Introduction
To select patients with hepatocellular carcinoma (HCC) for liver transplantation (LT), Milan criteria are still mainly used worldwide1. Several selection criteria that sought to expand the Milan criteria have been proposed and acceptable post-LT survival may be obtained2. However, none of the selection criteria challenged advanced HCC with macrovascular invasion. This is generally regarded as a contraindication for LT. Recently, several studies showed that combinations of locoregional and systemic treatment could significantly improve tumour response and overall survival for patients with advanced HCC3–5. This included promising results of lenvatinib, transcatheter arterial chemoembolization (TACE) and programmed cell death protein 1 (PD-1) inhibitor (LEN-TAP) for advanced HCC6. With the improvement of combination therapy, the authors wondered whether LT could serve as another option for patients with advanced HCC after successful downstaging. The present study described the perioperative and postoperative outcome of patients who opted for LT when they failed to receive salvage liver resection due to decompensated liver function after successful downstaging.
Methods
LEN-TAP is an investigator-initiated trial that was designed to investigate efficacy and safety of salvage liver resection after conversion therapy of lenvatinib, TACE and PD-1 inhibitor in patients with unresectable HCC7. The study protocol was approved by ethics committee of West China Hospital (2020-836) and the study was registered at ClinicalTrial.gov (NCT04997850). Details of LEN-TAP study design are available in Supplementary material. Clinical data of patients who opted for LT when they failed to receive salvage liver resection due to decompensated liver function after successful downstaging were reviewed and analysed. LT was suggested after several multidisciplinary team meetings based upon the following considerations: advanced stage disease was downstaged within Milan criteria and complete tumour necrosis was found8 on enhanced computed tomography; and decompensated liver function and high risk of posthepatectomy liver failure. Additional methods are shown in the supplementary file.
Results
Between October 2020 and March 2022, 142 patients were enrolled in the LEN-TAP trial, and 71 patients received the triple combination therapy. According to LEN-TAP study protocol, 62 (87.3%) patients were amenable to receive salvage liver resection after the conversion therapy. Finally, 42 (59.2%) patients successfully received salvage liver resection whereas 4 patients failed to received salvage liver resection due to decompensated liver function (Fig. S1). Finally, two patients received marginal donor LT and one patient received living donor LT. All these three patients had advanced HCC with portal vein tumour thrombus. Baseline characteristics and perioperative outcomes are shown in Table S1.
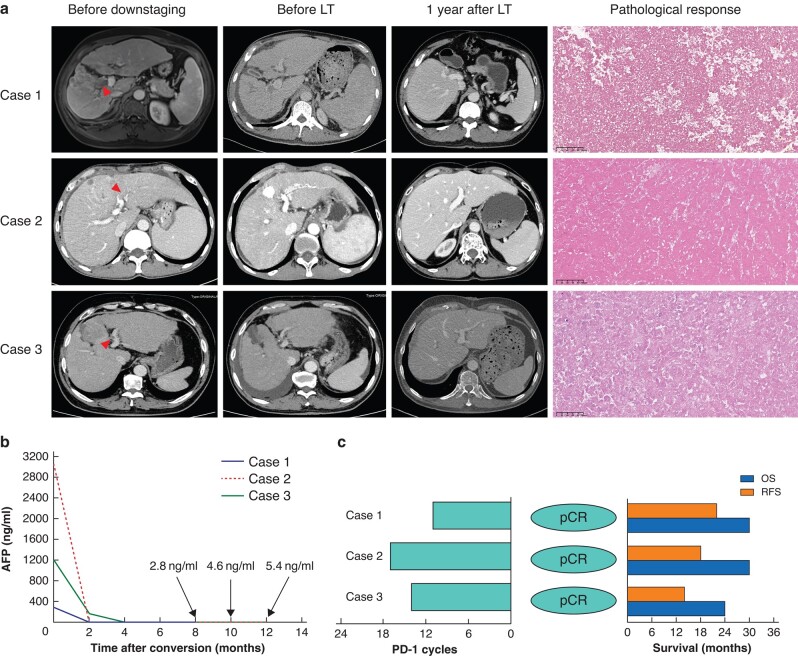
Images obtained before therapy, before LT and one year after LT, and pathological response of explanted liver and alpha-fetoprotein trends are shown in Fig. 1. All three patients remained alive with stable graft function and without tumour recurrence or metastasis (Fig. 1). Two patients had uneventful postoperative recoveries and one patient experienced acute rejection after LT. Timely administration of steroid pulse therapy and adjustment of the immunosuppression drug dosage led to a successful recovery.
