Abstract
Objective: To explore the effect of Shixiao Huoxue Decoction on pain and tumor necrosis factor (TNF)-α and interleukin (IL)-8 levels in patients with adenomyosis. Methods: A total of 65 patients with adenomyosis admitted to South District of Guang’anmen Hospital from January 2020 to December 2021 were divided into two groups according to the treatment methods. The control group was treated with pregnatrienone, and the study group was treated with additional Shixiaohuoxue decoction. The incidence of complications, treatment efficacy, levels of inflammatory factors, Traditional Chinese Medicine symptom score, dysmenorrhea score, menstrual volume score, dysmenorrhea symptom score, changes in uterine volume, level of insulin-like growth factor 1 (IGF-1), and changes in the level of carbohydrate antigen (CA125) were observed before and after treatment in both two groups. Univariate Logistic analysis showed that uterine volume, IGF-1, CA125, serum IL-8 and TNF-α were correlated with the short-term efficacy of Meixiaohuoxue Decoction in the treatment of uterine adenomyosis (P<0.05). Results: The levels of IL-8 and TNF-α in the study group were significantly lower than those in the control group after treatment (P<0.05). The scores of dyspareunia and non-menstrual pelvic pain in the study group were significantly lower than those in the control group (P<0.05). The overall response rate in the study group (93.75%) was significantly higher than that in the control group (66.66%) (P<0.05). The scores of Traditional Chinese Medicine symptoms, dysmenorrhea, menstrual volume, and dysmenorrhea symptoms in the study group were significantly lower than those in the control group after treatment (P<0.05). The IGF-1 and CA125 levels in the study group were significantly lower than those in the control group after treatment (P<0.05). However, no significant difference in uterine volume was found between the two groups after treatment (P>0.05). Conclusion: Xiaoxiao Huoxue Decoction demonstrated a better treatment efficacy in patients with adenomyosis through improving dysmenorrhea and Traditional Chinese Medicine symptoms, as well as reducing the levels of body inflammatory factors, non-menstrual pelvic pain, and dyspareunia, thus contributing to early recovery of patients. Therefore, Xiaoxiao Huoxue Decoction is worthy of promotion in clinical treatment of adenomyosis.
Keywords: Xiaoxiao Huoxue Decoction, adenomyosis, pain, tumor necrosis factor-α, interleukin-8
Introduction
Adenomyosis refers to the invasion of endometrial stroma and glands into the myometrium, accompanied by functional changes such as periodic bleeding, hyperplasia, and exfoliation [1]. Epidemiological data show that 30-50-year-old multiparous women are at high risk of the disease, about 15% of them have endometriosis of the genitals, and about half of them have fibroids of the genitals. The reported incidence of this disease is 10% to 65%. In recent years, women’s reproductive age has been delayed, resulting in a gradual increased incidence in nulliparous women [2]. However, the exact cause of the disease is currently not yet fully understood. Most scholars believe that the disease develops when the basal endometrium invades the myometrium. The main symptoms of patients are prolonged menstruation, increased menstrual volume, infertility, progressive dysmenorrhea, etc., reducing the quality of life of patients [3]. Drugs, surgery or other treatment methods can be suggested to patients according to their symptoms, age, and fertility requirements. For patients who have no symptoms and no fertility requirements, expectant observational treatment can be performed. Patients with mild symptoms, fertility requirements, and near-menopausal patients can try drug treatment (hormone therapy is the most common drug therapy), but there are potential adverse drug reactions and risk of reoccurrence after drug withdrawal [4]. Patients with severe symptoms and ineffective medical treatment can be treated with surgery. Surgical resection is the main treatment method in Western medicine, but most of the patients are women of childbearing age, so the acceptance of resection is low.
In recent years, Chinese medicine has been widely used in clinical treatment, with promising results. Traditional Chinese medicine believes that adenomyosis is related to infertility and dysmenorrhea. Current research has shown that adding traditional Chinese medicine to conventional Western medicine can improve the treatment effect of patients with adenomyosis, accelerate the patient’s recovery process, and improve the safety of treatment [5]. This paper aims to explore the therapeutic effect of Shixiaohuoxue decoction in patients with adenomyosis. The research is innovative and can provide reference for the treatment of adenomyosis and related diseases.
