a combination of time-lapse recordings of the dynamic behavior of the embryos, experimental reassessment under highly stringent conditions, and experimental manipulations. Our results provide the basis for a novel mechanism underlying establishment of first polarity in mouse preimplantation development.
Results and Discussion
SEP does not contribute to polarity specification
It has been proposed that SEP specifies not only the first cleavage plane but also which of the two blastomeres in the two-cell embryo divides first and contributes preferentially to the ICM lineage (Piotrowska and Zernicka-Goetz 2001, 2002). In contrast, our recent model of first cleavage specification (Hiiragi and Solter 2004) does not require any sperm contribution. Pronuclear transfer experiments have also suggested that the sperm component is dispensable in the determination of the first cleavage plane, since both the male and female pronuclei contribute equally to this process (Hiiragi and Solter 2004). Furthermore, analysis of parthenogenetically activated embryos showed that the two apposing pseudopronuclei in diploid parthenogenones define the first cleavage plane, whereas the cleavage plane in haploid parthenogenones with a single pseudopronucleus is essentially random (Hiiragi and Solter 2004). These data indicate that SEP or sperm components do not play any essential role in specifying the first cleavage plane.
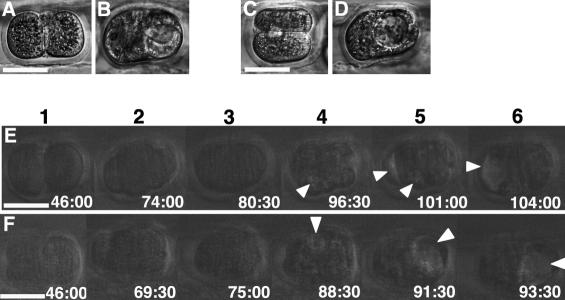
The recent controversies concerning the role of SEP in defining the future embryonic axis arise from uncertainty in marking the sperm or SEP as a consequence of different experimental approaches (Piotrowska and Zernicka-Goetz 2001; Davies and Gardner 2002; Plusa et al. 2002; Gardner and Davies 2003b; Zernicka-Goetz 2003). Our model anticipates that most embryos form the first cleavage plane such that only one of the two-cell blastomeres contains the SEP (SP) (Fig. 1A, blue; cf. NSP, red). Thus, the influence of SEP on the order in which two-cell stage blastomeres divide can be clearly assessed by time-lapse observations after the zona pellucida (ZP) has been marked with oil drops, one at the side of the SP-blastomere and two above the 2pb as a reference point (Fig. 1A). The embryos were first marked and then cultured in the incubator for up to 9 h until the second time-lapse recording. During this period, the two-cell embryos did not cleave and thus did not rotate significantly within the ZP (see Discussion below); in all the embryos the two marked points were clearly identified in the original position at the beginning of the second recording. In 10 embryos the SP-blastomere was the first to divide, whereas in nine embryos the NSP-blastomere divided first (see Fig. 1B,C, respectively; for full sequence, see Supplementary Movies S1–S4). These data indicate that SEP plays no role in determining which of the two blastomeres divides first, in contrast to the previous report (Piotrowska and Zernicka-Goetz 2001).
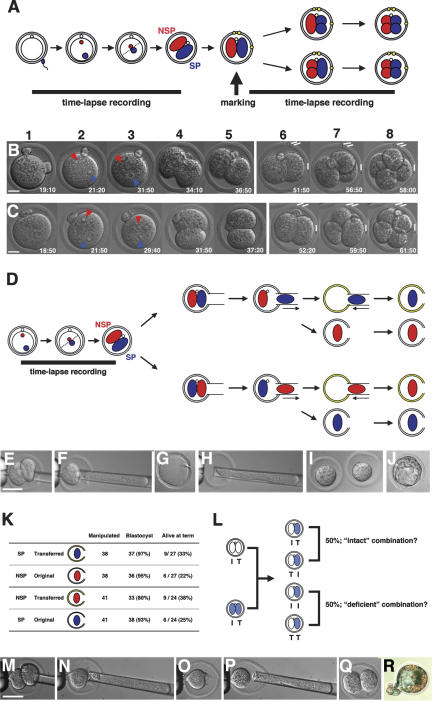
Figure 1.
Two-cell blastomeres do not differ in developmental potency. (A) Design of the experiment for analyzing the order in which two-cell stage blastomeres divide in relation to the SEP (see text). (B,C) An example for each type of embryo in which the SP-blastomere (B) and the NSP-blastomere (C) divides earlier. (1) Fertilization cone formation. (2) Pronuclei formation. (3) Two pronuclei apposing in the center just before pronuclear membrane breakdown. (4) First cleavage. (5) Nuclei formed in the two-cell embryo (end of first recording). (6) The same two-cell embryo after marking ZP with oil drops (white bars). (7) Earlier second cleavage. (8) Later second cleavage. (D) A scheme for the design of blastomere isolation experiments. Yellow ZP represents the empty ZP prepared from another embryo. In total, two pairs of isolated blastomeres, each originating from a single embryo, were produced. Each step of the manipulation is shown in E–J. (F) One blastomere is drawn into the pipette and detached from the other. (G) Empty ZP. (H) Transfer of one isolated blastomere into the empty ZP. (I) Isolated blastomeres in ZP. (J) A manipulated embryo developed to the blastocyst stage. (K) Result of the blastomere isolation experiments. (L) Design of the experiment for analyzing the development of chimeric two-cell stage embryos (see text). Each step of the manipulation is shown in M–Q; the isolated blastomere (N) is transferred into the ZP containing another isolated blastomere from a different mouse strain (P). (Q) A chimeric two-cell embryo formed by manipulation. (R) A blastocyst developed after manipulation and stained with X-gal to visualize the blastomere of ROSA strain origin. Bars: B,C, 20μm; E,M, 50 μm.
Two-cell blastomeres do not differ in developmental potency
To address the possible influence of the SEP on determination of developmental fate in the two-cell blastomeres, we conducted two sets of experimental manipulations. In the first set, time-lapse recordings from fertilization to two-cell stage served to identify the two blastomeres as either SP or NSP (Fig. 1D). In previous studies, it proved difficult to distinguish with absolute certainty the two blastomeres at the two-cell stage, thus confounding the interpretation of blastomere isolation experiments (Gardner 1996). The two-cell blastomeres identified as either SP or NSP in the time-lapse recording were isolated using a micromanipulator (Fig. 1E,F) and compared for their capacity to develop into blastocysts in vitro (Fig. 1J) and to term after transfer to surrogate mothers. Empty ZP (Fig. 1G) was prepared from another embryo to foster the blastomere obtained from the original ZP (Fig. 1H,I). Both transfer of the SP-blastomere (Fig. 1D, first row) and transfer of the NSP-blastomere (Fig. 1D, third row) were performed, producing a total of two types of embryo pairs, each originating from one single embryo. The results, summarized in Figure 1K, revealed no significant differences between the SP- and NSP-blastomeres in each pair (χ2-test with Yates' correction, p > 0.5). Thus, with respect to SEP, there is no difference in the developmental potency of the two blastomeres at the two-cell stage.
