Abstract
Lately, liver diseases were categorized as one of the most prevalent health problems globally as it causes a severe threat to mankind all over the world due to the wide range of occurrence. There are multiple factors causing hepatic disorders, such as alcohol, virus, poisons, adverse effects of drugs, poor diet, inherited conditions and obesity. Liver diseases have various types including alcoholic liver disease, non-alcoholic fatty liver disease, autoimmune hepatitis, liver cancer, hepatocellular carcinoma, liver fibrosis and hepatic inflammation. Therefore, it is imperative to find effective and efficacious agents in managing liver diseases.
Fusarium oxysporum, an endophytic fungus and containing many bioactive compounds, could be served as a forked medication for enormous number and types of maladies. It was characterized by producing biochemical compounds which had rare pharmacological properties as it may be found in a limit number of other medicinal plants. The majority of the past researches related to Fusarium oxysporum recited the fungal negative field either on the pathogenic effects of the fungus on economical crops or on the fungal chemical components to know how to resist it. The present review will highlight on the bright side of Fusarium oxysporum and introduce the functional activities of its chemical compounds for treating its target diseases. The key point of illustrated studies in this article is displaying wide range of detected bioactive compounds isolated from Fusarium oxysporum and in other illustrated studies it was elucidated the therapeutical and pharmacological potency of these biologically active compounds (isolated from medicinal plants sources) against different types of liver diseases including non-alcoholic fatty liver disease, alcoholic liver disease, cirrhosis and others. It was demonstrated that F. oxysporum contains unique types of isoflavones, flavonoids, phenols and another active chemical compounds, and these compounds showed recently a fabulous clinical contribution in the therapy of liver injury diseases, which opens new and unprecedented way for evaluating the maintaining efficacy of Fusarium oxysporum bioactive compounds in dealing with hepatic complications and its remedy impacting on liver diseases and injured hepatocytes through recommending implement a practical study.
Keywords: Fusarium oxysporum, Hepatic disorders, Alcoholic liver disease, Non-alcoholic fatty liver disease, Liver inflammatory disease, Cirrhosis, Oxidative stress, Fungal bioactive compounds, Therapeutic effects, Efficacy
1. Introduction
Over the last century human life style and food habits had drastically changed which led to emergence of various chronic diseases. The beginning of 1950s was the first discover of liver injury diseases and alcoholic drinks were rated as a main reason for hepatic disorders [1] as it was described by World Health Organization (WHO) [2] hepatic diseases was considered in India and England as one of the main 10th causes of death after cancer whereas each one in five Indians susceptible to injury by different types of liver diseases. Chronic alcoholic liver disease as a severe malady responsibly caused more than 200 maladies. The danger of hepatic diseases lies in being a death cause globally exceeding diabetes and road accidents where these types of diseases don't show any obvious symptoms or signs until all hepatocytes damaged totally and at this level of cirrhosis treating become hopeless case as informed by S. Sivakrishnan [3]. Liver injury diseases defined as it is any disease negatively effects on normal hepatocytes functions and liver healthy performance causing dereliction in its biological functions [4]. Authors [5] classified liver diseases to alcoholic liver disease, cirrhosis, autoimmune hepatitis, liver fibrosis, chronic viral infection, non-alcoholic fatty liver disease and hepatic cancer as it was mentioned by Seitz et al. [6] & Mantovani et al. [7]. Liver diseases passed by five stages starting by healthy liver till the last level of injury causing chorionic liver damage, these elucidated stages were: 1. Inflammation (primary stage), 2. fibrosis, 3. cirrhosis, 4. liver failure (end-stage liver disease), and 5. liver cancer. It was also noticed that curative transactions showed stronger effects in firstly stages than that in lastly stages which remedy efficacy become poorer as reported by Zhang et al. [4]. The chronic hepatic diseases symptoms were classified to two types of signs are non-specific and specific symptoms as elucidated by Ref. [8], non-specific signs including weight loss, fatigue and anorexia while the specific signs such as hepatocellular carcinoma, portal hypertension (ascites and esophageal varices) and hepatocellular insufficiency (hepatic encephalopathy and jaundice). On other side [9], authors mentioned that portal hypertension known as one of the severe complications of chronic liver diseases that leading progress of portal vein-systemic collateral circulation which comprises varices of gastric and esophageal and portal hypertensive gastropathy.
After spreading liver injury diseases at different ages and caused a huge number of death cases, discovering a medication became foregone conclusion and all scientific jurisprudence at that time was directed for producing an effective hepatic disorder therapy, as it was indicated that some of those discovered medications investigated a good effectiveness [10] but on other hand it led to another mischievous side effects or caused rapidly regression in liver injury case, which made scientists heading towards natural sources of medications such as medicinal herbals (Green tea, Turmeric, Garlic, Danshen, Ginseng, Ginger and Licorice) [11] and microorganisms (Lactobacillus genus) [12]. Fusarium oxysporum is considered one of the undervalued microorganisms and deserves to be highlighted in a positive way, it has acquired some therapeutic properties from the medicinal host plants and could synthesize a wide variety of phytochemical compounds that were used to conduct important biological functions [13]. The Fusarium oxysporum capacity of yielding various classes of secondary metabolites represented in a wide range of bioactive compounds including terpenoids, jasmonates, xanthones, alkaloids, cyclic depsipeptides, anthranilates, quinones and cyclic peptides that possesses effective actions such as antioxidant, antimicrobial, antiangiogenic, phytotoxicity, insecticidal and cytotoxicity activities [14], as the authors [15] mentioned the fungal ability to produce secondary metabolic compounds abundantly like flavonoids, alkaloids, tannins and terpenoids, in which its quantitively content reached to 148.48, 20.48, 9.39 and 3.18 μg/mL, respectively. Lipid content in Fusarium oxysporm fungus was determined by Matsakas et al. in 2017 [16], who declared that the fungal production capacity could be elevated by changing its medium content while edited synthetic media (contained different concentrations of carbon and nitrogen sources) effected on lipid content to become 42.6% (lipid concentration was 4.4 g/L). Furthermore, the previous practical studies proved that Fusarium oxysporum is not only a wealthy source in lipids but also it contained high concentrations of unsaturated fatty acids and exceeded its saturated fatty acids content twice as displayed [17]. Not just that but also many studies displayed the high production capacity of Fusarium oxysporum to diverse kinds for enzymes that could participate effectively in industrial and biotechnological applications, where the detected enzymes from Fusarium oxysporum are nitric oxide reductase [18], fructosyl amino acid oxidase [19], laccases, lipoxygenase, keratinase, decarboxylases, triosephosphate isomerase, phospholipase B [20], xylanases [21], α-galactopyranosidases [22] and fucosidase [23] and these enzymes possesses biotechnological, industrial, pharmaceutical, nanotechnological and medicinal values such as the applications of cutinases, glycoside hydrolases, nitrilases and also it could be used in synthesizing various types of metal nanoparticles [24] which hold promising effects in the agricultural, pharmaceutical and medicinal industries [25].
Caicedo et al. [26] analyzed the antioxidant capacity of Fusarium oxysporum by DPPH assay and the results revealed that the fungus had miraculous inhibitory activity that reached its scavenging activity of 51.5%. Moreover, the author [27] mentioned that F. oxysporum contained various biologically active compounds and listed 12 distinct chemical compounds. As a result, the fungus became qualified to treat different and numerous complications as it owned many biological activities. F. oxysporum had anti-diabetic, anti-inflammatory, anticancer, antimicrobial and insecticidal effectiveness [28] due to its diverse content from anthranilic acid derivates (dianthramide B, dianthalexin, hydroxyanilide B, hydroxydianthramide S, methoxy dianthramide B etc.) in a previous report [29]. The authors [30] separated podophyllotoxin from Fusarium oxysporum strain (YP9B) isolated from Juniperus recurve and evaluated its content quantitatively by HPLC, LC-MS and LC-MS/MS, this aromatic compound showed antiviral potency against the HSV type-1 virus, antibacterial potency against M. smegmatis, B. cereus, E. faecalis, S. aureus and S. mutans and cytotoxic potency against PC-3, A549 and MCF-7. Moreover, authors [31] isolated a pyran lactone derivative and identified it as chlamydosporol which assessed its antibacterial activity towards MRSA and MDRSA. It possesses other activities as: antifungal [32], antibacterial activity [33], antimicrobial activity [30], antioxidant activity [31], cytotoxicity [34] and insecticidal activity [35], which opens the way for searching outside studied fields to discover more scientific facts about Fusarium oxysporum fungus was not known or not focused on studying it yet.
1.1. Biologically active compounds produced by Fusarium oxysporum
-
1.
Phenols and flavonoids
Authors [36] declared that Fusarium oxysporum is a rich endophytic fungus by phenolic and flavonoid compounds as they were identified and analyzed qualitatively and quantitatively. The phytochemical screening showed that the fungus contained up to 2.87 μg/mg DW (0.29%) of phenols and 2.01 μg/mg DW (0.2%) of flavonoids while the phytochemical analysis was performed by HPLC and detected seven phenolic compounds and four flavonoid compounds. HPLC chromatogram indicated that detected phenols were chlorogenic acid (153.59 μg/g DW), catechin (16.62 μg/g DW), gallic acid 14.94 (μg/g DW), gallate (7.31 μg/g DW), coumaric acid (7.74 μg/g DW), ferulic acid (5 μg/g DW) and vanillin (2.14 μg/g DW) whilst the detected fungal flavonoids were naringenin (33.37 μg/g DW), daidzein (5.38 μg/g DW), kaempferol (3.22 μg/g DW) and rutin (1.31 μg/g DW) (Shalapy N.M. and Kang W.).
-
2.
Isoflavones
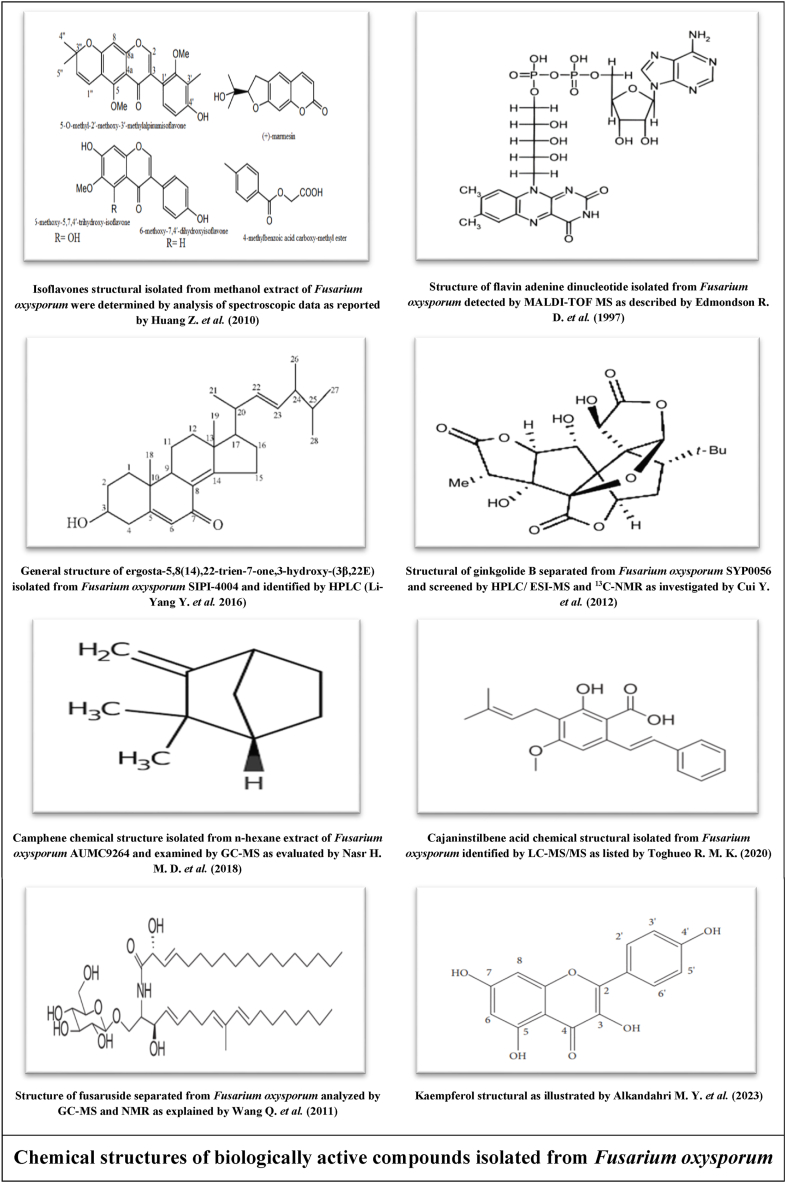
Authors [37] isolated and identified five isoflavones from Fusarium sp. ZZF41 by analysis of spectroscopic data and elucidated their chemical structures as shown in figure below. This fungus isolated from Kandelia candel (mangrove tree) grown in Dong Zai, Hainan, China. The first isoflavone was 5-O-methyl-2′-methoxy-3′-methylalpinumisoflavone as it was a new discovered fungal isoflavone. The second was 6-methoxy-5,7,4′-trihydroxy-isoflavone, and followed by third one 6-methoxy-7,4′-dihydroxyisoflavone, fourth one (+)-marmesin and the fifth 4-methylbenzoic acid carboxy-methyl ester. The quantitative analysis showed its concentrations were 8, 11, 7, 9 and 12 mg, respectively.
