Abstract
An inward Shaker K+ channel identified in Zea mays (maize), ZmK2.1, displays strong regulation by external K+ when expressed in Xenopus laevis (African clawed frog) oocytes or COS cells. ZmK2.1 is specifically activated by K+ with an apparent Km close to 15 mM independent of the membrane hyperpolarization level. In the absence of K+, ZmK2.1 appears to enter a nonconducting state. Thus, whatever the membrane potential, this maize channel cannot mediate K+ influx in the submillimolar concentration range, unlike its relatives in Arabidopsis thaliana. Its expression is restricted to the shoots, the strongest signal (RT-PCR) being associated with vascular/bundle sheath strands. Based on sequence and gene structure, the closest relatives of ZmK2.1 in Arabidopsis are K+ Arabidopsis Transporter 1 (KAT1) (expressed in guard cells) and KAT2 (expressed in guard cells and leaf phloem). Patch-clamp analyses of guard cell protoplasts reveal a higher functional diversity of K+ channels in maize than in Arabidopsis. Channels endowed with regulation by external K+ similar to that of ZmK2.1 (channel activity regulated by external K+ with a Km close to 15 mM, regulation independent of external Ca2+) constitute a major component of the maize guard cell inward K+ channel population. The presence of such channels in maize might reflect physiological traits of C4 and/or monocotyledonous plants.
INTRODUCTION
Potassium can constitute up to 10% of total plant dry weight. Being the most abundant cation in the cytosol, it plays a role in basic cellular functions such as control of osmotic pressure and membrane potential. Several types of K+ transport systems, differing in transport affinity, energetic coupling, voltage sensitivity, or ionic selectivity, have been identified in plant cell membranes by (electro)physiological analyses. The differences in functional properties and spatial distribution of the transport systems are thought to allow the fine tuning of K+ transport under varying environmental conditions in different plant tissues. During the past decade, considerable progress has been made in the molecular identification of these transport systems. Coupling molecular approaches with functional analyses has allowed the identification and characterization of several families of K+ channels and K+ carriers, coding for at least 28 transport systems in Arabidopsis thaliana, for example (Mäser et al., 2001; Véry and Sentenac, 2003).
Until now, the most extensively characterized family of plant K+ transport systems is the Shaker family (Véry and Sentenac, 2003). Shaker-like genes, which code for highly K+-selective channels, have been identified in animals, plants, fungi, and prokaryotes (Jan and Jan, 1997; Chalot et al., 2002; Ruta et al., 2003). In plants, Shaker channels provide major pathways for wholesale K+ uptake or secretion in several tissues and cell types. For instance, they have been shown to play crucial roles in root K+ uptake from the soil (Hirsch et al., 1998), K+ secretion into the xylem sap (Gaymard et al., 1998), K+ transport into or from guard cells during stomatal movements (Kwak et al., 2001; Hosy et al., 2003), and K+ uptake in pollen for tube elongation (Mouline et al., 2002). In Arabidopsis, several inward Shakers have been shown to contribute to K+ uptake over a wide range of external K+ concentrations, from micromolar to millimolar (Hirsch et al., 1998; Dennison et al., 2001; Mouline et al., 2002). This was unexpected because earlier electrophysiological analyses had led to the hypothesis that inward K+ channels contributed to K+ uptake only from high external concentrations (Kochian and Lucas, 1988; Maathuis and Sanders, 1996), in the range corresponding to the so-called Epstein mechanism II (Epstein and Rains, 1963).
To date, Arabidopsis is the only plant species for which the complete set of Shaker channels has been identified and almost entirely characterized. It is also the only plant species in which the functions of plant Shakers have been analyzed by reverse genetics approaches. In other plant species, only a few Shakers have been thoroughly studied. They all have a close relative within the Arabidopsis Shaker family, and none of them has been identified as displaying major differences in functional properties compared with its Arabidopsis counterpart (Véry and Sentenac, 2003). This absence of specific functional features has reinforced the role of model that Arabidopsis plays in this field.
Here, we characterize a maize (Zea mays) inward Shaker, ZmK2.1, and show that it is strictly devoted to K+ uptake in the concentration range corresponding to Epstein mechanism II, in contrast with its Arabidopsis relatives. This inward channel is endowed with a regulation not observed previously in related Shakers from other plant species. Indeed, ZmK2.1 is strongly regulated by the external concentration of K+: a decrease in external K+ decreases the channel activity, leading, in the absence of K+, to a complete nonconducting state. We show that this regulation, which is independent of channel gating by membrane hyperpolarization, occurs within the physiological external K+ range, restricting the role of this maize channel to low-affinity K+ uptake. This work suggests that significant functional differences exist between related Shaker channels from different plant species, monocots and dicots, which may underlie differences in plant development and physiology.
RESULTS
Identification of the Maize ZmK2.1 Shaker Channel Gene
Low-stringency screening of a maize cDNA library using a probe derived from the Arabidopsis K+ channel KAT1 (for K+ Arabidopsis Transporter 1) led to the identification of a partial cDNA incomplete at the 3′ end. A full-length cDNA (GenBank accession number AY461584; length of the 5′ untranslated region, open reading frame, and 3′ untranslated region: 100, 2175, and 124 bp, respectively) was obtained by 3′ rapid amplification of cDNA ends. PCR experiments performed on maize genomic DNA allowed us to check the cDNA sequence and to determine the structure of the corresponding gene (GenBank accession number AY461583). Sequence analysis and functional characterization of the encoded channel (see below) led us to name this gene ZmK2.1 (for Zea mays K+ group 2 Shaker, according to the nomenclature proposed by Pilot et al. [2003]; see below). DNA gel blot analyses (data not shown) using the radiolabeled full-length cDNA as a probe suggested that ZmK2.1 is present as a single copy in the maize genome.
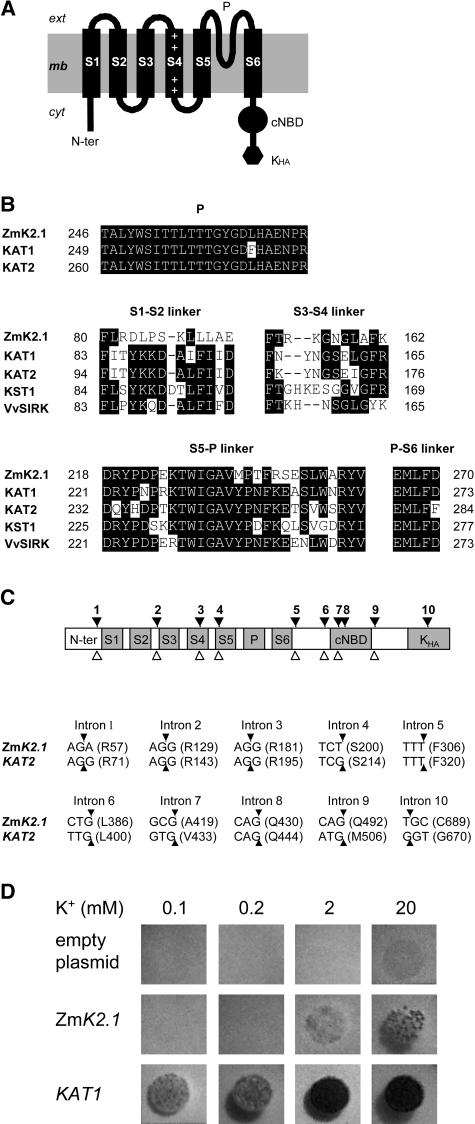
Sequence analyses indicated that ZmK2.1 is a member of the plant Shaker family (Mäser et al., 2001; Véry and Sentenac, 2003). The deduced polypeptide (725 amino acids, 83 kD) displays the typical Shaker channel hydrophobic core (Figure 1A). The Shaker core consists of six probable transmembrane segments named S1 to S6. S4 carries positive residues, a typical feature allowing this segment to act as a voltage sensor. A pore domain, named P (including the pore helix and the selectivity filter), is present between S5 and S6. The P domain of ZmK2.1 harbors a GYG motif (Figure 1B), a hallmark of highly K+-selective channels. The cytosolic C-terminal region of ZmK2.1 comprises a putative cyclic nucleotide binding domain and a KHA domain (rich in hydrophobic and acidic residues), which are found in all plant Shakers (Véry and Sentenac, 2003). ZmK2.1, like most of its closest relatives in the Shaker family (group 2 channels), does not harbor any ankyrin domain.
Figure 1.
Identification of ZmK2.1, a Maize Inward K+ Channel of the Shaker Family.
(A) Topology of the ZmK2.1 polypeptide. The hydrophobic core of Shaker channels typically displays six transmembrane segments, S1 to S6. S4, the channel voltage sensor, carries positively charged residues (+). The pore-forming domain (P) is present between S5 and S6. A putative cyclic nucleotide binding domain (cNBD) and a domain rich in hydrophobic and acidic residues (Kha) are present in the cytoplasmic C-terminal region.
(B) Comparison of the ZmK2.1 amino acid sequence with that of group 2 Shakers from other plant species (KAT1 and KAT2 from Arabidopsis, KST1 from potato, and VvSIRK from grapevine). Top, Alignment of the pore domains. Middle and bottom, Alignment of the S1-S2, S3-S4, S5-P, and P-S6 extracellular linkers.
(C) Structure of ZmK2.1. Top, The positions of the 10 introns identified in the ZmK2.1 gene, indicated by black arrowheads, strictly correspond to those of the 10 introns identified in the Arabidopsis KAT2 Shaker channel gene. The open arrowheads below indicate the positions of the eight introns in the Arabidopsis KAT1 Shaker gene. Bottom, Conservation of intron positions within the coding sequence between ZmK2.1 and KAT2. For each intron, the interrupted codon, if any (the arrowheads indicate the positions of the interrupting introns), or the codon just upstream of the intron is given. The corresponding amino acid (single-letter code) and the position of this residue in the predicted sequence are indicated in parentheses.
(D) Yeast complementation tests. The yeast Wagf2 strain deficient for K+ uptake was transformed with either the empty pFL61 plasmid (control), ZmK2.1 cDNA in pFL61, or the Arabidopsis KAT1 cDNA in pFL61. Drop tests were performed on selective agar media containing 0.1, 0.2, 2, or 20 mM K+ (added as KCl). The plates were photographed after 3 d of incubation at 28°C.
Based on sequence and gene structure analyses, the plant Shaker family (nine members in Arabidopsis) has been divided into five groups named groups 1 to 5 (Arabidopsis group leaders: Arabidopsis K+ Transport System 1 [AKT1], KAT1, AKT2, K+ channel 1, and Stelar K+ Outward Rectifier, respectively) (Pilot et al., 2003). Phylogenetic analysis indicates that ZmK2.1 belongs to group 2. Three Shakers have been characterized in maize: Z. mays K+ channel 1 (ZMK1), ZMK2, and K+ channel Z. mays 1 (KZM1) (Philippar et al., 1999, 2003). In addition, a cDNA sequence of a fourth maize Shaker has been deposited in GenBank (accession number AJ558238) and named KZM2. ZmK2.1 polypeptide shares approximately 40% identity with ZMK1 and ZMK2, which belong to groups 1 and 3, respectively, and 51% identity with the (as yet uncharacterized) KZM2 sequence, which belongs to group 2. The percentage of identity is much higher with KZM1 (94%), which also belongs to group 2. Although sequence alignment indicates that ZmK2.1 is shorter than KZM1 by 32 amino acids at its N-terminal end, the high percentage of identity between the two deduced polypeptides (isolated from different cultivars) and common features in the expression patterns (see below) suggest that ZmK2.1 and KZM1 are different alleles of the same gene. ZmK2.1 shares approximately 50% identity over the entire polypeptide sequence with the other group 2 channels characterized to date: KAT1 (Anderson et al., 1992) and KAT2 (Pilot et al., 2001) from Arabidopsis, K+ channel of Solanum tuberosum 1 (KST1) from potato (Müller-Röber et al., 1995), and Stomatal Inward Rectifying K+ channel from Vitis vinifera (VvSIRK; grapevine) (Pratelli et al., 2002). Within the transmembrane region, the percentage of identity between ZmK2.1 and the latter channels is 70 to 80%. The pore domain and the voltage sensor are especially conserved. ZmK2.1 has exactly the same P domain as KAT2 and differs from KAT1 in this region by only one residue (Figure 1B). The percentage of identity in the voltage sensor reaches 89 to 93%. The most divergent regions within the hydrophobic core are the two extracellular linkers S1-S2 and S3-S4 (less than 25 and 40% identity, respectively; Figure 1B).
