Abstract
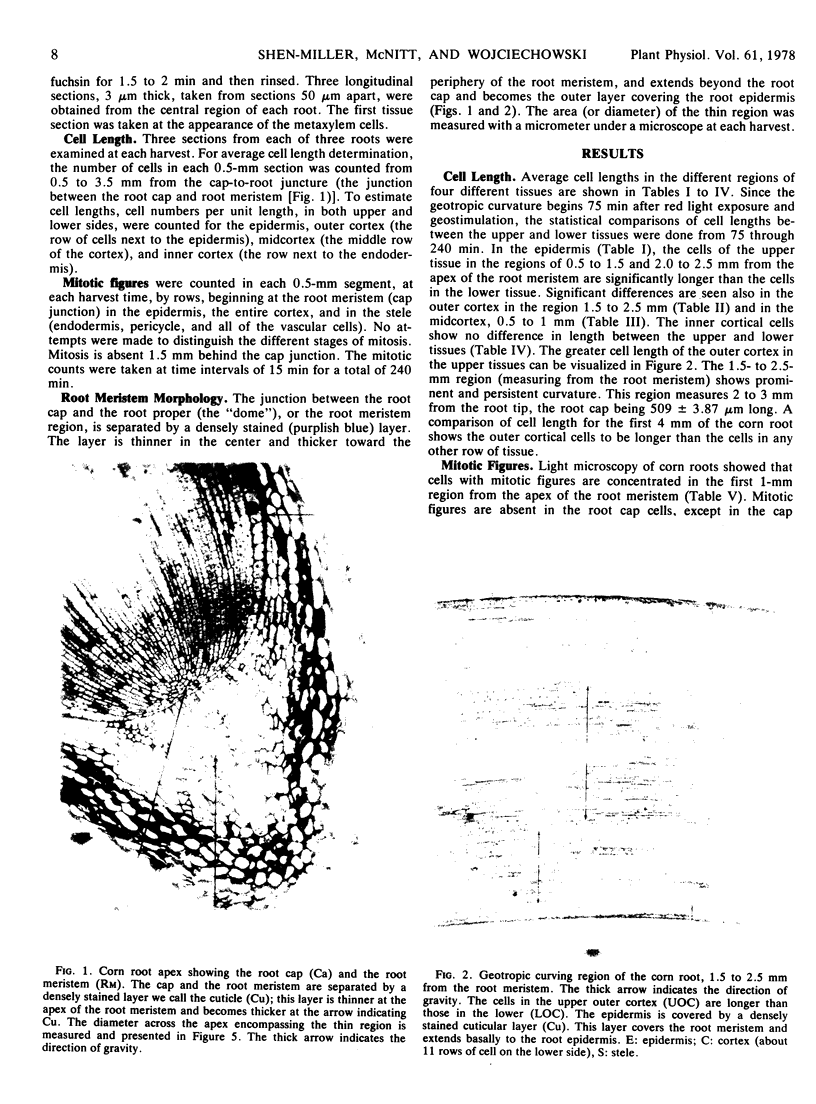
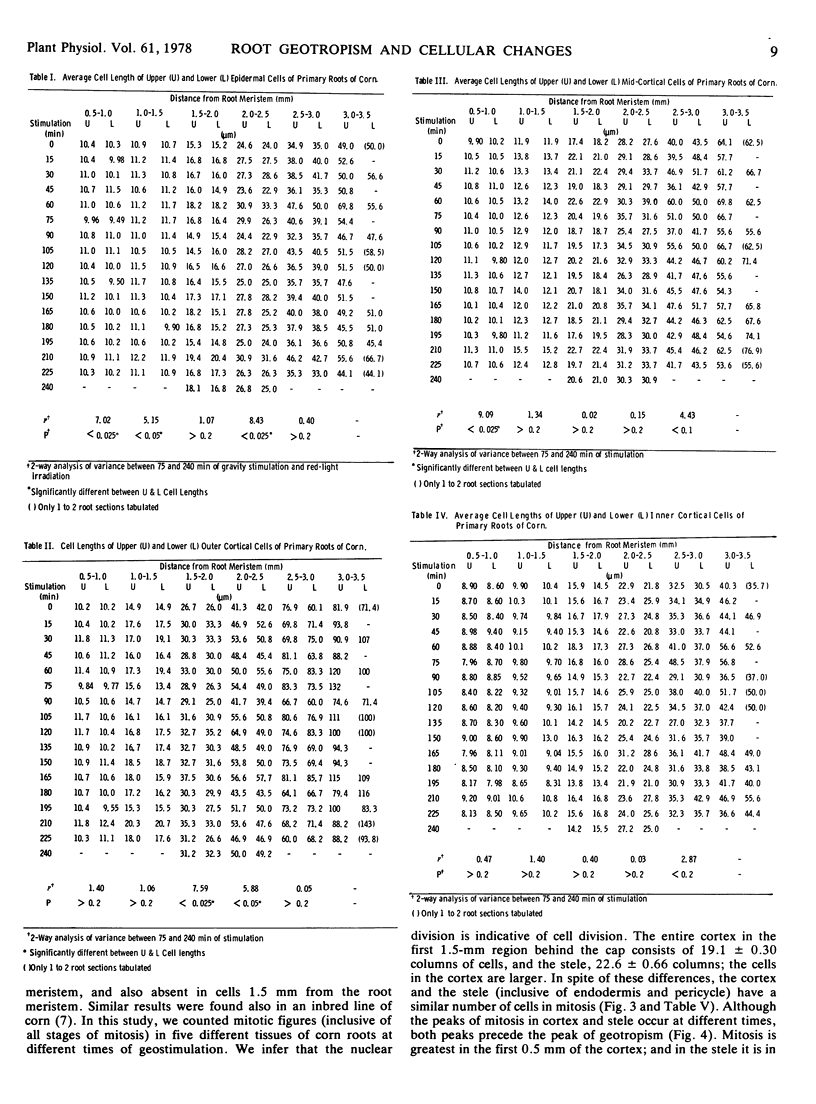
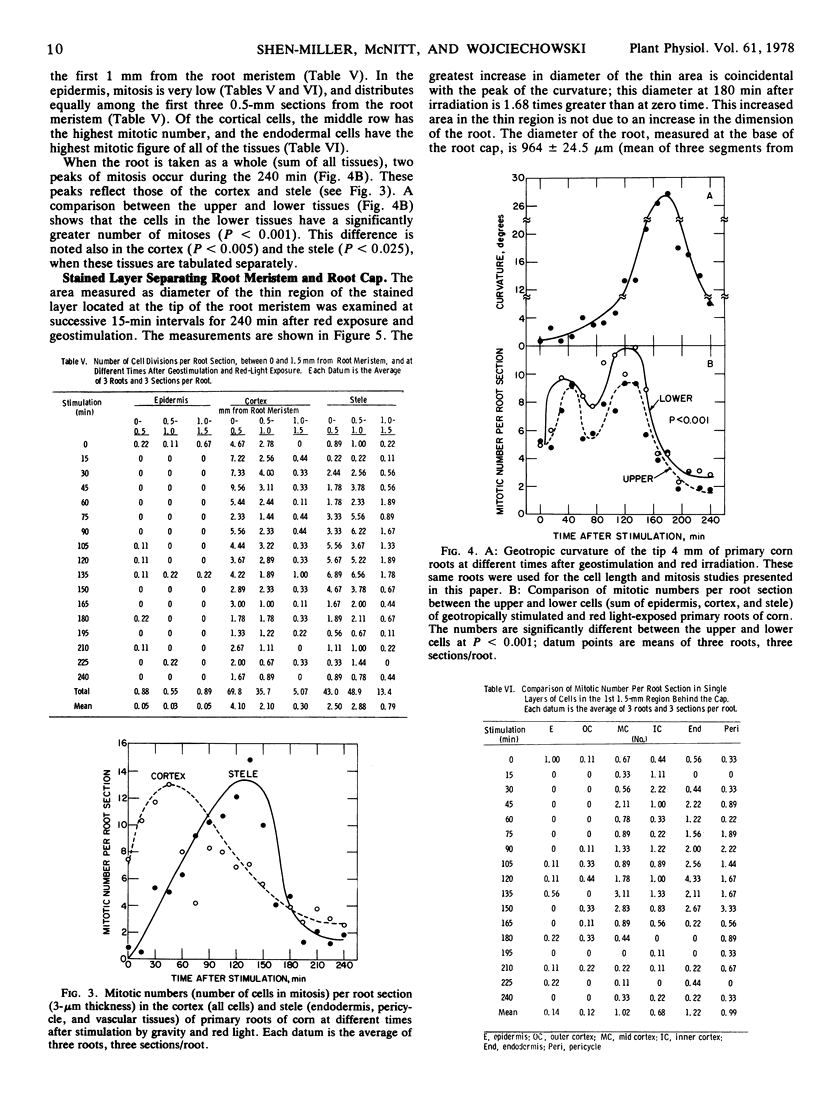
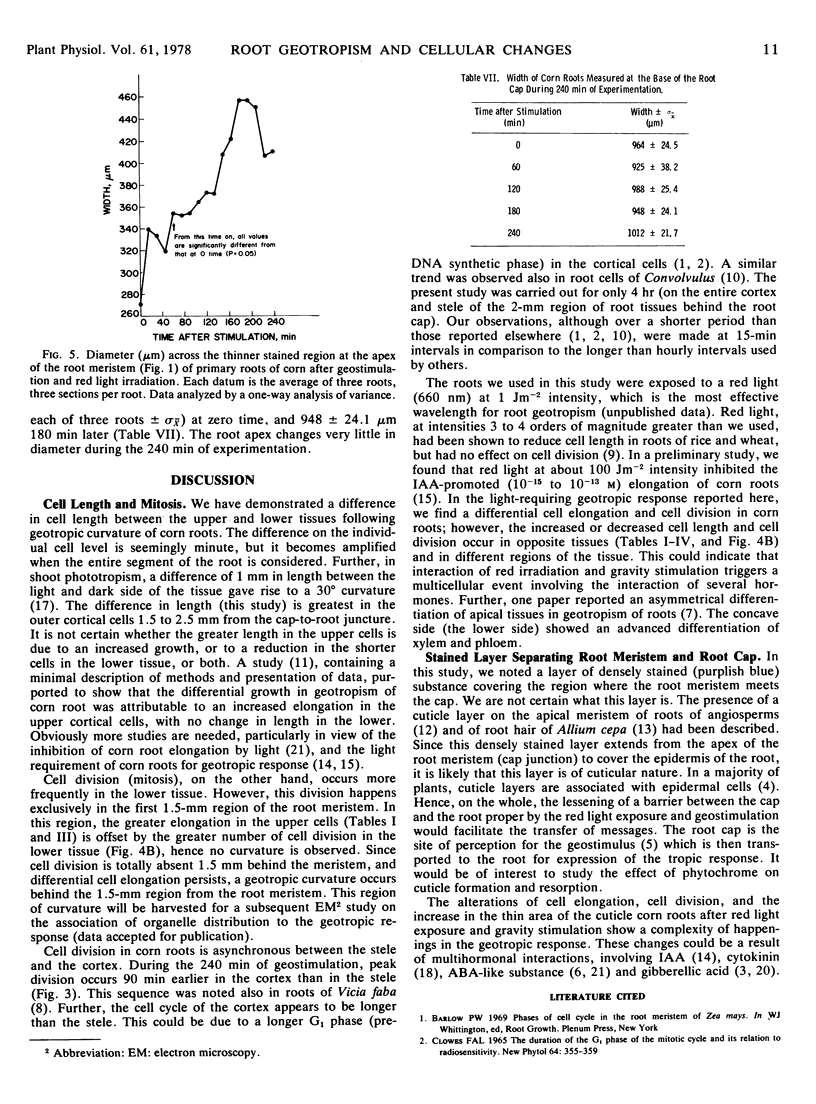
We examined cell length, mitosis, and root meristem “cuticle” in different tissues of geostimulated, red light-exposed primary roots of corn (Zea Mays, Wisconsin hybrid 64A × 22R). The examination was done at 15-minute intervals for a period of 240 minutes. Differences in cell elongation between the upper and lower sides were most prominent between 1.5 and 2.5 mm from the root meristem; the outer cortex had the greatest elongation growth, and the upper cells showed a significant increase in length compared to the lower. A differential mitosis was also found, with the lower tissue being greater. We infer that the