Abstract
TNF, a pleiotropic proinflammatory cytokine, is important for protective immunity and immunopathology during Mycobacterium tuberculosis (Mtb) infection, which causes tuberculosis (TB) in humans. TNF is produced primarily by phagocytes in the lungs during the early stages of Mtb infection and performs diverse physiological and pathological functions by binding to its receptors in a context-dependent manner. TNF is essential for granuloma formation, chronic infection prevention, and macrophage recruitment to and activation at the site of infection. In animal models, TNF, in cooperation with chemokines, contributes to the initiation, maintenance, and clearance of mycobacteria in granulomas. Although anti-TNF therapy is effective against immune diseases such as rheumatoid arthritis, it carries the risk of reactivating TB. Furthermore, TNF-associated inflammation contributes to cachexia in patients with TB. This review focuses on the multifaceted role of TNF in the pathogenesis and prevention of TB and underscores the importance of investigating the functions of TNF and its receptors in the establishment of protective immunity against and in the pathology of TB. Such investigations will facilitate the development of therapeutic strategies that target TNF signaling, which makes beneficial and detrimental contributions to the pathogenesis of TB.
Keywords: TNF-alpha, Mycobacterium tuberculosis, Host microbial interactions, Autophagy, Cell death, Pathogenesis
INTRODUCTION
Mycobacterium tuberculosis (Mtb), the pathogen of human tuberculosis (TB), is the primary cause of death attributable to a single bacterial pathogen globally. Mtb is an intracellular pathogen that infects the lungs upon the inhalation of bacteria-laden droplets (1). More than 90% of individuals infected with Mtb experience latent TB, an asymptomatic and prolonged stage of the disease. Active TB manifests in less than 10% of infected individuals, underscoring the importance of the interplay between host protection and bacterial pathogenesis in the prevention of active disease (2). Despite notable advancements, the mechanisms of host–pathogen interactions and their effects on the outcomes of TB infection remain unclear.
The production of TNF, a proinflammatory cytokine, protects against and promotes Mtb infection. Granuloma formation, a feature of Mtb infection, requires the recruitment of immune cells to the site of infection, driven predominantly by proinflammatory immune mediators, particularly TNF. In response to Mtb or its Ags, innate immune cells, including macrophages and dendritic cells, produce TNF following the stimulation of pattern-recognition receptors (PRRs) via intracellular signaling pathways involving NF-κB activation (3). The recognition of TNF by the producing cell or its neighbors via TNF receptors (TNFRs) initiates a variety of biological responses, including inflammation, oxidative stress, antimicrobial mechanisms, and cell death (4). Single-cell RNA sequencing analysis has shown that TNF has multiple cellular sources, e.g., clusters of blood cells, including myeloid-like and CD8+ human cells (5).
The strict control of TNF production is important for the maintenance of homeostasis during infection and the prevention of pathological inflammation and necrotic cell death while promoting host protective responses. The association of TNF with TB dates to its discovery as cachectin (6), although the mechanisms by which TNF modulates body mass and disease outcomes during different stages of TB infection are unknown. Thus, the investigation of the regulatory mechanisms of protective TNF responses will facilitate the development of inflammation-based host-directed therapies against TB.
This review focuses on the functions of TNF in the pathogenesis and prevention of TB. It highlights the importance of these functions in the activation of M1-macrophage responses, immunometabolic remodeling, trained immunity, and apoptosis, which are crucial for protective immunity against TB. By contrast, TNF-related mitochondrial oxidative stress and damage are associated with necrotic cell death and TB progression. Further investigation could enable the development of therapeutic strategies for TB through targeting TNF signaling.
OVERVIEW OF TNF AND TNFRs
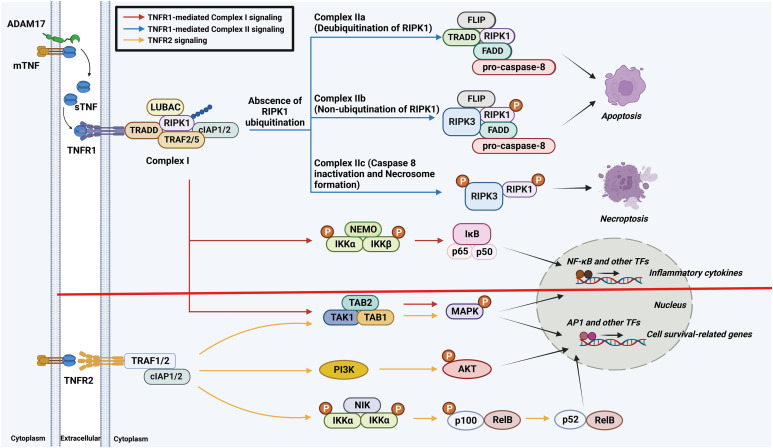
In 1975, the Carswell group at the Sloan–Kettering Institute discovered TNF. They found that it was released by activated macrophages in the serum of Bacillus Calmette-Guérin (BCG)-infected mice treated with endotoxin, inducing endotoxin-mediated tumor necrosis (7). This cytokine was given the name “TNF” because it exerts lethal effects on tumors via hemorrhagic necrosis (8). It is involved in acute and chronic inflammatory responses to microbial infections and autoimmune conditions. TNF also modulates diverse physiological and pathological processes, encompassing embryonic development, germinal center formation, and tissue degeneration and repair (4,9). In this section, we explore the properties of TNF and TNFRs, including their relationships in signaling cascades (Fig. 1).
Figure 1. Schematic diagram of the synthesis and receptor signaling pathways of TNF. TNFR1 signaling involves the formation of complexes I and II. Complex I initiates proinflammatory responses and promotes cell survival via the canonical NF-κB and MAPK pathways. In the absence of RIPK1 ubiquitination, complex II formation occurs, leading to caspase-dependent apoptosis or MLKL-dependent necroptosis, depending on caspase-8 activity. TNFR2 signaling begins with the binding of TRAF proteins and promotes cell survival and proliferation via the PI3K/AKT pathway. It also induces non-canonical NF-κB activation mediated by NIK.
PI3K, phosphoinositide 3-kinase; NIK, NF-κB–inducing kinase; ADAM17, a disintegrin and metalloprotease 17; FLIP, FADD–like IL-1β–converting enzyme–like inhibitory protein; IKK, inhibitor of NF-κB kinase; NEMO, NF-κB essential modulator; TAK1, transforming growth factor-β–activated kinase 1; TAB, TGF-β–activated kinase 1–binding protein; AP1, activator protein 1; TF, transcription factor.
TNF expression, synthesis, and secretion
Human TNF was initially purified from culture supernatants of HL-60 promyelocytic leukemia cells stimulated with PMA (10). It is produced primarily by activated immune cells, including macrophages and monocytes. In response to microbial products, it is generated at lower levels by activated non-immune cells, such as certain subsets of endothelial cells, fibroblasts, adipose cells, cardiac myocytes, and astrocytes (11).
The synthesis and secretion of TNF are controlled by a series of protein- and enzyme-mediated steps (Fig. 1). Initially, TNF is translated as a 26-kDa membrane-bound form (mTNF) with N-terminal intracellular and transmembrane and C-terminal extracellular domains arranged into non-covalently bound homotrimers, and serves as an external signal receptor and a ligand. Subsequently, mTNF is converted to 17-kDa soluble TNF (sTNF) via cleavage of the extracellular domain by the TNF-converting enzyme metalloprotease (TACE; a disintegrin and metalloprotease 17). This strictly regulated process releases sTNF homotrimers into the extracellular milieu (12) and is required for TNF functions and signaling in a variety of physiological and pathological contexts.
TNFR expression levels, structures, and functions
The bioactive homotrimeric form of TNF interacts with two cognate type-I transmembrane receptors, TNFR1 (also known as TNFRSF1A, CD120a, and p55) and TNFR2 (also known as TNFRSF1B, CD120b, and p75), triggering its pleiotropic functions (13). TNFR1 is expressed in a variety of cell types and interacts with mTNF and sTNF, thereby promoting inflammation and tissue injury. TNFR2 is expressed primarily in immune and endothelial cells and is implicated in immune modulation and tissue regeneration. Notably, TNFR2 is activated exclusively by mTNF, despite being capable of interacting with mTNF and sTNF. Additionally, transmembrane TNFR1 and TNFR2 are cleaved into soluble forms (sTNFR1 and sTNFR2) by TACE (11). Such complex receptor interactions and conversions contribute to the regulation of the TNF signaling pathway.
TNFR1 and TNFR2 are composed of extracellular, transmembrane, and intracellular domains. Their extracellular domains are similar and harbor cysteine-rich motifs containing two to six repeats, typically with six cysteine residues involved in three disulfide bonds (12). In contrast, the intracellular domains of TNFR1 and TNFR2 do not have homologous sequences, and they activate both common and distinct signaling pathways. TNFR1-deficient mice with Mycobacterium bovis BCG infection showed a massive increase in the bacterial burden and succumbed to infection, whereas TNFR2-deficient mice with the same infection showed a reduced delayed-type hypersensitivity response and impaired granuloma formation (14).
