Abstract
Cryptochromes (CRY) are blue light receptors that share sequence similarity with photolyases, flavoproteins that catalyze the repair of UV light–damaged DNA. Transgenic Arabidopsis thaliana seedlings expressing the C-terminal domains of the Arabidopsis CRY fused to β-glucuronidase (GUS) display a constitutive photomorphogenic (COP) phenotype, indicating that the signaling mechanism of Arabidopsis CRY is mediated through the C-terminal domain. The role of the Arabidopsis CRY N-terminal photolyase-like domain in CRY action remains poorly understood. Here, we report the essential role of the Arabidopsis CRY1 N-terminal domain (CNT1) in the light activation of CRY1 photoreceptor activity. Yeast two-hybrid assay, in vitro binding, in vivo chemical cross-linking, gel filtration, and coimmunoprecipitation studies indicate that CRY1 homodimerizes in a light-independent manner. Mutagenesis and transgenic studies demonstrate that CNT1-mediated dimerization is required for light activation of the C-terminal domain of CRY1 (CCT1). Transgenic data and native gel electrophoresis studies suggest that multimerization of GUS is both responsible and required for mediating a COP phenotype on fusion to CCT1. These results indicate that the properties of the GUS multimer are analogous to those of the light-modified CNT1 dimer. Irradiation with blue light modifies the properties of the CNT1 dimer, resulting in a change in CCT1, activating CCT1, and eventually triggering the CRY1 signaling pathway.
INTRODUCTION
Arabidopsis thaliana CRYPTOCHROME 1 (CRY1) mediates a variety of blue light–induced responses, including inhibition of hypocotyl elongation, enhancement of cotyledon expansion, and anthocyanin accumulation (Ahmad and Cashmore, 1993; Lin et al., 1995a, 1996, 1998; Ahmad et al., 1998a). CRY2, the second member of the Arabidopsis CRY family, also affects hypocotyl elongation (Ahmad et al., 1998a; Lin et al., 1998). Mutations in cry1 and cry2 affect flowering time (Bagnall et al., 1996; Guo et al., 1998; Mockler et al., 1999, 2003). An additional property of Arabidopsis CRY, together with the red/far-red light receptor phytochromes, is that they serve to entrain the circadian clock (Somers et al., 1998).
CRY typically have an N-terminal domain that shares sequence similarity with photolyase, a family of flavoproteins that catalyze the repair of UV light–damaged DNA, and a distinguishing C-terminal domain that is absent in photolyase and has no strong sequence similarity with known protein domains (Sancar, 1994; Cashmore et al., 1999). Insight into the signaling mechanism of Arabidopsis CRY was obtained through the demonstration that transgenic plants expressing the C-terminal domain of CRY1 or CRY2 (CCT1 or CCT2) fused to β-glucuronidase (GUS) display a constitutive photomorphogenic (COP) phenotype (Yang et al., 2000) that is similar to that of mutants of both COP1 and the COP9 signalosome, the negative regulators of photomorphogenesis (Deng et al., 1991, 1992; Misera et al., 1994; Wei et al., 1994). These data suggest that CRY1 and CRY2 signaling in response to light activation is mediated through their C-terminal domains. CCT was shown to bind to COP1, a RING finger and WD-40 repeat protein that functions like a component of an E3 ubiquitin ligase (Osterlund et al., 2000; Wang et al., 2001; Yang et al., 2001). Recent studies demonstrate that the initial photochemistry underlying CRY function and regulation involves the blue light–dependent phosphorylation of Arabidopsis CRY1 and CRY2 (Shalitin et al., 2002, 2003; Bouly et al., 2003) and that the primary photochemical reaction involves intraprotein electron transfer from Trp and Tyr residues to the excited flavin adenine dinucleotide cofactor (Giovani et al., 2003).
CRY is the primary circadian photoreceptor in Drosophila. The original cryb mutant of Drosophila is deficient in some circadian responses (Hall, 2000). In contrast with the Arabidopsis findings, in which the CCT interacts with the signaling partner COP1 (Wang et al., 2001; Yang et al., 2001), the N-terminal domain of Drosophila CRY interacts with its signaling partners, PERIOD (PER) and TIMELESS (TIM). Reminiscent of the activity of CCT in transgenic Arabidopsis, this N-terminal domain fragment of Drosophila CRY (in contrast with full-length CRY) binds PER constitutively in yeast (Rosato et al., 2001). Analysis of a newly isolated Drosophila cry mutant, which contains a premature stop codon that truncates CRY's last 19 amino acids, leaving the photolyase-like domain intact, indicates that the N-terminal domain is essential and sufficient for light detection and phototransduction in Drosophila (Busza et al., 2004). Consistent with these studies, transgenic Drosophila lines expressing the DsCRY lacking the C-terminal domain display a constitutive circadian response to dim light (Dissel et al., 2004).
To date, the role of the N-terminal domain of Arabidopsis CRY in CRY action remains largely unknown. Here, we show, by yeast two-hybrid, transgenic, and biochemical studies, that CRY1 constitutively forms a dimer through N-terminal domain of CRY1 (CNT1) and that CNT1-mediated dimerization is required for the light activation of CCT1 activity. We also show that GUS forms a multimer and that its multimerization is required to constitutively activate CCT1. We interpret these findings to indicate that activation of CCT1 is mediated through a blue light–dependent change in the properties of the CNT1 dimer.
RESULTS
Transgenic Plants Expressing the Arabidopsis CNT1 Display a Dominant-Negative Phenotype
Ethyl methanesulfonate mutagenesis screens gave rise to more than 22 missense cry1 mutant alleles that express full-length CRY1 apoprotein (Ahmad et al., 1995; Shalitin et al., 2003). At least 13 of them contain mutations that are distributed throughout CNT1, indicating an essential role of CNT1 in CRY1 action. To explore the CNT1 role, we prepared a construct expressing Myc-tagged CNT1 (Figure 1A, construct 1) and overexpressed it in wild-type Arabidopsis plants. We obtained 40 independent transgenic lines, 32 of which displayed a cry1 mutant phenotype of unexpanded cotyledons as well as long hypocotyls under blue light (Figure 1B, seedling 1), whereas 8 showed a wild-type phenotype in the dark and in red and far-red light (Table 1; data not shown). We examined, by protein gel blot analysis, extracts from the Myc-CNT1 lines showing a cry1 mutant phenotype using anti-Myc and anti-CCT1 antibodies. Some lines showing a cry1 mutant phenotype expressed neither Myc-CNT1 nor endogenous CRY1 (Figure 1C, lanes 8 and 9), indicating a result of cosuppression. However, some lines showing a cry1 mutant phenotype expressed both Myc-CNT1 and endogenous CRY1 at a level similar to that in wild-type plants (Figure 1C, lanes 4 to 7 and 10), indicating a negative effect of Myc-CNT1 on CRY1-mediated photomorphogenic development. Overexpression of Myc-CNT1 in the cry1 mutant background did not mediate blue light responses (Figure 1B, seedling 1*). Analyses of the progeny of a representative plant heterozygous for a single T-DNA insertion locus revealed that the negative effect of Myc-CNT1 was genetically dominant (data not shown).
Figure 1.
Expression of Myc-Tagged CNT1 Protein in Arabidopsis.
(A) Schemes displaying the Myc-tagged chimeric genes. In this and other figures, a number is given for each construct shown at left.
(B) Dominant-negative effect of CNT1 on CRY1-mediated photomorphogenic development. Six-day-old Arabidopsis seedlings grown under blue light are shown. In this and other figures, the number shown under each seedling represents a transgenic plant expressing the correspondingly numbered construct and is directly cited in the text. 1, transgenic seedling expressing Myc-CNT1 in the wild-type background; 1*, transgenic seedling expressing Myc-CNT1 in the cry1 mutant background. Measurements of the hypocotyl lengths of all the transgenic lines used in this study are given in Table 1.
(C) Protein gel blot analysis of the Myc-CNT1 lines made in the wild-type background showing a cry1 mutant phenotype. The same membrane was first probed with anti-Myc antibody (top), then reprobed with anti-CCT1 antibody (middle), and finally probed with anti-CUL1 antibody as a loading control (bottom). Some lines (lanes 4 to 7 and 10) expressed both Myc-CNT1 and endogenous CRY1 at a level similar to that seen in the wild type. A seedling of Myc-CNT1 line 33 (lane 10) is shown in (B); some lines (lanes 8 and 9) expressed neither Myc-CNT1 nor endogenous CRY1. A seedling of Myc-CRY1 line 5 (lane 1) is shown in (B). Asterisks indicate a band nonspecifically recognized by the antibody.
(D) Immunoblot showing the expression of Myc-CNT1 in the cry1 mutant background (lanes 1 and 2) and Myc-GUS in the wild-type background (lanes 5 and 6) using anti-Myc antibody. Seedlings of cry1 Myc-CNT1 line 3 (lane 1) and Myc-GUS line 12 (lane 5) are shown in (B). Myc-CNT1 line 33 (in the wild-type background) was used as a control (lane 3).
Table 1.