Materials and methods
General data
This is a retrospective study. A total of 65 cases of adenomyosis in patients admitted to the South District of Guang’anmen Hospital from January 2020 to December 2021 were divided into two groups according to different treatment methods. The control group (n=33) was treated with pregnatrienone, and the study group (n=32) was treated with additional Shixiaohuoxue decoction. In the control group, the patients had a disease course of 2-9 years (average: 5.09 ± 1.64 years), an age range of 28-47 years (average: 37.95 ± 1.94 years), parity of 2-5 times (average: 2.53 ± 0.59 times), BMI of 18-24 kg/m2 (average: 20.36 ± 1.46 kg/m2), and 2-6 times of being pregnant (average: 3.08 ± 0.62 times). In the study group, the patients had a disease course of 2-9 years (average: 5.24 ± 1.57 years), an age range of 28-47 years (average: 38.76 ± 2.01 years), parity of 1-5 times (average: 2.67 ± 0.44 times), BMI of 18-24 kg/m2 (average: 20.33 ± 1.45 kg/m2), and 2-6 times of being pregnant (average: 3.05 ± 0.61 times). The general data of the two groups were comparable (P>0.05).
Inclusion criteria: (1) patients with adenomyosis of the uterus confirmed by B-ultrasound and clinical manifestations [6]; (2) patients with complete clinical data; (3) patients who provided signed informed consent; (4) patients with normal cognitive and language function.
Exclusion criteria: (1) patients with a history of gynecological surgery; (2) patients with a history of mental illness; (3) patients with a history of autoimmune diseases or liver disease; (4) patients with pelvic inflammation or suspected malignant diseases; (5) patients with amenorrhea duration over 3 months; (6) patients who used hormone drugs three months before admission; (7) patients with serious diseases in vital organs.
Methods
The control group was treated with gestrinone (Manufacturer: China Resources Zizhu Pharmaceutical Co., LTD., Batch number: 20201015), starting on the first day of menstruation, with subsequent doses administered three days apart and continued weekly thereafter. A total of three courses of treatment was administered, and the therapeutic effect was observed.
The usage and dosage of gestrinone in the study group were the same as those in the control group. Additionally, Shixiao Huoxue Decoction was given. The prescription consisted of 15 g Luba Seed, 15 g Wulingzhi, 5 g Evodia, 15 g Puhuang, 15 g Guizhi, 6 g Sanleng, 30 g white peony root, 10 g curcuma, 10 g salvia, 20 g chicken ginseng, 10 g angelica, 9 g turmeric, 15 g codonopsis, 10 g salvia, and 12 g chuanxiong. (1) In cases where the patient exhibits liver stagnation and qi stagnation, 6 g of Cyperus radix and 10 g of Bupleurum were added to the prescription; if the patients experienced severe pain, 10 g of Yuanhu and 20 g of Daxueteng were added to the prescription. (2) If the patient presents with anal bulge, 10 g of Qianghuo was included. (3) For patients with chills, 9 g of aconite and 10 g of dried ginger were added to the prescription. (4) For patients with qi deficiency and heavy menstrual flow, 20 g of yam, 30 g of astragalus, and 10 g of Zuozhimu were included. Each prescription was decocted with water to obtain a decoction of 250 ml. One warm dose per day was administered, both in the morning and evening. The decoction was continuously used during the menstrual period. All the patients were given three courses of treatment, with one course of treatment being three menstrual cycles.
Observation indicators
The changes of inflammatory factors (interleukin (IL)-8 and tumor necrosis factor-α (TNF-α)) before and after treatment were compared between the two groups. The kits were purchased from R&D Systems, with batch numbers of H20190019 and H20221811, respectively. Before and after treatment, 3 ml of fasting venous blood was drawn from patients in the morning, centrifuge at 3000 rpm to obtain serum, and measured using enzyme-linked immunosorbent assay.