TNFR1 signaling pathway
The binding of TNF to TNFR1 activates several signaling pathways via intracellular complexes I and II. Complex I is responsible for the activation of genes associated with cell survival and the generation of proinflammatory cytokines via the canonical NF-κB and MAPK pathways. By contrast, the activation of complex II promotes programmed cell death (PCD), including apoptosis and necroptosis (3,13). This dual signaling determines the cellular response to TNF and balances pro-survival and pro-death pathways in different cellular contexts (Fig. 1).
TNFR1 contains a death domain (DD) in its cytoplasmic region. Upon the binding of TNF to TNFR1, a conformational change that facilitates interaction with the TNF receptor 1–associated DD (TRADD) occurs, leading to the formation of complex I. This process involves the recruitment of TNF receptor–associated factors (TRAF) 2 and 5 (and receptor-interacting serine/threonine-protein kinase 1 (RIPK1). Notably, RIPK1 governs the direction of TNFR1-mediated signal transduction. Its ubiquitination by cellular inhibitor of apoptosis proteins 1 and 2 (cIAP1/2) and the linear ubiquitin assembly complex (LUBAC) is important in this process, as it activates the NF-κB and MAPK signaling pathways via the assembly of IκB kinase complex and TGF-β–activated kinase 1 complexes, respectively (9). This signaling network fine tunes the cellular response to TNF, the outcomes of which depend on the context and ubiquitination state of RIPK1.
Complex II forms in the cytoplasm in the absence of RIPK1 ubiquitination and interacts with Fas-associated DD protein (FADD) and procaspases 8 and 10. In the absence of cIAP1/2, RIPK1 ubiquitination is prevented, leading to the recruitment of RIPK3, procaspase 8, and cellular FADD–like IL-1β–converting enzyme–inhibitory protein, ultimately resulting in caspase-dependent apoptotic cell death (4). TNFR1-mediated apoptosis, but not that mediated by TNFR2, is important for the activation of innate protective immunity in M. avium–infected mice (15). TNFR1 signaling triggers the formation of a necrosome involving phosphorylated RIPK1, RIPK3, and mixed-lineage kinase domain-like protein (MLKL), leading to caspase-independent necroptosis (13). Roca and Ramakrishnan (16) investigated the TNF-mediated pathogenesis of TB using M. marinum–infected zebrafish, finding that excess TNF promoted the mitochondrial ROS (mitoROS)-mediated activation of necroptosis via RIPK1- and RIPK3-dependent pathways in infected macrophages, resulting in increased macrophage microbicidal activity during the early stage and vigorous extracellular mycobacterial growth in the late stage. In summary, the TNFR1 signaling pathways dictate cellular responses to TNF, influencing cell fate and immunity during infection. Research on these pathways will promote the development of targeted therapies for diseases involving dysregulated TNF signaling.
TNFR2 signaling pathway
In contrast to TNFR1, TNFR2 lacks a DD and thus does not interact directly with TRADD. It does interact directly with TRAF2 via a TRAF-binding motif, leading to the indirect recruitment of TRAF2-associated proteins, such as TRAF1, cIAP1/2, and LUBAC. This recruitment promotes ubiquitin-mediated protein degradation, thereby suppressing apoptosis (17,18). The engagement of TNF with TNFR2 activates diverse signaling pathways, including the reciprocal phosphoinositide-3 kinase/Akt pathway, the apoptosis signal–regulating kinase 1–mediated c-Jun N-terminal kinase pathway, and the non-canonical NF-κB pathway, by activating NF-κB–inducing kinase. These pathways collectively promote cell proliferation and survival (18).
In summary, TNFR2 promotes cell survival and proliferation via diverse signaling pathways (Fig. 1). The interplay between TNFR1 and TNFR2 signaling is important for the regulation of cellular responses to TNF, and the understanding of these pathways is essential for the decipherment of their functions in a variety of physiological and pathological contexts.
TNF: A CONTENTIOUS FACTOR DURING HOST-Mtb INTERACTION IN TB
Mtb infection is initiated when the bacteria enter the lungs via inhalation and target alveolar macrophages in the lower respiratory tract (2). These macrophages internalize Mtb via receptor-mediated phagocytosis. Thereafter, Mtb employs sophisticated immune evasion strategies, such as the suppression of phagosome-lysosome fusion. It also disrupts the phagosomal membrane via its 6-kDa early secretory antigenic target secretion system 1 (ESX-1), thereby releasing various bacterial components, such as mycobacterial DNA, into the macrophage cytosol (19).
Mtb infiltrates the lungs, prompting the recruitment of immune cells and culminating in the formation of granulomas—localized clusters of immune cells that contain the infection and coordinate immune responses (1). During the initial phase of infection, the innate immune system detects Mtb, triggering a cascade of pro- and anti-inflammatory responses. Alveolar macrophages are important for the primary immune defense because of their specialized PRRs and communication with epithelial cells (20). The activation of innate immune signaling pathways triggered by PRR recognition results in the activation of the transcription factor NF-κB, which triggers the expression of TNF and other proinflammatory cytokines (21). TNF and TNFR signaling is linked to the production of other inflammatory cytokines and chemokines, M1 macrophage responses, oxidative stress induction, cell death initiation, and the adaptive immune response. In this section, we discuss TNF-mediated granuloma formation and innate immune activation.
TNF and granuloma formation
TNF regulates host immune responses, including granuloma formation. Granulomas are important for the control of TB, but can also contribute to Mtb proliferation and dissemination (22). Granuloma formation, quantity, and morphology differ between active and latent TB infections. Additionally, individual granulomas contain distinct populations of activated and differentiated immune cells, including macrophages, which have varying abilities to control bacterial growth. This heterogeneity reflects the diversity of TB progression and the local microenvironment, which is associated with host immune and pathological statuses at the site of infection and systemically (23,24).
The granulomatous inflammation triggered by Mtb infection can have protective effects and destructive consequences. From a protective standpoint, it entails the infiltration of inflammatory cells, including macrophages, T cells, and B cells, to the primary site of lung infection. These cells exert important effects on intracellular Mtb, and fibrous encapsulation promotes Mtb eradication and the establishment of a mechanical and functional barrier that prevents its dissemination (25). Mtb has evolved strategies to persist in the face of the host response, enabling its long-term survival. These adaptations subvert host immunity, contribute to latent TB development, and promote antimicrobial drug tolerance. Oxygen reduction is the most characteristic adaptation; the direct, in-vivo assessment of other environmental factors is challenging because the host response to Mtb evolves (26,27).
The precise regulation of TNF production is essential for protective granuloma formation and the maintenance of granuloma structure, as excessive proinflammatory responses during early Mtb infection can lead to extensive tissue damage. Thus, a balanced response is needed to promote protection, minimize tissue damage, reduce bacterial persistence, and enhance the efficacy of antimicrobial treatment (25). The investigation of the mechanisms of protective granuloma formation will enable the development of novel therapeutics that enhance protection and reduce damage.
TB and cachexia
Malnutrition is linked to cachexia, a wasting symptom associated with chronic illnesses (28). The factors underlying wasting in TB are unclear. Studies have linked cytokine production to cachexia prevalence in specific cancer types (29). Cytokine (IL-6, TNF, IL-8, TGF-β, and macrophage inhibitory cytokine 1/growth differentiation factor 15) production has been linked to the cachexia prevalence in certain cancer types (30,31). Interestingly, the sex of wasted TB patients impacts post-treatment weight regain, with men showing a higher rate of regain of the lean mass index relative to the fat mass index and women exhibiting the opposite pattern (32). Furthermore, B cells may protect in part against chronic TB, despite debate over their contribution to anti-Mtb immunity. In TB-susceptible I/St mice, the decline in B cells between weeks 12 and 16 post-infection aligned with intensified lung inflammation and elevated expression of IL-1, IL-11, IL-17α, and TNF. B-cell depletion at week 16 post-infection resulted in accelerated cachexia, reduced lifespan, heightened infiltration of CD8+ T cells, elevated IL-6 expression, and the upregulation of genes associated with neutrophil recruitment and tissue damage (33). Research on the mechanisms underlying the relationships among cachexia, inflammation, and sex-dependent anti-TB responses will facilitate the development of personalized interventions for TB.
TB and anti-TNF therapy
TNF has a variety of physiological and homeostatic functions, and anti-TNF therapies are used to treat a range of inflammatory and autoimmune diseases, including rheumatoid arthritis, psoriatic arthritis, and inflammatory bowel disease (13,34). Five TNF blockers—infliximab, golimumab, adalimumab, certolizumab pegol, and etanercept—have received regulatory approval to date. Infliximab is a chimeric monoclonal anti-TNF Ab with a human IgG1 fragment crystallizable (Fc) region and a murine variable region, whereas golimumab and adalimumab are fully human anti-TNF monoclonal Abs. Certolizumab pegol is a polyethylene glycol–ylated Ag-binding recombinant fragment of a humanized monoclonal Ab against TNF. Etanercept is a human TNFR Fc fusion protein comprising the extracellular ligand-binding domain of TNFR2/p75 and the Fc domain of human IgG1 (35).