Fig. 1.
Outcome for patients who underwent LT in LEN-TAP trial
a Radiological and pathological response for three patients who underwent LT. Target lesion and tumour thrombus (indicated by red arrows) necrosis and ascites were found after treatment; tumour-free images were obtained one year after LT; no residual tumour cells were found in explanted liver. b AFP trends between initiation of LEN-TAP therapy and pre-transplant. AFP decreased dramatically in these three patients after downstaging therapy. c Programmed cell death protein 1 inhibitor cycles before transplant, pathological response and survival time for patients received LT. LT, liver transplantation; LEN-TAP, lenvatinib, transarterial chemoembolization and PD-1 inhibitor therapy; AFP, alpha-fetoprotein; RFS, recurrence-free survival; OS, overall survival; pCR, pathological complete response.
Discussion
Combination of locoregional and systemic therapies has shown synergistic effects and plays a significant role in conversion therapy for unresectable HCC. The purpose is salvage resection for the tumour9. Several retrospective studies reported promising response rates and salvage resection rate following triple combination of TACE, tyrosine kinase inhibitor (TKI) and PD-1 inhibitor for unresectable HCC10,11. The authors’ pilot study and interim results of LEN-TAP trial showed promising tumour response and conversion resection rate6,7. Here, the authors reported the outcomes of salvage LT after successful downstaging by triple combination of lenvatinib, TACE and PD-1 inhibitors for advanced HCC.
Due to the potential induction of immune-related hepatitis and potentially fatal allograft rejection, the use of immunotherapy is controversial in the pre-transplant setting12. Therefore, aggressive induction immunosuppression with intravenous methylprednisolone and basiliximab was implemented prior to LT. The occurrence of acute rejection may be related to the relatively short interval between the last dose of PD-1 inhibitor and LT. The timing of deceased donor LT is uncertain, which complicates the decision of when to discontinue immunotherapy before LT. Studies have shown that discontinuing immunotherapy for at least 3 months prior to LT may be helpful to prevent acute rejection and graft loss13.
Continuing systemic therapy has been the standard of care for these patients. Then, disease progression usually occurs within 1–1.5 years after achieving a response14. For patients with resectable HCC it is generally accepted that complete removal of tumour provides the best opportunity of cure and offers favourable survival15. It is reasonable to believe that salvage LT for patients who were not feasible for salvage liver resection after successful downstaging might provide an acceptable post-LT survival. Further prospectively designed studies are needed to provide solid evidence.
Supplementary Material
Acknowledgements
Written approval has been obtained from all individuals. No preregistration exists for the study reported in this article. W.P. and Y.W. contributed equally to this study.
Contributor Information
Wei Peng, Division of Liver Surgery, Department of General Surgery, West China Hospital, Sichuan University, Chengdu, China; Chinese Evidence-based Medicine Center, West China Hospital, Sichuan University, Chengdu, China.
Youwei Wu, Division of Liver Surgery, Department of General Surgery, West China Hospital, Sichuan University, Chengdu, China.
Xiaoyun Zhang, Division of Liver Surgery, Department of General Surgery, West China Hospital, Sichuan University, Chengdu, China.
Chuan Li, Division of Liver Surgery, Department of General Surgery, West China Hospital, Sichuan University, Chengdu, China.
