Abstract
Intramedullary spinal cord abscess is a rare and severe infectious disease characterized by devastating neurological deficits. We report a case of cervical intramedullary spinal cord abscess in a 74-year-old diabetic male with a 3-day history of neck pain and weakness in the right lower extremity. Magnetic resonance imaging revealed a ring-shaped contrast lesion in C3-C6 of the cervical spinal cord with extensive edema. Further, 1 day after admission, he became comatose (Glasgow Coma Scale E1VtM1), and a computed tomography head scan revealed hydrocephalus. Despite emergency ventricular drainage, the patient's level of consciousness remained unchanged. Magnetic resonance imaging performed 1 day after surgery revealed bilateral intracranial extension of the abscess into the thalamus and caudate nucleus. The patient died 19 days after admission. Our report is the first case of extensive brain abscess development over a short period. Based on our experience, prompt administration of antibiotics and emergency abscess drainage of the cervical cord (and ventricular drainage, if necessary) are recommended in cases of neurological deterioration in patients with cervical intramedullary spinal cord abscess.
Keywords: intramedullary spinal cord abscess, cervical, infectious disease
Introduction
An intramedullary spinal cord abscess (ISCA) is a rare and life-threatening infectious disease, and an incorrect diagnosis can result in fatal outcomes.
Approximately 140 cases of ISCA have been reported in the literature.1) Clinical manifestations can vary depending on the spinal level and location of the abscess. A review of 137 cases showed that the clinical symptoms are motor (98.5%), sensory (87.1%), pain (55.7%), and urinary dysfunction (50%).2) ISCA results from infection of the dermal sinus (29%), sepsis (27%), and endocarditis (1%); 43% of the cases are considered cryptogenic.3) Differential diagnoses of ISCA include malignant lymphoma, neoplastic infiltration, transverse myelitis due to multiple sclerosis, neuromyelitis optica, tuberculosis, sarcoidosis, and viral myelitis.4) The magnetic resonance imaging (MRI) features of ISCA include high signals on DWI, ring-shaped contrast effects, and common upper and lower high-signal areas on T2-weighted images, implying edema, which are useful diagnostic features.5)
Here, we describe a rapidly progressive course of cervical ISCA in a 74-year-old male that resulted in death.
Case Report
A 74-year-old male presented to a regional hospital with a 3-day right shoulder and neck pain. He had diabetes and had been consuming oral anti-diabetic medications for the past 10 years. Subsequently, he developed right lower limb weakness and gait disturbances. He visited the same physician who had performed an MRI of his neck, which revealed cervical lesions. The patient was then referred to a neurosurgeon for further evaluation. However, given his worsening symptoms, he was urgently admitted to the same hospital 4 days after the first consultation. The patient could barely walk. After admission, he showed decreased spontaneous respiration and required intubation. Hence, the patient was transferred to our specialized hospital with a suspected cervical tumor. At the time of arrival, his blood pressure was 98/64 mmHg, pulse rate was 59 beats per min, body temperature was 35.5°C, and respiration rate was 20 beats per min. Even when intubated, the patient communicated by blinking, and no cranial nerve impairment was observed. Manual muscle testing of the upper and lower limbs revealed grade 1 muscle strength. His sense of touch, temperature, and pain markedly decreased below the Th2 level, and he had neck stiffness. Blood tests revealed an elevated white blood cell count (10,120/μL; neutrophils 86.6%) and a C-reactive protein level of 0.940 mg/dL. His blood glucose level was 189 mg/dL, and his hemoglobin A1c level was 7.7%. Tests for tumor markers (carcinoembryonic antigen, α-fetoprotein, carbohydrate antigen 19-9, squamous cell carcinoma antigen, prostate-specific antigen, and soluble interleukin-2 receptor) were all negative. Cerebrospinal fluid (CSF) examination revealed an elevated cell count (1,098/μL) and a protein level of 588 mg/dL; however, the CSF glucose level was within the standard limits (84 mg/dL). The CSF culture indicated the presence of Streptococcus intermedius, which is sensitive to meropenem and vancomycin.