TB infection has been reported in individuals receiving anti-TNF therapy. Infliximab, approved for clinical use in 1998, had caused 70 reported cases of TB and the death of 12 of these patients by 2001 (36). Harris et al. (37) reported that the three TNFα-suppressing drugs infliximab, adalimumab, and etanercept inhibited IFN-γ–induced phagosome maturation in human THP-1 cells treated with PMA, whereas the treatment of macrophages with TNF-α induced the maturation of phagosomes containing M. bovis BCG or Mtb H37Rv. Mezouar et al. (38) reported that etanercept treatment slightly delayed granuloma formation and reduced the proliferation of multinuclear giant cells by triggering the expression of M1 polarization genes and the generation of IL-10 in an in-vitro model of human tuberculous granuloma; adalimumab also attenuated the formation of multinuclear giant cells in granulomas. Thus, the understanding of the mechanism by which anti-TNF therapy modulates immune dynamics would provide insight into the pathogenesis of TB and promote the development of anti-TB interventions.
TNF and Mtb-mediated innate immune signaling
In response to Mtb infection, human macrophages initiate intracellular innate-immune signaling pathways. Macrophages engulf Mtb, resulting in the activation of antimicrobial systems and eradication of the bacteria by indirect opsonization and direct detection. Indirect detection involves soluble factors such as collectins and complements, which facilitate Mtb internalization by macrophages. Additionally, a variety of PRRs on macrophages identify pathogen-associated molecular patterns (PAMPs) on the cell surfaces and in macrophage phagolysosomes and cytosol (39). Among membrane-bound receptors, TLR2 and 4 are implicated in the innate immune response to Mtb in the respiratory tract (40). The recognition of Mtb components by TLR2 activates innate immune signaling mediated by the pivotal adaptor protein myeloid differentiation primary response 88 (MyD88), leading to the production of proinflammatory cytokines like TNF and IL-12 (40,41). Several mycobacterial Ags transmit TLR4 signaling via two pathways, regulated by the Toll/IL-1 receptor (TIR) homology domain–containing adaptor protein–MyD88 and TIR homology domain–containing adapter-inducing IFN-β (TRIF)-related adaptor molecule–TRIF pairs. These pathways stimulate the production of proinflammatory cytokines and type I IFNs (40).
Upon activation, adaptor molecules orchestrate the recruitment and activation of several kinases, including IL-1 receptor–associated kinases, and ubiquitin ligases such as TRAF6 and Pellino1. Subsequently, these activated kinases and ubiquitin ligases facilitate the liberation of NF-κB, specifically the RelA and p50 domains, from IκB or promote the nuclear translocation of IFN-regulatory factors (21). The MAPK pathway is activated in response to the recognition of Mtb by PRRs and enhances antimicrobial immune reactions during Mtb infection. These signaling cascades trigger the production of a range of proinflammatory cytokines and chemokines, including TNF, IL-1β, IL-6, IL-23, and GM-CSF, to promote antimicrobial responses (23). Furthermore, the immune signals mediated by TLR2 and TLR4, in collaboration with other PRRs, are important in mycobacterial infections, although further studies are required to elucidate the mechanisms underlying their cooperative functions (23,40).
TLR9 recognizes Mtb DNA in phagolysosomes, and the ESX-1 secretion system disrupts the integrity of the phagosomal membrane, enabling the recognition of cytosolic Mtb DNA and activation of the cyclic GMP-AMP synthase/stimulator of IFN genes pathway (19,42). This recognition of pathogenic Mtb DNA by TLR9 amplifies M1 macrophage responses, leading to the production of TNF and induction of autophagy (42). Moreover, Mtb coinfection with HIV triggers the activation of the TLR-3, -7, and -9 signaling pathways, although the underlying mechanisms are unclear (43). The functions of individual TLRs, as well as their combined effects, in the context of HIV-Mtb coinfection warrant further investigation. Furthermore, strategies that regulate TLR signaling, such as vaccination, are needed to control Mtb infection (44). Other efforts should be directed at the interruption of the sustained activation of TLR signaling during chronic mycobacterial infection, with the aim of delaying disease progression.
ROLE OF TNF IN PROTECTIVE IMMUNITY DURING MYCOBACTERIAL INFECTION
The innate immune responses triggered by Mtb involve primarily the production of proinflammatory cytokines, notably TNF, by macrophages. In this section, we discuss TNF and M1 macrophage responses, the immunometabolic remodeling in macrophages that influences TNF production, trained immunity and TNF, autophagy and TNF responses, and the apoptosis associated with TNF during mycobacterial infection (Fig. 2). Investigation of the function of TNF in protective immunity will promote the development of novel TB interventions and vaccines that target TNF-mediated pathways.
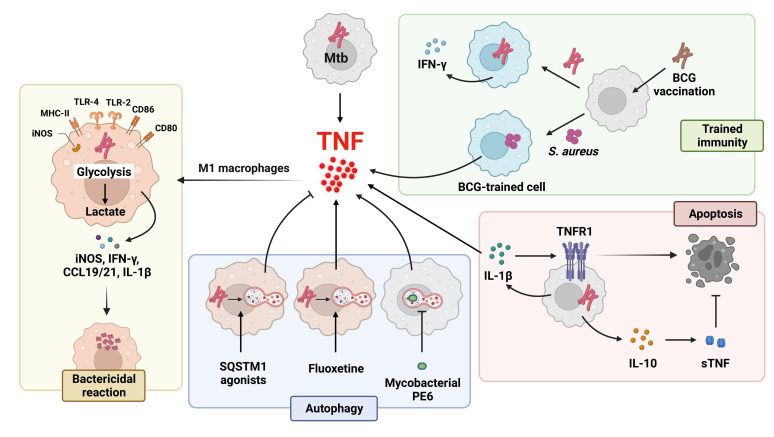
Figure 2. Regulatory pathways of TNF-α production in response to Mtb infection. TNF-α induces Mtb-infected macrophage polarization to M1 macrophages, which exert a bactericidal effect by producing iNOS, IFN-γ, T-bet, CCR7, CCL19/21, and IL-1β. M1 macrophages also produce lactate via enhanced aerobic glycolysis, which inhibits bacterial growth. After Mycobacterium bovis BCG vaccination, the production of IFN-γ and TNF-α increases in response to Mtb and Staphylococcus aureus infection, respectively. SQSTM1 agonists activate autophagy and reduce the TNF-α level. By contrast, the Mtb protein PE6 inhibits autophagy and stimulates TNF-α production during Mtb infection. Fluoxetine, an antidepressant, enhances autophagy and increases Mtb-induced TNF-α level. During Mtb infection, IL-1β increases TNFR1 expression and TNF-α secretion, leading to apoptosis to regulate intracellular Mtb growth. However, Mtb induces IL-10 secretion and soluble TNFR2 release, thereby suppressing apoptosis.
T-bet, T-box transcription factor TBX21; CCL19/21, chemokine (C-C motif) ligands 19 and 21.
TNF and M1 macrophage responses
Macrophages can be classified into M1 and M2 types based on their effector molecules; M1 macrophages have proinflammatory features. The TLR ligands IFN-γ, TNF, and GM-CSF induce M1 macrophages (45), which exert bactericidal effects by producing inducible nitric oxide synthase (iNOS), IFN-γ, T-box transcription factor TBX21, C-C chemokine receptor (CCR) 7 (, chemokine (C-C motif) ligands 19 and 21, and antimicrobial peptides (46). However, the distinction of M1 and M2 macrophages is hampered by the presence of host and microbial factors at the site of infection. Furthermore, macrophage responses are heterogeneous because of the co-evolutionary dynamics of Mtb and host–cell reactions (46). Indeed, the rate of intracellular bacterial growth is associated closely with intracellular iNOS activity (47).
Numerous Mtb protein and lipid effectors regulate the antimicrobial functions of macrophages and subvert the inflammatory process (48). For example, Mtb phthiocerol dimycoceroserate lipids mask PAMPs and inhibit TLR-dependent macrophage recruitment to suppress microbicidal reactive nitrogen species, thereby evading M1-mediated killing (49). In addition, phenolic glycolipids and CCR2 promote the recruitment of permissive macrophages during infection (49). Further studies should focus on the mechanisms by which Mtb components suppress the TLR-mediated recruitment of bactericidal macrophages in the respiratory tract.
TNF and immunometabolic rewiring toward aerobic glycolysis
Mtb alters the mitochondrial morphology, metabolism, and functions, markedly impacting macrophage immunometabolism and thereby influencing the effectiveness of host antimicrobial defenses and the outcomes of infection (50). Metabolic reprogramming occurs in alveolar and bone marrow–derived macrophages during Mtb infection (51). Alveolar macrophages have a limited ability to transition to aerobic glycolysis, resulting in the insufficient control of infection. Pharmacological reprogramming using metformin can promote glycolysis, leading to reduced necrosis and enhanced antimicrobial activity (51). The immunometabolic remodeling of immune cells orchestrates host resistance and disease tolerance during Mtb infection (52). In their resting state, alveolar macrophages rely on oxidative phosphorylation fueled by fatty acid oxidation due to the abundance of lipids in the lung. However, Mtb infection triggers M1 macrophage responses, characterized by enhanced aerobic glycolysis and increased lactate production, bolstering the immune functions that restrict Mtb growth (52). Indeed, caloric restriction, not malnutrition, protected DBA/2 mice against pulmonary Mtb infection. Such restriction reduces the bacterial load, lung damage, and foam-cell formation by inducing a metabolic shift toward glycolysis, decreased fatty acid oxidation and mTOR activity, and increased autophagy in immune cells. These findings underscore the importance of immunometabolic reprogramming toward aerobic glycolysis for the control of Mtb infection and enhancement of immunity (53). Further investigations of the effect of TNF on immunometabolism and its modulation of mycobacterial pathogenesis and protective immune responses are needed, given its association with M1 macrophage responses with an aerobic glycolysis metabolic signature.