The scores of non-menstrual pelvic pain and dyspareunia were compared between the two groups. The visual analog scale, encompassing a score range of 0-10 points, was employed for evaluation after treatment. The scale included five levels: no pain (0 point), mild pain (1-3 points), moderate pain (4-6 points), severe pain (7-9 points), extremely severe pain (10 points) [7].
The therapeutic effect was compared between the two groups. Markedly effective: the patient’s disease symptoms disappeared after treatment; effective: the disease symptoms were significantly improved after treatment; ineffective: the disease symptoms were not improved or aggravated after treatment. Response rate = cases of (markedly effective + effective)/ineffective cases * 100%. Higher response rate indicated a better therapeutic effect.
The scores of Traditional Chinese Medicine symptom, dysmenorrhea, menstrual volume, and dysmenorrhea symptom of the two groups were compared between before and after treatment. The dysmenorrhea score was evaluated by VAS [7], with a total score of 10 points: 0 representing no pain, and 10 representing extremely painful. This study used a self-made traditional Chinese medicine symptom scale in the hospital for effectiveness evaluation [8], which includes non-menstrual abdominal pain, normal lumbosacral pain, limb coldness, premenstrual breast pain, menstrual back pain, nerve fatigue, and menstrual pain. Bleeding, anal protrusion, pain during sexual intercourse, and decreased libido are 10 items, with a total score of 80 points. Each item has a score of 0-8 points, and the higher the score, the more severe the symptoms of the subject. The severity of menstrual flow symptoms was assessed by a self-made menstrual flow scale [9]. The higher the score, the more severe the condition. The severity of dysmenorrhea symptoms was assessed by the dysmenorrhea symptom score (COX score) [10], using a scale of 0-4, and the score was positively correlated with the severity.
The changes in uterine volume, insulin-like growth factor (IGF-1), and serum carbohydrate antigen 125 (CA125) levels were compared between before and after treatment in the two groups: 3 ml of fasting axial venous blood was drawn from patients before and after treatment for chemical analysis. CA125 was determined by luminescence method and automatic immunoassay [11], and IGF-1 was determined by enzyme-linked immunosorbent assay. The ELISA kits were Shanghai Haring Biotechnology Co., LTD., batch number: 180635.
Statistical methods
The measurement data, such as pain scores, changes in uterine volume, and changes in serum IGF-1 and CA125 levels, were represented by (x̅ ± s) and processed by t test. The χ2 test was used for comparison of counting data. A P<0.05 was considered statistically significant. The data visualization was conducted using GraphPadPrism8.
Results
Comparison of general data between the two groups
The general data, such as disease course, age, were comparable between the two groups (P>0.05) (Table 1).
Table 1.
Comparison of general data between the two groups
| Group | Cases | Course of disease | Age | Parity |
|---|---|---|---|---|
| Control group | 33 | 5.09 ± 1.64 | 37.95 ± 1.94 | 2.53 ± 0.59 |
| Study group | 32 | 5.24 ± 1.57 | 38.76 ± 2.01 | 2.67 ± 0.44 |
| t | / | 0.377 | 1.653 | 1.082 |
| P | / | 0.708 | 0.103 | 0.284 |
Comparison of inflammatory factor levels
After treatment, the levels of IL-8 and TNF-α inflammatory factors in the study group were lower than those in the control group, and the differences were significant (P<0.05) (Figure 1).
Figure 1.
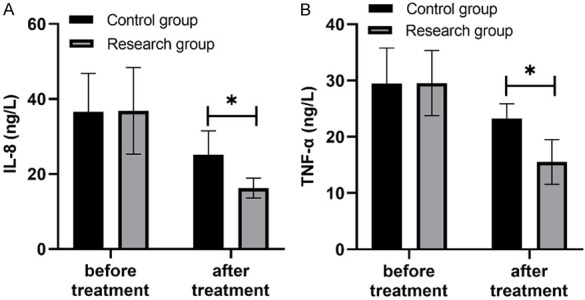
Comparison of inflammatory factor levels (x̅ ± s, ng/L). Note: There was no significant difference in the levels of IL-8 (A) and TNF-α (B) between the two groups before treatment, but the levels of IL-8 and TNF-α in the study group were lower than those in the control group after treatment. The symbol * stands for P<0.05.
