Abstract
NF-κB corresponds to an inducible eukaryotic transcription factor complex that is negatively regulated in resting cells by its physical assembly with a family of cytoplasmic ankyrin-rich inhibitors termed IκB. Stimulation of cells with various proinflammatory cytokines, including tumor necrosis factor alpha (TNF-α), induces nuclear NF-κB expression. TNF-α signaling involves the recruitment of at least three proteins (TRADD, RIP, and TRAF2) to the type 1 TNF-α receptor tail, leading to the sequential activation of the downstream NF-κB-inducing kinase (NIK) and IκB-specific kinases (IKKα and IKKβ). When activated, IKKα and IKKβ directly phosphorylate the two N-terminal regulatory serines within IκBα, triggering ubiquitination and rapid degradation of this inhibitor in the 26S proteasome. This process liberates the NF-κB complex, allowing it to translocate to the nucleus. In studies of NIK, we found that Thr-559 located within the activation loop of its kinase domain regulates NIK action. Alanine substitution of Thr-559 but not other serine or threonine residues within the activation loop abolishes its activity and its ability to phosphorylate and activate IKKα. Such a NIK-T559A mutant also dominantly interferes with TNF-α induction of NF-κB. We also found that ectopically expressed NIK both spontaneously forms oligomers and displays a high level of constitutive activity. Analysis of a series of NIK deletion mutants indicates that multiple subregions of the kinase participate in the formation of these NIK-NIK oligomers. NIK also physically assembles with downstream IKKα; however, this interaction is mediated through a discrete C-terminal domain within NIK located between amino acids 735 and 947. When expressed alone, this C-terminal NIK fragment functions as a potent inhibitor of TNF-α-mediated induction of NF-κB and alone is sufficient to disrupt the physical association of NIK and IKKα. Together, these findings provide new insights into the molecular basis for TNF-α signaling, suggesting an important role for heterotypic and possibly homotypic interactions of NIK in this response.
The eukaryotic NF-κB/Rel family of transcription factors plays an essential role in the regulation of both inflammatory and immune responses (2, 3). One of the distinguishing characteristics of the NF-κB/Rel transcription factor is its posttranslational regulation through interactions with a series of cytoplasmic inhibitory proteins termed IκB. IκBα corresponds to the major IκB species bound to the prototypic NF-κB (p50-p65) heterodimeric complex. A variety of signals induce nuclear expression of NF-κB, including the proinflammatory cytokines tumor necrosis factor alpha (TNF-α) and interleukin-1β (IL-1β), bacterial lipopolysaccharide, phorbol myristate acetate, CD3 and CD28 costimulation, phosphatase inhibitors such as okadaic acid and calyculin, and various viral proteins, including human T-cell leukemia virus type 1 (HTLV-1) Tax (for a review, see references 34 and 37). These stimuli lead to the phosphorylation of IκBα on serines 32 and 36 (4, 5, 33, 36, 39). The phosphorylated IκBα is then rapidly ubiquitinated on lysine 20 or 21 and subsequently degraded by the 26S proteasome complex (8, 30). The degradation of IκBα unmasks the nuclear localization signal of the NF-κB heterodimer, allowing its rapid translocation into the nucleus, where it engages cognate κB enhancer elements and modulates the transcription of various NF-κB-responsive target genes.
By using biochemical fractionation techniques, Chen et al. (9) first showed that IκB kinase activity is associated with a 700- to 900-kDa multiprotein complex and, further, suggested that activation of this kinase was dependent upon both ubiquitination (9) and phosphorylation by MEKK1 (19). More recently, two cytokine-induced IκB-specific kinases, IKKα and IKKβ, have been isolated and characterized (12, 24, 27, 40, 43). IKKα corresponds to an 85-kDa protein containing an amino-terminal kinase domain and two protein-protein interaction motifs situated at the carboxyl terminus, specifically, a leucine zipper and a helix-loop-helix domain. IKKα was originally cloned as CHUK (conserved helix-loop-helix ubiquitous kinase) (11). IKKβ is an 87-kDa protein that shares 52% homology and overall structural similarity with IKKα. IKKα and IKKβ form both homo- and heterodimers in vivo through their leucine zipper domains (40, 43), although heterodimer formation may be favored. Both IKKα and IKKβ phosphorylate IκBα at both serines 32 and 36 in response to the proinflammatory cytokines TNF-α and IL-1β
TNF-α binding to the type I TNF-α receptor (TNFR1) promotes receptor trimerization and recruitment of the TRADD adapter molecule and the serine/threonine kinase RIP to its cytoplasmic tail (14, 16, 35). However, kinase-deficient forms of RIP are as effective as the wild-type kinase in activating NF-κB, suggesting that this protein plays a structural rather than an enzymatic role in the TNF-α response (14, 35). TRADD also stimulates TRAF2 association with the TNFR1 complex (15). In contrast, TRAF2 is directly recruited to the cytoplasmic tail of TNFR2, leading to its oligomerization (28, 29). TRAF2 binds to a serine/threonine-specific NF-κB-inducing kinase (NIK) (23). NIK either directly or indirectly activates the IKKα-IKKβ complex, leading to IκB phosphorylation and degradation and NF-κB activation (23, 27, 40). Although IL-1 binds to a different membrane receptor, adapter proteins are similarly recruited in a ligand-dependent manner. Specifically, the MyD88 adaptor and the serine/threonine kinases IRAK and IRAK-2 (6, 25, 38) bind to the tail of the IL-1 receptor and its accessory protein, ACP. These proteins recruit TRAF6, a homologue of TRAF2, to the IL-1 receptor complex (7), leading to the activation of NIK. Members of the TRAF family may similarly mediate signaling through other TNF receptor superfamily members, including CD40 (10, 17), CD30 (13, 20), and the lymphotoxin β receptor (26). The fact that NIK is able to interact with all of the known members of the TRAF family (32) suggests that multiple TRAF-dependent receptor signaling pathways may converge on NIK. Moreover, the finding that TNF continues to activate NF-κB in TRAF2-deficient cells (42) raises the possibility of functional redundancy at the level of TRAFs. However, TNF-α signaling leading to NF-κB activation is maintained in cells from transgenic animals expressing a dominant negative mutant form of TRAF2 (21), arguing that TRAF2 may be dispensable for NF-κB activation by TNF-α. In contrast, cells from RIP knockout animals display a clear deficiency in TNF-α induction of NF-κB, arguing that this protein is centrally involved (18).
NIK shares homology with mitogen-activated protein (MAP) kinase kinase kinases (MAP3Ks) (23). NIK physically associates with and activates both IKKα and IKKβ (27, 40). However, NIK only phosphorylates IKKα, and not IKKβ, in vitro (22). The precise mechanism by which NIK is activated and regulated remains elusive. Further, the nature of the association of NIK with IKKα is poorly understood. In this paper, we also explore molecular determinants in NIK which regulate its activation and physical interplay with IKKα. We show that Thr-559 within the activation loop of NIK plays a critical role in regulating the activation of this kinase. We further show that biologically active NIK forms dimers and oligomers. Finally, we demonstrated that the carboxyl-terminal domain of NIK mediates its assembly with IKKα.
MATERIALS AND METHODS
Expression vectors, biological reagents, and cell lines.
Plasmid κB-TATA-luciferase has been previously described (33). The LacZ reporter construct containing the Rous sarcoma virus long terminal repeat (6RZ) was obtained from D. Pearce (University of California, San Francisco) and has been previously described.
