Abstract
Follicular helper T cells (Tfh) play a crucial role in generating high-affinity antibodies (Abs) and establishing immunological memory. Cytokines, among other functional molecules produced by Tfh, are central to germinal center (GC) reactions. This review focuses on the role of cytokines, including IL-21 and IL-4, in regulating B cell responses within the GC, such as differentiation, affinity maturation, and plasma cell development. Additionally, this review explores the impact of other cytokines like CXCL13, IL-10, IL-9, and IL-2 on GC responses and their potential involvement in autoimmune diseases, allergies, and cancer. This review highlights contributions of Tfh-derived cytokines to both protective immunity and immunopathology across a spectrum of diseases. A deeper understanding of Tfh cytokine biology holds promise for insights into biomedical conditions.
Keywords: Follicular helper T cell, Germinal center, B cell, Plasma cell, Cytokine
INTRODUCTION
The adaptive immune response is a complex and highly regulated process that relies on the coordinated efforts of various immune cell populations. One critical aspect of adaptive immunity involves the formation and maintenance of germinal centers (GCs), specialized microenvironments within secondary lymphoid organs where B cells undergo clonal expansion, affinity maturation, and differentiation into Ab-secreting plasma cells (PCs) or memory B cells (1,2). The regulation of these processes is orchestrated by a specialized subset of CD4+ T cells known as follicular helper T cells (Tfh). Tfh cells are distinguished by their expression of the chemokine receptor CXCR5, costimulatory receptor ICOS, and transcription factor (TF) Bcl6, and their capacity to assist B cells throughout the GC reaction (1). Within the GCs, B cells oscillate between two functionally distinct compartments: the dark zone (DZ), where Ag-binding Ab affinity matures, and the light zone (LZ), where B cell selection occurs. GC Tfh cells, predominantly located in the LZ, collaborate with follicular dendritic cells (FDC) to support GC B cells and facilitate their selection. B cells that are selected undergo somatic hypermutation and proliferate in the DZ. Subsequently, these B cells migrate back to the LZ (2). Tfh and GC Tfh cells provide indispensable support to B cells throughout this cycle, including the secretion of cytokines, which are essential for the comprehensive GC response.
The primary molecules secreted from Tfh cells include IL-21, IL-4, and CXCL13 (1). This review aims to provide an overview of the pivotal role played by cytokines produced by Tfh cells, with a particular focus on IL-21 and IL-4, and their multifaceted functions within the context of GC reactions. We will discuss the regulation and functions of IL-21 and IL-4 in GC B cell responses, including processes such as B cell differentiation, affinity maturation, class switch recombination, and PC differentiation. Additionally, this review covers the roles of other cytokines produced by Tfh or Tfh-like CD4 T cells, such as CXCL13, IL-10, IL-9, and IL-2, in modulating GC responses and their potential pathologic roles in autoimmune diseases, allergies, and the other type of diseases. Understanding the complex interplay of these cytokines and their impact on the immune system is essential for unraveling the mechanisms underlying adaptive immunity and developing novel therapeutic strategies for immune-related disorders.
CYTOKINES PRODUCED BY Tfh AND THEIR REGULATION
IL-21
The central roles of IL-21 in GC B cell responses
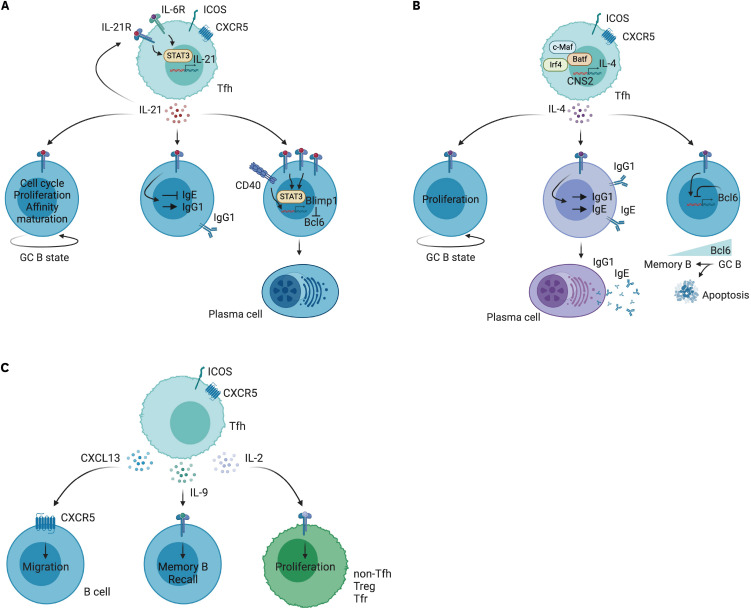
IL-21 is a cytokine predominantly produced by CD4+ T cells, specifically Tfh and Th17 cells, as well as by NKT cells. The best understood functions of IL-21 are in the context of Tfh. IL-21 plays a pivotal role in GC B cell differentiation and affinity maturation, essential processes for the adaptive immune response (Fig. 1A) (3,4). Studies in murine models demonstrate that IL-21 promotes B cell proliferation, isotype class switching, especially to IgG, and maturation into plasmablasts (5). Mice lacking IL-21 or its receptor show compromised antigenic responses, characterized by impaired GC maintenance and a tendency to develop low-affinity memory B cells, leading to reduced production of high-affinity Abs (3,6). Recent research suggests that the initial scale of the GC is influenced by the quantity of Tfh and IL-21 levels, with the availability of IL-21 determining the magnitude, persistence, and output of the GC response (7).
Figure 1. Influences of cytokines from Tfh on germinal center B cell dynamics. (A) Tfh cells release IL-21, which stimulates both the proliferation and the affinity maturation of B cells, culminating in their differentiation into PCs that synthesize Abs. (B) IL-4 promotes the generation of IgG1 or IgE and the differentiation into IgG1- or IgE-secreting PCs. (C) CXCL13 directs the migration of B cells in humans, IL-9 influences the expansion of certain B cell subsets, and IL-2 enhances the expansion of non-Tfh, Treg, and Tfr cells. See text for comprehensive details. Illustration produced with BioRender (https://BioRender.com/).
Furthermore, IL-21's role extends to the regulation of GC B cell selection and differentiation into either PCs or memory B cells. IL-21 plays a pivotal role in the facilitation of GC B cell entry into the cell cycle, thereby enhancing their proliferation (8). While the influence of IL-21 on cell cycle progression in the DZ B cells is limited, IL-21 prevents G1 stage accumulation in the LZ, which is crucial for the selection and maturation of high-affinity B cells (8). It is a nuanced process that is influenced by the combined signals from the B cell receptor (BCR), CD40, and IL-4.
The mechanism of IL-21's action is intrinsically linked to its influence on gene expression within B cells. IL-21 upregulates Bcl6, critical for the sustenance and optimal affinity maturation of the B cell receptor, and interestingly, IL-21 can also induce the expression of Blimp1 and Bcl6 simultaneously (5,9,10). This duality suggests that IL-21 may engage both STAT3 and STAT5 signaling pathways to modulate Bcl6 expression in GC B cells (3,11). Bcl6 and Blimp1 are reciprocal transcriptional regulators in B cells, with the former associated with cell survival, proliferation, and SHM, while the latter is associated with the differentiation of B cells into PCs (12). Further investigation is necessary to uncover the precise mechanisms by which IL-21 governs GC B cell differentiation via the concurrent upregulation of Bcl6 and Blimp1.
A recent study has provided insights into how IL-21 regulates the positive selection of GC B cells by modulating its availability and signal strength (13). Reduced IL-21 signals, possibly due to partial expression of the IL-21 receptor, can impede the differentiation of Ab-secreting PCs. In contrast, stronger IL-21 signals, which can be achieved through exogenous IL-21 treatment, appear to favor PC differentiation and concurrently reduce the total population of GC B cells (13). Collectively, the development of GC zones as a function of time and B cell proliferation highlights IL-21 as a key regulator of these intricate processes.
Roles of IL-21 in PC differentiation
The differentiation of PC is multifaceted in a complex process influenced by various cytokines, including IL-21, signaling from CD40 and BCR, and TFs to govern the complex transition of B cells to Ab-secreting PCs (14). Early GC-derived plasmablasts, which are still proliferative as denoted by Ki67 expression, are predominantly induced by IL-21 from Tfh cells. This process synergizes with IL-6 from stromal cells, suggesting the cooperative nature of cytokine signaling in early PC differentiation (1,15). Furthermore, IL-21 specifically promotes DZ B cell centroblast identity and PC differentiation. Interestingly, this specific IL-21 action on PC differentiation may be independent of T-B synaptic interactions (7).