Comparison of dyspareunia and non-menstrual pelvic pain scores
The scores of dyspareunia and non-menstrual pelvic pain in the study group were lower than those in the control group, and the differences were significant (P<0.05) (Table 2).
Table 2.
Comparison of dyspareunia and non-menstrual pelvic pain scores (points, x̅ ± s)
| Group | Cases | Dyspareunia | Non-menstrual pelvic pain |
|---|---|---|---|
| Control group | 33 | 4.29 ± 1.05 | 5.67 ± 1.22 |
| Study group | 32 | 1.03 ± 0.02 | 1.33 ± 0.22 |
| t | / | 17.390 | 19.809 |
| P | / | 0.000 | 0.000 |
Comparison of treatment efficacy
The total response rate of treatment in the study group (93.75%) was higher than that in the control group (66.66%) (P<0.05) (Table 3).
Table 3.
Comparison of treatment efficacy (cases, %)
| Group | Cases | Ineffective | Effective | Markedly effective | Total response rate |
|---|---|---|---|---|---|
| Control group | 33 | 9 (27.27) | 13 (39.39) | 11 (33.33) | 66.66% |
| Study group | 32 | 16 (50.00) | 14 (43.75) | 2 (6.25) | 93.75% |
| t | / | / | / | / | 8.140 |
| P | / | / | / | / | 0.004 |
Note: The treatment response rate of the study group was higher than that of the control group. *P<0.05 indicates that the difference between the groups was significant.
Comparison of Traditional Chinese Medicine symptom score, dysmenorrhea score, menstrual volume score, dysmenorrhea symptom score
After treatment, the Traditional Chinese Medicine symptom score, dysmenorrhea score, menstrual volume score, and dysmenorrhea symptom score in the study group were all lower than those in the control group, and the differences were significant (P<0.05) (Table 4; Figure 2).
Table 4.
Comparison of Traditional Chinese Medicine symptom score, dysmenorrhea score, menstrual volume score, and dysmenorrhea symptom score (points, x̅ ± s)
| Group | Before treatment | After treatment | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Study group | Control group | t | P | Study group | Control group | t | P | |
| Chinese Medicine Symptom score | 71.95 ± 5.21 | 70.21 ± 5.36 | 0.516 | 0.665 | 30.12 ± 2.06 | 52.33 ± 4.23 | 26.778 | 0.000 |
| Dysmenorrhea score | 7.72 ± 1.33 | 6.75 ± 1.32 | 0.321 | 0.816 | 3.06 ± 0.96 | 5.81 ± 1.06 | 12.856 | 0.000 |
| Menstrual flow score | 2.61 ± 0.69 | 2.56 ± 0.71 | 0.126 | 0.862 | 1.20 ± 0.44 | 2.26 ± 0.63 | 9.296 | 0.000 |
| Dysmenorrhea Symptom score | 3.38 ± 0.25 | 3.15 ± 0.32 | 0.069 | 0.991 | 1.06 ± 0.08 | 2.69 ± 0.22 | 39.451 | 0.000 |
Figure 2.