Junyi Shen, Division of Liver Surgery, Department of General Surgery, West China Hospital, Sichuan University, Chengdu, China.
Weixia Chen, Department of Radiology, West China Hospital, Sichuan University, Chengdu, China.
Qiu Li, Department of Medical Oncology, Cancer Center, West China Hospital, Sichuan University, Chengdu, China.
Ji Ma, Department of Medical Oncology, Cancer Center, West China Hospital, Sichuan University, Chengdu, China.
Yu Yang, Department of Medical Oncology, Cancer Center, West China Hospital, Sichuan University, Chengdu, China.
Wusheng Lu, Division of Liver Surgery, Department of General Surgery, West China Hospital, Sichuan University, Chengdu, China.
Zuojin Liu, Department of Hepatobiliary Surgery, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, China.
Xin Sun, Chinese Evidence-based Medicine Center, West China Hospital, Sichuan University, Chengdu, China.
Jiayin Yang, Transplant Center & Laboratory of Liver Transplantation, West China Hospital, Sichuan University, Chengdu, China.
Yongjie Zhou, Transplant Center & Laboratory of Liver Transplantation, West China Hospital, Sichuan University, Chengdu, China.
Tianfu Wen, Division of Liver Surgery, Department of General Surgery, West China Hospital, Sichuan University, Chengdu, China.
Funding
This study was funded by National Natural Science Foundation of China (Grant No. 82200691) and Key Research and Development Program of Sichuan Province (Grant No. 2022YFS0377).
Disclosure
Dr Wei Peng reports receiving grant support from Zelgen and lecture fees from Bayer, Merk, Roche, Hengrui and SciClone. Dr Xiaoyun Zhang receives grant support from Innovent, Eisai and lecture fees from Bayer, Merk, Roche and Hengrui. Dr Chuan Li receives grant support from Eisai and Merk, and advisor board fees from Bayer, Merk and Roche. Dr Tianfu Wen reports receiving grant support from AstraZeneca, Zelgen, Merk, Roche, Eisai and Innovent, and advisory board fees and lecture fees from Bayer, Merk, Roche and Hengrui. No other potential conflict of interest was reported.
Supplementary material
Supplementary material is available at BJS online.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Author contributions
Wei Peng (Conceptualization, Data curation, Methodology, Writing—original draft, Writing—review & editing), Youwei Wu (Methodology, Software, Validation, Writing—review & editing), Xiaoyun Zhang (Investigation, Writing—review & editing), Chuan Li (Investigation, Writing—review & editing), Junyi Shen (Data curation, Investigation, Writing—review & editing), Weixia Chen (Supervision, Writing—review & editing), Qiu Li (Project administration, Supervision, Writing—review & editing), Ji Ma (Writing—review & editing), Yu Yang (Methodology, Writing—review & editing), Wu-sheng Lu (Data curation, Supervision, Writing—review & editing), Zuojin Liu (Data curation, Investigation, Writing—review & editing), Xin Sun (Investigation, Methodology, Project administration), Jiayin Yang (Supervision, Writing—review & editing), Yongjie Zhou (Conceptualization, Formal analysis, Funding acquisition, Writing—review & editing), and Tianfu Wen (Conceptualization, Funding acquisition, Investigation, Project administration, Writing—original draft, Writing—review & editing)
References
- 1. Brown ZJ, Tsilimigras DI, Ruff SM, Mohseni A, Kamel IR, Cloyd JM et al. Management of hepatocellular carcinoma: a review. JAMA Surg 2023;158:410–420 [DOI] [PubMed] [Google Scholar]