NIK cDNA was generated in three fragments by reverse transcription-PCR by using Jurkat E6-1 mRNA and the following three pairs of primers based on the sequence published by Malinin et al. (23): 1, 5′-CCTGGGATCCATGGCAGTGATGGAAATGGCCTGCCCAG-3′ and 5′-GATTCTAGAGCCCCGTGCTGCCCAGGTCTTGGC-3′; 2, 5′-CGGTCTAGATCCCGGGAGCCCAGCCCCAAAACT-3′ and 5′-GACGTCGACCTTGGCGTCGCAGCTCCTGCC-3′; 3, 5′-AAGGTCGACGTCTGGAGCAGCTGCTGTATG-3′ and 5′-TTAGAATTCAACTAGTCATGGGCCTGTTCTCCAGCTGGCC-3′. The PCR fragments were subcloned into pBluescript as follows: 1, BamHI/XbaI; 2, XbaI/SalI; 3, SalI/EcoRI. The full-length NIK cDNA was reconstituted by sequentially subcloning the BamHI/XbaI, XbaI/SalI, and SalI/EcoRI fragments into the pRK vector (provided by Allan Hall, University College London) in frame with an N-terminal myc epitope tag, thus producing a full-length NIK expression vector designated pRK-Myc-NIK. The BamHI/SpeI fragment, which contains the full-length NIK cDNA, was further subcloned into the BamHI/NheI sites of pEV3S in frame with a C-terminal T7 epitope tag and was designated pEV-NIK-T7. Two NIK kinase domain mutant proteins containing alanine substitutions for two lysines involved in ATP binding in the kinase domain (NIK-KK429/430AA) or three serine and threonine residues located within the activation loop of NIK (NIK-S549A/T552A/T559A) were generated by overlapping PCR. Murine IKKα/CHUK was amplified by PCR from a previously described vector (11) and subcloned into pEV3S in frame with a C-terminal T7 epitope to generate pEV-IKKα-T7. Recombinant human TNF-α was purchased from Endogen (Cambridge, Mass.). HeLa and 293 cell lines were maintained in Dulbecco modified Eagle medium supplemented with 10% heat-inactivated fetal bovine serum, penicillin, and streptomycin.
Transfections and reporter assays.
293 cells (5 × 105 per well) were seeded into six-well (35-mm-diameter) plates and transfected the following day with 4 μg of DNA by the calcium phosphate precipitation method. After 15 to 20 h, selected cultures were stimulated with TNF-α (20 ng/ml) for 6 h. Luciferase activity was typically measured 20 to 25 h after transfection by using the enhanced luciferase assay kit and a Monolight 2010 luminometer (Analytical Luminescence Laboratory, Ann Arbor, Mich.). All transfections included the 6RZ plasmid to normalize for differences in gene transfer efficiency by assay of β-galactosidase activity.
Immunoprecipitation.
293 cells (5 × 105 per well) or HeLa cells (2 × 105 per well) were transfected in six-well plates and lysed 24 h later in ELB buffer containing 1.5% Nonidet P-40, 250 mM NaCl, 50 mM HEPES (pH 7.4), 1 mM EDTA, and the following protease inhibitors: 1 mM phenylmethylsulfonyl fluoride, 5-μg/ml antipain, 5-μg/ml aprotinin, 5-μg/ml leupeptin, 0.5-μg/ml pepstatin, 7.5-μg/ml bestatin, 4-μg/ml phosphoroamidon, and 5-μg/ml trypsin inhibitor. Lysates were immunoprecipitated with anti-T7-Tag antibody linked to agarose beads (Novagen, Madison, Wis.). Immunoprecipitates were washed three times in lysis buffer and then subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), followed by transfer to nitrocellulose membranes and immunoblotting with anti-Myc-Tag antibodies (Santa Cruz Biotechnology, Santa Cruz, Calif.).
Immune complex kinase assays.
293 cells were transfected in six-well plates, lysed 12 to 18 h posttransfection, and immunoprecipitated with antibodies and protein A-agarose beads as described above. Immunoprecipitated beads were further washed with kinase buffer containing 10 mM HEPES (pH 7.4), 1 mM MnCl2, 5 mM MgCl2, 12.5 mM β-glycero-2-phosphate, 50 μM Na3VO4, 2 mM NaF, and 50 μM dithiothreitol. After suspension in 20 μl of kinase buffer, the immunoprecipitates were incubated with 5 μCi of [γ-32P]ATP (6,000 Ci/mmol) with or without 1 μg of recombinant glutathione S-transferase (GST)–IκBα(1-62) as an exogenous substrate for 30 min at 30°C. The reaction was terminated by the addition of SDS sample buffer. The samples were analyzed by SDS-PAGE, followed by transfer to nitrocellulose membranes and exposure to Hyperfilm MP (Amersham Life Sciences). The membranes were subsequently probed with antibodies to determine the amount of immunoprecipitated kinases.
In vivo radiolabeling with 32P-labeled orthophosphoric acid.
293 cells were transfected with pRK-NIK or the kinase domain mutant forms of NIK (KK429/430AA, STT549/552/559AAA). Twenty-four hours after transfection, the cells were washed once in phosphate-free Dulbecco modified Eagle medium (Life Technologies) supplemented with 10% dialyzed, heat-inactivated fetal bovine serum and starved for 1 h in the same medium. 32P-labeled orthophosphoric acid (0.5 mCi) was then added to the cells. After incubation for 2 h, the cells were lysed in ELB buffer and immunoprecipitated with anti-Myc-Tag antibody and protein A-agarose as described above. Immunoprecipitates were analyzed by SDS-PAGE, followed by transfer to nitrocellulose membranes and exposure to Hyperfilm MP. Immunoblotting was performed with anti-Myc-Tag antibodies to assess the levels of immunoprecipitated NIK proteins.
RESULTS
Residues in the activation loop of NIK’s kinase domain are critical for NIK function.
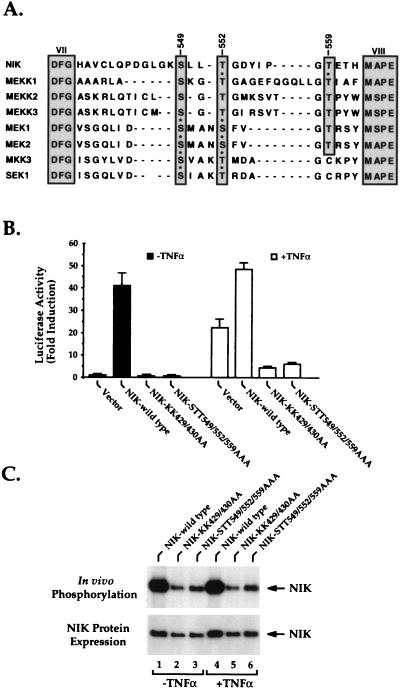
NIK is homologous to the MAP3Ks. Since many MAP kinases are regulated by phosphorylation of serine or threonine residues within the activation loop located between subdomains VII and VIII in their kinase domains (1, 31, 41, 44), we compared the sequence of the activation loop of NIK with those of several MAP3Ks (Fig. 1A). We observed that one serine and two threonine residues are highly conserved among these MAP3Ks, corresponding to amino acids Ser-549, Thr-552, and Thr-559 in NIK (Fig. 1A). To address the question of whether these three residues are involved in the regulation of NIK function, we generated a mutant NIK in which Ser-549, Thr-552, and Thr-559 of the activation loop were replaced with alanine residues. This mutant NIK and the wild-type kinase were compared for the ability to stimulate κB-dependent luciferase reporter activity in transfected human 293 embryonic kidney cells (Fig. 1B). In agreement with earlier studies, wild-type NIK strongly stimulated luciferase activity (closed bars in Fig. 1B) (23). Additional stimulation of these cultures with TNF-α (open bars in Fig. 1B) only weakly augmented the κB-luciferase activity, suggesting that the ectopically expressed NIK was almost fully induced. In contrast, NIK-STT549/552/559AAA altered in the activation loop and NIK-KK429/430AA altered at the ATP binding site of the NIK kinase domain displayed very little spontaneous functional activity, and both mutant proteins functioned as dominant-negative inhibitors of TNF-α (Fig. 1B)- and NIK (data not shown)-mediated induction of NF-κB.
