Abstract
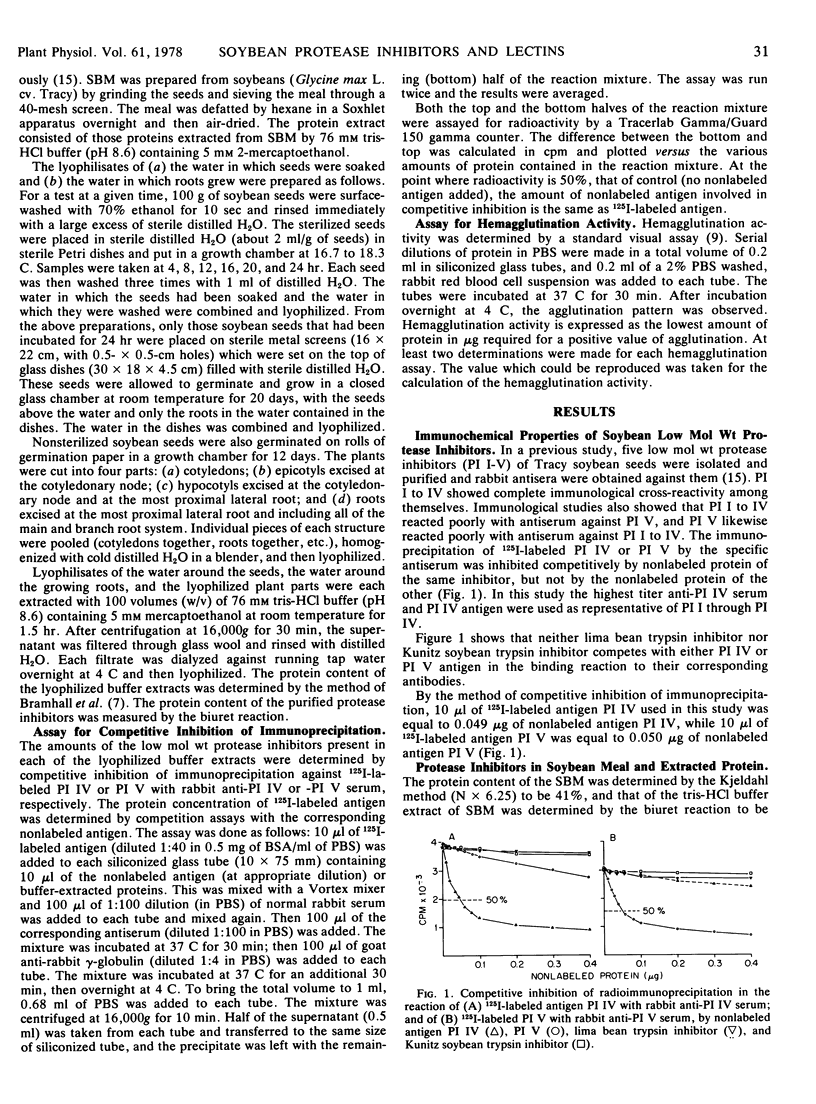
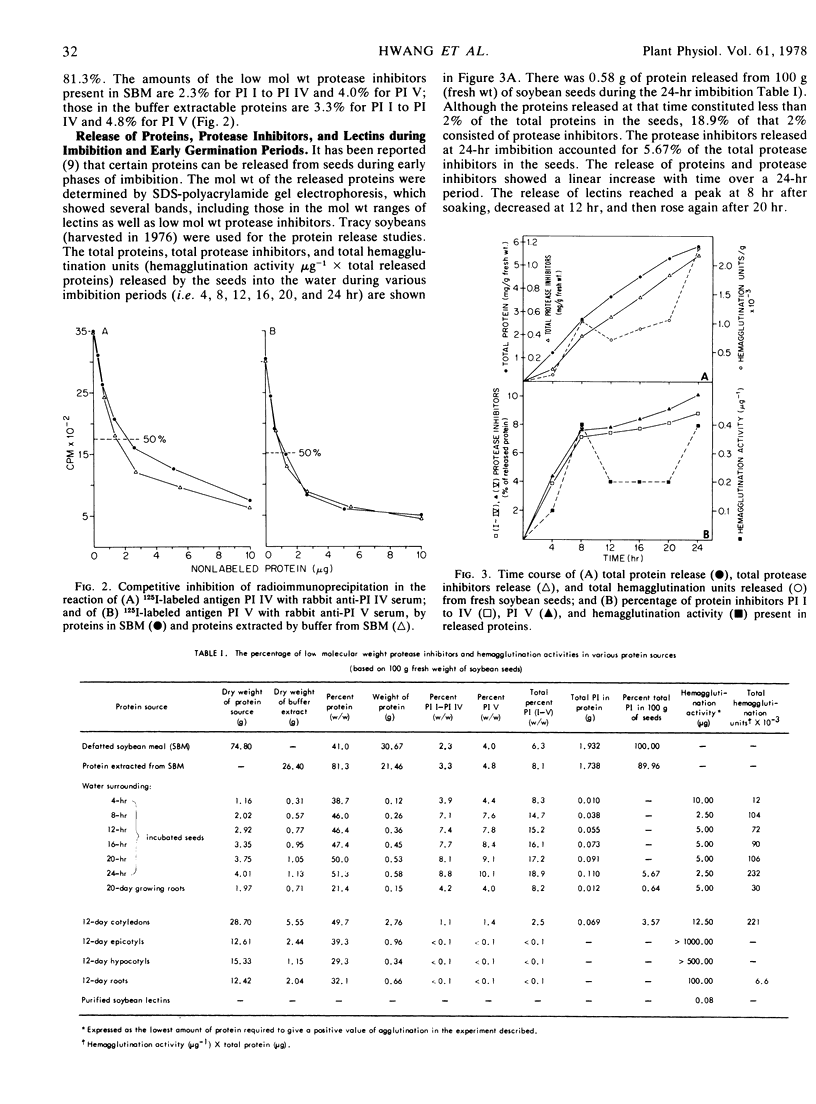
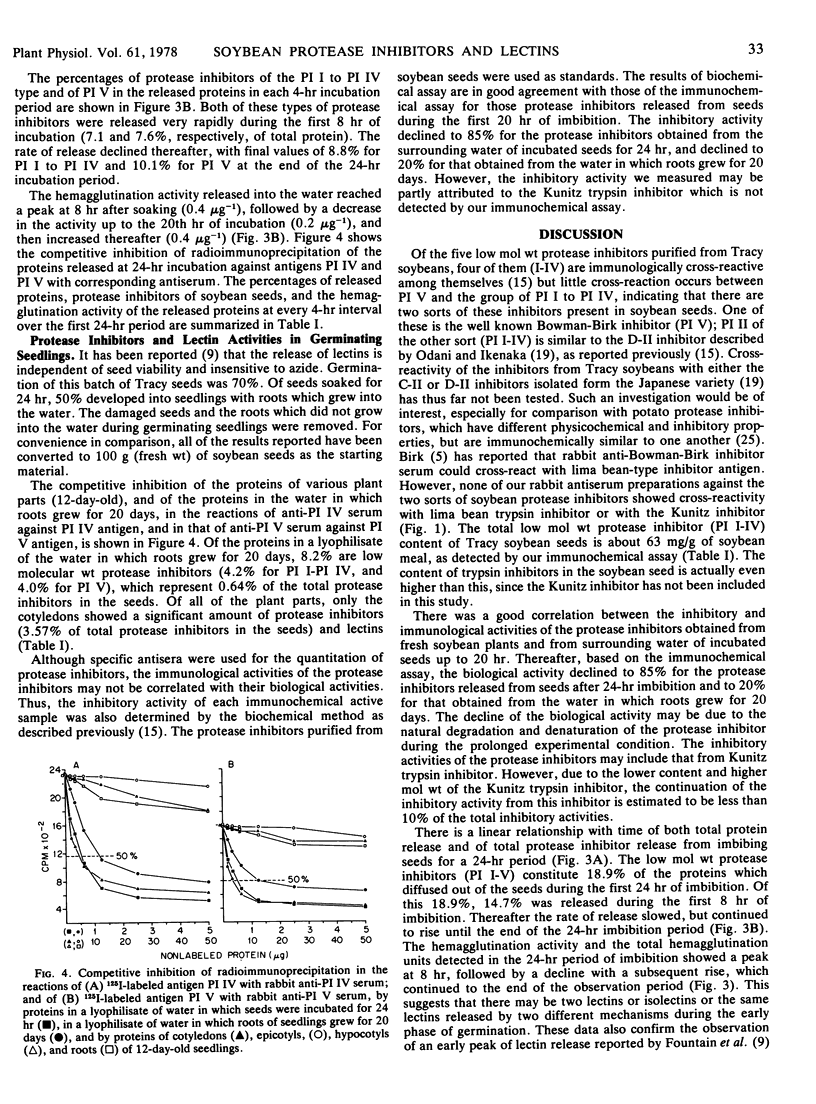
Specific antisera were prepared against the Bowman-Birk trypsin inhibitor and four other trypsin inhibitors of low molecular weight isolated from soybeans (Glycine max L. cv. Tracy). These antisera were used to detect the presence and amount of the inhibitors in: (a) seeds and protein extracts of soybean meal; (b) seedlings; and (c) the water surrounding the seeds and roots of seedlings. Lectin activities in seeds, seedlings, and water were also determined at the same time as the protease inhibitor activities. By competitive inhibition of immunoprecipitation, the combined five low molecular weight protease inhibitors were found to constitute the following percentages of proteins (w/w): 6.3% in defatted soybean meal; 8.1% of the protein extracted from the meal by a buffer of pH 8.6; 8.3, 14.7, 15.2, 16.1, 17.2, and 18.9% of the protein in a lyophilisate of water in which seeds were incubated for 4, 8, 12, 16, 20, and 24 hours, respectively; 8.2% in a lyophilisate of water in which roots of seedlings grew for 20 days; 1.5% in cotyledons; and less than 0.1% in epicotyls, hypocotyls, and roots of 12-day-old seedlings. Hemagglutination activities, expressed as the lowest amount of protein required to give a positive agglutination of 0.2 ml of 2% rabbit red blood cells, were as follows: purified soybean lectin, 0.08 μg; lyophilisate of water in which seeds were incubated for 4, 8, 12, 16, 20, and 24 hours, 10, 2.5, 5, 5, and 2.5 μg, respectively; lyophilisate