-
3.
Anthranilic acid analogues
Authors [29] illustrated the massive content of Fusarium oxysporum fungus to derivatives of anthranilic acid as it was mentioned many names of these compounds, which reached to twenty-one compounds of anthranilic acid analogues including Methoxydianthalexin S, Dianthalexin B, Dianthalexin, Hydroxydianthramide M, Hydroxydianthramide R, Hydroxydianthramide S, Hydroxydianthramide B, Methoxydianthramide A, Methoxydianthramide B, Methoxydianthramide R, Methoxydianthramide S, Methoxydianthramide M, Dianthramide, Dianthramide B, Hydroxyanilide R, Hydroxyanilide B, Hydroxyanilide S, Hydroxydianthalexin S, Hydroxydianthramide S ethyl ester, Methoxydianthramide A methyl ester and Hydroxydianthramide S methyl ester.
-
4.
Alkaloids
Fusarium oxysporum known by its diversified storage to a wide range of alkaloids as it is considered one of its major contents. Authors isolated and identified one of these alkaloids with MALDI-TOF MS technique to identify the isolated active compounds from Fusarium oxysporum [38]. The obtained data elucidated that the primary identification showed a naturally occurring substituted compound which is named as adenine dinucleotide alkaloid in advanced stages of analysis as its chemical structural illustrated in listed figure below and identified as redox-active coenzyme.
-
5.
Naphthoquinone derivatives
It was found that Fusarium oxysporum contained varied types of naphthoquinones analogues, and previous studies gathered on the abundant fungal content to different derivatives of naphaquinone. Authors mentioned that F. oxysporum ITCC 3636 enabled to produce three bioactive compounds classified as naphthoquinones compounds [39], and the detected chemical components from fungus were 3-O-methyl-8-O-methylfusarubin, fusarubin and 9-O-methylfusarubin. On this approach, 8-O-methylsolaniol, 8-O-methyljavanicin, 8-O-methyl-2-hydroxyjavanicin and 9-O-methylanhydrofusarubin are analogues of naphthoquinones identified and isolated from F. oxysporum [40]. Additionally, F. oxysporum had nectriafurone [41], naphthoquinone nectriafurone-8-methy ether [42], and isolated rhodolamprometrin [43].
-
6.
Terpenoids
The fungal storage from terpenoid compounds in Fusarium oxysporm could not be underestimated. Many studies were performed on determining and identifying the fungal content of terpenoids over the last years and from these experiments Li-Yang et al. [44] separated ergosta-5,8 (14),22-trien-7-one,3-hydroxy-(3β,22E) from a Chinese Fungal strain identified as F. oxysporum SIPI-4004 as elucidated in the figure below. Starratt et al. [45] isolated three fungal compounds from F. oxysporum CM 192679 which were Ergosterol peroxide, Ergosterol and Cerevisterol; as Mohamed et al. [46] identified other three different terpenoid compounds isolated from Egyptian strain of F. oxysporum called (22E,24R)-stigmasta-5,7,22-trien-3-β-ol, wortmannin and stigmasta-4,6,8 (14),22-tetraen-3-one. Complementary to above, fusariumin D and fusariumin C were extracted from Fusarium oxysporum ZZP-R1 [47] while other experiment [48] confirmed the existence of F. oxysporum SC0002 to cosmosporaside G, cosmosporaside F and cosmosporaside H. Kim et al. [49] identified isoverrucarol as fungal terpenoid extracted from F. oxysporum CJS-12 strain while (1R,2S,3R)-3-((S)-2-hydroxy-6-methylhept-5-en-2-yl)-1,2-dimethylcyclopentanol was detected from Korean isolate of F. oxysporum identified as LBKURCC41 [50]. The listed figure display structure of ginkgolide B terpenoid obtained from F. oxysporum SYP0056 was isolated from Ginkgo biloba in China [51]. Camphene is one of the monoterpenes as it was shown in the figure below, it is widespread in medicinal plants and some limited species of fungi. It was separated from n-hexane extract of Fusarium oxysporum AUMC9264 (isolated from Lupinustermis L.) and identified the fungal chemical composition using GC-MS equipment. The antimicrobial assay was examined and showed significant positive indicators [52].
-
7.
Unsaturated fatty acids and their analogues
Undoubtedly the unsaturated fatty acids showed a wide range from biological activities as studying its medicinal applications still in progress. Authors [53] isolated Fusarium oxysporum R1 from China and extracted its bioactive compounds using nonpolar solvent then identified its compositions through spectroscopic techniques. The chromatogram detected two unsaturated fatty acids identified as α-linolenic acid and palmitoleic acid. On other hand, Authors [36] separated eight unsaturated fatty acids from chloroform extract of Fusarium oxysporum strain isolated from roots of Egyptian tomato species and identified its chemical composition by liquid chromatograph-mass spectroscopy. Chromatographic data displayed unsaturated fatty acids as: oleic acid, linoleic acid, palmitoleic acid, cis-11-eicosenoate, cis −5,8,11,14,17-eicosapentaenoate, gamma linoleic acid, cis-5,8,11,14-eicosatetraenoate (arachidonate) and nervonate (cis-15-tetracosanoate). The total unsaturated fatty acids represented 70.84% from whole fungal nonpolar extract; as the hexanoic extract of Fusarium oxysporum SS50 isolated from a medicinal plant (Smallanthus sonchifolius) was analyzed spectrophotometrically by GC-MS and twenty-one compounds identified among these active compounds was methyl palmitate [54].
-
8.
Saccharide and its derivatives
A novel glucoside inositol derivative which was isolated from French fungal strain of Fusarium oxysporum L. was identified as α-d-Mannopyranosyl-(1→X)-inositol as announced by Nita-Lazar et al. [55] who reviewed the fungus ability to produce significant quantities of alcoholic sugar [α-d-Mannopyranosyl-(1→X)-inositol] via changing in its medium composition to reinforcement of F. oxysporum to excrete this aforementioned compound by adding wheat straw to fungal media. Furthermore, Authors [56] analyzed and identified the methanolic extract of Fusarium oxysporum using GC-MS as the data sheet elucidated twenty-one secondary metabolic bioactive compounds including two saccharides identified as dihydroxyacetone and DL-arabinose.
-
9.
Biologically active compounds separated from Fusarium oxysporum
Cajaninstilbene acid is sorted as biological active stilbene as it also may be known as CSA or 3-hydroxy-4-prenyl-5-methoxystilbene-2-carboxylic acid. It was separated from Fusarium oxysporum (ERP-10) hosted on pigeon pea leaves and the fungal content for cajaninstilbene was screened quantitatively and qualitatively by liquid chromatography-tandem mass spectrometry (LC-MS/MS). In the listed figure below, it was indicated the structure of cajaninstilbene as the fungal cajaninstilbene antioxidant activity was examined compared to standard antioxidant by DPPH radical-scavenging assay and the resulted data showed remarkable inhibition percentage of fungal cajaninstilbene as same as the standard one and herein Fusarium oxysporum considered as natural source for antioxidant CSA as explained by Zhao et al. [57] and Toghueo et al. [58]. Gas chromatography mass spectrometry mechanism was performed on Fusarium oxysporum methanolic extract for screening its secondary metabolic compounds content depending on molecular weight of the fungal bioactive compounds. GC-MS results identified many secondary metabolites comprising isosorbide dinitrate which belongs to nitrate group and 5- hydroxymethylfurfural (an organic compound) [56]. In another study, Fuasrium oxysporum strain was cultivated on fermented rice media [33] and its content of biological active compounds was analyzed by GC-MS and NMR. Nine compounds were detected and fusaruside was one of them as it is known as kind of sphingolipid and its structure showed in below figure.
Pharmaceutical effects of Fusarium oxysporum bioactive compounds against liver injury diseases.
Many features were characterized for the drugs obtained by endophytes, comprising season/weather independent, reproducible, plentiful, unlimited and fast production (specially through endophytic fermentation process) and easy controlled whereas we can get different produced compounds (drug) by changing in cultivation conditions or hosting endophytes on plants contained the target bioactive compounds as alteration in genetic engineering elevated the microbial capacity as studied by Grover et al. [59] and Strobel et al. [60].
-
1.
Phenols and flavonoids effectiveness
The authors [61] attribute the reason of most medicinal plants’ vital effectiveness that were used from the ancient period of time to its content of phenols and flavonoids as a result to its various therapeutic actions as main secondary metabolic compounds present in plants and microorganisms abundantly, numerous studies emphasis that phenolic and flavonoid compounds possess magical therapeutical potency against diverse human diseases where these bioactive compounds elucidated markedly protective effects towards management and development of cancer, diabetes, neurodegenerative diseases, cardiovascular diseases, osteoporosis, and more due to its unrivaled properties comprising features as antioxidant, antitumor, antidiabetic, antifungal and antibacterial, that qualify the rich sources of phenols and flavonoids to be human alternative drugs against different diseases and disorders.
-
A.
Chlorogenic acid
An experimental study was conducted to evaluate chlorogenic acid effectiveness on non-alcoholic fatty liver disease (NAFLD) induced by high-fat diet (HFD) [62]. Western blot and inflammatory cytokines were the assays to analyze Toll like receptor 4 (TLR4) signaling pathway and gut microbiota content was examined with real-time PCR as it was determined expressions of intestine tight junctional protein. Therapeutic effect of chlorogenic acid was displayed in decreasing serum transaminase, elevating insulin sensitivity, alleviating inflammation and steatosis in liver and reducing blood lipids and FBG, as it was capable to reverse activation of expression of interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) in liver and TLR4 signaling pathway in HFD-induced NAFLD. These results indicated the protective effect of chlorogenic acid against hepatic steatosis and inflammation of HFD mice due to its high anti-inflammatory and antioxidant activities, making it potent remedy for preventing and treating NAFLD.
-
B.
Catechin
Authors [63] focused on the therapeutic effect of catechin on liver disorders. Non-alcoholic fatty liver disease is one of the hepatic diseases that affects negatively on hepatic lipid metabolism and liver functions. Catechins were isolated from green tea and examined its remedy activity practically depending on liver injury markers, lipid and carbohydrates metabolism, clinicopathologic outcomes and inflammatory oxidative stress. The obtained data showed that catechins had a magical efficiency in regulating glucose and lipid metabolism as it was capable of modifying expressions of genes related to lipid synthesis. Moreover, benefitable effects were displayed on pathways of oxidative stress that responsible for activating pro-inflammatory responses causing hepatic damage. The previous findings proved the significant influence of catechin on non-alcoholic fatty liver disease and could be recommended as a potential treatment option for hepatic disorders.
-
C.
Gallic acid
Due to its high antioxidant capacity, gallic acid was considered as one of phenolic compounds able to treat hepatic disorders fluently. A study was investigated for the improvement efficacy of gallic acid on ischemia-reperfusion induced hepatic injury through estimating its effect on histopathological alterations, microRNAs (122 and 34a) and biochemical evaluation of liver function. The anti-liver injury activity of gallic acid was observed clearly for it diminished concentrations of liver enzymes, microRNAs-122 and microRNAs-34a in serum and conserved liver cells changing in ischemia-reperfusion induced liver injury, which demonstrated the positive indicators of gallic acid on liver damage induced by ischemia-reperfusion, suggesting that the administration of gallic acid in advance before hepatic surgeries could prohibit the previous mentioned complications [64].
-
D.
Coumaric acid
A study on estimating the biologically active effectiveness of coumaric acid was implemented by Parvizi et al. [65] who presented the potent beneficial actions of coumaric acid for maintaining liver functions. Rats were inoculated by coumaric acid with dosage 100 mg/kg for seven days in advance before induction with Ischemia-reperfusion induced liver injury of rats to evaluate the protective impact of coumaric acid on damaged hepatocytes. Functional liver tests, gene expression of caspase-3, liver antioxidants levels and staining of hematoxylin and eosin were examined to estimate the hepatoprotective influence of coumaric acid. Results exhibited modifying in liver functional enzymes, antioxidants and liver inflammation concentrations to normal levels. Coumaric acid led to significant reduction in AST and ALT levels while SOD, GSH and catalase were elevated concretely compared to the reduced gene expression of caspase-3 in the Ischemia-reperfusion control group.
