Abstract
To investigate the potential role of protein kinase C-δ (PKC-δ) in insulin-like growth factor I receptor (IGF-IR)-mediated cell transformation, an oncogenic gag-IGF-IR β-fusion receptor lacking the entire extracellular domain, which was designated NM1, and a full-length IGF-IR were coexpressed with either wild-type PKC-δ (PKC-δWT) or an ATP-binding mutant of PKC-δ (PKC-δK376R) in NIH 3T3 fibroblasts. While overexpression of PKC-δWT did not affect NM1- and IGF-IR-induced focus and colony formation of NIH 3T3 cells, expression of PKC-δK376R severely impaired these events. In contrast, NM1-mediated cell growth in monolayer was not affected by coexpressing PKC-δK376R. PKC-δWT and PKC-δK376R were constitutively phosphorylated on a tyrosine residue(s) in the NM1- and IGF-IR-expressing cells and were associated with them in an IGF-I-independent manner. Activated IGF-IR was able to phosphorylate purified PKC-δ in vitro and stimulated its kinase activity. Furthermore, the level of endogenous PKC-δ protein was up-regulated through transcriptional activation in response to long-term IGF-IR activation. Taken together, our results demonstrate that PKC-δ plays an important role in IGF-IR-mediated cell transformation, probably via association of the receptor with PKC-δ and its activation through protein up-regulation and tyrosine phosphorylation. Competition with endogenous PKC-δ for NM1 and IGF-IR association by PKC-δK376R is probably an important mechanism underlying the PKC-δK376R-mediated inhibition of cell transformation by NM1 and IGF-IR.
Insulin-like growth factor I receptor (IGF-IR) is a type II tyrosine kinase receptor which is composed of two extracellular α subunits and two membrane-spanning β subunits linked by disulfide bonds (49, 50). IGF-I stimulation of IGF-IR results in receptor autophosphorylation and phosphorylation of certain signaling molecules such as Shc, phosphatidylinositol 3′ kinase (PI 3′K), Grb2, Grb10, insulin receptor substrate 1 (IRS-1), and interleukin 4-phosphorylated substrate (4PS)/IRS-2 (5). Activation of the IGF-IR signaling pathway leads to proliferation, differentiation, and inhibition of apoptosis in different model systems (5). The importance of IGF-IR in cell growth and development has been demonstrated by the targeted disruption of the IGF-IR gene (2, 29). The size of newborn mice lacking the IGF-IR was reduced by 70% in comparison to that of wild-type littermates. Moreover, IGF-IR activation has been demonstrated to play an important role in transformation of cultured cells and in tumor progression in syngeneic animals and nude mice (3). Embryonic fibroblasts established from IGF-IR−/− mice (R− cells) are resistant to transformation induced by a variety of oncogenes, growth factor receptors, and viral proteins, including v-ras, raf, platelet-derived growth factor β receptor (PDGF-βR), epidermal growth factor receptor (EGFR), simian virus 40 (SV40) T antigen, and the E5 protein of bovine papillomavirus (6, 7, 32, 33, 41, 42). Reconstitution of R− cells with wild-type IGF-IR restored the susceptibility of transformation by those oncogenes and growth factor receptors. Inhibition of the IGF-IR signaling pathway by expression of anti-sense IGF-I (48) or IGF-IR (17, 34, 36, 40, 43), by expression of dominant-negative IGF-IR (18, 39), or by microinjection of neutralizing antibody against IGF-IR (1, 15) has been shown to abolish or delay the progression of a variety of tumors in animal models. For example, down-regulation of the IGF-IR by antisense oligonucleotide blocking has been demonstrated to reduce the tumorigenicity of human glioblastoma T98G, rat glioblastoma C6, human breast carcinoma MC-F7, and mouse melanoma B16-F10 cells (5).
Protein kinase C-δ (PKC-δ) is a serine/threonine kinase whose activation has been tightly linked to monocytic differentiation of the 32D myeloid progenitor cell line in response to 12-O-tetradecanoylphorbol-13-acetate (TPA) stimulation (31). PKC-δ has also been demonstrated to be phosphorylated on a tyrosine residue(s) both in vitro and in vivo in response to a variety of stimuli (9, 22). Recently, PKC-δ has been identified as an important downstream signaling molecule of the PDGF-βR (23, 24). Autophosphorylation, membrane translocation, and membrane-associated kinase activity of PKC-δ increased in response to PDGF stimulation in NIH 3T3 fibroblasts overexpressing PKC-δ and in 32D cells coexpressing PDGF-βR and PKC-δ. PKC-δ was tyrosine phosphorylated both in vitro and in vivo by the activated PDGF-βR (20, 22, 24). Coexpression of an ATP binding mutant of PKC-δ (PKC-δK376R) (25) with the sis oncogene which encodes the PDGF-B chain significantly inhibited sis/PDGF-βR-mediated cell transformation of NIH 3T3 fibroblasts, strongly suggesting that PKC-δ is a physiological substrate involved in PDGF-βR-mediated cell transformation (21).
We have previously constructed a gag-IGF-IR β fusion receptor by deleting the entire extracellular domain of human IGF-IR and fusing the remaining sequence to the avian sarcoma virus UR2 gag and have designated it NM1 (14, 27, 28). Expression of NM1 in chicken embryo fibroblasts (CEF) resulted in constitutive receptor autophosphorylation and cell transformation, as reflected in morphological alteration and colony formation in soft agar. NM1 also induced tumors in chicken efficiently. In this study, we investigated the potential role of PKC-δ in the native and fusion IGF-IR-induced transformation of NIH 3T3 cells. Our results demonstrate that transformation of NIH 3T3 cells by NM1 is severely impaired by coexpression of PKC-δK376R. The PKC-δK376R mutant is also capable of blocking full-length IGF-IR-mediated focus formation in response to exogenous IGF-I. Furthermore, we show that PKC-δ is tyrosine phosphorylated in NM1- and IGF-IR-expressing cells and is associated with these receptor tyrosine kinases in vivo. In addition, purified PKC-δ is tyrosine phosphorylated in vitro by NM1 and IGF-IR, and this phosphorylation results in increased PKC-δ activity. Finally, we present evidence that endogenous PKC-δ is up-regulated in protein and RNA levels upon long-term NM1 and IGF-IR activation, thus providing a potential link between IGF-IR and PKC-δ in the NM1- and IGF-IR-mediated cell transforming pathway.
MATERIALS AND METHODS
cDNA construction.
The construction of oncogenic NM1 gag-IGF-IR and T6 gag-IR has been described elsewhere (27, 28). The NM1 mutant F1136, containing the Y1136-to-F1136 mutation of NM1, has been described elsewhere (14). Construction of the mutant dS2, from which 19 amino acids in the subtransmembrane region of NM1 were deleted, will be described elsewhere. All of these fusion receptor genes have been subcloned into a Moloney murine leukemia virus long terminal repeat-based expression vector pMEXneo as described previously (14, 27, 28). The full-length IGF-IR in pMEXneo was obtained from William Rutter. Cloning of PKC-δWT and PKC-δK376R into the pLTRgpt vector was reported before (25).
Transfection and focus formation assay.
Transfection of NIH 3T3 cells was performed by the calcium phosphate precipitation, as previously described (21). Briefly, 1.5 × 105 cells were plated on 10-cm tissue culture plates 1 day before transfection. Equal amounts of precipitated DNA were added to two separate plates for each transfecting sample. One of these plates was cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, BRL) containing 5% of calf serum for the focus-forming assay, while the other plate was selected in medium containing geneticin and/or mycophenolic acid, depending on the selectable marker gene present in the expression vectors. The number of drug-resistant colonies formed per plate was determined in order to ensure that equivalent amounts of DNA were utilized and that comparable transfection efficiencies were obtained. Media were changed twice a week following the transfection. Three weeks after transfection, the nonselected plates were fixed, stained with Giemsa dye (Fisher Scientific), and photographed for assessing focus formation. The selected plates were enumerated for the drug-resistant colonies, and cells of combined colonies from drug-resistant plates were used for further biochemical studies and for colony-forming assays (see below). When the full-length IGF-IR (coding for both the α and β subunits) cloned in pMEXneo was utilized in the focus formation assay, media containing 1% of calf serum instead of 5% were included for the nonselective plates either in the absence or in the presence of 50 ng of human IGF-I per ml for focus induction. The electroporation method for 32D cell transfection has been reported before (31).
Soft agar colony formation assay.
The soft agar assay measuring anchorage-independent growth has been reported elsewhere (21). Briefly, 105 NIH 3T3 stable transfectants were suspended in 4 ml of DMEM supplemented with 10% calf serum and 0.4% Seaplaque agarose in 6-cm tissue culture plates containing 4 ml of 0.8% agarose containing DMEM underlay. Cultures were fed with 0.2 ml of DMEM containing 10% of calf serum twice a week for 2 weeks. The colonies were stained with p-iodonitrotetrazolium violet (Sigma) after 2 weeks and photographed on an inverted light.