Measurements of Hypocotyl Lengths of Transgenic Seedlings Expressing Various Constructs
| Construct | Genotypes of Plants | Hypocotyl Length in the Dark (mm) | Hypocotyl Length in Blue Light (mm) |
|---|---|---|---|
| Wild type (Col) | 14.1 ± 0.6 | 3.8 ± 0.6 | |
| cry1 | 14.2 ± 0.9 | 11.6 ± 0.8 | |
| 1 | Myc-CNT1 (Col) | 14.2 ± 0.7 | 10.6 ± 1.1 |
| 1* | Myc-CNT1 | 14.0 ± 0.6 | 11.1 ± 0.7 |
| 2 | Myc-GUS (Col) | 14.1 ± 0.9 | 3.8 ± 0.6 |
| 3 | Myc-CRY1 | 13.9 ± 0.6 | 0.8 ± 0.3 |
| 4 | CRY1 | 14.0 ± 0.8 | 1.1 ± 0.2 |
| 5 | CNT1Δ401-489-CCT1 | 14.1 ± 0.6 | 11.0 ± 0.9 |
| 6 | CNT1Δ1-99-CCT1 | 13.9 ± 0.8 | 11.9 ± 0.7 |
| 7 | Myc-CNT1-305 (Col) | 14.0 ± 0.6 | 3.6 ± 0.7 |
| 8 | Myc-CNT1-375 (Col) | 14.2 ± 0.9 | 3.7 ± 0.7 |
| 9 | Myc-CNT1-388 (Col) | 13.9 ± 0.7 | 3.8 ± 0.6 |
| 10 | Myc-CRY1-375 | 14.0 ± 0.9 | 11.2 ± 0.8 |
| 11 | GFP-CCT1 | 13.9 ± 0.6 | 11.2 ± 0.7 |
| 12 | UVR3-CCT1 | 14.1 ± 0.9 | 11.3 ± 0.6 |
| 13 | GUS-CCT1 | 2.0 ± 0.9 | 1.0 ± 0.2 |
| 14 | GUSΔ402-603-CCT1 | 14.1 ± 0.6 | 11.6 ± 0.9 |
| 15 | GUSΔ1-200-CCT1 | 14.2 ± 0.6 | 11.8 ± 0.6 |
| 16 | GUS-CRY1 | 2.1 ± 0.9 | 1.0 ± 0.3 |
Hypocotyl lengths were measured for 6-d-old seedlings after growth in the dark or under blue light. Measurements were performed on 30 seedlings of each genotype. Transgenic lines made in the wild-type background are indicated as Columbia (Col), and those made in the cry1 background are not indicated. Error values represent se.
To define the specificity of the dominant-negative phenotype mediated by Myc-CNT1, we made a construct expressing Myc-tagged GUS (Figure 1A, construct 2) and overexpressed it in the wild-type background. No phenotype change was observed in the Myc-GUS lines (Figure 1B, seedling 2). Protein gel blot analysis indicated that the Myc-GUS fusion protein was expressed at normal levels in transgenic plants (Figure 1D, lanes 5 and 6). By contrast, overexpression of Myc-tagged full-length CRY1 in the cry1 mutant background conferred enhanced blue light responses (Figure 1B, seedling 3). Therefore, the dominant-negative effect of Myc-CNT1 was mediated by CNT1.
CNT1 Interacts with Both Full-Length CRY1 and CNT1 Itself in Yeast Cells
At least two mechanisms could explain the dominant-negative effect of CNT1. First, CNT1 might interact with endogenous CRY1 to form a CNT1–CRY1 dimer, which is not active or is less active than the native CRY1–CRY1 dimer; second, CNT1 might compete with the endogenous CRY1 for the downstream targets. Because CNT1 does not interact with COP1 (Yang et al., 2001), we tested the former possibility by the yeast two-hybrid assay (Figures 2A to 2C), although it is possible that CNT1 interacts with the unknown CRY1 targets. Strong CNT1–CRY1 and CNT1–CNT1 interactions were obtained (Figure 2C, samples 1 and 2). Similarly, clear CNT2–CRY2 and CNT2–CNT2 interactions were observed (Figure 2C, samples 9 and 10). CNT1 did not interact with CCT1 (Figure 2C, sample 4). Furthermore, we examined whether full-length CRY1 interacts with CRY1 itself. Although the bait construct expressing the LexA-CRY1 fusion protein showed significant background (Figure 2C, sample 7), full-length CRY1 clearly interacted strongly with CRY1 itself (Figure 2C, sample 3). No interaction was observed among the negative control pairs (Figure 2C, samples 5, 8, and 11). In contrast with CNT, CCT did not interact with the full-length CRY (Figure 2C, samples 6 and 12). Using the plate assay, we found that the CNT1–CRY1 interaction was light-independent in yeast (Figure 2D).
Figure 2.
Interaction of CNT with Full-Length CRY and CNT Itself in Yeast Cells.
(A) CNT and CCT bait constructs. All proteins are fusions with the LexA DNA binding domain (LexA) fused to CNT and CCT.
(B) Prey proteins. All proteins are fusions to the B42 activation domain (AD).
(C) Yeast two-hybrid assay showing CNT–CRY and CNT–CNT interactions. The interaction strength was determined by quantitative yeast two-hybrid interaction assays. In this and other figures, all vector combinations are given as bait/prey. Ten independent original transformants were analyzed for each vector combination. Standard deviations are indicated by error bars.
(D) Light-independent CNT1–CRY1 interaction. Blue precipitate represents cumulative β-galactosidase activity resulting from the activation of the lacZ reporter gene by protein–protein interaction. Quadruplicate yeast patches expressing the indicated LexA hybrid (rows) and the indicated AD hybrid (columns) were derived from four independent transformants. BL, blue light; DK, dark.
Arabidopsis CNT and Full-Length CRY Interact with CRY in Vitro
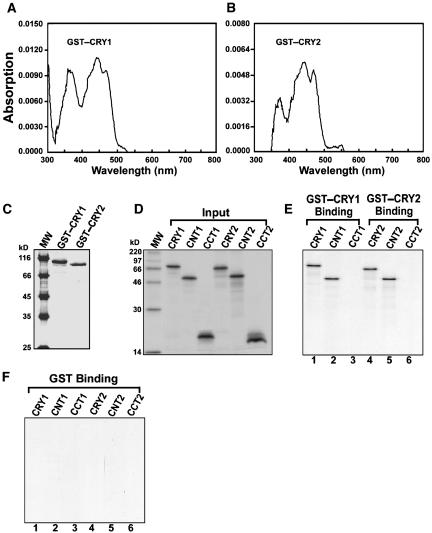
To confirm the interaction observed in the yeast two-hybrid assay, we performed protein interaction studies in vitro using glutathione S-transferase (GST)–CRY1 and GST–CRY2 fusion proteins purified from Schizosaccharomyces pombe (Figure 3C), which were soluble and had absorption spectra similar to that of the recombinant CRY1 purified from Sf9 cells (Figures 3A and 3B) (Lin et al., 1995b). CNT, CCT, and full-length CRY were synthesized as radioactively labeled polypeptides by in vitro transcription/translation (Figure 3D). Pulldown assay using these proteins showed that both CRY and CNT bound to CRY under blue light (Figure 3E, lanes 1, 2, 4, and 5) and in darkness (data not shown), whereas CCT did not bind to CRY (Figure 3E, lanes 3 and 6). None of the CRY domains and full-length CRY bound to GST (Figure 3F). These results, together with the data from the yeast two-hybrid assays, indicate that CRY might dimerize through CNT.
Figure 3.
In Vitro Binding of Arabidopsis CRY to CNT and CRY.
(A) and (B) Absorption spectra of the GST-CRY fusion proteins. GST-CRY1 and GST-CRY2 fusion proteins were purified from S. pombe. Their absorption spectra are similar to that of the recombinant CRY1 protein purified from Sf9 cells (Lin et al., 1995b).
(C) GST-CRY1 and GST-CRY2 fusion proteins purified from S. pombe. Purified proteins were separated by SDS-PAGE and stained with Coomassie blue. MW, molecular weight marker.
(D) In vitro transcription/translation of CRY, CNT, and CCT polypeptides in the presence of [35S]Met.
(E) Pulldown assay and SDS-PAGE showing binding of CRY and CNT to CRY. The GST fusion proteins were incubated with glutathione Sepharose 4B beads, and then the CRY, CNT, and CCT proteins were added and incubated in the dark and in blue light. The beads were isolated, and bound proteins were identified by SDS-PAGE and autoradiography. Both CRY and CNT bound to CRY in the dark and in blue light, and the result with blue light is shown.
(F) Pulldown assay and SDS-PAGE showing no binding of CRY, CNT, and CCT to GST.
CRY1 Constitutively Homodimerizes in Arabidopsis Seedlings
To confirm whether CRY1 dimerizes in vivo, native gel electrophoresis was performed using protein extracts from both light- and dark-grown transgenic Arabidopsis seedlings expressing Myc-CRY1, followed by protein gel blot analysis using monoclonal anti-Myc antibody. Myc-CRY1 was found to be a monomer (data not shown). The failure to detect a CRY1 dimer might reflect the fact that the CRY1 dimer is prone to disassociate during the process of electrophoresis. In an attempt to circumvent this problem, we used the chemical cross-linker formaldehyde to covalently preserve the possible CRY1 dimer in vivo. After treatment with formaldehyde, a novel high molecular mass band, approximately twice as high as that of the Myc-CRY1 monomer, recognized by anti-Myc antibody, was observed after SDS-PAGE separation of the protein extracts prepared from dark-grown and blue light–grown, and blue light–irradiated seedlings expressing Myc-CRY1 (Figure 4A, lanes 2 to 6).
Figure 4.
Light-Independent Homodimerization of CRY1 in Vivo.
(A) In vivo chemical cross-linking studies showing dimerization of CRY1. Formaldehyde treatment (+) of transgenic seedlings expressing Myc-CRY1 generates a high molecular mass protein on SDS-PAGE that is recognized by the anti-Myc antibody. B, blue light exposure (15, 30, and 60 min); BL, continuous blue light; D, putative CRY1 dimer; DK, dark; M, CRY1 monomer.
(B) In vivo chemical cross-linking studies showing that the CRY1 mutant protein does not dimerize. After treatment with formaldehyde, SDS-PAGE separation, and protein gel blot analysis using anti-Myc antibody (left) or anti-CCT1 antibody (right), the putative CRY1 dimer was absent in protein extracts prepared from seedlings expressing the Myc-CRY1-375 mutant protein.