Similar to the observation that the LPS-induced enhancement of aerobic glycolysis is associated with the elevated production of TNF and IL-1β (54), M1 macrophages infected with BCG produce M1-specific cytokines, including TNF and IL-1β, and upregulate aerobic glycolysis (55). Importantly, lactate, the end product of aerobic glycolysis, significantly improved the intracellular clearance of Mtb in human macrophages, in part by promoting autophagy (56). However, lactate also suppresses the secretion of TNF and IL-1β by Mtb-infected human macrophages, indicating the existence of a negative feedback effect on inflammatory responses (56). Consequently, although Mtb infection promotes M1 macrophage responses, accompanied by the upregulation of TNF and aerobic glycolysis, lactate prevents an excessive inflammatory response and enhances bacterial killing. Investigation of the regulatory mechanisms of macrophage differentiation, inflammation, and metabolic reprogramming may enable the identification of new therapeutic targets for human TB and other intracellular bacterial infections.
TNF and trained immunity
The concept of trained immunity encompasses the provision of medium-term protection by the innate immune system via the reprogramming of immune cells by epigenetic and metabolic changes. The process involves an initial challenge followed by a period of rest, leading to an altered response upon subsequent challenge. Notably, the functional reprogramming of monocytes during trained immunity involves distinct epigenetic modifications, particularly stable alterations in histone trimethylation at histone H3 lysine K4 (H3K4) (57). Additionally, trained immunity influences the generation of proinflammatory mediators, including IL-1β, TNF, and IL-6, as well as factors that impact Ag presentation to T cells and IFN-γ production (58,59).
An example of trained immunity in the context of mycobacterium–host responses is the protection conferred by BCG vaccination, which induces immune metabolic rewiring and drives long-term epigenetic modifications involving chromatin remodeling. Glycolysis, central carbon metabolism, and cholesterol synthesis are key metabolic contributors to this immune response (57,60). The genetic or pharmacological modulation of glycolysis enzymes not only inhibits trained immunity but also modifies the histone marks (H3K4me3 and H3K9me3) associated with BCG-induced trained immunity (61). Circulating metabolites shape BCG-induced trained immunity in humans. Taurine metabolism, the tricarboxylic acid cycle, and glutamine metabolism were associated with such immunity in 325 BCG-vaccinated individuals (62). Furthermore, BCG vaccination alters the gut microbiome composition and BCG-induced protective immunity and the accompanying cytokine production are influenced by the abundance of metabolites produced by immunomodulatory gut microbes (63). Thus, the manipulation of the gut microbiota and metabolic pathways could enable the development of novel vaccines and therapies for TB.
BCG-induced antimycobacterial responses rely on macrophages and neutrophils, rather than trained immunity. Furthermore, BCG vaccination induced sustained protection in TNF-deficient mice (64). These findings emphasize the importance of innate immune pathways, beyond TNF production and trained immunity, for mycobacterial clearance. Further studies are needed to evaluate the potential of trained immunity for use in the development of next-generation vaccines for human TB.
TNF and autophagy during mycobacterial infection
Autophagy is a self-digestion process by which lysosomes degrade cellular components. It is vital for quality control, energy supply, and immune defenses against pathogens, including Mtb (65). In macroautophagy, unc-51–like autophagy-activating kinase 1 and Beclin-1 initiate the formation of a cup-shaped membrane, which expands to capture cytoplasmic targets, resulting in the formation of an autophagosome in a process dependent on the conjugates autophagy-related gene (Atg) 12–Atg5 and light chain 3 (LC3)–phosphatidylethanolamine. Autophagic adaptors/receptors with LC3-interacting region domains bind to LC3, thereby capturing cytoplasmic cargo. Autophagosomes fuse with lysosomes, leading to the degradation of their contents. Autophagy is repressed by the mammalian target of rapamycin (mTOR) and regulated by other pathways (66). The relationship between autophagy and inflammation differs depending on the context. Mitophagy, a selective form of autophagy that removes damaged mitochondria, is required for the controlled production of inflammatory cytokines, including TNF and IL-6, which sustains immune-cell homeostasis (67).
Autophagy and inflammation are interrelated in the host defense against mycobacteria. The inhibition of autophagy in Mtb-stimulated peripheral-blood mononuclear cells reduced TNF production and increased IL-1β production (68). Additionally, exogenous vitamin D, an autophagy activator, restored the compromised macrophage responses of HIV-seropositive patients, as indicated by increased TNF release as a result of enhanced TLR signaling (69). Dehydroepiandrosterone, which also activates autophagy, reduced the bacterial load in macrophages independently of effects on proinflammatory cytokine production (70).
The effectors produced by Mtb suppress autophagy and activate proinflammatory responses. For instance, the PE6 (Rv0335c) protein of Mtb promotes mTOR signaling and impedes autophagy activation while concurrently activating the canonical NF-κB signaling pathway to stimulate TNF production via the triggering of TLR4 signaling (71). Several small-molecule activators of autophagy also induce TNF production to eradicate intracellular Mtb. Fluoxetine, a selective serotonin reuptake inhibitor, promotes TNF secretion and autophagy (72). In contrast, chemical mimics of N-terminal-arginine N-degron degrade intracellular Mtb by activating xenophagy, but significantly reduce pathological inflammation and the expression of chemokines and TNF by targeting p62/SQSTM1 (73). Thus, these autophagy activators modulate Mtb-induced inflammation in a context-dependent manner. Moreover, autophagy is critical for the suppression of spontaneous pulmonary inflammation and the induction of an effective immune response during respiratory infection. However, uncontrolled or ineffective autophagy can have detrimental effects on lung epithelial cells, thereby causing lung injury (74). The relationship between autophagy and inflammation differs depending on the context. Thus, further studies should focus on the involvement of autophagy in inflammatory responses at different stages of TB and the potential for the development of therapeutic strategies targeting autophagy and inflammation.
TNF and apoptosis during mycobacterial infection
PCD, which encompasses apoptosis and autophagy, defends against Mtb infection by sequestering bacteria in dead macrophages for phagocytosis. In contrast to necrosis, apoptosis eliminates cells without causing inflammation. Non-PCD necrotic death is a pathogenic event that promotes Mtb release into the extracellular milieu during infection (75). Although most mycobacterial taxa trigger apoptosis, virulent strains show a decreased tendency to induce the apoptosis of macrophages relative to that of H37Ra (76). Among the factors involved, TNF initiates extrinsic apoptotic cell death by binding to its receptor, triggering receptor trimerization and the recruitment of adaptor proteins (TRADD/FADD) to the cytoplasmic end. Subsequently, caspase-8 is activated, initiating a cascade of events that leads to the activation of effector caspases-3, -6, and -7, resulting in apoptosis (77). Furthermore, IL-1β enhances TNF signaling in macrophages by increasing TNF secretion and TNFR1 expression, leading to caspase-3 activation. This IL-1β–mediated pathway, combined with downstream TNF production, contributes to the caspase-dependent limitation of intracellular Mtb growth (78).
Interestingly, Mtb evades apoptosis by inducing the release of sTNFRs to neutralize the activity of TNF and evade TNF-mediated cell death (79). TNF shedding is facilitated by the secretion of IL-10, which triggers the release of sTNFRs, thereby deactivating TNF (79). Further investigation of the molecular regulatory mechanisms of TNF production is needed, and may enable the development of therapeutics based on host-protective cell death that ameliorate lung pathology.
DETRIMENTAL EFFECTS OF TNF DURING MYCOBACTERIAL INFECTION
In this section, we explore the paradoxical function of TNF in the progression and pathological inflammation of chronic Mtb infection (Table 1). The deleterious effects of TNF stem predominantly from excessive mitoROS production, necrosis, and necroptosis, as opposed to apoptosis.