Monolayer cell growth determination.
Each NM1 transfectant coexpressing the various PKC-δ constructs was plated in a six-well Coaster plate at 1 × 104 to 5 × 104 cells/well with DMEM containing 10% calf serum. On the following day, the cells were washed once with DMEM and maintained in DMEM containing either 1% or 10% calf serum. Cell numbers were counted from one of these wells on day 0 and were continuously counted every other day from each of these wells until day 8 by using an automatic cell counter (Coulter Corporation). Two wells from each sample were counted at each time, and the mean values were calculated and expressed in the corresponding figure.
Protein extraction, immunoprecipitation, and immunoblot analysis.
Overnight serum starvation, growth factor stimulation, and lysis of cells have been described elsewhere (24, 25). For direct anti-PKC-δ and anti-IGF-IR immunoblot analysis, equivalent amounts of total cellular proteins (100 μg per sample) extracted with the lysis buffer containing 1% Triton X-100, 50 mM HEPES (pH 7.5), 5 mM EDTA, 50 mM NaCl, 10 mM sodium pyrophosphate, 50 mM sodium fluoride, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, 10-μg/ml aprotinin, and 10-μg/ml leupeptin were immunoblotted with anti-PKC-δ serum (1 to 1,000 dilution [R&D Aba.]) or with anti-IGF-IR serum (1 to 1,000 dilution [27]). For measuring IGF-IR tyrosine phosphorylation and PKC-δ tyrosine phosphorylation and for coimmunoprecipitation of IGF-IR with PKC-δ, cells were serum starved overnight and were either untreated or stimulated with human IGF-I (10 ng/ml) for 10 min at 37°C and lysed with the same lysis buffer described above. Equivalent amounts of cell lysates (2 to 5 mg per sample) were immunoprecipitated with either anti-IGF-IR or anti-PKC-δ. Proteins were fractionated and transferred to Immobilon membranes (Millipore) and immunoblotted with antiphosphotyrosine (anti-pTyr [UBI; 2 μg/ml]), anti-PKC-δ, or anti-IGF-IR antibody as described in the corresponding figures.
Immune complex assay for IGF-IR autophosphorylation.
Cells were either untreated or stimulated with IGF-I for 10 min after overnight serum starvation and lysed in the lysis buffer described above. Equal amounts of protein from cell lysates (2 mg per sample) were immunoprecipitated with anti-IGF-IR serum. The immunoprecipitates were washed with the lysis buffer and incubated in a reaction solution containing 25 mM HEPES (pH 7.5), 0.1% Nonidet P-40 (NP-40), 10 mM MgCl2, 3 mM MnCl2, and 30 μM Na3VO4 and 10 μCi of [γ-32P]ATP at room temperature for 10 min. The reaction was stopped by adding an equal volume of 2× sample loading buffer, and the mixture was boiled and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The dried gel was autoradiographed.
In vitro PKC-δ phosphorylation by IGF-IR and PKC-δ activity assay.
IGF-IR from NIH 3T3 cells and various transfectants was immunoprecipitated with anti-IGF-IR serum. The immunoprecipitates were incubated with the baculovirus-derived PKC-δ for 20 min at room temperature in cold-ATP-containing buffer as reported elsewhere (22). After reaction, 2 μl of each reaction mixture was used as a PKC-δ source to measure its activity by using PKC-δ pseudosubstrate region-derived peptide in the presence of [γ-32P]ATP (22). Briefly, purified PKC-δ before and after tyrosine phosphorylation was incubated at room temperature in 40 μl of reaction buffer containing 10 μM PKC-δ substrate derived from the PKC-δ pseudosubstrate region, 20 mM Tris-HCl (pH 7.5), 1 mM CaCl2, 10 μM magnesium acetate, 1 μM TPA, 50-μg/ml phosphatidylserine (Sigma), 30 μM cold ATP, and 30 μCi of [γ-32P]ATP for 20 min. The reaction tube was centrifuged, and 20 μl of the supernatant was spotted on phosphocellulase disks (Life Technologies, Inc./BRL). The disks were washed twice with 1% phosphoric acid and twice with distilled water, and samples were analyzed by liquid scintillation. The remaining reaction mixture was subjected to SDS-PAGE and immunoblotted with anti-pTyr or anti-PKC-δ.
Northern blot analysis.
The method with full-length mouse PKC-δ cDNA as the probe in Northern blot analysis has been described before (24).
RESULTS
Expression of PKC-δK376R mutant severely impairs NM1-, IGF-IR-, and T6-induced focus formation of NIH 3T3 fibroblasts.
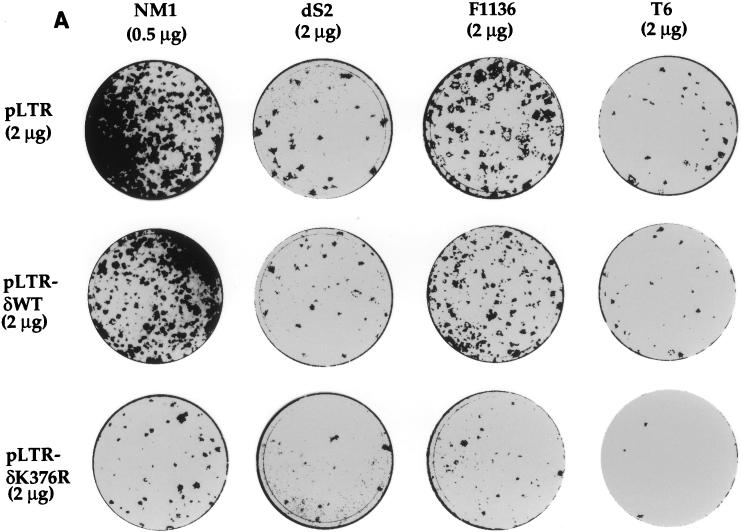
To investigate the potential role played by PKC-δ in IGF-IR-mediated cell transformation of NIH 3T3 fibroblasts, we cotransfected expression vectors containing various PKC-δ cDNAs together with NM1 plasmid. Consistent with its transforming activity in CEF (27, 28), NM1 was able to induce focus formation of NIH 3T3 fibroblasts with an efficiency of 6 × 102 foci/μg of DNA (Fig. 1A). Coexpression of PKC-δWT with NM1 did not affect the focus-forming activity induced by NM1 (Fig. 1A). In striking contrast, coexpression of PKC-δK376R with NM1 reduced its focus-forming activity by 90%. dS2 contains a 19-amino-acid deletion in the juxtamembrane region of NM1, and its transforming activity in CEF was dramatically reduced compared to that of NM1 (14a). Similarly, the focus-forming activity of dS2-transfected NIH 3T3 cells was only one-fifth of that of NM1-transfected cells (Fig. 1A, panel 2). Tyrosine 1136 of NM1 or IGF-IR has been demonstrated to be important for their transforming activity, as measured by colony formation of CEF, NIH 3T3, and mouse embryonic cells in soft agar (12, 14, 18). However, expression of the NM1 F1136 mutant in NIH 3T3 cells showed that it still retained about 50 to 60% of the focus-forming activity of NM1 (Fig. 1A, panel 3). Both dS2- and F1136-induced focus-forming activities were also severely inhibited by coexpression of PKC-δK376R. T6 is a gag-insulin receptor (gag-IR) fusion protein that is similar in structure to NM1 (38). Although T6 has a transforming activity comparable to that of NM1 in CEF (38), its focus-forming activity was remarkably reduced in comparison with that of NM1 in NIH 3T3 cells (Fig. 1A, panel 4). The focus-forming activity of T6 was nearly abolished in the presence of PKC-δK376R, which was a result consistent with that of NM1.
FIG. 1.
Expression of PKC-δK376R inhibits focus formation induced by NM1 and full-length IGF-IR. (A) NM1, dS2, and F1136 of NM1 and T6 were all cloned in pMEXneo vector and cotransfected with pLTR vector containing either PKC-δWT or PKC-δK376R with the amounts indicated. The plates were fixed and stained with Giemsa dye 3 weeks after transfection and photographed. Inhibition of NM1-induced focus formation by coexpressing PKC-δK376R had been observed more than three times. This panel represents one of those experiments. (B) Two micrograms of pMEX-IGF-IR (fusion of α and β chains) was cotransfected with 2 μg of PKC-δ cDNAs into NIH 3T3 fibroblasts. Twenty-four hours after transfection, the plates were kept in DMEM containing 1% calf serum in the absence or in the presence of 50 ng of human IGF-I per ml for 3 weeks. The plates were fixed, stained, and photographed.
