Abstract
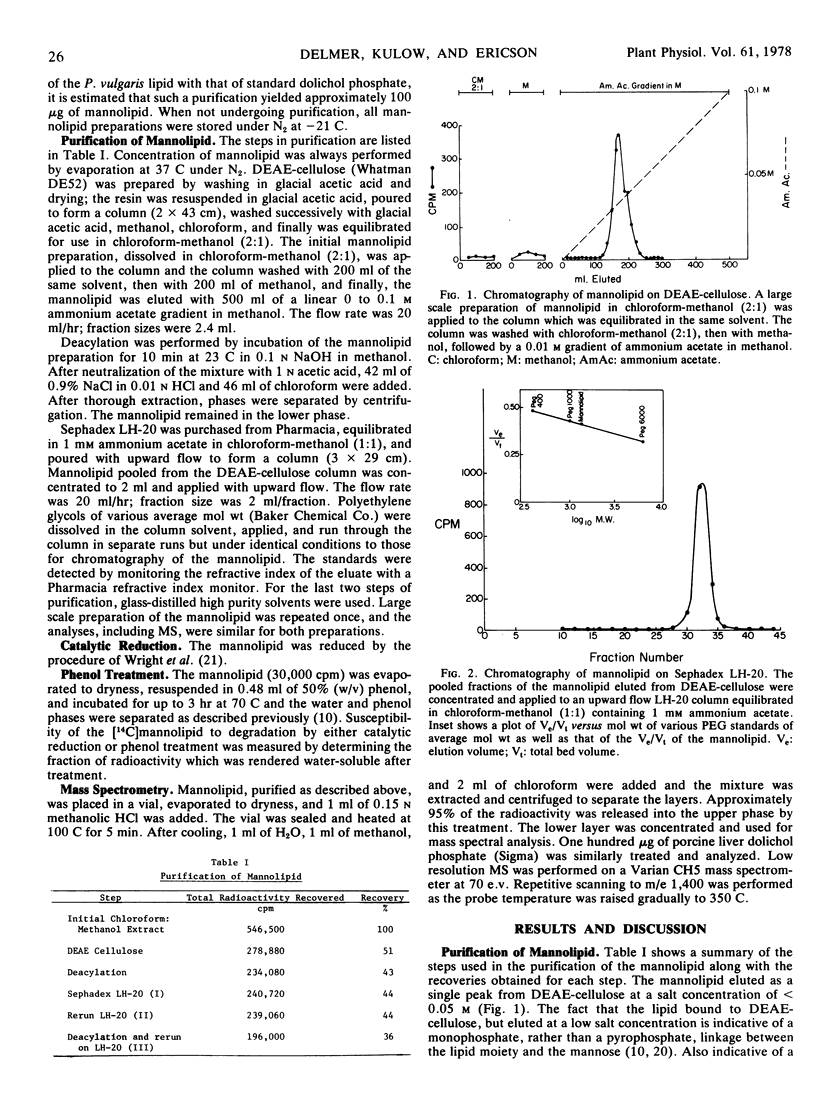
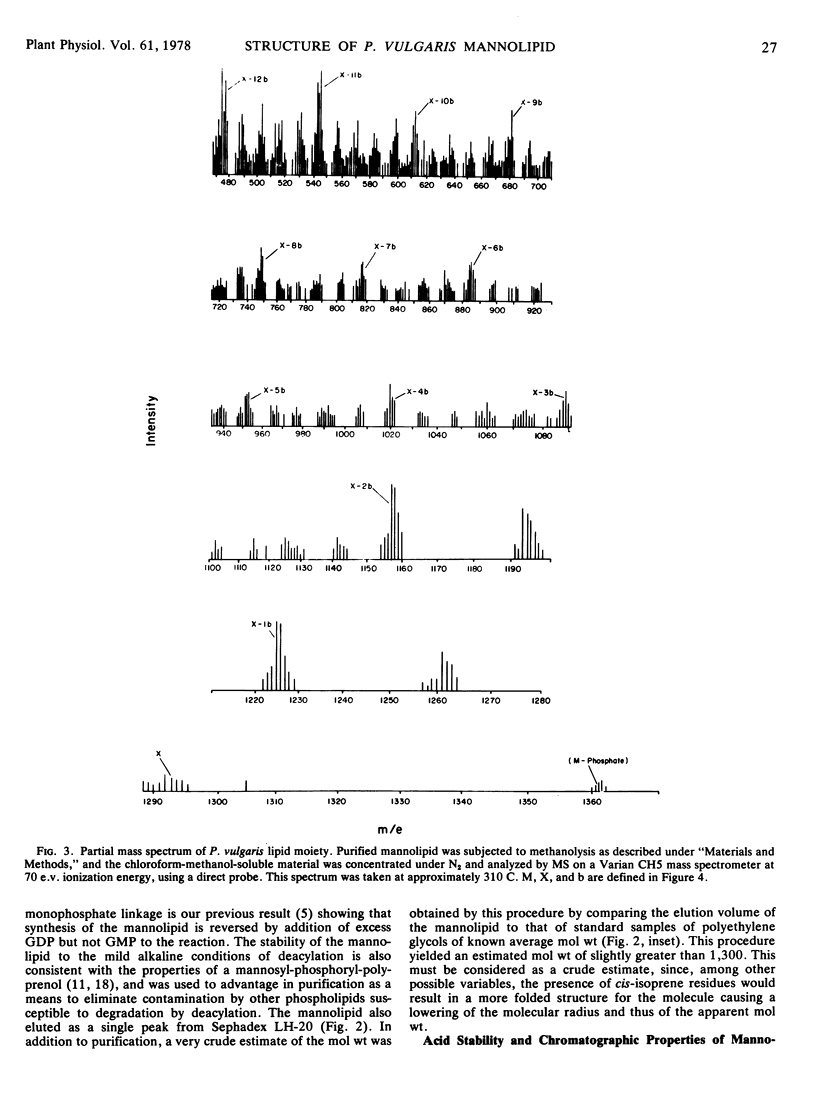
This paper reports the purification and structural determination of the mannolipid shown previously (Ericson and Delmer 1977 Plant Physiol 59: 341-347) to serve as an intermediate in glycoprotein synthesis in cotyledons of Phaseolus vulgaris. The mannolipid was purified by chromatography in organic solvents on diethylaminoethyl-cellulose, followed by repeated steps of deacylation and rechromatography on Sephadex LH-20. Binding and elution behavior on diethylaminoethyl-cellulose was consistent with the presence of a monophosphate residue. Lability of the mannolipid to mild acid treatment as well as its resistance to hot phenol treatment or catalytic hydrogenation are consistent with the structure of a polyprenol having a saturated α-residue. After methanolysis, the chloroform-methanol-soluble portion of the mannolipid was analyzed by mass spectrometry. The fragmentation pattern obtained was nearly identical to that obtained from standard dolichol-phosphate. An intense ion at m/e 69 represented the ω-terminal isoprenoid residue, and repeating fragments separated by 68 mass units were observed up to m/e of > 1,200. All evidence supports the conclusion that the mannolipid is dolichol-monophosphate-mannose and thus provides further support for the concept that the processes involved in the glycosylation of protein in higher plants are similar to those known to occur in the animal kingdom.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baynes J. W., Hsu A. F., Heath E. C. The role of mannosyl-phosphoryl-dihydropolyisoprenol in the synthesis of mammalian glycoproteins. J Biol Chem. 1973 Aug 25;248(16):5693–5704. [PubMed] [Google Scholar]