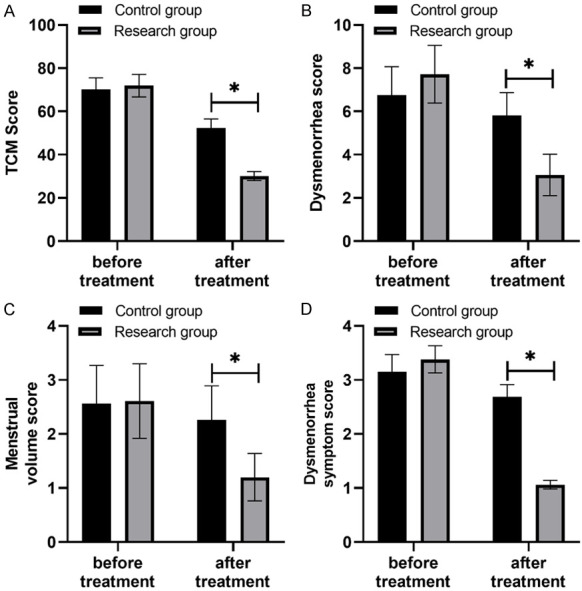
Comparison of Traditional Chinese Medicine symptom score, dysmenorrhea score, menstrual volume score, and dysmenorrhea symptom score. Note: After treatment, the Traditional Chinese Medicine symptom score, dysmenorrhea score, menstrual flow score and dysmenorrhea symptom score of the study group were lower than those of the control group, and the symbol * represents statistically significant difference between the two groups.
Comparison of uterine volume, IGF-1 and CA125 levels
The levels of IGF-1 and CA125 in the study group were lower than those in the control group after treatment, and there were statistical significances between the two groups (P<0.05). However, no significant difference in the uterine volume was found between the two groups after treatment (P>0.05). See Figure 3.
Figure 3.
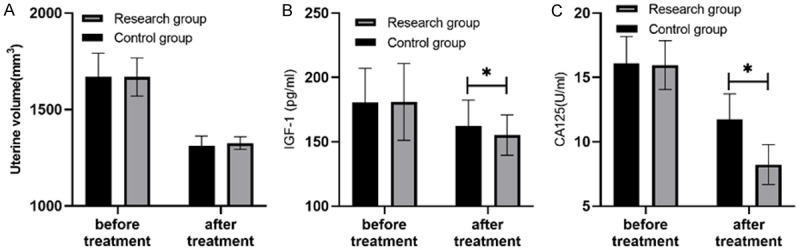
Comparison of uterine volume, IGF-1 and CA125 levels (x̅ ± s). Note: Before treatment, there was no significant difference in uterine volume, IGF-1 and CA125 levels between the two groups. After treatment, the levels of IGF-1 and CA125 in the study group were lower than those in the control group (P<0.05), and the symbol * indicates a significant difference between the two groups. IGF-1, insulin-like growth factor 1; CA125, carbohydrate antigen.
Discussion
Adenomyosis invades the myometrium but does not extend beyond the uterus. Adenomyosis has previously been called intrinsic endometriosis, while endometriosis at other sites has been called extrinsic endometriosis [12]. At present, estrogen levels and pathological changes are clinically considered to be the main causes of dysmenorrhea in patients with adenomyosis, seriously reducing the quality of life of patients [13]. The preferred treatment for such patients is conservative treatment with drugs. Although western medicines like Mirena and have good efficacy, there is a potential risk of adverse reactions and recurrence rate, so the application is limited [14].
In recent years, traditional Chinese medicine has been widely used in clinical practice, and it has certain advantages in the treatment of patients with adenomyosis and pain, because it can not only reduce the incidence of adverse reactions in patients, but also obtain long-term and stable efficacy and avoid disease recurrence [15]. Adenomyosis belongs to the category of “infertility”, “menstrual disease” and “dysmenorrhea” in traditional Chinese medicine. It is considered that blood stasis and weakness of kidney qi are the main factors leading to the occurrence of the disease, so the principles of supplementing qi, dispersing blood stasis and activating blood circulation should be followed in the treatment. Xiaoxiao Huoxue Decoction is mainly composed of Shengpu Huang, Tablet Curcuma longa, Sanleng, Curcuma zedoaria, Wulingzhi, Ji Nei Jin, Danshen, Chuanxiong, Paeonia lactiflora, Codonopsis pilosula, Angelica sinensis, Lubazi, Guizhi, and Evodia rutaecarpa. Among them, Shengpu Huang has the effects of detumescence, removing blood stasis, and stopping bleeding; Curcuma longa and Sanleng have the effects of relieving pain through meridians and facilitating blood circulation, and promoting qi; Curcuma zedoaria has the effects of eliminating accumulation and relieving pain, and facilitating blood circulation; Luba seed has the effect of promoting qi and relieving pain, warming yang, and tonifying kidney. Therefore, the synthesis of Shixiao Huoxue Decoction can achieve the effects of invigorating Qi and promoting yang energy [16].