We then tested whether full-length IGF-IR-induced transformation of NIH 3T3 cells could be affected by expression of PKC-δK376R. NIH 3T3 cells were cotransfected with full-length IGF-IR and the PKC-δWT or PKC-δK376R plasmid. The transfected cells were maintained in DMEM containing 1% calf serum in the absence or presence of 50 ng of human IGF-1 per ml. As shown in Fig. 1B, fewer than 30 foci were observed in cells cotransfected with IGF-IR and pLTR in the absence of exogenous IGF-I. IGF-I stimulation of the same transfectant for 3 weeks resulted in more than 200 foci. Similar numbers of foci were observed in the IGF-IR and pLTR-δWT cotransfectant upon IGF-I stimulation. In contrast, expression of PKC-δK376R totally abolished focus formation by spontaneous and IGF-I-stimulated IGF-IR activation.
Anchorage-independent growth of NIH 3T3 cells induced by NM1 is inhibited by PKC-δK376R.
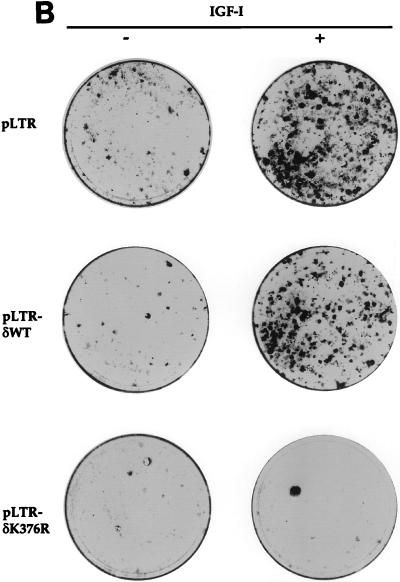
To test whether expression of PKC-δK376R also affected NM1-mediated anchorage-independent growth, we generated stable NIH 3T3 transfectants coexpressing NM1 and PKC-δK376R. As shown in Fig. 2, parental NIH 3T3 cells formed only a few spontaneous colonies in media containing 10% calf serum. NM1-expressing NIH 3T3 cells formed more than 200 colonies. In striking contrast, expression of PKC-δK376R significantly reduced the number of NM1-induced colonies in soft agar. Consistent with the results of the focus formation assay, expression of PKC-δWT had no effect on the colony-inducing activity of NM1 compared to that of NM1/pLTR-cotransfected cells. Taken together, these results demonstrate that PKC-δ plays a pivotal role in NM1-, IGF-IR-, and T6-mediated transformation of NIH 3T3 fibroblasts.
FIG. 2.
Anchorage-independent growth induced by NM1 is suppressed when PKC-δK376R is coexpressed. Stable NM1 transfectants coexpressing the various PKC-δ constructs or the parental NIH 3T3 fibroblasts were plated in soft agar-containing media with 10% calf serum and maintained for 2 weeks. The dishes were stained and photographed. Inhibition of NM1-induced colony formation in the soft agar assay had been observed more than three times. This represents one of those experiments.
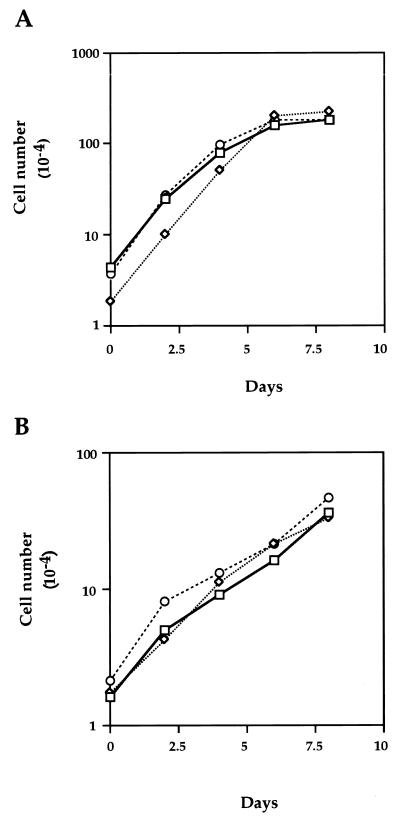
PKC-δK376R expression does not affect the growth rate of NM1-expressing cells in monolayer.
The growth rates of cells stably cotransfected with NM1 and PKC-δWT or PKC-δK376R in monolayer culture were measured in the presence of two serum concentrations. Transfectants cultured in 10% serum reached confluence in about 6 days, whereas those in 1% serum grew much more slowly, even though they were transformed by NM1 (Fig. 3). Nevertheless, neither coexpression of PKC-δWT nor that of PKC-δK376R affected the growth rate of NM1-expressing cells at either serum concentration. Since the same transfectants were utilized for both the soft agar colony formation assay and the monolayer growth assay, these data provide the evidence that PKC-δ-mediated signaling is important for IGF-IR-induced anchorage-independent growth and escape from contact inhibition, but not for proliferation of cells in monolayer culture. Segregation of signaling pathways leading to cell growth on monolayer versus those for focus and colony formation has been observed previously in other oncogene systems, including the differential effect exerted by various Ros mutants (52). However, the result here represents the initial observation that PKC-δ plays a differential role in distinct biological pathways mediated by IGF-IR activation.
FIG. 3.
Monolayer cell growth mediated by NM1 is not affected by PKC-δK376R expression. NM1/pLTR (squares), NM1/pLTR-δWT (diamonds), and NM1/pLTR-δK376R (circles) transfectants were plated in six-well Coaster plates and maintained in DMEM containing either 10% (A) or 1% (B) calf serum. Cell numbers were counted every other day until day 8.
IGF-IR expression and kinase activity are not affected by coexpression of the PKC-δK376R mutant.
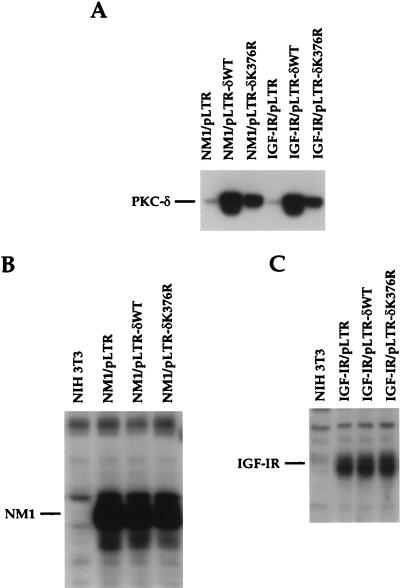
PKC-δ expression levels among the various transfectants were measured by direct immunoblot analysis with anti-PKC-δ serum. The expression levels of PKC-δWT and PKC-δK376R were increased by five- and threefold, respectively, over that of the endogenous PKC-δ (Fig. 4A). The protein level for the PKC-δK376R mutant was lower than that of PKC-δWT in the different transfectants, despite the use of the same expression vector. This was also observed in our previous studies, in which we attempted to express this mutant in 32D myeloid progenitor cells and to coexpress it with the sis oncogene in NIH 3T3 cells (21, 25). The expression levels of the 53-kDa NM1 protein in the control and PKC-δ transfectants were very similar (Fig. 4B). IGF-IR transfectants displayed a fivefold increase in IGF-IR expression over that of the endogenous IGF-IR, as judged by the expression of the 97-kDa IGF-IR β subunit (Fig. 4C). Again, no differences in IGF-IR protein levels were detected among the various PKC-δ transfectants.
FIG. 4.
Expression of PKC-δ, NM1, and IGF-IR proteins in NIH 3T3 transfectants. Equal amounts of cell lysates (100 μg per lane) were loaded on SDS-PAGE gels and immunoblotted with anti-PKC-δ serum (A) or with anti-IGF-IR sera (B and C).
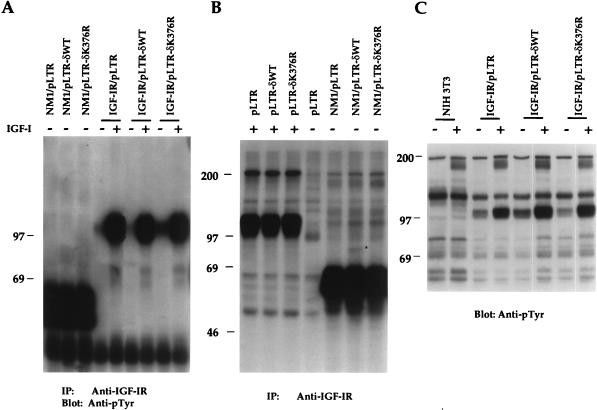
To determine whether overexpression of PKC-δWT or PKC-δK376R would affect NM1 and IGF-IR tyrosine kinase activities, the extents of receptor tyrosine phosphorylation and in vitro kinase activities of NM1 and IGF-IR were measured. Expression of NM1 resulted in a constitutively tyrosine-phosphorylated protein, which migrated as a broad band of 53 to 60 kDa (Fig. 5A). We did not observe any significant changes in the level of NM1 protein tyrosine phosphorylation upon coexpression of either PKC-δWT or PKC-δK376R. Overexpression of the full-length IGF-IR resulted in basal phosphorylation of the receptor. Ligand stimulation greatly increased tyrosine phosphorylation of the receptor (Fig. 5A). Again, ligand-dependent phosphorylation of IGF-IR was not affected by coexpression of PKC-δK376R.