Analysis of the intron positions showed that the gene structure is strongly conserved between ZmK2.1 and the Arabidopsis KAT1 and KAT2 genes. Eight introns can be identified in KAT1 and 10 in KAT2, each of the 8 KAT1 introns having a counterpart, strictly at the same location, in KAT2 (Pilot et al., 2003). Interestingly, 10 introns can be identified in ZmK2.1, exactly at the same positions, at the nucleotide level in the sequence alignment, as in KAT2 (Figure 1C). Thus, based on gene structure analysis, ZmK2.1 is closer to KAT2 than to KAT1, and ZmK2.1 and KAT2 are closer to each other than are the two Arabidopsis genes KAT1 and KAT2.
All plant Shaker channels from group 2 characterized to date are inward rectifiers. As a first step toward functional characterization, ZmK2.1 was expressed in a Saccharomyces cerevisiae (yeast) mutant strain defective for K+ uptake and displaying reduced growth on media containing less than ∼50 mM K+. Control experiments were performed using the Arabidopsis KAT1 channel, known to allow yeast growth rescue in the presence of submillimolar K+ concentrations (Anderson et al., 1992). Expression of ZmK2.1, like that of KAT1, was able to rescue yeast growth (Figure 1D), indicating that ZmK2.1, like other group 2 Shakers, can mediate wholesale K+ uptake. However, growth rescue in cells expressing ZmK2.1 occurred at a much higher external K+ concentration (in the 2 to 20 mM concentration range) than in cells expressing KAT1.
Localization of ZmK2.1 Expression in the Plant
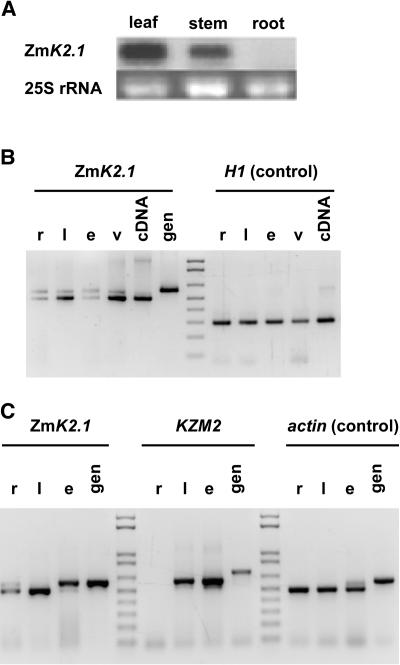
RNA gel blot experiments revealed the presence of ZmK2.1 transcripts in shoots (leaves and stem) but not in roots (Figure 2A). Further analysis of ZmK2.1 expression was performed by RT-PCR (Figure 2B), again showing higher transcript levels in leaves than in roots. In leaves, ZmK2.1 transcripts were more abundant in vascular/bundle sheath strands (central vein) than in epidermis (Figure 2B).
Figure 2.
Expression of ZmK2.1 in the Plant.
(A) RNA gel blot analysis. The blot (10 μg of total RNA per lane) was hybridized with 32P-labeled ZmK2.1 probe.
(B) RT-PCR analysis. r, root; l, leaf without central vascular/bundle sheath strand; e, epidermis; v, central vascular/bundle sheath strand; gen, genomic DNA. Histone H1 was used as a control.
(C) Comparison of ZmK2.1 and KZM2 expression by RT-PCR analysis. l, leaf; r, e, and gen, same as in (B). Actin was used as a control. The actual amplification of KZM2 (GenBank accession number AJ558238) cDNA was checked by sequencing the PCR products.
The expression pattern of KZM2, the as yet uncharacterized likely paralog of ZmK2.1, was analyzed in parallel experiments, revealing similar features. As observed for ZmK2.1, the transcript level was much higher in leaves than in roots (Figure 2C). In the former organs, it was abundant in vascular/bundle sheath strands (data not shown) and in epidermis (Figure 2C). Comparison of the relative intensities of signals revealed that ZmK2.1 transcripts were less abundant than KZM2 transcripts in the epidermis extracts (Figure 2C). Quantification of the transcript levels (Image Gauge 4.0; Fuji Photo Film, Tokyo, Japan) using actin or histone H1 as a control led to epidermis-to-whole leaf transcript ratios of 0.3 ± 0.05 (n = 5) for ZmK2.1 and 1.6 ± 0.16 (n = 4) for KZM2.
Functional Characterization of ZmK2.1 in Xenopus Oocytes and COS Cells
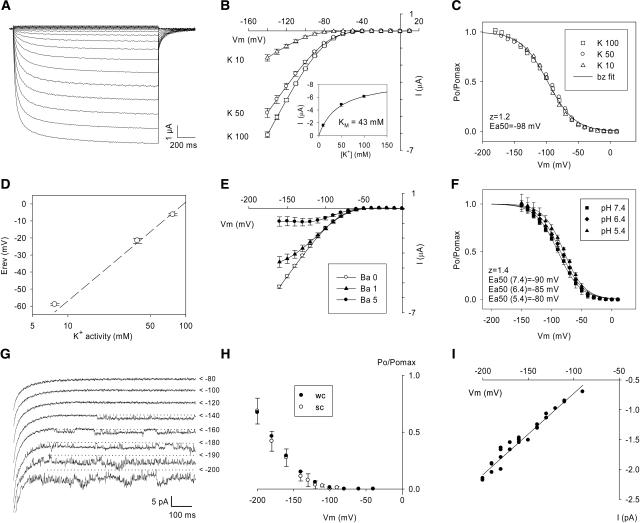
Expressed in Xenopus laevis oocytes, ZmK2.1 mediated inwardly rectifying currents when the membrane was clamped at potentials more negative than −50 mV (Figure 3A). The magnitude of the current was dependent on the external K+ concentration (Figure 3B). The relationship between current intensity and K+ concentration suggested a saturation mechanism with an apparent Km of approximately 40 mM (Figure 3B). ZmK2.1 gating was voltage-dependent. The voltage dependence of the relative open probability was independent of the external K+ concentration in the 10 to 100 mM range (Figure 3C), as already reported for the other plant inwardly rectifying Shakers (Pratelli et al., 2002; Véry and Sentenac, 2002). Analysis of zero-current potentials in bath solutions containing both K+ and Na+ indicated, as expected from the presence of the GYG motif in the pore sequence, that ZmK2.1 is selective for K+ over Na+ (Figure 3D). ZmK2.1 currents were blocked by Ba2+ in a voltage-dependent manner (Figure 3E). Tetraethylammonium (6 mM) or Cs+ (0.4 mM) inhibited 50% of ZmK2.1 currents recorded in 50 mM K+, independent of voltage (n = 3; data not shown). ZmK2.1 was weakly sensitive to external pH: a 10-mV positive shift in voltage dependence of activation was observed upon acidification from pH 7.4 to 5.4 (Figure 3F).
Figure 3.
Conduction and Gating Properties of ZmK2.1 Expressed in Xenopus Oocytes ([A] to [F]) or COS Cells ([G] to [I]).
(A) Inwardly rectifying currents recorded in an oocyte expressing ZmK2.1. Voltages applied from a holding potential of −40 mV ranged from −140 to +10 mV with an increment of 10 mV. K+ concentration in the bath was 100 mM (pH 7.4).
(B) Current-voltage relationships at steady state in different bath K+ concentrations (10, 50, or 100 mM, pH 7.4). Inset, Mean currents recorded at −140 mV plotted versus the bath K+ concentration were fitted with a Michaelis-Menten equation (solid line), leading to an apparent Km of 43 mM.
(C) Analysis of ZmK2.1 activation at different external K+ concentrations (10, 50, or 100 mM [90, 50, and 0 mM NaCl, respectively], pH 7.4). The solid line is a Boltzmann (bz) fit of the relative open probability (Po/Pomax) versus membrane potential (Véry et al., 1995). z and Ea50, equivalent gating charge and half-activation potential, respectively, obtained from the Boltzmann fit.
(D) Analysis of ZmK2.1 selectivity. Zero-current potentials (Erev) were determined in bath solutions differing in K+ and Na+ concentrations (10, 50, or 100 mM K+, along with 90, 50, or 0 mM Na+, respectively, pH 7.4).
(E) ZmK2.1 blockage by Ba2+ at steady state. BaCl2 was present in the bath at 0, 1, or 5 mM. K+ concentration was 50 mM (pH 7.4).
(F) Effect of external pH on ZmK2.1 activation. K+ concentration in the bath was 50 mM. Lines are Boltzmann fits.
Data in (B), (D), (E), and (F) are means ± sd (n = 3).
(G) Single-channel current traces recorded in COS cells in the outside-out patch-clamp configuration. External and internal K+ concentrations were 50 and 150 mM, respectively. Applied voltages from a holding potential of 0 mV are indicated at right of the traces. Carets mark the current levels corresponding to closed ZmK2.1 channels.
(H) Comparison of ZmK2.1 voltage dependence determined either at the single-channel (sc) or whole cell (wc) level in COS cells. Analyzed single-channel (outside-out patch configuration) and whole cell data were from the same cell. Ionic conditions were as described for (G). The relative open probability data at the single-channel level are means from six successive recordings ± se.
(I) Single-channel conductance (recording conditions as described for [G]).
ZmK2.1 was also expressed in mammalian COS cells. Its functional properties in this expression system were basically very similar to those depicted in Xenopus oocytes. Slight differences in activation parameters were observed, however, as frequently reported when the functional properties of a given channel are compared in different expression systems (Dreyer et al., 1999; Decher et al., 2003). In COS cells, ZmK2.1 activation occurred at more negative membrane potentials than in oocytes (mean half-activation potential ± sd [n = 11]: −105 ± 7 mV in oocytes versus −141 ± 19 mV in COS cells). Also, the slope of the voltage dependence of activation was slightly reduced in COS cells (mean apparent gating charge ± sd [n = 11]: 1.3 ± 0.1 in oocytes versus 1.1 ± 0.15 in COS cells). Single-channel features of ZmK2.1 were investigated in COS cells in the outside-out patch configuration (Figures 3G to 3I). ZmK2.1 was identified at the single-channel level on the basis of a voltage dependence similar to that obtained at the whole cell level (Figures 3G and 3H) and a selectivity for K+. The channel conductance was 14 pS in the presence of 50 mM external K+ (Figure 3I). In subsequent experiments, the COS cell expression system was preferred to the Xenopus oocyte system because it generally produced fewer endogenous currents at very hyperpolarized membrane potentials (see Figure 5A, which shows absence of endogenous current down to at least −200 mV).