- 2. Li J, Yan LN, Yang J, Chen ZY, Li B, Zeng Y et al. Indicators of prognosis after liver transplantation in Chinese hepatocellular carcinoma patients. World J Gastroenterol 2009;15:4170–4176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhu HD, Li HL, Huang MS, Yang WZ, Yin GW, Zhong BY et al. Transarterial chemoembolization with PD-(L)1 inhibitors plus molecular targeted therapies for hepatocellular carcinoma (CHANCE001). Signal Transduct Target Ther 2023;8:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Peng Z, Fan W, Zhu B, Wang G, Sun J, Xiao C et al. Lenvatinib combined with transarterial chemoembolization as first-line treatment for advanced hepatocellular carcinoma: a phase III, randomized clinical trial (LAUNCH). J Clin Oncol 2023;41:117–127 [DOI] [PubMed] [Google Scholar]
- 5. Chiang CL, Chiu KWH, Chan KSK, Lee FAS, Li JCB, Wan CWS et al. Sequential transarterial chemoembolisation and stereotactic body radiotherapy followed by immunotherapy as conversion therapy for patients with locally advanced, unresectable hepatocellular carcinoma (START-FIT): a single-arm, phase 2 trial. Lancet Gastroenterol Hepatol 2023;8:169–178 [DOI] [PubMed] [Google Scholar]
- 6. Peng W, Zhang X, Li C, Zhu X, Li Q, Chen W et al. Programmed cell death protein 1 and tyrosine kinase inhibition plus transcatheter arterial chemoembolization of advanced hepatocellular carcinoma. Br J Surg 2023;110:746–748 [DOI] [PubMed] [Google Scholar]
- 7. Xiaoyun Z, Zhu X, Feng X, Han W, Yan ML, Xie F et al. 715P the safety and efficacy of lenvatinib combined with TACE and PD-1 inhibitors (Len-TAP) versus TACE alone in the conversion resection for initially unresectable hepatocellular carcinoma: interim results from a multicenter prospective cohort study. Ann Oncol 2022;33:S870 [Google Scholar]
- 8. Yao FY, Fidelman N, Mehta N. The key role of staging definitions for assessment of downstaging for hepatocellular carcinoma. Semin Liver Dis 2021;41:117–127 [DOI] [PubMed] [Google Scholar]
- 9. Sun HC, Zhou J, Wang Z, Liu X, Xie Q, Jia W et al. Chinese expert consensus on conversion therapy for hepatocellular carcinoma (2021 edition). Hepatobiliary Surg Nutr 2022;11:227–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Qu WF, Ding ZB, Qu XD, Tang Z, Zhu GQ, Fu XT et al. Conversion therapy for initially unresectable hepatocellular carcinoma using a combination of toripalimab, lenvatinib plus TACE: real-world study. BJS Open 2022;6:zrac114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu JY, Yin ZY, Bai YN, Chen YF, Zhou SQ, Wang SJ et al. Lenvatinib combined with anti-PD-1 antibodies plus transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: a multicenter retrospective study. J Hepatocell Carcinoma 2021;8:1233–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nordness MF, Hamel S, Godfrey CM, Shi C, Johnson DB, Goff LW et al. Fatal hepatic necrosis after nivolumab as a bridge to liver transplant for HCC: are checkpoint inhibitors safe for the pretransplant patient? Am J Transplant 2020;20:879–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schnickel GT, Fabbri K, Hosseini M, Misel M, Berumen J, Parekh J et al. Liver transplantation for hepatocellular carcinoma following checkpoint inhibitor therapy with nivolumab. Am J Transplant 2022;22:1699–1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim T-Y et al. IMbrave150: updated overall survival (OS) data from a global, randomized, open-label phase III study of atezolizumab (atezo) + bevacizumab (bev) versus sorafenib (sor) in patients (pts) with unresectable hepatocellular carcinoma (HCC). J Clin Oncol 2021;39:267–267 [Google Scholar]
- 15. Lau WY, Lai EC. Salvage surgery following downstaging of unresectable hepatocellular carcinoma—a strategy to increase resectability. Ann Surg Oncol 2007;14:3301–3309 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.