Recent findings elucidate how IL-21R signaling reprograms GC B cells, steering their differentiation, selection, and class switching in coordination with CD40 and BCR signals. Signaling through both IL-21R+CD40 and BCR+CD40 pathways in GC B cells promotes the expression of Blimp1—a transcriptional repressor pivotal for PC differentiation. However, only IL-21R+CD40 signaling culminates in the differentiation of PCs. In contrast, simultaneous IL-21R+CD40+BCR signaling steers GC B cells towards a memory-like fate, illustrating the specific role of IL-21R in determining B cell destiny (16). IL-21 also exerts its influence on class switch recombination, notably inhibiting IgE production while promoting IgG1 production (17). The inhibition of IgE class switch recombination by IL-21 was attenuated by CD40 signaling (18). IL-4, in contrast, is crucial for IgE production, particularly during helminth infections, highlighting the intricate balance between IL-21 and other cytokines in immune regulation (18,19).
The differentiation of PCs from GC B cells involves complex, multi-step transcriptional reprogramming. This process requires a decrease in the expression of Bcl6 and an increase in Irf4, followed by the expression of Blimp1 (20,21). The induction of Blimp1 in Irf4hiBcl6lo early PCs is a hallmark of IL-21's influence, indicating its vital role in the functionality and longevity of PCs (1,20). The development of long-lived PCs is dependent on IL-21, which activates STAT3 signaling. Notably, the expression of constitutively active STAT3 in human B cells has been shown to induce PC differentiation. This highlights the crucial role of IL-21-STAT3-mediated upregulation of BLIMP1, which works in coordination with the down-regulation of BCL6, in controlling the differentiation of human PCs (22). One complexity associated with IL-21 is its capacity to induce the expression of both Blimp1 and Bcl6 (5,10,22). Combinatorial signals with CD40 and BCR results in the regulation of the expression of Blimp1 and Bcl6. It is reported that the ligation of CD40 triggers NF-kB and Irf4 activation, leading to a decrease in Bcl6 expression (23). In vitro stimulation of human GC B cells with IL-21 and anti-CD40 reduced the expression of BCL6 and increased the frequency of Irf4hiCD138+ PC, indicating that the combination of IL-21R and CD40 signaling in GC B cells directs PC differentiation through the regulation of BCL6 and BLIMP1 TFs (16). This process underscores the tight regulatory network where IL-21-induced signals are critical for the terminal differentiation of B cells.
IL-21 in Tfh cell development
IL-21 is a key player in the development and maintenance of Tfh cells, alongside other cytokines. The generation of Tfh cells is driven by IL-21, which induces Tfh differentiation independently of Th1, Th2, or Th17 cell lineages in mice (24). It is known that IL-21 is also produced by Th17 and that it enhances Th17 differentiation through autoregulation. Differing from Th17 cells, murine Tfh cell differentiation does not require TGF-β, and IL-21-producing Tfh cells do not express IL-17 (24,25). Research employing IL-21-/- mice and a neutralizing monoclonal Ab against IL-6 has verified that both IL-21 and IL-6 are essential for the optimal generation of Tfh cells (26), highlighting the synergistic effect of these cytokines in GC immunity. IL-21, together with IL-6, redundantly promotes the preferential differentiation of Tfh cells.
Studies utilizing IL-21R-deficient mice support the importance of IL-21 in Tfh development. Deficiency of IL-21R in T cells results in a modest decrease in the generation of Tfh cells after immunization in mice or no defect in virus infection (3,25,27), suggesting potential redundant STAT3 pathways, such as IL-6R signaling, and IL-21 may be more critical for the maintenance of Tfh than initial differentiation. A recent study further shows that IL-21 promotes CD4+ T cell expansion and differentiation in a paracrine manner and also enhances its own production (7). IL-21-deficient OTII CD4+ T cells significantly reduce the number of Tfh cells in IL-21 and IL-21R double-deficient host mice, indicating that IL-21 directly promotes Tfh differentiation independently of B cells (7). These findings collectively suggest IL-21's central role in orchestrating the complex interplay of cellular mechanisms that support the successful differentiation and maintenance of Tfh cells, which are pivotal to the adaptive immune response.
Molecular mechanisms of IL-21 production by Tfh cells
Multiple different signals are involved in IL-21 production from CD4 T cells. The initial strength of TCR binding influences the production of IL-21. It is reported that stronger TCR signaling augments IL-21 production in vitro, potentially offering autocrine sustenance for Tfh cells with higher Ag affinity (28,29). Furthermore, ICOS, an essential Tfh costimulatory molecule, signaling is a crucial component for IL-21 production, influencing c-Maf binding to Il21 locus during Tfh and Th17 cell development (30,31). The TF c-Maf is a known inducer of IL-21 expression in both murine and human CD4+ T cells, achieving this by binding to the Il21 promoter and the CNS2 enhancer. This activation is counterbalanced by TGF-β, which diminishes c-Maf-mediated IL-21 synthesis, delineating a negative feedback loop (32,33). The level of c-Maf expression in Tfh cells correlates with increased levels of CXCR5, IL-21, and IL-4, indicating a significant association with Tfh cell activity (32,34,35,36).
In addition to TCR and costimulation, cytokine signaling is an important factor for induction of IL-21 expression. IL-6 is the most potent inducer of IL-21 production in murine CD4 T cells (1). Both IL-6 and IL-21 activate STATs, particularly STAT3, which is essential for IL-21 expression in murine CD4+ T cells (37). In humans, the IL-6 effect on human CD4 T cells remains unclear (1). Instead, IL-12 is an inducer of IL-21 in human CD4+ T cells (38,39). IL-23, another IL-12 family member, also can induce IL-21 production in human Tfh cells. In concert with TGF-β, IL-12 or IL-23 can generate human Tfh in vitro (38). IL-12-mediated activation of STAT4 and IL-23-mediated activation of STAT3 are involved in IL-21 regulation in human counterparts (40,41,42), suggesting a differential regulatory mechanism across species. Recent research suggests that Klf2 and Tcf1, both downstream of Bcl6, play a key role in fine-tuning IL-21 expression in mouse Tfh cells (43). These findings indicate their role in the elaborate regulatory network that governs IL-21 production, highlighting the multifaceted control of Tfh cell functionality (44).
In addition to positive regulators, IL-21 expression is also negatively mediated by Foxp1 and the orphan nuclear receptor NR2F6. Foxp1 directly represses IL-21 expression in Tfh cells, while NR2F6 limits the accumulation of Tfh cells, thereby indirectly affecting IL-21 production (9,45). Our recent studies have shown that Mef2d functions as a transcriptional repressor of the Il21 gene, influencing IL-21 production through DNA binding. Mef2d binds to potential Mef2d binding sites in the Il21 gene, demonstrated by luciferase assays, and introducing Mef2d into CD4 T cells from naive mice markedly decreases IL-21 production (46). Overall, the regulation of IL-21 production in Tfh cells involves a complex network of TFs responding to various extracellular signals.
IL-4
Role of IL-4 in GC response
IL-4 plays pleiotropic roles in the immune response, including the proliferation and differentiation of B cells. IL-4, predominantly secreted by Tfh and Th2 cells after antigenic sensitization, provides antiapoptotic support to B cells, crucial for the survival of GC B cells prone to apoptosis (Fig. 1B) (47). Initially, Tfh cells provide IL-21 and CD40L signals to stimulate B cell proliferation and differentiation (48). As the GC response progresses, Tfh cells decrease IL-21 production and shift towards promoting IL-4 production. IL-4, in synergy with IL-21, supports GC maintenance and plasmablast differentiation (2,4). IL-4 also expands the population of rare GC B cells that recognize common epitopes during influenza infection (49). A recent study has shown that excess availability of IL-4 limits memory B cell differentiation from a specific subset of GC B cells (50). Single-cell RNA sequencing has identified an IL-4Ra+ CD23+ GC B cell subset in the LZ, and Tfh-derived IL-4 prevents this subset from evolving into memory cells. Interestingly, FDC can limit GC IL-4 availability through IL-4Ra expression, enhancing memory B cell generation (50). IL-4 also triggers a negative autoregulatory mechanism of Bcl6 expression in GC B cells, although it initially enhances Bcl6 in naive B cells to facilitate the generation of GC (51). This reduction in Bcl6 by IL-4 contributes to a selective process: pre-memory GC B cells with access to supplementary survival signals are granted permission to exit the GC, while those devoid of such signals undergo apoptosis (51). These findings suggest that IL-4 acts on B cell differentiation at multiple stages, including GC B cell and memory B cell differentiation.