A second set of experiments was carried out to exclude the possibility that the developmental fate of a single blastomere is altered by its isolation. The premise that two-cell blastomeres do differ in developmental potency, that is, that one blastomere tends to produce the ICM and the other the TE, predicts that exchange of one two-cell blastomere with one from another two-cell embryo (Fig. 1M–Q) will result in deficiency of either ICM or TE in half the combination (Fig. 1L). Moreover, at least 50% of these exchanged embryos would not develop into intact blastocysts if normal development to the blastocyst stage actually requires the descendants of both “ICM” and “TE” blastomeres (Fig. 1R). However, all 43 chimeric embryos developed to blastocysts, and 60% of those (six of 10 whose foster mothers became pregnant) were born alive, comparable to the rates for unmanipulated controls (data not shown). This result clearly supports the idea that two-cell blastomeres, at least with respect to SEP, do not differ in their developmental potency.
Time-lapse recording of development from two-cell to blastocyst stage
As a basis for further analysis, we observed the development of the embryos from two-cell to blastocyst in time-lapse recordings. Using a micromanipulator, we closely aligned two-cell embryos and exploited the adhesive nature of the ZP to anchor the embryos to each other at two to four points. These adhesive properties of the ZP also enable anchoring of the embryos to the bottom of the glass chamber, thus preventing any rotation of the ZP. As a control for the possibility that ZP adherence might promote immobilization of the embryo, we also analyzed the recordings of embryos not attached to each other but only to the bottom of the chamber. In this case, we observed similar behavior as described below (data not shown).
Under optimized conditions, 72 of 105 embryos (69%) developed to blastocyst stage II (see Terminology in Materials and Methods) after 60 h of recording starting from the two-cell stage, and 26% (10 of 38 blastocysts whose foster mothers became pregnant) developed to term after transfer into foster mothers. Thus, our time-lapse observation closely reflects the in vivo situation, and the dynamic behavior of each of these individual 72 embryos was analyzed (Fig. 2A–D; the corresponding Supplementary Movies S5–S8). A striking observation was that, once the embryos start dividing, they rotate extensively inside the ZP and change their orientation with no apparent regularity (Fig. 2A,D; Supplementary Movies S5, S8). This is in contrast to a previous “failure to detect obvious net rotation of conceptuses within this glycoprotein coat in time-lapse records of cleavage in vitro” (Gardner 2001). In fact, 28 of 35 embryos (80%) whose 2pb survived and could be traced until the blastocyst stage rotated so extensively that their 2pb reached the non-PB hemispheric area inside the ZP (Fig. 2A4,D4). This finding clearly invalidates the methods in a previous report (Gardner 2001) that rely on ZP marking in relation to 2pb to trace the orientation of the embryo. It is interesting to note that 33 of 70 embryos (47%; two were excluded from analysis because the Em–Ab axis was so close to perpendicular to the observation plane that it was difficult to judge its orientation) formed their ICM–TE boundary parallel to the first cleavage plane (within 30°; yellow broken lines in Fig. 2B–D), in statistical agreement with the previous report (Gardner 2001) (see discussion of the model below).
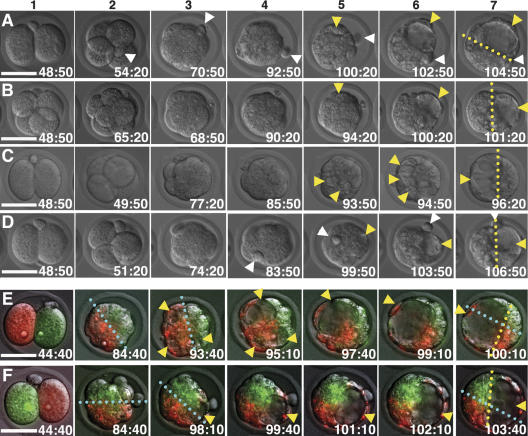
Figure 2.
Dynamic behavior of the embryo developing from two-cell to blastocyst stage. (A–D) Sequential DIC images of the embryos cultured in vitro. (1) Two-cell, except for B, in which the embryo divided before the start of the time-lapse recording (see Materials and Methods). (2) Four-cell, except for B, which is eight-cell. (3) Compacted eight-cell. (4) Morula. (5) Blastocyst I stage in which blastocoel formation has started. (6) Blastocyst I stage, with cavity(ies) enlarging and shifting position. (7) Blastocyst II stage. (E,F) Sequential DIC image, superimposed with fluorescent signal, of embryos marked with fluorescence (green or red) for each blastomere at the two-cell stage and cultured in vitro. (1) Two-cell, after marking and alignment on the chamber. (2) Morula, beginning of the time-lapse recording after in vitro culture in the incubator from the two-cell stage. (3) Blastocyst I stage, when blastocoel formation starts. (4–7) Blastocyst I–II–III stages, when blastocoel formation advances with gradual rotation of the embryo and relocation of the blastocoels. (A–F) White and yellow arrowheads indicate the 2pb and blastocoels, respectively. Yellow and cyan broken lines indicate the ICM–TE boundary and the clonal boundary, respectively. In each frame, time is given in hours:minutes after hCG injection. Bars, 50 μm.
In search of a mechanism to correlate the first cleavage plane with the subsequent embryonic axis, we carefully observed blastocoel formation from morula to blastocyst. Interestingly, we found that the blastocoel was not formed in a predefined manner from one of the two poles of the future Em–Ab axis, but instead originated from various points and eventually localized on only one of the two poles of the long axis of the ellipsoidal ZP. Some embryos rotated themselves while orienting the blastocoel along the long axis (Fig. 2B,D; Supplementary Movies S6, S8), whereas others gradually relocated the smaller blastocoels to one end of their long axis (Fig. 2C; Supplementary Movie S7). Note that in 47 embryos (65%) time-lapse recordings showed blastocoels that originated from multiple points (Calarco and Brown 1969; Wiley and Eglitis 1981; Garbutt et al. 1987; Aziz and Alexandre 1991), merged with each other, and increased in size, and eventually shifted to one end of the long axis (Fig. 2C; Supplementary Movies S7, S8). In addition, the 2pb in 19 (54%) embryos was localized near the ICM–TE border of the blastocyst II, statistically consistent with the previous report (Gardner 1997), although it did not remain at the original position relative to ZP or embryo but instead moved around the surface of the embryo (Fig. 2D; Supplementary Movie S8) (see discussion of the model).