(C) Gel filtration studies of protein extracts from transgenic plants expressing native CRY1 and TAPa-tagged CRY1. Fractions of protein extracts from 4-d-old seedlings grown under continuous darkness (top) or continuous blue light (bottom) expressing TAPa-CRY1 in the wild-type background were subjected to immunoblot analysis using antibody to CRY1. The fraction numbers are indicated at top. T indicates the unfractionated total protein extract. The positions of the molecular weight standards are labeled at bottom. The peak fractions containing the endogenous CRY1 dimer (D) and monomer (M) are indicated.
(D) IgG precipitation of TAPa-tagged proteins. Protein extracts from 4-d-old dark-grown transgenic seedlings expressing TAPa-CRY1 and TAPa-GFP fusions were used for IgG precipitation. Immunoblot analysis was performed using anti-Myc antibody. A size shift is observed in all fusion bands after IgG precipitation, as shown when elution was performed using 3C human rhinovirus protease to cleave part of the TAPa tag.
(E) CRY1 and TAPa-CRY1 in vivo interaction. The protein extracts and IgG precipitates described in (D) were subjected to immunoblot analysis using antibody against CRY1. The cry1 mutant protein extract was used as a negative control. The asterisk marks a nonspecific band cross-reacting with the anti-CRY1 antibody, which is used as a loading control for the total protein extracts.
To further confirm the homodimerization of CRY1 in vivo, we generated transgenic plants expressing an N-terminal fusion of the TAPa tag to the CRY1 protein (TAPa-CRY1) (Saijo et al., 2003; Rubio et al., 2005) in the wild-type background and conducted gel filtration studies. In both dark and blue light conditions, approximately equal amounts of dimer and monomer of the native CRY1 were obtained (Figure 4C), whereas the TAPa-CRY1 was found almost all as a dimer. Furthermore, coimmunoprecipitation assays showed that TAPa-CRY1 bound endogenous CRY1, whereas a TAPa–green fluorescent protein (GFP) fusion did not (Figures 4D and 4E). Therefore, these data indicate that CRY1 homodimerizes in vivo in a light-independent manner.
Deletions and Point Mutations within CNT1 Compromise the Ability to Interact with CRY1
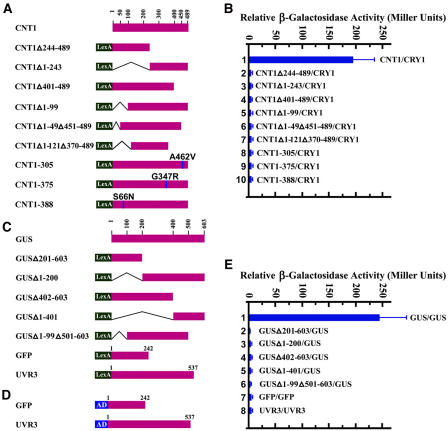
In an attempt to map the domains within CNT1 that are required for interaction with CRY1, we prepared bait constructs corresponding to a series of CNT1 deletion fragments (Figure 5A). A protein gel blot experiment, conducted on extracts from yeast cells coexpressing CRY1 and any one of the CNT1 fragments, demonstrated that all of the CNT1 fragments were expressed at similar protein levels (data not shown). Unexpectedly, all deletion fragments failed to show an interaction with CRY1 (Figure 5B, samples 2 to 7). Reminiscent of the CRY1 mutagenesis study results indicating that missense mutations throughout CNT1 compromise CRY1 activity, these negative results might reflect the fact that the overall structure of CNT1 is important for CRY1 action. To examine this possibility, we prepared bait vectors expressing the CNT1 mutants [CNT1-305, containing an A462V mutation corresponding to the previously described cry1-305 allele; CNT1-375, containing a G347R mutation corresponding to six cry1 alleles (cry1-375.1, -375.2, -375.3, -375.4, hy4-15, and hy4-16); and CNT1-388, containing an S66N mutation corresponding to the cry1-388 allele (Ahmad et al., 1995; Shalitin et al., 2003)] (Figure 5A). Protein gel blot analysis of extracts from yeast cells coexpressing CRY1 and any one of the CNT1 mutants demonstrated that all of the CNT1 mutants were expressed at a similar protein level (data not shown). Yeast two-hybrid assays showed that all of these point mutations abolished CNT1–CRY1 interaction (Figure 5B, samples 8 to10). Therefore, these results indicate that loss of CRY1 function in these cry1 mutant alleles might be attributable to a lack of CRY1 dimerization.
Figure 5.
Quantitative Yeast Two-Hybrid Interaction Assay Showing Effects of Mutations or Deletions within CNT1 and GUS on the Interaction with CRY1 and GUS.
(A) Bait constructs composed of either CNT1 mutants or CNT1 deletion fragments fused to the LexA DNA binding domain (LexA). The CNT1 mutants (CNT1-305, -375, and -388) contain point mutations within CNT1 corresponding to previously identified cry1 mutant alleles (Ahmad et al., 1995; Shalitin et al., 2003).
(B) The yeast two-hybrid assay showing that deletions and point mutations within CNT1 all abolished the interaction with CRY1.
(C) Bait constructs composed of either GUS deletion fragments or GFP or UVR3 fused to the LexA DNA binding domain. UVR3 is an Arabidopsis (6–4) photolyase (Nakajima et al., 1998).
(D) Prey constructs composed of either GFP or UVR3 fused to the B42 activation domain (AD).
(E) Effects of deletions within GUS on the interaction with GUS. Full-length GUS interacted with GUS itself strongly, whereas deletion fragments of GUS did not interact with GUS. GFP and UVR3 did not self-associate.
CNT1-Mediated Dimerization Is Required for CRY1 Activity
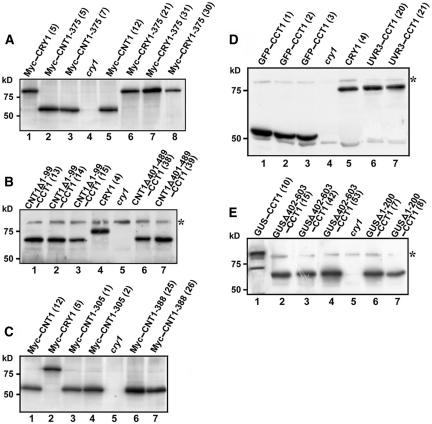
To further determine the relationship between the structure of CNT1 and CRY1 function, we first prepared vectors expressing CCT1 fused to CNT1 deletion fragments (Figure 6A, constructs 5 and 6) and overexpressed them in the cry1 mutant background. None of these transgenic lines showed enhanced blue light responses (Figure 6C, seedlings 5 and 6). Protein gel blot analysis using antibody against CCT1 indicated that the deletions within CNT1 did not affect the expression level of the truncated CRY1 proteins (Figure 7B). We then prepared constructs expressing Myc-tagged CNT1 mutants (Figure 6A, constructs 7 to 9) and expressed them in wild-type Arabidopsis. If mutations within CNT1 eliminate CRY1's ability to dimerize in vivo, the corresponding CNT1 mutants would not be able to mediate a dominant-negative effect when expressed in the wild-type background. Indeed, transgenic seedlings overexpressing these CNT1 mutants all displayed a wild-type phenotype under blue light (Figure 6C, seedlings 7 to 9). Protein gel blot analysis using anti-Myc antibody indicated that these point mutations did not affect the expression level of CNT1 proteins (Figures 7A and 7C).
Figure 6.
Essential Roles of the Integral CNT1 Dimer and GUS Multimer in Activating CCT1.
(A) Constructs composed of either CNT1 mutants or CNT1 deletion fragments or GFP and UVR3 fused to CCT1.
(B) Constructs composed of various GUS fragments fused to CCT1 and CRY1.
(C) Effects of CNT1 structure on CRY1 activity in transgenic Arabidopsis plants. Point mutations and deletions within CNT1 abolished CRY1 activity, as seen by the lack of enhanced light responses observed for full-length CRY1. Point mutations within CNT1 also compromised the ability to mediate a dominant-negative effect. Transgenic seedlings expressing GFP-CCT1 and UVR3-CCT1 fusion proteins did not show enhanced blue light responses.
(D) Effects of GUS structure on CCT1 activity in transgenic Arabidopsis plants. Transgenic seedlings expressing GUS-CCT1 and GUS-CRY1 showed a COP phenotype in the dark (top) and enhanced blue light responses (bottom), whereas those expressing GUS deletion fragments fused to CCT1 did not show either a COP phenotype in the dark (top) or enhanced blue light responses (bottom).
Figure 7.
Protein Gel Blot Analysis of Transgenic Plants Expressing Various CRY1 Fragments.
(A) Protein gel blots showing expression of Myc-CNT1-375 and Myc-CRY1-375 in transgenic plants using anti-Myc antibody. Lanes 2 and 7 show samples from the transgenic lines shown in Figure 6C, seedlings 8 and 10, respectively.
(B) Protein gel blot showing expression of deletion CNT1 fragments fused to CCT1 in transgenic plants using antibody against CCT1. Lanes 1, 4, and 7 show samples from the transgenic lines shown in Figure 6C, seedlings 6, 4, and 5, respectively. Asterisks indicate a band nonspecifically recognized by the antibody.
(C) Protein gel blots showing expression of Myc-CNT1-305 and Myc-CNT1-388 in transgenic plants using anti-Myc antibody. Lanes 4 and 6 show samples from the transgenic lines shown in Figure 6C, seedlings 7 and 9, respectively.
(D) Protein gel blot showing expression of GFP and UVR3 fused to CCT1 in transgenic plants using antibody against CCT1. Lanes 1 and 6 show samples from transgenic lines shown in Figure 6C, seedlings 11 and 12, respectively.
