SUMMARY
The European Society of Clinical Microbiology and Infectious Disease (ESCMID) has advised against the use of metronidazole for fulminant Clostridioides difficile (C. difficile) infection (CDI) in their latest guidelines. They suggest using oral vancomycin alone instead. This recommendation is based on a few retrospective studies, which have multiple biases. We evaluated the three studies that led ESCMID to advise against intravenous metronidazole for fulminant CDI and performed a meta-analysis.
The meta-analysis revealed a mild (2.7%), not statistically significant (p=0.8) difference in mortality between the two groups. The high heterogeneity (I2= 89%) should also be noted. The decision to add or remove metronidazole should be discussed in the near future. In the meantime, combination therapy could be a cautious treatment for fulminant CDI.
Keywords: Metronidazole, oral vancomycin, Clostridioides difficile
INTRODUCTION
Clostridioides difficile infection (CDI) is a global issue, with an increasing incidence in the community [1]. In hospital settings, CDI spreads due to antimicrobial overuse, lack of infection control, and the burden of high-virulence strains [2–5]. Diagnostic and treatment approaches for mild and severe forms are similar in American and European guidelines. However, the treatment of fulminant (or “severe-complicated”) forms remains a topic of debate.
In the most recent update of CDI guidelines, the Infectious Disease Society of America (IDSA) confirmed that combination therapy with oral vancomycin and intravenous metronidazole is the preferred treatment for fulminant CDI [6].
A few months after the IDSA focused update on CDI, the European Society of Clinical Microbiology and Infectious Disease (ESCMID) updated its CDI guidelines [7]. The suggested treatment for fulminant C. difficile infection is either oral vancomycin or fidaxomicin monotherapy. This choice is based on four observational retrospective studies. In a recent letter to the editor of the official ESC-MID journal, we highlighted the pros and cons of these four observational studies [8]. We conducted a meta-analysis and expanded the article’s discussion to achieve completion.
The purpose of this study is to examine the observational studies referenced in the ESCMID and IDSA guidelines that assess the effectiveness of adding metronidazole to oral vancomycin for severe/fulminant C. difficile infection, and to integrate it with a meta-analysis.
MATERIALS AND METHODS
To conduct the statistical analysis, data from three [9–11] out of the four [9–12] studies cited by ESCMID and IDSA guidelines were extracted. The purpose was to compare the mortality of C. difficile patients who were treated with vancomycin alone or combination therapy. The excluded study did not compare vancomycin alone versus metronidazole plus oral vancomycin [12]. The meta-analysis was performed using SPSS© ver. 28.0.1.0 by a random effect model to assess the mortality risk associated with the treatment. The statistical analysis did not consider the dosage of vancomycin as all the studies had aggregated data for this variable.
RESULTS
The study conducted by Rokas K.E. and colleagues was a retrospective analysis of patients with severe or fulminant CDI [9]. The study included 88 patients who were admitted to Intensive Care Unit (ICU) units, with 44 patients in the combination group (oral vancomycin plus i.v. metronidazole) and 44 patients in the oral vancomycin group. The diagnosis was based on clinical suspicion and confirmed by a positive polymerase chain reaction (PCR) or CD toxin in faeces. The ESCMID guidelines cited this study, which highlighted a larger number of oncologic patients (11 in the monotherapy group vs 6 in the combination group, p=0.18) and neutropenic patients (8 in the monotherapy group vs 3 in the combination group, p=0.2) in the monotherapy group. However, these differences were not statistically significant and can be disregarded. However, there were statistically significant differences in the number of patients with higher white blood cells (WBC) (p=0.004), lower mean arterial pressure (p=0.004), and a higher number of moderate to severe renal disease (p=0.02) in the combination group. Despite this, the combination group had a lower mortality rate.
The multicentric observational retrospective study conducted by Wang Y. et al. compared the effectiveness of vancomycin plus intravenous metronidazole to oral vancomycin in treating C. difficile infection (CDI) [10]. The study included 2114 patients with CDI, including those with non-severe, severe, and fulminant CDI, both in the ICU and non-ICU settings. The diagnosis was confirmed with a positive PCR and clinical suspicion.