IL-4 is crucial for class switch recombination to IgG1 in mice and IgG4 in humans, as well as IgE in both species (52). Notably, IL-4 from Tfh cells, rather than classical Th2 cells, predominantly governs IgE and IgG1 Ab responses, suggesting its vital role in humoral immunity. IL-4 and IL-21 appear to have distinct roles in class switch recombination. While IL-21 inhibits IgE production, IL-4 is essential for IgE production and plasmablast formation during helminth infection (18,19). This suggests that Tfh cell-derived IL-4 is key in orchestrating type 2 immune responses in reactive lymph nodes during parasitic infections.
Molecular mechanisms of IL-4 production by Tfh cells
The regulation of IL-4 expression in Tfh cells is a highly controlled process. IL-4 expression is a later development in fully differentiated GC Tfh cells (4,53). The mechanisms governing IL-4 production in GC Tfh cells are diverse. Unlike classical Th2 cells, Tfh cells utilize unique pathways and molecular mechanisms for IL-4 regulation, crucial for both humoral and cellular immune responses. GC Tfh IL-4 production requires signaling lymphocytic activation molecule (SLAM) receptor signaling (53), indicating that the SLAM-associated protein is a key mediator for IL-4 expression in Tfh cells.
In classical Th2 cells, TF GATA-3 controls IL-4 gene transcription (34,54,55). However, IL-4 expression in Tfh cells does not seem to be regulated by GATA-3. A critical aspect of IL-4 regulation in Tfh cells involves CNS2, also known as the enhancer hypersensitivity site V (HS V), within the Il4 locus (56). This site is crucial for IL-4 production, as demonstrated by the significant defects in type 2 humoral immune responses in mice lacking HS V, including reduced IgE and IgG1 production (57). The TF Batf plays a crucial role in initiating IL-4 expression in Tfh cells. It achieves this by binding to the CNS2 region and forming a complex with Irf4 in mice (58). In collaboration with Irf4, STAT3, and STAT6, Batf actively stimulates the CNS2 region within the Il4 locus, thereby initiating IL-4 production in Tfh cells. Additionally, c-Maf and Irf4, along with JunB and NFATc2, respectively, induce IL-4 expression in a GATA-3-independent manner (55,59,60).
Batf also can form a Batf-Bach2 complex, which counteracts the recruitment of the Batf-Irf4 complex to AP-1 motifs, thus inhibiting Th2 cytokine production (61). Bach2 modulates Batf and Batf3 expressions by suppressing IL-4 production and direct binding to Batf and Batf3 gene loci (61). STAT3 represses both Bcl6 and IL-4 expression in Tfh cells, indicating its critical role in regulating multiple key genes in these cells (62). IL-4 repression in Th1 cells involves the Runx/Cbf beta complex binding to the Il4 silencer (63). Bcl6 suppresses Runx3 in Tfh cells to induce multiple Tfh genes, including CXCR5 (43), suggesting a potential role for Runx3 in IL-4 regulation. Notably, Klf2, another downstream target TF of Bcl6, shows a repressive function on IL-4 and IL-21 expression (43), indicating that Bcl6 represses Klf2 to induce important Tfh cytokines, IL-4 and IL-21 (44).
Other cytokines
CXCL13
CXCL13 is one of the most important chemokines in GC biology. CXCL13 is predominantly produced by stromal cells and FDCs in both humans and mice (1). The primary role of CXCL13 in the GC is attracting activated Ag-specific B cells and Tfh cells to B cell follicles and further into GC. In contrast to mice where Tfh does not produce CXCL13, human Tfh can produce CXCL13 (64). This is another substantial difference between humans and mice (1). CXCL13 produced by Tfh may facilitate GC B cell migration to the LZ to enhance interactions between GC Tfh, GC B, and FDC trifecta (Fig. 1C) (47). Although CXCL13 is considered the most important chemokine in the GC dynamics, including the localization of cells, the particular role of CXCL13 from Tfh cells is still unclear. Further study is required to elucidate the proper role of Tfh-derived CXCL13 at the immune synapse in humans.
IL-10
IL-10 is recognized as a significant factor in the differentiation of PC in humans (65). In various contexts, IL-10 is acknowledged as an immunosuppressive or anti-inflammatory cytokine. Some studies have indicated that IL-10-producing Tfh cells in mice assist B cells (66). Nevertheless, the presence of IL-10-producing Tfh cells is closely associated with specific disease conditions, as discussed below. For instance, IL-10-producing Tfh cells tend to accumulate with age and are linked to inflammation in the context of age-related immune suppression (67).
IL-9
Germinal-center development of memory B cells driven by IL-9 by Tfh cells (68). Deletion of IL-9 from GC Tfh cells results in impaired memory formation of B cells. Consequently, IL-9 originating from Tfh cells promotes the generation of memory B cells (68). Additionally, IL-9 is thought to play a role in the recall of memory B cells, but there are conflicting reports in this context (68,69,70).
IL-2
IL-2 is widely recognized as the most potent suppressor of Tfh differentiation through STAT5 signaling, leading to the subsequent downregulation of Bcl6 expression (71). TCR activation triggers the expression of both IL-2 and IL-2Rα in CD4 T cells. Interestingly, CD4+ T cells that produce IL-2 tend to become Tfh cells, while those that do not produce IL-2 develop into non-Tfh cells (72). Despite the high expression of IL-2 in Tfh cells, these cells maintain a state of hyposensitivity to IL-2 (73). In a recent study involving mice infected with influenza, IL-6 was found to play a negative role in regulating the expression of IL-2Rβ. This regulation prevents the initiation of a negative feedback loop involving TCR and IL-2, which in turn inhibits the generation of GC Tfh cells during the transition phase from non-GC to GC Tfh (73). This suggests that an imbalance between IL-2-producing Tfh precursors and IL-2-nonproducing non-Tfh precursors can either promote or inhibit Tfh differentiation (74).
PATHOLOGIC ROLES OF CYTOKINES PRODUCED BY Tfh OR Tfh-LIKE CD4 T CELLS
IL-21
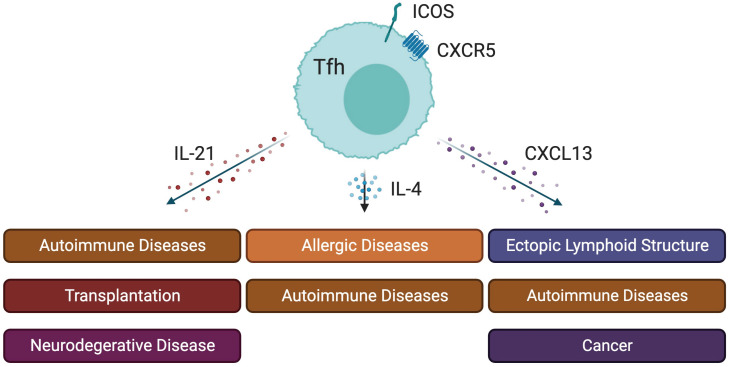
Tfh-derived cytokines have been associated with a range of diseases, including autoimmune disorders, allergies, and even cancer (Fig. 2) (1). Specifically, IL-21 has been associated with various autoimmune diseases, such as systemic lupus erythematosus (SLE), Sjögren’s syndrome, allograft rejection, and certain neuronal diseases. IL-21 contributes to the differentiation of an autoreactive CD11chi T-bet+ B cell subset into Ig-secreting autoreactive PCs in SLE (75). IL-21 has been identified as a pathogenic factor in lupus-prone MRL-FasLpr mice. Blocking it with IL-21R-Fc has been demonstrated to mitigate disease progression (76,77). In the B6.Sle1.Yaa lupus-prone mouse model, targeting IL-21 with an anti-IL-21 Ab led to reduced autoantibody levels, delayed disease progression, and reduced GC B and PC frequency (78). Furthermore, in a pristane-induced lupus-like model, IL-21 was found to upregulate the expression of a tumor suppressor gene, absent in melanoma 2 (AIM2) by recruiting Tet2 to the AIM2 promoter. This upregulation enhances c-Maf expression, which in turn promotes Tfh differentiation, further exacerbating lupus pathogenesis (79). Collectively, these findings indicate the detrimental role of IL-21 in SLE pathogenesis and highlight its potential as a therapeutic target to alleviate lupus progression.