FIG. 1.
(A) Alignment of the amino acid sequences of the activation loop between subdomains VII (DFG) and VIII (M(A/S)PE) of NIK and other MAP kinases. Asterisks denote residues shown to be phosphorylated and/or implicated in the activation of these kinases. (B) Biological function of wild-type NIK and kinase domain mutant forms of NIK. Expression vectors encoding wild-type NIK or the indicated mutant forms of NIK were cotransfected into 293 cells together with κB-luciferase and β-galactosidase reporter plasmids. Twenty hours later, the cultures were stimulated with or without TNF-α (20 ng/ml) for 6 h. Cell lysates were then prepared, and the luciferase activities of these lysates were determined. β-Galactosidase activities in these lysates were measured for normalization of differences in transfection efficiency occurring in the cultures. Data are presented as fold induction of luciferase activity ± the standard deviations derived from independent triplicate transfections. The closed bars show the biological activity of wild-type NIK or the mutant forms of NIK, while the open bars show the effect of these mutants in the presence of TNF-α stimulation. (C) In vivo phosphorylation of wild-type NIK and the mutant forms of NIK. 293 cells were transfected with wild-type NIK or the indicated mutant forms of NIK containing an N-terminal Myc epitope tag. These cells were then incubated in phosphate-free medium for 1 h, radiolabeled for 2 h with 32P-labeled orthophosphoric acid, and either cultured in medium alone or stimulated with TNF-α (20 ng/ml) for 15 min, and then NIK was immunoprecipitated with anti-Myc antibodies. The resulting immunoprecipitates were subjected to SDS-PAGE, followed by transfer to a nitrocellulose membrane and autoradiography. The lower part of the panel shows the levels of the wild-type and mutant NIK proteins present in the samples.
To further explore the molecular basis for the spontaneous activity of the ectopically expressed NIK protein, in vivo phosphorylation of the biologically active wild-type and inactive mutant NIK proteins was compared by metabolic radiolabeling with 32P-labeled orthophosphoric acid (Fig. 1C). These studies revealed significant in vivo phosphorylation of wild-type NIK but sharply diminished phosphorylation of the biologically inactive NIK-KK429/430AA and NIK-STT549/552/559AAA mutant proteins (Fig. 1C, top). Addition of TNF-α did not enhance the level of phosphorylation of wild-type NIK or the mutant NIKs (lanes 4 to 6, Fig. 1C). The observed decrease in phosphorylation of the two mutant NIKs was not explained by marked instability of these proteins, since immunoblotting revealed only slightly lower levels of expression for each (Fig. 1C, bottom) and the half-lives of these mutant proteins are virtually identical to that of the wild-type protein (data not shown). Together, these findings suggest that NIK induction of NF-κB correlates with its ability to undergo auto- or transphosphorylation in vivo. Further, such phosphorylation likely involves either inter- or intramolecular phosphorylation of the activation loop of NIK. This result is consistent with the known regulatory function of the activation loop in many other kinases (1, 31, 41, 44).
Threonine 559 plays a critical role in the regulation of NIK function.
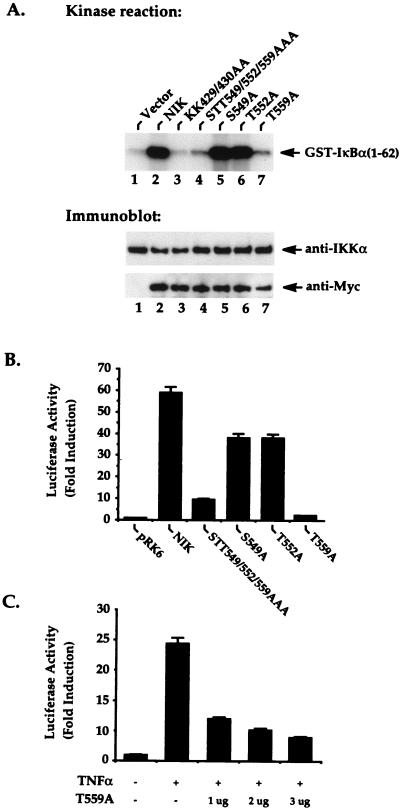
To further determine which Ser or Thr residue in the activation loop of NIK is critical for the regulation of its function, we generated a set of mutant proteins in which alanine was substituted for the individual serine or threonine residues. These mutant proteins, NIK S549A, T552A, and T559A, were transfected into 293 cells and evaluated for the ability to activate the downstream kinase IKKα (Fig. 2A) and to induce NF-κB-dependent transcription (Fig. 2B). It is noteworthy that while the NIK S549A and T552A mutant proteins functioned similarly to wild-type NIK, the T559A NIK mutant protein failed to induce IKKα activity (Fig. 2A) or κB-luciferase activity (Fig. 2B). Expression of the T559A NIK mutant protein also dominantly interfered with TNF-α-induced NF-κB activation in a dose-related manner (Fig. 2C).
FIG. 2.
The NIK-T559A mutant protein fails to activate IKKα- and NF-κB-dependent transcription. (A) 293 cells were seeded at 5 × 105/well in six-well plates and transfected 24 h later with 4 μg of plasmid DNA encoding Myc-tagged NIK or the indicated mutant proteins. Twenty hours after transfection, in vitro kinase reactions were performed by using anti-IKKα immunoprecipitates prepared from these cell lysates; GST–IκBα(1-62) was added as an exogenous substrate. The kinase reactions were analyzed by SDS-PAGE, followed by transfer to a nitrocellulose membrane and autoradiography. The phosphorylated GST–IκBα(1-62) substrate is indicated on the right. The lower panels show the amounts of immunoprecipitated IKKα and expressed NIK present in each of the cell lysates. One microgram of expression vector DNA encoding wild-type NIK or the indicated mutants form of NIK (B) or 1, 2, or 3 μg of an expression vector encoding NIK-T559A (C) was cotransfected into 293 cells together with 200 ng of κB-luciferase and 100 ng of β-galactosidase reporter plasmids. The total DNA concentration was held constant at 4 μg by supplementation with the parental pRK vector. Twenty hours after transfection, the cultures were stimulated with or without TNF-α (20 ng/ml) for 6 h, as indicated in panels B and C. Cell lysates were then prepared from the cultures, and the luciferase activity present in these lysates was determined. The β-galactosidase activities of these lysates were also measured for normalization of differences in transfection efficiency occurring in the cultures.