of water in which roots grew for 20 days, 5 μg; 12-day-old cotyledons, roots, epicotyls, and hypocotyls, 12.5, 100, >1,000, and >500 μg, respectively. The results indicate that a large amount of protease inhibitors as well as lectins are released from seeds during the first 8 hours of imbibition. Neither lima bean trypsin inhibitor (mol wt, 10,000) nor Kunitz soybean trypsin inhibitor (mol wt, 21,500) showed competitive inhibition in tests with antisera against low molecular weight soybean protease inhibitors.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bramhall S., Noack N., Wu M., Loewenberg J. R. A simple colorimetric method for determination of protein. Anal Biochem. 1969 Oct 1;31(1):146–148. doi: 10.1016/0003-2697(69)90251-6. [DOI] [PubMed] [Google Scholar]
- Clarke A. E., Knox R. B., Jermyn M. A. Localization of lectins in legume cotyledons. J Cell Sci. 1975 Oct;19(1):157–167. doi: 10.1242/jcs.19.1.157. [DOI] [PubMed] [Google Scholar]
- Fountain D. W., Foard D. E., Replogle W. D., Yang W. K. Lectin release by soybean seeds. Science. 1977 Sep 16;197(4309):1185–1187. doi: 10.1126/science.197.4309.1185. [DOI] [PubMed] [Google Scholar]
- Frattali V. Soybean inhibitors. 3. Properties of a low molecular weight soybean proteinase inhibitor. J Biol Chem. 1969 Jan 25;244(2):274–280. [PubMed] [Google Scholar]
- Green T. R., Ryan C. A. Wound-Induced Proteinase Inhibitor in Plant Leaves: A Possible Defense Mechanism against Insects. Science. 1972 Feb 18;175(4023):776–777. doi: 10.1126/science.175.4023.776. [DOI] [PubMed] [Google Scholar]