- Brett C. T., Leloir L. F. Dolichyl monophosphate and its sugar derivatives in plants. Biochem J. 1977 Jan 1;161(1):93–101. doi: 10.1042/bj1610093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daleo G. R., Pont Lezica R. Synthesis of dolichol phosphate by a cell-free extract from pea. FEBS Lett. 1977 Mar 1;74(2):247–250. doi: 10.1016/0014-5793(77)80856-9. [DOI] [PubMed] [Google Scholar]
- Ericson M. C., Delmer D. P. Glycoprotein synthesis in plants: I. Role of lipid intermediates. Plant Physiol. 1977 Mar;59(3):341–347. doi: 10.1104/pp.59.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans P. J., Hemming F. W. The unambiguous characterization of dolichol phosphate mannose as a product of mannosyl transferase in pig liver endoplasmic reticulum. FEBS Lett. 1973 May 1;31(3):335–338. doi: 10.1016/0014-5793(73)80135-8. [DOI] [PubMed] [Google Scholar]
- Forsee W. T., Elbein A. D. Biosynthesis of mannosyl- and glucosyl-phosphoryl-polyprenols in cotton fibers. J Biol Chem. 1973 Apr 25;248(8):2858–2867. [PubMed] [Google Scholar]
- Forsee W. T., Elbein A. D. Glycoprotein biosynthesis in plants. Demonstration of lipid-linked oligosaccharides of mannose and N-acetylglucosamine. J Biol Chem. 1975 Dec 25;250(24):9283–9293. [PubMed] [Google Scholar]
- Forsee W. T., Valkovich G., Elbein A. D. Glycoprotein biosynthesis in plants. Formation of lipid-linked oligosaccharides of mannose and N-acetylglucosamine by mung bean seedlings. Arch Biochem Biophys. 1976 Jun;174(2):469–479. doi: 10.1016/0003-9861(76)90375-1. [DOI] [PubMed] [Google Scholar]
- García R. C., Recondo E., Dankert M. Polysaccharide biosynthesis in Acetobacter xylinum. Enzymatic synthesis of lipid diphosphate and monophospate sugars. Eur J Biochem. 1974 Mar 15;43(1):93–105. doi: 10.1111/j.1432-1033.1974.tb03389.x. [DOI] [PubMed] [Google Scholar]