Figure 2. Impact of cytokines from Tfh cells in pathological conditions. See text for comprehensive details. Illustration produced with BioRender (https://BioRender.com/).
IL-21 influences other diseases through its modulation of immune cell pathways. The suppression of the Pax3-Id3 pathway by IL-21 exacerbates the development of Sjögren’s syndrome by promoting the activity of Tfh cells, leading to heightened severity of disease symptoms (80). In the context of transplantation, IL-21-producing effector Tfh cells have been shown to drive B cell alloimmunity in lymph nodes and kidney allografts, suggesting a mechanism for transplant rejection (81). Furthermore, the role of IL-21 in neurodegenerative diseases has been identified, impacting neuro-immune communication between peripheral systems and the brain, which affects Alzheimer's disease neuropathology and may influence the progression and severity of the disease (82). Virus-specific brain-resident CD8 and CD4 T cells are associated with protection against pathological brain diseases, including progressive multifocal leukoencephalopathy. During persistent viral infections, IL-21 from high-affinity CD4 T cells drives the differentiation of brain-resident CD8 T cells, indicating its critical role in the immune response within the central nervous system (83). This body of evidence collectively highlights IL-21 as a pivotal factor in various autoimmune and neurodegenerative diseases, as well as in immune responses following organ transplantation.
IL-4
In the complex field of allergic immunology, Tfh cells emerge as key players of B cell function and IgE responses, distinct from GATA3hi Th2 cells predominantly found in non-lymphoid tissues (84,85). IL-4 produced by Tfh cells primarily influences B cell differentiation and IgE production, which are critical factors in IgE-mediated allergic diseases (85). The essential role of Tfh cell-derived IL-4 in IgE class switching and Ab production has been demonstrated in helminth models in mice with a Tfh-specific Il4 deficiency, suggesting the specificity of this cytokine's function in allergic responses (19). Tfh2 cells, which predominantly generate IL-4 under specific conditions, promote B cell differentiation into PCs, thereby facilitating IgE class switching and Ab production (86). Additionally, research has unveiled a strong correlation between the number of circulating Tfh2 cells and elevated levels of IgG4 and IL-4, along with an increase in plasmablast numbers in patients with IgG4-related disease (87). Furthermore, IL-4+IL13+CXCR5+ Tfh2 cells have been observed in SLE patients, and IL-4+ Tfh2 cells in Ets1-deficient mice exacerbate SLE with heightened IgE production (88). A distinct population of Tfh cells, referred to as ‘Tfh13’, which is characterized by high levels of IL-4, IL-5, and IL-13 but low IL-21, has been crucial for producing high-affinity IgE and triggering allergen-induced anaphylaxis in both mice and humans sensitive to allergens. These Tfh13 cells, characterized by a unique cytokine profile and simultaneous expression of Bcl6 and GATA3, are absent in cases without allergen-specific IgE or with only low-affinity IgE (89). This association highlights the specialized role of IL-4-producing Tfh cells in humoral immunity, allergic disease, and autoimmune disease pathogenesis. Collectively, these studies provide a comprehensive understanding of how IL-4, particularly when produced by Tfh2 or Tfh13 cells, contributes to the immunological landscape in allergic diseases, offering potential targets for therapeutic interventions.
Other cytokines
CXCL13
CXCL13 is implicated in the formation of ectopic lymphoid-like structures (ELSs) at inflammatory sites in autoimmune diseases (90). These structures functionally resemble GCs and can promote autoreactive B-cell responses and local autoantibody production (91). The CXCL13/CXCR5 axis plays a pivotal role in ELS development, as evidenced by the increased expression of CXCL13 in synovial tissues of rheumatoid arthritis (RA) patients and salivary glands of individuals with Sjögren's syndrome (92,93). This expression correlates with the size and organization of lymphoid aggregates. In humans, GC Tfh cells play a central role in producing CXCL13 (94). Interestingly, in the joints of individuals with RA, CD4+ T cells that express CXCL13 are mainly negative for CXCR5 and BCL6 (95), suggesting that CXCR5lo Tfh-like cells in ELSs may function similarly to CXCL13-expressing Tfh cells. The role of CXCL13 in ELS is similarly active in other autoimmune diseases, including SLE and multiple sclerosis (96,97), indicating that CXCL13 contributes to the pathogenesis of various autoimmune diseases by regulating the development and activity of ELSs.
CXCL13 is also strongly associated with cancer. CXCL13-producing Tfh cells establish a link between immune suppression and adaptive memory in human breast cancer (94). These CXCL13-producing Tfh cells potentially trigger ELS formation and generate GC B cell responses at the tumor site. Interestingly, CXCL13-producing Tfh cells in the ELS are also CXCR5lo CD4 T cells in cancer, such as nasopharyngeal carcinoma and cutaneous melanoma (98,99). In summary, peripheral CXCR5lo Tfh-like cells and Tfh cells that have migrated into the tumor microenvironment are potent CXCL13 producers, leading to the formation of ELSs, and the attraction of more B cells and CXCR5+ T cells to the chronic autoimmune inflammation and tumor microenvironment.
IL-2 and IL-10
Although IL-2 and IL-10 cytokines are known to be produced by Tfh cells, their roles remain unclear, as IL-2 is produced predominantly by activated T cells, and IL-10 is primarily produced by Treg and follicular Tregs (Tfr). In SLE, IL-2 drives the conversion of Tfh to Tfr through transcriptional regulation (100). Thus, low-dose IL-2 has been considered as a therapeutic strategy for SLE because IL-2 is a potent inhibitor of Tfh cells and facilitates the conversion of autoreactive Tfh cells into Tfr cells (101).
IL-10-producing CCR6+ CD4 T cells have been observed in the human tonsil and a pristane-induced SLE mouse model (102). These tonsillar IL-10-producing CCR6+ helper T cells exhibit distinct phenotypes from Tfh cells and do not express BCL6. While these IL-10-producing cells are considered to originate extrafollicular, they provide assistance for Ab production in mouse models and SLE patients (102). Recent research has shown that IL-10-producing Tfh cells accumulate with age and establish a connection between inflammation and age-related immune suppression (67). In aged mice, IL-10-producing CD4 T cells are predominately Tfh cells, and these cells also accumulate during aging in humans. Moreover, IL-10 limits Tfh-dependent vaccine responses in aged mice (67). IL-10 is considered a potent regulatory cytokine of Treg or Tfr, while IL-2 is regarded as a critical factor for the differentiation and maintenance of Treg or Tfr and for the proliferation of conventional CD4 T cells. Consequently, IL-2 and IL-10 may play roles in adjusting the balance between Tfh and Tfr or Tfh and non-Tfh cells in various pathophysiological conditions. Further research is necessary to elucidate the specific functions of IL-2-producing Tfh or IL-10-producing Tfh cells in different disease conditions.
CONCLUDING REMARKS
Cytokines generated by Tfh cells are implicated in humoral immunity and a broad spectrum of diseases, whose etiology is strongly associated with immunological functions of Abs (Table 1 and Fig. 2). A comprehensive exploration of the cytokines produced by both Tfh and Tfh-like CD4 T cells is imperative for elucidating their roles in orchestrating the adaptive immune response and their relevance to various medical conditions. Over the past decade, substantial progress has been achieved in comprehending Tfh cytokines, encompassing both cellular and molecular facets, as well as their implications in disease. Nevertheless, unraveling the precise contributions of individual cytokines or the complex interactions among them to specific diseases remains a formidable and intricate challenge.
Table 1. Summary of cytokine functions in Tfh cell development and B cell regulation.
| Cytokines | Development of Tfh cells | Regulation of B cells |
|---|---|---|
| IL-21 | CD4 T cell expansion Tfh differentiation | B cell proliferation |
| GC B cell differentiation & selection | ||
| Affinity maturation | ||
| Class switching: IgG | ||
| Plasmablasts maturation | ||
| IL-4 | - | GC B cell survival |
| Plasmablast differentiation | ||
| Class switching: IgE, IgG1 (mouse), IgG4 (human) | ||
| Memory B cell generation | ||
| CXCL13 | Attracting CD4 T cells to B cell follicle | Attracting B cells to B cell follicle |
| GC B cell migration to the LZ | ||
| IL-10 | - | Plasma cell differentiation |
| IL-9 | - | Memory B cell generation |
| IL-2 | Tfh suppression | - |
A fuller comprehension of the functions of these cytokines in both health and disease is pivotal for the development of targeted therapeutic interventions. Disease modeling in animal subjects plays a critical role in researching fundamental Tfh biology and discovering potential therapeutics. While Tfh differentiation and function exhibit substantial similarities across species, noteworthy disparities in Tfh biology exist between mice and humans. For instance, human Tfh cells are primary producers of the CXCL13 chemokine, which is not produced by murine Tfh cells (1). Another disparity is the utilization of IL-6 and IL-12 cytokines for the differentiation of mouse and human Tfh cells, respectively (1). Further research endeavors are imperative to establish more accurate animal models for human diseases.