Lineage of the two-cell blastomeres in the blastocyst
We carried out a lineage analysis of the two blastomeres at the two-cell stage to examine the specification of the ICM/TE lineage in relation to the clonal descendants of these blastomeres (Piotrowska et al. 2001; Alarcon and Marikawa 2003; Fujimori et al. 2003; Chroscicka et al. 2004; Plusa et al. 2005). The membrane of the two blastomeres was marked by two different lipophilic fluorescent dyes to avoid disturbing embryo development (Piotrowska et al. 2001; Plusa et al. 2005). Analysis was conducted more rigorously than in the other studies (Piotrowska et al. 2001; Alarcon and Marikawa 2003; Fujimori et al. 2003; Chroscicka et al. 2004; Plusa et al. 2005), based on the following modifications: (1) Embryos at the same stage of development (blastocyst II) were selected, since the clonal descendants tend to intermingle with each other as the embryo develops further at the blastocyst stages (see Fig. 2E,F, and discussion below; T. Bauer and T. Hiiragi, unpubl.). (2) Both confocal scanning (Fig. 3A; Piotrowska et al. 2001; Plusa et al. 2005) and 3D-reconstruction software (Fig. 3B) were used (see Blastomere labeling, immunofluorescence staining, and confocal microscopy in Materials and Methods for detailed description) to measure the 3D-tilt angle between the clonal boundary plane of the two blastomeres (Fig. 3A [cyan broken lines], B [gray plane marked with white arrow]) and the plane of the ICM–TE boundary or “anatomical” boundary (see Terminology in Materials and Methods; Fig. 3A [yellow broken lines], B [gray plane marked with white arrowhead]). Note that the angle measured in the 2D scanned images (Piotrowska et al. 2001) varies depending on the confocal section (Fig. 3A, cf. the angle between cyan and yellow broken lines) and that it differs from the real angle in 3D reconstruction (Fig. 3B), which could explain the discrepancy between our current results and those previously reported (Piotrowska et al. 2001) (see below). (3) For each embryo, the number of blastomeres crossing the clonal boundary was also counted for each of the clonal descendants, and the relationship of this number with the 3D-tilt angle was analyzed (Fig. 3C).
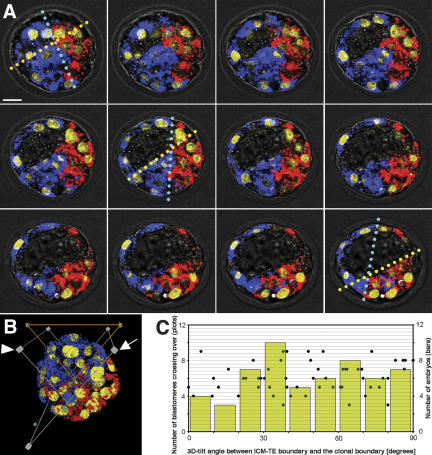
Figure 3.
Lineage analysis of two-cell blastomeres in the blastocyst. (A) Sequential confocal microscopy images (2 μm) of embryos marked with two fluorescent dyes (blue and red) for the blastomeres at the two-cell stage and stained for DNA (yellow) at the blastocyst stage. Yellow and cyan broken lines indicate the ICM–TE boundary and the clonal boundary, respectively. Note that the direction of the cyan lines varies, depending on the scanned level (see text). Bar, 20 μm. (B) Reconstructed 3D-image of the same embryo as in A showing the method of tilt-angle measurement (orange triangle lines) between the anatomical boundary and the clonal boundary (both on the gray planes). (C) Summary of the tilt-angle analysis. Each green dot represents the 3D-tilt angle for each embryo in relation to the number of blastomeres crossing the clonal boundary of the two-cell blastomeres. Yellow bars show the number of embryos in each range of the tilt angle.
Confocal microscopy was used to scan 2-μm slices (Fig. 3A) of a total of 116 blastocyst II-stage embryos that had been marked with two fluorescent dyes at the two-cell stage and had developed normally. 3D reconstruction (Fig. 3B; Supplementary Movie S11) enabled delineation of the clonal boundary plane between the two colors in 80 embryos (69%), while extensive intermingling of the two clonal descendants prevented this in the remaining 36 embryos. Based on Hoechst DNA staining, we counted the number of blastomeres crossing the clonal boundary from each side (Fig. 3A,B); if this number exceeded a total of 10 for both sides or five for each side, the embryo was excluded from further analysis, since extensive intermingling of the descendants renders the clonal boundary plane setting ambiguous. Under these strict conditions, 56 embryos (48%) showed a defined boundary between the two-cell blastomere's clonal descendants at the blastocyst II stage. Graphical analysis of the 3D-tilt angle plotted against the number of the blastomeres crossing the clonal boundary (Fig. 3C) revealed a uniform distribution of the observed values (Kolmogorov-Smirnov test, p = 0.4529). There was no correlation between the tilt angle and the extent to which the blastomeres crossed the clonal boundary. Our findings are in sharp contrast to a previous report that “in the great majority of blastocysts (which appeared to be around 68%) the angle between these planes was less than 30 degrees” (Piotrowska et al. 2001). Our finding for this category was 25%, consistent with recent work by others (Alarcon and Marikawa 2003).
Lineage marking combined with time-lapse recordings was used to examine the relationship between blastocoel formation and the two clonal descendants of the two-cell blastomeres. Embryos marked with two different fluorescent dyes for each blastomere were aligned on a glass chamber, photographed, and cultured in vitro in an incubator to minimize UV exposure. After development to the morula stage (40 h of culture), the embryos were returned to the microscope for time-lapse recordings to observe blastocoel formation (Fig. 2E,F; Supplementary Movies S9, S10). These recordings, in which the adhesive nature of the ZP ensured that the conceptuses were kept in the same position, clearly demonstrated the extensive net rotation of the embryo inside the ZP, as well as the initiation of blastocoel formation (yellow arrowheads in Fig. 2E,F) irrespective of the clonal area of the two-cell blastomeres. Thus, the blastocoel can originate from multiple points within either or both clonal areas (Fig. 2E; Supplementary Movie S9) as well as from the boundary area (Fig. 2F; Supplementary Movie S10). This may explain, at least in part, why intermingling of the two clonal descendants occurs and increases especially after blastocoel formation (Fig. 2E5–7,F5–7).