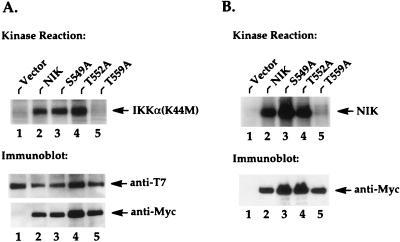
To determine whether the inability of the NIK T559A mutant protein to activate IKKα and NF-κB was due to its failure to phosphorylate IKKα, we cotransfected kinase-deficient (K44M) IKKα with wild-type NIK or each of the activation loop mutant proteins. IKKα was then immunoprecipitated and subjected to an immunocomplex kinase reaction to test the ability of coimmunoprecipitated NIK to phosphorylate IKKα (22). As shown in Fig. 3A, the S549A and T552A mutant proteins, like wild-type NIK, effectively phosphorylated the coimmunoprecipitated IKKα(K44M) protein in vitro (Fig. 3A). In contrast, despite assembling normally with IKKα (Fig. 3A, bottom), the NIK T559A mutant protein failed to phosphorylate the coprecipitating IKKα (Fig. 3A, lane 5). Consistent with this result, the NIK T559A mutant protein also lacked autophosphorylation ability compared to the other activation loop mutant proteins (Fig. 3B, lane 5). Together, these results suggest that Thr-559 corresponds to a key regulatory residue within the activation loop of NIK that is essential for NIK function.
FIG. 3.
The NIK-T559A mutant protein fails to phosphorylate IKKα and to undergo autophosphorylation. (A) 293 cells were seeded at 5 × 105/well in six-well plates and transfected 24 h later with 2 μg of T7-tagged IKKα(K44M) expression vectors and 2 μg of Myc-tagged NIK expression vectors or vectors encoding the indicated mutant proteins. Twenty hours after transfection, in vitro kinase reactions were performed on anti-T7 immunoprecipitates from these cell lysates. The resulting kinase reactions were separated by SDS-PAGE, transferred to nitrocellulose membranes, and analyzed by autoradiography. Phosphorylated IKKα(K44M) is indicated on the right in the upper part of the panel. The lower parts of the panel show the levels of immunoprecipitated IKKα and the levels of coimmunoprecipitated NIK proteins. (B) Two micrograms of Myc-tagged NIK expression vectors or the indicated mutants was transfected into 293 cells, and in vitro kinase reactions were performed on anti-Myc immunoprecipitates as described for panel A. Autophosphorylated NIK proteins are indicated on the right. The lower parts of the panel show the levels of immunoprecipitated NIK proteins present in the kinase reactions.
Ectopically expressed NIK spontaneously forms homotypic oligomers in vivo.
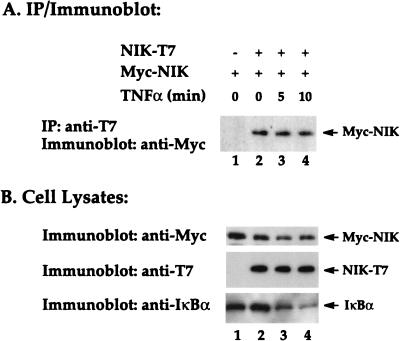
Several adapter proteins, including TRADD, RIP, and TRAF2, that associate with TNFR1 contain protein-protein interaction or self-association domains. When ectopically expressed, each of these proteins activates NF-κB (14, 16, 29, 35). One explanation for these results is that overexpression of these adapter proteins may lead to the formation of oligomers through their self-association domains. Consequently, this aggregation may lead to oligomerization of downstream signaling components which, in fact, may mimic physiological ligand-induced receptor oligomerization. Having shown that ectopic expression of NIK potently activates NF-κB (Fig. 1) (23), we investigated whether these active NIK molecules were monomeric or had assembled into homotypic oligomers in vivo. For these studies, two different epitope-tagged versions of NIK (Myc-NIK and NIK-T7) were prepared and cotransfected into HeLa cells. Lysates from these transfected cells were then sequentially immunoprecipitated with anti-T7 antibodies and immunoblotted with anti-Myc antibodies. As expected, the anti-T7 antibodies did not immunoprecipitate Myc-NIK (Fig. 4A, lane 1); however, when NIK-T7 and Myc-NIK were coexpressed, the two proteins were effectively coimmunoprecipitated (Fig. 4A, lane 2). These findings support the spontaneous dimerization or higher-order oligomerization of biologically active NIK proteins in vivo. Immunoblotting of the total cell lysates confirmed that the Myc-NIK and NIK-T7 proteins were expressed at comparable levels in the transfected cultures (Fig. 4B, lane 2). It is noteworthy that stimulation of these cultures for 5 or 10 min with TNF-α did not further enhance NIK oligomerization (Fig. 4A, lanes 3 and 4). However, the added TNF-α was biologically active, since it induced the partial degradation of endogenous IκBα observed at 10 min (Fig. 4B). The spontaneous oligomerization of ectopically expressed NIK may contribute importantly to the high level of constitutive NF-κB-inducing activity found for this enzyme (Fig. 1B). However, intramolecular phosphorylation of NIK molecules is not a prerequisite for oligomerization, since kinase-deficient versions of NIK were detected as oligomers (data not shown). Instead, oligomerization may favor subsequent phosphorylation reactions.
FIG. 4.
Biologically active NIK spontaneously forms homotypic oligomers in vivo. (A) HeLa cells were transiently transfected with plasmids encoding T7- and Myc epitope-tagged versions of NIK, as indicated. After 24 h of culture, a portion of the cells was treated with TNF-α for 5 or 10 min as indicated. Cells lysates were prepared from the cultures and subjected to immunoprecipitation (IP) with anti-T7 antibody conjugated to agarose beads. The immunoprecipitates were then analyzed by immunoblotting with anti-Myc antibodies. (B) Aliquots of the whole-cell lysates (10 μl) were subjected to SDS-PAGE and immunoblotted with anti-T7 or anti-Myc antibodies to determine the levels of Myc-NIK and NIK-T7 expression. In addition, the biological activity of the added TNF-α was confirmed by induced degradation of endogenous IκBα detected by immunoblotting with antibodies specific for the C terminus of this cytoplasmic inhibitor.
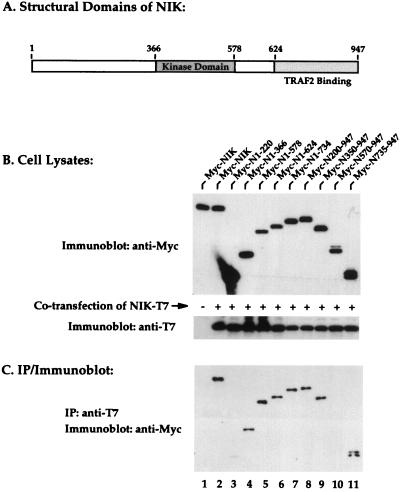
Mapping of domains involved in homotypic oligomerization of NIK.