Relevant studies have pointed out [17] that patients with adenomyosis have significant immunoregulatory dysfunction, which leads to ineffective removal of the myometrial endometrium, increased levels of inflammatory mediators and disease aggravation. Serum IL-8 stimulates neutrophil chemotaxis and stromal cell proliferation, aggravates immune responses, and induces the body to produce new blood vessels, while it accelerates ectopic endometrial implantation under the combined action of serum TNF-α and IL-8 [18,19]. Some scholars have found that TNF-α levels are higher in adenomyosis patients than in healthy women, and the reason may be due to increased monocyte activity in the peripheral blood [20]. Our results showed that the Traditional Chinese Medicine symptom score, dysmenorrhea score, menstrual volume score, and dysmenorrhea symptom score in the study group were lower than those in the control group, and the treatment response rate was higher in the study group than that in the control group (P<0.05), indicating that the Xiaoxiao Huoxue Decoction can reduce the degree of dysmenorrhea. Modern pharmacology has confirmed that Xiaoxiao Huoxue Decoction can also enhance microcirculatory status, increase hormone levels in the body while reducing blood viscosity, which helps to enhance microvascular perfusion in patients [21].
IGF-1, as a multifunctional factor similar to the structure of insulin, can regulate the differentiation and proliferation of body cells. Related studies have pointed out that IGF-1 is a factor that induces cell proliferation, and it is highly expressed in lesions with more active cell proliferation [22]. CA125 is a glycoprotein macromolecule surface antigen distributed in renal ducts and mesothelial cells. It is a kind of inflammatory marker and is highly expressed in gynecological inflammatory diseases or benign uterine tumors [23]. In recent years, related studies have found that the expression of CA125 is increased in adenomyosis, which has a certain value in the treatment and diagnosis of this disease [24]. The results also showed that after treatment, there was no significant differences in the uterine volume between the two groups (P>0.05), and the levels of IGF-1 and CA125 in the study group were lower than those in the control group (P<0.05), indicating the Shixiao Huoxue Decoction can effectively decrease the CA125 and IGF-1 levels in patients with adenomyosis pain. The reason is that the active ingredients in Shixiao Huoxue Decoction have a positive effect on the down-regulation of CA125 and IGF-1 levels, and can reduce the regulation of cell proliferation by these two factors [25]. Gu analyzed the efficacy of the combination of Guizhi Pachycaria decoction and mifepristone in the treatment of adenomyosis with dysmenorrhea. The results showed that the total response rate of the study group was 94.67%, higher than 82.67% in the control group (P<0.05). It is indicated that the treatment effect of the combination was significantly better than that of mifepristone alone.
Conclusion
Shixiao Huoxue Decoction has a good therapeutic effect in patients with adenomyosis, because it can effectively improve dysmenorrhea and Traditional Chinese Medicine symptoms, as well as reduce non-menstrual pelvic pain and dyspareunia, thus contributing to the early recovery of patients. Therefore, Shixiao Huoxue Decoction is worthy of popularization and application for the treatment of adenomyosis.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant number 82104921), and the Science and Technology Innovation Project of the Chinese Academy of Traditional Chinese Medicine (Grant number CI2021A02405).
Disclosure of conflict of interest
None.