Mechanism of blastocoel formation
Our present analyses of developmental potency, morphological aspects (blastocoel formation and thus Em–Ab axis specification), and fate specification (tilt angle between the ICM–TE boundary and the clonal boundary) indicate the absence of lineage specification at the two-cell stage. The nature of blastocoel formation is especially important for our understanding of how the first obvious embryonic axis, that is, the Em–Ab axis, is established in mammalian development (Garbutt et al. 1987). In the light of our finding that blastocoels arise from multiple points apparently independent of the two clonal descendants of the two-cell blastomeres (Fig. 2E,F; Supplementary Movies S9, S10), we studied the formation of the blastocoel in earlier stage I blastocysts. Immunofluorescent actin staining for cortical actin beneath the cell membrane enabled us to visualize clearly the cell–cell boundary as well as the small intercellular spaces in the embryo. Serial confocal scanning every 1 μm identified tiny blastocoel cavities (Fig. 4A), according to the following criteria: (1) a green signal for cortical actin (cell membrane) surrounding the blastocoel; (2) absence of the gray signal caused by the laser light reflected from the embryo (see Materials and Methods); and (3) continuity of the cavity along the Z-axis in several consecutive sections and delineation of the cavity from the area of the blastomere cytoplasm containing the nucleus (stained yellow with DAPI). This staining protocol was combined with lineage marking to identify the clonal origin of the blastomeres (Fig. 4A, red and blue signals). Finally, the contour of the cavities was marked in each scanned picture, and their distribution in the whole embryo was visualized after 3D reconstruction (Fig. 4B; Supplementary Movies S12, S13).
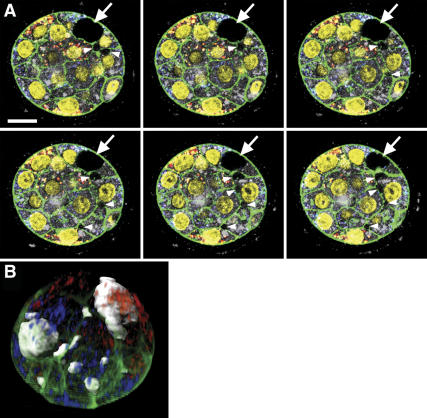
Figure 4.
Mechanism of blastocoel formation. (A) Sequential images of the embryos marked with two fluorescent dyes (blue and red) for the blastomeres at the two-cell stage, stained for actin (green) and DNA (yellow) at the blastocyst stage and scanned by confocal microscopy every 1 μm. The arrow indicates the relatively larger cavity visible in the stereomicroscope. The arrowhead indicates smaller cavities in the intercellular spaces. Bar, 20 μm. (B) Reconstructed 3D-image of the same embryo as in A, showing 10 cavities (gray), constructed by marking the contour in each section in A.
In all of the 11 blastocyst I embryos, multiple small cavities (Fig. 4A, white arrowheads), ranging from four to 16 per embryo, were identified in addition to the relatively larger cavities visible by stereomicroscopy during their initial identification as blastocyst I stage (Fig. 4A, white arrow). The position of the cavities was apparently random (Fig. 4B; Supplementary Movies S12, S13) but mainly distributed over more than three intercellular junctions and on the basal layer of the outer blastomeres. It is important to note that in not a single embryo did all cavities originate within just one of the two clonal areas.
Novel model of first embryonic polarity determination
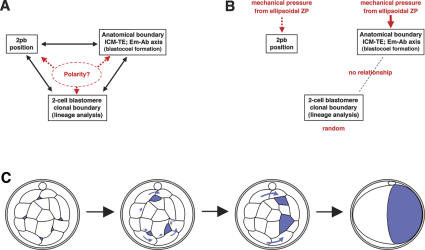
Our data point to the necessity for reinterpretation of recent findings (Gardner 1997, 2001; Piotrowska et al. 2001; Fujimori et al. 2003). Three independent reports (Gardner 2001; Piotrowska et al. 2001; Fujimori et al. 2003) suggest that the Em–Ab axis of the blastocyst is predominantly perpendicular to the first cleavage plane; however, there are differences in the experimental evidence and methods of analysis supporting these claims. One group (Gardner 2001) used oil-drop marking in the ZP but discounted the possibility of embryo rotation within the ZP. The other two groups (Piotrowska et al. 2001; Fujimori et al. 2003; Plusa et al. 2005) used blastomere labeling and lineage analysis but did not include 3D reconstruction, which, based on our experience, is crucial for the correct positioning of the clonal and anatomical boundaries. An assumed close relationship between the 2pb position, first cleavage plane, and the anatomical and clonal boundaries was then used as evidence for the preconceived notion of polarity determination in the two-cell stage embryo (Fig. 5A). Thus, although two independent approaches appeared to support the idea that the first cleavage plane somehow presages the location of the Em–Ab axis, serious flaws in each approach invalidate their conclusion. A re-examination of the present and previous results, but without any preconceived notion of predetermination, leads to a new model that may reconcile all the data (Fig. 5B).
Figure 5.
A proposed mechanism for polarity determination in the mouse preimplantation embryo. (A) View proposed by others (Gardner 1997, 2001; Piotrowska et al. 2001; Fujimori et al. 2003) concerning the relationships between the landmarks for polarity in the preimplantation embryo. (B) Our proposed view and the mechanism responsible for establishing each landmark. (C) Mechanism of blastocoel (blue) formation leading to the Em–Ab axis aligned with the long axis of the ellipsoidal ZP.
In this model, the two blastomeres at the two-cell stage are undetermined in their developmental fate toward either ICM or TE; instead, blastocoel formation leads to the definition of first embryonic polarity, the Em–Ab axis, and thus to further lineage specification in concert with the ICM–TE specification (Tarkowski and Wroblewska 1967; Graham and Deussen 1978; Garbutt et al. 1987; Johnson and McConnell 2004) in the mouse embryo. As discussed above, there is no predetermined polarity or asymmetry in the embryo itself before the blastocyst stage. The ZP adopts an ellipsoidal shape with its long axis determined by the alignment of the two-cell blastomeres (the ratio of the long axis to the short axis is, on average, 1.07 in 75 embryos analyzed), which is retained until the blastocyst stage (the corresponding ratio is 1.06) (Gardner 2001). The orientation of the long axis of the ZP is maintained during development from two-cell to blastocyst II stage (average deviation of 2.8° for the 75 embryos). However, we found that the ZP is pliable rather than rigid, and once the blastocyst has developed to the expanded blastocyst III stage, the ratio of the long axis to the short axis decreases to 1.02 due to the expansion of the blastocoel in essentially all directions. The pliable nature of the ZP is compatible with our model, as discussed below.