Furthermore, although T helper subsets are typically considered terminally differentiated, they possess a degree of plasticity that allows them to differentiate into other types of helper T cells (103) or multifunctional helper T cells capable of producing various cytokines, particularly in disease conditions. Transcriptional and epigenetic changes play a role in regulating this context-dependent plasticity (104). In-depth mechanistic studies are essential to elucidate the complex molecular interactions that control the expression of cytokines derived from Tfh cells, including the roles of TFs, chromatin regulators, and epigenetic modifications.
In summary, cytokines emanating from Tfh and Tfh-like CD4 T cells serve as pivotal players in shaping the adaptive immune response, influencing various facets of GC biology, and contributing to the pathogenesis of a multitude of diseases. Continued research into the precise mechanisms and regulatory networks governing these cytokines will continue to enhance our understanding of immune regulation and open new avenues for therapeutic interventions.
ACKNOWLEDGEMENTS
This work was supported by the Catholic Medical Center Research Foundation made in the program year 2022 and 2023 (Choi J), by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (2023R1A2C1007319 to Choi J; RS-2023-00258956 to Choi YS), and by National Institutes of Health (NIH) grant, including the National Institute of Allergy and Infectious Diseases (NIAID) P01 AI145815, and internal LJI institutional funds to Crotty S.
Abbreviations
- AIM2
absent in melanoma 2
- BCR
B cell receptor
- DZ
dark zone
- ELS
ectopic lymphoid-like structure
- FDC
follicular dendritic cells
- GC
germinal center
- LZ
light zone
- PC
plasma cell
- RA
rheumatoid arthritis
- SLAM
signaling lymphocytic activation molecule
- SLE
systemic lupus erythematosus
- TF
transcription factor
- Tfh
follicular helper T cells
- Tfr
follicular Tregs
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
- Conceptualization: Choi J, Crotty S, Choi YS.
- Writing - original draft: Choi J.
- Writing - review & editing: Choi J, Crotty S, Choi YS.
References
- 1.Crotty S. T follicular helper cell biology: a decade of discovery and diseases. Immunity. 2019;50:1132–1148. doi: 10.1016/j.immuni.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Victora GD, Nussenzweig MC. Germinal centers. Annu Rev Immunol. 2022;40:413–442. doi: 10.1146/annurev-immunol-120419-022408. [DOI] [PubMed] [Google Scholar]
- 3.Linterman MA, Beaton L, Yu D, Ramiscal RR, Srivastava M, Hogan JJ, Verma NK, Smyth MJ, Rigby RJ, Vinuesa CG. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J Exp Med. 2010;207:353–363. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinstein JS, Herman EI, Lainez B, Licona-Limón P, Esplugues E, Flavell R, Craft J. TFH cells progressively differentiate to regulate the germinal center response. Nat Immunol. 2016;17:1197–1205. doi: 10.1038/ni.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ozaki K, Spolski R, Ettinger R, Kim HP, Wang G, Qi CF, Hwu P, Shaffer DJ, Akilesh S, Roopenian DC, et al. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J Immunol. 2004;173:5361–5371. doi: 10.4049/jimmunol.173.9.5361. [DOI] [PubMed] [Google Scholar]
- 6.Zotos D, Coquet JM, Zhang Y, Light A, D’Costa K, Kallies A, Corcoran LM, Godfrey DI, Toellner KM, Smyth MJ, et al. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J Exp Med. 2010;207:365–378. doi: 10.1084/jem.20091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quast I, Dvorscek AR, Pattaroni C, Steiner TM, McKenzie CI, Pitt C, O’Donnell K, Ding Z, Hill DL, Brink R, et al. Interleukin-21, acting beyond the immunological synapse, independently controls T follicular helper and germinal center B cells. Immunity. 2022;55:1414–1430.e5. doi: 10.1016/j.immuni.2022.06.020. [DOI] [PubMed] [Google Scholar]
- 8.Zotos D, Quast I, Li-Wai-Suen CSN, McKenzie CI, Robinson MJ, Kan A, Smyth GK, Hodgkin PD, Tarlinton DM. The concerted change in the distribution of cell cycle phases and zone composition in germinal centers is regulated by IL-21. Nat Commun. 2021;12:7160. doi: 10.1038/s41467-021-27477-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vinuesa CG, Linterman MA, Yu D, MacLennan ICM. Follicular helper T cells. Annu Rev Immunol. 2016;34:335–368. doi: 10.1146/annurev-immunol-041015-055605. [DOI] [PubMed] [Google Scholar]
- 10.Kwon H, Thierry-Mieg D, Thierry-Mieg J, Kim HP, Oh J, Tunyaplin C, Carotta S, Donovan CE, Goldman ML, Tailor P, et al. Analysis of interleukin-21-induced Prdm1 gene regulation reveals functional cooperation of STAT3 and IRF4 transcription factors. Immunity. 2009;31:941–952. doi: 10.1016/j.immuni.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scheeren FA, Naspetti M, Diehl S, Schotte R, Nagasawa M, Wijnands E, Gimeno R, Vyth-Dreese FA, Blom B, Spits H. STAT5 regulates the self-renewal capacity and differentiation of human memory B cells and controls Bcl-6 expression. Nat Immunol. 2005;6:303–313. doi: 10.1038/ni1172. [DOI] [PubMed] [Google Scholar]
- 12.Crotty S, Johnston RJ, Schoenberger SP. Effectors and memories: Bcl-6 and Blimp-1 in T and B lymphocyte differentiation. Nat Immunol. 2010;11:114–120. doi: 10.1038/ni.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Z, Cui Y, Yao Y, Liu B, Yunis J, Gao X, Wang N, Cañete PF, Tuong ZK, Sun H, et al. Heparan sulfate regulates IL-21 bioavailability and signal strength that control germinal center B cell selection and differentiation. Sci Immunol. 2023;8:eadd1728. doi: 10.1126/sciimmunol.add1728. [DOI] [PubMed] [Google Scholar]
- 14.Moens L, Tangye SG. Cytokine-mediated regulation of plasma cell generation: IL-21 takes center stage. Front Immunol. 2014;5:65. doi: 10.3389/fimmu.2014.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Tech L, George LA, Acs A, Durrett RE, Hess H, Walker LS, Tarlinton DM, Fletcher AL, Hauser AE, et al. Plasma cell output from germinal centers is regulated by signals from Tfh and stromal cells. J Exp Med. 2018;215:1227–1243. doi: 10.1084/jem.20160832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo W, Conter L, Elsner RA, Smita S, Weisel F, Callahan D, Wu S, Chikina M, Shlomchik M. IL-21R signal reprogramming cooperates with CD40 and BCR signals to select and differentiate germinal center B cells. Sci Immunol. 2023;8:eadd1823. doi: 10.1126/sciimmunol.add1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suto A, Nakajima H, Hirose K, Suzuki K, Kagami S, Seto Y, Hoshimoto A, Saito Y, Foster DC, Iwamoto I. Interleukin 21 prevents antigen-induced IgE production by inhibiting germ line C(ε) transcription of IL-4-stimulated B cells. Blood. 2002;100:4565–4573. doi: 10.1182/blood-2002-04-1115. [DOI] [PubMed] [Google Scholar]
- 18.Yang Z, Wu CM, Targ S, Allen CDC. IL-21 is a broad negative regulator of IgE class switch recombination in mouse and human B cells. J Exp Med. 2020;217:e20190472. doi: 10.1084/jem.20190472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meli AP, Fontés G, Leung Soo C, King IL. T follicular helper cell-derived IL-4 is required for IgE production during intestinal helminth infection. J Immunol. 2017;199:244–252. doi: 10.4049/jimmunol.1700141. [DOI] [PubMed] [Google Scholar]
- 20.Ise W, Fujii K, Shiroguchi K, Ito A, Kometani K, Takeda K, Kawakami E, Yamashita K, Suzuki K, Okada T, et al. T follicular helper cell-germinal center B cell interaction strength regulates entry into plasma cell or recycling germinal center cell fate. Immunity. 2018;48:702–715.e4. doi: 10.1016/j.immuni.2018.03.027. [DOI] [PubMed] [Google Scholar]