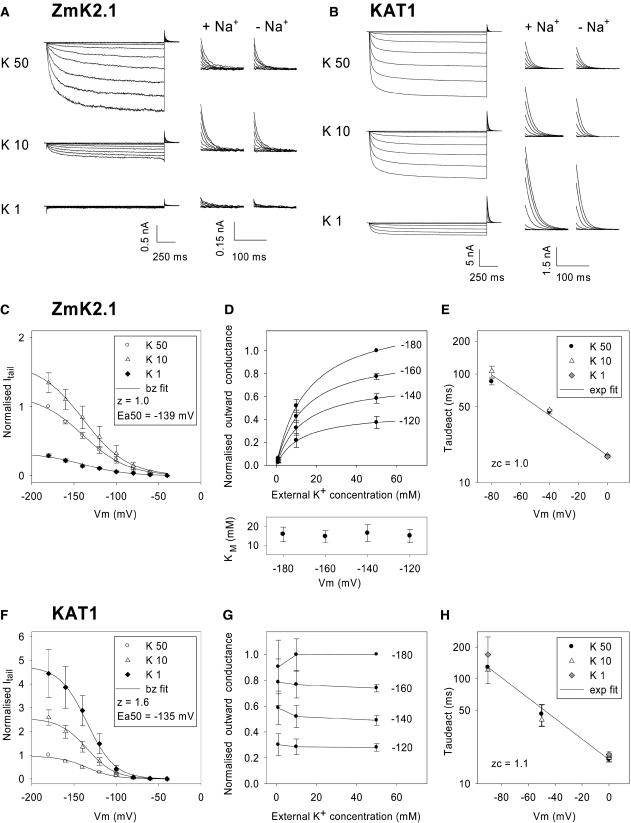
Figure 5.
Effect of External K+ Concentration on ZmK2.1 and KAT1 Activity.
(A) and (B) Current traces recorded in COS cells expressing either ZmK2.1 (A) or KAT1 (B) in the presence of different external K+ concentrations: 50, 10, or 1 mM. The internal K+ concentration was 150 mM. The voltage-clamp protocol consisted of a channel activation step, with applied voltages ranging from −40 to −200 mV, followed by a deactivation step at 0 mV. At left, full traces are shown. The solutions containing 10 or 1 mM K+ were supplemented with 40 or 49 mM NaCl, respectively, for ionic and osmotic strength adjustment. Middle and right, Zoom on deactivation currents recorded at 0 mV after the activation step. In the middle panels (+ Na+), the ionic strength of the bath solutions containing 10 or 1 mM K+ was adjusted with NaCl (as at left). In the right panels (− Na+), no NaCl was added to the bath solutions, the decrease in KCl concentration being compensated for at the osmotic level with mannitol.
(C) to (H) Analysis of ZmK2.1 ([C] to [E]) and KAT1 ([F] to [H]) deactivation currents in the presence of 50, 10, or 1 mM external K+. Na+, N-methyl-d-glucamine, or mannitol was added to compensate for the changes in external K+ concentrations. No difference being observed between the three conditions, the data were pooled. Values shown are means ± se, with n = 6 in (C) to (E) and n = 5 in (F) to (H).
(C) and (F) Deactivation (tail) currents, recorded at 0 mV at 6 ms after the start of the step, plotted against the activation voltage. Deactivation currents were normalized for each cell by the current recorded in 50 mM K+ at −180 mV to suppress current variability caused by differences in cell size and level of expression. Solid lines are Boltzmann (bz) fits.
(D) and (G) Effect of external K+ on the GKout in ZmK2.1 (D) or KAT1 (G). Initial (outward) deactivation currents were assumed to follow the modified Ohm's law (Blatt, 1992; Hille, 1992): I = GK (E – EK), where GK is the macroscopic K+ conductance, E is the membrane potential (here 0 mV), and EK is the K+ equilibrium potential. The GK values were calculated from deactivation currents recorded after activation at −120, −140, −160, or −180 mV. For each cell, the resulting GK values were normalized by the value determined in 50 mM external K+ at −180 mV to suppress variability attributable to differences in cell size and level of expression. For ZmK2.1, normalized GK values plotted versus the external K+ concentration were fitted with a Michaelis-Menten equation for each of the four activation potentials. Resulting apparent Km values are shown at the bottom of (D) (means ± se, n = 5). Solid lines at the top of (D) are mean Michaelis-Menten adjustments (using the mean Km values indicated at bottom).
(E) and (H) Effect of external K+ on the kinetics of current deactivation in ZmK2.1 (E) and KAT1 (H). Deactivation time constants (“Taudeact”; means ± se, n = 6) were obtained from monoexponential fits of deactivation currents recorded at membrane potentials ranging from −90 to 0 mV. Fits of the voltage dependence of deactivation time constants with a monoexponential function (exp fit; solid lines) were used to determine zc, the equivalent charge involved in channel-closing transitions near the open state (Liman et al., 1991).
ZmK2.1 Requires K+ or Rb+ in the External Medium to Be in a Conducting State
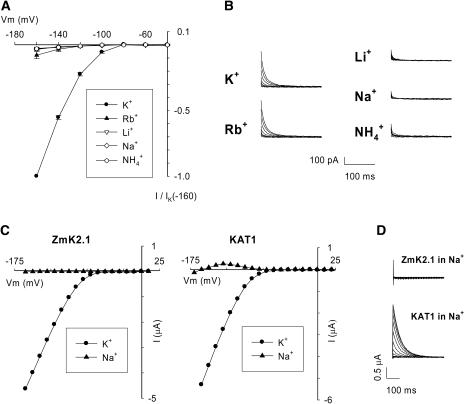
The effect of external K+ substitution by another monovalent cation (Rb+, Li+, Na+, or NH4+) on ZmK2.1 currents was examined in COS cells (Figures 4A and 4B) and in oocytes (shown for K+/Na+ substitution: Figures 4C and 4D). Similar results were obtained in both expression systems. ZmK2.1 steady state inward currents were strongly reduced when 50 mM Rb+ replaced 50 mM K+. For instance, at −160 mV in COS cells, the ratio of current in the presence of Rb+ to that in the presence of K+ was 0.08 ± 0.02 (Figure 4A). No significant inward current was detected in the presence of 50 mM Li+, Na+, or NH4+ (Figures 4A and 4C). Large outward deactivation currents were recorded when the membrane potential was switched from activating hyperpolarized values to 0 mV in the presence of 50 mM external K+ or Rb+ (Figure 4B), as expected, because the reversal potential of current through ZmK2.1 was −25 mV in the presence of K+ (K+ equilibrium potential [EK]) or more negative than −25 mV in the presence of Rb+ (this ion being less permeant than K+) (Figure 4A). Interestingly, using the same protocol, no deactivation current was recorded in the presence of Li+, Na+, or NH4+. The disappearance of inward and outward ZmK2.1 current upon replacement of K+ (or Rb+) by Li+, Na+, or NH4+ was a reversible phenomenon: currents similar in magnitude to those recorded before the substitution were observed when K+ was reintroduced in the bath (replacing Na+, Li+, or NH4+). Absence of deactivation current in the presence of Li+, Na+, or NH4+ was striking in that the predicted reversal potential of current through ZmK2.1 should be more negative in the presence of Li+, Na+, or NH4+ than in the presence of K+ or Rb+ (the former ions being less permeant than the latter ones) (Figure 4A), and thus K+ efflux would be expected to be greater in the presence of the former ions. Together, the absence of inward steady state current and of outward deactivation current when K+ was replaced by Li+, Na+, or NH4+ in the external medium could indicate either that the three latter cations blocked the channel or that ZmK2.1 was no longer in a conducting state in the absence of K+ or Rb+ in the external medium—that is, that no ionic flux could occur, whether inward (Li+, Na+, or NH4+ inward currents) or outward (outward K+ current). The use of bath solutions in which a decrease in K+ concentration was compensated for by Na+, N-methyl-d-glucamine, or mannitol allowed us to exclude the former hypothesis (see Figure 5). Thus, ZmK2.1 seemed to require K+ or Rb+ ions at its external face to be in a conducting state, or “active.” The term “activity” is used (here and throughout the text) at the macroscopic level. Therefore, a loss of activity includes the possibility of an inhibition of the outward unitary conductance or of a reduction in the number of open channels, which could result from a decrease in either the open probability or the number of channels available for conduction (see Figure 5E). Whatever the origin of the loss of activity in the absence of K+ or Rb+, this behavior is in sharp contrast with that of the Arabidopsis closest homologs of ZmK2.1, KAT1 (Figures 4C and 4D) (Véry et al., 1995) and KAT2 (Lacombe, 2000), the activity of which is not prevented by the absence of external K+. Indeed, KAT1 and KAT2 remain active when only poorly permeant ions such as Na+ are present in the bath (or in the presence of submillimolar K+ concentrations), resulting in outward steady state K+ currents at membrane potentials ranging from the channel activation threshold to the current reversal potential (e.g., from −80 to −160 mV for KAT1 in oocytes in 100 mM NaCl [Figure 4C]) (Véry et al., 1995). On the contrary, such steady state outward currents were not observed in ZmK2.1 in the presence of Na+, Li+, or NH4+ (Figures 4A and 4C).
Figure 4.
Effect of the Nature of the External Monovalent Cation on ZmK2.1 Conductance and Activity.
(A) and (B) Currents mediated by ZmK2.1 in COS cells were recorded in bath solutions successively containing KCl, RbCl, LiCl, NaCl, and NH4Cl at a concentration of 50 mM.
(A) Current-voltage relationships at steady state. Currents were normalized for each cell by current level in the presence of K+ at −160 mV. Values shown are means ± se (n = 5).
(B) Examples of deactivation currents recorded at 0 mV after activation steps at voltages ranging from −40 to −160 mV. The recordings shown were obtained from the same cell.
(C) and (D) Comparison of currents in oocytes expressing either ZmK2.1 or KAT1 in bath solutions containing 100 mM KCl or 100 mM NaCl.
(C) Current-voltage relationships at steady state.
(D) Deactivation currents recorded at −40 mV after activation steps at voltages ranging from +10 to −160 mV in a bath containing NaCl. The steady state and deactivation currents shown for each channel are from the same oocyte.
Analysis of ZmK2.1 Activation by External K+ Reveals a Voltage-Independent Km in the 10 mM K+ Concentration Range
The dependence of ZmK2.1 activity on external K+ was analyzed in COS cells using different external K+ concentrations: 50, 10, and 1 mM (Figure 5A). Parallel experiments were performed on the Arabidopsis KAT1 channel (Figure 5B). As in the experiment described in Figure 4, both inward currents recorded during activation steps at hyperpolarized membrane potentials and deactivation currents recorded at 0 mV immediately after activation were analyzed. The deactivation current at 0 mV could be predicted to be outward in all three ionic conditions, because the EK was −25, −65, and −123 mV when external K+ was 50, 10, and 1 mM, respectively. Furthermore, the magnitude of the outward current could be predicted to increase upon reduction in external K+ concentration (in the absence of channel sensitivity to external K+) as a result of the increase in driving force for K+ efflux. KAT1 deactivation current followed these predictions: the current was 2.5 times greater in 10 mM external K+ and 4.5 to 5 times greater in 1 mM K+ than in 50 mM K+ (Figures 5B and 5F). Results obtained for ZmK2.1 were quite different. The deactivation current was only slightly greater in 10 mM K+ than in 50 mM K+ (∼1.4 times) and strongly reduced in 1 mM K+ (∼3.5 times less than in 50 mM K+) (Figures 5A and 5C). In conjunction with the very small deactivation current, no steady state inward current was detected in 1 mM K+ (Figure 5A). Loss of current was immediate upon external K+ reduction to 1 mM and was fully reversible when external K+ concentration was increased again (data not shown). In these experiments, the decreases in external K+ concentration were compensated for at the ionic and osmotic strength levels by NaCl or N-methyl-d-glucamine or only at the osmotic level by mannitol (Figure 5). Very similar results were obtained in the three conditions, excluding the hypothesis that the loss of ZmK2.1 current upon external K+ reduction was attributable to channel block by the compensating cations. Thus, the whole set of results indicated that ZmK2.1 activity is dependent on external K+ concentration.
