Abstract
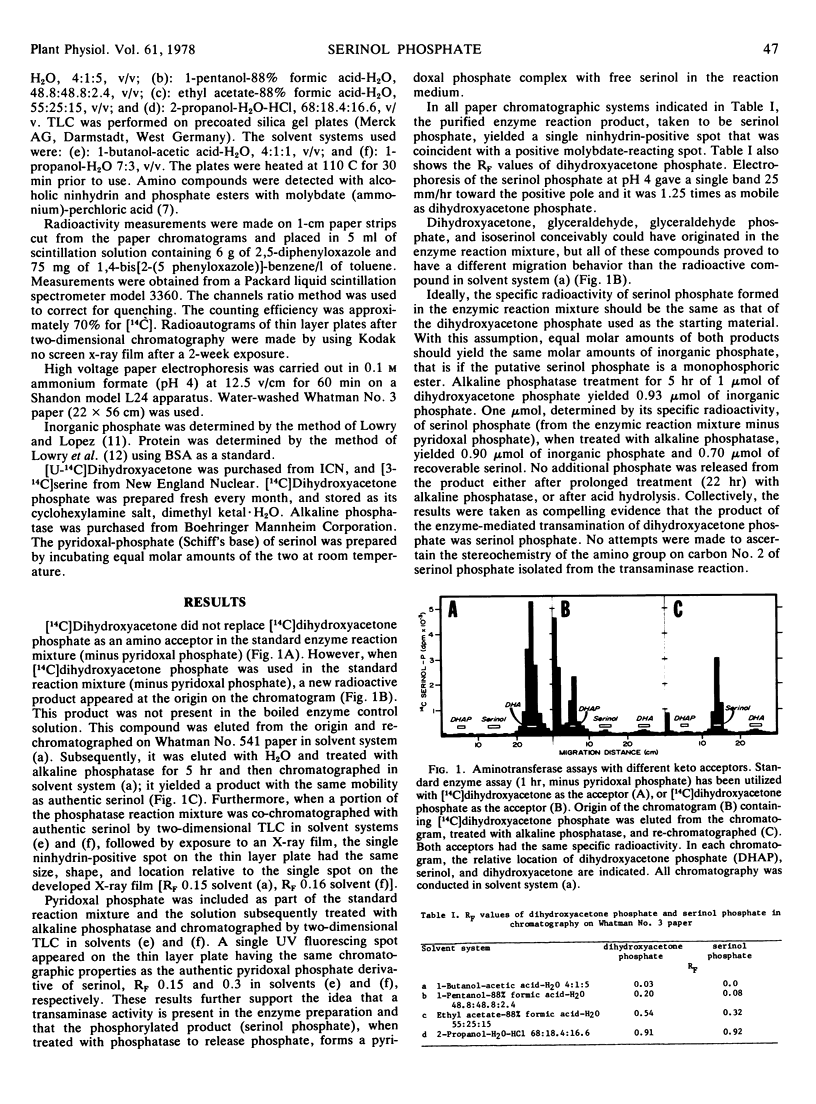
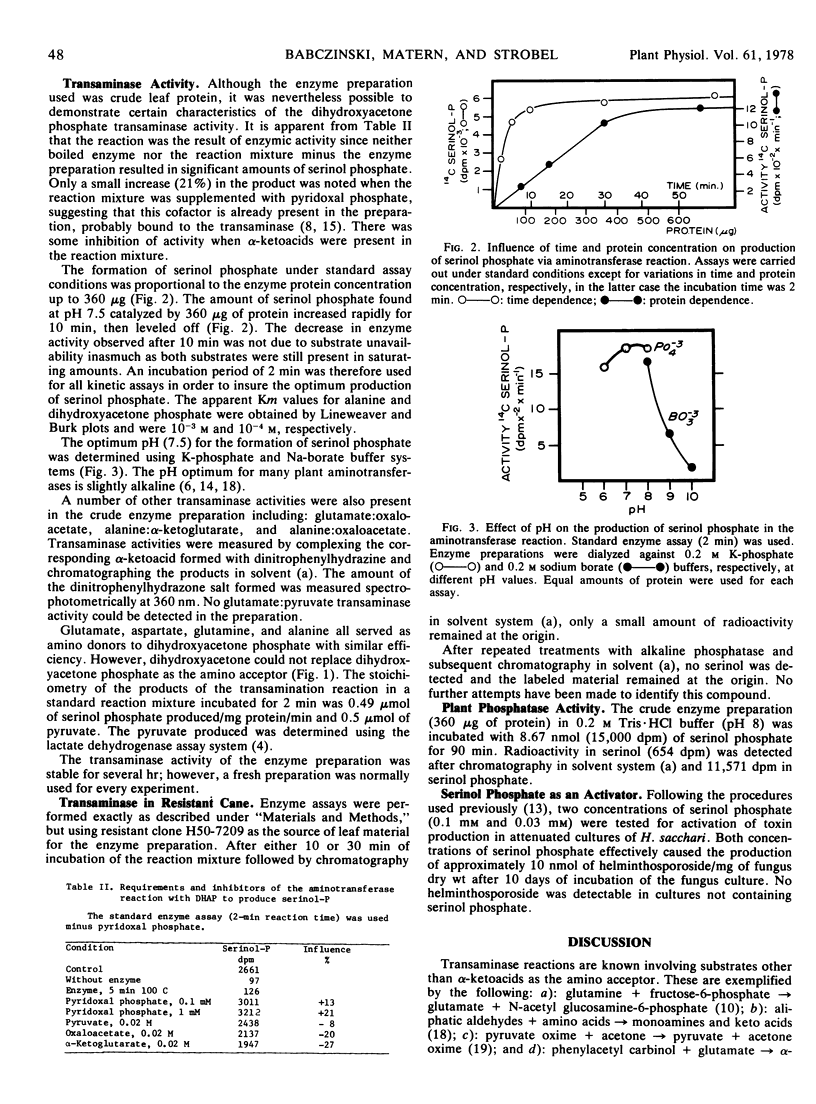
A novel compound, serinol phosphate, was identified in sugarcane (Saccharum officinarum) clone 51NG97. It was produced by an enzyme-mediated transamination of dihydroxyacetone phosphate with either alanine, glutamate, aspartate, or glutamine serving equally well as an amino donor. Some detectable phosphatase activity was present in crude leaf enzyme preparation that hydrolyzed serinol phosphate. A proposal for a pathway of the biosynthesis of serinol in sugarcane was formulated.
Serinol can serve as an “activator” of toxin production in attenuated cultures of the sugarcane pathogen Helminthosporium sacchari and it is present in susceptible clone 51NG97. Resistant clone H50-7209 does not possess serinol and likewise no dihydroxyacetone phosphate transaminase activity could be demonstrated in enzyme preparations of this clone. The concept of toxin activation in attenuated fungus cultures is briefly discussed relative to disease resistance and susceptibility.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., HORECKER B. L. The biosynthesis of histidine: imidazoleacetol phosphate transaminase. J Biol Chem. 1956 May;220(1):113–128. [PubMed] [Google Scholar]
- BUBLITZ C., KENNEDY E. P. Synthesis of phosphatides in isolated mitochondria. III. The enzymatic phosphorylation of glycerol. J Biol Chem. 1954 Dec;211(2):951–961. [PubMed] [Google Scholar]