To determine which subregions of NIK mediate the observed homotypic interaction, a series of nested deletion mutant forms of NIK were prepared and tested. An outline of the structural organization of NIK is provided in Fig. 5A. Each of the mutant forms of NIK contains an N-terminal Myc epitope tag, and each was stably expressed in transfected 293 cells (Fig. 5B, lanes 3 to 11). When cotransfected with NIK-T7, both wild-type NIK and each of the mutant forms of NIK, with the notable exceptions of Myc-N1-220 and Myc-N570-947, effectively formed oligomers with NIK-T7, as indicated by their coimmunoprecipitation with anti-T7 antibodies (Fig. 5C, lanes 2 to 11). No oligomerization of NIK-T7 and Myc-PAK1 (p21-activated kinase) was detected in parallel assays, indicating the specificity of the observed protein-protein interaction (data not shown). These findings suggest that multiple domains within NIK, excluding its N terminus, participate in homotypic assembly. It is noteworthy that we do not fully understand why NIK amino acids 570 to 947 fail to form oligomers, since binding was observed with the shorter amino acid 735 to 947 NIK fragment. The region between amino acids 672 and 759 is characterized by the presence of multiple proline residues, which might lead to an altered protein conformation within the 570-to-947 fragment not recapitulated in the shorter peptide.
FIG. 5.
Multiple domains of NIK participate in homotypic oligomerization. (A) Schematic overview of the structural organization of NIK. (B) 293 cells were transiently transfected with plasmids encoding Myc-tagged NIK and the indicated series of deletion mutant forms of NIK similarly epitope tagged with Myc. After 24 h, cell lysates were prepared, subjected to SDS-PAGE, and blotted with anti-Myc or anti-T7 antibodies to verify protein expression levels. (C) To assess the ability of the mutant NIK proteins to form oligomers with NIK-T7, aliquots of the cell lysates were subjected to immunoprecipitation (IP) with anti-T7 antibody conjugated to agarose beads. The immunoprecipitates were subjected to SDS-PAGE and immunoblotted with anti-Myc antibodies.
Functional effects of the mutant forms of NIK on TNF-α and wild-type NIK-induced NF-κB activation.
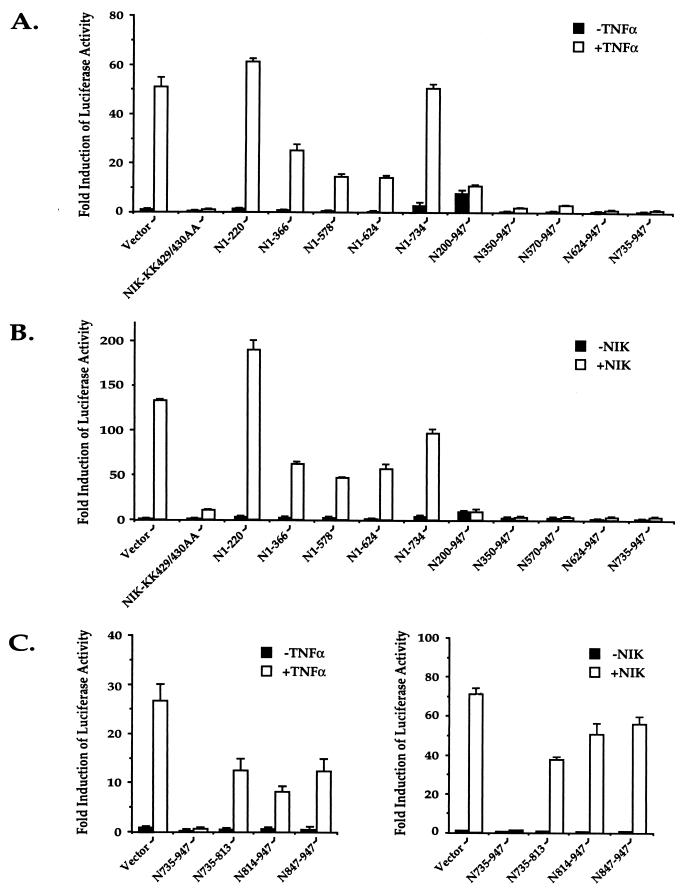
Studies were next performed to assess both the intrinsic biological function of each of the deletion mutant forms of NIK and their effects on TNF-α and NIK activation of NF-κB. When cotransfected into 293 cells with the κB-luciferase reporter plasmid, only the N200-947 mutant form of NIK retained significant, albeit weak, stimulatory activity (Fig. 6A and B, closed bars). When tested in the presence of TNF-α or wild-type NIK as an inducer, the C-terminal deletion mutant forms of NIK (N1-366, N1-578, and N1-624) produced only modest and various degrees of inhibition (Fig. 6A and B, open bars). In contrast, each of the N-terminal deletion mutant forms of NIK (N350-947, N578-947, N624-947, and N735-947) markedly inhibited both TNF-α and wild-type NIK induction of κB-luciferase activity (Fig. 6A and B). These results confirm and extend the studies of Malinin et al. (23), who showed that residues 624 to 947 of NIK also exert inhibitory effects on TNF-α signaling. To determine which sequences of the 213-amino-acid C-terminal region of NIK are required for this inhibition, additional deletion mutant forms of NIK (735-813, 814-947, and 847-947) were prepared and analyzed (Fig. 6C). As expected, none of these smaller fragments induced an increase in κB-luciferase activity when added alone (Fig. 6C, closed bars). Further, none of the smaller C-terminal fragments of NIK exerted potent inhibitory effects on TNF-α (Fig. 6C, left)- or wild-type NIK (Fig. 6C, right)-induced NF-κB-driven luciferase activity. Thus, the carboxyl terminus of NIK, composed of amino acids 735 to 947, represents the smallest fragment identified which potently inhibits TNF-α-mediated activation of NF-κB.
FIG. 6.
Analysis of the functional effects of various deletion mutant forms of NIK in the presence and absence of TNF-α (A) or wild-type NIK (B). (A) Three micrograms of expression vectors encoding the indicated NIK mutant proteins were cotransfected into 293 cells together with κB-luciferase and β-galactosidase reporter plasmids. Twenty hours after transfection, the cultures were stimulated with or without TNF-α (20 ng/ml) for 6 h. Cell lysates were then prepared from the cultures, and the luciferase activities of these lysates were determined. The β-galactosidase activities of these lysates were measured for normalization of differences in transfection efficiency occurring in the cultures. (B) Three micrograms of expression vectors encoding the indicated NIK mutant proteins in the absence or presence of 0.3 μg of a wild-type NIK expression vector were cotransfected into 293 cells together with κB-luciferase and β-galactosidase reporter plasmids. Twenty-four hours after transfection, cell lysates were prepared from the cultures. The luciferase and β-galactosidase activities of these lysates were determined. (C) Analysis of the inhibitory effect of the C terminus of NIK (amino acids 735 to 947) on TNF-α (left graph) and NIK (right graph) induction of κB-luciferase activity. Experiments were performed as described for panels A and B for the smaller fragments of NIK. All data are presented as mean fold induction of luciferase activity ± the standard deviation derived from independent triplicate transfections. The closed bars show the biological activity of wild-type or mutant NIK alone, while the open bars show the effect of mutant NIK on TNF-α or wild-type NIK stimulation.
The C terminus of NIK mediates binding to the IκB-specific kinase IKKα.
