Abstract
The influence of O2 availability on the rate of protein synthesis, the levels of RNA and of adenylates, and the value of the energy charge in squash (Cucurbita maxima) cotyledons isolated from seeds germinated for 15 or 28 hours at different O2 concentration (3% or 20% O2) has been investigated.
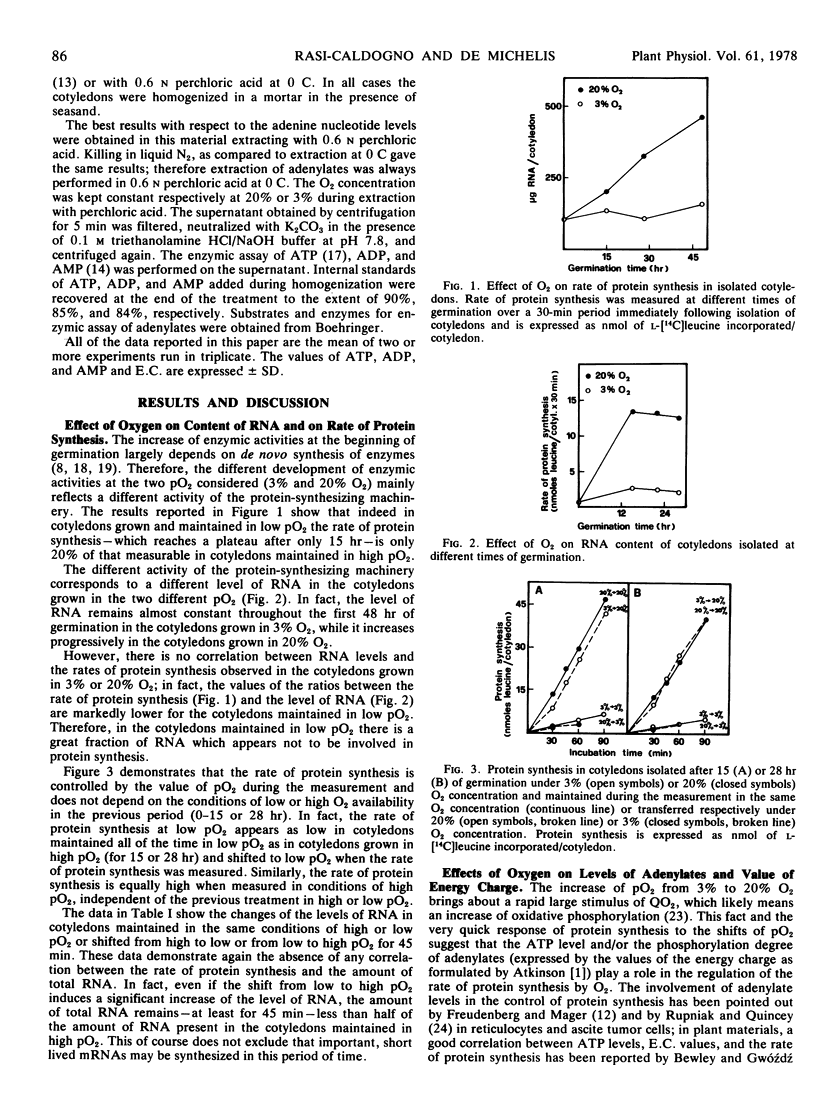
The rate of protein synthesis is five times lower in cotyledons maintained in 3% O2 than in those maintained in 20% O2. Also net RNA synthesis is almost blocked in 3% O2, while in 20% O2 it proceeds almost linearly for 48 hours.
The different RNA contents cannot explain the different rates of protein synthesis.
The results of shift experiments (cotyledons shifted from 20% to 3% O2 or vice versa) show that the rate of protein synthesis is strictly correlated with actual O2 availability and is largely independent of the one in the previous period. O2 controls the development of the adenylate pool and particularly the increase of ATP level. Thus, both the adenylate pool and the values of the energy charge ratio are lower in cotyledons grown in 3% than in 20% O2.