(E) Protein gel blot showing expression of deletion GUS fragments fused to CCT1 in transgenic plants using antibody against CCT1. Lanes 1, 2, and 6 show samples from the transgenic lines shown in Figure 6D, seedlings 13, 14, and 15, respectively.
Next, we constructed a vector expressing Myc-tagged full-length CRY1 containing a G347R mutation (Myc-CRY1-375) (Figure 6A, construct 10) and overexpressed it in the cry1 mutant background. We analyzed 40 independent transgenic lines, and none of them showed light responses (Figure 6C, seedling 10). Protein gel blot analysis using anti-Myc antibody indicated that Myc-CRY1-375 was expressed normally (Figure 7A, lanes 6 to 8). In vivo chemical cross-linking studies indicated that Myc-CRY1-375 did not form a homodimer in Arabidopsis seedlings (Figure 4B, lanes 4 and 5). Thus, this result is consistent with CNT1-375 being unable to interact with full-length CRY1 in yeast cells (Figure 5B, sample 9) and supports the novel band with a molecular mass twice as high as that of the CRY1 monomer being CRY1 dimer (Figure 4A). Together, these data demonstrate that CNT1-mediated dimerization is required for CRY1 activity.
Transgenic Plants Expressing Monomeric Proteins Fused to CCT1 Do Not Show Light Responses
To extend our findings, we next assessed whether CCT1 is active on fusion with two monomeric proteins. One is GFP and the other is UVR3, an Arabidopsis (6–4) photolyase that shares sequence homology with CNT1 (Nakajima et al., 1998). Yeast two-hybrid assays suggested that both GFP and UVR3 were unable to self-associate (Figure 5E, samples 7 and 8), consistent with GFP and photolyases being monomers (Sancar et al., 1987; Lumb et al., 1999). We prepared constructs expressing CCT1 fused to GFP and UVR3 (Figure 6A, constructs 11 and 12) and overexpressed them in the cry1 mutant background. None of these GFP-CCT1 and UVR3-CCT1 lines showed blue light responses (Figure 6C, seedlings 11 and 12). Protein gel blot analysis using antibody to CCT1 indicated that these fusion proteins were expressed normally in transgenic plants (Figure 7D). These results indicate that GFP and photolyase are unable to perform the CNT1 role in activating CCT1.
The Constitutive Light Response Mediated by the GUS-CCT Fusion Protein Mechanistically Involves Multimerization of GUS
With the demonstrations that dimerization of CNT1 is required for the light activation of CCT1 and that GFP-CCT1 and UVR3-CCT1 are not able to mediate light responses, we entertained the possibility that the COP phenotype observed for transgenic plants expressing the GUS-CCT fusion protein (Yang et al., 2000) mechanistically involves multimerization of GUS. To test this possibility, we performed the yeast two-hybrid assay and found that GUS indeed interacted strongly with GUS itself (Figure 5E, sample 1). In parallel, we generated transgenic lines expressing GUS-CCT1 in the cry1 mutant background and obtained a COP phenotype in the dark (Figure 6D, top, seedling 13) and enhanced blue light responses (Figure 6D, bottom, seedling 13). Then we showed, by native gel electrophoresis and protein gel blot analysis of protein extracts prepared from transgenic seedlings expressing Myc-GUS and GUS-CCT1 using anti-Myc and anti-CCT1 antibodies, that both Myc-GUS and GUS-CCT1 multimerized in vivo in a light-independent manner (Figures 8A, lanes 1 and 2, 8B, lanes 1 and 2, and 8C).
Figure 8.
GUS and GUS Fusion Proteins Constitutively Multimerize in Vivo.
(A) Protein gel blot showing that Myc-GUS was a multimer. Protein extracts prepared from dark-grown and blue light–grown transgenic plants expressing Myc-GUS were fractionated by native gel electrophoresis, and the blot was probed with anti-Myc antibody. The positions of mass markers are indicated at left. The asterisk indicates a degradation band.
(B) Native gel electrophoresis and protein gel blot analysis showing multimerization of GUS fusion proteins. Protein extracts from transgenic plants expressing GUS-CCT1, GUS-CRY1, and deletion GUS fragments fused to CCT1 were fractionated by native gel electrophoresis, and the blot was probed with antibody to CCT1. GUS-CCT1 and GUS-CRY1 were multimers, whereas deletion GUS fragments fused to CCT1 were monomers. Lanes 5 and 6 show the GUS-CRY1 line shown in Figure 6D. Asterisks indicate degradation bands.
(C) Native molecular masses of the various proteins were determined by Ferguson plots (Hedrick and Smith, 1968). Monomeric mass was calculated from the deduced amino acid sequence. Ratios of the native size to the monomeric mass are shown.
Multimerization of GUS Is Required to Constitutively Activate CCT1
In an attempt to map the domains within GUS that are required for interaction with GUS, we prepared bait constructs corresponding to a series of GUS deletion fragments (Figure 5C). Protein gel blot analysis, conducted on extracts from yeast cells coexpressing GUS and any one of these deletion fragments, demonstrated that all of the GUS fragments were expressed at a similar protein level (data not shown). Unexpectedly, all deletion fragments failed to show an interaction with GUS (Figure 5E, samples 2 to 6). To examine whether multimerization of GUS is necessary to mediate a COP phenotype on fusion with CCT1, we prepared constructs expressing CCT1 joined to the GUS deletion fragments (Figure 6B, constructs 14 and 15) and overexpressed them in the cry1 mutant background. None of the transgenic seedlings expressing these truncated GUS-CCT1 fusion proteins showed either a COP phenotype in the dark (Figure 6D, top, seedlings 14 and 15) or enhanced blue light responses (Figure 6D, bottom, seedlings 14 and 15). Protein gel blot analysis using antibody against CCT1 indicated that these truncated fusion proteins were expressed normally (Figure 7E). Furthermore, native gel electrophoresis and protein gel blot analysis of the protein extracts prepared from these transgenic plants indicated that the truncated GUS-CCT1 fusion protein was a monomer (Figures 8B, lanes 3 and 4, and 8C). Therefore, the multimerization property of GUS is required to activate CCT1 on fusion to CCT1.
Transgenic Plants Expressing GUS Fused to the N Terminus of Full-Length CRY1 Display a COP Phenotype
In contrast to GUS-CCT1 plants, transgenic seedlings overexpressing full-length CRY1 are fully etiolated in darkness (Lin et al., 1995a, 1996). It was postulated that CNT represses CCT activity in the dark and that the removal of CNT thus relieves its repression and results in constitutive light activation of CCT (Yang et al., 2000; Cashmore, 2003). However, the absence of a COP phenotype observed for plants expressing the various truncated GUS-CCT1 and GFP-CCT1 fusion proteins argued against this hypothesis (Figure 6, Table 1). Therefore, we proposed that CNT1 in the dark simply does not have activity and that the role of CNT1 likely positively regulates CCT1 activity in response to light. To further test this proposition, we constructed a vector expressing GUS fused to the N terminus of the full-length CRY1 (Figure 6B, construct 16) and overexpressed it in the cry1 mutant background. Indeed, transgenic seedlings expressing GUS-CRY1 showed a COP phenotype in the dark (Figure 6D, top, seedling 16) and enhanced blue light responses (Figure 6D, bottom, seedling 16), similar to those observed for plants expressing GUS-CCT1 fusion protein. By contrast, the COP phenotype was not observed for transgenic plants expressing GFP-CRY1 fusion protein and missense CRY1 mutants fused to GUS [GUS-CRY1-10 (R576K), -19 (E515K), and -20 (E531K), corresponding to the previously described cry1 mutant alleles (Ahmad et al., 1995)] (data not shown), indicating that the COP phenotype observed for plants expressing GUS-CRY1 is physiologically meaningful. Similar to GUS-CCT1, GUS-CRY1 was shown to multimerize in vivo (Figures 8B, lanes 5 and 6, and 8C). Therefore, these combined data suggest that GUS is fully capable of constitutively activating CCT1 in the presence of CNT1 and that the role of CNT1 is essentially to activate CCT1 in response to light.
DISCUSSION
Arabidopsis CNT1-Mediated Dimerization Is Required for Light Activation of CCT1
Ethyl methanesulfonate mutagenesis screening resulted in a large number of missense cry1 mutants that contain point mutations throughout both CNT1 and CCT1 (Ahmad et al., 1995; Shalitin et al., 2003). It has been demonstrated that transgenic plants expressing either CCT1 or CCT2 fused to GUS display a COP phenotype (Yang et al., 2000). These data suggest that CRY1 and CRY2 signaling in response to light activation is mediated through their C-terminal domains. This phenotype was not observed for transgenic plants expressing mutant CCT1 proteins corresponding to the missense cry1 alleles that contain point mutations within CCT1. CCT1 and CCT2 have been shown to interact with COP1 (Wang et al., 2001; Yang et al., 2001), and it has been demonstrated that missense mutations within CCT1 affect its negative regulation of COP1 (Yang et al., 2001). Our transgenic, yeast two-hybrid, in vivo chemical cross-linking, gel filtration, and coimmunoprecipitation studies revealed that CRY1 homodimerizes through CNT1 in Arabidopsis in a light-independent manner and that the point mutations corresponding to the loss-of-function alleles that contain mutations within CNT1 affect CRY1 dimerization. These findings are further strengthened by the demonstrations that truncated CRY1 proteins with deletions within CNT1 do not have CRY1 activity and that the COP phenotype mediated by GUS-CCT1 and GUS-CRY1 mechanistically involve multimerization of GUS; additionally, they are supported by yeast two-hybrid and coimmunoprecipitation studies showing that Arabidopsis CRY2 homodimerizes in vivo (C. Lin, personal communication). Arabidopsis phytochrome has been shown to homodimerize (Edgerton and Jones, 1992; Wagner et al., 1996), and the blue light receptor phototropin 1, which mediates phototropism (Huala et al., 1997; Sakai et al., 2001) and blue light–induced stomatal opening (Kinoshita et al., 2001), has also been shown to dimerize through its LOV1 domain (Salomon et al., 2004). Interestingly, the CRY signaling partner COP1 has been demonstrated to form a dimer (Torii et al., 1998). Combining the earlier CRY1 mutagenesis analysis (Ahmad et al., 1995; Shalitin et al., 2003) and our transgenic data with the biochemical data indicates that CRY1 constitutively forms a homodimer, that CNT1 is responsible for its dimerization, and that light activation of Arabidopsis CCT1 requires CNT1-mediated dimerization.