To further explore the biological basis for the significant inhibitory effects of the amino acid 735 to 947 C-terminal region of NIK on TNF-α signaling, we examined the potential role of this region in NIK binding to IKKα. IKKα-T7 was coexpressed with the various Myc-NIK deletion mutants followed by coimmunoprecipitation with anti-T7 antibodies and immunoblotting with anti-Myc antibodies (Fig. 7). In these studies, enzymatically deficient NIK-KK429/430AA was used in place of the wild-type enzyme to avoid potential phosphorylation of IKKα, which might weaken the NIK-IKKα interaction. All of the deletion mutant forms of NIK were comparably expressed in these transfection studies (Fig. 7A). As previously reported (27), wild-type NIK effectively interacted with IKKα, as evidenced by their coimmunoprecipitation (Fig. 7B, lane 2). It is noteworthy that none of the C-terminal deletion mutant forms of NIK assembled with IKKα (Fig. 7B, lanes 3 to 7), while all of the N-terminal deletion mutant proteins, including N735-947, effectively associated with IKKα (Fig. 7B, lanes 8 to 11).
FIG. 7.
The C terminus of NIK mediates heterotypic oligomerization with IKKα. 293 cells were cotransfected with IKKα-T7 and Myc-NIK or the series of deletion mutant forms of Myc-NIK. (A) Twenty-four hours after transfection, cell lysates were prepared, subjected to SDS-PAGE, and immunoblotted with anti-Myc antibodies or anti-T7 antibodies to assess the overall level of expression of the individual proteins. (B) Aliquots of the cell lysates were subjected to immunoprecipitation (IP) with anti-T7 antibodies conjugated to agarose beads. The immunoprecipitates were then subjected to SDS-PAGE and immunoblotted with anti-Myc antibodies.
Expression of the C-terminal 213-amino-acid segment of NIK interrupts the assembly of NIK and IKKα.
Studies were next performed to determine whether coexpression of the 213-amino-acid C-terminal fragment of NIK (N735-947) is sufficient to disrupt the interaction of NIK and IKKα. The comparably sized amino acid 1 to 220 N-terminal fragment of NIK was used as a control in these studies. When coexpressed with Myc-NIK and IKKα-T7, the Myc-N735-947 peptide (Fig. 8A, lane 4), but not the Myc-N1-220 peptide (Fig. 8A, lane 3), effectively blocked the physical association of IKKα and NIK. Both Myc-NIK and the two NIK fragments were comparably expressed in these transfected cultures (Fig. 8B). Together with the results presented in Fig. 7 and 8, these findings indicate that the region between amino acids 735 and 945 at the carboxyl terminus of NIK corresponds to a binding domain for IKKα that is both necessary and sufficient for NIK binding to IKKα. The marked inhibitory effect of the peptide corresponding to this 213-residue C-terminal region in the TNF-α signaling assays likely derives from its ability to prevent the effective assembly of NIK and IKKα.
FIG. 8.
(A) Expression of a C-terminal fragment (amino acids 735 to 947) of NIK inhibits the interaction of wild-type NIK and IKKα. 293 cells were cotransfected with IKKα-T7 and Myc-NIK in the presence of either N-terminal amino acids 1 to 220 or C-terminal amino acids 735 to 947 of NIK, as indicated. Twenty-four hours after transfection, cell lysates were prepared from the transfected cultures. (A) Aliquots of the cell lysates were subjected to immunoprecipitation (IP) with anti-T7 antibodies conjugated to agarose beads. The immunoprecipitates were then subjected to SDS-PAGE and immunoblotted with anti-Myc antibodies. (B) Aliquots of the cell lysates were subjected to SDS-PAGE and immunoblotted with anti-Myc antibodies to assess the expression of the individual proteins.
DISCUSSION
The intracellular signaling events that translate TNF-α binding at the plasma membrane into the induction of NF-κB in the nucleus represent an area of considerable recent discovery. It is now known that ligand binding induces trimerization of TNFR1 and the recruitment of two death domain-containing proteins, TRADD and RIP, to its cytoplasmic tail (14, 16, 35). This ensemble of proteins promotes association of the TRAF2 adapter protein (15), and the complex associates with NIK, a MAP3K level enzyme (23). In turn, NIK activates the recently characterized IKKα and IKKβ enzymes (12, 24, 27, 40, 43), leading to phosphorylation of IκBα on both of its N-terminal regulatory serines at residues 32 and 36 (4, 5, 33, 36, 39). By an unknown mechanism, phosphorylation of IκBα triggers its ubiquitination at lysine 20 or 21 (8, 30), which in turn promotes its proteolytic degradation in the 26S proteasome (8). The degradation of IκBα both liberates the heterodimeric NF-κB transcription factor complex (p50-p65) and unmasks the nuclear localization signal of p65, allowing rapid translocation of the NF-κB complex into the nucleus. Once in the nucleus, NF-κB engages its cognate DNA enhancer elements and activates NF-κB-responsive target gene expression.
NIK has strong homology with other MAP3Ks in its enzymatic domain. Since protein phosphorylation has been shown to be critical for the activation of MAP kinases (1, 31, 41, 44), we investigated whether Ser and/or Thr residues in the activation loop located between subdomains VII and VIII of the kinase domain of NIK play a regulatory role. Our studies show that ectopically expressed NIK is phosphorylated in vivo and that this posttranslational modification is abrogated by the introduction of point mutations replacing certain Ser and Thr residues within either the activation loop or the ATP binding site of the NIK kinase domain. Both of these classes of kinase-deficient mutations lead to a loss of NIK function in vivo. Our results further indicate that a single threonine residue at position 559 in the activation loop of NIK is essential for catalytic activity. Replacement of Thr-559 of the activation loop with Ala in NIK completely abolishes the ability of NIK to phosphorylate its substrate, IKKα, and its ability to undergo autophosphorylation. The functional defect of this mutant protein cannot be attributed to a grossly altered protein conformation, since it retains its ability to assemble with IKKα (Fig. 3A, bottom, lane 5) and functions as a dominant negative inhibitor of TNF-α signaling (Fig. 2C). We do not know whether NIK phosphorylation reflects inter- or intramolecular phosphorylation. However, residues within the activation loop appear to be involved in this phosphorylation reaction.
It is interesting that Thr-559 of NIK is homologous to Thr-572 of ΔMEKK1 (or Thr-1393 of the full length MEKK1 protein) (Fig. 1A). However, in MEKK1, both Thr-572 and Thr-560 must be phosphorylated for full kinase activity (31). Additionally, the critical Ser and Thr residues in MAP2K level kinases appear to correspond to Ser-549 and Thr-552 in NIK (Fig. 1A), which are dispensable for NIK function (Fig. 2 and 3). These data suggest that different MAP kinases are regulated through the phosphorylation of one or more different serine and threonine residues that are topographically represented in a conserved manner within the activation loop. Determination of whether all MAP3K level kinases are preferentially phosphorylated at threonine residues homologous to those in MEKK1 and NIK will await similar analysis of other family members.
Several adapter proteins, including TRADD, RIP, and TRAF2, which can directly or indirectly bind to TNF-α receptor complexes, can activate NF-κB when ectopically expressed. Although NIK has been shown to associate with upstream adapter protein TRAF2 (23) and other TRAF family members (32), it is not clear how these adapter proteins activate NIK. One possible mechanism that could account for NIK activation by adapter proteins could be induced oligomerization. This could mimic physiologically ligand-induced aggregation of receptor complexes at the plasma membrane. Such aggregation of receptor complexes and adapters may lead to further oligomerization of downstream components of the kinase cascade and induce changes in the protein conformation of these kinases, leading to activation of the kinase cascade. In this study, we observed that ectopically expressed NIK both was constitutively active and spontaneously formed homotypic oligomers in vivo. We have not identified a mutant form of NIK which displays a high level of biological activity but does not form homotypic oligomers. However, such an analysis is complicated by the fact that multiple domains of NIK are sufficient to mediate its oligomerization (Fig. 5). It is noteworthy that both NIK activity and its oligomerization were not further induced by the addition of TNF-α (Fig. 1 and 4). These results are consistent with the above-described hypothesis that homo-oligomerization of NIK molecules may lead to conformational changes that alter the activation state of this kinase, mimicking ligand-induced oligomerization of TNF-α receptor complexes and associated proteins. Kinase activity does not appear to be required for NIK oligomerization but may, instead, be the result of such conformational changes that lead to phosphorylation of threonine 559. To better explore the possibility of signal-induced oligomerization, the establishment of a cell line stably expressing two different epitope-tagged versions of NIK would be required.