Table 1. Infection-associated roles of TNF-α during mycobacterial infections.
| Study model | Infection status | TNF-α level | Outcome | Ref. | |
|---|---|---|---|---|---|
| Infection-associated biomarker | |||||
| BCG-vaccinated South African infants | Mtb infection | ↑ | Identification of markers that are associated with susceptibility to Mtb infection | (80) | |
| Pulmonary TB patients | TB therapy for 6 months | ↓ | Good lung recovery | (82) | |
| TB patients | LTBI, DS-TB, DR-TB | ↑ | Usage of biomarkers across the TB spectrum | (83) | |
| DR-TB patients | Anti-TB therapy for 6 months | ↑ | Usage of biomarkers and host-directed therapy | (84) | |
| Disease pathogenesis | |||||
| TB-DM patients | Anti-TB treatment | ↑ | Non-resolving systemic and local inflammation compared to TB patients | (85) | |
| Human PBMCs; Human lung TB granulomas and sputum | Anti-PD1 immunotherapy | ↑ | Mtb growth↑ | (86) | |
| TB-susceptible I/St mice | Mtb infection | ↑ | Rapid cachexia | (33) | |
| Cell death and mitochondrial oxidative stress | |||||
| TB patients | Severe TB | NS | TNF-induced apoptosis↑ | (87) | |
| Zebrafish macrophages; Zebrafish | Mycobacterial infections | ↑ | Cyclophilin D- and ceramide-mediated necrosis↑ | (55) | |
| THP-1 cells; Zebrafish | Mycobacterial infections | ↑ | Programmed necrosis↑ | (88) | |
| THP-1 cells; Zebrafish | Mycobacterial infections and metformin treatment | ↑ | TNF-induced mtROS and necrosis↓ | (89) | |
| BMDMs; Sirt3+/+ and Sirt3−/− mice; human PBMCs from TB patients; human MDMs | Mtb infection | ↑ | ∙ Inflammation↑ | (90) | |
| ∙ Mitochondrial damage↑ | |||||
| ∙ Autophagy ↓ | |||||
| ∙ Mtb growth↑ | |||||
| Murine macrophages; mice | Mtb protease Rv3090 | ↑ | Late-stage apoptosis↑ | (91) | |
LTBI, latent tuberculosis infection; DS-TB, drug-sensitive TB; DR-TB, drug-resistant TB; TB-DM, TB patients with diabetes mellitus; BMDM, bone marrow-derived macrophage; Sirt, sirtuin; MDM, human monocyte-derived macrophages; NS, not significant.
Role of TNF in the pathogenesis of mycobacterial infection
Among BCG-vaccinated South African infants who were determined to be positive by QuantiFERON testing but did not develop active TB, inflammation, immune activation, and the risk of Mtb infection were correlated. Infants subsequently infected with Mtb showed an elevated level of Ag85A-specific IgG, upregulated expression of Ig-associated genes and type-I IFN, and higher plasma levels of IFN-α2, TNF, CXCL10/IFN-γ–induced protein 10, and complement C2 (80). A systematic review and meta-analysis confirmed that the cerebrospinal fluid levels of TNF, other proinflammatory cytokines, and IFN-γ are higher in patients with TB meningitis than in healthy controls (81). In addition, patients with improved lung recovery had lower levels of certain mediators (including TNF) after 6 months of therapy (82). Hence, the investigation of therapeutic agents targeting TNF and proinflammatory mediators is warranted.
TNF and TNFRs are related to the different immune responses to drug-susceptible and -resistant TB. Research based on multiplex assays identified hyperinflammatory cytokine signatures associated with drug-resistant TB. Increased plasma levels of IFN-γ, TNF, and IL-6 differentiated patients with drug-resistant TB, latent TB, and healthy individuals (83). Additionally, patients with drug-resistant TB had reduced frequencies of certain T-cell subpopulations and showed systemic inflammation characterized by elevated TNF levels after 6 months of treatment (84). These data suggest that TNF signaling and inflammatory status is closely associated with the severity of TB and the drug sensitivity in patients receiving anti-TB therapy. Furthermore, the transcript levels of the proinflammatory cytokines IL-1β and TNF in the peripheral blood were higher after anti-TB treatment in patients with pulmonary TB and diabetes mellitus (DM) than in those with TB alone (85). This finding suggests that TNF sustains inflammation, thereby increasing disease severity, in patients with TB and DM.
Immune-checkpoint therapy for cancer can activate TB infection. PD-1 is expressed in Mtb-infected lung tissue, but not in areas of immunopathology. The inhibition of PD-1 signaling enhances Mtb growth in a TNF-dependent manner. The increased pulmonary TNF immunoreactivity in human TB and the negative correlation between the circulating PD-1 and sputum TNF levels support these findings. Thus, PD-1 regulates the immune response to TB and its inhibition can cause excessive TNF secretion, thereby contributing to TB pathology and accelerated Mtb growth (86). A single-cell RNA transcriptome study in which data from the healthy control and active TB patients with diverse severity were used that showed that patients with severe disease had elevated numbers of inflammatory immune cells and fewer lymphocytes; they exhibited immune exhaustion and the activation of TNF-induced apoptosis, as well as high cytotoxicity in T helper 1, CD8+ T, and NK cells (87). These findings shed light on the dysregulated expression of TNF and its roles in TB immunopathogenesis and clinical severity, which will aid the design of novel effective therapies against severe TB.
TNF, mitochondrial oxidative stress, and cell death
High-level TNF production induces mitoROS in infected macrophages via RIPK1–RIPK3–MLKL-dependent pathways. During Mtb infection, ROS initially enhance microbicidal activity but thereafter trigger necroptosis, releasing mycobacteria into the extracellular milieu. Interestingly, the repression of RIPK3 or MLKL results in a switch from TNF-induced necroptosis to delayed RIPK1-dependent apoptosis (88). Avirulent Mtb strains induce apoptosis, whereas virulent strains promote apoptosis–-necroptosis conversion, benefiting pathogen dissemination. Virulent strains induce greater TNF production, upregulate anti-apoptotic B-cell lymphoma 2 proteins, and trigger the secretion of a caspase-8 inhibitor (89). Although apoptosis functions as a protective mechanism against Mtb infection, late-stage apoptosis is associated with bacterial spread (76). The cell wall–associated protease Rv3090 is reported to be a virulence factor of Mtb. Rv3090 induced the late apoptosis of macrophages, hepatocytes, and lung cells; stimulated the secretion of proinflammatory cytokines; and promoted Mtb survival, thereby contributing to Mtb pathogenicity and dissemination (90).
Nondegradable materials such as silica, as well as Mtb, evade degradation in lysosomes, leading to persistent macrophage activation (54). The result is the upregulation of nicotinamide adenine dinucleotide phosphate hydrogen oxidase activation and increased mitoROS production, ultimately resulting in macrophage death (16,54,91). During Mtb infection, excessive TNF production promotes necrosis, which is characterized by the lysis of infected cells, leading to the release of viable bacteria and damage to the surrounding tissue (77). Elevated sTNF levels can induce necroptosis via two pathways. The first pathway involves the regulation of mitochondrial cyclophilin D, which is associated with the formation of the mitochondrial permeability transition pore. The second pathway entails acid sphingomyelinase–mediated ceramide production (16). TNF initiated the programmed necrosis of mycobacterium-infected macrophages by stimulating mitoROS production in an RIPK1/3-dependent fashion; excessive TNF production triggered an increase in the mitoROS level in these macrophages via reverse electron transport through complex I (91). TNF-induced glutamine uptake elevated the succinate level, thereby driving reverse electron transport and generating mitoROS. Metformin, an antidiabetic medication that inhibits complex I, prevented the TNF-induced production of mitoROS and subsequent necrosis in Mtb-infected macrophages and a zebrafish model, suggesting therapeutic potential for TB (92). Furthermore, sirtuin 3 (SIRT3) regulated the excessive inflammation induced by mitoROS, strengthening the host defense against Mtb infection (93). Macrophages lacking SIRT3 exhibit heightened oxidative stress, which exacerbated inflammation. Mechanistically, the SIRT3–peroxisome proliferator–activated receptor-α–transcription factor EB pathway is implicated in the activation of autophagy, and thus the enhancement of the host defense against Mtb infection. Indeed, honokiol, a SIRT3 agonist, maintained mitochondrial homeostasis and promoted autophagy and antimicrobial activity (93). Further research is needed to identify the key components that co-ordinate mitochondrial and autophagic responses to Mtb.
CONCLUSION
The effects of TNF on host immunobiological processes suggest the potential for the development of host-directed therapeutics against TB. Granulomas confine infections to localized sites. The precise regulation of TNF production is crucial for the establishment and maintenance of granulomas; an excessive proinflammatory response during Mtb infection can result in extensive tissue damage. TNF is implicated in multiple aspects of protective immunity against Mtb infection, encompassing M1 macrophage responses, immunometabolic remodeling toward aerobic glycolysis, the enhancement of trained immunity, and involvement in autophagy and apoptosis. However, uncontrolled TNF production, coupled with mitochondrial damage and oxidative stresses, contributes to disease progression, pathological inflammation, necrotic cell death, and cachexia during chronic Mtb infection. Further investigation of the mechanisms underlying TNF production to enhance protective immunity is needed to enable the development of effective TB interventions and vaccines targeting TNF-mediated pathways. Additionally, the functional characterization of the TNF immune networks that orchestrate host defense mechanisms will facilitate the identification of novel drug targets and the development of new therapeutics. Further research should focus on the enhancement of TNF-based host defense mechanisms and targeting of the mechanisms by which Mtb evades TNF-mediated immunity, which have important implications for combating TB.
ACKNOWLEDGEMENTS
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Korea government (MSIT) (NRF-2017R1A5A2015385, NRF-2022R1C1C1004346, and RS-2023-00255021).