Arabidopsis CRY1 and Photolyase Require Different Protein Structures to Perform Their Roles
Although CNT1 shows sequence similarity with photolyase, CRY1 does not have photolyase activity (Lin et al., 1995b; Malhotra et al., 1995). Conversely, Arabidopsis (6–4) photolyase is not able to perform the role of CNT1 in the light activation of CCT1 (this report). By contrast, the domains of CRY1 and CRY2 are functionally interchangeable, because transgenic plants overexpressing either CNT2-CCT1 or CNT1-CCT2 fusion proteins show blue light–dependent responses (Ahmad et al., 1998a). These earlier results can be explained by previous findings that both CCT1 and CCT2 are able to interact with COP1 (Wang et al., 2001; Yang et al., 2001) and our findings that both CNT1 and CNT2 are capable of mediating self-association. It was postulated that CRY and photolyases are monomeric proteins with similar structures (Sancar, 2004). The demonstrations that Arabidopsis CRY1 dimerizes and its activity requires dimerization of the protein, and that UVR3 is unable to substitute for CNT1 to activate CCT1, suggest that CRY and photolyases perform their distinct roles on a different basis of protein structure (homodimer versus monomer).
CNT1-Mediated Dimerization May Affect CCT1 Phosphorylation
The light activation of Arabidopsis CRY1 and CRY2 involves blue light–dependent phosphorylation (Shalitin et al., 2002, 2003; Bouly et al., 2003). Interestingly, only one of the missense mutations in the newly isolated cry1 mutants affects phosphorylatable residues (Shalitin et al., 2003). It has been demonstrated that GUS-CCT2 undergoes constitutive phosphorylation and that CNT2 is not required for CCT2 phosphorylation (Shalitin et al., 2002). However, it was later demonstrated that missense mutations within CNT1 compromise CRY1 phosphorylation (Shalitin et al., 2003). Based on our results, we propose that the integral structure of the CNT1 dimer is critical for CCT1 phosphorylation. In the case of GUS-CCT1 fusion proteins, the properties of the GUS multimer are likely similar to those of the light-modified CNT1 dimer. Therefore, the GUS multimer is able to confer a change in CCT1 and result in the constitutive phosphorylation of CCT1. Details of the relationship between CNT1 structure and CCT1 phosphorylation remain to be determined.
CNT1 and the Phytochrome B N-Terminal Domain Have Contrasting Roles
CRY, along with the red/far-red light photoreceptor phytochromes, regulate photomorphogenesis and flowering time and entrain the circadian clock in Arabidopsis (Zhong et al., 1997; Casal and Mazzella, 1998; Guo et al., 1998; Neff and Chory, 1998; Somers et al., 1998; Mockler et al., 1999, 2003). It has been shown that Arabidopsis CRY and phytochromes interact (Ahmad et al., 1998b; Mas et al., 2000). Phytochromes have an N-terminal photosensory domain with a chromophore and a C-terminal domain that carries dimerization sites (Edgerton and Jones, 1992; Park et al., 2000; Smith, 2000), and both domains are necessary for the photoreceptor activity of phytochrome B (Wagner et al., 1996). GUS has been demonstrated to be able to substitute for the C-terminal domain of phytochrome B, with the N-terminal domain of phytochrome B fused to GUS being able to trigger much stronger phytochrome B responses than the full-length phytochrome B (Matsushita et al., 2003). Thus, in contrast with the roles of CNT1 and CCT1, it is the C-terminal domain of phytochrome B that mediates dimerization and the N-terminal domain of phytochrome B that transduces light signal. Consistently, the CCT interacts with the CRY signaling partner COP1 (Wang et al., 2001; Yang et al., 2001), whereas the N-terminal domain of phytochrome B interacts with its signaling partner PIF3 (Ni et al., 1999; Shimizu-Sato et al., 2002). Nevertheless, it is known now that both kinds of photoreceptors contain two functionally distinguishing domains, with one mediating dimerization and the other transducing light signal, and that GUS can perform the role of the domain that mediates dimerization and confer enhanced photoreceptor activity.
The Role of Arabidopsis CNT1 Is Different from That of Drosophila CNT
A recent study of a new Drosophila cry mutant allele (crym) expressing the photolyase homology domain shows that Drosophila CNT (CRYM) is sufficient for light detection and phototransduction, whereas CCT regulates CRY stability (Busza et al., 2004). Transgenic Drosophila lines overexpressing Drosophila CNT show constitutive circadian photoresponses (Dissel et al., 2004). In keeping with these findings, it is CNT of Drosophila CRY that interacts with its signaling partners (PER and TIM) (Rosato et al., 2001; Busza et al., 2004). Sharply contrasting with these findings in Drosophila, mutations in Arabidopsis CRY1 that lead to a premature stop codon that truncates CCT1 compromise CRY1 activity (Ahmad et al., 1995); transgenic lines overexpressing CNT1 do not show CRY1 activity (this report); it is CCT of Arabidopsis CRY that transduces light signal downstream (Yang et al., 2000) and CNT1 that is responsible for the regulation of CCT1 activity (this report). These studies suggest that plants and insects have acquired strikingly different mechanisms during evolution to regulate CRY activity. It will be of interest to examine whether CNT of Drosophila CRY mediates homodimerization, and if so, whether dimerization is required for its activity.
A Model Describing the Role of CNT1 in the Activation of CCT1
The capacities of the GUS-CCT1 and GUS-CRY1 fusion proteins to mediate a COP phenotype (in contrast with the monomeric truncated GUS-CCT1 fusion proteins) and the light-activated CRY1 to mediate enhanced light responses (in contrast with the CRY1 molecule in the dark) allow us to predict that the properties of the GUS multimer are analogous to those of the light-modified CNT1 dimer in principle (Figures 9A and 9C) and that the Arabidopsis CNT1 dimer must have contrasting properties in the dark and in the light (Figure 9A). Based on our findings, we tentatively conclude that irradiation with blue light modifies the properties of the CNT1 dimer, resulting in a change in CCT1 and eventually activating CCT1. In this manner, the CNT1 dimer exerts its positive regulation on CCT1. Missense mutations within CNT1 that abolish dimerization also abolish CNT1's ability to undergo a blue light–induced property change, as a result of which CCT1 cannot be activated (Figure 9B). A recent study presents the crystal structure of Arabidopsis CNT1, which shows a fold similar to that of photolyase (Brautigam et al., 2004). Although it was postulated in this study that CRY1 functionality requires two closely contacting CRY1 molecules, analysis of the CNT1 crystal structure did not reveal homodimerization of CNT1 and the presumptive light-dependent property change in CNT1 proposed here (Figure 9A), both of which are observed in the phytochrome and the phototropin photoreceptors (Crosson and Moffat, 2002; Harper et al., 2003; van der Horst and Hellingwerf, 2004). Unraveling the “elusive” nature of CRY awaits future analyses of the crystal structures of the dark- and blue light–treated full-length CRY proteins.
Figure 9.
Model Describing the Blue Light–Dependent Regulation of CCT1 by CNT1.
(A) The CNT1 dimer presumably has contrasting properties in the dark (left) and in the light (right). Perception of blue light by flavin in CNT1 initiates modification of the CNT1 dimer, resulting in a change in the CNT1 dimer, and in turn altering the properties of CCT1, eventually activating CCT1. BL, blue light; F, flavin adenine dinucleotide.
(B) Mutations within CNT1 eliminate CNT1 dimerization, resulting in an inability to mediate a proper change in CCT1, which is required for its activation.
(C) The properties of the GUS multimer are analogous to those of the light-modified CNT1 dimer in principle, resulting in constitutively activating CCT1.
METHODS
Construction of Vectors for the LexA Yeast Two-Hybrid System
Bait Constructs
pLexA bait vectors expressing GUS, Arabidopsis thaliana CNT1 and CCT1, and Arabidopsis CCT2 were made previously (Yang et al., 2001). The PCR-amplified fragments of Arabidopsis CNT2, six CNT1 deletion fragments [CNT1Δ244-489 (lacking amino acids 244 to 489), CNT1Δ1-243 (lacking amino acids 1 to 243), CNT1Δ401-489 (lacking amino acids 401 to 489), CNT1Δ1-99 (lacking amino acids 1 to 99), CNT1Δ1-49Δ451-489 (lacking amino acids 1 to 49 and 451 to 489), and CNT1Δ1-121Δ370-489 (lacking amino acids 1 to 121 and 370 to 489)], five GUS deletion fragments [GUSΔ201-603 (lacking amino acids 201 to 603), GUSΔ1-200 (lacking amino acids 1 to 200), GUSΔ402-603 (lacking amino acids 402 to 603), GUSΔ1-401 (lacking amino acids 1 to 401), and GUSΔ1-99Δ501-603 (lacking amino acids 1 to 99 and 501 to 603)], GFP, and UVR3 were cloned into EcoRI and XhoI sites of pLexA. The bait constructs expressing CNT1 mutants (CNT1-305, containing an A462V mutation; CNT1-375, containing a G347R mutation; and CNT1-388, containing an S66N mutation) were made with the Stratagene (La Jolla, CA) in vitro mutagenesis kit using pLexA-CNT1 as template. All of the constructs used were confirmed by DNA sequencing.