Prior studies have demonstrated that TRAF2 assembly with NIK involves C-terminal sequences located between amino acids 624 and 948 (23). Our studies now demonstrate that NIK assembly with IKKα similarly involves the C terminus of NIK, specifically, an overlapping subregion encompassing residues 735 to 947. It is noteworthy that NIK contains a proline-rich region between amino acids 712 and 728 and that, overall, 28% of the amino acids between residues 672 and 759 are prolines. What role, if any, these proline residues play in the NIK-TRAF2 and NIK-IKKα interactions remains to be determined. The interaction of NIK with IKKα may serve to recruit NIK into the macromolecular environment of the signalsome (27, 40). Although TRAF2 and IKKα interact with an overlapping subregion of NIK, it appears that the trimolecular complex TRAF2-NIK-IKKα exists in vivo, although this complex may be transient (27). Even though NIK and IKKβ appear to assemble in vivo (40), it seems that IKKβ may not be the direct downstream target of NIK phosphorylation, since NIK only phosphorylates IKKα but not IKKβ in vitro (22). It is noteworthy that the carboxyl terminus of NIK is both necessary and sufficient for the assembly of NIK with IKKα. Indeed, the 213-amino-acid C-terminal peptide region not only binds effectively to IKKα but also potently blocks TNF-α and NIK induction of NF-κB. Due to the critical nature of the NIK-IKKα interaction in the TNF-α and IL-1 proinflammatory responses, the identification of small-molecule inhibitors that prevent the assembly of these kinases could provide a novel alternative approach to the development of kinase domain inhibitors for interference with TNF-α and IL-1 signaling. If achievable, these oligomerization inhibitors would form a new and complementary class of anti-inflammatory agents.
ACKNOWLEDGMENTS
E.T.C. is supported by an NIH grant (K08-EY00352). R.G. is supported by a Centennial Fellowship from the Medical Research Council of Canada.
This work was supported by grants from the UCSF Center for AIDS Research (P30A127763) and from Pfizer.
REFERENCES
- 1.Alessi D R, Saito Y, Campbell D G, Cohen P, Sithanandam G, Rapp U, Ashworth A, Marshall C J, Cowley S. Identification of the sites in MAP kinase kinase-1 phosphorylated by p74raf-1. EMBO J. 1994;13:1610–1619. doi: 10.1002/j.1460-2075.1994.tb06424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baeuerle P A, Baltimore D. NF-κB: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 3.Baldwin A J. The NF-κB and IκB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 4.Brockman J A, Scherer D C, McKinsey T A, Hall S M, Qi X, Lee W Y, Ballard D W. Coupling of a signal response domain in IκBα to multiple pathways for NF-κB activation. Mol Cell Biol. 1995;15:2809–2818. doi: 10.1128/mcb.15.5.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown K, Gerstberger S, Carlson L, Franzoso G, Siebenlist U. Control of IκB-α proteolysis by site-specific, signal-induced phosphorylation. Science. 1995;267:1485–1488. doi: 10.1126/science.7878466. [DOI] [PubMed] [Google Scholar]
- 6.Cao Z, Henzel W J, Gao X. IRAK: a kinase associated with the interleukin-1 receptor. Science. 1996;271:1128–1131. doi: 10.1126/science.271.5252.1128. [DOI] [PubMed] [Google Scholar]
- 7.Cao Z, Xiong J, Takeuchi M, Kurama T, Goeddel D V. TRAF6 is a signal transducer for interleukin-1. Nature. 1996;383:443–446. doi: 10.1038/383443a0. [DOI] [PubMed] [Google Scholar]
- 8.Chen Z, Hagler J, Palombella V J, Melandri F, Scherer D, Ballard D, Maniatis T. Signal-induced site-specific phosphorylation targets I κ B α to the ubiquitin-proteasome pathway. Genes Dev. 1995;9:1586–1597. doi: 10.1101/gad.9.13.1586. [DOI] [PubMed] [Google Scholar]
- 9.Chen Z J, Parent L, Maniatis T. Site-specific phosphorylation of IκBα by a novel ubiquitination-dependent protein kinase activity. Cell. 1996;84:853–862. doi: 10.1016/s0092-8674(00)81064-8. [DOI] [PubMed] [Google Scholar]
- 10.Cheng G, Cleary A M, Ye Z S, Hong D I, Lederman S, Baltimore D. Involvement of CRAF1, a relative of TRAF, in CD40 signaling. Science. 1995;267:1494–1498. doi: 10.1126/science.7533327. [DOI] [PubMed] [Google Scholar]
- 11.Connelly M A, Marcu K B. CHUK, a new member of the helix-loop-helix and leucine zipper families of interacting proteins, contains a serine-threonine kinase catalytic domain. Cell Mol Biol Res. 1995;41:537–549. [PubMed] [Google Scholar]
- 12.DiDonato J A, Hayakawa M, Rothwarf D M, Zandi E, Karin M. A cytokine-responsive IκB kinase that activates the transcription factor NF-κB. Nature. 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 13.Duckett C S, Gedrich R W, Gilfillan M C, Thompson C B. Induction of nuclear factor κB by the CD30 receptor is mediated by TRAF1 and TRAF2. Mol Cell Biol. 1997;17:1535–1542. doi: 10.1128/mcb.17.3.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu H, Huang J, Shu H B, Baichwal V, Goeddel D V. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity. 1996;4:387–396. doi: 10.1016/s1074-7613(00)80252-6. [DOI] [PubMed] [Google Scholar]
- 15.Hsu H, Shu H B, Pan M G, Goeddel D V. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell. 1996;84:299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- 16.Hsu H, Xiong J, Goeddel D V. The TNF receptor 1-associated protein TRADD signals cell death and NF-κB activation. Cell. 1995;81:495–504. doi: 10.1016/0092-8674(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 17.Ishida T K, Tojo T, Aoki T, Kobayashi N, Ohishi T, Watanabe T, Yamamoto T, Inoue J. TRAF5, a novel tumor necrosis factor receptor-associated factor family protein, mediates CD40 signaling. Proc Natl Acad Sci USA. 1996;93:9437–9442. doi: 10.1073/pnas.93.18.9437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelliher M A, Grimm S, Ishida Y, Kuo F, Stanger B Z, Leder P. The death domain kinase RIP mediates the TNF-induced NF-κB signal. Immunity. 1998;8:297–303. doi: 10.1016/s1074-7613(00)80535-x. [DOI] [PubMed] [Google Scholar]
- 19.Lee F S, Hagler J, Chen Z J, Maniatis T. Activation of the IκBα kinase complex by MEKK1, a kinase of the JNK pathway. Cell. 1997;88:213–222. doi: 10.1016/s0092-8674(00)81842-5. [DOI] [PubMed] [Google Scholar]