MRI revealed a ring-shaped contrast lesion in C3-C6 of the cervical spinal cord with extensive edema (Fig. 1a, b). Hence, we diagnosed it as a cervical neoplasm with a differential diagnosis of an infectious disease. Treatment with meropenem (6 g/day) and vancomycin (2 g/day) was initiated and surgery to remove the lesion was scheduled in the next 3 days. Subsequently, there was a temporary improvement in muscle strength to grade 2 in both upper extremities and a shape change in the contrast effect on MRI 1 day after admission (Fig. 1c). However, after MRI, the patient's level of consciousness suddenly decreased (Glasgow Coma Scale E1VtM1), and a computed tomography (CT) head scan revealed hydrocephalus (Fig. 2a) and a low-density area in the posterior inferior cerebellar artery region of the right cerebellum (Fig. 2b). Three-dimensional CT angiography revealed no evidence of posterior circulation occlusion (Fig. 2c). The patient's spontaneous respiration had completely disappeared, and his blood pressure could no longer be maintained without medication; thus, an emergency ventricular drainage was performed. However, despite ventricular drainage, his consciousness level remained unchanged (Glasgow Coma Scale E1VtM1), and a CT scan revealed a markedly high symmetric low-density signal in diffusion-weighted imaging (DWI) areas, including the thalamus and caudate nucleus (Fig. 2d, e, f, g). At this point, intracranial extension of a cervical spinal cord abscess was diagnosed. While administration of antibiotics was continued, no improvement was observed, and the patient died 19 days after admission.
Fig. 1.

Contrast-enhanced (a and c) and T2-weighted (b and d) MR images acquired in the sagittal plane. a, b: Ring-shaped contrast lesions at C3-C6 with extensive edema. c: MR images after starting antibiotic treatment. A change in the shape of the contrasting lesion is observed. d: Widening of the upper and lower edematous regions. MR: magnetic resonance
Fig. 2.

Imaging test findings. a: Head CT scan at the time of a sudden loss of consciousness revealing acute hydrocephalus. b: Low-density areas of the right cerebellar hemisphere. c: No vascular occlusive findings on 3D-CTA. d: CT performed 1 day after ventricular drainage, revealing symmetrical low-density areas in the caudate nucleus and thalamus. e, f: Diffusion-weighted MR images leading to abscess diagnosis. g: T1-weighted contrast-enhanced MR. CT: computed tomography. 3D-CTA: 3-dimensional computed tomography angiography. MR: magnetic resonance
A pathological autopsy revealed intramedullary abscesses throughout the spinal cord, gliosis around the abscesses, and circumferential inflammatory cell proliferation around the anterior spinal artery and vasa corona (Fig. 3).
Fig. 3.
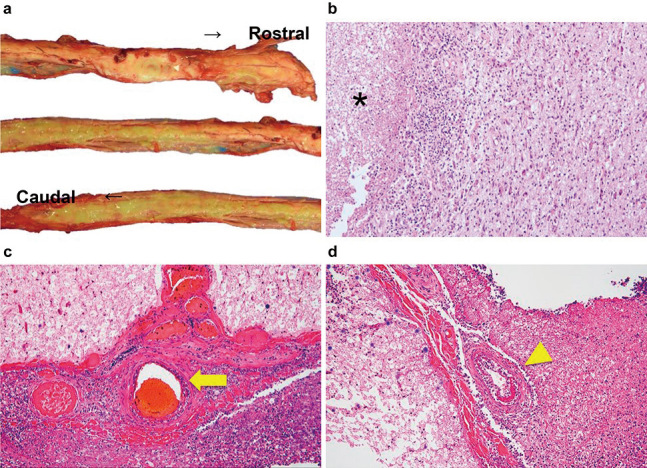
Macrograph of the spinal cord (a) and photomicrographs of hematoxylin and eosin staining (b, c, and d). Magnification: ×100 (b) and ×400 (c, d).
a: Yellowish intramedullary abscesses throughout the spinal cord. b: Reactive gliosis surrounding the abscess (*). c, d: Inflammatory cell infiltration around the anterior spinal artery (arrow) and vasa corona (arrowhead).