Abbreviations
- Atg
autophagy-related gene
- BCG
Bacillus Calmette-Guérin
- CCR
C-C chemokine receptor
- cIAP1/2
cellular inhibitor of apoptosis protein 1 and 2
- DD
death domain
- DM
diabetes mellitus
- ESX-1
6-kDa early secretory antigenic target secretion system 1
- Fc
fragment crystallizable
- H3K4
H3 lysine K4
- iNOS
inducible nitric oxide synthase
- LC3
light chain 3
- LUBAC
linear ubiquitin assembly complex
- mitoROS
mitochondrial ROS
- MLKL
mixed lineage kinase domain-like protein
- Mtb
Mycobacterium tuberculosis
- mTNF
membrane-bound TNF
- MyD88
myeloid differentiation primary response 88
- PAMP
pathogen-associated molecular pattern
- PCD
programmed cell death
- PRR
pattern-recognition receptor
- RIPK
receptor-interacting serine/threonine-protein kinase
- sTNF
soluble TNF
- sTNFR
soluble TNF receptor
- TACE
TNF-converting enzyme metalloprotease
- TB
tuberculosis
- TNFR
TNF receptor
- TRAF
TNF receptor-associated factor
- TRADD
TNFR1-associated death domain
- TRIF
TIR-domain-containing adapter-inducing interferon-β
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
- Conceptualization: Yuk JM, Jo EK.
- Data curation: Kim JK, Yuk JM.
- Formal analysis: Kim JK, Kim IS.
- Funding acquisition: Yuk JM, Jo EK.
- Supervision: Jo EK.
- Writing - original draft: Yuk JM, Jo EK.
- Writing - review & editing: Yuk JM, Jo EK.
References
- 1.Russell DG. Mycobacterium tuberculosis and the intimate discourse of a chronic infection. Immunol Rev. 2011;240:252–268. doi: 10.1111/j.1600-065X.2010.00984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alsayed SSR, Gunosewoyo H. Tuberculosis: pathogenesis, current treatment regimens and new drug targets. Int J Mol Sci. 2023;24:5202. doi: 10.3390/ijms24065202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruiz A, Palacios Y, Garcia I, Chavez-Galan L. Transmembrane TNF and its receptors TNFR1 and TNFR2 in mycobacterial infections. Int J Mol Sci. 2021;22:5461. doi: 10.3390/ijms22115461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dostert C, Grusdat M, Letellier E, Brenner D. The TNF family of ligands and receptors: communication modules in the immune system and beyond. Physiol Rev. 2019;99:115–160. doi: 10.1152/physrev.00045.2017. [DOI] [PubMed] [Google Scholar]
- 5.Coppola M, Villar-Hernández R, van Meijgaarden KE, Latorre I, Muriel Moreno B, Garcia-Garcia E, Franken KL, Prat C, Stojanovic Z, De Souza Galvão ML, et al. Cell-mediated immune responses to in vivo-expressed and stage-specific Mycobacterium tuberculosis antigens in latent and active tuberculosis across different age groups. Front Immunol. 2020;11:103. doi: 10.3389/fimmu.2020.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beutler B, Greenwald D, Hulmes JD, Chang M, Pan YC, Mathison J, Ulevitch R, Cerami A. Identity of tumour necrosis factor and the macrophage-secreted factor cachectin. Nature. 1985;316:552–554. doi: 10.1038/316552a0. [DOI] [PubMed] [Google Scholar]
- 7.Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975;72:3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Malley WE, Achinstein B, Shear MJ. Action of bacterial polysaccharide on tumors. Iii. repeated response of sarcoma 37, in tolerant mice, to serratia marcescens endotoxin. Cancer Res. 1963;23:890–895. [PubMed] [Google Scholar]
- 9.Baud V, Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001;11:372–377. doi: 10.1016/s0962-8924(01)02064-5. [DOI] [PubMed] [Google Scholar]
- 10.Aggarwal BB, Kohr WJ, Hass PE, Moffat B, Spencer SA, Henzel WJ, Bringman TS, Nedwin GE, Goeddel DV, Harkins RN. Human tumor necrosis factor. Production, purification, and characterization. J Biol Chem. 1985;260:2345–2354. [PubMed] [Google Scholar]
- 11.Yang S, Wang J, Brand DD, Zheng SG. Role of TNF-TNF receptor 2 signal in regulatory T cells and its therapeutic implications. Front Immunol. 2018;9:784. doi: 10.3389/fimmu.2018.00784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bodmer JL, Schneider P, Tschopp J. The molecular architecture of the TNF superfamily. Trends Biochem Sci. 2002;27:19–26. doi: 10.1016/s0968-0004(01)01995-8. [DOI] [PubMed] [Google Scholar]
- 13.Chédotal H, Narayanan D, Povlsen K, Gotfredsen CH, Brambilla R, Gajhede M, Bach A, Clausen MH. Small-molecule modulators of tumor necrosis factor signaling. Drug Discov Today. 2023;28:103575. doi: 10.1016/j.drudis.2023.103575. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs M, Brown N, Allie N, Chetty K, Ryffel B. Tumor necrosis factor receptor 2 plays a minor role for mycobacterial immunity. Pathobiology. 2000;68:68–75. doi: 10.1159/000028116. [DOI] [PubMed] [Google Scholar]
- 15.Shundo Y, On R, Matsumoto T, Ouchi H, Fujita M. TNFR1 mediated apoptosis is protective against Mycobacterium avium in mice. Microorganisms. 2023;11:778. doi: 10.3390/microorganisms11030778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roca FJ, Ramakrishnan L. TNF dually mediates resistance and susceptibility to mycobacteria via mitochondrial reactive oxygen species. Cell. 2013;153:521–534. doi: 10.1016/j.cell.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Souza RF, Caetano MAF, Magalhães HIR, Castelucci P. Study of tumor necrosis factor receptor in the inflammatory bowel disease. World J Gastroenterol. 2023;29:2733–2746. doi: 10.3748/wjg.v29.i18.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naudé PJ, den Boer JA, Luiten PG, Eisel UL. Tumor necrosis factor receptor cross-talk. FEBS J. 2011;278:888–898. doi: 10.1111/j.1742-4658.2011.08017.x. [DOI] [PubMed] [Google Scholar]
- 19.Wong KW. The role of ESX-1 in Mycobacterium tuberculosis pathogenesis. Microbiol Spectr. 2017;5 doi: 10.1128/microbiolspec.tbtb2-0001-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu CH, Liu H, Ge B. Innate immunity in tuberculosis: host defense vs pathogen evasion. Cell Mol Immunol. 2017;14:963–975. doi: 10.1038/cmi.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 22.Ray JC, Flynn JL, Kirschner DE. Synergy between individual TNF-dependent functions determines granuloma performance for controlling Mycobacterium tuberculosis infection. J Immunol. 2009;182:3706–3717. doi: 10.4049/jimmunol.0802297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chai Q, Lu Z, Liu CH. Host defense mechanisms against Mycobacterium tuberculosis . Cell Mol Life Sci. 2020;77:1859–1878. doi: 10.1007/s00018-019-03353-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cadena AM, Fortune SM, Flynn JL. Heterogeneity in tuberculosis. Nat Rev Immunol. 2017;17:691–702. doi: 10.1038/nri.2017.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiran D, Podell BK, Chambers M, Basaraba RJ. Host-directed therapy targeting the Mycobacterium tuberculosis granuloma: a review. Semin Immunopathol. 2016;38:167–183. doi: 10.1007/s00281-015-0537-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsai MC, Chakravarty S, Zhu G, Xu J, Tanaka K, Koch C, Tufariello J, Flynn J, Chan J. Characterization of the tuberculous granuloma in murine and human lungs: cellular composition and relative tissue oxygen tension. Cell Microbiol. 2006;8:218–232. doi: 10.1111/j.1462-5822.2005.00612.x. [DOI] [PubMed] [Google Scholar]
- 27.Patel K, Jhamb SS, Singh PP. Models of latent tuberculosis: their salient features, limitations, and development. J Lab Physicians. 2011;3:75–79. doi: 10.4103/0974-2727.86837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tonko S, Baty F, Brutsche MH, Schoch OD. Length of hospital stay for TB varies with comorbidity and hospital location. Int J Tuberc Lung Dis. 2020;24:948–955. doi: 10.5588/ijtld.19.0759. [DOI] [PubMed] [Google Scholar]
- 29.Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab. 2012;16:153–166. doi: 10.1016/j.cmet.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 30.Paval DR, Patton R, McDonald J, Skipworth RJE, Gallagher IJ, Laird BJ Caledonian Cachexia Collaborative. A systematic review examining the relationship between cytokines and cachexia in incurable cancer. J Cachexia Sarcopenia Muscle. 2022;13:824–838. doi: 10.1002/jcsm.12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balsano R, Kruize Z, Lunardi M, Comandatore A, Barone M, Cavazzoni A, Re Cecconi AD, Morelli L, Wilmink H, Tiseo M, et al. Transforming growth factor-beta signaling in cancer-induced cachexia: from molecular pathways to the clinics. Cells. 2022;11:2671. doi: 10.3390/cells11172671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mupere E, Malone L, Zalwango S, Okwera A, Nsereko M, Tisch DJ, Parraga IM, Stein CM, Mugerwa R, Boom WH, et al. Wasting among Uganda men with pulmonary tuberculosis is associated with linear regain in lean tissue mass during and after treatment in contrast to women with wasting who regain fat tissue mass: prospective cohort study. BMC Infect Dis. 2014;14:24. doi: 10.1186/1471-2334-14-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Linge I, Kondratieva E, Apt A. Prolonged B-lymphocyte-mediated immune and inflammatory responses to tuberculosis infection in the lungs of TB-resistant mice. Int J Mol Sci. 2023;24:1140. doi: 10.3390/ijms24021140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopetuso LR, Cuomo C, Mignini I, Gasbarrini A, Papa A. Focus on anti-tumour necrosis factor (TNF)-α-related autoimmune diseases. Int J Mol Sci. 2023;24:8187. doi: 10.3390/ijms24098187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wallis RS. Tumour necrosis factor antagonists: structure, function, and tuberculosis risks. Lancet Infect Dis. 2008;8:601–611. doi: 10.1016/S1473-3099(08)70227-5. [DOI] [PubMed] [Google Scholar]