- 21.Kallies A, Hasbold J, Fairfax K, Pridans C, Emslie D, McKenzie BS, Lew AM, Corcoran LM, Hodgkin PD, Tarlinton DM, et al. Initiation of plasma-cell differentiation is independent of the transcription factor Blimp-1. Immunity. 2007;26:555–566. doi: 10.1016/j.immuni.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Diehl SA, Schmidlin H, Nagasawa M, van Haren SD, Kwakkenbos MJ, Yasuda E, Beaumont T, Scheeren FA, Spits H. STAT3-mediated up-regulation of BLIMP1 is coordinated with BCL6 down-regulation to control human plasma cell differentiation. J Immunol. 2008;180:4805–4815. doi: 10.4049/jimmunol.180.7.4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klein U, Dalla-Favera R. Germinal centres: role in B-cell physiology and malignancy. Nat Rev Immunol. 2008;8:22–33. doi: 10.1038/nri2217. [DOI] [PubMed] [Google Scholar]
- 24.Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, Wang YH, Watowich SS, Jetten AM, Tian Q, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vogelzang A, McGuire HM, Yu D, Sprent J, Mackay CR, King C. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 2008;29:127–137. doi: 10.1016/j.immuni.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Eto D, Lao C, DiToro D, Barnett B, Escobar TC, Kageyama R, Yusuf I, Crotty S. IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PLoS One. 2011;6:e17739. doi: 10.1371/journal.pone.0017739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rasheed MAU, Latner DR, Aubert RD, Gourley T, Spolski R, Davis CW, Langley WA, Ha SJ, Ye L, Sarkar S, et al. Interleukin-21 is a critical cytokine for the generation of virus-specific long-lived plasma cells. J Virol. 2013;87:7737–7746. doi: 10.1128/JVI.00063-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fazilleau N, McHeyzer-Williams LJ, Rosen H, McHeyzer-Williams MG. The function of follicular helper T cells is regulated by the strength of T cell antigen receptor binding. Nat Immunol. 2009;10:375–384. doi: 10.1038/ni.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Linterman MA, Vinuesa CG. Signals that influence T follicular helper cell differentiation and function. Semin Immunopathol. 2010;32:183–196. doi: 10.1007/s00281-009-0194-z. [DOI] [PubMed] [Google Scholar]
- 30.Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, Foucat E, Dullaers M, Oh S, Sabzghabaei N, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34:108–121. doi: 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, Mitsdoerffer M, Strom TB, Elyaman W, Ho IC, et al. IL-4 inhibits TGF-β-induced Foxp3+ T cells and, together with TGF-β, generates IL-9+ IL-10+ Foxp3(-) effector T cells. Nat Immunol. 2008;9:1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hiramatsu Y, Suto A, Kashiwakuma D, Kanari H, Kagami S, Ikeda K, Hirose K, Watanabe N, Grusby MJ, Iwamoto I, et al. c-Maf activates the promoter and enhancer of the IL-21 gene, and TGF-beta inhibits c-Maf-induced IL-21 production in CD4+ T cells. J Leukoc Biol. 2010;87:703–712. doi: 10.1189/jlb.0909639. [DOI] [PubMed] [Google Scholar]
- 33.Bocharnikov AV, Keegan J, Wacleche VS, Cao Y, Fonseka CY, Wang G, Muise ES, Zhang KX, Arazi A, Keras G, et al. PD-1hiCXCR5- T peripheral helper cells promote B cell responses in lupus via MAF and IL-21. JCI Insight. 2019;4:e130062. doi: 10.1172/jci.insight.130062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bauquet AT, Jin H, Paterson AM, Mitsdoerffer M, Ho IC, Sharpe AH, Kuchroo VK. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat Immunol. 2009;10:167–175. doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ise W, Kohyama M, Schraml BU, Zhang T, Schwer B, Basu U, Alt FW, Tang J, Oltz EM, Murphy TL, et al. The transcription factor BATF controls the global regulators of class-switch recombination in both B cells and T cells. Nat Immunol. 2011;12:536–543. doi: 10.1038/ni.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kroenke MA, Eto D, Locci M, Cho M, Davidson T, Haddad EK, Crotty S. Bcl6 and Maf cooperate to instruct human follicular helper CD4 T cell differentiation. J Immunol. 2012;188:3734–3744. doi: 10.4049/jimmunol.1103246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suto A, Kashiwakuma D, Kagami S, Hirose K, Watanabe N, Yokote K, Saito Y, Nakayama T, Grusby MJ, Iwamoto I, et al. Development and characterization of IL-21-producing CD4+ T cells. J Exp Med. 2008;205:1369–1379. doi: 10.1084/jem.20072057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmitt N, Liu Y, Bentebibel SE, Munagala I, Bourdery L, Venuprasad K, Banchereau J, Ueno H. The cytokine TGF-β co-opts signaling via STAT3-STAT4 to promote the differentiation of human TFH cells. Nat Immunol. 2014;15:856–865. doi: 10.1038/ni.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Locci M, Wu JE, Arumemi F, Mikulski Z, Dahlberg C, Miller AT, Crotty S. Activin A programs the differentiation of human TFH cells. Nat Immunol. 2016;17:976–984. doi: 10.1038/ni.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma CS, Suryani S, Avery DT, Chan A, Nanan R, Santner-Nanan B, Deenick EK, Tangye SG. Early commitment of naïve human CD4(+) T cells to the T follicular helper (T(FH)) cell lineage is induced by IL-12. Immunol Cell Biol. 2009;87:590–600. doi: 10.1038/icb.2009.64. [DOI] [PubMed] [Google Scholar]
- 41.Ma CS, Avery DT, Chan A, Batten M, Bustamante J, Boisson-Dupuis S, Arkwright PD, Kreins AY, Averbuch D, Engelhard D, et al. Functional STAT3 deficiency compromises the generation of human T follicular helper cells. Blood. 2012;119:3997–4008. doi: 10.1182/blood-2011-11-392985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmitt N, Ueno H. Blood Tfh cells come with colors. Immunity. 2013;39:629–630. doi: 10.1016/j.immuni.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi J, Diao H, Faliti CE, Truong J, Rossi M, Bélanger S, Yu B, Goldrath AW, Pipkin ME, Crotty S. Bcl-6 is the nexus transcription factor of T follicular helper cells via repressor-of-repressor circuits. Nat Immunol. 2020;21:777–789. doi: 10.1038/s41590-020-0706-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choi J, Crotty S. Bcl6-mediated transcriptional regulation of follicular helper T cells (TFH) Trends Immunol. 2021;42:336–349. doi: 10.1016/j.it.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Olson WJ, Jakic B, Labi V, Schoeler K, Kind M, Klepsch V, Baier G, Hermann-Kleiter N. Orphan nuclear receptor NR2F6 suppresses T follicular helper cell accumulation through regulation of IL-21. Cell Reports. 2019;28:2878–2891.e5. doi: 10.1016/j.celrep.2019.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim YJ, Oh J, Jung S, Kim CJ, Choi J, Jeon YK, Kim HJ, Kim JW, Suh CH, Lee Y, et al. The transcription factor Mef2d regulates B:T synapse-dependent GC-TFH differentiation and IL-21-mediated humoral immunity. Sci Immunol. 2023;8:eadf2248. doi: 10.1126/sciimmunol.adf2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 48.Lee SK, Rigby RJ, Zotos D, Tsai LM, Kawamoto S, Marshall JL, Ramiscal RR, Chan TD, Gatto D, Brink R, et al. B cell priming for extrafollicular antibody responses requires Bcl-6 expression by T cells. J Exp Med. 2011;208:1377–1388. doi: 10.1084/jem.20102065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miyauchi K, Adachi Y, Tonouchi K, Yajima T, Harada Y, Fukuyama H, Deno S, Iwakura Y, Yoshimura A, Hasegawa H, et al. Influenza virus infection expands the breadth of antibody responses through IL-4 signalling in B cells. Nat Commun. 2021;12:3789. doi: 10.1038/s41467-021-24090-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duan L, Liu D, Chen H, Mintz MA, Chou MY, Kotov DI, Xu Y, An J, Laidlaw BJ, Cyster JG. Follicular dendritic cells restrict interleukin-4 availability in germinal centers and foster memory B cell generation. Immunity. 2021;54:2256–2272.e6. doi: 10.1016/j.immuni.2021.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shehata L, Thouvenel CD, Hondowicz BD, Pew LA, Rawlings DJ, Choi J, Pepper M. IL-4 downregulates BCL6 to promote memory B cell selection in germinal centers. 2023. [DOI] [PMC free article] [PubMed]