Prey Constructs
The PCR-amplified fragments of GUS and Arabidopsis CRY1, CNT1, CCT1, CRY2, CNT2, GFP, and UVR3 were cloned into EcoRI and XhoI sites of the prey vector pJG4-5.
Yeast Two-Hybrid Assay
Yeast transformation and the calculation of relative β-galactosidase activities were as described previously (Yang et al., 2001). The expression of LexA and B42 transcriptional activation domain fusion proteins was detected by gel blot analysis using antibodies against LexA and hemagglutinin. Ten clones for each combination of prey and bait constructs were taken randomly and analyzed to generate the data in Figures 2C, 5B, and 5E. For all of the two-hybrid experiments described in Figures 2C, 5B, and 5E, the yeast cells were grown under standard laboratory lighting conditions. The plate assay shown in Figure 2D was performed according to the user manual for the Matchmaker LexA two-hybrid system (Clontech, Palo Alto, CA). Briefly, the yeast cells were inoculated on induction synthetic defined plates containing 20 mg/mL 5-bromo-4-chloro-3-indoyl-β-d-galactopyranoside, grown at 30°C either in darkness or under blue light (25 μmol·s−1·m−2) for 15 h, and photographed.
Expression and Purification of GST-CRY1 and GST-CRY2 Fusion Proteins
Full-length CRY1 and CRY2 were amplified by PCR, digested with SpeI, and then fused to the C terminus of GST in pESP2. Plasmid transformation, induction of expression, and purification of the fusion proteins were performed according to the manufacturer's instructions for the ESP Yeast Protein Expression and Purification System in Schizosaccharomyces pombe (Stratagene). The absorption spectra and the contents of the purified GST-CRY1 and GST-CRY2 fusion proteins were measured with a Beckman Instruments (Fullerton, CA) DU-640 nucleic acid and protein analyzer.
In Vitro Transcription/Translation and in Vitro Binding Assay
CRY1, CNT1, CCT1, CRY2, CNT2, and CCT2 polypeptides were synthesized and labeled with [35S]Met in the TNT T7 Quick for PCR DNA transcription/translation system (Promega, Madison, WI). Five micrograms of GST, GST-CRY1, and GST-CRY2 proteins were incubated separately with 15 μL of glutathione Sepharose 4B beads (Amersham, Buckinghamshire, UK) in 300 μL of binding buffer [50 mM Tris-HCl, pH 7.4, 100 mM NaCl, 5 mM EDTA, 0.5% Triton X-100, 1 mM phenylmethylsulfonyl fluoride (PMSF), and 1 mM DTT] containing 1 × complete protease inhibitor cocktail (Roche, Basel, Switzerland) at 4°C for 1 h, and then the beads were washed three times with 1 mL of binding buffer. Five microliters of radiolabeled prey proteins was then added and incubated in a 300-μL reaction on a rotating shaker at 4°C overnight in the dark or under blue light (30 μmol·s−1·m−2). After centrifugation, the beads were pelleted and washed four times with the ice-cold binding buffer described above. The washed pellet was resuspended in 2× SDS sample buffer, and the proteins were analyzed by SDS-PAGE and autoradiography.
Construction of Expression Cassettes, Transformation, and Growth of Plants
pKYL71-GUS-CCT1 was made previously (Yang et al., 2000). The 6×Myc tag was cloned into XhoI and PstI sites of pBluescript SK+ (pBS; Stratagene). PCR-amplified fragments of CNT1 and CRY1 were cloned into EcoRI and SacI sites of pBS-6×Myc, and the resulting constructs (pBS-Myc-CNT1 and pBS-Myc-CRY1) were used as templates to make the pBS constructs containing Myc-tagged CNT1 and CRY1 mutants (Myc-CNT1-305, Myc-CNT1-375, Myc-CNT1-388, and Myc-CRY1-375) with the Stratagene in vitro mutagenesis kit. The PCR-amplified GUS fragment was cloned into SpeI and SacI sites of pBS-6×Myc. To make pBS-GUS-CRY1, the PCR-amplified GUS fragment was first cloned into EcoRI and SpeI sites of pBS, and then the PCR-amplified CRY1 fragment was cloned into SpeI and SacI sites of pBS-GUS. The PCR-amplified CCT1 fragment was cloned into SpeI and SacI sites of pBS. The PCR-amplified fragments encoding CNT1Δ401-489, GUSΔ402-603, and GFP were cloned into XhoI and SpeI sites of pBS-CCT1. The PCR-amplified UVR3 fragment was cloned into XhoI and XbaI sites of pBS-CCT1. The PCR-amplified fragments encoding full-length CRY1, CNT1Δ1-99-CCT1, and GUSΔ1-200-CCT1 were cloned into XhoI and SacI sites of pBS. After confirmation by sequencing, the fragments encoding Myc-CNT1, Myc-GUS, Myc-CRY1, CRY1, CNT1Δ401-489-CCT1, CNT1Δ1-99-CCT1, Myc-CRY1-375, Myc-CNT1-305, Myc-CNT1-375, Myc-CNT1-388, GUSΔ402-603-CCT1, GUSΔ1-200-CCT1, GUS-CRY1, CRY1-GUS, GFP-CCT1, and UVR3-CCT1 were excised with XhoI and SacI and cloned into pKYL71.
All constructs were transferred into Agrobacterium tumefaciens strain GV3101. The in planta method was used to conduct transformation of Arabidopsis, Columbia ecotype (Clough and Bent, 1998). The cry1 mutant used in this study was hy4-104 (Bruggemann et al., 1996). T1 seeds were screened on 15-cm MS plates supplemented with 100 mg/L kanamycin under blue light (20 μmol·s−1·m−2), and blue light responses of the T2 seedlings expressing each construct were initially evaluated on 200 to 300 independent transformants. Then, 30 to 50 independent T2 lines expressing each transgene were transferred to soil, and protein gel blot analysis was performed on 8 to 16 independent transgenic lines. Homozygous T4 seeds were used for final analyses. Arabidopsis seeds were sterilized with 20% bleach and were put in a 4°C refrigerator for 3 to 5 d, induced for germination in white light for 24 h, and finally transferred to the dark and continuous blue light (20 μmol·s−1·m−2).
Antibodies
The PCR-amplified fragments encoding CCT1 (amino acids 490 to 681) of Arabidopsis CRY1 and the full-length Arabidopsis CUL1 were cloned into pET-21a(+). These vectors were transformed into Escherichia coli BL21 (DE3). Protein expression was induced with 1 M isopropyl-β-d-thiogalactopyranoside for 4 h at 37°C, and fusion proteins were purified using a nickel affinity column (Qiagen, Valencia, CA). The purified proteins were further purified by SDS-PAGE and dialysis. Rabbits or mice were immunized, and the CCT1 and CUL1 antisera were analyzed by protein gel blot using protein extracts prepared from the cry1 and fus6-1 mutant plants, respectively. The monoclonal antibodies against LexA, hemagglutinin, and Myc were from Santa Cruz Biotechnology (Santa Cruz, CA).
Protein Expression Studies
Protein gel blot analysis was performed as described previously with minor modifications (Yang et al., 2000). Approximately 50 μg of total protein, determined with the DC Protein Assay Kit (Bio-Rad, Hercules, CA), was fractionated on a 10% SDS-PAGE mini-gel and blotted to a polyvinylidene difluoride membrane (Amersham). The blots were probed with the primary antibodies [diluted in PBST (80 mM Na2HPO4, 20 mM NaH2PO4, 100 mM NaCl, and 0.1% Tween 20)], washed in PBST three times, reacted with goat anti-rabbit or goat anti-mouse IgG conjugated with horseradish peroxidase (1:4000 to 1:5000; Amersham), washed, and exposed to x-ray film using the enhanced chemiluminescence method according to the manufacturer's instructions (Amersham). The same blot sometimes was reprobed with different antibodies. The exposure time for enhanced chemiluminescence of different immunoblots was not controlled precisely, so the signal intensities from different immunoblots are not directly comparable.
Native Gel Electrophoresis
Fifty to 70 7-d-old dark-grown or blue light–grown seedlings were collected, and the total soluble proteins were extracted with the extraction buffer (50 mM Tris-HCl, pH 7.4, 100 mM NaCl, 10 mM MgCl2, 1 mM EDTA, and 10% glycerol) containing 1 mM PMSF and 1× complete protease inhibitor cocktail (Roche). The protein extracts were separated on gels of different acrylamide concentrations (6, 7, 8, and 9%), and the immunoblots were probed with the primary antibodies. The result of the 8% gel is shown in Figures 8A and 8B. The ratio of the native size to the monomeric mass is shown in Figure 8C. The negative slope of 100 (log [RF × 100]) was used to determine the retardation coefficient. The High Molecular Weight Calibration Kit for native electrophoresis was used as a standard (Amersham).
In Vivo Cross-Linking
Formaldehyde cross-linking was performed as described previously with minor modifications (Serino et al., 2003). Fifty to 70 7-d-old dark-grown or blue light–grown seedlings or etiolated seedlings exposed to blue light (30 μmol·s−1·m−2) for a period of time (15, 30, and 60 min) were incubated in 600 μL of PBSM buffer (1.76 mM KH2PO4, 10 mM Na2HPO4, 136 mM NaCl, 2.6 mM KCl, 5 mM MgCl2, and 10% glycerol) containing 1% formaldehyde under vacuum for 30 min. The reaction was stopped by adding 150 μL of 1 M Tris-HCl, pH 7.5, for 5 min. The seedlings were washed with distilled, deionized water, frozen with liquid nitrogen, and ground with protein extraction buffer. Protein extracts were subjected to SDS-PAGE and immunoblot analysis with anti-Myc or anti-CCT1 antibody.