- 36.Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, Siegel JN, Braun MM. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345:1098–1104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 37.Harris J, Hope JC, Keane J. Tumor necrosis factor blockers influence macrophage responses to Mycobacterium tuberculosis . J Infect Dis. 2008;198:1842–1850. doi: 10.1086/593174. [DOI] [PubMed] [Google Scholar]
- 38.Mezouar S, Diarra I, Roudier J, Desnues B, Mege JL. Tumor necrosis factor-alpha antagonist interferes with the formation of granulomatous multinucleated giant cells: new insights into Mycobacterium tuberculosis infection. Front Immunol. 2019;10:1947. doi: 10.3389/fimmu.2019.01947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bo H, Moure UAE, Yang Y, Pan J, Li L, Wang M, Ke X, Cui H. Mycobacterium tuberculosis-macrophage interaction: molecular updates. Front Cell Infect Microbiol. 2023;13:1062963. doi: 10.3389/fcimb.2023.1062963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pattanaik KP, Sengupta S, Jit BP, Kotak R, Sonawane A. Host-mycobacteria conflict: immune responses of the host vs. the mycobacteria TLR2 and TLR4 ligands and concomitant host-directed therapy. Microbiol Res. 2022;264:127153. doi: 10.1016/j.micres.2022.127153. [DOI] [PubMed] [Google Scholar]
- 41.Gopalakrishnan A, Salgame P. Toll-like receptor 2 in host defense against Mycobacterium tuberculosis: to be or not to be-that is the question. Curr Opin Immunol. 2016;42:76–82. doi: 10.1016/j.coi.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruiz A, Guzmán-Beltrán S, Carreto-Binaghi LE, Gonzalez Y, Juárez E. DNA from virulent M. tuberculosis induces TNF-α production and autophagy in M1 polarized macrophages. Microb Pathog. 2019;132:166–177. doi: 10.1016/j.micpath.2019.04.041. [DOI] [PubMed] [Google Scholar]
- 43.Nguyen H, Gazy N, Venketaraman V. A role of intracellular toll-like receptors (3, 7, and 9) in response to Mycobacterium tuberculosis and co-infection with HIV. Int J Mol Sci. 2020;21:6148. doi: 10.3390/ijms21176148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kwon KW, Kang TG, Lee A, Jin SM, Lim YT, Shin SJ, Ha SJ. Protective efficacy and immunogenicity of Rv0351/Rv3628 subunit vaccine formulated in different adjuvants against Mycobacterium tuberculosis infection. Immune Netw. 2023;23:e16. doi: 10.4110/in.2023.23.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang LX, Zhang SX, Wu HJ, Rong XL, Guo J. M2b macrophage polarization and its roles in diseases. J Leukoc Biol. 2019;106:345–358. doi: 10.1002/JLB.3RU1018-378RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahmad F, Rani A, Alam A, Zarin S, Pandey S, Singh H, Hasnain SE, Ehtesham NZ. Macrophage: a cell with many faces and functions in tuberculosis. Front Immunol. 2022;13:747799. doi: 10.3389/fimmu.2022.747799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rutschmann O, Toniolo C, McKinney JD. Preexisting heterogeneity of inducible nitric oxide synthase expression drives differential growth of Mycobacterium tuberculosis in macrophages. MBio. 2022;13:e0225122. doi: 10.1128/mbio.02251-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chandra P, Grigsby SJ, Philips JA. Immune evasion and provocation by Mycobacterium tuberculosis . Nat Rev Microbiol. 2022;20:750–766. doi: 10.1038/s41579-022-00763-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cambier CJ, Takaki KK, Larson RP, Hernandez RE, Tobin DM, Urdahl KB, Cosma CL, Ramakrishnan L. Mycobacteria manipulate macrophage recruitment through coordinated use of membrane lipids. Nature. 2014;505:218–222. doi: 10.1038/nature12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mohareer K, Banerjee S. Mycobacterial infection alters host mitochondrial activity. Int Rev Cell Mol Biol. 2023;377:87–119. doi: 10.1016/bs.ircmb.2023.01.007. [DOI] [PubMed] [Google Scholar]
- 51.Mendonca LE, Pernet E, Khan N, Sanz J, Kaufmann E, Downey J, Grant A, Orlova M, Schurr E, Krawczyk C, et al. Human alveolar macrophage metabolism is compromised during Mycobacterium tuberculosis infection. Front Immunol. 2023;13:1044592. doi: 10.3389/fimmu.2022.1044592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tukiman MH, Norazmi MN. Immunometabolism of immune cells in mucosal environment drives effector responses against Mycobacterium tuberculosis . Int J Mol Sci. 2022;23:8531. doi: 10.3390/ijms23158531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Palma C, La Rocca C, Gigantino V, Aquino G, Piccaro G, Di Silvestre D, Brambilla F, Rossi R, Bonacina F, Lepore MT, et al. Caloric restriction promotes immunometabolic reprogramming leading to protection from tuberculosis. Cell Metab. 2021;33:300–318.e12. doi: 10.1016/j.cmet.2020.12.016. [DOI] [PubMed] [Google Scholar]
- 54.Marrocco A, Ortiz LA. Role of metabolic reprogramming in pro-inflammatory cytokine secretion from LPS or silica-activated macrophages. Front Immunol. 2022;13:936167. doi: 10.3389/fimmu.2022.936167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mahla RS, Kumar A, Tutill HJ, Krishnaji ST, Sathyamoorthy B, Noursadeghi M, Breuer J, Pandey AK, Kumar H. NIX-mediated mitophagy regulate metabolic reprogramming in phagocytic cells during mycobacterial infection. Tuberculosis (Edinb) 2021;126:102046. doi: 10.1016/j.tube.2020.102046. [DOI] [PubMed] [Google Scholar]
- 56.Ó Maoldomhnaigh C, Cox DJ, Phelan JJ, Mitermite M, Murphy DM, Leisching G, Thong L, O’Leary SM, Gogan KM, McQuaid K, et al. Lactate alters metabolism in human macrophages and improves their ability to kill Mycobacterium tuberculosis . Front Immunol. 2021;12:663695. doi: 10.3389/fimmu.2021.663695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van der Meer JW, Joosten LA, Riksen N, Netea MG. Trained immunity: a smart way to enhance innate immune defence. Mol Immunol. 2015;68:40–44. doi: 10.1016/j.molimm.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 58.Murphy DM, Mills KHG, Basdeo SA. The effects of trained innate immunity on T cell responses; clinical implications and knowledge gaps for future research. Front Immunol. 2021;12:706583. doi: 10.3389/fimmu.2021.706583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Netea MG, Domínguez-Andrés J, Barreiro LB, Chavakis T, Divangahi M, Fuchs E, Joosten LA, van der Meer JW, Mhlanga MM, Mulder WJ, et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol. 2020;20:375–388. doi: 10.1038/s41577-020-0285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rawat BS, Kumar D, Soni V, Rosenn EH. Therapeutic potentials of immunometabolomic modulations induced by tuberculosis vaccination. Vaccines (Basel) 2022;10:2127. doi: 10.3390/vaccines10122127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arts RJ, Carvalho A, La Rocca C, Palma C, Rodrigues F, Silvestre R, Kleinnijenhuis J, Lachmandas E, Gonçalves LG, Belinha A, et al. Immunometabolic pathways in BCG-induced trained immunity. Cell Reports. 2016;17:2562–2571. doi: 10.1016/j.celrep.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koeken VA, Qi C, Mourits VP, de Bree LC, Moorlag SJ, Sonawane V, Lemmers H, Dijkstra H, Joosten LA, van Laarhoven A, et al. Plasma metabolome predicts trained immunity responses after antituberculosis BCG vaccination. PLoS Biol. 2022;20:e3001765. doi: 10.1371/journal.pbio.3001765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stražar M, Mourits VP, Koeken VA, de Bree LC, Moorlag SJ, Joosten LA, van Crevel R, Vlamakis H, Netea MG, Xavier RJ. The influence of the gut microbiome on BCG-induced trained immunity. Genome Biol. 2021;22:275. doi: 10.1186/s13059-021-02482-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bickett TE, McLean J, Creissen E, Izzo L, Hagan C, Izzo AJ, Silva Angulo F, Izzo AA. Characterizing the BCG induced macrophage and neutrophil mechanisms for defense against Mycobacterium tuberculosis . Front Immunol. 