-
E.
Ferulic Acid
Authors [66] studied the amelioration impacts of ferulic acid on fibrosed liver and evaluated the hepatic stellate cells activation, transforming growth factor β1 on human liver stellate cell lines inducing hepatic damage in LX-2 cells while CCl4 was used to induce hepatic fibrosis for experimental rats to estimate the therapeutic effect of ferulic acid through in vivo and in vitro experiments. It was determined the localization of Smad4 by fluorescence staining method and the gene expression by RT-qPCR technique while α-smooth muscle actin, p-JNK, p-p38, protein levels of collagen I, p-Smad2 and p-Smad3 parameters were estimated by Western blot process, as ELISA procedure was used to measure hydroxyproline, hexadecenoic acid, ALT and AST values. Acquired data showed obvious perfection in fibronectin, p-Smad2, Smad4, p-Smad3, α-SMA and collagen I levels in LX-2 cells which decreased significantly contrast to transforming growth factor β1control group, biochemical parameters (Hyp, HA, ALT and AST) were reduced as p-Smad2 and p-Smad3 protein levels in liver cells were elevated markedly in administrated group with ferulic acid.
-
F.
Vanillin
Thioacetamide were used to induce hepatic fibrosis for rat model to define the hepatoprotective effectiveness of vanillin in a survey was carried out by Ghanim et al. [67]. Vanillin was given at dosage 100 mg/kg body weight to rats injected with thioacetamide induced liver injury for eight weeks. This experimentation produced remarkable amendment in hepatic function and histology whereas inoculation with vanillin led to emphatic excessing in cyclin D1 expression and hepatic HGF & VEGF values accompanied with marked reduction in TIMP-1 level. These conclusions explained the role of vanillin in regression of stimulating fibrosis and enhancement the recovery of rats’ liver treated with thioacetamide induced liver damage through inhibiting ECM accumulation and improved its degradation.
-
G.
Naringenin
Naringenin was known as strong antioxidant, anticancer, anti-inflammatory and antifibrogenic flavonoid in preventing liver fibrosis [68]. Hepato-therapeutic effects of naringenin due to its capability to inhibit oxidative stress, mutating growth factor (TGF-β) pathway, prohibiting hepatic stellate cells transdifferentiation which led to diminution in collagen synthesis, inhibition toll-like receptor, TGF-β non-canonical pathways and mitogen activated protein kinase (MAPK). Naringenin adjusted the synthesis and oxidation of serum lipids and cholesterol as it regulated lipid metabolism to accomplish hepatic remedy actions against nonalcoholic fatty liver disease, which made naringenin an important candidate curative drug for different liver disorders.
-
H.
Daidzein
Authors [69] evaluated the antisteatotic properties of daidzein supplementation against non-alcoholic fatty liver disease in C57BL/6J mice through adipocyte metabolism and liver gene expression profiles. Administration with daidzein at different dosage (0.1, 0.5, 1 and 2 g/kg bw) for twelve weeks showed salient remedy effectiveness against liver injury diseases involving significant alleviation in hepatic steatosis, liver lipid concentrations and element binding protein of carbohydrate response that led to considering daidzein as drug prohibiting hepatic steatosis and resistance of insulin. Additionally, it was capable of upregulating genes responsible for fatty acid β-oxidation and anti-adipogenesis to inhibit adiposity as it reduced the concentration of steatotic tumor necrosis (ghrelin and alpha) and enhanced levels of adiponectin mRNA and antisteatohepatitic leptin. Preceding findings illustrated that daidzein regulate de novo lipogenesis of liver and insulin signaling (direct way) and variate adipocyte metabolism to control in adipocytokines and adiposity (indirect way) for alleviating the deterioration effects of non-alcoholic fatty liver disease.
-
I.
Kaempferol
A study was conducted to explain the hepatoprotective properties of kaempferol against liver fibrosis as it indicated its molecular mechanism of action in resisting the effects of damaging hepatic cells in different liver diseases cases. Above figure showed structure of kaempferol that had multi-therapeutic actions against various disorders such as diabetes, cancers and cardiovascular complications, otherwise it possesses capability to resist against acute cirrhosis due to its huge antioxidant and anti-inflammatory potent which made it able to combat diverse hepatotoxicity protocols comprising non-alcoholic fatty liver disease (NAFLD), carbon tetrachloride (CCL4), lipopolysaccharide (LPS), acetaminophen (APAP), alcoholic liver disease (ALD) and hepatocellular carcinoma (HCC) induced chronic liver injury. It was discussed about kaempferol anti-hepatic injury activity that it had several therapeutic pathways including diminishing phosphorylation of Smad2 and Smad3, inhibiting TGF-β stimulated HSCs, reduction collagen density in the liver tissue and alleviation α-SMA production as it could inhibit the pathway of TGF-β/Smads and linked with ALK5; thus, this treatise promoting usage of kaempferol in liver disorders cases instead of chemical drugs which could not treat liver complications definitively as it may create adverse side effects [70].
-
J.
Rutin
Authors [71] performed an experiment to evaluate the hepatoprotective actions of rutin on high-cholesterol diet fed male Wistar rats induced hepatotoxicity, it was used 2% of rutin concentration as a drug for hepatotoxic of hypercholesterolemia rats for six consecutive weeks then liver functions and lipid profile parameters were determined in experimental rats’ serum. Results investigated considerable improvement in ALT, AST, TC, TG, LDL, and HDL levels (liver function and lipid profile values) in serum of hypercholesterolemic rats administrated with rutin; likewise, it was a significant minifying in Mothers Against Decapentaplegic Homolog 2, Mothers Against Decapentaplegic Homolog 4, Interleukin-6, caspase-3, mRNA expression of transforming growth factor beta, P53 and Bcl-2-binding component 3 rates, otherwise an observed excessing in interleukin-3 and Cyclin depended kinase inhibitor levels in group of high-cholesterol diet induced hepatotoxicity treated with rutin contrasted to control group of high-cholesterol diet induced hepatotoxicity.
-
2.
Isoflavones effectiveness
Computational approaches were conduct to survey the remedy effects of isoflavones against alcoholic liver disease [72]. Deficiency of aldehyde dehydrogenase enzyme in liver increase susceptibility to injury with liver complexation started by hepatic inflammatory to hepatic fibrosis and cirrhosis, thus aldehyde dehydrogenase was deemed as most vital enzyme controlling in liver diseases infection through producing acetate from acetaldehyde, as many treatises announced the role of inhibition of aldehyde dehydrogenase enzyme in developing cancers in the liver. Sequels to above, analogues of isoflavone have beneficial actions on the progression of alcoholic liver disease and notable enhancement in liver pathophysiology. Similar observations were noticed by Lee et al. [73], who discussed in details about the curative role of isoflavone analogues dietary on liver injury and praised by its essential protective effects against harmful actions of drug induced hepatic disorders. Interestingly, therapeutic strategies of isoflavone derivatives had many pathways as diminishing signaling pathways of inflammatory nuclear factor-κB, binding with signal transducer, farnesoid X activation receptor and transcription factor 3 phosphorylation activator in hepatoprotection DILI, prohibiting cytochromes P450 enzyme expression during metabolism of drug, adjusting equilibrium between cell survival and death, elevating nuclear factor of oxidative stress-responsive erythroid 2-like 2 and modifying specific genes binding with inflammatory responses and control of cellular redox homeostasis.
-
3.
Anthranilic acid analogues effectiveness
Authors [74] discussed in details about the powerful antiviral efficiency of anthranilic acid derivatives versus hepatic virus C and described its biological actions in managing several metabolic disorders. Analogues of anthranilic acid were reported its noticeable inhibition activity for hepatitis C virus NS5B polymerase through targeting the crucial viral proteins for hepatitis C virus (HCV) replication, and controlling in the viral protein NS5B which responsible for viral RNA replication using the viral positive RNA strand. Likewise, favorable interactions of anthranilic compounds were offered with lipophilic pocket located on HCV NS5B polymerase binding site that promoted the antiviral impact of anthranilic derivatives containing this lipophilic pocket against hepatitis virus C. Additionally, many analogues of the anthranilic acid capable of inhibiting Thumb Pocket 2 hepatitis C virus NS5B polymerase for developing their anti-HCV therapeutic efficacy which predicted the promising hepatitis virus C antiviral performance.
-
4.
Alkaloids effectiveness
Flavin adenine dinucleotide is one of well-known Fusarium oxysporum alkaloids as it had a wide range of biological active effects, a mixture of flavin adenine dinucleotide and liver extract were prepared to examine its antiviral effect as therapeutic paradigm on chronic hepatitis C patients receiving interferon (IFN). This mixture was widely used by Japanese on chronic liver disorders patients as it contained 15 μL of liver nucleic acid phenol-soluble phase fraction added to 10 mg flavin adenine dinucleotide, which was injected intravenously or intramuscularly once per day for five consecutive days as it was determined hepatitis C virus (HCV) RNA levels, ALT concentration and 2′5′-oligoadenylate synthetase activity in serum regularly to evaluate the effect of the mixture on hepatitis C patients. The biochemical analysis of patients' serum showed significant elevation in 2′5′-oligoadenylate synthetase activity while that patients receiving interferon alone did not change their serum enzyme activity. Intravenous injection by the previous prepared mixture enhanced the production of 2′5′-oligoadenylate synthetase due to excessing mitochondrial adenosine triphosphate production that may lead to the antiviral potency of the prepared mixture on chronic hepatitis C patients [75].
-
5.
Naphthoquinone derivatives effectiveness
A survey was performed by Wang et al. [76] to study hepatic anticancer activity of derivatives of naphthoquinone in liver cancer cells as a result to its remarkable effectiveness as antioxidant and anti-inflammatory factor. MTT cell viability and flow cytometry assays were used to determine the effect of naphthoquinone analogous on accumulation and apoptosis of reactive oxygen species (ROS) in liver cancer cells; otherwise, Western blot analysis was used to evaluate the expression levels of activated protein kinase of mitochondrial and mitogen as it also used to analyze signaling pathway-associated proteins of signal transducer and activator of transcription 3 in Hep3B liver cancer cells. Acquired data demonstrated tangible inhibition in proliferation of liver cancer Huh7, Hep3B and HepG2 cell lines as it was noticed normal results in stomach GES-1 and lung IMR-90 cell lines due to naphthoquinone analogues prodigious actions, notable increase in ROS levels and number of apoptotic cells after processing with naphthoquinone derivatives. Moreover, downregulating extracellular signal-regulated kinase and STAT3 while upregulating c-Jun N-terminal kinase and protein expression of p38 MAPK due to naphthoquinone analogues induced apoptosis through signaling pathways of STAT3 and ROS-modulated MAPK in Hep3B cells, thus proved antitumor effect of naphthoquinone derivatives on treating liver cancer.
-
6.
Terpenoids effectiveness
According to Yang et al. [77] who defined terpenoids as the most important secondary metabolic compounds that play important roles in diverse biological and physiological processes related to plant and human, as they discussed the vast ambit of pharmacological and therapeutical effectiveness of terpenoids against various disorders. Terpenoids possesses numerous of bio-vital applications comprising neuroprotection, insect resistance, antiaging, immunoregulation, hypoglycemic effect, promote transdermal absorption, treating and preventing cardiovascular diseases due to its complex structure which causing different effects with diverse mechanisms of action. Complementary, this bioactive group consists of a large number of chemical compounds had promising activities including antiviral, antitumor, antioxidant, anti-inflammatory, antidiabetic, antibacterial, antimalarial and anti-insect influences.
-
A.
Ergosta-5,8 (14),22-trien-7-one,3-hydroxy-(3β,22E)
Ergosta-5,8 (14),22-trien-7-one,3-hydroxy-(3β,22E) is a terpenoid isolated from Chinese strain of Fusarium oxysporum grown on fermented media and estimated its biotherapeutic efficacy on hepatitis C virus. Bioactive compounds of fungal extract were separated by several methods including silica-gel column chromatography, reverse-phase silica-gel column chromatography, high performance liquid chromatography (semi-preparative reverse-phase) and spectroscopic analysis to elucidate molecular structure of fungal bioactive compounds. Ergosta-5,8 (14),22-trien-7-one,3-hydroxy-(3β,22E) is one of isolated fungal bioactive compounds as it was examined its antiviral activity against hepatitis C virus (HCV) and resulting elucidated prodigious action of mentioned terpenoid in inhibiting hepatitis C virus NS3 protease inhibitory activity. Therefore, the isolated (H1-A, 1) ergosta-5, 8 (14),22-trien-7-one, 3-hydroxy-(3β, 22E) from Fusarium oxysporum could act as hepatoprotective agent against hepatitis C virus as demonstrated by Ref. [78].