However, it is important to consider that there is a statistically significant difference in the distribution of non-severe, severe, and fulminant patients between the two groups (X2 (2, N=2114)=115.588, p< 0.000001). A potential bias was found in the lower proportion of non-severe patients among those receiving ‘dual therapy’ compared to ‘monotherapy’ (25% vs 42%, p<0.0001), as well as the higher proportion of fulminant CDI among those receiving “dual therapy” compared to “monotherapy” (35% vs 15%, p<0.0001). This resulted in a higher proportion of patients in the “dual therapy” group being stratified with “death” (25% vs 16%, p<0.01) or “death and colectomy” (28% vs 18%, p<0.01). The aOR 1.07 (95% CI: 0.79–1.45) of the primary outcome may be affected by these factors.
The retrospective observational study conducted by Vega A. et al among ICU patients with CDI showed similar mortality rates between the two groups (monotherapy versus combination therapy) [11]. The authors included 138 patients, 60 in the combination group and 78 in the monotherapy group. Diagnosis was based on clinical suspicion plus a positive PCR. In this study, only patients in the ICU with severe but non-fulminant C. difficile infection were included (fulminant CDI was an exclusion criterion). To avoid bias, patients were included in the combination group only if metronidazole was started within 72 hours of vancomycin. However, it is important to note that the prognosis of ICU patients can irreversibly change within 72 hours. The baseline characteristics showed a statistically significant higher proportion of patients with WBC <4000 or ≥15000 cells/mm3 (p=0.019) among the combination group. Furthermore, there were significantly higher numbers of patients treated with rectal vancomycin and a higher vancomycin dose (>125 mg) in the combination group (p<0.001 and p=0.015, respectively). The worse patients conditions at baseline in the combination group could be a possible reason for this. Additionally, a higher number of patients with one, three, four, and six severity criteria in the combination group could introduce bias. The Cox regression model indicated an association between overall severity criteria and mortality, with an adjusted hazard ratio of 2.15 (95% CI, 1.49–3.1).
The study results suggest a difference in all-cause mortality between the dual therapy group and the monotherapy group (respectively 30% vs 14.1%, p=0.02). However, there was no significant difference in 30-day mortality between the two groups (12.8% vs 18.3%, p=0.37).
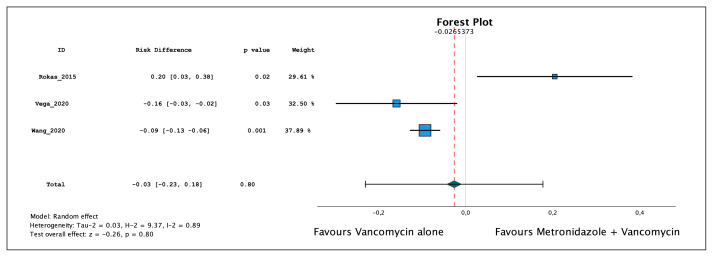
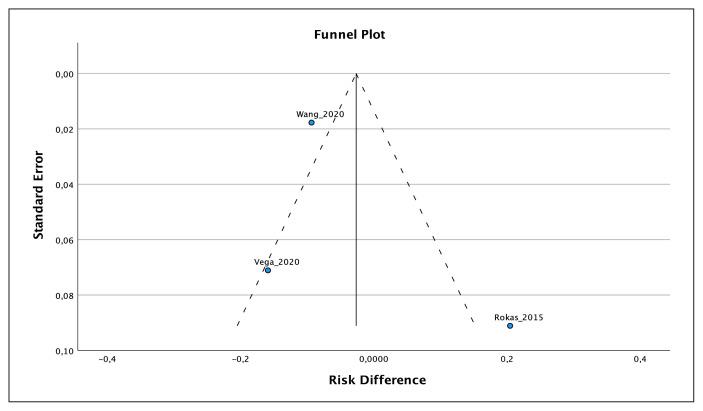
A meta-analysis was conducted using data from three out of four cited studies [9–11]. The meta-analysis showed a mild risk difference of 2.7% in favor of vancomycin monotherapy (−0.027, 95% CI: −0.23, 0.18), which was not statistically significant (p= 0.8) (Figure 1). Additionally, the studies exhibited significant heterogeneity (I2=89%), which was confirmed by the funnel plot (Figure 2). The data utilized in the studies is presented in Table 1.