FIG. 5.
NM1 and IGF-IR activities are not inhibited by PKC-δK376R expression. (A) NIH 3T3 transfectants were serum starved overnight in DMEM and either untreated or stimulated with 10 ng of IGF-I per ml for 10 min. Equivalent cell lysates were immunoprecipitated with anti-IGF-IR serum, and transferred proteins were immunoblotted with anti-pTyr. (B) NIH 3T3 transfectants were treated in a manner similar to that described for panel A. Equivalent cell lysates were immunoprecipitated with anti-IGF-IR serum and subjected to an immune complex assay as described in Materials and Methods. The dried gel was autoradiographed. An 80-kDa phosphoprotein associated with NM1 is indicated by the asterisk. (C) NIH 3T3 transfectants were treated in a manner similar to that described for panel A. Equivalent cell lysates (100 μg per lane) were subjected to immunoblot analysis with anti-pTyr. Marker proteins are given in kilodaltons. IP, immunoprecipitation.
The in vitro kinase assay was performed to further examine the effect of PKC-δK376R on NM1 and IGF-IR kinase activities. As seen in Fig. 5B, the autophosphorylation activities in all NM1 transfectants were indistinguishable. An 80-kDa phosphorylated protein was coprecipitated by anti-IGF-IR only in lysates from the NM1/pLTR-δWT cotransfectant (Fig. 5B [indicated by the asterisk]), but not from the NM1/pLTR or NM1/pLTR-δK376R cotransfectant. The ligand-dependent activation of the endogenous IGF-IR in various PKC-δ single transfectants was also measured by the kinase assay (Fig. 5B). Expression of PKC-δK376R did not affect activation of the endogenous IGF-IR. These results indicate that the inhibitory effect of PKC-δK376R on NM1 and IGF-IR transformation is not due to its effect on receptor activation and expression.
Tyrosine phosphorylation of cellular proteins in response to IGF-I stimulation was also examined by anti-pTyr immunoblot analysis. As seen in Fig. 5C, a relatively high level of IGF-IR β subunit tyrosine phosphorylation was observed in all three IGF-IR-transfected lines, but not in NIH 3T3 cells. Ligand stimulation resulted in increased tyrosine phosphorylation of the overexpressed IGF-IR to a similar extent in PKC-δWT and PKC-δK376R cotransfectants. Interestingly, a 180-kDa tyrosine phosphorylated protein, which may represent endogenous IRS-1, was detected in response to IGF-I stimulation in all the lines tested. Again, no obvious difference was observed in tyrosine phosphorylation of cellular proteins among all of the PKC-δ cotransfectants.
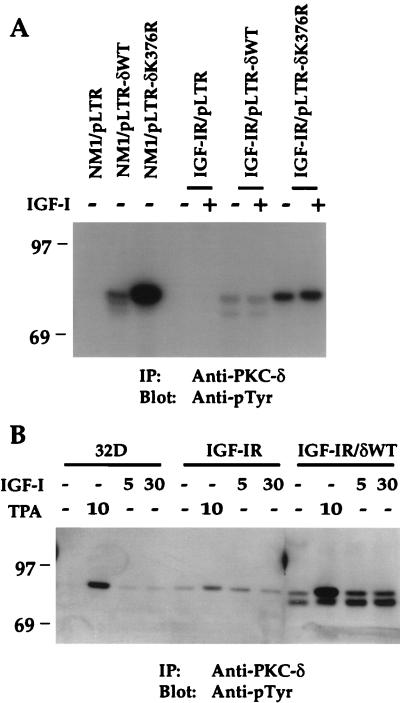
Overexpressed PKC-δ is constitutively phosphorylated on a tyrosine residue(s) in NM1- or IGF-IR-cotransfected NIH 3T3 cells.
PKC-δ has been previously demonstrated by our laboratory and several others to be phosphorylated on a tyrosine residue(s) in vivo and in vitro (8, 9, 11, 16, 20, 22, 24, 44, 45, 51). Tyrosine phosphorylation of PKC-δ was considered an indicator of its activation, since only the membrane-associated PKC-δ was found to be phosphorylated (24). We were interested to know whether activation of IGF-IR was able to induce tyrosine phosphorylation of PKC-δ. As shown in Fig. 6A, coexpression of NM1 with PKC-δWT resulted in constitutive tyrosine phosphorylation of PKC-δWT. Phosphorylation of the PKC-δK376R mutant protein by NM1 was much higher than that of PKC-δWT, even though mutant protein expression was two- to threefold lower (Fig. 4A). This is consistent with our previous finding that PKC-δK376R protein was constitutively and highly phosphorylated on tyrosine, which may be due to its exclusive localization in the membrane fraction of the cell. Surprisingly, PKC-δWT overexpression in an IGF-IR/pLTR-δWT cotransfectant resulted in constitutive tyrosine phosphorylation of PKC-δ independent of IGF-I, although IGF-IR tyrosine phosphorylation was greatly increased upon IGF-I stimulation (Fig. 5A). Likewise, PKC-δK376R was constitutively phosphorylated on tyrosine at a level higher than that of PKC-δWT in the IGF-IR/pLTR-δK376R cotransfectant.
FIG. 6.
PKC-δWT and PKC-δK376R proteins are constitutively tyrosine phosphorylated in NM1- or IGF-IR-cotransfected NIH 3T3 cells. (A) NM1 and IGF-IR cotransfectants were serum starved overnight in DMEM and either untreated or stimulated with 10 ng of IGF-I per ml for 10 min. (B) 32D cells and transfectants were serum starved for 2 h and either untreated or stimulated with 100 ng of TPA per ml for 10 min or with 10 ng of IGF-I per ml for either 5 or 30 min. Equivalent cell lysates were immunoprecipitated with anti-PKC-δ serum. Transferred proteins were immunoblotted with anti-pTyr. Marker proteins are indicated in kilodaltons. IP, immunoprecipitation.
The constitutive phosphorylation of PKC-δWT by overexpressed IGF-IR was in sharp contrast to PKC-δ activation by the PDGF-βR, in which PKC-δ was tyrosine phosphorylated in a PDGF-dependent manner (24). IGF-IR overexpression led to a relatively high level of autophosphorylation in the absence of addition of exogenous ligand (Fig. 5A and C). Furthermore, we have previously shown that overexpression of PKC-δ resulted in its partial localization in the membrane fraction of the cell before stimulation (25). To test whether constitutive tyrosine phosphorylation of PKC-δWT prior to IGF-I stimulation was due to its overexpression, we chose to stimulate 32D cells with IGF-I or TPA. 32D cells express functional IGF-IR (data not shown) and higher endogenous PKC-δ than NIH 3T3 cells whose tyrosine phosphorylation in response to PDGF (24) or TPA (see below) was easily detected in vivo. As shown in Fig. 6B, tyrosine phosphorylation of endogenous PKC-δ from 32D cells in response to IGF-I stimulation for 5 and 30 min was increased by at least two- to threefold, strongly suggesting that PKC-δ is a physiological substrate of IGF-IR. Overexpression of IGF-IR in 32D cells led to some constitutive PKC-δ tyrosine phosphorylation, although a onefold increase of PKC-δ tyrosine phosphorylation was still observed in response to IGF-I stimulation for 5 min. These data clearly indicate that overexpression of IGF-IR can cause constitutive tyrosine phosphorylation of endogenous PKC-δ, which may be contributed by the leaky IGF-IR due to its overexpression. Tyrosine phosphorylation of PKC-δWT became fully independent of IGF-I when both IGF-IR and PKC-δ were overexpressed in 32D cells. This result mimics the phenomenon observed with NIH 3T3 cells coexpressing IGF-IR with PKC-δ (Fig. 6A). As reported elsewhere (22, 24), endogenous PKC-δ and overexpressed PKC-δ were tyrosine phosphorylated in response to TPA stimulation (Fig. 6B). We conclude that overexpression of PKC-δ with NM1 or IGF-IR overexpressed in NIH 3T3 cells results in constitutive tyrosine phosphorylation of PKC-δ. Based on the data obtained from the 32D cell system, it is speculated that endogenous PKC-δ of NIH 3T3 cells may also be tyrosine phosphorylated and activated by IGF-IR in vivo in a ligand-dependent fashion when it reaches a certain expression level during the IGF-IR transformation process. This hypothesis is further supported by the up-regulation of endogenous PKC-δ through long-term IGF-IR activation in NIH 3T3 cell system (see Fig. 9).