The development of abscesses in the brain could be attributed to two mechanisms. First, the ruptured abscess may have spread from the spinal cord to the brain ventricles via the CSF. Second, a hematogenous mechanism may have caused the abscess to spread to the brain because the intracranial abscess was symmetrical, and pathological examination revealed inflammatory cell infiltration around the blood vessels. The pathologist also noted extensive histological edema and ischemic changes in the brain parenchyma, suggesting that the brain herniation may have caused circulatory disturbances. Consent was obtained from the patient's family.
Discussion
The reported patient had a rapidly progressing cervical ISCA and had been consuming oral diabetic medications but not steroids or immunosuppressive drugs. Blood tests and a whole-body CT scan did not reveal any neoplastic or other causative diseases.
In a review of 26 ISCA cases by Kurita et al., 21 patients had spinal fluid cultures that yielded pathogenic organisms, Staphylococcus aureus (n = 3).6) Another review of 20 ISCA cases by Jabbar et al. revealed that S. intermedius was the causative agent in one case (5%).2) S. intermedius, a beta-hemolytic gram-positive bacterium from the Streptococcus anginosus group, is a component of the normal microflora and one of the most common pathogens associated with brain and liver abscesses.7) The potential risk factors for S. intermedius infection included a history of dental treatment (18.8%), sinusitis (11.9%), and diabetes (7.9%).7) Although our patient had diabetes, the mechanism of the abscess formation was considered cryptogenic.
After the initiation of antibiotic therapy, our patient showed temporary improvement in paralysis in both upper extremities and a change in the shape of the contrast effect on MRI (Fig. 1c) due to decompression of the ruptured abscess. MRI revealed enlarged high-signal areas on T2-weighted images around the abscess (Fig. 1d), and the patient soon developed a sudden loss of consciousness and acute hydrocephalus. Only two cases of ISCA have been reported to have acute hydrocephalus, both with cervical ISCA.8,9) Sinha et al. considered that obstructive hydrocephalus is caused by edema in the upper cervical spine region, including the craniocervical junction, due to mass effects.8) One of the two patients who survived underwent emergency ventricular and abscess drainage. Thus, hydrocephalus and impaired consciousness in patients with cervical ISCA may require simultaneous ventricular and abscess drainage. To the best of our knowledge, only four cases of ISCA with concomitant brain abscesses have been reported in the literature,9-12) and intracranial abscesses were relatively small nodular lesions. Some of these patients were cured with medical management using antibiotics alone. Our report is the first case of an extensive brain abscess development over a short period. Although the risk factors for the intracranial extension of ISCA are unknown, clinicians should always bear in mind that some cases of ISCA can have such a dramatic course.
Five deaths caused by adult ISCA have been reported,13-16) four of which were associated with cervical ISCA. The causes of death included ventriculitis, cerebral infarction due to vasculitis, respiratory failure, and sudden cardiac arrest. Cervical ISCA has a poor prognosis because of its anatomical proximity to the brainstem and propensity for intracranial extension. In our case, the rapid deterioration and subsequent death of the patient were attributed to delayed treatment. The initial antibiotic administration and emergency drainage of the cervical cord abscess after transfer to our facility may have improved the outcome. Based on our experience, prompt antibiotic administration and urgent drainage of the cervical cord abscess (and ventricular drainage, if necessary) are recommended for patients with cervical ISCA and neurological deterioration.
ISCA is a rare and severe disease requiring prompt diagnosis and treatment. Clinicians should familiarize themselves with its clinical features, diagnostic considerations, and treatment outcomes.
Abbreviations
intramedullary spinal cord abscess (ISCA), magnetic resonance imaging (MRI), computed tomography (CT), cerebrospinal fluid (CSF), diffusion-weighted imaging (DWI) areas
Informed Consent
Consent was obtained from the patient's family.