- 20.Lee S Y, Lee S Y, Kandala G, Liou M L, Liou H C, Choi Y. CD30/TNF receptor-associated factor interaction: NF-κB activation and binding specificity. Proc Natl Acad Sci USA. 1996;93:9699–9703. doi: 10.1073/pnas.93.18.9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee S Y, Reichlin A, Santana A, Sokol K A, Nussenzweig M C, Choi Y. TRAF2 is essential for JNK but not NF-κB activation and regulates lymphocyte proliferation and survival. Immunity. 1997;7:703–713. doi: 10.1016/s1074-7613(00)80390-8. [DOI] [PubMed] [Google Scholar]
- 22.Ling L, Cao Z, Goeddel D V. NF-κB-inducing kinase activates IKK-α by phosphorylation of Ser-176. Proc Natl Acad Sci USA. 1998;95:3792–3797. doi: 10.1073/pnas.95.7.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malinin N L, Boldin M P, Kovalenko A V, Wallach D. MAP3K-related kinase involved in NF-κB induction by TNF, CD95 and IL-1. Nature. 1997;385:540–544. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- 24.Mercurio F, Zhu H, Murray B W, Shevchenko A, Bennett B L, Li J, Young D B, Barbosa M, Mann M, Manning A, Rao A. IKK-1 and IKK-2: cytokine-activated IκB kinases essential for NF-κB activation. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 25.Muzio M, Ni J, Feng P, Dixit V M. IRAK (Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL-1 signaling. Science. 1997;278:1612–1615. doi: 10.1126/science.278.5343.1612. [DOI] [PubMed] [Google Scholar]
- 26.Nakano H, Oshima H, Chung W, Williams A L, Ware C F, Yagita H, Okumura K. TRAF5, an activator of NF-κB and putative signal transducer for the lymphotoxin-β receptor. J Biol Chem. 1996;271:14661–14664. doi: 10.1074/jbc.271.25.14661. [DOI] [PubMed] [Google Scholar]
- 27.Regnier C H, Song H Y, Gao X, Goeddel D V, Cao Z, Rothe M. Identification and characterization of an IκB kinase. Cell. 1997;90:373–383. doi: 10.1016/s0092-8674(00)80344-x. [DOI] [PubMed] [Google Scholar]
- 28.Rothe M, Pan M G, Henzel W J, Ayres T M, Goeddel D V. The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell. 1995;83:1243–1252. doi: 10.1016/0092-8674(95)90149-3. [DOI] [PubMed] [Google Scholar]
- 29.Rothe M, Wong S C, Henzel W J, Goeddel D V. A novel family of putative signal transducers associated with the cytoplasmic domain of the 75 kDa tumor necrosis factor receptor. Cell. 1994;78:681–692. doi: 10.1016/0092-8674(94)90532-0. [DOI] [PubMed] [Google Scholar]
- 30.Scherer D C, Brockman J A, Chen Z, Maniatis T, Ballard D W. Signal-induced degradation of IκBα requires site-specific ubiquitination. Proc Natl Acad Sci USA. 1995;92:11259–11263. doi: 10.1073/pnas.92.24.11259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siow Y L, Kalmar G B, Sanghera J S, Tai G, Oh S S, Pelech S L. Identification of two essential phosphorylated threonine residues in the catalytic domain of Mekk1. Indirect activation by Pak3 and protein kinase C. J Biol Chem. 1997;272:7586–7594. doi: 10.1074/jbc.272.12.7586. [DOI] [PubMed] [Google Scholar]
- 32.Song H Y, Regnier C H, Kirschning C J, Goeddel D V, Rothe M. Tumor necrosis factor (TNF)-mediated kinase cascades: bifurcation of nuclear factor-κB and c-jun N-terminal kinase (JNK/SAPK) pathways at TNF receptor-associated factor 2. Proc Natl Acad Sci USA. 1997;94:9792–9796. doi: 10.1073/pnas.94.18.9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun S-C, Elwood J, Greene W C. Both amino- and carboxyl-terminal sequences within IκBα regulate its inducible degradation. Mol Cell Biol. 1996;16:1058–1065. doi: 10.1128/mcb.16.3.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thanos D, Maniatis T. NF-κB: a lesson in family values. Cell. 1995;80:529–532. doi: 10.1016/0092-8674(95)90506-5. [DOI] [PubMed] [Google Scholar]
- 35.Ting A T, Pimentel M F, Seed B. RIP mediates tumor necrosis factor receptor 1 activation of NF-κB but not Fas/APO-1-initiated apoptosis. EMBO J. 1996;15:6189–6196. [PMC free article] [PubMed] [Google Scholar]
- 36.Traenckner E B, Pahl H L, Henkel T, Schmidt K N, Wilk S, Baeuerle P A. Phosphorylation of human IκB-α on serines 32 and 36 controls IκB-α proteolysis and NF-κB activation in response to diverse stimuli. EMBO J. 1995;14:2876–2883. doi: 10.1002/j.1460-2075.1995.tb07287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verma I M, Stevenson J K, Schwarz E M, Van A D, Miyamoto S. Rel/NF-κB/IκB family: intimate tales of association and dissociation. Genes Dev. 1995;9:2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- 38.Wesche H, Henzel W J, Shillinglaw W, Li S, Cao Z. MyD88: an adapter that recruits IRAK to the IL-1 receptor complex. Immunity. 1997;7:837–847. doi: 10.1016/s1074-7613(00)80402-1. [DOI] [PubMed] [Google Scholar]
- 39.Whiteside S T, Ernst M K, LeBail O, Laurent-Winter C, Rice N, Israël A. N- and C-terminal sequences control degradation of MAD3/IκBα in response to inducers of NF-κB activity. Mol Cell Biol. 1995;15:5339–5345. doi: 10.1128/mcb.15.10.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Woronicz J D, Gao X, Cao Z, Rothe M, Goeddel D V. IκB kinase-beta: NF-κB activation and complex formation with IκB kinase-α and NIK. Science. 1997;278:866–869. doi: 10.1126/science.278.5339.866. [DOI] [PubMed] [Google Scholar]
- 41.Yan M, Templeton D J. Identification of 2 serine residues of MEK-1 that are differentially phosphorylated during activation by raf and MEK kinase. J Biol Chem. 1994;269:19067–19073. [PubMed] [Google Scholar]
- 42.Yeh W C, Shahinian A, Speiser D, Kraunus J, Billia F, Wakeham A, de la Pompa J, Ferrick D, Hum B, Iscove N, Ohashi P, Rothe M, Goeddel D V, Mak T W. Early lethality, functional NF-κB activation, and increased sensitivity to TNF-induced cell death in TRAF2-deficient mice. Immunity. 1997;7:715–725. doi: 10.1016/s1074-7613(00)80391-x. [DOI] [PubMed] [Google Scholar]
- 43.Zandi E, Rothwarf D M, Delhase M, Hayakawa M, Karin M. The IκB kinase complex (IKK) contains two kinase subunits, IKKα and IKKβ, necessary for IκB phosphorylation and NF-κB activation. Cell. 1997;91:243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]
- 44.Zheng C F, Guan K L. Activation of MEK family kinases requires phosphorylation of two conserved Ser/Thr residues. EMBO J. 1994;13:1123–1131. doi: 10.1002/j.1460-2075.1994.tb06361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]