The blastocoel is initially formed as cytoplasmic vesicles or droplets, followed by secretion of the fluid into the intercellular space, thus creating extracellular cavities (Calarco and Brown 1969; Wiley and Eglitis 1981; Fleming and Pickering 1985; Garbutt et al. 1987; Manejwala et al. 1989; Aziz and Alexandre 1991; Watson and Barcroft 2001). This takes place in essentially all the outer blastomeres, which have the characteristics of epithelial cells with apical and basolateral membrane asymmetry. Since the outer layer is sealed by the epithelial tight junctions, the minute intercellular fluid spaces coalesce to gradually form larger cavities (Fig. 5C), compressing the fluid to the base of the cells accompanied by the deformation of the blastomeres (see Fig. 4A). This secretion of fluid can produce a relative high-pressure area in the embryo, causing the fluid to move to areas with more free space, that is, to one end of the long axis, by relocation through the intercellular spaces and by net rotation of the embryo (Fig. 5C). Ultimately, most embryos have one blastocoel at one end of the long axis of the ellipsoidal ZP, thus forming an Em–Ab axis perpendicular to the first cleavage plane. However, this axis relationship is not absolute, since not all embryos have ellipsoidal ZP and the extent of asymmetry of the ZP long and short axis is embryo-dependent. We found that the Em–Ab axis in embryos with spherical ZP tends to form without any regularity with respect to the first cleavage plane (data not shown), explaining why the Em–Ab axis is perpendicular to the first cleavage plane in only 50%–60% of embryos (Gardner 2001). This relationship does not imply that the first two blastomeres and their descendants can be superimposed on the blastocyst along the Em–Ab axis. Because the embryos rotate extensively during cleavage, the plane representing the clonal boundary between descendants of the first two blastomeres becomes independent of the plane of first cleavage.
It was also claimed that “cell division order does influence the establishment of the Em–Ab axis, early dividing cells tending to be associated with the nascent blastocoel and the site of the nascent blastocoel tending to mark the site of the abembryonic pole,” while “a counteracting influence (or confounding effect) of division order arising from its effects on the allocation of cells to the inner cell mass” was acknowledged (Garbutt et al. 1987). We did not observe any significant relationship between the site of the nascent blastocoel and the site of the Ab-pole (time-lapse recordings as exemplified in Fig. 2), nor between the site of the nascent blastocoel and the descendants of the two blastomeres at the two-cell stage (Figs. 2E,F, 4). Our observation strongly suggests that the blastocoel originates from multiple points, essentially at random and irrespective of the clonal area of the two-cell blastomeres. It is difficult to directly compare our results obtained using normal embryos with those of others using chimeric constructs (Garbutt et al. 1987; Johnson and McConnell 2004). Nevertheless, our model proposes that the site of the nascent blastocoel, if any, has no causal relationship with the future Ab-pole, since spatial constraints and mechanical pressure exerted by the ZP in conjunction with the outer epithelial seal are the driving forces to coalesce and localize the multiple blastocoel to the final position.
Neither the ZP nor its asymmetry is essential for blastocoel formation at one end of the blastocyst resulting in the Em–Ab axis: It is well known that the embryo develops to the blastocyst stage and to term without the ZP. Proper blastocoel formation depends on the integrity of the outer epithelial cell layer, with apical and basolateral membrane asymmetry enabling directed fluid secretion, and on the ability of the outer cell layer to seal the inside from the outside of the embryo (Watson and Barcroft 2001). The mechanical pressure model explains the effect of ZP shape on blastocoel formation. When the ZP is ellipsoidal, the blastocoel localizes according to the spatial constraints exerted by the shape of the ZP, whereas when the ZP is round (in 41 of 75 embryos, 55%, the long and short diameters of the ZP differ by <5%), the embryo forms the Em–Ab axis with no relationship to the first cleavage plane, which would also be the case for an embryo without ZP or with an unusually fragile or larger ZP. This model also explains the extensive rotation of the embryo inside the ZP (see Fig. 2), since in each successive division the embryo may be forced to simply accommodate the spatial constraints imposed by the ellipsoidal ZP on its ever-changing geometry. Moreover, our model may explain why the 2pb is frequently found along the short axis in the blastocyst on the ICM–TE boundary (Gardner 1997) instead of along the high-pressured long axis; our preliminary data show that the 2pb can move quite independently from its “tethering” structure, a remnant of the last meiotic division.
Experimental proof for the proposed model
Our model of Em–Ab polarity formation in the preimplantation embryo predicts that in essentially all embryos, mechanical compression exerted through the ZP as well as spatial constraints will induce formation of the Em–Ab axis perpendicular to the short axis of the ZP. Using a micromanipulator to place individual embryos into a slit in an agar plate, we successfully increased pressure by maintaining the compressed shape of the ZP from the two-cell to the blastocyst. Of 18 embryos subjected to increased pressure in the same direction as the original pressure, that is, along the first cleavage plane (Fig. 6A), 17 (94%) formed the Em–Ab axis perpendicular to the short axis, in this case the first cleavage plane (Fig. 6B); two of these embryos had two blastocoels at both ends of the long axis at blastocyst II stage and then developed further to blastocyst III by forming ICM at one of the two ends or at a different site. When pressure was applied perpendicular to the original direction, that is, to the first cleavage plane (Fig. 6C), 12 of 13 embryos (92%) formed the Em–Ab axis perpendicular to the short axis (parallel to the first cleavage plane) (Fig. 6D); two of these embryos had two blastocoels at both ends of the long axis at the blastocyst II stage. Time-lapse recordings of these manipulated embryos clearly show the distinct movement of the blastocoel from the original position to one end of the long axis of the compressed ZP (Fig. 6E,F; Supplementary Movie S14). These experimental manipulations strongly support the conclusion that the position of the blastocoel, and thus the Em–Ab axis, is directed by the mechanical pressure and spatial constraints imposed by the ZP and not by any intrinsic predetermined polarity. This is in agreement with previous data that alginate-embedded embryos form their Em–Ab axis perpendicular to the first cleavage plane more consistently than do normal embryos (see Fig. 4F in Gardner 2001).
Figure 6.