To quantify the sensitivity of ZmK2.1 activity to external K+, the outward macroscopic K+ conductance (GKout) was extracted from deactivation currents using the simple modified Ohm's law equation [GK = I/(E – EK)] (see legend to Figures 5D and 5G). The channels having been activated at a given potential, the GKout measured at a membrane potential sufficiently depolarized from EK should be independent of the external K+ concentration, unless external K+ regulates the number of open channels or the outward unitary conductance. In Figures 5D (for ZmK2.1) and 5G (for KAT1), the GKout values obtained at 0 mV after activation of the channel at −120, −140, −160, or −180 mV are plotted versus the external concentration of K+ (1, 10, or 50 mM). In both ZmK2.1 and KAT1, in agreement with the voltage gating (activation by hyperpolarization) of these channels, GKout was sensitive to the activation voltage: the more negative the voltage the greater the number of open channels and hence the larger the macroscopic conductance, whatever the external concentration of K+. Despite this common feature, the two channels clearly differed in sensitivity of GKout toward external K+. The GKout values derived for KAT1 at a given activation voltage appeared weakly dependent on the external K+ concentration (Figure 5G), suggesting that neither the number of open channels nor the outward unitary conductance (at 0 mV) was noticeably sensitive to external K+ concentration in this channel (at least in the 1 to 50 mM range). By contrast, the GKout of ZmK2.1 increased rapidly when the external K+ concentration was increased to greater than 1 mM (Figure 5D). Michaelis-Menten hyperbolic functions were used to fit the data. The resulting apparent Km value was close to 15 mM, whatever the voltage activation of the channel (Figure 5D, bottom). Such a Km value means that the remaining conductance, expressed in percentage of the maximal value that would be observed at saturating K+ concentrations, is 7% in 1 mM K+ and 43% in 10 mM K+. In other words, these results indicate that the external concentration of K+ does exert a strong control on ZmK2.1 activity.
The kinetics of ZmK2.1 current deactivation in the presence of different external K+ concentrations were analyzed to test the hypothesis that the control of ZmK2.1 activity by external K+ involved an effect of the cation on the channel open probability. A corollary to this hypothesis is that K+ affects transition rate constants near the open state, a phenomenon likely to show up in K+ sensitivity of the macroscopic current activation and deactivation kinetics (Blatt, 1990; Zagotta et al., 1994). Activation kinetics were not analyzed because ZmK2.1 inward currents were below the detection level in low (≤1 mM) external K+ conditions. Deactivation current kinetics were analyzed in both ZmK2.1 (Figure 5E) and KAT1 (Figure 5H) for comparison. Deactivation currents recorded in ZmK2.1 and KAT1 at membrane potentials in the −90- to 0-mV range could be well fitted with monoexponential functions, the time constant decreasing exponentially with depolarization (Figures 5E and 5H), as classically observed in voltage-gated inwardly rectifying channels (Blatt, 1992; Fairley-Grenot and Assmann, 1993; Véry et al., 1995). For both channels, the time constants were independent of external K+ in the 1 to 50 mM range (Figures 5E and 5H), indicating that the effect of external K+ on ZmK2.1 macroscopic activity was probably not the result of an effect on ZmK2.1 open probability.
Sensitivity to External K+ of ZmK2.1 Activity Is Not Attributable to Block by External Ca2+
Inwardly rectifying K+ channels displaying some permeability to Ca2+ and blocked by this cation have been reported in maize (Fairley-Grenot and Assmann, 1992a, 1992b). Furthermore, external Ca2+ is a common blocker of plant inwardly rectifying K+ channels (Zimmermann et al., 1999). Therefore, we examined whether a block of ZmK2.1 channels by external Ca2+, which is likely to be more pronounced in the presence of low K+ concentrations, could explain the observed sensitivity to external K+ of ZmK2.1 activity.
A first set of experiments revealed that Ca2+, in the presence of 10 mM K+, is very weakly permeant through ZmK2.1 (PCa/PK < 0.05). Increasing the external concentration of Ca2+ from 0.1 to 10 mM in the presence of 10 mM K+ did not significantly shift the zero current potential from the EK (Figure 6A).
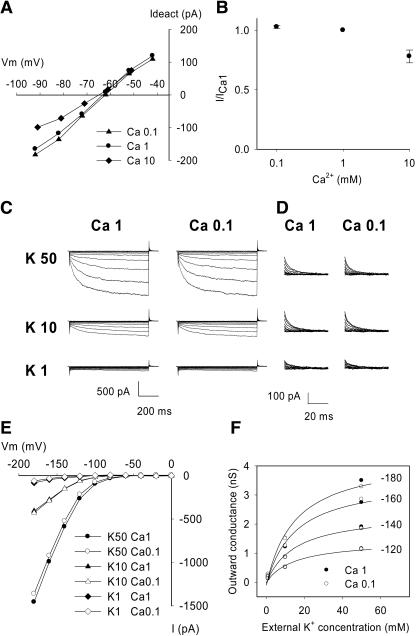
Figure 6.
Effect of External Ca2+ on ZmK2.1 Currents in COS Cells.
(A) ZmK2.1 permeability to Ca2+. Example of deactivation currents recorded, after an activation step at −140 mV, at membrane potentials ranging from −92 to −42 mV in bath solutions containing 10 mM K+ and successively 0.1, 1, and 10 mM Ca2+ [Ca2+ added as Ca(OH)2/Mes, pH 6.0]. EK was −64 mV when the cell was bathed with the solution containing 0.1 or 1 mM Ca2+ and was −65.5 mV in the solution containing 10 mM Ca2+. Mean zero-current potentials (±se, n = 4) recorded in the latter solutions were −63 ± 0.5 mV, −63 ± 1 mV, and −65 ± 1 mV, respectively.
(B) Sensitivity of ZmK2.1 steady state inward current to external Ca2+. ZmK2.1 whole cell currents were recorded in bath solutions containing 10 mM KCl and successively 0.1, 1, and 10 mM Ca2+ (same solutions as in [A]). I/ICa1, magnitude of the steady state currents recorded at −140 mV in 0.1, 1, and 10 mM Ca2+ standardized by the magnitude of the corresponding current in 1 mM Ca2+ (means ± se; n = 5).
(C) to (F) Effects of external Ca2+ on ZmK2.1 sensitivity to external K+. Typical examples from four experiments are shown.
(C) Example of whole cell currents recorded successively in different external K+ concentrations (50, 10, and 1 mM) in the presence of either 1 or 0.1 mM external Ca2+ (Ca 1 and Ca 0.1, respectively). In solutions in which K+ concentration was 10 or 1 mM or Ca2+ was 0.1 mM, NaCl was added for ionic and osmotic strength adjustment. The voltage-clamp protocol was the same as described for Figure 5A.
(D) Magnification of deactivation currents recorded at 0 mV.
(E) Effect of external Ca2+ on ZmK2.1 I/V relationships at steady state. The bath solution contained 50, 10, or 1 mM K+ and either 1 or 0.1 mM Ca2+.
(F) Effect of external Ca2+ on the outward macroscopic conductance at 0 mV (see legend to Figure 5).
Solid lines indicate Michaelis-Menten adjustments using the mean Km value (15 mM) determined for ZmK2.1 in COS cells (see Figure 5D).
A second set of experiments was aimed at testing whether active ZmK2.1 channels were sensitive to Ca2+. In the presence of 10 mM K+, an increase in Ca2+ concentration from 0.1 to 1 mM (i.e., an increase in the Ca2+/K+ concentration ratio from 0.01 to 0.1) had very little effect (3% ± 1% decrease) on ZmK2.1 steady state inward current at −140 mV (Figure 6B). A further increase in Ca2+ concentration, from 1 to 10 mM (Ca2+/K+ concentration ratio increase from 0.1 to 1), induced a current decrease of 22% ± 5% at −140 mV (Figure 6B). Thus, ZmK2.1 channels were moderately sensitive to external Ca2+. This finding suggested that the absence of inward current at low external K+ (1 mM) in the presence of 1 mM Ca2+ (Figure 5A) did not merely result from Ca2+-induced block.
This hypothesis was further assessed by comparing ZmK2.1 activity in the presence of 1 mM K+ and either 1 or 0.1 mM Ca2+, the latter condition (Ca2+/K+ concentration ratio of 0.1) being expected to result in little if any Ca2+ block (from the data shown in Figure 6B). Reduction of external Ca2+ led neither to the reappearance of ZmK2.1 inward currents in 1 mM external K+ (Figures 6C and 6E) nor to a significant increase in outward deactivation currents (Figure 6D). Thus, the absence of inward current recorded in these conditions mostly resulted from a Ca2+-independent process. Furthermore, the absence of an effect of Ca2+ on outward deactivation currents suggested that external Ca2+ did not block outward currents; therefore, the regulation by external K+ of ZmK2.1 activity (indicated by analysis of outward deactivation currents) was completely independent of external Ca2+ (Figure 6F).
Activity of Inwardly Rectifying Channels in Maize Guard Cells Is Regulated by External K+
The strong sensitivity to external K+ displayed by ZmK2.1 appeared as a unique feature among the plant inwardly rectifying Shakers (groups 1 and 2) (Pilot et al., 2003) cloned and characterized to date. Furthermore, no indication of in planta activity of a channel endowed with such regulation could be identified in published electrophysiological analyses. To search for an in planta K+ channel activity similar to that of ZmK2.1, the effect of external K+ on inwardly rectifying channel activity was investigated in maize guard cell protoplasts (Figures 7A to 7C). Guard cells appeared as a good candidate for the expression of ZmK2.1 or ZmK2.1-like channels, because all group 2 Shakers characterized in other plant species are expressed in this cell type (Véry and Sentenac, 2003).
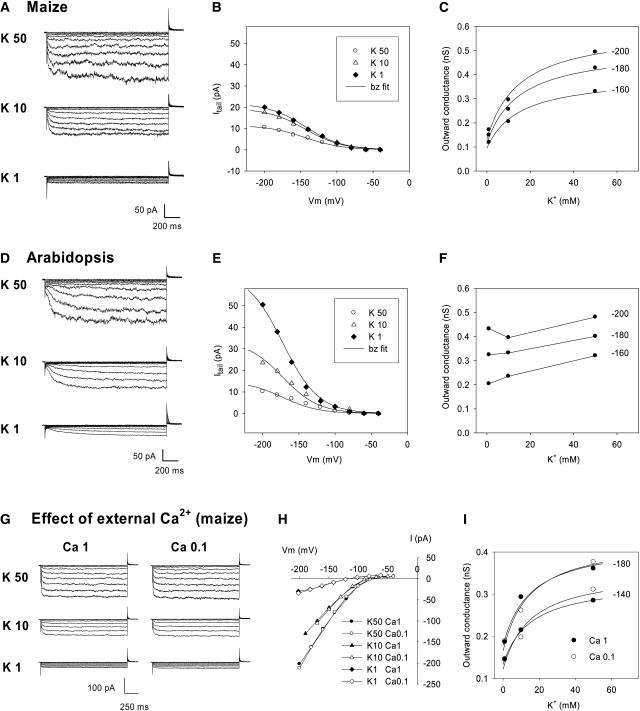
Figure 7.