-
B.
Ergosterol peroxide
Another survey [79] illustrated the effectiveness of ergosterol peroxide as antiviral paradigm against hepatitis B virus (HBV) as its latest stages of injury caused cirrhosis and hepatocellular carcinoma as it was recommended usage bioactive natural compounds instead of chemical drugs (nucleotide, nucleoside analogues and interferons) because it may cause squeaky side effects in patients. The antiviral activity of ergosterol peroxide could be summarized in its inhibition ability to hepatitis virus C entrying into primary human hepatocytes cells of DMSO-differentiated immortalization, which plays important roles in overexpressing sodium taurocholate cotransporting polypeptide (NTCP), as it could interfere with interaction of NTCP– B surface protein (LHBsAg) directly. The therapeutic potential of ergosterol peroxide against infection with hepatitis B virus genotypes A–D were highlighted to its fighting activity.
-
C.
Ginkgolide B
Considered as one of the important terpenoids, ginkgolide B had a wide remedy usage as a result to its elegant inhibition activity on oxidative stress and lipid peroxidation which known as two main reasons responsible for chronic hepatic complications as confirmed by Yang et al. [80] who examined ginkgolide B efficacy in recovering nonalcoholic fatty liver disease in obese mice to normal case. At the dosage 20 and 30 mg/kg bw of ginkgolide B, the expression levels of ferroptosis-related proteins in vivo and in vitro by western blotting assay as the impacts of ginkgolide B on Nrf2 pathway were analyzed. Experimental findings indicated presumed increasing in Nrf2 expression and Nrf2 pathway activation leading to exerting anti-ferroptosis effects, hence ginkgolide B had specific influence on oxidative stress and lipid accumulation induced ferroptosis in nonalcoholic fatty liver disease through pathway of Nrf2 signaling. Similar conclusions were obtained by Wang et al., [81] related to ginkgolide B magical efficacy in ameliorating nonalcoholic fatty liver disease for high fat diet induced mice obesity by its prodigious capability in reducing hepatic oxidative stress and lipid accumulation in liver.
-
D.
Camphene
Study on male C57BL/6 N mice fed with high fat diet (fat and cholesterol) induced hepatic steatosis and insulin resistance [82] to investigate the camphene hepatoprotective effects and to expounded the mechanism of action of camphene, the protective effectiveness of camphene was elucidated in alleviating in hepatic lipid levels and in liver weight of mice while circulating adiponectin levels were elevated. In vivo data showed significant increase in 3T3-L1 adipocytes secretion and adiponectin expression as it caused a predicted augmentation in AMP-activated protein kinase (AMPK) activation and hepatic adiponectin receptor expression. Concordant results showed excessing in fatty acid oxidation-related genes hepatic expression and notable reduction in lipogenesis-related genes in hepatic steatosis mice, as the curative potency of camphene was kept on accretion of stimulated glucose transporter-2 translocation and activation of insulin-signaling molecules to the plasma membrane in liver Therefore, camphene had a potential hepatoprotective influences against hepatic steatosis and insulin resistance in mice fed with high fat diet, which may be mediated via the adiponectin-AMPK signaling activation.
-
7.
Unsaturated fatty acids and its analogues effectiveness
The illustrated survey performed by Selvaraj [83] confirmed the anticancer activity of unsaturated fatty acids and showed its capability in inhibiting tumor development and modulating the tumor cell response to chemotherapeutic drugs, where many practical studies proved that up-taking unsaturated fatty acids leaded to a great enhancement in cancered cells exceed the expected results. Treating with omega‐3 fatty acids and omega‐6 polyunsaturated fatty acids like linoleic acid displayed a magical potency in the cancer treatment and showed a promising influence a in cancer prevention. Chemical drugs up-taking generally causing mischievous side effects while unsaturated fatty acids nutrition deemed as anticancer factors preferentially damage tumor cells without causing adverse impacts on normal cells. As a result, unsaturated fatty acids categorized under antitumor nutraceuticals (functional foods) that were explored lately for treating cancer without harmful effects and become a secured chemotherapeutic drug. In other study, unsaturated fatty acids achieved another therapeutic aim as antibacterial agent and that qualify unsaturated fatty acids to participate strongly in improving the bactericidal properties through traditional and non-traditional mechanisms of unsaturated fatty acids as explained by Casillas-Vargas et al. [84]. Therefore, unsaturated fatty acids could act as defense system against enormous number of pathogens due to its various mechanisms of action.
-
A.
Oleic acid
Regularization role of dietary oleic acid on hepatic lipogenesis as a therapeutic unsaturated fatty acid through signaling of liver X receptor-dependent were studied by Ducheix et al. [85] to define its enhancement ability on hepatic gene expression of wild-type mice. Study observations demonstrated that lipogenic genes expression in liver and hepatic-lipid accumulation were enhanced significantly of mice fed with oleic acid enriched diet. Furthermore, the curative impacts of oleic acid were not limited to that but also it alleviated cholesterolemia strikingly in mice that had inflamed liver and damaged LXR signs. Hence, it was announced the hypocholesterolemic effect of oleic dietary on deficient mice for both α and β Liver X Receptor isoforms without prejudice influence for liver cells.
-
B.
Linoleic acid
It was reported the hepatoprotective activity of conjugated linolenic acid supplementation on liver damage due to hepatic steatosis or steatohepatitis and explain its ameliorated effect on Zucker rats injured by non-alcoholic liver disease. The beneficial biological activity of linolenic acid was observed after eight weeks of administration on hepatic steatosis rats as leakage in hepatic triglyceride, alleviated markers of hepatic injury in plasma and enhancement in hepatomegaly, as the plasma adiponectin were raised up in group of linolenic acid fed rat, which improved insulin sensitivity. Markedly results were expressed the hepato-therapeutic activity of linolenic acid as suppressing inflammatory cytokine and the mRNA expression of tumor necrosis factor-α, as well it was noticed a significant elevation in plasmatic adiponectin concentration which responsible for impeding progression and development of non-alcoholic liver disease [86].
-
C.
Palmitoleic acid
A practical survey was carried out on the potential nutraceutical usage of palmitoleic acid versus non-alcoholic fatty liver disease by Souza et al. [87] who explained the improvement actions of hepatic steatosis mice administrated with palmitoleic through in vivo an in vitro study. Dietary unsaturated fatty acid at dosage 300 mg/kg orally for fifteen days to high fat diet fed mice showed a fabulous influence including hereinafter: reducing serum insulin and improving insulin tolerance, exhibiting less steatosis and inflammation, diminishing in liver macrophages (F4/80+; CD11c+; CD86+; LM) as anti-inflammatory action and upregulating lipogenic transcription factor in liver. In contrast, inoculation with palmitoleic caused dwindling in fatty acid binding scavenger receptor expression and significant augmentation in activity of hepatic AMPK which enhance nomination this unsaturated fatty acid in treating non-alcoholic liver diseases due to controlling liver inflammation edgy by fat accumulation via induction of M2a macrophages as the anti-inflammatory impacts of palmitoleic are rely on macrophages PPAR-γ.
-
D.
Methyl palmitate
Authors [88] described the defended role of supplementing methyl palmitate on non-alcoholic steatohepatitis, as liver function parameters, hepatic antioxidant enzymes and lipid profile values were determined to evaluate the enhancement effect of methyl palmitate in treating non-alcoholic fatty liver induction of PPAR-α. Biochemical analysis including ALT and AST levels were determined in mice serum while TC, TG, SOD, MDA and GSH parameters were estimated in liver tissue, conversely in vivo test comprising gene expression levels and liver protein were evaluated by qPCR and Western blot assays, respectively. Generally, results of biochemical tests showed a remarkable improvement in serum levels of ALT and AST and hepatic content TC, TG, MDA, GSH and SOD; on other hand, administrating methyl palmitate to hepatic steatosis mice promoted gene expressions and β-oxidation protein, activated PPARα expression, participating in prohibition of non-alcoholic steatohepatitis and suppressed levels of gene expression, Colla 1 protein, TNFα, TGFβ1 and MCP1; thence, methyl palmitate plays a successful role in preventing non-alcoholic steatohepatitis via induction of PPAR-α pathway as it was elucidated its pivotal therapeutic influences in overcoming etiology of malady.
-
8.
Saccharide and its derivatives effectiveness
It was reported that sugars and its derivatives possess numerous biological actions, as it was promoted the influence of pharmacological activities of many therapeutically important compounds as a result to its content to saccharides moieties and its analogues and that interprets the reason of participating sugar moieties in the structure of many clinically used drugs. Saccharides and its modified or synthetic derivatives investigated markedly therapeutic activities against different pathogens such as anti-diabetic, anti-cancer, analgesic, antiviral, antimicrobial and anti-inflammatory effects which indicates the large impact of sugar moieties therapeutic activity in facing various diseases [89].
-
A.
Inositols
Liver lipid accumulation showed unorthodox improvement after supplementation of inositols, which led to its wide usage in treatment of non-alcoholic fatty liver disease as reported [90]. Administrating animal models complaining accumulation of hepatic lipid by inositols diminished hepatic cholesterol accumulation and triglycerides in liver as it modulated histopathology of normal ultrastructural liver. Additionally, most affected parameters by hepatic lipogenesis were maintained due to therapeutic actions of inositols dietary comprising AST values, triglycerides levels, lipid peroxidation excessing activity of glutathione peroxidase which give positive indicators for inositols administration for therapy liver complications related to lipid accumulation.
-
B.
Dihydroxyacetone
A new promising agent was discovered lately to examine hepatic metabolisms. This compound was identified by hyperpolarized dihydroxyacetone as explained by Kirpich et al. [91] who elucidated definitely its magical effectiveness in expounding different liver metabolic fats paths including oxidation in the tricarboxylic acid cycle, glucose production and glycerol production and inclusion into triglycerides. Potentially, these pathways were regulated by multiple enzymes. Dihydroxyacetone effectively identifies alterations hepatic metabolism between glycogenolytic and gluconeogenic models of liver function.
-
C.
DL-Arabinose
Hypercholesterolemia related to enterohepatic circulation was enhanced through regulating metabolism of bile acid by the action of arabinose in mice fed on high-fat-high-sucrose diet [92]. Practical survey was operated to study the hypolipidemic impacts of arabinose on hepatic lipid accumulation. Supplementation of arabinose to mice suffering from hepatic hypercholesterolemia at dosage 400 mg/kg daily for twelve weeks showed a positive signals as minifying body weight gain, elevating circulation of high-density lipoprotein cholesterol and reducing low-density lipoprotein cholesterol levels that accordingly alleviated lipid accumulations in liver and hepatic inflammation in group of high-fat-high-sucrose diet mice. Sequels to above, arabinose caused downregulation of 3-hydroxy-3-methylglutaryl-CoA reductase and that leads to efficiently inhibition of cholesterol synthesis, as it facilitates reversing cholesterol transport due to scavenging receptor class B type 1 and increasing mRNA expressions of low-density lipoprotein receptor, it also downregulating ileal bile acid-binding protein and transporter of apical sodium-dependent bile acid. These findings confirmed synthesizing of bile acids in liver through cholesterol-7α-hydroxylase upregulations and this regulation to related pathways of bile acids metabolism exhibited hepatoprotective efficacy of arabinose on hepatic lipid accumulation and inflammation.
-
9.
Biologically active compounds separated from Fusarium oxysporum effectiveness
Several studies confirmed to containing Fusarium oxysporum to various bioactive compounds had wide range of pharmacological and therapeutical effectiveness, Xu et al. [93] in 2023 listed the separated biochemical compounds from Fusarium spp. by different extraction methods then identify them by chromatographic assays, as most of these compounds were classified as secondary metabolic compounds had different chemical structures and a vast ambit of biological properties. The isolated secondary metabolites were analyzed by spectroscopic analytics in order to identify them depending on its molecular structures, the obtained data demonstrated the effective efficient of Fusarium strains metabolites which possesses significant activities as antibacterial, antimicrobial, antifungal, antiparasitic and antiviral factors and that prove the effectiveness broad spectrum of biochemical compounds separated from Fusarium.
-
A.