Experimental proof for our model. The eventual position of the blastocoel, thus Em–Ab axis, is specified by external mechanical pressure imposed by the ellipsoidal ZP. (A,C) Two-cell embryos were compressed by placing them into a slit in an agar plate, in the same direction as (A) or perpendicular to (C) the original direction. (B,D) Blastocysts developed (B from A and D from C) after in vitro culture under compression. (E,F) Sequential images of the time-lapse recordings of the compressed embryos. (1, E) Two-cell. (F) Three-cell. (2) Compacted eight-cell. (3) Morula. (4) Blastocyst I, cavity formation started. (5) Blastocyst I, cavity(ies) shifting to the long axis. (6) Blastocyst II, blastocoel relocated at one end of the long axis. White arrowheads indicate the blastocoels. In each frame, time is given in hours:minutes after hCG injection. Bars, 50 μm.
Conclusion
We have presented a novel model in which first polarity during mouse development (the Em–Ab axis) is not specified until the embryo forms the blastocoel, and in which the eventual position of the blastocoel (the abembryonic pole) is determined by the external pressure and spatial constraints imposed by the ZP in conjunction with the epithelial seal in the outer cell layer. The fluid cavities arise essentially from all intercellular spaces beneath the blastomeres in the outer layer and gradually coalesce to form larger cavities as a result of the integrity of these blastomeres as epithelial cells. In most, but not all, cases the blastocoel eventually settles at one of the two ends of the long axis in the ellipsoidal ZP. There is no predetermined embryonic polarity before the blastocyst stage and no specified lineage in the two-cell blastomeres. The SEP does not contribute to polarity specification. All experimental manipulations carried out to date, which indicate the highly regulative nature of the mammalian preimplantation embryo, are consistent with our model.
The mammalian embryo gradually acquires axes of asymmetry starting with the Em–Ab axis, initially through a random process or using available, but not essential, cues. The Em–Ab axis in the blastocyst is the first axis of polarity in mammalian development, and seems to be unique to mammals, as it is the basis for the extraembryonic tissue (placenta) formation. It remains largely uncertain if the Em–Ab axis at the blastocyst stage has any relationship to the future “embryonic axes” (dorsal–ventral, anterior–posterior, and left–right), and if there is any asymmetry inside the ICM relevant to the further developmental process (for review, see Beddington and Robertson 1999; Rossant and Tam 2004). The specification of the Em–Ab axis should be conceptually differentiated from the cell fate commitment to either ICM or TE. Commitment to lineage fate begins earlier, at the 16-cell stage, most likely in response to environmental clues (inside–outside hypothesis) (Tarkowski and Wroblewska 1967; Johnson and McConnell 2004) The two processes—formation of the Em–Ab axis and cell fate commitment—proceed hand in hand until in the late blastocyst the morphology and early cell differentiation are essentially completed. Differential gene expression, cued by cell–cell and cell–environment interactions, proceeds in parallel, stabilizes the fate of ICM and TE compartments, and subsequently directs further specification in a manner reminiscent of the development of most nonmammalian embryos (Johnson and McConnell 2004). In terms of genetically driven developmental mechanisms, the late blastocyst or the early egg cylinder of the mouse (but not the zygote) should be considered as conceptually equivalent to the egg of most nonmammalian species (e.g., Caenorhabditis elegans, Drosophila, Xenopus) (O'Farrell et al. 2004).
At present, all evidence points to the absence of predetermination in the mammalian egg, although no experiments can conclusively eliminate its presence. Only a gene whose product is specifically localized in the mammalian egg and whose absence has defined developmental consequences could serve as positive proof for predetermination. Until such a gene is identified, it makes sense to base our future experiments on the premise that the mammalian egg neither has nor requires predetermination for successful development.
Materials and methods
Terminology
We propose redesignation of “animal (A)- and vegetal (V)-pole” in the mouse egg (Hiiragi and Solter 2004) to “PB-pole and non-PB-pole,” respectively. The half-spheres of the egg, “animal-half and vegetal-half,” are designated “PB-half and non-PB-half,” respectively. For strict analysis, we delineate three blastocyst stages: blastocyst I, with one or several visible cavities, which in total do not exceed half the volume of the embryo; blastocyst II, with the blastocoel occupying half the volume of the embryo; and blastocyst III, with the blastocoel occupying more than half the volume of the embryo. This classification enables a precise identification of embryos at the blastocyst II stage. We also designate the boundary between ICM and TE in the blastocyst as the “anatomical” boundary.
Time-lapse recording of the embryos
For all experiments, two-cell embryos were recovered by superovulating (C57BL/6 × C3H) F1 female mice with human chorionic gonadotropin (hCG, 5IU), followed by mating with (C57BL/6 × DBA/2) F1 male mice and flushing the oviducts with H-KSOM-AA (KSOM with amino acids and 21 mM HEPES) 41–46 h after hCG injection.
Time-lapse recording was conducted as described (Hiiragi and Solter 2004) with a slight modification. Prior to the recording, embryos were aligned on the glass chamber one-by-one using a micromanipulator so that their first cleavage planes were all parallel. Since very-late-stage two-cell embryos (still in interphase when selected) were used to obtain the best possible development and since usually 20–30 min was required to align 25–35 embryos, some of these had already begun second division while others were still being aligned. Thus, in some recordings, the time lapse started from the three- or four-cell stage (see Fig. 2B), although these were originally aligned in the same direction as the other two-cell embryos.
Experimental manipulations
Blastomere isolation in the two-cell embryo After time-lapse recording from the zygote to the two-cell stage, a slit was made in the ZP next to either the SP- or NSP-blastomere based on the analysis of the time-lapse recordings. After a 5-min incubation in Ca,Mg-free KSOM-AA containing 5 μg/mL cytochalasin B (CB; Sigma, C6762), one blastomere was isolated from a two-cell embryo in a drop of the same medium using a pipette attached to a micromanipulator (see Fig. 1F). An empty ZP was prepared from another two-cell embryo by removing both blastomeres using a pipette in H-KSOM-AA containing 5 μg/mL CB. One blastomere was transferred into the empty ZP. A total of four different combinations of the isolated blastomere, two pairs each corresponding to the original embryo, were produced (see Fig. 1D).
Chimeric embryo formation Two-cell embryos were recovered from superovulated (C57BL/6 × C3H) F1 female mice mated with (C57BL/6 × DBA/2) F1 or ROSA26 male mice. An isolated blastomere of one strain was transferred into the ZP containing a blastomere isolated from a different strain, and vice versa. Chimeric two-cell embryos were cultured in 6% CO2 at 37°C in KSOM-AA until the blastocyst stage. To examine the relative contribution of the two different strains, the blastocyst was fixed for 5 min at room temperature in 0.25% glutaraldehyde (Sigma, G5882) in PBS and stained overnight at 37°C in 1 mM K3Fe(CN)6 (Sigma, P8131), 1 mM K4Fe(CN)6 (Sigma, P9387), and 0.1 mM MgCl2 in PBS containing 1 mg/mL X-gal (Sigma, B4252).