FIG. 9.
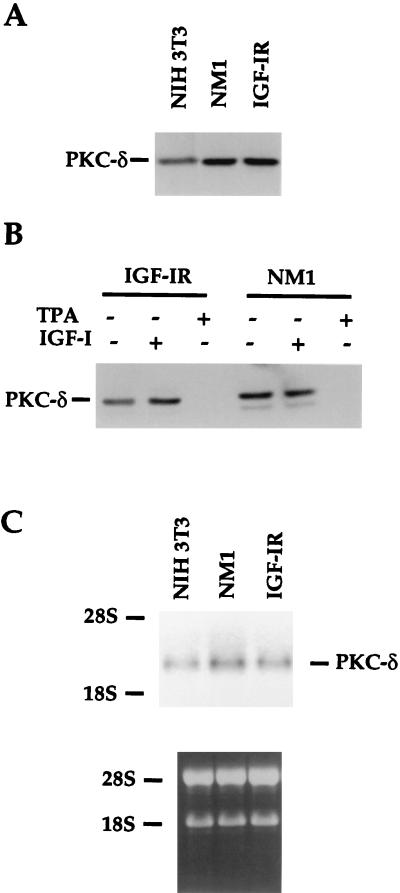
Endogenous PKC-δ protein and RNA are up-regulated by long-term IGF-IR activation. (A) NIH 3T3 cells and transfectants were maintained in media containing 10% calf serum and lysed. Equivalent amounts of proteins were loaded on SDS-PAGE gels. The transferred proteins were immunoblotted with anti-PKC-δ serum. (B) NIH 3T3 transfectants were serum starved for the first 8 h and either untreated or exposed to IGF-I (50 ng/ml) or TPA (100 ng/ml) for another 16 h. The cells were lysed, and transferred proteins from SDS-PAGE gels were immunoblotted with anti-PKC-δ. (C) Fifteen micrograms of total RNA from the parental NIH 3T3 and transfectants was isolated from normal cultured cells and loaded into agarose gel. Equivalent amounts of loading were demonstrated by ethidium bromide staining, as shown in the lower panel. The specific PKC-δ messages were detected with the full-length mouse PKC-δ as a probe (top panel). 18S and 28S rRNAs were used as markers.
Purified PKC-δ can be phosphorylated by NM1 and IGF-IR with increased activity in vitro.
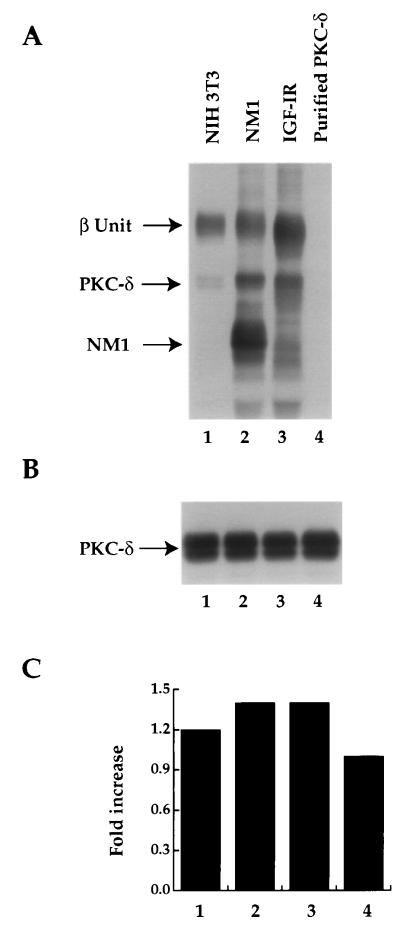
Having demonstrated that PKC-δ was tyrosine phosphorylated in the NM1 or IGF-IR cotransfectant, we were interested to know whether activated IGF-IR was able to phosphorylate PKC-δ directly and affect PKC-δ kinase activity. For this purpose, IGF-IR from the NM1 or IGF-IR transfectant and NIH 3T3 parental line was immunoprecipitated and subjected to an in vitro kinase assay in the presence of purified PKC-δ derived from baculovirus and cold ATP (22). After reaction, some of the supernatant containing the purified PKC-δ was assayed for PKC-δ activity, while the remaining reaction mixture was subjected to immunoblot analysis with anti-pTyr. As shown in Fig. 7A, coincubation of the purified PKC-δ in vitro with immunoprecipitated endogenous IGF-IR resulted in PKC-δ tyrosine phosphorylation. The level of PKC-δ tyrosine phosphorylation was increased with immunoprecipitates from NM1- or IGF-IR-overexpressed cells (Fig. 7A; compare lanes 1 to 2 and 3). The same amount of purified PKC-δ was used for the in vitro tyrosine phosphorylation reaction in all of the samples, as determined by reprobing the same membrane shown in Fig. 7A with anti-PKC-δ (Fig. 7B). Subsequent analysis of PKC-δ activity demonstrated that its activity was increased by 1.2- to 1.4-fold after it was tyrosine phosphorylated, compared to the non-tyrosine-phosphorylated PKC-δ (Fig. 7C; compare lanes 1 to 3 to 4). These results suggest that PKC-δ is phosphorylated by NM1 and IGF-IR in vitro and that this phosphorylation increases PKC-δ activity.
FIG. 7.
Purified PKC-δ is tyrosine phosphorylated and activated by the activated IGF-IR from normal and transfected NIH 3T3 cells. (A) Cell lysates from NIH 3T3 and its transfectants were immunoprecipitated with anti-IGF-IR. Washed immunoprecipitates were subjected to an in vitro kinase assay by including the purified PKC-δ as a substrate, together with cold ATP (lanes 1 to 3). Purified PKC-δ was also incubated with the kinase assay buffer alone as a control (lane 4). After phosphorylation reaction, one portion of the supernatant was used as a PKC-δ source for the subsequent PKC-δ activity assay (see panel C). The remainder of the reaction mixture was resolved by SDS-PAGE and immunoblotted with anti-pTyr. (B) The membrane from panel A was reblotted with anti-PKC-δ serum. (C) Two microliters of the mixture from the in vitro reaction performed in panel A was subjected to an in vitro PKC-δ activity assay in the presence of [γ-32P]ATP as described in Materials and Methods. The fold increases were calculated by counts per minute from lanes 1 to 3 divided by counts per minute from lane 4. A similar result was also obtained in another independent experiment.
PKC-δ associates with NM1 and IGF-IR intracellularly.
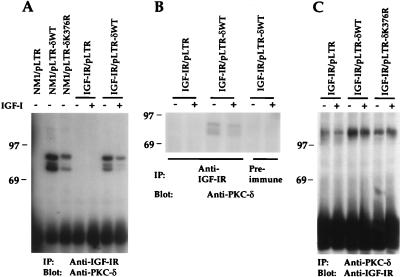
Association of tyrosine kinase receptors with their substrates in vivo occurs frequently via Src homology 2 (SH2) or phosphotyrosine binding (PTB) domains of these substrates (37). Although PKC-δ does not possess an SH2 or PTB domain, it has been shown to be tyrosine phosphorylated by various protein tyrosine kinase receptors, including PDGF-βR, IR, and EGFR (8, 22, 24), and to associate with p85 subunit of PI 3′K (10) and v-src (51) in vivo. An 80-kDa phosphoprotein from the NM1/pLTR-δWT cotransfectant was reproducibly detected in the in vitro IGF-IR kinase assay (Fig. 5B). Since PKC-δ possesses autophosphorylation capacity and is known to migrate as an 80-kDa protein by SDS-PAGE, we suspected that the 80-kDa phosphoprotein could be PKC-δ. Failure to detect the same 80-kDa protein from the NM1/pLTR-δK376R cotransfectant further suggested its identity as PKC-δ, since PKC-δK376R is not able to undergo autophosphorylation (25). To test our hypothesis, we performed reciprocal immunoprecipitation and immunoblotting analyses using anti-IGF-IR and anti-PKC-δ sera. As shown in Fig. 8A, both PKC-δWT and PKC-δK376R proteins were detected in anti-IGF-IR immunoprecipitates from lysates of NM1-cotransfected cells. The amounts of PKC-δ associated with NM1 protein were proportional to the expression levels of PKC-δWT and PKC-δK376R in the respective cotransfectants (Fig. 4A). PKC-δWT and PKC-δK376R coimmunoprecipitated by anti-IGF-IR migrated as doublets or triplets on SDS-PAGE gels, which may be due to existence of multiple phosphorylated forms of PKC-δ as reported elsewhere (25). Endogenous PKC-δ was not detected under such condition. PKC-δWT in the IGF-IR/pLTR-δWT cotransfectant was constitutively associated with IGF-IR in this assay (Fig. 8A). PKC-δK376R was also detected from anti-IGF-IR immunoprecipitates in the IGF-IR/pLTR-δK376R cotransfectant, albeit at a much lower level (data not shown). To exclude any possibility of nonspecific coimmunoprecipitation of overexpressed PKC-δ from anti-IGF-IR immunoprecipitates, we immunoprecipitated IGF-IR/pLTR-δWT transfectant with preimmune serum. As seen in Fig. 8B, preimmune serum did not precipitate PKC-δ from the transfectant, suggesting that detection of PKC-δ from anti-IGF-IR immunoprecipitates is due to the specific interaction of PKC-δ with the IGF-IR.