Gel Filtration Studies
To generate the TAPa-CRY1– and TAPa-GFP–expressing transgenic lines, the cDNA encoding a full-length version of CRY1 or GFP was amplified by PCR and cloned into pDONR201. The transfer of genes from pDONR201 to pN-TAPa vector (Saijo et al., 2003; Rubio et al., 2005) was performed using the recombination reaction (Gateway; Invitrogen, Carlsbad, CA). Both TAPa constructs were transformed into wild-type plants by the floral dip method. T1 seeds were screened on MS plates supplemented with 200 mg/L gentamycin. TAPa-CRY1 seedlings were grown for 4 d under continuous blue light (5 μmol·s−1·m−2) or continuous darkness. Plants were harvested and protein extracts were obtained using buffer containing 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 10% glycerol, 0.1% Nonidet P-40, 1 mM DTT, 1 mM PMSF, and 1× complete protease inhibitor cocktail (Roche). Protein extracts (200 μg) were injected onto a Superdex 200 column (Amersham). Twenty-four fractions of 0.5 mL were collected. Proteins on each fraction were concentrated using Strataresin (Stratagene), run on 10% SDS-PAGE gels, and blotted using the anti-CRY1 antibody.
Coimmunoprecipitation Studies
TAPa-CRY1– and TAPa-GFP–expressing seedlings (0.4 g fresh weight) grown in complete medium for 4 d in continuous darkness were ground in liquid nitrogen, thawed in 2 volumes of extraction buffer, and centrifuged at 12,000g for 10 min at 4°C. Extracts containing equal amounts of total protein were incubated with 200-μL IgG beads (bed volume; Amersham) for 2 h at 4°C with gentle rotation. After centrifugation at 150g for 3 min at 4°C, the IgG beads were recovered and washed three times with 1 mL of washing buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 10% glycerol, and 0.1% Nonidet P-40) and once with 1 mL of cleavage buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 10% glycerol, 0.1% Nonidet P-40, and 1 mM DTT). Elution from the IgG beads was performed by incubation with 10 μL (20 units) of 3C protease (Prescission protease; Amersham) in 1 mL of cleavage buffer at 4°C with gentle rotation. Elutes were recovered after centrifugation at 150g for 3 min at 4°C, and proteins were concentrated using Strataresin (Stratagene). Protein extracts and immunoprecipitated samples were separated on 8% SDS-PAGE gels. Immunoblotting was performed using anti-Myc and anti-CRY1 antibodies.
Light Source
All experiments involving blue light illumination were performed in an E-30 LED growth chamber (Percival, Boone, IA) using the blue diodes (λmax 469 nm) at 22°C in continuous light unless stated otherwise. Light spectra and fluence rates were measured using a HandHeld spectroradiometer (ASD, Boulder, CO) and a Li250 quantum photometer (Li-Cor, Lincoln, NE).
Acknowledgments
Several constructs were initially made while H.-Q.Y. was a postdoctoral fellow in A.R. Cashmore's laboratory. H.-Q.Y. is indebted to A.R. Cashmore for his generosity and support. We thank C. Lin for kindly allowing us to cite CRY2 homodimerization data; K. Yamamoto, Q. Xie, C. Lin, and T. Tsuge for providing us with an Arabidopsis UVR3 cDNA clone, a Myc vector, anti-CRY1 antibody, and anti-Myc antibody, respectively; X.Y. Chen and Z.R. Sung for critical reading of the manuscript; and S.F. Gong for technical assistance. This work was supported by National Natural Science Foundation of China Grants 90208005, 30325007, and 30421001 to H.-Q.Y. and by the Chinese Academy of Sciences, Shanghai-Hong Kong-Anson Research Foundation, and grants from the National Institutes of Health (GM-047850) and the National Science Foundation 2010 program (MCB-0115870) to X.-W.D. V.R. is supported by a Human Frontiers Science Program long-term fellowship.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Hong-Quan Yang (hqyang@sibs.ac.cn).
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.104.029645.
References
- Ahmad, M., and Cashmore, A.R. (1993). HY4 gene of A. thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature 366, 162–166. [DOI] [PubMed] [Google Scholar]
- Ahmad, M., Jarillo, J.A., and Cashmore, A.R. (1998. a). Chimeric proteins between cry1 and cry2 Arabidopsis blue light photoreceptors indicate overlapping functions and varying protein stability. Plant Cell 10, 197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad, M., Jarillo, J.A., Smirnova, O., and Cashmore, A.R. (1998. b). The CRY1 blue light photoreceptor of Arabidopsis interacts with phytochrome A in vitro. Mol. Cell 1, 939–948. [DOI] [PubMed] [Google Scholar]
- Ahmad, M., Lin, C., and Cashmore, A.R. (1995). Mutations throughout an Arabidopsis blue-light photoreceptor impair blue-light-responsive anthocyanin accumulation and inhibition of hypocotyl elongation. Plant J. 8, 653–658. [DOI] [PubMed] [Google Scholar]
- Bagnall, D.J., King, R.W., and Hangarter, R.P. (1996). Blue-light promotion of flowering is absent in hy4 mutants of Arabidopsis. Planta 200, 278–280. [DOI] [PubMed] [Google Scholar]
- Bouly, J.P., Giovani, B., Djamei, A., Mueller, M., Zeugner, A., Dudkin, E.A., Batschauer, A., and Ahmad, M. (2003). Novel ATP-binding and autophosphorylation activity associated with Arabidopsis and human cryptochrome-1. Eur. J. Biochem. 270, 2921–2928. [DOI] [PubMed] [Google Scholar]
- Brautigam, C.A., Smith, B.S., Ma, Z., Palnitkar, M., Tomchick, D.R., Machius, M., and Deisenhofer, J. (2004). Structure of the photolyase-like domain of cryptochrome 1 from Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 101, 12142–12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruggemann, E., Handwerger, K., Essex, C., and Storz, G. (1996). Analysis of fast neutron-generated mutants at the Arabidopsis thaliana HY4 locus. Plant J. 10, 755–760. [DOI] [PubMed] [Google Scholar]
- Busza, A., Emery-Le, M., Rosbash, M., and Emery, P. (2004). Roles of the two Drosophila CRYPTOCHROME structural domains in circadian photoreception. Science 304, 1503–1506. [DOI] [PubMed] [Google Scholar]
- Casal, J.J., and Mazzella, M.A. (1998). Conditional synergism between cryptochrome 1 and phytochrome B is shown by the analysis of phyA, phyB, and hy4 simple, double, and triple mutants in Arabidopsis. Plant Physiol. 118, 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashmore, A.R. (2003). Cryptochromes: Enabling plants and animals to determine circadian time. Cell 114, 537–543. [PubMed] [Google Scholar]
- Cashmore, A.R., Jarillo, J.A., Wu, Y.J., and Liu, D. (1999). Cryptochromes: Blue light receptors for plants and animals. Science 284, 760–765. [DOI] [PubMed] [Google Scholar]
- Clough, S.J., and Bent, A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Crosson, S., and Moffat, K. (2002). Photoexcited structure of a plant photoreceptor domain reveals a light-driven molecular switch. Plant Cell 14, 1067–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, X.W., Caspar, T., and Quail, P.H. (1991). cop1: A regulatory locus involved in light-controlled development and gene expression in Arabidopsis. Genes Dev. 5, 1172–1182. [DOI] [PubMed] [Google Scholar]
- Deng, X.W., Matsui, M., Wei, N., Wagner, D., Chu, A.M., Feldmann, K.A., and Quail, P.H. (1992). COP1, an Arabidopsis regulatory gene, encodes a protein with both a zinc-binding motif and a G beta homologous domain. Cell 71, 791–801. [DOI] [PubMed] [Google Scholar]
- Dissel, S., Codd, V., Fedic, R., Garner, K.J., Costa, R., Kyriacou, C.P., and Rosato, E. (2004). A constitutively active cryptochrome in Drosophila melanogaster. Nat. Neurosci. 7, 834–840. [DOI] [PubMed] [Google Scholar]
- Edgerton, M.D., and Jones, A.M. (1992). Localization of protein-protein interactions between subunits of phytochrome. Plant Cell 4, 161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovani, B., Byrdin, M., Ahmad, M., and Brettel, K. (2003). Light-induced electron transfer in a cryptochrome blue-light photoreceptor. Nat. Struct. Biol. 10, 489–490. [DOI] [PubMed] [Google Scholar]
- Guo, H., Yang, H., Mockler, T.C., and Lin, C. (1998). Regulation of flowering time by Arabidopsis photoreceptors. Science 279, 1360–1363. [DOI] [PubMed] [Google Scholar]
- Hall, J.C. (2000). Cryptochromes: Sensory reception, transduction, and clock functions subserving circadian systems. Curr. Opin. Neurobiol. 10, 456–466. [DOI] [PubMed] [Google Scholar]
- Harper, S.M., Neil, L.C., and Gardner, K.H. (2003). Structural basis of a phototropin light switch. Science 301, 1541–1544. [DOI] [PubMed] [Google Scholar]
- Hedrick, J.L., and Smith, A.J. (1968). Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch. Biochem. Biophys. 126, 155–164. [DOI] [PubMed] [Google Scholar]
- Huala, E., Oeller, P.W., Liscum, E., Han, I.S., Larsen, E., and Briggs, W.R. (1997). Arabidopsis NPH1: A protein kinase with a putative redox-sensing domain. Science 278, 2120–2123. [DOI] [PubMed] [Google Scholar]