Cajaninstilbene acid
Evaluation experiment for cajaninstilbene acid therapeutic effectiveness on hepatocytes of mice administrated with acetaminophen induced liver injury in vivo and in vitro was carried out by Yan et al. [94] who administrated dosages of 50 and 75 mg/kg of cajaninstilbene acid to male C57BL/6 N mice infected with chronic liver injury. Hepatic damage and cell death were estimated via several assays comprising hematoxylin and eosin staining, propidium iodide staining, serum transaminases and TUNEL analysis, as the primary mouse hepatocytes used to test cajaninstilbene acid direct effect on hepatocytes. Aforementioned compound reduced serum aspartate aminotransferase and alanine aminotransferase levels significantly, which ameliorated the prejudicing influences of hepatic injury, as it decreased oxidative stress and interrupted the activation loop of sustained JNK-Sab-ROS. In addition, overdose of acetaminophen induction of chronic liver inflammation was maintained by cajaninstilbene acid as it reduced p62 expression and increased mitochondrial Parkin expression, PINK1, PGC-1α, LC3-Ⅱ, TFAM; mitochondrial quality control (mitophagy and mitochondrial biogenesis) was promoted by cajaninstilbene acid. Herein, this survey proved the remedy activity in alleviating oxidative stress and enhancing mitochondrial quality control and that admitted by considering cajaninstilbene acid as protecting therapy to liver injury induction by acetaminophen. Cajaninstilbene acid and its derivatives are bioactive compounds separated from extract of pigeon pea leaves by Ref. [95] who studied its therapeutical effects to evaluate its potency as anti-inflammatory agent. In vitro studies were carried out to estimate anti-inflammatory effectiveness of cajaninstilbene acid and its derivatives through determining the production of interleukin-6, nitric oxide and factor-alpha of pro-inflammatory cytokines tumor necrosis while reverse transcription-polymerase chain reaction and Western blot assays were performed to elucidate anti-inflammatory mechanism of these biochemical compounds. A remarkable inhibition was noticed in releasing nitric oxide, pro-inflammatory cytokines tumor necrosis factor-alpha and interleukin-6 in lipopolysaccharides due to treatment of cajaninstilbene acid and its derivatives; as well, the phosphorylation of proteins involved in mitogen-activated protein kinase and nuclear factor kappa B signal pathways were inhibited notably which indicate the promising anti-inflammatory efficacy of cajaninstilbene acid and its derivatives that qualify it to be a potential medication for hepatic inflammation.
-
B.
Isosorbide dinitrate
Hemodynamic actions of isosorbide dinitrate on seventeen chronic patients with cirrhosis and portal hypertension were operated to define its therapeutic activity at dosage 5 mg administrated sublingually on cirrhotic patients (hepatic and renal). Observed improvement was investigated after 5 min of administration of isosorbide dinitrate showed decreasing in right atrial mean pressure, pulmonary arterial wedge pressure, cardiac index and the mean arterial pressure while it caused augmenting in heart rate. The hepatic potency was appeared in reduction of hepatic venous pressure gradient as the renal blood flow reduced; these results indicated the therapeutic efficacy of isosorbide dinitrate on patients with liver failure which decreased portal hypertension in cirrhosis patients [96]. An experimental study was performed on ten patients with liver cirrhosis and esophageal varices were administrated isosorbide dinitrate orally four times per day for fourteen days to estimate the slow-release effect of isosorbide dinitrate in blood. At the end of therapy period, it was noticed significant increase in portal blood flow rate reached to 54.3 % and blood flow velocity elevated to 35.4% also it was observed a remark dilation in the portal vein reached to 11.9 %; moreover, the average wedged hepatic vein pressure was reduced significantly to 60.5 %. Isosorbide dinitrate treatment caused a prodigious influence in ameliorating portohepatic gradient to be 9.5 mmHg which was exceed the normal range by four times before drug treating. Thereby, isosorbide dinitrate considered as a valuable drug investigated great therapeutical impacts for hepatic cirrhosis patients suffering from esophageal varices as reported by Ref. [97].
-
C.
5- Hydroxymethylfurfural
In vitro study was designed to evaluate the hepatoprotective efficacy of 5-hydroxymethylfurfural on L02 hepatocytes of human inducted by d-galactosamine and tumor necrosis factor-α, as its mechanism of action was explored by Jiang et al. [98]. The viability of L02 cells injured by d-galactosamine and tumor necrosis factor-α was excessed significantly while morphological and flow cytometric analyses showed notable regression of apoptotic cell death in injured-administrated group with 5-hydroxymethylfurfural, as it caused remarkable improvement in the activation of protein kinase RNA-like ER kinase, expression of CHOP and ATF4 proteins, eukaryotic initiation factor 2 alpha phosphorylation and concentration of intracellular Ca2+ in the treated group which was analyzed using Western blot and immunofluorescence assays. Balancing the Bcl-2 family members expression by 5-hydroxymethylfurfural led to marked anti-apoptotic activity which suppressed phospho-eIF2α, CHOP, ATF4 and phospho-PERK expression due to PERK knockdown. Conclusions demonstrated a pivotal role for 5-hydroxymethylfurfural against ER stress-induced apoptosis that enhanced impact of PERK knockdown which protected L02 cells against the galactosamine and tumor necrosis factor-α via adjusting PERK-eIF2α signaling pathway. Authors [99] separated 5-hydroxymethylfurfural (5-HMF) from the methanolic extract of Schisandra chinensis fruits and estimated it qualitatively and quantitively by high performance liquid chromatography to evaluate its hepato-therapeutic effects and to assess its ameliorative potency on alcoholic liver oxidative injury mice. In experimental examination, 5-HMF was inoculated for seven days at doses of 7.5, 15, and 30 mg/kg and then oxidative stress levels, inflammation levels, liver functions and total lipids parameters were determined in serum and liver tissue to show the influence of 5-HMF on biochemical markers of cirrhosis mice compared to positive and negative control groups. The obtained results illustrated a markedly regression in ALT, AST, TC, TG, LDL levels in serum and MDA concentration in hepatocytes while the values of CAT, GSH-Px and SOD in liver tissue were elevated significantly in treated mice group with 5-hydroxymethylfurfural in comparison with non-treated alcoholic mice group, as it showed a markedly control in TNF-α and IL-1β levels in hepatic cells. These findings and the histopathological results confirming undoubtedly the hepatoprotective and hepato-therapeutical effectiveness of 5-hydroxymethylfurfural on alcohol-induced liver oxidative injury.
-
D.
Fusaruside
The rebuilding capability to the balance of STAT1 and STAT3 signaling by fusaruside in T-cell-mediated fulminant hepatitis of mice inducted by concanavalin A was operated by Wu et al. [100] to evaluate the therapeutic actions of fusaruside as useful approach for curing immune hepatitis complications which usually associated with dysregulation of signal transducer and activator of transcription. Fusaruide was separated from Fusarium sp. IFB-121 and its biological remedy effectiveness against immune hepatitis disorders was illustrated through ameliorating concanavalin A (induced hepatitis in mice), regulating the equilibrium of Th1/Th2/Th17/Treg cytokines and conserving hepatocyte from apoptosis, inhibiting the activation of liver NKT cells and CD4 (+) T cells and improving histopathological parameters. In vitro results displayed marked inhibition for T-cell proliferation in fusaruside administrated group, as it elucidated up-regulating in Bcl-X(L) expression and STAT3 activation in hepatocytes while T-bet expression and STAT1 activation were down-regulated in liver CD4 (+) T cells in injured-treated hepatocytes, hence, it was promoted usage of fusaruside as potential effective medication for treating T-cell-mediated human hepatic injury. In agreement with above, the authors [101] defined fusaruside as an unsaturated immunosuppressive fungal sphingolipid which had valuable properties and biologically important functions. Fusaruside possesses magical therapeutic potency as anti-hepatic injury factor but its pharmacological usage is minimalistic due to its relative scarcity. In this paper, fusaruside isolated from Fusarium graminearum and it was performed a lot of chemical and genetic experiments on the fungus and the fungal media to produce the fusaruside compound in abundant quantities due to its medical potentials for curing liver injury and colitis as reported in this study.
List of biologically active compounds isolated from Fusarium oxysporum and its hepatic protective and therapeutic activities
| Serial no. | Compound name | Molcular formula | Chemical class | Compound ref. | Activity | Activity ref. |
|---|---|---|---|---|---|---|
| 1 | Chlorogenic acid | C16H18O9 | Phenols | [36] | Treatment of non-alcoholic fatty liver disease | [62] |
| 2 | Catechin | C15H14O6 | Phenols | [36] | Treatment of non-alcoholic fatty liver disease | [63] |
| 3 | Gallic acid | C7H6O5 | Phenols | [36] | Anti-liver injury activity | [64] |
| 4 | Coumaric acid | C9H8O | Phenols | [36] | Modulating liver functions | [65] |
| 5 | Ferulic acid | C10H10O4 | Phenols | [36] | Treatment of hepatic fibrosis | [66] |
| 6 | Vanillin | C8H8O3 | Phenols | [36] | Inhibiting fibrosis stimulating | [67] |
| 7 | Naringenin | C15H12O5 | Flavonoids | [36] | Hepato-therapeutic activity | [68] |
| 8 | Daidzein | C15H10O4 | Flavonoids | [36] | Antisteatotic properties against non-alcoholic fatty liver disease | [69] |
| 9 | Kaempferol | C15H10O6 | Flavonoids | [36] | Hepatoprotective activity | [70] |
| 10 | Rutin | C27H30O16 | Flavonoids | [36] | Hepato-therapeutic actions against hepatotoxicity | [71] |
| 11 | 5-O-methyl-2′-methoxy-3′-methylalpinumisoflavone/6-methoxy-5,7,4′-trihydroxy-isoflavone/6-methoxy-7,4′-dihydroxyisoflavone/(+)-marmesin/4-methylbenzoic acid carboxy-methyl ester | C23H24O7 C16H13O5 C16H12O C14H14O4 C10H12O2 |
Isoflavones | [37] | Treatment of alcoholic liver disease | [72,73] |
| 12 | Methoxydianthalexin S/Dianthalexin B/Dianthalexin/Hydroxydianthramide M, …etc. | C15H11NO4 C14H9NO2 C14H9NO3 C16H15NO6 |
Anthranilic acid analogues | [29] | Antiviral activity against hepatic virus C | [74] |
| 13 | Flavin adenine dinucleotide | C27H33P2N9O15 | Alkaloids | [38] | Antiviral activity against virus C | [75] |
| 14 | 3-O-methyl-8-O-methylfusarubin/fusarubin/9-O-methylfusarubin | C17H19O8 C15H14O7 C16H16O7 |
Naphthoquinone derivatives | [39] | Anti-hepatic cancer activity | [76] |
| 15 | Ergosta-5,8 (14),22-trien-7-one,3-hydroxy-(3β,22E) | C28H44O | Terpenoids | [44] | Inhibition activity of hepatitis C virus | [78] |
| 16 | Ergosterol peroxide | C28H44O3 | Terpenoids | [45] | Antiviral activity against hepatic virus B | [79] |
| 17 | Ginkgolide B | C20H24O10 | Terpenoids | [51] | Inhibition activity of oxidative stress and lipid peroxidation | [80,81] |
| 18 | Camphene | C10H16 | Terpenoids | [52] | Anti-hepatic steatosis activity | [82] |
| 19 | Oleic acid | C18H34O2 | Fatty acids and analogues | [36] | Anti-hepatic lipogenesis potency | [85] |
| 20 | Linoleic acid | C18H32O2 | Fatty acids and analogues | [36,53] | Anti-steatohepatitis effectiveness | [86] |
| 21 | Palmitoleic acid | C16H30O2 | Fatty acids and analogues | [36,53] | Anti-inflammatory activity in hepatic steatosis | [87] |
| 22 | Methyl palmitate | C17H34O2 | Fatty acids and analogues | [54] | Therapeutic effect against non-alcoholic steatohepatitis | [88] |
| 23 | Inositol | C6H12O6 | Saccharides and analogues | [55] | Treatment of non-alcoholic fatty liver disease | [90] |
| 24 | Dihydroxyacetone | C3H6O3 | Saccharides and analogues | [56] | Expounding different liver metabolic fats paths | [91] |
| 25 | DL-Arabinose | C5H10O5 | Saccharides and analogues | [56] | Hepatoprotective activity against hepatic lipid accumulation and inflammation | [92] |
| 26 | Cajaninstilbene acid | C21H22O | Stilbenes | [57,58] | Therapeutic effectiveness against liver injury | [94,95] |
| 27 | Isosorbide dinitrate | C6H8N2O8 | Nitrates | [56] | Hemodynamic activity in chronic cirrhosis cases | [96,97] |
| 28 | 5-Hydroxymethylfurfural | C6H6O3 | Organic compounds | [56] | Anti-hepatic apoptotic activity | [98,99] |
| 29 | Fusaruside | C₄₃H₇₇NO₉ | Sphingolipids | [33] | Inhibiting proliferation of T-cell in hepatitis cases | [100,101] |
Some species of Fusarium oxysporum strain are toxic due to its high content of alkaloids or other toxic compounds (depend on species) so it had to be examined its toxicity percentage in advance to evaluate the fungal toxicity levels in acceptable limits that qualify the usage of the fungus extracts in pharmaceutical and medicinal fields. In case if the chosen bioactive compound was secreted by non-abundant quantities for experimental usage, Fusarium oxysporum had to be grown on fermented media which its content forced the fungus to focus on secreting the target bioactive compound and also the hosting plant that used in isolating the fungus has to be accurately studied for its history due to its effect on producing fungal metabolic compounds. It was recommended to isolate a new Fusarium oxysporum strain and perform a general chemical screening for its bioactive compounds then identify its detected compounds to examine its content for one or more of aforementioned compounds and also to search for new chemical compounds possess anti-hepatic injury activity was not detected before in Fusarium oxysporum to become unrivaled experimental study.