- 52.Kubo M. The role of IL-4 derived from T follicular helper cells and TH2 cells. Int Immunol. 2021;33:717–772. doi: 10.1093/intimm/dxab080. [DOI] [PubMed] [Google Scholar]
- 53.Yusuf I, Kageyama R, Monticelli L, Johnston RJ, DiToro D, Hansen K, Barnett B, Crotty S. Germinal center T follicular helper cell IL-4 production is dependent on signaling lymphocytic activation molecule receptor (CD150) J Immunol. 2010;185:190–202. doi: 10.4049/jimmunol.0903505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim JI, Ho IC, Grusby MJ, Glimcher LH. The transcription factor c-Maf controls the production of interleukin-4 but not other Th2 cytokines. Immunity. 1999;10:745–751. doi: 10.1016/s1074-7613(00)80073-4. [DOI] [PubMed] [Google Scholar]
- 55.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harada Y, Tanaka S, Motomura Y, Harada Y, Ohno S, Ohno S, Yanagi Y, Inoue H, Kubo M. The 3′ enhancer CNS2 is a critical regulator of interleukin-4-mediated humoral immunity in follicular helper T cells. Immunity. 2012;36:188–200. doi: 10.1016/j.immuni.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 57.Vijayanand P, Seumois G, Simpson LJ, Abdul-Wajid S, Baumjohann D, Panduro M, Huang X, Interlandi J, Djuretic IM, Brown DR, et al. Interleukin-4 production by follicular helper T cells requires the conserved Il4 enhancer hypersensitivity site V. Immunity. 2012;36:175–187. doi: 10.1016/j.immuni.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sahoo A, Alekseev A, Tanaka K, Obertas L, Lerman B, Haymaker C, Clise-Dwyer K, McMurray JS, Nurieva R. Batf is important for IL-4 expression in T follicular helper cells. Nat Commun. 2015;6:7997. doi: 10.1038/ncomms8997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li B, Tournier C, Davis RJ, Flavell RA. Regulation of IL-4 expression by the transcription factor JunB during T helper cell differentiation. EMBO J. 1999;18:420–432. doi: 10.1093/emboj/18.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rengarajan J, Mowen KA, McBride KD, Smith ED, Singh H, Glimcher LH. Interferon regulatory factor 4 (IRF4) interacts with NFATc2 to modulate interleukin 4 gene expression. J Exp Med. 2002;195:1003–1012. doi: 10.1084/jem.20011128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kuwahara M, Ise W, Ochi M, Suzuki J, Kometani K, Maruyama S, Izumoto M, Matsumoto A, Takemori N, Takemori A, et al. Bach2-Batf interactions control Th2-type immune response by regulating the IL-4 amplification loop. Nat Commun. 2016;7:12596. doi: 10.1038/ncomms12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu H, Xu LL, Teuscher P, Liu H, Kaplan MH, Dent AL. An Inhibitory Role for the Transcription Factor Stat3 in Controlling IL-4 and Bcl6 Expression in Follicular Helper T Cells. J Immunol. 2015;195:2080–2089. doi: 10.4049/jimmunol.1500335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Naoe Y, Setoguchi R, Akiyama K, Muroi S, Kuroda M, Hatam F, Littman DR, Taniuchi I. Repression of interleukin-4 in T helper type 1 cells by Runx/Cbf beta binding to the Il4 silencer. J Exp Med. 2007;204:1749–1755. doi: 10.1084/jem.20062456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rasheed AU, Rahn HP, Sallusto F, Lipp M, Müller G. Follicular B helper T cell activity is confined to CXCR5(hi)ICOS(hi) CD4 T cells and is independent of CD57 expression. Eur J Immunol. 2006;36:1892–1903. doi: 10.1002/eji.200636136. [DOI] [PubMed] [Google Scholar]
- 65.Bryant VL, Ma CS, Avery DT, Li Y, Good KL, Corcoran LM, de Waal Malefyt R, Tangye SG. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J Immunol. 2007;179:8180–8190. doi: 10.4049/jimmunol.179.12.8180. [DOI] [PubMed] [Google Scholar]
- 66.Xin G, Zander R, Schauder DM, Chen Y, Weinstein JS, Drobyski WR, Tarakanova V, Craft J, Cui W. Single-cell RNA sequencing unveils an IL-10-producing helper subset that sustains humoral immunity during persistent infection. Nat Commun. 2018;9:5037. doi: 10.1038/s41467-018-07492-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Almanan M, Raynor J, Ogunsulire I, Malyshkina A, Mukherjee S, Hummel SA, Ingram JT, Saini A, Xie MM, Alenghat T, et al. IL-10-producing Tfh cells accumulate with age and link inflammation with age-related immune suppression. Sci Adv. 2020;6:eabb0806. doi: 10.1126/sciadv.abb0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Y, Shi J, Yan J, Xiao Z, Hou X, Lu P, Hou S, Mao T, Liu W, Ma Y, et al. Germinal-center development of memory B cells driven by IL-9 from follicular helper T cells. Nat Immunol. 2017;18:921–930. doi: 10.1038/ni.3788. [DOI] [PubMed] [Google Scholar]
- 69.Takatsuka S, Yamada H, Haniuda K, Saruwatari H, Ichihashi M, Renauld JC, Kitamura D. IL-9 receptor signaling in memory B cells regulates humoral recall responses. Nat Immunol. 2018;19:1025–1034. doi: 10.1038/s41590-018-0177-0. [DOI] [PubMed] [Google Scholar]
- 70.Do-Thi VA, Lee JO, Lee H, Kim YS. Crosstalk between the producers and immune targets of IL-9. Immune Netw. 2020;20:e45. doi: 10.4110/in.2020.20.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ballesteros-Tato A, León B, Graf BA, Moquin A, Adams PS, Lund FE, Randall TD. Interleukin-2 inhibits germinal center formation by limiting T follicular helper cell differentiation. Immunity. 2012;36:847–856. doi: 10.1016/j.immuni.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.DiToro D, Winstead CJ, Pham D, Witte S, Andargachew R, Singer JR, Wilson CG, Zindl CL, Luther RJ, Silberger DJ, et al. Differential IL-2 expression defines developmental fates of follicular versus nonfollicular helper T cells. Science. 2018;361:eaao2933. doi: 10.1126/science.aao2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Papillion A, Powell MD, Chisolm DA, Bachus H, Fuller MJ, Weinmann AS, Villarino A, O’Shea JJ, León B, Oestreich KJ, et al. Inhibition of IL-2 responsiveness by IL-6 is required for the generation of GC-TFH cells. Sci Immunol. 2019;4:eaaw7636. doi: 10.1126/sciimmunol.aaw7636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sheikh AA, Groom JR. Transcription tipping points for T follicular helper cell and T-helper 1 cell fate commitment. Cell Mol Immunol. 2021;18:528–538. doi: 10.1038/s41423-020-00554-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang S, Wang J, Kumar V, Karnell JL, Naiman B, Gross PS, Rahman S, Zerrouki K, Hanna R, Morehouse C, et al. IL-21 drives expansion and plasma cell differentiation of autoreactive CD11chiT-bet+ B cells in SLE. Nat Commun. 2018;9:1758. doi: 10.1038/s41467-018-03750-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Herber D, Brown TP, Liang S, Young DA, Collins M, Dunussi-Joannopoulos K. IL-21 has a pathogenic role in a lupus-prone mouse model and its blockade with IL-21R.Fc reduces disease progression. J Immunol. 2007;178:3822–3830. doi: 10.4049/jimmunol.178.6.3822. [DOI] [PubMed] [Google Scholar]