- Dougall D. K., Fulton M. M. Biosynthesis of Protein Amino Acids in Plant Tissue Culture IV Isotope Competition Experiments using Glucose-U-C and Potential Intermediates. Plant Physiol. 1967 Jul;42(7):941–945. doi: 10.1104/pp.42.7.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANES C. S., ISHERWOOD F. A. Separation of the phosphoric esters on the filter paper chromatogram. Nature. 1949 Dec 31;164(4183):1107-12, illust. doi: 10.1038/1641107a0. [DOI] [PubMed] [Google Scholar]
- HASSE K., SCHMID G. [Synthesis and degradation of biogenic amines by enzymatic transamination]. Biochem Z. 1963;337:69–79. [PubMed] [Google Scholar]
- LELOIR L. F., CARDINI C. E. The biosynthesis of glucosamine. Biochim Biophys Acta. 1953 Sep-Oct;12(1-2):15–22. doi: 10.1016/0006-3002(53)90119-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Pinkerton F., Strobel G. Serinol as an activator of toxin production in attenuated cultures of Helminthosporium sacchari. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4007–4011. doi: 10.1073/pnas.73.11.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehfeld D. W., Tolbert N. E. Aminotransferases in peroxisomes from spinach leaves. J Biol Chem. 1972 Aug 10;247(15):4803–4811. [PubMed] [Google Scholar]
- Strobel G. A. The helminthosporoside-binding protein of sugarcane. Its properties and relationship to susceptibility to the eye spot disease. J Biol Chem. 1973 Feb 25;248(4):1321–1328. [PubMed] [Google Scholar]
- YAMAFUJI K., OMURA H., MIURA M. On the transoximase. Enzymologia. 1953 Jun 15;16(2):75–80. [PubMed] [Google Scholar]