Effect of External K+ Concentration on Maize and Arabidopsis Guard Cell Inwardly Rectifying Channel Activity.
(A) and (D) Typical current traces recorded in maize (A) and Arabidopsis (D) guard cell protoplasts in bath solutions successively containing 50, 10, and 1 mM K+ (decrease in K+ concentration adjusted at the osmotic level with mannitol). The internal K+ concentration was 125 mM. The voltage-clamp protocol was identical to that described for Figure 5.
(B), (C), (E), and (F) Analysis of deactivation currents in maize ([B] and [C]) and Arabidopsis ([E] and [F]). Data presented in (B) and (C) (and [E] and [F], respectively) were obtained from the recordings shown in (A) (and [D], respectively).
(B) and (E) Deactivation (tail) currents measured 10 ms after the start of the deactivation pulse, plotted versus the activation potential. Solid lines are Boltzmann (bz) fits.
(C) and (F) Outward macroscopic conductance (GKout = Itail/(E – EK)) at 0 mV plotted versus external K+ concentration (see legend to Figure 5). Conductance values were calculated for the three activation potentials −200, −180, and −160 mV. In (C), the data were fitted (solid lines) based on the assumption that the conductance results from the activity of two kinds of voltage-gated channels: ZmK2.1-like channels displaying regulation by external K+ with the same Km as that determined for ZmK2.1 in COS cells (15 mM; see Figure 5D and text), and channels not regulated by external K+, providing a constant contribution to the conductance over the entire K+ concentration range.
(G) to (I) Effect of external Ca2+ on inwardly rectifying channel activity in maize guard cells.
(G) Comparison of protoplast inward current in bath solutions containing either 1 or 0.1 mM Ca2+. The bath K+ concentration was successively 50, 10, and 1 mM. The voltage-clamp protocol was as described for (A).
(H) Effect of external Ca2+ on I/V relationships at steady state.
(I) Effect of external Ca2+ on the outward macroscopic conductance at 0 mV (same analysis as described for [C]).
Experiments similar to those performed in COS cells (Figure 5) were performed on maize guard cell protoplasts (Figure 7A). Analysis of deactivation currents (Figures 7B and 7C) revealed that the activity of maize guard cell inwardly rectifying channels was, like that of ZmK2.1, regulated by external K+ concentration. Analysis of the derived values of GKout (Figure 7C), however, indicated that the loss of channel activity upon reduction of external K+ was less pronounced in guard cells than in COS cells expressing ZmK2.1: the decrease in GKout attributable to reduction of external K+ from 50 to 1 mM was 48% in maize guard cells versus 72% in ZmK2.1-expressing COS cells (percentage computed from the experimental data shown in Figures 7C and 5D, respectively). One possible explanation for the lower sensitivity to external K+ of inwardly rectifying channel activity in guard cells is that ZmK2.1 or ZmK2.1-like channels are coexpressed in this cell type with other inward K+ channels that are not sensitive to external K+. The presence of at least two types of inwardly rectifying K+ channels in maize guard cells was suggested by the observation that the level of remaining current in 1 mM external K+ compared with the current level in 50 mM external K+ was quite variable within a 10-fold range (from 1.5 to 15.5% [n = 10]; mean, 7%; corresponding value for the recordings shown in Figure 7A, 9.5%). Within the framework of the hypothesis described above, two components were assumed to contribute to GKout in maize guard cells: one component (offset) was strictly insensitive to the external concentration of K+, and a second component was sensitive to this concentration and followed a Michaelis-Menten hyperbolic function with the same Km value (15 mM) as that derived for ZmK2.1 in COS cells (Figure 5D). This assumption provided satisfactory fits to the GKout values (Figure 7C). Thus, channels with sensitivity to external K+ are active in maize guard cells. Their relative contribution to the guard cell inward K+ conductance is important at high K+ concentrations, reaching 70% at saturating concentrations in the example shown in Figure 7A. In Arabidopsis, on the other hand, the guard cell inwardly rectifying K+ channel activity was found, in parallel experiments, to be poorly sensitive to external K+ (Figures 7D to 7F), in agreement with the fact that the channels thought to be the two major contributors to the guard cell inward K+ conductance, KAT1 and KAT2 (Pilot et al., 2001; Szyroki et al., 2001), are not sensitive to external K+.
As in many other plant species, the guard cell inwardly rectifying K+ conductance of maize is sensitive to external Ca2+ (Fairley-Grenot and Assmann, 1992a, 1992b). Calcium block of the inward K+ current was observed in our experiments. In the presence of 50 mM K+, a clear voltage-dependent block of the inward K+ current resulting in a decrease in current intensity of 11% ± 5% at −200 mV (n = 5; data not shown) appeared when the concentration of the bivalent was increased to 10 mM (Ca2+/K+ concentration ratio of 0.2). In presence of 1 mM K+, the inward K+ current appeared less sensitive to Ca2+, the voltage-dependent block being 7% ± 2% at −200 mV (21% ± 2% at −220 mV [n = 4]) in presence of 1 mM Ca2+ (Ca2+/K+ concentration ratio of 1). This difference in external Ca2+ sensitivity provides further support for the hypothesis of two types of inwardly rectifying K+ channels in maize guard cells, one with strong contribution to the K+ conductance at low external K+ and the other at high external K+, as discussed above.
In this context, we examined whether the sensitivity to external K+ of the inwardly rectifying K+ channel activity in guard cells (Figure 7C) was, like that of ZmK2.1 in COS cells, independent of external Ca2+ (Figures 7G to 7I). Consistent with the results described above, decreasing the external concentration of Ca2+ from 1 to 0.1 mM had little effect on the inward K+ current when external K+ was 10 or 50 mM, and it suppressed the (slight) voltage-dependent block when external K+ was 1 mM (Figures 7G and 7H). Deactivation currents recorded in these two external Ca2+ conditions were very similar, which indicated that external Ca2+ did not block outward deactivation K+ currents. Thus, activity regulation by external K+ of inward K+ channels in maize guard cells indicated by the analysis of deactivation currents was, like that of ZmK2.1 in COS cells, an external Ca2+-independent process (Figure 7I).
In conclusion, K+ channels endowed with regulation by external K+ similar to that of ZmK2.1 (hyperbolic relationships between channel activity and external K+ concentration with a Km value close to 15 mM, regulation mechanism independent of external Ca2+) can be detected in planta. In maize guard cells, such channels constitute a major component of the inwardly rectifying K+ channel population.
DISCUSSION
Comparison of ZmK2.1 with Other Plant Shaker Genes of the Group 2 Subfamily
ZmK2.1 belongs to group 2 of the plant Shaker-like K+ channel family. At least one other group 2 channel, named KZM2 (GenBank accession number CAD90161), as yet uncharacterized, exists in maize. KZM1 (Philippar et al., 2003) is likely to be encoded by an allele of ZmK2.1, because the two sequences display high identity (94%) and common features in their expression patterns (see below). It should be noted, however, that the two channels differ in their sensitivity to external pH, because KZM1 is not activated by acidification (Philippar et al., 2003).
Only a few genes from Shaker group 2 have been characterized to date: KAT1 and KAT2 from Arabidopsis, VvSIRK from grapevine, and ZmK2.1. The gene structure of group 2 plant Shakers has weakly evolved since the separation between dicots and monocots (Figure 1C). ZmK2.1 displays the same structure as KAT2 and VvSIRK, with the same number of introns (10) and the same intron positions. KAT1 has the same structure except that it has lost two introns (Pilot et al., 2003).
All group 2 Shakers characterized to date are expressed in aerial parts of the plant. Detailed expression patterns have been obtained for a few genes, KAT1, KAT2, VvSIRK, and the potato KST1, using the β-glucuronidase reporter gene and/or in situ hybridization methods. KAT1 is essentially expressed in guard cells (Nakamura et al., 1995). KAT2 is expressed in guard cells and in minor vein phloem tissues (Pilot et al., 2001). Expression of VvSIRK seems to be restricted to guard cells, and that of KST1 to guard cells and tissues at the flower base (Müller-Röber et al., 1995; Plesch et al., 2001). Thus, the four group 2 Shaker K+ channel genes for which detailed expression pattern information is available are all expressed in guard cells, where they are believed to play major roles in K+ uptake during stomatal opening (Assmann and Wang, 2001; Véry and Sentenac, 2003). Like these group 2 Shakers, ZmK2.1 and KZM2 are expressed mainly in the aerial parts of the plant (Figure 2). The transcript levels of both ZmK2.1 and KZM2 were high in vascular/bundle sheath strands (Figure 2B for ZmK2.1, as reported previously for KZM1 [Philippar et al., 2003]), suggesting both that the expression patterns of ZmK2.1 and KZM2 might be similar to those of the Arabidopsis KAT2 gene and that expression in phloem vasculature might correspond to an ancient trait of this channel type. Expression of ZmK2.1 in guard cells cannot be ascertained based on our expression data, but ZmK2.1-type channels displaying regulation of activity by external K+ are major components of the guard cell inwardly rectifying channel population (see below).
ZmK2.1 Displays Unique Functional Properties among Plant Inward Shakers
Comparison of functional properties between ZmK2.1 and other members of Shaker group 2 reveals both similarities and differences. Like all group 2 Shakers characterized to date, ZmK2.1 is a K+-selective, voltage-gated, inwardly rectifying channel (Figure 3). The sequence of its pore domain is exactly the same as that of KAT2 (Figure 1B). However, the single-channel conductance of ZmK2.1 is more than twice that of KAT2 (Figures 3G and 3I) (Pilot et al., 2001). Another difference is the sensitivity to Ba2+, which is strongly voltage dependent in ZmK2.1 (Figure 3E) but weakly voltage dependent in KAT2 (Pilot et al., 2001), suggesting that Ba2+ enters deeper into the pore in ZmK2.1 than in KAT2. This further highlights the fact that the P domain is not alone in determining conduction properties such as unitary conductance or voltage-dependent block (Aiyar et al., 1994; Lopez et al., 1994; Liu and Joho, 1998; Zei et al., 1999; Pilot et al., 2001).
The most striking property of ZmK2.1 is certainly the strong sensitivity of its activity to the concentration of K+ in the external medium (Figure 5). For instance, the channel displays only 7% residual activity in 1 mM K+ (with respect to activity at saturating K+). Total absence of K+ from the external solution (a nonphysiological situation) was shown to result in the loss of outward deactivating currents in the maize KZM1 channel (Philippar et al., 2003), suggesting that this channel is endowed, like ZmK2.1, with sensitivity to external K+, consistent with the hypothesis that the two proteins are encoded by different alleles of the same gene. To date, sensitivity of channel activity to external K+ has not been reported for any other cloned inwardly rectifying (group 1 or 2) plant Shaker.