2. Conclusion
The existent survey reported the predicted hepatoprotective and hepato-therapeutic potency of Fusarium oxysporum due to its massive content of bioactive compounds that had remedy effects against various types of liver complications. The pivotal target of this study is to highlight the mediational usage of F. oxysporum bioactive components to combat mischievous influences of hepatic injury. This review was basically performed to elucidate that F. oxysporum possess a significant anti-liver injury activity uniquely to face various types of hepatic disorders as a result of its abundant content to enormous chemical bioactive compounds that had therapeutical liver efficacy. Previous studies showed chemical composition of F. oxysporum and identified vast ambit of fungal biochemical compounds and these compounds was isolated from medicinal plants in other lately studies, which demonstrated tremendous hepato-therapeutic effectiveness, indicating the treatment potency of F. oxysporum against various hepatic complications and that is the main purpose of this survey study. Despite of containing the fungal content to a wide range of biologically active compounds for protecting and treating liver diseases, it was underestimated for its valuable and remarkable performance in studying and researching fields. All the aforesaid compounds isolated from F. oxysporum and most of them are capable of contribution in effective medications by reversing the harmful effects triggered by different hepatic maladies and recovering injured hepatocytes through numerous and varied pathways. But until now it was not applied in experimental work to assess its curative efficacy in confronting hepatic diseases and ameliorating infected hepatocytes situation practically. Therefore, it could nominate Fusarium oxysporum as anti-hepatic injury agent for preventing and resisting diverse kinds of liver diseases due to its copious production of biochemical compounds famed by its prodigious therapeutic impacts and its prophylactic actions against hepatic complications.
CRediT authorship contribution statement
Nashwa M. Shalapy: Writing – review & editing, Writing – original draft. Ming Liu: Formal analysis. Wenyi Kang: Writing – review & editing.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Wenyi Kang reports financial support was provided by Research on Precision Nutrition and Health Food, Department of Science and Technology of Henan Province (CXJD2021006). Wenyi Kang reports a relationship with Research on Precision Nutrition and Health Food, Department of Science and Technology of Henan Province (CXJD2021006). that includes: funding grants. There is no room for wrong interpretation by the reader as conflict of interest.
Contributor Information
Nashwa M. Shalapy, Email: nashwa.mustafa410@yahoo.com.
Wenyi Kang, Email: kangweny@hotmail.com.
Abbreviations
- DPPH
2,2-Diphenyl-1-picrylhydrazyl
- MALDI-TOF MS
Matrix-assisted laser desorption/ionization-time of flight mass spectrometry
- GC-MS
Gas chromatography–mass spectrometry
- HPLC
High-performance liquid chromatography
- HPLC/ESI-MS
Fast and sensitive high-performance liquid chromatography/electrospray ionization tandem mass spectrometry
- 13C-NMR
Carbon-13 nuclear magnetic resonance
- LC-MS/MS
Liquid chromatography-tandem mass spectrometry
- ALD
Alcoholic liver diseases
- NAFLD
Non-alcoholic fatty liver disease
- HFD
High-fat diet high-fat diet
- PCR
Polymerase chain reaction
- qPCR
Quantitative polymerase chain reaction
- FBG
Fasting blood glucose
- AST
Aspartate aminotransferase
- ALT
Alanine transaminase
- SOD
Superoxide dismutase
- GSH
Glutathione
- MDA
Malondialdehyde
- ELISA
Enzyme-linked immunosorbent assay
- HGF
Hepatocyte growth factor
- VEGF
Vascular endothelial growth factor
- TIMP-1
Tissue inhibitor of metalloproteinases level-1
- ECM
Extracellular matrix
- TGF-β
Transforming growth factor β1
- CCL4
Carbon tetrachloride
- LPS
Lipopolysaccharide
- APAP
Acetaminophen
- HCC
Hepatocellular carcinoma
- HSCs
hematopoietic stem cells
- α-SMA
Alpha-Smooth Muscle Actin
- ALK5
Activin receptor-like kinase 5
- TC
Total cholesterol
- TG
Triglycerides
- LDL
Low density lipoprotein
- HDL
High density lipoprotein
- P53
Tumor protein/cellular tumor antigen
- DILI
Drug-induced liver injury
- HCV
hepatitis C virus
- HBV
hepatitis B virus
- TNF-α
Tumour necrosis factor alpha
- IFN
Interferon
- MTT
Assay to measure cellular metabolic activity
- Huh7/Hep3B/HepG2
Hepatocellular carcinoma cell lines
- GES-1
Human gastric epithelial cell line
- ROS
Reactive oxygen species
- STAT1
Signal transducer and activator of transcription 1
- STAT3
Signal transducer and activator of transcription 3
- p38 MAPK
p38 Mitogen-activated protein kinases
- DMSO
Dimethyl sulfoxide
- NTCP
Sodium taurocholate cotransporting polypeptide
- HBsAg
Hepatitis B surface antigen
- Nrf2
Nuclear factor erythroid 2–related factor 2
- 3T3-L1
Continuous sub-strain of 3T3 developed through clonal isolation
- AMPK
Adenosine mono phosphate-activated protein kinase
- LXRs
Liver X Receptors (α and β)
- PPAR-γ
Peroxisome proliferator-activated receptor gamma
- PPAR-α
Peroxisome proliferator-activated receptor alpha
- TNFα
Tumor necrosis factor α
- TGFβ1
Transforming growth factor β1
- MCP-1
Monocyte chemoattractant protein-1
- TUNEL
Terminal deoxynucleotidyl transferase dUTP nick end labeling
- JNK-Sab-ROS
c-Jun N-terminal kinases- SH3 domain-binding protein 5 -Reactive oxygen species
- P62
Ubiquitin-binding protein P62
- PINK1
PTEN Induced Kinase 1
- PGC-1α
Peroxisome proliferator-activated receptor-gamma coactivator-1 alpha
- LC3-Ⅱ
Microtubule-associated protein 1A/1B-light chain 3
- TFAM
Transcription Factor A Mitochondrial
- ER kinase
Endoplasmic reticulum kinase
- CHOP
C/EBP homologous protein
- ATF4
Activating Transcription Factor 4
- Phospho-PERK
Phosphorylation of Protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK)
- Phospho-eIF2α
Phosphorylation of eukaryotic initiation factor-2α
- Th1/Th2/Th17
T helper cells type 1/2/17
- Treg
Regulatory T cells
- NKT cells
Natural killer T cells
- CD4(+) T cells
CD4 T lymphocytes
- Bcl-xL
B-cell lymphoma-extra large
- T-bet
Transcription factor
- CAT
Catalase
- IL-1β
interleukin 1-beta
- 5-HMF
5-hydroxymethylfurfural
References
- 1.Seitz H.K., Neuman M.G. The history of alcoholic liver disease: from an unrecognized disease to one of the most frequent diseases in hepatology. J. Clin. Med. 2021;10(4):858. doi: 10.3390/jcm10040858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization (Who) 2014. Global Status Report on Alcohol and Health. [Google Scholar]
- 3.Sivakrishnan S. Liver diseases-an overview. World J. Pharm. Pharmaceut. Sci. 2019;8(1):1385–1395. [Google Scholar]
- 4.Zhang C., Liu S., Yang M. Antioxidant and anti-inflammatory agents in chronic liver diseases: molecular mechanisms and therapy. World J. Hepatol. 2023;15(2):180–200. doi: 10.4254/wjh.v15.i2.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Embade N., Millet O. Molecular determinants of chronic liver disease as studied by NMR-Metabolomics. Curr. Top. Med. Chem. 2017;17:2752–2766. doi: 10.2174/1568026617666170707124539. [DOI] [PubMed] [Google Scholar]
- 6.Seitz H.K., Bataller R., Cortez-Pinto H., Gao B., Gual A., Lackner C., Mathurin P., Mueller S., Szabo G., Tsukamoto H. Alcoholic liver disease. Nat. Rev. Dis. Prim. 2018;4:16. doi: 10.1038/s41572-018-0014-7. [DOI] [PubMed] [Google Scholar]
- 7.Mantovani A., Beatrice G., Dalbeni A. Coronavirus disease 2019 and prevalence of chronic liver disease: a meta-analysis. Liver Int. 2020;40:1316–1320. doi: 10.1111/liv.14465. [DOI] [PubMed] [Google Scholar]
- 8.Munoz S.J., Maddrey W.C. Major complications of acute and chronic liver disease. Gastroenterol. Clin. N. Am. 1988;17(2):265–287. [PubMed] [Google Scholar]
- 9.Arshad M., Asif H., John A., Pasha M.O. Chronic liver disease and it's complications. J. Zool. 2021;4(1):3–7. [Google Scholar]
- 10.Hayward K.L., Weersink R.A. Improving medication‐related outcomes in chronic liver disease. Hepatol Commun. 2020;4(11):1562–1577. doi: 10.1002/hep4.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luper S. A review of plants used in the treatment of liver disease: part 1. Alternative Med. Rev. 1998;3(6):410–421. [PubMed] [Google Scholar]
- 12.Jeong J., Park H.J., Cha M.G., Park E., Won S., Ganesan R., Gupta H., Gebru Y.A., Sharma S.P., Lee S.B., Kwon G.H., Jeong M.K., Min B.H., Hyun J.Y., Eom J.A., Yoon S.J., Choi M.R., Kim D.J., Suk K.T. The lactobacillus as a probiotic: focusing on liver diseases. Microorg. 2022;10(2):288. doi: 10.3390/microorganisms10020288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gustavo G., Marianella R., Magdalena M., Eduardo P., María P.A. Endophytic fungi in medicinal plants of northeast of Argentina: I: morphotaxonomic approach of their foliar community. Bol. Micol. 2010;25:15–27. [Google Scholar]
- 14.Ibrahim S.R.M., Sirwi A., Eid B.G., Mohamed S.G.A., Mohamed G.A. Bright side of Fusarium oxysporum: secondary metabolites bioactivities and industrial relevance in biotechnology and nanotechnology. J Fungi (Basel). 2021;7(11):943. doi: 10.3390/jof7110943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hastutia U.S., Al Asna P.M., Rahmawati D. Histologic observation, identification, and secondary metabolites analysis of endophytic fungi isolated from a medicinal plant, hedychium acuminatum roscoe. AIP Conf. Proc. 2002;(1) (2018) 020070-1–020070-8. [Google Scholar]
- 16.Matsakas L., Giannakou M., Vörös D. Effect of synthetic and natural media on lipid production from Fusarium oxysporum. Electron. J. Biotechnol. 2017;30:95–102. [Google Scholar]
- 17.Brodhun F., Cristobal-Sarramian A., Zabel S., Newie J., Hamberg M., Feussner I. An iron 13S-lipoxygenase with an α-linolenic acid specific hydroperoxidase activity from Fusarium oxysporum. PLoS One. 2013;8(5) doi: 10.1371/journal.pone.0064919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quarters A.B.M., Wirgau N.E., Lehnert N. Model complexes of key intermediates in fungal cytochrome P450 nitric oxide reductase (P450nor) Curr. Opin. Chem. Biol. 2014;19:82–89. doi: 10.1016/j.cbpa.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 19.Sakai Y., Yoshida N., Isogai A., Tani Y., Kato N. Purification and properties of fructosyl lysine oxidase from Fusarium oxysporum S-1F4. Biosci. Biotechnol. Biochem. 1995;59:487–491. doi: 10.1271/bbb.59.487. [DOI] [PubMed] [Google Scholar]