FIG. 8.
PKC-δWT and PKC-δK376R are constitutively associated with NM1 and IGF-IR in vivo. (A) Various NIH 3T3 transfectants were serum starved overnight in DMEM and either untreated or stimulated with 10 ng of IGF-I per ml for 10 min. Equivalent cell lysates were immunoprecipitated with anti-IGF-IR serum. Transferred proteins were immunoblotted with anti-PKC-δ. (B) Cell lysates were immunoprecipitated either with anti-IGF-IR or with preimmune serum. Transferred proteins were immunoblotted (Blot) with anti-PKC-δ. (C) The same lysates from panel A were immunoprecipitated with anti-PKC-δ serum. Transferred proteins were immunoblotted with anti-IGF-IR. Marker proteins are indicated in kilodaltons. IP, immunoprecipitation.
In the reciprocal experiment, we found that IGF-IR was detected in the anti-PKC-δ immunoprecipitates from the IGF-IR/pLTR, IGF-IR/pLTR-δWT, or IGF-IR/pLTR-δK376R cotransfectant, with the most abundant association detected from the IGF-IR/pLTR-δWT cotransfectant (Fig. 8C). Like tyrosine phosphorylation of PKC-δ by IGF-IR, its association with IGF-IR appeared to be mainly ligand independent. These results demonstrate that PKC-δWT and PKC-δK376R are associated with both IGF-IR and NM1 in a ligand-independent manner in NIH 3T3 cells overexpressing these proteins.
Endogenous PKC-δ protein and RNA levels are increased upon constitutive or long-term IGF-IR activation.
To further explore the role of endogenous PKC-δ in NM1- and IGF-IR-mediated cell transformation, we examined the endogenous PKC-δ protein levels in NM1- and IGF-IR-overexpressing NIH 3T3 cells. As shown in Fig. 9A, when the cells were cultured in media containing 10% calf serum, overexpression of NM1 or IGF-IR resulted in a twofold increase in the PKC-δ protein level compared to that of the parental NIH 3T3 cells. When NM1 and IGF-IR transfectants were serum starved for 8 h and then treated with IGF-I for another 16 h, endogenous PKC-δ from IGF-I-treated IGF-IR transfectant was up-regulated by twofold (Fig. 9B). As expected, TPA treatment for 16 h completely degraded endogenous PKC-δ protein. The level of PKC-δ in the NM1 transfectant was equivalent to that of the IGF-I-treated IGF-IR transfectant and was independent of IGF-I stimulation, indicating that constitutively activated NM1 was able to up-regulate the PKC-δ protein level even in the absence of serum.
When total RNAs were isolated from NIH 3T3 cells and NM1 or IGF-IR transfectant cultured in the presence of serum, up-regulation of PKC-δ messages by one- to twofold in NM1 and IGF-IR transfectants compared to that of NIH 3T3 cells was clearly observed (Fig. 9C). Taken together, our results suggest that association with and tyrosine phosphorylation of PKC-δ by IGF-IR and up-regulation of the expression of PKC-δ by long-term IGF-IR overexpression and activation play an important role in NM1- and IGF-IR-mediated cell transformation.
DISCUSSION
Although activation of IGF-IR due to its mutations was not reported in samples from tumor patients, overexpression of functional IGF-IR has been repeatedly documented in different cancers (3–5). In the present study, we provide evidence that overexpression of native and oncogenic IGF-IR can lead to NIH 3T3 cell transformation, clearly indicating the causal role of overexpressed IGF-IR in cell transformation. The IGF-IR- and oncogenic IR-mediated transformation is inhibited by coexpression of an ATP binding mutant of PKC-δ (PKC-δK376R). Since PKC-δWT overexpression did not enhance IGF-IR-mediated transformation, it is likely that the level of endogenous PKC-δ is not limiting for relaying IGF-IR transformation signals. It is also possible that downstream signaling molecules of PKC-δ are limiting. Therefore, transformation would not be enhanced when PKC-δWT is overexpressed together with NM1 or IGF-IR. To date, we have shown that c-Sis (PDGF-B)-, IGF-IR-, and IR-induced, but not v-H-Ras- and v-Raf-induced, transformation of NIH 3T3 cells can be blocked by coexpressing PKC-δK376R, indicating specificity in the dominant inhibitory effect of this mutant on oncogene-mediated cell transformation. PKC-δK376R expression did not affect NM1 or IGF-IR tyrosine kinase activities, indicating that PKC-δK376R must exert its inhibitory effect downstream of receptor activation.
Our data show association of IGF-IR with PKC-δ and tyrosine phosphorylation of PKC-δ in NM1- and IGF-IR-transfected cells coexpressing PKC-δ. The activated IGF-IR was also demonstrated to phosphorylate purified PKC-δ in vitro, leading to increased PKC-δ enzymatic activity. In addition, PKC-δ tyrosine phosphorylation correlated with its association with NM1 or IGF-IR in vivo. All of these results strongly suggest that PKC-δ is a direct in vivo substrate of IGF-IR. That the endogenous PKC-δ was tyrosine phosphorylated in response to short-term IGF-I stimulation in 32D cells further substantiates the role of PKC-δ as a physiological substrate of IGF-IR in vivo. Our previous study also demonstrated that the IR was able to phosphorylate purified PKC-δ in vitro and that tyrosine phosphorylation of PKC-δ by IR increased PKC-δ kinase activity (22). Inhibition of oncogenic IR-induced transformation by the PKC-δK376R mutant correlates with these observations.
Although our data did not provide evidence that PKC-δ is directly activated by short-term IGF-I stimulation in the NIH 3T3 cell system, it has been reported that insulin stimulation leads to diacylglycerol production and subsequent PKC activation (46, 47). In addition, long-term stimulation by NM1 or IGF-I treatment of IGF-IR in NIH 3T3 transfectants results in up-regulation of PKC-δ protein levels. In addition, endogenous PKC-δ of 32D cells is tyrosine phosphorylated by the activated IGF-IR (Fig. 6B), an indicator of PKC-δ activation (24). Thus, endogenous PKC-δ may be regulated in an IGF-I-dependent manner during the transformation process. Inhibition of NM1 and IGF-IR transformation by the PKC-δK376R mutant and its association with these receptors further substantiate the specific role of endogenous PKC-δ in IGF-IR-mediated cell transformation. The exact mechanism underlying PKC-δK376R inhibition of NM1 and IGF-IR transformation is still unclear. However, the association of PKC-δK376R with NM1 and IGF-IR in vivo strongly suggests that PKC-δK376R might compete with endogenous PKC-δ for IGF-IR binding. Thus, PKC-δK376R competition may block the PKC-δ-mediated signal transduction pathway utilized by IGF-IR by sequestering important substrates whose activation requires phosphorylation by endogenous PKC-δ.
Our results utilizing the PKC-δ ATP binding mutant indicate that IGF-IR-mediated cell proliferation can be segregated from focus and colony formation. It appears that PKC-δ activation is involved in cell transformation but not in matrix-attached cell growth induced by IGF-IR. Systematic deletion and point mutation of the cytoplasmic domain of IGF-IR have also suggested that IGF-IR-mediated soft agar growth, mitogenicity, and inhibition of apoptosis are separable (35). A cluster of serine residues at the COOH terminus of IGF-IR has been identified as important for IGF-IR-induced transformation, but not for its mitogenicity (19). Whether these serines are phosphorylated by PKC-δ remains to be determined.
Recently, conflicting results concerning the role played by PKC-δ in cellular transformation have been observed in different cell systems. PKC-δ was suggested to be a tumor suppressor gene in c-Src-transfected 3Y1 fibroblasts (30). c-Src transformed 3Y1 cells only when TPA was present. It was proposed that the synergistic effect between TPA and c-Src on 3Y1 cell transformation was due to the down-regulation of functional PKC-δ. Expression of a dominant-negative mutant of PKC-δ (13), similar to that generated in our laboratory and used in our present study, enhanced the colony-forming activity of c-Src-expressing cells. In contrast to this study, a positive role for PKC-δ in malignant transformation was demonstrated in a separate report (26). When rat embryo fibroblasts were transformed by SV40 T antigen, endogenous PKC-δ levels were increased by more than threefold. When clones capable of growing in soft agar were analyzed, their endogenous PKC-δ levels were found to be further increased compared to those of the original SV40-transformed cells. Expression of the NH2 terminus of PKC-δ, which appeared to act in a dominant inhibitory fashion, completely suppressed soft agar colony formation by SV40-transformed cells (26). More recently, this group further demonstrated that PKC-δ may play an important role in determining the metastatic property of SV40-transformed cells (13a). In our hands, both PDGF and IGF-I, two important mitogens for cell proliferation, are able to up-regulate endogenous PKC-δ levels (Fig. 9) (24). Whether the role of PKC-δ in cellular transformation is cell type specific or oncogene specific remains to be determined.