- Howard I. K., Sage H. J., Horton C. B. Studies on the appearance and location of hemagglutinins from a common lentil during the life cycle of the plant. Arch Biochem Biophys. 1972 Mar;149(1):323–326. doi: 10.1016/0003-9861(72)90328-1. [DOI] [PubMed] [Google Scholar]
- Hwang D. L., Foard D. E., Wei C. H. A soybean trypsin inhibitor. Crystallization and x-ray crystallographic study. J Biol Chem. 1977 Feb 10;252(3):1099–1101. [PubMed] [Google Scholar]
- Mirelman D., Galun E., Sharon N., Lotan R. Inhibition of fungal growth by wheat germ agglutinin. Nature. 1975 Jul 31;256(5516):414–416. doi: 10.1038/256414a0. [DOI] [PubMed] [Google Scholar]
- Odani S., Ikenaka T. Studies on soybean trypsin inhibitors. IV. Complete amino acid sequence and the anti-proteinase sites of Bowman-Birk soybean proteinase inhibitor. J Biochem. 1972 May;71(5):839–848. doi: 10.1093/oxfordjournals.jbchem.a129833. [DOI] [PubMed] [Google Scholar]
- Odani S., Ikenaka T. The amino acid sequences of two soybean double headed proteinase inhibitors and evolutionary consideration on the legume proteinase inhibitors. J Biochem. 1976 Sep;80(3):641–643. doi: 10.1093/oxfordjournals.jbchem.a131321. [DOI] [PubMed] [Google Scholar]
- Plant C. G., Jones I. H., Wilson H. J. Setting characteristics of lining and cementing materials. Br Dent J. 1972 Jul 4;133(1):21–24. doi: 10.1038/sj.bdj.4802869. [DOI] [PubMed] [Google Scholar]
- Ryan C. A., Santarius K. Immunological similarities of proteinase inhibitors from potatoes. Plant Physiol. 1976 Nov;58(5):683–685. doi: 10.1104/pp.58.5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]