Initially, low-affinity uptake (Epstein mechanism II) was proposed to involve channels, whereas high-affinity uptake, from concentrations below a few tens to a few hundred micromolar (Epstein mechanism I), was believed to involve cotransporters (Kochian and Lucas, 1988; Maathuis and Sanders, 1996). It is now known that Arabidopsis inward rectifiers from Shaker groups 1 and 2 can play a role in cell K+ uptake in a wide range of external K+ concentrations, from tens of millimolar to a few micromolar, provided that the membrane potential is more negative than EK (Anderson et al., 1992; Sentenac et al., 1992; Hirsch et al., 1998; Brüggemann et al., 1999; Mouline et al., 2002). On the other hand, such inward rectifiers can also mediate outward K+ fluxes, as shown in Figure 4C for KAT1, when they are faced with submillimolar K+ concentrations and opened by a membrane potential more negative than their activation potential but less negative than EK (e.g., more negative than −80 mV and less negative than approximatively −170 mV for KAT1 in Figure 4C). In these conditions, such channels can behave as leak-like channels at membrane potentials around EK, a behavior that might allow the channel to play a role in the control of cell membrane polarization close to EK. Facing the same conditions, ZmK2.1 does not mediate K+ efflux (Figure 4C) because of its sensitivity to external K+. In agreement with the electrophysiological analysis, yeast complementation tests showing no growth rescue by ZmK2.1 in the presence of submillimolar external K+ concentrations (Figure 1D) provide further support for the conclusion that ZmK2.1 activity is restricted to K+ concentrations in the high (millimolar) range. Thus, by sensitizing the activity of an inward Shaker to both the membrane potential and the external concentration of K+, the plant has produced a channel strictly devoted to K+ uptake from high concentrations, whatever the level of membrane hyperpolarization. This functional property is unique among the plant transport systems characterized to date.
As discussed above, ZmK2.1 is expressed in leaf tissues. The (local) concentration of K+ in the leaf apoplast is determined by the relative rates of import via the xylem, export via the phloem, and transport across plasma membrane into/from the symplast. Because the relative volume of the apoplast is small, large concentration changes can result from small changes in net membrane fluxes. Using selective microelectrodes to probe local K+ concentration in the leaf apoplast, it has been shown that steep concentration gradients can settle (Bowling, 1987). Three orders of magnitude separate the lowest values of local K+ activity recorded in the apoplast (in the 10 to 100 μM range) from the highest values (10 to 100 mM) (Blatt, 1985; Bowling, 1987; Bowling and Smith, 1990; Grignon and Sentenac, 1991). Using the infiltration–centrifugation method, which averages local differences, variations within 1 order of magnitude, from 1 to 10 mM, were detected in response to both plant K+ nutrition status and light/dark period (Mühling and Läuchli, 1999). In other words, because the apoplastic concentration of K+ in the leaf can vary in a wide range, regulation of ZmK2.1-type channels by external K+ with an apparent Km close to 15 mM can be predicted to have physiological meaning.
Regulation of ZmK2.1 Activity by External K+ Involves a Low-Affinity Binding Site Outside the P Domain
As the modulation of ZmK2.1 activity by external cations displays strong selectivity (for K+ and Rb+ and against Na+, Li+, and NH4+) and saturation at high external K+ concentration (Figures 4 and 5), we propose that a K+ binding site, the occupancy of which depends on external K+ concentration, is involved in this control. Two different sets of results strongly suggest that the effect of external K+ on ZmK2.1 activity is independent of the level of voltage activation of the channel. First, the same value of the Km parameter could be used to describe the increase in channel activity with external K+ regardless of the voltage of the activation prepulse (Figure 5D). Second, no effect of external K+ was observed on the voltage dependence of the relative open probability of the channel (Figure 3C). Thus, the control of ZmK2.1 activity by external K+ is not likely to be linked to voltage gating.
The Arabidopsis weak inward rectifier AKT2 (Lacombe et al., 2000), which belongs to plant Shaker group 3 (Pilot et al., 2003), has been shown to be inhibited by total withdrawal of K+ from the external solution (Geiger et al., 2002). A point mutation in the P domain of this channel, replacing a Ser with a Glu four residues downstream from the GYGD motif, suppressed the channel sensitivity to the absence of external K+. It is worth noting that, after this substitution, the corresponding region of the AKT2 P domain was identical to that of all characterized group 2 Shakers, including ZmK2.1. The molecular basis of K+ regulation of channel activity in ZmK2.1, therefore, is different from that seen in AKT2. In fact, because the entire P domain of ZmK2.1 is identical to that of KAT2, which does not display inhibition of activity by low external K+ (Lacombe, 2000), the molecular determinants of ZmK2.1 regulation by external K+ are certainly not situated in the P domain. Similarly, these determinants are not situated in the P-S6 linker, because this region is identical in ZmK2.1 and other group 2 Shakers that are not inhibited by low external K+ (KAT1, KST1, and VvSIRK; Figure 1B). The most divergent regions accessible to external K+ between ZmK2.1 and non-K+-regulated group 2 Shakers, in terms of global charge and residue identity, are the S1-S2 linker and, to a lesser extent, the S3-S4 linker (Figure 1B). These linkers have been proposed, in a few studies on animal and bacterial Shaker channels, to be located close to the channel pore, possibly overhanging the external vestibule (Blaustein et al., 2000; Jiang et al., 2003a, 2003b). Their involvement in channel conduction properties is unknown at present.
External K+ is known to regulate some animal K+ channels, among them several outwardly rectifying channels of the Shaker family. Several K+ binding sites have been identified in the narrow region forming the selectivity filter (Doyle et al., 1998; Harris et al., 1998; Vergara et al., 1999) but also in the outer and inner vestibules of K+ channels (Thompson and Begenisich, 2001; Consiglio et al., 2003; Kuo et al., 2003). It has been proposed that the occupancy of some of these K+ binding sites (e.g., the external “lock-in” site situated at the external side of the selectivity filter) is dependent on external K+ concentration (Kiss and Korn, 1998; Immke et al., 1999; Kiss et al., 1999; Vergara et al., 1999; Morais-Cabral et al., 2001). The absence of K+ binding would result in conformational changes within the pore, thereby modifying some of the channel conduction properties. Two positively charged residues, the first in the outer vestibule at a position close to the selectivity filter and the second in the so-called turret lining more external regions of the outer vestibule, have been shown in some animal Shakers to make the whole cell outward current highly sensitive to external K+ (Pardo et al., 1992; Jäger et al., 1998; Jäger and Grissmer, 2001), probably by controlling the occupancy of the external K+ lock-in site, a positive charge favoring expulsion of K+ from the binding site, and the resulting conformational changes affecting channel activity. However, the two amino acids involved in this phenomenon have no counterpart in the sequence of ZmK2.1. Furthermore, no other closely located positively charged residue (in the P domain, the S5-P linker or P-S6 linker) present in ZmK2.1 but not in other plant Shakers not regulated by external K+ have been found. Therefore, the molecular basis of activity control by external K+ in ZmK2.1 is certainly different from that seen in animal Shaker channels.
A Larger Diversity of K+ Channel Types in Maize Than in Arabidopsis
Sensitivity to external K+ is not a feature shared by every inwardly rectifying channel in maize. Indeed, the major inward conductance active in root cortical cells does not display any inhibition of activity upon reduction in external K+ concentration (Roberts and Tester, 1995). On the other hand, channels such as ZmK2.1, with activity regulated by external K+, can dominate the membrane conductance of some maize cells at high external K+ concentrations, as shown by patch-clamp recordings in guard cells (Figure 7).
Investigation with the patch-clamp technique of the diversity of K+ channels in maize guard cells indicates that channels regulated by K+, such as ZmK2.1, are active at the plasma membrane, together with channels that are not regulated by K+. The ZmK2.1-type channels are major components of the guard cell inward K+ channel population, although their relative contribution to membrane conductance is variable. They strongly contribute to whole cell inward K+ conductance at “high” external K+ (>10 mM), whereas channels that are not regulated by K+ have a preponderant role at low external K+ concentrations, compensating for the lack of ZmK2.1-type activity in these conditions. Furthermore, the fact that the guard cell inwardly rectifying conductance at high K+ concentrations is permeable to Ca2+ (Fairley-Grenot and Assmann, 1992a, 1992b) but ZmK2.1 is not permeable to this cation (Figure 6A) suggests that at least part of the inward K+ channels active at high external K+ in maize guard cells are different from ZmK2.1. Thus, it is clear that the situation is more complex in maize than in the model plant Arabidopsis, in which a single type of inward K+ channels, not regulated by external K+, is active (Figures 6D to 6F) and mediates K+ influx in the whole range of physiological situations, from micromolar to millimolar concentrations (Brüggemann et al., 1999).
At the molecular level, ZmK2.1 might contribute to the expression of guard cell inward conductance regulated by external K+, because RT-PCR analyses reveal that transcripts of this gene are present in leaf epidermal peels. However, a more significant contribution of KZM2 can be predicted. Indeed, owing to the sequence similarity in the transmembrane region and external linkers between ZmK2.1 and KZM2, it is likely that KZM2 is endowed, like ZmK2.1, with regulation by external K+. Furthermore, based on the RT-PCR data, KZM2 should be more strongly expressed in guard cells than ZmK2.1 (Figure 2C). Components of the guard cell inward conductance not regulated by external K+ might be encoded by Shaker genes from group 1, such as ZMK1 (Philippar et al., 1999), which has been reported to be highly expressed in leaf epidermis (Büchsenschütz et al., 2004).
In conclusion, the regulation of K+ transport seems to require a higher functional diversity of K+ channels in maize than in Arabidopsis, with regard to sensitivity to the apoplastic concentration of K+. Functional characterization of the set of inward Shakers in rice should reveal whether this type of diversity exists in other species and allow us to further analyze its physiological significance. If present in rice, it might be a common trait of monocots, linked for example to their lower cell wall cation-exchange capacity (Grignon and Sentenac, 1991). If absent, it might reflect a specific feature of C4 plants.
METHODS
Cloning of ZmK2.1 cDNA
A partial K+ channel cDNA was isolated by screening of a maize (Zea mays cv AM0406; Maïsadour, Mont-de-Marsan, France) cDNA library constructed using the ZAP-cDNA Gigapack II Gold cloning kit (Stratagene, La Jolla, CA) with a probe generated from transmembrane regions (S4 to S6) of the Arabidopsis thaliana KAT1 K+ channel cDNA. ZmK2.1 full-length cDNA was obtained by 3′ rapid amplification of cDNA ends.
In Planta Expression Studies
Maize seedlings were grown hydroponically on air-bubbled solution (pH 5.5) containing 1 mM KH2PO4, 1 mM Ca(NO3)2, 1 mM MgCl2, and micronutrients. Total RNAs were isolated from 15-d-old tissues. RNA gel blot analysis was performed according to Church and Gilbert (1984) using radiolabeled ZmK2.1 full-length cDNA as a probe. 25S rRNA staining on gel was used as a control for RNA loading. For RT-PCR experiments, 10 μg of total RNA was used for first-strand synthesis by Moloney murine leukemia virus RT (final volume, 25 μL; Promega, Madison, WI), and 0.5 μL of RT products was used for PCR. Specific primers surrounding introns (sequences available upon request) were used for ZmK2.1 and KZM2 (GenBank accession number AJ558238) amplification. Expression of histone H1 or actin was monitored as a control (Savino et al., 1997; Philippar et al., 2003).
Expression in Yeast
ZmK2.1 and KAT1 cDNAs were cloned into the yeast expression vector pFL61 (Bonneaud et al., 1991) and transferred into the K+ uptake–defective yeast strain Wagf2 (MATa; trk1Δ∷LEU2; trk2Δ∷TRP; ade2-101; his3-11, 15; leu2-3, 112; trp1-1; ura3-1; canr) (Ros et al., 1999). Complementation tests were performed on minimal medium supplemented with 20 mg/L adenine and different concentrations of KCl, with 15 μL of cell suspension (A600 = 0.3) being spotted on agar plates (drop tests).