Figure 1.
Forest Plot, Meta-analysis of the three studies cited by ESCMID guideline. Tau-2: Tau2. H-2: H2. I-2: I2.
Figure 2.
Funnel Plot of the three studies cited. Two studies (Wang et al. and Rokas et al.) out of three are out of the funnel plot, showing high heterogeneity, maybe due to publication bias.
Table 1.
Data extracted from the studies, used in the meta-analysis.
| Study ID | Year | Vancomycin+metronidazole | Vancomycin alone | ||
|---|---|---|---|---|---|
| Events | Total | Events | Total | ||
| Rokas 2015 | 2015 | 7 | 44 | 16 | 44 |
| Vega 2020 | 2020 | 18 | 60 | 11 | 78 |
| Wang 2020 | 2020 | 255 | 993 | 183 | 1121 |
DISCUSSION
All of the cited studies were observational and retrospective; therefore, the baseline level of evidence should be considered ‘low’ [13]. It is important to note that these studies may have biases and a high degree of heterogeneity. Each study had different inclusion criteria and criteria for ‘severe CDI’. In all three studies, diagnosis was made using PCR (only one study enrolled patients with both PCR and/or toxin [9]). Patients with severe CDI were treated with dual therapy, which may introduce selection bias. We also found differences in the number of severe patients between groups, which may introduce allocation bias. In one study, the severity criteria did not strictly follow the IDSA or ESCMID guidelines [11]. In the other two studies, no definition of the fulminant form was given, leading to misclassification bias [9, 10]. Outcomes were not blinded, and some studies had differences in patients who underwent rectal vancomycin or higher vancomycin doses. Additionally, only aggregate data were available for vancomycin and metronidazole doses, as well as for fulminant forms, leading to performance and detection bias. The funnel chart indicates a potential publication bias, therefore the level of evidence should be downgraded to “very low”.
Although a meta-analysis showed mild efficacy of vancomycin alone, this could be explained by the worse baseline conditions of patients in the combination groups. However, the risk difference is not statistically significant. Therefore, authorities should exercise caution in their judgment. A combination treatment with the addition of intravenous metronidazole or tigecycline to oral and/or rectal vancomycin should be considered as a careful approach in fulminant CDI. As suggested by Wilcox MH, in a fulminant CDI, an oral drug an oral drug may not reach therapeutic levels in the bowel [14]. Recent data suggest the usefulness of fecal microbiota transplantation (FMT) for fulminant forms [15, 16]. However, to evaluate the efficacy of FMT or vancomycin plus either intravenous metronidazole or tigecycline, randomized clinical trials (RCTs) are necessary. Additionally, attention should be given to the use of fidaxomicin for fulminant CDI. Unfortunately, there is a lack of literature on RCTs for this case.
CONCLUSIONS
When approaching a patient with severe or fulminant CDI, caution should be exercised. It is not advisable to remove a drug for the treatment of severely ill patients based solely on a few retrospective studies with a higher number of patients in worse clinical conditions in the combination group. RCTs are needed. Meanwhile, combination therapy with i.v. metronidazole or i.v. tigecycline to oral/rectal vancomycin or fidaxomycin should be preferred due to the risk of low intestinal drug levels in case of fulminant forms such as shock, megacolon, or ileus.
Footnotes
Conflict of interest: Authors declare no conflict of interest.
Authors’ contributions: GP, GG were involved in the study design; GP wrote the initial draft of the manuscript; GP and GG performed systematic review; GP performed statistical analysis; GP, GG, MS, CIa, AC were involved in development and methodology; CIm, AC critically review the manuscript; AG, CIm, CIa, LM visualization and validation; all authors have read the manuscript and agree to publish the present version of the manuscript.
Funding: Authors declare no funding.
Availability of data
Data are available on appropriate request to the corresponding author.