2020;11:1202. doi: 10.3389/fimmu.2020.01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Espert L, Beaumelle B, Vergne I. Autophagy in Mycobacterium tuberculosis and HIV infections. Front Cell Infect Microbiol. 2015;5:49. doi: 10.3389/fcimb.2015.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290:1717–1721. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu Y, Shen J, Ran Z. Emerging views of mitophagy in immunity and autoimmune diseases. Autophagy. 2020;16:3–17. doi: 10.1080/15548627.2019.1603547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kleinnijenhuis J, Oosting M, Plantinga TS, van der Meer JW, Joosten LA, Crevel RV, Netea MG. Autophagy modulates the Mycobacterium tuberculosis-induced cytokine response. Immunology. 2011;134:341–348. doi: 10.1111/j.1365-2567.2011.03494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Anandaiah A, Sinha S, Bole M, Sharma SK, Kumar N, Luthra K, Li X, Zhou X, Nelson B, Han X, et al. Vitamin D rescues impaired Mycobacterium tuberculosis-mediated tumor necrosis factor release in macrophages of HIV-seropositive individuals through an enhanced Toll-like receptor signaling pathway in vitro. Infect Immun. 2013;81:2–10. doi: 10.1128/IAI.00666-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bongiovanni B, Mata-Espinosa D, D’Attilio L, Leon-Contreras JC, Marquez-Velasco R, Bottasso O, Hernandez-Pando R, Bay ML. Effect of cortisol and/or DHEA on THP1-derived macrophages infected with Mycobacterium tuberculosis . Tuberculosis (Edinb) 2015;95:562–569. doi: 10.1016/j.tube.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 71.Sharma N, Shariq M, Quadir N, Singh J, Sheikh JA, Hasnain SE, Ehtesham NZ. Mycobacterium tuberculosis protein PE6 (Rv0335c), a novel TLR4 agonist, evokes an inflammatory response and modulates the cell death pathways in macrophages to enhance intracellular survival. Front Immunol. 2021;12:696491. doi: 10.3389/fimmu.2021.696491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stanley SA, Barczak AK, Silvis MR, Luo SS, Sogi K, Vokes M, Bray MA, Carpenter AE, Moore CB, Siddiqi N, et al. Identification of host-targeted small molecules that restrict intracellular Mycobacterium tuberculosis growth. PLoS Pathog. 2014;10:e1003946. doi: 10.1371/journal.ppat.1003946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee YJ, Kim JK, Jung CH, Kim YJ, Jung EJ, Lee SH, Choi HR, Son YS, Shim SM, Jeon SM, et al. Chemical modulation of SQSTM1/p62-mediated xenophagy that targets a broad range of pathogenic bacteria. Autophagy. 2022;18:2926–2945. doi: 10.1080/15548627.2022.2054240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Racanelli AC, Kikkers SA, Choi AM, Cloonan SM. Autophagy and inflammation in chronic respiratory disease. Autophagy. 2018;14:221–232. doi: 10.1080/15548627.2017.1389823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nisa A, Kipper FC, Panigrahy D, Tiwari S, Kupz A, Subbian S. Different modalities of host cell death and their impact on Mycobacterium tuberculosis infection. Am J Physiol Cell Physiol. 2022;323:C1444–C1474. doi: 10.1152/ajpcell.00246.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lam A, Prabhu R, Gross CM, Riesenberg LA, Singh V, Aggarwal S. Role of apoptosis and autophagy in tuberculosis. Am J Physiol Lung Cell Mol Physiol. 2017;313:L218–L229. doi: 10.1152/ajplung.00162.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mohareer K, Asalla S, Banerjee S. Cell death at the cross roads of host-pathogen interaction in Mycobacterium tuberculosis infection. Tuberculosis (Edinb) 2018;113:99–121. doi: 10.1016/j.tube.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 78.Jayaraman P, Sada-Ovalle I, Nishimura T, Anderson AC, Kuchroo VK, Remold HG, Behar SM. IL-1β promotes antimicrobial immunity in macrophages by regulating TNFR signaling and caspase-3 activation. J Immunol. 2013;190:4196–4204. doi: 10.4049/jimmunol.1202688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Balcewicz-Sablinska MK, Keane J, Kornfeld H, Remold HG. Pathogenic Mycobacterium tuberculosis evades apoptosis of host macrophages by release of TNF-R2, resulting in inactivation of TNF-alpha. J Immunol. 1998;161:2636–2641. [PubMed] [Google Scholar]
- 80.Satti I, Wittenberg RE, Li S, Harris SA, Tanner R, Cizmeci D, Jacobs A, Williams N, Mulenga H, Fletcher HA, et al. Inflammation and immune activation are associated with risk of Mycobacterium tuberculosis infection in BCG-vaccinated infants. Nat Commun. 2022;13:6594. doi: 10.1038/s41467-022-34061-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Saghazadeh A, Rezaei N. Central inflammatory cytokines in tuberculous meningitis: a systematic review and meta-analysis. J Interferon Cytokine Res. 2022;42:95–107. doi: 10.1089/jir.2021.0176. [DOI] [PubMed] [Google Scholar]
- 82.Muefong CN, Owolabi O, Donkor S, Charalambous S, Mendy J, Sey IC, Bakuli A, Rachow A, Geldmacher C, Sutherland JS. Major neutrophil-derived soluble mediators associate with baseline lung pathology and post-treatment recovery in tuberculosis patients. Front Immunol. 2021;12:740933. doi: 10.3389/fimmu.2021.740933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sampath P, Rajamanickam A, Thiruvengadam K, Natarajan AP, Hissar S, Dhanapal M, Thangavelu B, Jayabal L, Ramesh PM, Ranganathan UD, et al. Cytokine upsurge among drug-resistant tuberculosis endorse the signatures of hyper inflammation and disease severity. Sci Rep. 2023;13:785. doi: 10.1038/s41598-023-27895-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Téllez-Navarrete NA, Ramon-Luing LA, Muñoz-Torrico M, Preciado-García M, Medina-Quero K, Hernandez-Pando R, Chavez-Galan L. Anti-tuberculosis chemotherapy alters TNFR2 expression on CD4+ lymphocytes in both drug-sensitive and -resistant tuberculosis: however, only drug-resistant tuberculosis maintains a pro-inflammatory profile after a long time. Mol Med. 2021;27:76. doi: 10.1186/s10020-021-00320-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mily A, Sarker P, Taznin I, Hossain D, Haq MA, Kamal SMM, Raqib R. Slow radiological improvement and persistent low-grade inflammation after chemotherapy in tuberculosis patients with type 2 diabetes. BMC Infect Dis. 2020;20:933. doi: 10.1186/s12879-020-05473-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tezera LB, Bielecka MK, Ogongo P, Walker NF, Ellis M, Garay-Baquero DJ, Thomas K, Reichmann MT, Johnston DA, Wilkinson KA, et al. Anti-PD-1 immunotherapy leads to tuberculosis reactivation via dysregulation of TNF-α. eLife. 2020;9:e52668. doi: 10.7554/eLife.52668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang Y, Sun Q, Zhang Y, Li X, Liang Q, Guo R, Zhang L, Han X, Wang J, Shao L, et al. Systemic immune dysregulation in severe tuberculosis patients revealed by a single-cell transcriptome atlas. J Infect. 2023;86:421–438. doi: 10.1016/j.jinf.2023.03.020. [DOI] [PubMed] [Google Scholar]
- 88.Remijsen Q, Goossens V, Grootjans S, Van den Haute C, Vanlangenakker N, Dondelinger Y, Roelandt R, Bruggeman I, Goncalves A, Bertrand MJ, et al. Depletion of RIPK3 or MLKL blocks TNF-driven necroptosis and switches towards a delayed RIPK1 kinase-dependent apoptosis. Cell Death Dis. 2014;5:e1004. doi: 10.1038/cddis.2013.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xu G, Wang J, Gao GF, Liu CH. Insights into battles between Mycobacterium tuberculosis and macrophages. Protein Cell. 2014;5:728–736. doi: 10.1007/s13238-014-0077-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cui Y, Tang Y, Shao M, Zang X, Jiang Y, Cui Z, Dang G, Liu S. Mycobacterium tuberculosis protease Rv3090 is associated with late cell apoptosis and participates in organ injuries and mycobacterial dissemination in mice. Microb Pathog. 2022;173:105880. doi: 10.1016/j.micpath.2022.105880. [DOI] [PubMed] [Google Scholar]
- 91.Roca FJ, Whitworth LJ, Redmond S, Jones AA, Ramakrishnan L. TNF induces pathogenic programmed macrophage necrosis in tuberculosis through a mitochondrial-lysosomal-endoplasmic reticulum circuit. Cell. 2019;178:1344–1361.e11. doi: 10.1016/j.cell.2019.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Roca FJ, Whitworth LJ, Prag HA, Murphy MP, Ramakrishnan L. Tumor necrosis factor induces pathogenic mitochondrial ROS in tuberculosis through reverse electron transport. Science. 2022;376:eabh2841. doi: 10.1126/science.abh2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim TS, Jin YB, Kim YS, Kim S, Kim JK, Lee HM, Suh HW, Choe JH, Kim YJ, Koo BS, et al. SIRT3 promotes antimycobacterial defenses by coordinating mitochondrial and autophagic functions. Autophagy. 2019;15:1356–1375. doi: 10.1080/15548627.2019.1582743. [DOI] [PMC free article] [PubMed] [Google Scholar]