The shifts of O2 availability induce rapid changes of ATP, ADP, and AMP levels and thus of the values of the energy charge, which are about 0.7 at 3% O2 and higher than 0.8 at 20% O2, independent of previous O2 availability.
The rate of protein synthesis appears to be largely independent of the levels of the single nucleotides and better correlated to the energy charge values.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson D. E. Regulation of enzyme function. Annu Rev Microbiol. 1969;23:47–68. doi: 10.1146/annurev.mi.23.100169.000403. [DOI] [PubMed] [Google Scholar]
- Bewley J. D., Gwódź E. A. Plant Desiccation and Protein Synthesis: II. On the Relationship between Endogenous Adenosine Triphosphate Levels and Protein-synthesizing Capacity. Plant Physiol. 1975 Jun;55(6):1110–1114. doi: 10.1104/pp.55.6.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomsel J. L., Pradet A. Study of adenosine 5'-mono-,di- and triphosphates in plant tissues. IV. Regulation of the level of nucleotides, in vivo, by adenylate kinase: theoretical and experimental study. Biochim Biophys Acta. 1968 Aug 20;162(2):230–242. doi: 10.1016/0005-2728(68)90105-9. [DOI] [PubMed] [Google Scholar]
- Chapman A. G., Fall L., Atkinson D. E. Adenylate energy charge in Escherichia coli during growth and starvation. J Bacteriol. 1971 Dec;108(3):1072–1086. doi: 10.1128/jb.108.3.1072-1086.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching T. M., Ching K. K. Content of adenosine phosphates and adenylate energy charge in germinating ponderosa pine seeds. Plant Physiol. 1972 Nov;50(5):536–540. doi: 10.1104/pp.50.5.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenberg H., Mager J. Studies on the mechanism of the inhibition of protein synthesis induced by intracellular ATP depletion. Biochim Biophys Acta. 1971 Mar 25;232(3):537–555. doi: 10.1016/0005-2787(71)90608-3. [DOI] [PubMed] [Google Scholar]
- Hasson-Porath E., Poljakoff-Mayber A. Content of adenosine phosphate compounds in pea roots grown in saline media. Plant Physiol. 1971 Jan;47(1):109–113. doi: 10.1104/pp.47.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A. O., Larkins B. A. Influence of Ionic Strength, pH, and Chelation of Divalent Metals on Isolation of Polyribosomes from Tobacco Leaves. Plant Physiol. 1976 Jan;57(1):5–10. doi: 10.1104/pp.57.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrè E. Ribosome and enzyme changes during maturation and germination of the castor bean seed. Curr Top Dev Biol. 1967;2:75–105. doi: 10.1016/s0070-2153(08)60284-7. [DOI] [PubMed] [Google Scholar]
- Moreland D. E., Hussey G. G., Shriner C. R., Farmer F. S. Adenosine Phosphates in Germinating Radish (Raphanus sativus L.) Seeds. Plant Physiol. 1974 Oct;54(4):560–563. doi: 10.1104/pp.54.4.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obendorf R. L., Marcus A. Rapid Increase in Adenosine 5'-Triphosphate during Early Wheat Embryo Germination. Plant Physiol. 1974 May;53(5):779–781. doi: 10.1104/pp.53.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupniak H. T., Quincey R. V. Small changes in energy charge affect protein synthesis in reticulocyte lysates. FEBS Lett. 1975 Oct 15;58(1):99–101. doi: 10.1016/0014-5793(75)80234-1. [DOI] [PubMed] [Google Scholar]
- Verma D. P., Marcus A. Oxygen availability as a control factor in the density-dependent regulation of protein synthesis in cell culture. J Cell Sci. 1974 Mar;14(2):331–337. doi: 10.1242/jcs.14.2.331. [DOI] [PubMed] [Google Scholar]



