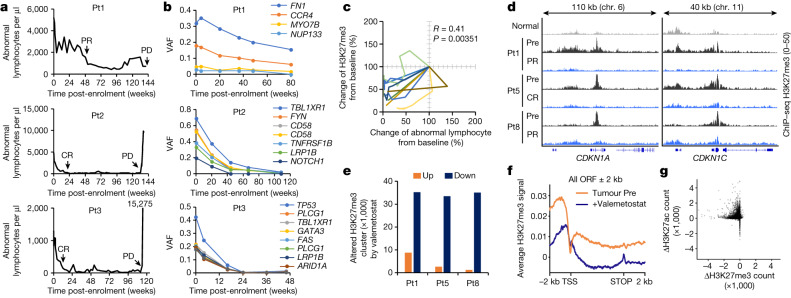
Fig. 1. Antitumour effect of valemetostat.
a, Changes in abnormal lymphocytes of three cases in a first-in-human valemetostat phase I study. Valemetostat was administered orally once daily (200 mg daily) until a sign of disease progression was observed. Clinical diagnoses (partial response (PR), complete response (CR) and progressive disease (PD)) are annotated. b, Changes in variant allele frequency (VAF) of major somatic mutations from the initiation of treatment identified by targeted deep sequencing of peripheral blood. c, Correlation between changes of abnormal lymphocyte and H3K27me3 from baseline (%) in nine patients. d, Representative tracks (CDKN1A and CDKN1C loci) for H3K27me3 in Pt1, Pt5 and Pt8 before and after valemetostat treatment. Chr., chromosome. e, The number of altered H3K27me3 clusters after treatment in three cases detected by ChIP–seq. f, Average ChIP–seq signal profiles for H3K27me3 in tumour baseline (Pre) and after treatment (48 weeks) around the TSS and across the gene body. ORF, open reading frame. g, Treatment-associated changes of H3K27me3 (x axis) and H3K27ac (y axis) at ChIP–seq-merged all peaks. Statistics and reproducibility are described in the Methods.