- 20.Bisakowski B., Kermasha S., Klopfenstein M. Partial purified lipoxygenase from Fusarium oxysporum: characterization and kinetic studies. Process Biochem. 1995;30:261–268. [Google Scholar]
- 21.Zhao Z., Ramachandran P., Kim T.S., Chen Z., Jeya M., Lee J.K. Characterization of an acid-tolerant β-1,4-glucosidase from Fusarium oxysporum and its potential as an animal feed additive. Appl. Microbiol. Biotechnol. 2013;97:10003–10011. doi: 10.1007/s00253-013-4767-3. [DOI] [PubMed] [Google Scholar]
- 22.Sakamoto T., Tsujitani Y., Fukamachi K., Taniguchi Y., Ihara H. Identification of two GH27 bifunctional proteins with β-L-arabinopyranosidase/α-D-galactopyranosidase activities from Fusarium oxysporum. Appl. Microbiol. Biotechnol. 2010;86:1115–1124. doi: 10.1007/s00253-009-2344-6. [DOI] [PubMed] [Google Scholar]
- 23.Yano T., Yamamoto K., Kumagai H., Tochikura T., Yokoyama T., Seno T., Yamaguchi H. Purification and characterization of a novel α-L-fucosidase from Fusarium oxysporum grown on sludge. Agric. Biol. Chem. 1985;49:3179–3187. [Google Scholar]
- 24.Ahmed A.A., Hamzah H., Maaroof M. Analyzing formation of silver nanoparticles from the filamentous fungus Fusarium oxysporum and their antimicrobial activity. Turk. J. Biol. 2018;42:54–62. doi: 10.3906/biy-1710-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohanpuria P., Rana N.K., Yadav S.K. Biosynthesis of nanoparticles: technological concepts and future applications. J. Nanoparticle Res. 2008;10:507–517. [Google Scholar]
- 26.Caicedo N.H., Davalos A.F., Puente P.A., Rodríguez A.Y., Caicedo P.A. Antioxidant activity of exo‐metabolites produced by Fusarium oxysporum: an endophytic fungus isolated from leaves of Otoba gracilipes. Microbiol. 2019;8(10):903. doi: 10.1002/mbo3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmed A.M., Mahmoud B.K., Millan-Aguinaga N., Abdelmohsen U.R., Fouad M.A. The endophytic Fusarium strains: a treasure trove of natural products. RSC Adv. 2023;13:1339–1369. doi: 10.1039/d2ra04126j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prasher P., Sharma M. Medicinal chemistry of anthranilic acid derivatives: a mini review. Drug Dev. Res. 2021;82(7):945–958. doi: 10.1002/ddr.21842. [DOI] [PubMed] [Google Scholar]
- 29.Niemann G.J., Liem J., Hoof A.V.D.K., Niessen W.M.A. Phytoalexins, benzoxazinones, N-aroylanthranilates and N-aroylanilines, from Fusarium-infected carnation stems. Phytochemistry. 1992;31:3761–3767. [Google Scholar]
- 30.Kılıç G., Tosun G., Bozdeveci A., Erik ˙I., Öztürk E., Reis R., Sipahi H., Cora M., Karaoglu S.A., Yaylı N. Antimicrobial, cytotoxic, antiviral effects, and spectroscopic characterization of metabolites produced by Fusarium oxysporum YP9B. Record Nat. Prod. 2021;15:547–567. [Google Scholar]
- 31.Nenkep V., Yun K., Son B.W. Oxysporizoline, an antibacterial polycyclic quinazoline alkaloid from the marine-mudflat-derived fungus Fusarium oxysporum. J. Antibiot. 2016;69:709–711. doi: 10.1038/ja.2015.137. [DOI] [PubMed] [Google Scholar]
- 32.Breinhold J., Ludvigsen S., Rassing B.R., Rosendahl C.N., Nielsen S.E., Olsen Oxysporidinone C.E. A novel, antifungal N-methyl-4-hydroxy-2-pyridone from Fusarium oxysporum. J. Nat. Prod. 1997;60:33–35. doi: 10.1021/np9605596. [DOI] [PubMed] [Google Scholar]
- 33.Wang Q.X., Li S.F., Zhao F., Dai H.Q., Bao L., Ding R., Gao H., Zhang L.X., Wen H.A., Liu H.W. Chemical constituents from endophytic fungus Fusarium oxysporum. Fitoterapia. 2011;82:777–781. doi: 10.1016/j.fitote.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 34.Lee H.S., Song H.H., Jeong J.H., Shin C.G., Choi S.U., Lee C. Cytotoxicities of enniatins H, I, and MK1688 from Fusarium oxysporum KFCC 11363P. Toxicon. 2008;51:1178–1185. doi: 10.1016/j.toxicon.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 35.Jarocka-Karpowicz I., Markowska A. Therapeutic potential of jasmonic acid and its derivatives. Int. J. Mol. Sci. 2021;22:8437. doi: 10.3390/ijms22168437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shalapy N.M., Kang W. Fusarium oxysporum & Fusarium solani: identification, characterization, and differentiation the fungal phenolic profiles by HPLC and the fungal lipid profiles by GC-MS. J. Food Qual. 2022;2022:1–12. [Google Scholar]
- 37.Huang Z., Yang J., She Z., Lin Y. Isoflavones from the mangrove endophytic fungus Fusarium sp. (ZZF41) Nat. Prod. Commun. 2010;5(11):1771. 1773. [PubMed] [Google Scholar]
- 38.Edmondson R.D., Gadda G., Fitzpatrick P.F., Russell D.H. Identification of native flavin adducts from Fusarium oxysporum using accurate mass matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Anal. Chem. 1997;69:2862–2865. [Google Scholar]
- 39.Kundu A., Saha S., Walia S., Dutta T.K. Anti-nemic secondary metabolites produced by Fusarium oxysporum f. sp. Ciceris. J. Asia Pac. Entomol. 2016;19:631–636. [Google Scholar]
- 40.Tatum J.H., Baker R.A., Berry R.E. Naphthoquinones produced Fusarium oxysporum isolated from citrus. Phytochemistry. 1985;24:457–459. [Google Scholar]
- 41.Baker R.A., Tatum J.H., Nemec S., Jr. Antimicrobial activity of naphthoquinones from Fusaria. Mycopathologia. 1990;111:9–15. doi: 10.1007/BF02277294. [DOI] [PubMed] [Google Scholar]
- 42.Tatum J.H., Baker R.A., Berry R.E. Naphthofurans produced Fusarium oxysporum isolated from citrus. Phytochemistry. 1987;26:2499–2500. [Google Scholar]
- 43.Zhan J., Burns A.M., Liu M.X., Faeth S.H., Gunatilaka A.A. Search for cell motility and angiogenesis inhibitors with potential anticancer activity: beauvericin and other constituents of two endophytic strains of Fusarium oxysporum. J. Nat. Prod. 2007;70:227–232. doi: 10.1021/np060394t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li-Yang Y., Lin J., Zhou B., Liu Y.G., Zhu B.Q. H1-A, a compound isolated from Fusarium oxysporum inhibits hepatitis C virus (HCV) NS3 serine protease. Chin. J. Nat. Med. 2016;14:299–302. doi: 10.1016/S1875-5364(16)30031-0. [DOI] [PubMed] [Google Scholar]
- 45.Starratt A.N., Madhosingh C. Sterol and fatty acid components of mycelium of Fusarium oxysporum. Can. J. Microbiol. 1967;13:1351–1355. doi: 10.1139/m67-181. [DOI] [PubMed] [Google Scholar]
- 46.Mohamed G.A., Ibrahim S.R.M., Alhakamy N.A., Aljohani O.S. Fusaroxazin, a novel cytotoxic and antimicrobial xanthone derivative from Fusarium oxysporum. Nat. Prod. Res. 2020;36(4):952–960. doi: 10.1080/14786419.2020.1855165. [DOI] [PubMed] [Google Scholar]
- 47.Chen J., Bai X., Hua Y., Zhang H., Wang H. Fusariumins C and D, two novel antimicrobial agents from Fusarium oxysporum ZZP-R1 symbiotic on Rumex madaio Makino. Fitoterapia. 2019;134:1–4. doi: 10.1016/j.fitote.2019.01.016. [DOI] [PubMed] [Google Scholar]
- 48.Fu Y., Wu P., Xue J., Zhang M., Wei X. Cosmosporasides F-H, three new sugar alcohol conjugated acyclic sesquiterpenes from a Fusarium oxysporum fungus. Nat. Prod. Res. 2020;36(13):3420–3428. doi: 10.1080/14786419.2020.1864366. [DOI] [PubMed] [Google Scholar]
- 49.Kim K.H., Lee Y.W., Mirocha C.J., Pawlosky R.J. Isoverrucarol production by Fusarium oxysporum CJS-12 isolated from corn. Appl. Environ. Microbiol. 1990;56:260–263. doi: 10.1128/aem.56.1.260-263.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Piska F., Teruna H.Y. Terpenoid as antibacterial produced by endophyte Fusarium oxysporum LBKURCC41 from Dahlia variabilis tuber. J. Phys. Conf. Ser. 2020;1655 [Google Scholar]
- 51.Cui Y., Yi D., Bai X., Sun B., Zhao Y., Zhang Y. Ginkgolide B produced endophytic fungus (Fusarium oxysporum) isolated from Ginkgo biloba. Fitoterapia. 2012;83:913–920. doi: 10.1016/j.fitote.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 52.Nasr H.M.D., Abdel-ghany R.O., Mousa S.A.S., Alasmaey M., Atalla A.A. Biological activity of crude extracts of endophytic Fusarium Oxysporum and its chemical composition by gas chromatography–mass spectrometry. Elixir Org. Chem. 2018;117:50565–50568. [Google Scholar]
- 53.Yu R., Li M., Wang Y., Bai X., Chen J., Li X., Wang H., Zhang H. Chemical investigation of a co-culture of Aspergillus fumigatus D and Fusarium oxysporum R1. Record Nat. Prod. 2021;15:130–135. [Google Scholar]
- 54.Nascimento A.M., Conti R., Turatti I.C.C., Cavalcanti B.C., Costa-Lotufo L.V., Pessoa C., Moraes M.O., Manfrim V., Toledo J.S., Cruz A.K., Pupo M.T. Bioactive extracts and chemical constituents of two endophytic strains of Fusarium oxysporum. Brazilian Journal of Pharmacognosy. 2012;22(6):1276–1281. [Google Scholar]
- 55.Nita-Lazar M., Heyraud A., Gey C., Braccini I., Lienart Y. Novel oligosaccharides isolated from Fusarium oxysporum L. rapidly induce PAL activity in Rubus cells. Acta Biochim. Pol. 2004;51:625–647. [PubMed] [Google Scholar]
- 56.Al-Rubaye A.F., Hameed I.H., Kamal S.A. Screening of metabolites products of Fusarium oxysporum and determination of its antibacterial and antifungal activity using medicinal plants extract. Indian Journal of Public Health Research & Develop. 2018;9(3):400–404. [Google Scholar]
- 57.Zhao J., Fu Y., Luo M., Zu Y., Wang W., Zhao C., Gu C. Endophytic fungi from pigeon pea [Cajanus cajan (L.) Mill sp.] produce antioxidant Cajaninstilbene acid. J. Agric. Food Chem. 2012;60(17):4314–4319. doi: 10.1021/jf205097y. [DOI] [PubMed] [Google Scholar]
- 58.Toghueo R.M.K. Bioprospecting endophytic fungi from Fusarium genus as sources of bioactive metabolites. Mycology. 2020;11(1):1–21. doi: 10.1080/21501203.2019.1645053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grover J.K., Yadav S., Vats V. Medicinal plants of India with anti-diabetic potential. J. Ethnopharmacol. 2002;81(1):81–100. doi: 10.1016/s0378-8741(02)00059-4. [DOI] [PubMed] [Google Scholar]
- 60.Strobel G., Daisy B., Castillo U. The biological promise of microbial endophytes and their natural products. Plant Pathol. J. 2005;4(2):161–176. [Google Scholar]
- 61.Mutha R.E., Tatiya A.U., Surana S.J. Flavonoids as natural phenolic compounds and their role in therapeutics: an overview. Futur J Pharm Sci. 2021;7(1):25. doi: 10.1186/s43094-020-00161-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shi A., Li T., Zheng Y., Song Y., Wang H., Wang N., Dong L., Shi H. Chlorogenic acid improves NAFLD by regulating gut microbiota and GLP-1. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.693048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abunofal O., Mohan C. Salubrious effects of green tea catechins on fatty liver disease: a Systematic Review. Medicines (Basel). 2022;9(3):20. doi: 10.3390/medicines9030020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Akbari G., Savari F., Mard S.A., Rezaie A., Moradi M. Gallic acid protects the liver in rats against injuries induced by transient ischemia-reperfusion through regulating microRNAs expressions. Iran J Basic Med Sci. 2019;22(4):439–444. doi: 10.22038/ijbms.2018.31589.7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Parvizi F., Yaghmaei P., Rohani S.A.H., Mard S.A. Hepatoprotective properties of p-coumaric acid in a rat model of ischemia-reperfusion. Avicenna J Phytomed. 2020;10(6):633–640. [PMC free article] [PubMed] [Google Scholar]