In summary, our results provide the first evidence that PKC-δ is a direct tyrosine substrate of IGF-IR and plays a pivotal role in IGF-IR-mediated transformation in NIH 3T3 cells. Association of the PKC-δ dominant-negative mutant with NM1 or IGF-IR may block IGF-IR-mediated signal transduction by competing with endogenous PKC-δ for receptor association and activation. The identification of PKC-δ as a substrate of tyrosine kinase receptors, such as the IGF-IR and PDGF-βR, will further allow us to evaluate novel mechanisms of receptor-substrate interaction and activation. Since PKC-δ does not possess SH2, PTB, or pleckstrin homology domains which have been documented as the important modules in protein-protein interactions (37), it will be interesting to determine whether association of PKC-δ with NM1 or IGF-IR in vivo is direct, and, if so, which regions of PKC-δ and IGF-IR are required for this association. We are also very interested to determine if endogenous PKC-δ is critical in tumorigenesis, in which the up-regulation of IGF-IR and activation of its signaling pathway are tightly involved.
ACKNOWLEDGMENTS
This work was supported in part by NIH grant CA55054.
We thank Nelson Ellmore for excellent technical assistance.
REFERENCES
- 1.Arteaga C L. Interference of the IGF system as a strategy to inhibit breast cancer growth. Breast Cancer Res Treat. 1992;22:101–106. doi: 10.1007/BF01833338. [DOI] [PubMed] [Google Scholar]
- 2.Baker J, Liu J P, Robertson E J, Efstraitiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;79:927–930. [PubMed] [Google Scholar]
- 3.Baserga R. The insulin-like growth factor I receptor: a key to tumor growth. Cancer Res. 1995;55:249–252. [PubMed] [Google Scholar]
- 4.Baserga R. Oncogenes and the strategy of growth factors. Cell. 1994;79:927–930. doi: 10.1016/0092-8674(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 5.Baserga R, Hongo A, Rubini M, Prisco M, Valentinis B. The IGF-I receptor in cell growth, transformation and apoptosis. Biochim Biophys Acta. 1997;1332:F105–F126. doi: 10.1016/s0304-419x(97)00007-3. [DOI] [PubMed] [Google Scholar]
- 6.Coppola D, Ferver A, Miura M, Sell C, D’Ambrosio C, Rubin R, Baserga R. A functional IGF-I receptor is required for the mitogenic and transforming activities of the epidermal growth factor receptor. Mol Cell Biol. 1994;14:4588–4595. doi: 10.1128/mcb.14.7.4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeAngelis T, Ferber A, Baserga R. Insulin-like growth factor I receptor is required for the mitogenic and transforming activities of the platelet-derived growth factor receptor. J Cell Physiol. 1995;164:214–221. doi: 10.1002/jcp.1041640126. [DOI] [PubMed] [Google Scholar]
- 8.Denning M F, Dlugosz A, Threadgill D W, Magnuson T, Yuspa S H. Activation of the epidermal growth factor receptor signal transduction pathway stimulates tyrosine phosphorylation of protein kinase C δ. J Biol Chem. 1996;271:26079–26081. doi: 10.1074/jbc.271.10.5325. [DOI] [PubMed] [Google Scholar]
- 9.Denning M F, Dlugosz A A, Howett M K, Yuspa S H. Expression of an oncogenic rasHa gene in murine keratinocytes induces tyrosine phosphorylation and reduced activity of protein kinase C δ. J Biol Chem. 1993;268:26079–26081. [PubMed] [Google Scholar]
- 10.Ettinger S L, Lauener R W, Duronio V. Protein kinase C δ specifically associates with phosphatidylinositol 3-kinase following cytokine stimulation. J Biol Chem. 1996;271:14514–14518. doi: 10.1074/jbc.271.24.14514. [DOI] [PubMed] [Google Scholar]
- 11.Gschwendt M, Kielbassa K, Kittstein W, Marks F. Tyrosine phosphorylation and stimulation of protein kinase Cδ from porcine spleen by src in vitro: dependence on the activated state of protein kinase Cδ. FEBS Lett. 1994;347:85–89. doi: 10.1016/0014-5793(94)00514-1. [DOI] [PubMed] [Google Scholar]
- 12.Hernandez-Sanchez C, Blakesley V, Kalebic T, Helman L, LeRoith D. The role of the tyrosine kinase domain of the insulin-like growth factor-I receptor in intracellular signaling, cellular proliferation, and tomorigenesis. J Biol Chem. 1995;270:29176–29181. doi: 10.1074/jbc.270.49.29176. [DOI] [PubMed] [Google Scholar]
- 13.Hirai S, Izumi Y, Higa K, Kaibuchi K, Mizuno K, Osada S, Suzuki K, Ohno S. Ras-dependent signal transduction is indispensable but not sufficient for the activation of AP1/Jun by PKCδ. EMBO J. 1994;13:2331–2340. doi: 10.1002/j.1460-2075.1994.tb06517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13a.Jaken, S. Personal communication.
- 14.Jiang Y, Chan J L-K, Zong C S, Wang L-H. Effect of tyrosine mutations on the kinase activity and transforming potential of an oncogenic human insulin-like growth factor I receptor. J Biol Chem. 1996;271:160–167. doi: 10.1074/jbc.271.1.160. [DOI] [PubMed] [Google Scholar]
- 14a.Jiang, Y.-X., and L.-H. Wang. Unpublished observations.
- 15.Kalebic T, Tsokos M, Helman L J. In vivo treatment with antibody against the IGF-I receptor suppresses growth of human rhabdomyosarcoma and down regulates p34/cdc2. Cancer Res. 1994;54:5531–5534. [PubMed] [Google Scholar]
- 16.Konishi H, Tanaka M, Takemura Y, Matsuzaki H, Ono Y, Kikkawa U, Nishizuka Y. Activation of protein kinase C by tyrosine phosphorylation in response to H2O2. Proc Natl Acad Sci USA. 1997;94:11233–11237. doi: 10.1073/pnas.94.21.11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee C-T, Wu S, Gabrilovich D, Chen H, Nadaf-Rahrov N, Ciernik I F, Carbone D P. Antitumor effects of an adenovirus expressing antisense insulin-like growth factor I receptor on human lung cancer cell lines. Cancer Res. 1996;56:3038–3041. [PubMed] [Google Scholar]
- 18.Li S, Ferber A, Miura M, Baserga R. Mitogenicity and transforming activity of the insulin-like growth factor I receptor with mutations in the tyrosine kinase domain. J Biol Chem. 1994;269:32558–32564. [PubMed] [Google Scholar]
- 19.Li S, Resnicoff M, Baserga R. Effect of mutations at serines 1280–1283 on the mitogenic and transforming activities of the insulin-like growth factor I receptor. J Biol Chem. 1996;271:12254–12260. doi: 10.1074/jbc.271.21.12254. [DOI] [PubMed] [Google Scholar]
- 20.Li W, Li W, Chen X-H, Kelley C A, Alimandi M, Zhang J, Chen Q, Bottaro D P, Pierce J H. Identification of tyrosine 187 as a protein kinase C-δ phosphorylation site. J Biol Chem. 1996;271:26404–26409. doi: 10.1074/jbc.271.42.26404. [DOI] [PubMed] [Google Scholar]
- 21.Li W, Michieli P, Alimandi M, Lorenzi M V, Wu Y, Wang L-H, Heidaran M A, Pierce J H. Expression of an ATP binding mutant of PKC-δ inhibits Sis-induced transformation of NIH3T3 cells. Oncogene. 1996;13:731–737. [PubMed] [Google Scholar]
- 22.Li W, Mischak H, Yu J-C, Wang L-M, Mushinski J F, Heidaran M A, Pierce J H. Tyrosine phosphorylation of protein kinase C-δ in response to its activation. J Biol Chem. 1994;269:2349–2352. [PubMed] [Google Scholar]
- 23.Li W, Pierce J H. Protein kinase C-δ, an important signaling molecule in the platelet-derived growth factor-β receptor pathway. Cur Top Microbiol Immunol. 1996;211:55–65. doi: 10.1007/978-3-642-85232-9_6. [DOI] [PubMed] [Google Scholar]
- 24.Li W, Yu J-C, Michieli P, Beeler J F, Ellmore N, Heidaran M A, Pierce J H. Stimulation of the platelet-derived growth factor β receptor signaling pathway activates protein kinase C-δ. Mol Cell Biol. 1994;14:6727–6735. doi: 10.1128/mcb.14.10.6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li W, Yu J-C, Shin D-Y, Pierce J H. Characterization of a protein kinase C-δ (PKC-δ) ATP binding mutant: an inactive enzyme that competitively inhibits wild type PKC-δ enzymatic activity. J Biol Chem. 1995;270:8311–8318. doi: 10.1074/jbc.270.14.8311. [DOI] [PubMed] [Google Scholar]