Expression in Xenopus Oocytes and COS Cells
ZmK2.1 and KAT1 cDNA open reading frames were amplified (PCR) and cloned into pCI vector (Promega). The pCI-ZmK2.1 construct was used for expression in both Xenopus laevis oocytes and COS cells. The pCI-KAT1 construct was used for expression in COS cells, whereas in vitro transcribed KAT1 copy RNA (pBSTA vector) (Véry et al., 1994) was used for oocyte studies. Oocytes were injected with 30 ng of pCI-ZmK2.1 or KAT1 copy RNA (1 μg/μL in water). Control oocytes were injected with 30 nL of water. Two-electrode voltage-clamp measurements on oocytes and analysis of the data were performed as described previously (Véry et al., 1995). Recording solutions contained 1.8 mM CaCl2, 1 mM MgCl2, 5 mM Hepes/NaOH, pH 7.4, or 5 mM Mes/NaOH, pH 6.4 or 5.4, and KCl as indicated. K+ was added as a Cl− salt, and the ionic strength (constant in all solutions) was adjusted with NaCl. Expression and patch-clamp experiments in COS cells were performed as described by Mouline et al. (2002). All patch-clamp solutions were adjusted to 300 mosmol with mannitol. The pipette solution (cell internal medium) contained 150 mM KCl, 2 mM MgCl2, 5 mM MgATP, 5 mM EGTA, and 10 mM Hepes/Tris, pH 7.2. All bath solutions contained a background of 2 mM MgCl2, 1 mM CaCl2 (unless otherwise stated), and 10 mM Mes/Tris, pH 6.0. K+ was added as a Cl− salt.
Patch Clamp on Guard Cell Protoplasts
Maize seedlings were grown hydroponically for 2 to 3 weeks as described above. Guard cell protoplasts were isolated from leaf epidermal peels using the enzymatic digestion protocol described by Fairley-Grenot and Assmann (1992a). Growth of Arabidopsis plants (Wassilewskija ecotype) and isolation of Arabidopsis guard cell protoplasts were performed as described by Hosy et al. (2003). Patch-clamp recordings on guard cell protoplasts were performed as described for COS cells (see above). Patch-clamp bath solutions contained 1 or 0.1 mM CaCl2, 4 or 4.9 mM MgCl2, respectively, 10 mM Mes/Tris, pH 5.8, and K+ glutamate as indicated. In experiments comparing maize and Arabidopsis, the pipette solution contained 100 mM K+ glutamate, 5 mM EGTA, 1 mM CaCl2 (45 nM free Ca2+), 0.5 mM MgCl2, 2 mM MgATP, 20 mM Hepes, and 25 mM KOH, pH 7.25. In experiments addressing the effect of external Ca2+, free Ca2+ in the pipette solution was less than 5 nM (no CaCl2 added), MgCl2 concentration was increased to 1 mM, and pH was decreased to 7.0 (KOH = 15 mM). The osmolarity of bath and pipette solutions was adjusted with mannitol to 500 and 530 mosmol, respectively.
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under accession numbers AY461583 and AY461584.
Acknowledgments
We thank Jossia Boucherez for technical assistance, Stéphane Lobréaux for the gift of the maize histone H1 cDNA, and Isabel Lefèvre and Sabine Zimmermann for critical reading of the manuscript. This work was supported in part by fellowships from the Institut National de la Recherche Agronomique and the Association Franco-Chinoise pour la Recherche Scientifique et Technique to Y.-H.S. and by the European Community BIOTECH Program (BIO4-CT96).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Anne-Aliénor Véry (very@ensam.inra.fr).
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.104.030551.
References
- Aiyar, J., Nguyen, A.N., Chandy, K.G., and Grissmer, S. (1994). The P-region and S6 of Kv3.1 contribute to the formation of the ion conduction pathway. Biophys. J. 67, 2261–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, J.A., Huprikar, S.S., Kochian, L.V., Lucas, W.J., and Gaber, R.F. (1992). Functional expression of a probable Arabidopsis thaliana potassium channel in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 89, 3736–3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmann, S.M., and Wang, X.Q. (2001). From milliseconds to millions of years: Guard cells and environmental responses. Curr. Opin. Plant Biol. 4, 421–428. [DOI] [PubMed] [Google Scholar]
- Blatt, M.R. (1985). Extracellular potassium activity in attached leaves and its relation to stomatal function. J. Exp. Bot. 36, 240–251. [Google Scholar]
- Blatt, M.R. (1990). Potassium channel currents in intact stomatal guard cells: Rapid enhancement by abscisic acid. Planta 180, 445–455. [DOI] [PubMed] [Google Scholar]
- Blatt, M.R. (1992). K+ channels of stomatal guard cells: Characteristics of the inward rectifier and its control by pH. J. Gen. Physiol. 99, 615–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein, R.O., Cole, P.A., Williams, C., and Miller, C. (2000). Tethered blockers as molecular ‘tape measures’ for a voltage-gated K+ channel. Nat. Struct. Biol. 7, 309–311. [DOI] [PubMed] [Google Scholar]
- Bonneaud, N., Ozier-Kalogeropoulos, O., Li, G.Y., Labouesse, M., Minvielle-Sebastia, L., and Lacroute, F. (1991). A family of low and high copy replicative, integrative and single-stranded S. cerevisiae/E. coli shuttle vectors. Yeast 7, 609–615. [DOI] [PubMed] [Google Scholar]
- Bowling, D.J.F. (1987). Measurement of the apoplastic activity of K+ and Cl− in the leaf epidermis of Commelina communis in relation to stomatal activity. J. Exp. Bot. 193, 1351–1355. [Google Scholar]
- Bowling, D.J.F., and Smith, G.N. (1990). Apoplastic transport in the leaf epidermis in relation to stomatal activity. Biochem. Physiol. Pflanz. (BPP) 186, 309–316. [Google Scholar]
- Brüggemann, L., Dietrich, P., Becker, D., Dreyer, I.I., Palme, K., and Hedrich, R. (1999). Channel-mediated high-affinity K+ uptake into guard cells from Arabidopsis. Proc. Natl. Acad. Sci. USA 96, 3298–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büchsenschütz, K., Philippar, K., Kranz, E., and Hedrich, R. (2004). Role of K+ channels in vegetative and reproductive organs of Zea mays. In Proceedings of the 13th International Workshop on Plant Membrane Biology. (Montpellier, France: Ecole Nationale Supérieure Agronomique Montpellier), p. 159, http://www.montpellier.inra.fr/PMB.
- Chalot, M., Javelle, A., Blaudez, D., Lambilliote, R., Cooke, R., Sentenac, H., Wipf, D., and Botton, B. (2002). An update on nutrient transport processes in ectomycorrhizas. Plant Soil 244, 165–175. [Google Scholar]
- Church, G.M., and Gilbert, W. (1984). Genomic sequencing. Proc. Natl. Acad. Sci. USA 81, 1991–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consiglio, J.F., Andalib, P., and Korn, S.J. (2003). Influence of pore residues on permeation properties in the Kv2.1 potassium channel. Evidence for a selective functional interaction of K+ with the outer vestibule. J. Gen. Physiol. 121, 111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decher, N., Bundis, F., Vajna, R., and Steinmeyer, K. (2003). KCNE2 modulates current amplitudes and activation kinetics of HCN4: Influence of KCNE family members on HCN4 currents. Pflügers Arch. 446, 633–640. [DOI] [PubMed] [Google Scholar]
- Dennison, K.L., Robertson, W.R., Lewis, B.D., Hirsch, R.E., Sussman, M.R., and Spalding, E.P. (2001). Functions of AKT1 and AKT2 potassium channels determined by studies of single and double mutants of Arabidopsis. Plant Physiol. 127, 1012–1019. [PMC free article] [PubMed] [Google Scholar]
- Doyle, D.A., Morais-Cabral, J., Pfuetzner, R.A., Kuo, A., Gulbis, J.M., Cohen, S.L., Chait, B.T., and MacKinnon, R. (1998). The structure of the potassium channel: Molecular basis of K+ conduction and selectivity. Science 280, 69–77. [DOI] [PubMed] [Google Scholar]
- Dreyer, I., Horeau, C., Lemaillet, G., Zimmermann, S., Bush, D.R., Rodríguez-Navarro, A., Schachtman, D.P., Spalding, E.P., Sentenac, H., and Gaber, R. F. (1999). Identification and characterization of plant transporters using heterologous expression systems. J. Exp. Bot. 50 (special issue), 1073–1087. [Google Scholar]
- Epstein, E., and Rains, D.W. (1963). Resolution of dual mechanisms of potassium absorption by barley roots. Proc. Natl. Acad. Sci. USA 49, 684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairley-Grenot, K.A., and Assmann, S.M. (1992. a). Whole-cell K+ current across the plasma membrane of guard cells from a grass: Zea mays. Planta 186, 282–293. [DOI] [PubMed] [Google Scholar]
- Fairley-Grenot, K.A., and Assmann, S.M. (1992. b). Permeation of Ca2+ through K+ channels in the plasma membrane of Vicia faba guard cells. J. Membr. Biol. 128, 103–113. [DOI] [PubMed] [Google Scholar]
- Fairley-Grenot, K.A., and Assmann, S.M. (1993). Comparison of K+-channel activation and deactivation in guard cells from a dicotyledon (Vicia faba L.) and a graminaceous monocotyledon (Zea mays). Planta 189, 410–419. [DOI] [PubMed] [Google Scholar]
- Gaymard, F., Pilot, G., Lacombe, B., Bouchez, D., Bruneau, D., Boucherez, J., Michaux-Ferriere, N., Thibaud, J.-B., and Sentenac, H. (1998). Identification and disruption of a plant Shaker-like outward channel involved in K+ release into the xylem sap. Cell 94, 647–655. [DOI] [PubMed] [Google Scholar]
- Geiger, D., Becker, D., Lacombe, B., and Hedrich, R. (2002). Outer pore residues control the H+ and K+ sensitivity of the Arabidopsis potassium channel AKT3. Plant Cell 14, 1859–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grignon, C., and Sentenac, H. (1991). pH and ionic conditions in the apoplast. Annu. Rev. Plant Physiol. Plant Mol. Biol. 42, 103–128. [Google Scholar]
- Harris, R.E., Larsson, H.P., and Isacoff, E.Y. (1998). A permanent ion binding site located between two gates of the Shaker K+ channel. Biophys. J. 74, 1808–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille, B. (1992). Ionic Channels of Excitable Membranes, 2nd ed. (Sunderland, MA: Sinauer Associates).