Conflicts of Interest Disclosure
The authors declare that there are no conflicts of interest.
References
- 1). Szmyd B, Jabbar R, Lusa W, et al. : What is currently known about intramedullary spinal cord abscess among children? A concise review. J Clin Med 11: 4549, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). Jabbar R, Szmyd B, Jankowski J, et al. : Intramedullary spinal cord abscess with concomitant spinal degenerative diseases: a case report and systematic literature review. J Clin Med 11: 5148, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Verdier EP, Konsol O, Portillo S: Intramedullary cervical abscess mimicking a spinal cord tumor in a 10-year-old girl: a case-based review. Childs Nerv Syst 34: 2143-2147, 2018 [DOI] [PubMed] [Google Scholar]
- 4). Higuchi K, Ishihara H, Okuda S, Kanda F: A 51-year-old man with intramedullary spinal cord abscess having a patent foramen ovale. BMJ Case Rep 2011: bcr1120103512, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Murphy KJ, Brunberg JA, Quint DJ, Kazanjian PH: Spinal cord infection: myelitis and abscess formation. AJNR Am J Neuroradiol 19: 341-348, 1998 [PMC free article] [PubMed] [Google Scholar]
- 6). Kurita N, Sakurai Y, Taniguchi M, Terao T, Takahashi H, Mannen T: Intramedullary spinal cord abscess treated with antibiotic therapy-case report and review. Neurol Med Chir (Tokyo) 49: 262-268, 2009 [DOI] [PubMed] [Google Scholar]
- 7). Issa E, Salloum T, Tokajian S: From normal flora to brain abscess: a review of streptococcus intermedius. Front Microbiol 11: 826, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Sinha P, Parekh T, Pal D: Intramedullary abscess of the upper cervical spinal cord. Unusual presentation and dilemmas of management: case report. Clin Neurol Neurosurg 115: 1845-1850, 2013 [DOI] [PubMed] [Google Scholar]
- 9). Erlich JH, Rosenfeld JV, Fuller A, Brown GV, Wodak J, Tress BP: Acute intramedullary spinal cord abscess: case report. Surg Neurol 38: 287-290, 1992 [DOI] [PubMed] [Google Scholar]
- 10). Luo W, Yin Y, Liu W, Ren H: Intramedullary spinal cord abscess with brain abscess due to subacute infective endocarditis. BMC Neurol 23: 18, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Virtanen PS, Jimenez MJD, Horak VJ, Desai VR, Manaloor JJ, Raskin JS: Concomitant brain abscess and spinal cord abscess in an immunocompetent teenage male: illustrative case. J Neurosurg Case Lessons 5: CASE22458, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Durmaz R, Atasoy MA, Durmaz G, et al. : Multiple nocardial abscesses of cerebrum, cerebellum and spinal cord, causing quadriplegia. Clin Neurol Neurosurg 103: 59-62, 2001 [DOI] [PubMed] [Google Scholar]
- 13). Agyei JO, Qiu J, Fabiano AJ: Fusarium species intramedullary spinal cord fungus ball: case report. J Neurosurg Spine 31: 440-446, 2019 [DOI] [PubMed] [Google Scholar]
- 14). McCaslin AF, Lall RR, Wong AP, Lall RR, Sugrue PA, Koski TR: Thoracic spinal cord intramedullary aspergillus invasion and abscess. J Clin Neurosci 22: 404-406, 2015 [DOI] [PubMed] [Google Scholar]
- 15). Samkoff LM, Monajati A, Shapiro JL: Teaching neuroImage: Nocardial intramedullary spinal cord abscess. Neurology 71: e5, 2008 [DOI] [PubMed] [Google Scholar]
- 16). Vora YA, Raad II, McCutcheon IE: Intramedullary abscess from group F Streptococcus. Surg Infect (Larchmt) 5: 200-204, 2004 [DOI] [PubMed] [Google Scholar]