Compression of the embryo Two percent agar (Biowhittaker Molecular Applications, 50080) solution in H-KSOM-AA was dispensed into a 35-mm culture dish (Falcon, 3001). The agar dish was kept at 4°C before use. Coverglasses of two different thicknesses (0.085–0.115 mm and 0.13–0.16 mm; Marienfeld; #0 and #1, respectively) were inserted vertically to make a groove in the agar plate for each orientation of the two-cell embryos (see Fig. 6A,C), and the plate was filled with 2 mL of H-KSOM-AA covered with mineral oil (Sigma, M8410). After incubation of the plate for at least 1 h in 5% CO2 at 37°C, two-cell embryos were embedded into the groove using a thick needle to maintain the compressed shape. Some of the embedded embryos were cultured in a 5% CO2 incubator at 37°C, and others were time-lapse recorded until the blastocyst stage.
Blastomere labeling, immunofluorescence staining, and confocal microscopy
Blastomere labeling was carried out essentially as described (Piotrowska et al. 2001). Briefly, the lipophilic fluorescent dyes, DiI, DiD, and DiA (Molecular Probes, D3899, D307, and D3897, respectively), were dissolved in 2 mL of virgin olive oil (final concentrations of 2.5 mg/mL, 12.5 mg/mL, and 5 mg/mL, respectively) at 37°C–60°C for 3–120 min depending on the dye. Two blastomeres of the two-cell embryos recovered from female mice at 41–46 h after hCG injection were labeled with DiI and DiD, respectively, for clonal boundary analysis (Fig. 3) and for blastocoel formation analysis (Fig. 4), or with DiI and DiA for time-lapse recording (Fig. 2E,F). The dyes were applied by placing a microdroplet by microinjector (Eppendorf, 5242) next to the blastomere membranes, where the oil drop was spontaneously incorporated into the inner layer of the blastomere membrane within 1–2 min. The labeled embryos were cultured in KSOM-AA covered with mineral oil in 6% CO2 at 37°C until they reached the required developmental stage.
For clonal boundary analysis, embryos were observed every 30 min under the stereomicroscope beginning at 87 h after hCG injection and recovered from the KSOM culture at the blastocyst II stage. Embryos were further incubated in H-KSOM-AA containing 10 μg/mL Hoechst 33342 (Sigma, B2261) for 5–10 min at 37°C, washed several times with 1% bovine serum albumin (BSA; Sigma, A9647) in PBS, fixed in 4% paraformaldehyde (PFA; Sigma, P6148) in PBS for 30 min at room temperature, and mounted in microdrops of 5–10 μL 1% BSA in PBS on glass-bottom dishes (World Precision Instruments, 500864) covered with mineral oil. Confocal analysis was performed using a Leica TCS SP2 UV laser scan head attached to a Leica DM IRE2 inverted microscope equipped with Leica Confocal software version 2.5 and taking optical sections every 2 μm. 3D images were reconstructed in IMARIS imaging software version 4.05 (Bitplane AG), and the ICM–TE boundary plane (gray plane marked with white arrowhead in Fig. 3B) and the clonal boundary plane (gray plane marked with white arrow in Fig. 3B) were fitted in a rotating 3D image. By placing three measurement points, one on the center of the intersecting line of the two planes and the other two on the lines perpendicular to the intersection, a triangle was formed (orange triangle, Fig. 3B), in which one of the angles corresponded to the 3D-tilt angle and was calculated on the basis of measurement of the triangle perimeter. The number of blastomeres crossing the clonal boundary was determined based on DNA staining (Hoechst) in the 3D reconstructed image.
For blastocoel analysis, embryos were observed hourly, beginning at 85 h after hCG injection, and recovered from the KSOM culture at the blastocyst I stage as soon as the cavities were detected by stereomicroscopy. Embryos were fixed in 4% PFA in PBS for 30 min at room temperature, washed with 1% BSA in PBS, and stained by incubation with 0.005 U/μL Alexa Fluor 488 phalloidin (Molecular Probes, A12379) and 10 μM 4′,6-diamidino-2-phenylindole, dilactate (DAPI; Molecular Probes, D3571) in 1% BSA in PBS overnight at room temperature. After several washes totaling at least 1 h with 1% BSA in PBS, embryos were mounted on glass-bottom dishes and analyzed by confocal scanning as described above but at 1-μm intervals. In addition to the emission signal from the fluorochrome, the reflected signal (620–645 nm) from the embryo itself by HeNe laser (633 nm) was also detected to help distinguish the cytoplasm from the blastocoel cavities, which should not reflect the laser or emit a signal (black area in Fig. 4A marked with white arrow and arrowheads). The contour of the blastocoels in each section was marked, and a 3D image was reconstructed in IMARIS software to visualize the distribution of the cavities in the embryo.
Acknowledgments
We thank N. Bobola, F. Chen, M. Hoffman, H. Kirk, P. Nielsen, M. Takeichi, A. Tomilin, and Sh. Tsukita for critical reading of the manuscript and discussions; S. Kuppig for assistance with confocal microscopy analysis; and B. King and W. Zhang for assistance with statistical analysis. The statistical analysis in this work was partially supported by the National Cancer Institute Cancer Center Support Grant NIH-P30-CA034196.
Supplemental material is available at http://www.genesdev.org.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.1304805.