- Lehle L., Fartaczek F., Tanner W., Kauss H. Formation of polyprenol-linked mono- and oligosaccharides in Phaseolus aureus. Arch Biochem Biophys. 1976 Aug;175(2):419–426. doi: 10.1016/0003-9861(76)90529-4. [DOI] [PubMed] [Google Scholar]
- Lezica R. P. Membrane-bound UDP-Glucose: Lipid Glucosyltransferases from Peas. Plant Physiol. 1976 Nov;58(5):675–680. doi: 10.1104/pp.58.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm D. L., Hassid W. Z. The Role of a d-Mannosyl-Lipid as an Intermediate in the Synthesis of Polysaccharide in Phaseolus aureus Seedlings. Plant Physiol. 1972 Oct;50(4):473–476. doi: 10.1104/pp.50.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waechter C. J., Lennarz W. J. The role of polyprenol-linked sugars in glycoprotein synthesis. Annu Rev Biochem. 1976;45:95–112. doi: 10.1146/annurev.bi.45.070176.000523. [DOI] [PubMed] [Google Scholar]
- Warren C. D., Jeanloz R. W. The characterization of glycolipids derived from long-chain polyprenols: chemical synthesis of -D-mannopyranosyl dolichyl phosphate. FEBS Lett. 1973 May 1;31(3):332–334. doi: 10.1016/0014-5793(73)80134-6. [DOI] [PubMed] [Google Scholar]
- Wellburn A. R., Stevenson J., Hemming F. W., Morton R. A. The characterization and properties of castaprenol-11, -12 and -13 from the leaves of Aesculus hippocastanum (horse chestnut). Biochem J. 1967 Jan;102(1):313–324. doi: 10.1042/bj1020313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright A., Dankert M., Fennessey P., Robbins P. W. Characterization of a polyisoprenoid compound functional in O-antigen biosynthesis. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1798–1803. doi: 10.1073/pnas.57.6.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]