- 66.Mu M., Zuo S., Wu R., Deng K., Lu S., Zhu J., Zou G., Yang J., Cheng M., Zhao X. Ferulic acid attenuates liver fibrosis and hepatic stellate cell activation via inhibition of TGF-β/Smad signaling pathway. Drug Des. Dev. Ther. 2018;12:4107–4115. doi: 10.2147/DDDT.S186726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ghanim A.M.H., Younis N.S., Metwaly H.A. Vanillin augments liver regeneration effectively in Thioacetamide induced liver fibrosis rat model. Life Sci. 2021;286 doi: 10.1016/j.lfs.2021.120036. [DOI] [PubMed] [Google Scholar]
- 68.Hernández-Aquino E., Muriel P. Beneficial effects of naringenin in liver diseases: molecular mechanisms. World J. Gastroenterol. 2018;24(16):1679–1707. doi: 10.3748/wjg.v24.i16.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim M., Park J., Jung J., Byun K., Kang K., Lee Y. Daidzein supplementation prevents non-alcoholic fatty liver disease through alternation of hepatic gene expression profiles and adipocyte metabolism. Int. J. Obes. 2011;35(8):1019–1030. doi: 10.1038/ijo.2010.256. [DOI] [PubMed] [Google Scholar]
- 70.Alkandahri M.Y., Pamungkas B.T., Oktoba Z., Shafirany M.Z., Sulastri L., Arfania M., Anggraeny E.N., Pratiwi A., Astuti F.D., Dewi S.Y.I., Hamidah S.Z. Hepatoprotective effect of kaempferol: a review of the dietary sources, bioavailability, mechanisms of action, and safety. Adv Pharmacol Pharm Sci. 2023;2023 doi: 10.1155/2023/1387665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.AlSharari S.D., Al-Rejaie S.S., Abuohashish H.M., Ahmed M.M., Hafez M.M. Rutin attenuates hepatotoxicity in high-cholesterol-diet-fed rats. Oxid. Med. Cell. Longev. 2016;2016 doi: 10.1155/2016/5436745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee W., Kim S. Protective effects of isoflavones on alcoholic liver diseases: computational approaches to investigate the inhibition of ALDH2 with isoflavone analogues. Front. Mol. Biosci. 2023;10:2023. doi: 10.3389/fmolb.2023.1147301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yao L., Nisar M.F., Yan T., Wan C. Potential effects of dietary isoflavones on drug-induced liver injury. J. Food Qual. 2021;2021 [Google Scholar]
- 74.Prasher P., Sharma M. Medicinal chemistry of anthranilic acid derivatives: a mini review. Drug Dev. Res. 2021;82:945–958. doi: 10.1002/ddr.21842. [DOI] [PubMed] [Google Scholar]
- 75.Saito H., Ebinuma H., Tadal S., Tsunematsu S., Atsukawa K., Masuda T., Tsuchiya M., Ishii H. Enhancing effect of the liver extract and flavin adenin dinucleotide mixture on anti-viral efficacy of interferon in patients with chronic hepatitis C. Keio J. Med. 1996;45(1):48–53. doi: 10.2302/kjm.45.48. [DOI] [PubMed] [Google Scholar]
- 76.Wang Y., Luo Y., Piao X., Shen G., Meng L., Zhang Y., Wang J., Li J., Wang H., Xu W., Liu Y., Zhang Y., Zhang T., Wang S., Sun H., Han Y., Jin M., Zang Y., Zhang D., Jin C. Novel 1,4-naphthoquinone derivatives induce reactive oxygen species-mediated apoptosis in liver cancer cells. Mol. Med. Rep. 2019;19:1654–1664. doi: 10.3892/mmr.2018.9785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang W., Chen X., Li Y., Guo S., Wang Z., Yu X. Advances in pharmacological activities of terpenoids. Nat. Prod. Commun. 2020;15(3):1–13. [Google Scholar]
- 78.Li-Yuan Y., Jun L., Bin Z., Yan-Gang L., Bao-Quan Z. H1-A, a compound isolated from Fusarium oxysporum inhibits hepatitis C virus (HCV) NS3 serine protease. Chin. J. Nat. Med. 2016;14(4):299–302. doi: 10.1016/S1875-5364(16)30031-0. [DOI] [PubMed] [Google Scholar]
- 79.Huang H., Huang H., Chiou W., Lin L., Chen J., Liu H., Lai Y., Huang C. Ergosterol peroxide inhibits HBV infection by inhibiting the binding of the pre-S1 domain of LHBsAg to NTCP. Antivir. Res. 2021;195 doi: 10.1016/j.antiviral.2021.105184. [DOI] [PubMed] [Google Scholar]
- 80.Yang Y., Chen J., Gao Q., Shan X., Wang J., Lv Z. Study on the attenuated effect of Ginkgolide B on ferroptosis in high fat diet induced nonalcoholic fatty liver disease. Toxic. 2020;445 doi: 10.1016/j.tox.2020.152599. [DOI] [PubMed] [Google Scholar]
- 81.Wang C., Liou C., Wu S., Wei C., Huang W. Ginkgolide B reduces hepatic lipid accumulation and ameliorates nonalcoholic fatty liver disease in high-fat diet–induced obese mice. Oncotarget. 2018;1949:2553. [Google Scholar]
- 82.Kim S., Choi Y., Choi S., Choi Y., Park T. Dietary camphene attenuates hepatic steatosis and insulin resistance in mice. Obesity. 2014;22(2):408–417. doi: 10.1002/oby.20554. [DOI] [PubMed] [Google Scholar]
- 83.Selvaraj J. Fatty Acids. InTech.; 2017. Fatty acids and their analogues as anticancer agents. [DOI] [Google Scholar]
- 84.Casillas-Vargas G., Ocasio-Malavé C., Medina S., Morales-Guzmán C., Valle R.G.D., Carballeira N.M., Sanabria-Ríos D.J. Antibacterial fatty acids: an update of possible mechanisms of action and implications in the development of the next-generation of antibacterial agents. Prog. Lipid Res. 2021;82 doi: 10.1016/j.plipres.2021.101093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ducheix S., Montagner A., Polizzi A., Lasserre F., Régnier M., Marmugi A., Benhamed F., Bertrand-Michel J., Mselli-Lakhal L., Loiseau N., Martin P.G., Lobaccaro J., Ferrier L., Postic C., Guillou H. Dietary oleic acid regulates hepatic lipogenesis through a liver X receptor-dependent signaling. PLoS One. 2017;12(7) doi: 10.1371/journal.pone.0181393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nagao K., Inoue N., Wang Y., Shirouchi B., Yanagita T. Dietary conjugated linoleic acid alleviates nonalcoholic fatty liver disease in Zucker (fa/fa) Rats. J. Nutr. 2005;135(1):9–13. doi: 10.1093/jn/135.1.9. [DOI] [PubMed] [Google Scholar]
- 87.Souza C.O., Teixeira A.A.S., Biondo L.A., Silveira L.S., Breda C.N.S., Braga T.T., Camara N.O.S., Belchior T., Festuccia W.T., Diniz T.A., Ferreira G.M., Hirata M.H., Chaves-Filho A.B., Yoshinaga M.Y., Miyamoto S., Calder P.C., Sethi J.K., Neto J.C.R. Palmitoleic acid reduces high fat diet-induced liver inflammation by promoting PPAR-γ-independent M2a polarization of myeloid cells. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2020;1865(10) doi: 10.1016/j.bbalip.2020.158776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang L., Li H., Pan W., Khan F.U., Qian C., Qi-Li F., Xu X. Administration of methyl palmitate prevents non-alcoholic steatohepatitis (NASH) by induction of PPAR-α. Biomed. Pharmacother. 2019;111:99–108. doi: 10.1016/j.biopha.2018.12.059. [DOI] [PubMed] [Google Scholar]
- 89.Spriha S.E., Abdur-Rahman S.M. A review on biological activities of sugars and sugar derivatives. J. Pharmaceut. Sci. 2022;20(3):381–394. [Google Scholar]
- 90.Pani A., Giossi R., Menichelli D., Fittipaldo V.A., Agnelli F., Inglese E., Romandini A., Roncato R., Pintaudi B., Sole F.D., Scaglione F. Inositol and non-alcoholic fatty liver disease: a systematic review on deficiencies and supplementation. Nutrients. 2020;12(11):3379. doi: 10.3390/nu12113379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kirpich A., Ragavan M., Bankson J.A., McIntyre L.M., Merritt M.E. Kinetic analysis of hepatic metabolism using hyperpolarized dihydroxyacetone. J. Chem. Inf. Model. 2019;59(1):605–614. doi: 10.1021/acs.jcim.8b00745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang Y., Zhao J., Li Q., Liu J., Sun Y., Zhang K., Fan M., Qian H., Li Y., Wang L. L-Arabinose improves hypercholesterolemia via regulating bile acid metabolism in high-fat-high-sucrose diet-fed mice. Nutr. Metab. 2022;2022:19–30. doi: 10.1186/s12986-022-00662-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xu M., Huang Z., Zhu W., Liu Y., Bai X., Zhang H. Fusarium-derived secondary metabolites with antimicrobial effects. Molecules. 2023;28(8):3424. doi: 10.3390/molecules28083424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yan M., Jin S., Liu Y., Wang L., Wang Z., Xia T., Chang Q. Cajaninstilbene acid ameliorates acetaminophen-induced liver injury through enhancing sestrin2/AMPK-mediated mitochondrial quality control. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.824138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Huang M., Lin J., Lu K., Xu H., Geng Z., Sun P., Chen W. Anti-Inflammatory effects of cajaninstilbene acid and its derivatives. J. Agric. Food Chem. 2016;64(14):2893–2900. doi: 10.1021/acs.jafc.6b00227. [DOI] [PubMed] [Google Scholar]
- 96.Mols P., Hallemans R., Melot C., Lejeune P., Naeije R. Systemic and regional hypodynamic effects of isosorbide dinitrate in patients with liver cirrhosis and portal hypertension. J. Hepatol. 1989;8(3):316–324. doi: 10.1016/0168-8278(89)90029-9. [DOI] [PubMed] [Google Scholar]
- 97.Cervinka J., Kordac V., Kalab M. Effect of peroral administration of isosorbide dinitrate on portal pressure and blood flow in patients with cirrhosis of the liver. J. Int. Med. Res. 1989;17:560–564. doi: 10.1177/030006058901700610. [DOI] [PubMed] [Google Scholar]
- 98.Jiang Z., Ma Y., Li M., Zhan X., Zhang X., Wang M. 5-Hydroxymethylfurfural protects against ER stress-induced apoptosis in GalN/TNF-α-injured L02 hepatocytes through regulating the PERK-eIF2α signaling pathway. Chin. J. Nat. Med. 2015;13(12):896–905. doi: 10.1016/S1875-5364(15)30095-9. [DOI] [PubMed] [Google Scholar]
- 99.Li W., Qu X., Han Y., Zheng S., Wang J., Wang Y. Ameliorative effects of 5-hydroxymethyl-2-furfural (5-HMF) from Schisandra chinensis on alcoholic liver oxidative injury in mice. Int. J. Mol. Sci. 2015;16:2446–2457. doi: 10.3390/ijms16022446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wu X., Sun Y., Guo W., Gu Y., Wu X., Tan R., Xu Q. Rebuilding the balance of STAT1 and STAT3 signalings by fusaruside, a cerebroside compound, for the treatment of T-cell-mediated fulminant hepatitis in mice. Biochem. Pharmacol. 2012;84(9):1164–1173. doi: 10.1016/j.bcp.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 101.Tian Y., Zhao G.Y., Fang W., Xu Q., Tan R.X. Δ10(E)-sphingolipid desaturase involved in fusaruside mycosynthesis and stress adaptation in Fusarium graminearum, Sci Rep. 2015;5 doi: 10.1038/srep10486. [DOI] [PMC free article] [PubMed] [Google Scholar]