References
- Alarcon V.B. and Marikawa, Y. 2003. Deviation of the blastocyst axis from the first cleavage plane does not affect the quality of mouse postimplantation development. Biol. Reprod. 69: 1208–1212. [DOI] [PubMed] [Google Scholar]
- Aziz M. and Alexandre, H. 1991. The origin of the nascent blastocoele in preimplantation mouse embryos: Ultrastructural cytochemistry and effect of chloroquine. Roux's Arch. Dev. Biol. 200: 77–85. [DOI] [PubMed] [Google Scholar]
- Beddington R.S. and Robertson, E.J. 1999. Axis development and early asymmetry in mammals. Cell 96: 195–209. [DOI] [PubMed] [Google Scholar]
- Calarco P.G. and Brown, E.H. 1969. An ultrastructural and cytological study of preimplantation development of the mouse. J. Exp. Zool. 171: 253–283. [DOI] [PubMed] [Google Scholar]
- Chroscicka A., Komorowski, S., and Maleszewski, M. 2004. Both blastomeres of the mouse 2-cell embryo contribute to the embryonic portion of the blastocyst. Mol. Reprod. Dev. 68: 308–312. [DOI] [PubMed] [Google Scholar]
- Davies T.J. and Gardner, R.L. 2002. The plane of first cleavage is not related to the distribution of sperm components in the mouse. Hum. Reprod. 17: 2368–2379. [DOI] [PubMed] [Google Scholar]
- Fleming T.P. and Pickering, S.J. 1985. Maturation and polarization of the endocytotic system in outside blastomeres during mouse preimplantation development. J. Embryol. Exp. Morphol. 89: 175–208. [PubMed] [Google Scholar]
- Fujimori T., Kurotaki, Y., Miyazaki, J., and Nabeshima, Y. 2003. Analysis of cell lineage in two- and four-cell mouse embryos. Development 130: 5113–5122. [DOI] [PubMed] [Google Scholar]
- Garbutt C.L., Chisholm, J.C., and Johnson, M.H. 1987. The establishment of the embryonic–abembryonic axis in the mouse embryo. Development 100: 125–134. [DOI] [PubMed] [Google Scholar]
- Gardner R.L. 1996. Can developmentally significant spatial patterning of the egg be discounted in mammals? Hum. Reprod. Update 2: 3–27. [DOI] [PubMed] [Google Scholar]
- ____. 1997. The early blastocyst is bilaterally symmetrical and its axis of symmetry is aligned with the animal–vegetal axis of the zygote in the mouse. Development 124: 289–301. [DOI] [PubMed] [Google Scholar]
- ____. 2001. Specification of embryonic axes begins before cleavage in normal mouse development. Development 128: 839–847. [DOI] [PubMed] [Google Scholar]
- Gardner R.L. and Davies, T.J. 2003a. The basis and significance of pre-patterning in mammals. Philos. Trans. R Soc. Lond. B Biol. Sci. 358: 1331–1338; discussion 1338–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ____. 2003b. Is the plane of first cleavage related to the point of sperm entry in the mouse? Reprod. Biomed. Online 6: 157–160. [DOI] [PubMed] [Google Scholar]
- Graham C.F. and Deussen, Z.A. 1978. Features of cell lineage in preimplantation mouse development. J. Embryol. Exp. Morphol. 48: 53–72. [PubMed] [Google Scholar]
- Gray D., Plusa, B., Piotrowska, K., Na, J., Tom, B., Glover, D.M., and Zernicka-Goetz, M. 2004. First cleavage of the mouse embryo responds to change in egg shape at fertilization. Curr. Biol. 14: 397–405. [DOI] [PubMed] [Google Scholar]
- Hiiragi T. and Solter, D. 2004. First cleavage plane of the mouse egg is not predetermined but defined by the topology of the two apposing pronuclei. Nature 430: 360–364. [DOI] [PubMed] [Google Scholar]
- Johnson M.H. and McConnell, J.M. 2004. Lineage allocation and cell polarity during mouse embryogenesis. Semin. Cell Dev. Biol. 15: 583–597. [DOI] [PubMed] [Google Scholar]
- Louvet-Vallee S., Vinot, S., and Maro, B. 2005. Mitotic spindles and cleavage planes are oriented randomly in the two-cell mouse embryo. Curr. Biol. 15: 464–469. [DOI] [PubMed] [Google Scholar]
- Manejwala F.M., Cragoe Jr., E.J., and Schultz, R.M. 1989. Blastocoel expansion in the preimplantation mouse embryo: Role of extracellular sodium and chloride and possible apical routes of their entry. Dev. Biol. 133: 210–220. [DOI] [PubMed] [Google Scholar]
- O'Farrell P.H., Stumpff, J., and Su, T.T. 2004. Embryonic cleavage cycles: How is a mouse like a fly? Curr. Biol. 14: R35–R45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotrowska K. and Zernicka-Goetz, M. 2001. Role for sperm in spatial patterning of the early mouse embryo. Nature 409: 517–521. [DOI] [PubMed] [Google Scholar]
- ____. 2002. Early patterning of the mouse embryo—Contributions of sperm and egg. Development 129: 5803–5813. [DOI] [PubMed] [Google Scholar]
- Piotrowska K., Wianny, F., Pedersen, R.A., and Zernicka-Goetz, M. 2001. Blastomeres arising from the first cleavage division have distinguishable fates in normal mouse development. Development 128: 3739–3748. [DOI] [PubMed] [Google Scholar]
- Plusa B., Piotrowska, K., and Zernicka-Goetz, M. 2002. Sperm entry position provides a surface marker for the first cleavage plane of the mouse zygote. Genesis 32: 193–198. [DOI] [PubMed] [Google Scholar]
- Plusa B., Hadjantonakis, A.K., Gray, D., Piotrowska-Nitsche, K., Jedrusik, A., Papaioannou, V.E., Glover, D.M., and Zernicka-Goetz, M. 2005. The first cleavage of the mouse zygote predicts the blastocyst axis. Nature 434: 391–395. [DOI] [PubMed] [Google Scholar]
- Rossant J. 1976. Postimplantation development of blastomeres isolated from 4- and 8-cell mouse eggs. J. Embryol. Exp. Morphol. 36: 283–290. [PubMed] [Google Scholar]
- Rossant J. and Tam, P.P. 2004. Emerging asymmetry and embryonic patterning in early mouse development. Dev. Cell 7: 155–164. [DOI] [PubMed] [Google Scholar]
- Tarkowski A.K. 1959. Experiments on the development of isolated blastomers of mouse eggs. Nature 184: 1286–1287. [DOI] [PubMed] [Google Scholar]
- ____. 1961. Mouse chimaeras developed from fused eggs. Nature 190: 857–860. [DOI] [PubMed] [Google Scholar]
- Tarkowski A.K. and Wroblewska, J. 1967. Development of blastomeres of mouse eggs isolated at the 4- and 8-cell stage. J. Embryol. Exp. Morphol. 18: 155–180. [PubMed] [Google Scholar]
- Watson A.J. and Barcroft, L.C. 2001. Regulation of blastocyst formation. Front. Biosci. 6: D708–D730. [DOI] [PubMed] [Google Scholar]
- Wiley L.M. and Eglitis, M.A. 1981. Cell surface and cytoskeletal elements: Cavitation in the mouse preimplantation embryo. Dev. Biol. 86: 493–501. [DOI] [PubMed] [Google Scholar]
- Zernicka-Goetz M. 2003. Determining the first cleavage of the mouse zygote. Reprod. Biomed. Online 6: 160–163. [DOI] [PubMed] [Google Scholar]