mitotic activity is indicative of cell division, and this division occurs strictly in the first 1.5 mm of the root meristem. The combined effect of differential cell elongation and cell division results in the localization of the geotropic curvature in the 1.5- to 2.5-mm region from the root meristem. Mitosis that occurs primarily in the cortex and stele were asynchronous; the peak of cortical division preceded that of the stele. Both peaks occurred before the peak of geotropism. A densely stained layer separates the cap from the root meristem. This layer is thinner at the apex of the root meristem. The area of the thin region increased with time and peaked at 180 minutes after geostimulation, which was coincidental with the peak of the geotropic response.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Mattingly E. Differences in mitotic cycle in various tissues of Vicia faba roots as revealed by 5-amino uracil synchronization of cell division. Exp Cell Res. 1966 May;42(2):274–280. doi: 10.1016/0014-4827(66)90290-4. [DOI] [PubMed] [Google Scholar]
- Shen-Miller J., Gordon S. A. Gravitational compensation and the phototropic response of oat coleoptiles. Plant Physiol. 1967 Mar;42(3):352–360. doi: 10.1104/pp.42.3.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen-Miller J., Gordon S. A. Hormonal Relations in the Phototropic Response: III. The Movement of C-labeled and Endogenous Indoleacetic Acid in Phototropically Stimulated Zea Coleoptiles. Plant Physiol. 1966 Jan;41(1):59–65. doi: 10.1104/pp.41.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]