- Kinoshita, T., Doi, M., Suetsugu, N., Kagawa, T., Wada, M., and Shimazaki, K. (2001). Phot1 and phot2 mediate blue light regulation of stomatal opening. Nature 414, 656–660. [DOI] [PubMed] [Google Scholar]
- Lin, C., Ahmad, M., and Cashmore, A.R. (1996). Arabidopsis cryptochrome 1 is a soluble protein mediating blue light-dependent regulation of plant growth and development. Plant J. 10, 893–902. [DOI] [PubMed] [Google Scholar]
- Lin, C., Ahmad, M., Gordon, D., and Cashmore, A.R. (1995. a). Expression of an Arabidopsis cryptochrome gene in transgenic tobacco results in hypersensitivity to blue, UV-A, and green light. Proc. Natl. Acad. Sci. USA 92, 8423–8427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, C., Robertson, D.E., Ahmad, M., Raibekas, A.A., Jorns, M.S., Dutton, P.L., and Cashmore, A.R. (1995. b). Association of flavin adenine dinucleotide with the Arabidopsis blue light receptor CRY1. Science 269, 968–970. [DOI] [PubMed] [Google Scholar]
- Lin, C., Yang, H., Guo, H., Mockler, T., Chen, J., and Cashmore, A.R. (1998). Enhancement of blue-light sensitivity of Arabidopsis seedlings by a blue light receptor cryptochrome 2. Proc. Natl. Acad. Sci. USA 95, 2686–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumb, M.J., Drake, A.F., and Danpure, C.J. (1999). Effect of N-terminal alpha-helix formation on the dimerization and intracellular targeting of alanine:glyoxylate aminotransferase. J. Biol. Chem. 274, 20587–20596. [DOI] [PubMed] [Google Scholar]
- Malhotra, K., Kim, S.T., Batschauer, A., Dawut, L., and Sancar, A. (1995). Putative blue-light photoreceptors from Arabidopsis thaliana and Sinapis alba with a high degree of sequence homology to DNA photolyase contain the two photolyase cofactors but lack DNA repair activity. Biochemistry 34, 6892–6899. [DOI] [PubMed] [Google Scholar]
- Mas, P., Devlin, P.F., Panda, S., and Kay, S.A. (2000). Functional interaction of phytochrome B and cryptochrome 2. Nature 408, 207–211. [DOI] [PubMed] [Google Scholar]
- Matsushita, T., Mochizuki, N., and Nagatani, A. (2003). Dimers of the N-terminal domain of phytochrome B are functional in the nucleus. Nature 424, 571–574. [DOI] [PubMed] [Google Scholar]
- Misera, S., Muller, A.J., Weiland-Heidecker, U., and Jurgens, G. (1994). The FUSCA genes of Arabidopsis: Negative regulators of light responses. Mol. Gen. Genet. 244, 242–252. [DOI] [PubMed] [Google Scholar]
- Mockler, T., Yang, H., Yu, X., Parikh, D., Cheng, Y.C., Dolan, S., and Lin, C. (2003). Regulation of photoperiodic flowering by Arabidopsis photoreceptors. Proc. Natl. Acad. Sci. USA 100, 2140–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mockler, T.C., Guo, H., Yang, H., Duong, H., and Lin, C. (1999). Antagonistic actions of Arabidopsis cryptochromes and phytochrome B in the regulation of floral induction. Development 126, 2073–2082. [DOI] [PubMed] [Google Scholar]
- Nakajima, S., Sugiyama, M., Iwai, S., Hitomi, K., Otoshi, E., Kim, S.T., Jiang, C.Z., Todo, T., Britt, A.B., and Yamamoto, K. (1998). Cloning and characterization of a gene (UVR3) required for photorepair of 6-4 photoproducts in Arabidopsis thaliana. Nucleic Acids Res. 26, 638–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff, M.M., and Chory, J. (1998). Genetic interactions between phytochrome A, phytochrome B, and cryptochrome 1 during Arabidopsis development. Plant Physiol. 118, 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni, M., Tepperman, J.M., and Quail, P.H. (1999). Binding of phytochrome B to its nuclear signalling partner PIF3 is reversibly induced by light. Nature 400, 781–784. [DOI] [PubMed] [Google Scholar]
- Osterlund, M.T., Hardtke, C.S., Wei, N., and Deng, X.W. (2000). Targeted destabilization of HY5 during light-regulated development of Arabidopsis. Nature 405, 462–466. [DOI] [PubMed] [Google Scholar]
- Park, C.M., Bhoo, S.H., and Song, P.S. (2000). Inter-domain crosstalk in the phytochrome molecules. Semin. Cell Dev. Biol. 11, 449–456. [DOI] [PubMed] [Google Scholar]
- Rosato, E., Codd, V., Mazzotta, G., Piccin, A., Zordan, M., Costa, R., and Kyriacou, C.P. (2001). Light-dependent interaction between Drosophila CRY and the clock protein PER mediated by the carboxy terminus of CRY. Curr. Biol. 11, 909–917. [DOI] [PubMed] [Google Scholar]
- Rubio, V., Shen, Y., Saijo, Y., Liu, Y., Gusmaroli, G., Dinesh-Kumar, S.P., and Deng, X.W. (2005). An alternative tandem affinity purification strategy applied to Arabidopsis protein complex isolation. Plant J. 41, 767–778. [DOI] [PubMed] [Google Scholar]
- Saijo, Y., Sullivan, J.A., Wang, H., Yang, J., Shen, Y., Rubio, V., Ma, L., Hoecker, U., and Deng, X.W. (2003). The COP1–SPA1 interaction defines a critical step in phytochrome A-mediated regulation of HY5 activity. Genes Dev. 17, 2642–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai, T., Kagawa, T., Kasahara, M., Swartz, T.E., Christie, J.M., Briggs, W.R., Wada, M., and Okada, K. (2001). Arabidopsis nph1 and npl1: Blue light receptors that mediate both phototropism and chloroplast relocation. Proc. Natl. Acad. Sci. USA 98, 6969–6974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon, M., Lempert, U., and Rudiger, W. (2004). Dimerization of the plant photoreceptor phototropin is probably mediated by the LOV1 domain. FEBS Lett. 572, 8–10. [DOI] [PubMed] [Google Scholar]
- Sancar, A. (1994). Structure and function of DNA photolyase. Biochemistry 33, 2–9. [DOI] [PubMed] [Google Scholar]
- Sancar, A. (2004). Regulation of the mammalian circadian clock by cryptochrome. J. Biol. Chem. 279, 34079–34082. [DOI] [PubMed] [Google Scholar]
- Sancar, G.B., Smith, F.W., and Heelis, P.F. (1987). Purification of the yeast PHR1 photolyase from an Escherichia coli overproducing strain and characterization of the intrinsic chromophores of the enzyme. J. Biol. Chem. 262, 15457–15465. [PubMed] [Google Scholar]
- Serino, G., Su, H., Peng, Z., Tsuge, T., Wei, N., Gu, H., and Deng, X.W. (2003). Characterization of the last subunit of the Arabidopsis COP9 signalosome: Implications for the overall structure and origin of the complex. Plant Cell 15, 719–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalitin, D., Yang, H., Mockler, T.C., Maymon, M., Guo, H., Whitelam, G.C., and Lin, C. (2002). Regulation of Arabidopsis cryptochrome 2 by blue-light-dependent phosphorylation. Nature 417, 763–767. [DOI] [PubMed] [Google Scholar]
- Shalitin, D., Yu, X., Maymon, M., Mockler, T., and Lin, C. (2003). Blue light–dependent in vivo and in vitro phosphorylation of Arabidopsis cryptochrome 1. Plant Cell 15, 2421–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu-Sato, S., Huq, E., Tepperman, J.M., and Quail, P.H. (2002). A light-switchable gene promoter system. Nat. Biotechnol. 20, 1041–1044. [DOI] [PubMed] [Google Scholar]
- Smith, H. (2000). Phytochromes and light signal perception by plants: An emerging synthesis. Nature 407, 585–591. [DOI] [PubMed] [Google Scholar]
- Somers, D.E., Devlin, P.F., and Kay, S.A. (1998). Phytochromes and cryptochromes in the entrainment of the Arabidopsis circadian clock. Science 282, 1488–1490. [DOI] [PubMed] [Google Scholar]
- Torii, K.U., McNellis, T.W., and Deng, X.W. (1998). Functional dissection of Arabidopsis COP1 reveals specific roles of its three structural modules in light control of seedling development. EMBO J. 17, 5577–5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Horst, M.A., and Hellingwerf, K.J. (2004). Photoreceptor proteins, “star actors of modern times”: A review of the functional dynamics in the structure of representative members of six different photoreceptor families. Acc. Chem. Res. 37, 13–20. [DOI] [PubMed] [Google Scholar]
- Wagner, D., Koloszvari, M., and Quail, P.H. (1996). Two small spatially distinct regions of phytochrome B are required for efficient signaling rates. Plant Cell 8, 859–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H., Ma, L.G., Li, J.M., Zhao, H.Y., and Deng, X.W. (2001). Direct interaction of Arabidopsis cryptochromes with COP1 in light control development. Science 294, 154–158. [DOI] [PubMed] [Google Scholar]
- Wei, N., Chamovitz, D.A., and Deng, X.W. (1994). Arabidopsis COP9 is a component of a novel signaling complex mediating light control of development. Cell 78, 117–124. [DOI] [PubMed] [Google Scholar]
- Yang, H.Q., Tang, R.H., and Cashmore, A.R. (2001). The signaling mechanism of Arabidopsis CRY1 involves direct interaction with COP1. Plant Cell 13, 2573–2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, H.Q., Wu, Y.J., Tang, R.H., Liu, D., Liu, Y., and Cashmore, A.R. (2000). The C termini of Arabidopsis cryptochromes mediate a constitutive light response. Cell 103, 815–827. [DOI] [PubMed] [Google Scholar]
- Zhong, H.H., Resnick, A.S., Straume, M., and Robertson McClung, C. (1997). Effects of synergistic signaling by phytochrome A and cryptochrome1 on circadian clock-regulated catalase expression. Plant Cell 9, 947–955. [DOI] [PMC free article] [PubMed] [Google Scholar]