REFERENCES
- 1. Khanna S, Pardi DS, Aronson SL, et al. The epidemiology of community-acquired Clostridium difficile infection: a population-based study. Am J Gastroenterol. 2012;107(1):89–95. doi: 10.1038/ajg.2011.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bertolino L, Patauner F, Gagliardi M, et al. Diagnostic and infection control strategies for Clostridioides difficile infections in a setting of high antimicrobial resistance prevalence. Infez Med. 2021;29(1):70–78. [PubMed] [Google Scholar]
- 3. Mancini A, La Vigna G, Puciarelli S, et al. A three-year study entailing molecular characterization and epidemiology of Clostridium difficile in an Italian tertiary care hospital. Infez Med. 2018;26(3):204–209. [PubMed] [Google Scholar]
- 4. Aschbacher R, Indra A, Wiedermann CJ, et al. Predominance of Clostridium difficile 027 during a five-year period in Bolzano, Northern Italy. Infez Med. 2017;25(1):13–20. [PubMed] [Google Scholar]
- 5. Sedigh Ebrahim-Saraie H, Heidari H, Amanati A, et al. A multicenter-based study on epidemiology, antibiotic susceptibility and risk factors of toxigenic Clostridium difficile in hospitalized patients in southwestern Iran. Infez Med. 2018;26(4):308–3. [PubMed] [Google Scholar]
- 6. Johnson S, Lavergne V, Skinner AM, et al. Clinical Practice Guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 Focused Update Guidelines on Management of Clostridioides difficile Infection in Adults. Clin Infect Dis. 2021;73(5):e1029–e44. doi: 10.1093/cid/ciab549. [DOI] [PubMed] [Google Scholar]
- 7. van Prehn J, Reigadas E, Vogelzang EH, et al. European Society of Clinical Microbiology and Infectious Diseases: 2021 update on the treatment guidance document for Clostridioides difficile infection in adults. Clin Microbiol Infect. 2021;27(Suppl 2):S1–S21. doi: 10.1016/j.cmi.2021.09.038. [DOI] [PubMed] [Google Scholar]
- 8. Pipitone G, Granata G, Sartelli M, et al. Intravenous metronidazole for fulminant Clostridioides difficile infection. Clin Microbiol Infect. 2023;29(5):656–657. doi: 10.1016/j.cmi.2023.01.026. [DOI] [PubMed] [Google Scholar]
- 9. Rokas KE, Johnson JW, Beardsley JR, et al. The addition of intravenous metronidazole to oral vancomycin is associated with improved mortality in critically ill patients with Clostridium difficile infection. Clin Infect Dis. 2015;61(6):934–941. doi: 10.1093/cid/civ409. [DOI] [PubMed] [Google Scholar]
- 10. Wang Y, Schluger A, Li J, et al. Does Addition of intravenous metronidazole to oral vancomycin improve outcomes zClostridioides difficile Infection? Clin Infect Dis. 2020;71(9):2414–2420. doi: 10.1093/cid/ciz1115. [DOI] [PubMed] [Google Scholar]
- 11. Vega AHT, Heil E, Johnson J, et al. Oral vancomycin plus intravenous metronidazole for severe Clostridium difficile infection in critically ill patients. Open Forum Infectious Diseases. 2018;5(S181) [Google Scholar]
- 12. Wenisch JM, Schmid D, Kuo HW, et al. Prospective observational study comparing three different treatment regimes in patients with Clostridium difficile infection. Antimicrob Agents Chemother. 2012;56(4):1974–1978. doi: 10.1128/AAC.05647-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guyatt GH, Oxman AD, Santesso N, et al. GRADE guidelines: 12. Preparing summary of findings tables-binary outcomes. J Clin Epidemiol. 2013;66(2):158–172. doi: 10.1016/j.jclinepi.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 14. Wilcox MH. Editorial Commentary: critically ill patients with Clostridium difficile infection: are 2 antibiotics better than one? Clin Infect Dis. 2015;61(6):942–944. doi: 10.1093/cid/civ413. [DOI] [PubMed] [Google Scholar]
- 15. Cheng YW, Phelps E, Ganapini V, et al. Fecal microbiota transplantation for the treatment of recurrent and severe Clostridium difficile infection in solid organ transplant recipients: A multicenter experience. Am J Transplant. 2019;19(2):501–511. doi: 10.1111/ajt.15058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Krajicek E, Bohm M, Sagi S, et al. Fulminant Clostridium difficile infection cured by fecal microbiota transplantation in a bone marrow transplant recipient with critical neutropenia. ACG Case Rep J. 2019;6(8):e00198. doi: 10.14309/crj.0000000000000198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on appropriate request to the corresponding author.