- Hirsch, R.E., Lewis, B.D., Spalding, E.P., and Sussman, M.R. (1998). A role for the AKT1 potassium channel in plant nutrition. Science 280, 918–921. [DOI] [PubMed] [Google Scholar]
- Hosy, E., et al. (2003). The Arabidopsis outward K+ channel GORK is involved in regulation of stomatal movements and plant transpiration. Proc. Natl. Acad. Sci. USA 100, 5549–5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immke, D., Wood, M., Kiss, L., and Korn, S.J. (1999). Potassium-dependent changes in the conformation of the Kv2.1 potassium channel pore. J. Gen. Physiol. 113, 819–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäger, H., and Grissmer, S. (2001). Regulation of a mammalian Shaker-related potassium channel, hKv1.5, by extracellular potassium and pH. FEBS Lett. 488, 45–50. [DOI] [PubMed] [Google Scholar]
- Jäger, H., Rauer, H., Nguyen, A.N., Aiyar, J., Chandy, K.G., and Grissmer, S. (1998). Regulation of mammalian Shaker-related K+ channels: Evidence for non-conducting closed and non-conducting inactivated states. J. Physiol. 506, 291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan, L.Y., and Jan, Y.N. (1997). Cloned potassium channels from eukaryotes and prokaryotes. Annu. Rev. Neurosci. 20, 91–123. [DOI] [PubMed] [Google Scholar]
- Jiang, Y., Lee, A., Chen, J., Ruta, V., Cadene, M., Chait, B.T., and MacKinnon, R. (2003. a). X-ray structure of a voltage-dependent K+ channel. Nature 423, 33–41. [DOI] [PubMed] [Google Scholar]
- Jiang, Y., Ruta, V., Chen, J., Lee, A., and MacKinnon, R. (2003. b). The principle of gating charge movement in a voltage-dependent K+ channel. Nature 423, 42–48. [DOI] [PubMed] [Google Scholar]
- Kiss, L., and Korn, S.J. (1998). Modulation of C-type inactivation by K+ at the potassium channel selectivity filter. Biophys. J. 74, 1840–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss, L., LoTurco, J., and Korn, S.J. (1999). Contribution of the selectivity filter to inactivation in potassium channels. Biophys. J. 76, 253–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochian, L.V., and Lucas, W.J. (1988). Potassium transport in roots. Adv. Bot. Res. 15, 93–178. [Google Scholar]
- Kuo, A., Gulbis, J.M., Antcliff, J.F., Rahman, T., Lowe, E.D., Zimmer, J., Cuthbertson, J., Ashcroft, F.M., Ezaki, T., and Doyle, D.A. (2003). Crystal structure of the potassium channel KirBac1.1 in the closed state. Science 300, 1922–1926. [DOI] [PubMed] [Google Scholar]
- Kwak, J.M., Murata, Y., Baizabal-Aguirre, V.M., Merrill, J., Wang, M., Kemper, A., Hawke, S.D., Tallman, G., and Schroeder, J.I. (2001). Dominant negative guard cell K+ channel mutants reduce inward-rectifying K+ currents and light-induced stomatal opening in Arabidopsis. Plant Physiol. 127, 473–485. [PMC free article] [PubMed] [Google Scholar]
- Lacombe, B. (2000). Caractérisation Fonctionnelle en Système Hétérologue de Canaux Potassiques de Type Shaker Clonés chez Arabidopsis thaliana. PhD dissertation (Montpellier, France: ENSA Montpellier).
- Lacombe, B., Pilot, G., Michard, E., Gaymard, F., Sentenac, H., and Thibaud, J.-B. (2000). A shaker-like K+ channel with weak rectification is expressed in both source and sink phloem tissues of Arabidopsis. Plant Cell 12, 837–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liman, E.R., Hess, P., Weaver, F., and Koren, G. (1991). Voltage-sensing residues in the S4 region of a mammalian K+ channel. Nature 353, 752–756. [DOI] [PubMed] [Google Scholar]
- Liu, Y., and Joho, R.H. (1998). A side chain in S6 influences both open-state stability and ion permeation in a voltage-gated K+ channel. Pflügers Arch. 435, 654–661. [DOI] [PubMed] [Google Scholar]
- Lopez, G.A., Jan, Y.N., and Jan, L.Y. (1994). Evidence that the S6 segment of the Shaker voltage-gated K+ channel comprises part of the pore. Nature 367, 179–182. [DOI] [PubMed] [Google Scholar]
- Maathuis, F.J.M., and Sanders, D. (1996). Mechanisms of potassium absorption by higher plant roots. Physiol. Plant. 96, 158–168. [Google Scholar]
- Mäser, P., et al. (2001). Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiol. 126, 1646–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais-Cabral, J.H., Zhou, Y., and MacKinnon, R. (2001). Energetic optimization of ion conduction rate by the K+ selectivity filter. Nature 414, 37–42. [DOI] [PubMed] [Google Scholar]
- Mouline, K., Véry, A.-A., Gaymard, F., Boucherez, J., Pilot, G., Devic, M., Bouchez, D., Thibaud, J.-B., and Sentenac, H. (2002). Pollen tube development and competitive ability are impaired by disruption of a Shaker K+ channel in Arabidopsis. Genes Dev. 16, 339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühling, K.H., and Läuchli, A. (1999). Effect of K+ nutrition, leaf age and light intensity on apoplastic K+ in leaves of Vicia faba. J. Plant Nutr. Soil Sci. 162, 571–576. [Google Scholar]
- Müller-Röber, B., Ellenberg, J., Provart, N., Willmitzer, L., Busch, H., Becker, D., Dietrich, P., Hoth, S., and Hedrich, R. (1995). Cloning and electrophysiological analysis of KST1, an inward rectifying K+ channel expressed in potato guard cells. EMBO J. 14, 2409–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura, R.L., McKendree, W.L., Jr., Hirsch, R.E., Sedbrook, J.C., Gaber, R.F., and Sussman, M.R. (1995). Expression of an Arabidopsis potassium channel gene in guard cells. Plant Physiol. 109, 371–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo, L.A., Heinemann, S.H., Terlau, H., Ludewig, U., Lorra, C., Pongs, O., and Stühmer, W. (1992). Extracellular K+ specifically modulates a rat brain K+ channel. Proc. Natl. Acad. Sci. USA 89, 2466–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippar, K., Büchsenschütz, K., Abshagen, M., Fuchs, I., Geiger, D., Lacombe, B., and Hedrich, R. (2003). The K+ channel KZM1 mediates potassium uptake into the phloem and guard cells of the C4 grass Zea mays. J. Biol. Chem. 278, 16973–16981. [DOI] [PubMed] [Google Scholar]
- Philippar, K., Fuchs, I., Lüthen, H., Hoth, S., Bauer, C.S., Haga, K., Thiel, G., Ljung, K., Sandberg, G., Bottger, M., Becker, D., and Hedrich, R. (1999). Auxin-induced K+ channel expression represents an essential step in coleoptile growth and gravitropism. Proc. Natl. Acad. Sci. USA 96, 12186–12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilot, G., Lacombe, B., Gaymard, F., Chérel, I., Boucherez, J., Thibaud, J.-B., and Sentenac, H. (2001). Guard cell inward K+ channel activity in Arabidopsis involves expression of the twin channel subunits KAT1 and KAT2. J. Biol. Chem. 276, 3215–3221. [DOI] [PubMed] [Google Scholar]
- Pilot, G., Pratelli, R., Gaymard, F., Meyer, Y., and Sentenac, H. (2003). Five-group distribution of the Shaker-like K+ channel family in higher plants. J. Mol. Evol. 56, 418–434. [DOI] [PubMed] [Google Scholar]
- Plesch, G., Ehrhardt, T., and Mueller-Roeber, B. (2001). Involvement of TAAAG elements suggests a role for Dof transcription factors in guard cell-specific gene expression. Plant J. 28, 455–464. [DOI] [PubMed] [Google Scholar]
- Pratelli, R., Lacombe, B., Torregrosa, L., Gaymard, F., Romieu, C., Thibaud, J.-B., and Sentenac, H. (2002). A grapevine gene encoding a guard cell K+ channel displays developmental regulation in the grapevine berry. Plant Physiol. 128, 564–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, S.K., and Tester, M. (1995). Inward and outward K+-selective currents in the plasma membrane of protoplasts from maize root cortex and stele. Plant J. 8, 811–825. [Google Scholar]
- Ros, R., Lemaillet, G., Fonrouge, A.-G., Daram, P., Enjuto, M., Salmon, J.-M., Thibaud, J.-B., and Sentenac, H. (1999). Molecular determinants of the Arabidopsis AKT1 K+ channel ionic selectivity investigated by expression in yeast of randomly mutated channels. Physiol. Plant. 105, 459–468. [Google Scholar]
- Ruta, V., Jiang, Y., Lee, A., Chen, J., and MacKinnon, R. (2003). Functional analysis of an archaebacterial voltage-dependent K+ channel. Nature 422, 180–185. [DOI] [PubMed] [Google Scholar]
- Savino, G., Briat, J.-F., and Lobréaux, S. (1997). Inhibition of the iron-induced ZmFer1 maize ferritin gene expression by antioxidants and serine/threonine phosphatase inhibitors. J. Biol. Chem. 272, 33319–33326. [DOI] [PubMed] [Google Scholar]
- Sentenac, H., Bonneaud, N., Minet, M., Lacroute, F., Salmon, J.-M., Gaymard, F., and Grignon, C. (1992). Cloning and expression in yeast of a plant potassium ion transport system. Science 256, 663–665. [DOI] [PubMed] [Google Scholar]
- Szyroki, A., Ivashikina, N., Dietrich, P., Roelfsema, M.R.G., Ache, P., Reintanz, B., Deeken, R., Godde, M., Felle, H.H., Steinmeyer, R., Palme, K., and Hedrich, R. (2001). KAT1 is not essential for stomatal opening. Proc. Natl. Acad. Sci. USA 98, 2917–2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, J., and Begenisich, T. (2001). Affinity and location of an internal K+ ion binding site in Shaker K channels. J. Gen. Physiol. 117, 373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara, C., Alvarez, O., and Latorre, R. (1999). Localization of the K+ lock-in and the Ba2+ binding sites in a voltage-gated calcium-modulated channel. Implications for survival of K+ permeability. J. Gen. Physiol. 114, 365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Véry, A.-A., and Sentenac, H. (2002). Cation channels in the Arabidopsis plasma membrane. Trends Plant Sci. 7, 168–175. [DOI] [PubMed] [Google Scholar]
- Véry, A.-A., and Sentenac, H. (2003). Molecular mechanisms and regulation of K+ transport in higher plants. Annu. Rev. Plant Biol. 54, 575–603. [DOI] [PubMed] [Google Scholar]
- Véry, A.-A., Bosseux, C., Gaymard, F., Sentenac, H., and Thibaud, J.-B. (1994). Level of expression in Xenopus oocytes affects some characteristics of a plant inward-rectifying voltage-gated K+ channel. Pflügers Arch. 428, 422–424. [DOI] [PubMed] [Google Scholar]
- Véry, A.-A., Gaymard, F., Bosseux, C., Sentenac, H., and Thibaud, J.-B. (1995). Expression of a cloned plant K+ channel in Xenopus oocytes: Analysis of macroscopic currents. Plant J. 7, 321–332. [DOI] [PubMed] [Google Scholar]
- Zagotta, W.N., Hoshi, T., Dittman, J., and Aldrich, R.W. (1994). Shaker potassium channel gating. II. Transitions in the activation pathway. J. Gen. Physiol. 103, 279–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zei, P.C., Ogielska, E.M., Hoshi, T., and Aldrich, R.W. (1999). Effects on ion permeation with hydrophobic substitutions at a residue in Shaker S6 that interacts with a signature sequence amino acid. Ann. N. Y. Acad. Sci. 868, 458–464. [DOI] [PubMed] [Google Scholar]
- Zimmermann, S., Ehrhardt, T., Plesch, G., and Müller-Röber, B. (1999). Ion channels in plant signaling. Cell. Mol. Life Sci. 55, 183–203. [DOI] [PMC free article] [PubMed] [Google Scholar]