- 26.Liao L, Ramsey K, Jaken S. Protein kinase C isozyme in progressively transformed rat fibroblasts. Cell Growth Differ. 1994;5:1185–1194. [PubMed] [Google Scholar]
- 27.Liu D, Rutter W J, Wang L-H. Enhancement of transforming potential of human insulin-like growth factor I receptor N-terminal truncation and fusion to avian sarcoma virus. J Virol. 1992;66:374–385. doi: 10.1128/jvi.66.1.374-385.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu D, Rutter W, Wang L-H. Modulatory effects of the extracellular sequence of the human insulin-like growth I receptor on its transforming and tumorigenic potential. J Virol. 1993;67:9–18. doi: 10.1128/jvi.67.1.9-18.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu J P, Baker J, Perkins A S, Robertson E J, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- 30.Lu Z, Hornia A, Jiang Y-W, Zang Q, Ohno S, Foster D A. Tumor promotion by depleting cells of protein kinase Cδ. Mol Cell Biol. 1997;17:3418–3428. doi: 10.1128/mcb.17.6.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mischak H, Pierce J H, Goodnight J, Kazanietz M G, Blumberg P M, Mushinski J F. Phorbol ester-induced myeloid differentiation is mediated by protein kinase C-α and -δ and not protein kinase C-βII, -γ, -ζ, and -η. J Biol Chem. 1993;268:20110–20115. [PubMed] [Google Scholar]
- 32.Miura M, Surmacz E, Burgaud J-L, Baserga R. Different effects on mitogenesis and transformation of a mutation at tyrosine 1251 of the insulin-like growth factor I receptor. J Biol Chem. 1995;270:22639–22644. doi: 10.1074/jbc.270.38.22639. [DOI] [PubMed] [Google Scholar]
- 33.Morrione A, DeAngelis T, Baserga R. Failure of the bovine papillomavirus to transform mouse embryo fibroblasts with a targeted disruption of the insulin-like growth factor I receptor genes. J Virol. 1995;69:5300–5303. doi: 10.1128/jvi.69.9.5300-5303.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neuenschwander S, Roberts J C T, LeRoith D. Growth inhibition of MCF-7 breast cancer cells by stable expression of an insulin-like growth factor 1 receptor antisense ribonucleic acid. Endocrinology. 1995;136:4298–4303. doi: 10.1210/endo.136.10.7664648. [DOI] [PubMed] [Google Scholar]
- 35.O’Connor R, Kauffmann-Zeh A, Liu Y, Lehar S, Evan G I, Baserga R, Blatter W A. Identification of domains of the insulin-like growth factor I receptor that are required for protection from apoptosis. Mol Cell Biol. 1997;17:427–435. doi: 10.1128/mcb.17.1.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pass H I, Mew D J W, Carbone M, Matthews W A, Donington J S, Baserga R, Walker C L, Resnicoff M, Steinberg S M. Inhibition of hamster mesothelioma tumorigenesis by an antisense expression plasmid to the insulin-like growth factor I receptor. Cancer Res. 1996;56:4044–4048. [PubMed] [Google Scholar]
- 37.Pawson T. Protein modules and signalling networks. Nature. 1995;373:573–580. doi: 10.1038/373573a0. [DOI] [PubMed] [Google Scholar]
- 38.Poon B, Dixon D, Ellis L, Roth R A, Rutter W J, Wang L-H. Molecular basis of the activation of the tumorigenic potential of Gag-insulin receptor chimeras. Proc Natl Acad Sci USA. 1991;88:877–881. doi: 10.1073/pnas.88.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prager D, Li H L, Asa S, Melmed S. Dominant negative inhibition of tumorigenesis in vivo by human insulin-like growth factor-I receptor mutant. Proc Natl Acad Sci USA. 1994;91:2181–2185. doi: 10.1073/pnas.91.6.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Resnicoff M, Coppola D, Sell C, Rubin R, Ferrone S, Baserga R. Growth inhibition of human melanoma cells in nude mice by antisense strategies to the type 1 insulin-like growth factor receptor. Cancer Res. 1994;54:4848–4850. [PubMed] [Google Scholar]
- 41.Sell C, Dumenil G, Devaud C, Miura M, Coppola D, DeAngelis T, Rubin R, Efstratiadis A, Baserga R. Effect of a null mutation of the insulin-like growth factor I receptor gene on growth and transformation of mouse embryo fibroblasts. Mol Cell Biol. 1994;14:3604–3612. doi: 10.1128/mcb.14.6.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sell C, Rubini M, Rubin R, Liu J P, Efstratiadis A, Baserga R. Simian virus 40 large tumor antigen is unable to transform mouse embryonic fibroblasts lacking type I IGF receptor. Proc Natl Acad Sci USA. 1993;90:11217–11221. doi: 10.1073/pnas.90.23.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shapiro D N, Jones B G, Shapiro L H, Dias P, Houghton P J. Antisense-mediated reduction in insulin-like growth factor I receptor expression suppresses the malignant phenotype of a human alveolar rhabdomyosarcoma. J Clin Invest. 1994;94:1235–1242. doi: 10.1172/JCI117441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith H, Chang E-Y, Szallasi Z, Blumberg P M, Rivera J. Tyrosine phosphorylation of protein kinase C-δ in response to the activation of the high-affinity receptor for immunoglobulin E modifies its substrate recognition. Proc Natl Acad Sci USA. 1995;92:9112–9116. doi: 10.1073/pnas.92.20.9112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soltoff S P, Toker A. Carbachol, substance P, and phorbol ester promote the tyrosine phosphorylation of protein kinase C delta in salivary gland epithelial cells. J Biol Chem. 1995;270:13490–13495. doi: 10.1074/jbc.270.22.13490. [DOI] [PubMed] [Google Scholar]
- 46.Standaert M, Bandyopadhyay G, Zhou X, Galloway L, Farese R. Insulin stimulates phospholipase D-dependent phosphatidylcholine hydrolysis, Rho translocation, de novo phospholipid synthesis, and diacylglycerol/protein kinase C signaling in L6 myotubes. Endocrinology. 1996;137:3014–3020. doi: 10.1210/endo.137.7.8770926. [DOI] [PubMed] [Google Scholar]
- 47.Standaert M, Musunuru K, Yamada K, Cooper D, Farese R. Insulin-stimulated phosphatidylcholine hydrolysis, diacylglycerol/protein kinase C signalling, and hexose transport in pertussis toxin-treated BC3H-1 myocytes. Cell Signal. 1994;6:707–716. doi: 10.1016/0898-6568(94)90052-3. [DOI] [PubMed] [Google Scholar]
- 48.Trojan J, Johnson T R, Rudin S D, Ilan J, Tykocinski M L, Ilan I. Treatment and prevention of rat glioblastoma by immunogenic C6 cells expressing antisense insulin-like growth factor I RNA. Science. 1993;256:94–97. doi: 10.1126/science.8418502. [DOI] [PubMed] [Google Scholar]
- 49.Ullrich A, Gray A, Tam R W, Yang-Feng T, Tsubokawa M, Collins C, Henzel W, Le Bon T, Kathuria S, Chen E, Jacobs S, Franke U, Ramachandran J, Fujita-Yamaguchi Y. Insulin-like growth factor I receptor primary structure: comparison with insulin receptor suggests structural determinants that define functional specificity. EMBO J. 1986;5:2503–2512. doi: 10.1002/j.1460-2075.1986.tb04528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ullrich A, Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990;61:203–211. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- 51.Zang Q, Lu Z, Curto M, Barile N, Shalloway D, Foster D A. Interaction between v-Src and protein kinase C δ in v-Src-transformed fibroblasts. J Biol Chem. 1997;272:13275–13280. doi: 10.1074/jbc.272.20.13275. [DOI] [PubMed] [Google Scholar]
- 52.Zong C S, Chan J L K, Yang S K, Wang L-H. Mutation of Ros differentially effecting signal transduction pathways leading to cell growth versus transformation. J Biol Chem. 1997;272:1500–1506. doi: 10.1074/jbc.272.3.1500. [DOI] [PubMed] [Google Scholar]