- 77.Odegard JM, Marks BR, DiPlacido LD, Poholek AC, Kono DH, Dong C, Flavell RA, Craft J. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. J Exp Med. 2008;205:2873–2886. doi: 10.1084/jem.20080840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Choi JY, Seth A, Kashgarian M, Terrillon S, Fung E, Huang L, Wang LC, Craft J. Disruption of pathogenic cellular networks by IL-21 blockade leads to disease amelioration in murine lupus. J Immunol. 2017;198:2578–2588. doi: 10.4049/jimmunol.1601687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wu H, Deng Y, Long D, Yang M, Li Q, Feng Y, Chen Y, Qiu H, Huang X, He Z, et al. The IL-21-TET2-AIM2-c-MAF pathway drives the T follicular helper cell response in lupus-like disease. Clin Transl Med. 2022;12:e781. doi: 10.1002/ctm2.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Park JS, Kim SM, Choi J, Jung KA, Hwang SH, Yang S, Kwok SK, Cho ML, Park SH. Interleukin-21-mediated suppression of the Pax3-Id3 pathway exacerbates the development of Sjögren’s syndrome via follicular helper T cells. Cytokine. 2020;125:154834. doi: 10.1016/j.cyto.2019.154834. [DOI] [PubMed] [Google Scholar]
- 81.Zhang H, Cavazzoni CB, Podestà MA, Bechu ED, Ralli G, Chandrakar P, Lee JM, Sayin I, Tullius SG, Abdi R, et al. IL-21-producing effector Tfh cells promote B cell alloimmunity in lymph nodes and kidney allografts. JCI Insight. 2023;8:e169793. doi: 10.1172/jci.insight.169793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Agrawal S, Baulch JE, Madan S, Salah S, Cheeks SN, Krattli RP, Jr, Subramanian VS, Acharya MM, Agrawal A. Impact of IL-21-associated peripheral and brain crosstalk on the Alzheimer’s disease neuropathology. Cell Mol Life Sci. 2022;79:331. doi: 10.1007/s00018-022-04347-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ren HM, Kolawole EM, Ren M, Jin G, Netherby-Winslow CS, Wade Q, Shwetank, Rahman ZS, Evavold BD, Lukacher AE. IL-21 from high-affinity CD4 T cells drives differentiation of brain-resident CD8 T cells during persistent viral infection. Sci Immunol. 2020;5:eabb5590. doi: 10.1126/sciimmunol.abb5590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Reinhardt RL, Liang HE, Locksley RM. Cytokine-secreting follicular T cells shape the antibody repertoire. Nat Immunol. 2009;10:385–393. doi: 10.1038/ni.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liang HE, Reinhardt RL, Bando JK, Sullivan BM, Ho IC, Locksley RM. Divergent expression patterns of IL-4 and IL-13 define unique functions in allergic immunity. Nat Immunol. 2011;13:58–66. doi: 10.1038/ni.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Olatunde AC, Hale JS, Lamb TJ. Cytokine-skewed Tfh cells: functional consequences for B cell help. Trends Immunol. 2021;42:536–550. doi: 10.1016/j.it.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Akiyama M, Suzuki K, Yamaoka K, Yasuoka H, Takeshita M, Kaneko Y, Kondo H, Kassai Y, Miyazaki T, Morita R, et al. Number of circulating follicular helper 2 T cells correlates with IgG4 and interleukin-4 levels and plasmablast numbers in IgG4-related disease. Arthritis Rheumatol. 2015;67:2476–2481. doi: 10.1002/art.39209. [DOI] [PubMed] [Google Scholar]
- 88.Kim CJ, Lee CG, Jung JY, Ghosh A, Hasan SN, Hwang SM, Kang H, Lee C, Kim GC, Rudra D, et al. The transcription factor Ets1 suppresses T follicular helper type 2 cell differentiation to halt the onset of systemic lupus erythematosus. Immunity. 2018;49:1034–1048.e8. doi: 10.1016/j.immuni.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 89.Gowthaman U, Chen JS, Zhang B, Flynn WF, Lu Y, Song W, Joseph J, Gertie JA, Xu L, Collet MA, et al. Identification of a T follicular helper cell subset that drives anaphylactic IgE. Science. 2019;365:eaaw6433. doi: 10.1126/science.aaw6433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pan Z, Zhu T, Liu Y, Zhang N. Role of the CXCL13/CXCR5 axis in autoimmune diseases. Front Immunol. 2022;13:850998. doi: 10.3389/fimmu.2022.850998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bombardieri M, Lewis M, Pitzalis C. Ectopic lymphoid neogenesis in rheumatic autoimmune diseases. Nat Rev Rheumatol. 2017;13:141–154. doi: 10.1038/nrrheum.2016.217. [DOI] [PubMed] [Google Scholar]
- 92.Bugatti S, Manzo A, Vitolo B, Benaglio F, Binda E, Scarabelli M, Humby F, Caporali R, Pitzalis C, Montecucco C. High expression levels of the B cell chemoattractant CXCL13 in rheumatoid synovium are a marker of severe disease. Rheumatology (Oxford) 2014;53:1886–1895. doi: 10.1093/rheumatology/keu163. [DOI] [PubMed] [Google Scholar]
- 93.Colafrancesco S, Priori R, Smith CG, Minniti A, Iannizzotto V, Pipi E, Lucchesi D, Pontarini E, Nayar S, Campos J, et al. CXCL13 as biomarker for histological involvement in Sjögren’s syndrome. Rheumatology (Oxford) 2020;59:165–170. doi: 10.1093/rheumatology/kez255. [DOI] [PubMed] [Google Scholar]
- 94.Gu-Trantien C, Migliori E, Buisseret L, de Wind A, Brohée S, Garaud S, Noël G, Dang Chi VL, Lodewyckx JN, Naveaux C, et al. CXCL13-producing TFH cells link immune suppression and adaptive memory in human breast cancer. JCI Insight. 2017;2:e91487. doi: 10.1172/jci.insight.91487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Manzo A, Vitolo B, Humby F, Caporali R, Jarrossay D, Dell’accio F, Ciardelli L, Uguccioni M, Montecucco C, Pitzalis C. Mature antigen-experienced T helper cells synthesize and secrete the B cell chemoattractant CXCL13 in the inflammatory environment of the rheumatoid joint. Arthritis Rheum. 2008;58:3377–3387. doi: 10.1002/art.23966. [DOI] [PubMed] [Google Scholar]
- 96.Serafini B, Rosicarelli B, Magliozzi R, Stigliano E, Aloisi F. Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol. 2004;14:164–174. doi: 10.1111/j.1750-3639.2004.tb00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Steinmetz OM, Velden J, Kneissler U, Marx M, Klein A, Helmchen U, Stahl RA, Panzer U. Analysis and classification of B-cell infiltrates in lupus and ANCA-associated nephritis. Kidney Int. 2008;74:448–457. doi: 10.1038/ki.2008.191. [DOI] [PubMed] [Google Scholar]
- 98.Hoellwerth M, Koelblinger P, Lang R, Harrer A. Revisiting the role of the CXCL13/CXCR5-associated immune axis in melanoma: potential implications for anti-PD-1-related biomarker research. Life (Basel) 2023;13:553. doi: 10.3390/life13020553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li JP, Wu CY, Chen MY, Liu SX, Yan SM, Kang YF, Sun C, Grandis JR, Zeng MS, Zhong Q. PD-1+CXCR5-CD4+ Th-CXCL13 cell subset drives B cells into tertiary lymphoid structures of nasopharyngeal carcinoma. J Immunother Cancer. 2021;9:e002101. doi: 10.1136/jitc-2020-002101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hao H, Nakayamada S, Yamagata K, Ohkubo N, Iwata S, Inoue Y, et al. IL-2 drives conversion of T follicular helper cells to T follicular regulatory cells through transcriptional regulation in systemic lupus erythematosus. Arthritis Rheumatol. 2020;73:132–142. doi: 10.1002/art.41457. [DOI] [PubMed] [Google Scholar]
- 101.He J, Zhang X, Wei Y, Sun X, Chen Y, Deng J, Jin Y, Gan Y, Hu X, Jia R, et al. Low-dose interleukin-2 treatment selectively modulates CD4(+) T cell subsets in patients with systemic lupus erythematosus. Nat Med. 2016;22:991–993. doi: 10.1038/nm.4148. [DOI] [PubMed] [Google Scholar]
- 102.Facciotti F, Larghi P, Bosotti R, Vasco C, Gagliani N, Cordiglieri C, Mazzara S, Ranzani V, Rottoli E, Curti S, et al. Evidence for a pathogenic role of extrafollicular, IL-10-producing CCR6+B helper T cells in systemic lupus erythematosus. Proc Natl Acad Sci U S A. 2020;117:7305–7316. doi: 10.1073/pnas.1917834117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Crotty S. Do memory CD4 T cells keep their cell-type programming: plasticity versus fate commitment? complexities of interpretation due to the heterogeneity of memory CD4 T cells, including T follicular helper cells. Cold Spring Harb Perspect Biol. 2018;10:a032102. doi: 10.1101/cshperspect.a032102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Friedman MJ, Lee H, Lee JY, Oh S. Transcriptional and epigenetic regulation of context-dependent plasticity in T-helper lineages. Immune Netw. 2023;23:e5. doi: 10.4110/in.2023.23.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]