References
- 1.Hassanin AI, Youssef AA, Yousef AM, Ali MK. Comparison of dienogest versus combined oral contraceptive pills in the treatment of women with adenomyosis: a randomized clinical trial. Int J Gynaecol Obstet. 2021;154:263–269. doi: 10.1002/ijgo.13600. [DOI] [PubMed] [Google Scholar]
- 2.Hou X, Xing J, Shan H, Mei J, Sun Y, Yan G, Sun H, Wang J. The effect of adenomyosis on IVF after long or ultra-long GnRH agonist treatment. Reprod Biomed Online. 2020;41:845–853. doi: 10.1016/j.rbmo.2020.07.027. [DOI] [PubMed] [Google Scholar]
- 3.Crha Karel, Ješeta Michal, Pilka Radovan, Ventruba Pavel, Žáková Jana, Vodička Jan, Crha Tomáš. Adenomyosis - its possible effect on endometrial function and receptivity. Ceska Gynekol. 2021;86:205–209. doi: 10.48095/cccg2021205. [DOI] [PubMed] [Google Scholar]
- 4.Kim MS, Jang JH, Park S, Ahn EH, Jung SH. Effect of adenomyosis on adverse obstetrical outcomes in twin pregnancies achieved with assisted reproductive technology. J Obstet Gynaecol. 2021;41:1225–1229. doi: 10.1080/01443615.2020.1867969. [DOI] [PubMed] [Google Scholar]
- 5.Zhang S, Wang K, Di A, Yu D, Yao T. Ultrasound-guided percutaneous microwave ablation of adenomyosis: a narrative review. Ann Palliat Med. 2021;10:12003–12011. doi: 10.21037/apm-21-3133. [DOI] [PubMed] [Google Scholar]
- 6.Han W, Liu Q, Liu Y. To investigate the clinical value of transvaginal three-dimensional color ultrasound in the diagnosis of uterine fibroids and uterine adenomyosis. Maternal and Child Health Care in China. 2011;26:2. [Google Scholar]
- 7.Squillace ALA, Simonian DS, Allegro MC, Borges E Júnior, Bianchi PHM, Bibancos M. Adenomyosis and in vitro fertilization impacts - a literature review. JBRA Assist Reprod. 2021;25:303–309. doi: 10.5935/1518-0557.20200104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang S, Shi LY, Duan JY, Liu HH, Wang TT, Li CY. Celecoxib reduces inflammation and angiogenesis in mice with adenomyosis. Am J Transl Res. 2021;13:2858–2866. [PMC free article] [PubMed] [Google Scholar]
- 9.Qin X, Sun W, Wang C, Li M, Zhao X, Li C, Zhang H. Mifepristone inhibited the expression of B7-H2, B7-H3, B7-H4 and PD-L2 in adenomyosis. Reprod Biol Endocrinol. 2021;19:114. doi: 10.1186/s12958-021-00800-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cozzolino M, Tartaglia S, Pellegrini L, Troiano G, Rizzo G, Petraglia F. The effect of uterine adenomyosis on IVF outcomes: a systematic review and meta-analysis. Reprod Sci. 2022;29:3177–3193. doi: 10.1007/s43032-021-00818-6. [DOI] [PubMed] [Google Scholar]
- 11.Capmas P, Brun JL, Legendre G, Koskas M, Merviel P, Fernandez H. Ulipristal acetate use in adenomyosis: a randomized controlled trial. J Gynecol Obstet Hum Reprod. 2021;50:101978. doi: 10.1016/j.jogoh.2020.101978. [DOI] [PubMed] [Google Scholar]
- 12.Tian J, Kang N, Wang J, Sun H, Yan G, Huang C, Mei J. Transcriptome analysis of eutopic endometrium in adenomyosis after GnRH agonist treatment. Reprod Biol Endocrinol. 2022;20:13. doi: 10.1186/s12958-021-00881-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calderon L, Netter A, Grob-Vaillant A, Mancini J, Siles P, Vidal V, Agostini A. Progression of adenomyosis magnetic resonance imaging features under ulipristal acetate for symptomatic fibroids. Reprod Biomed Online. 2021;42:661–668. doi: 10.1016/j.rbmo.2020.11.012. [DOI] [PubMed] [Google Scholar]
- 14.Che X, Wang J, He J, Guo X, Li T, Zhang X. The new application of mifepristone in the relief of adenomyosis-caused dysmenorrhea. Int J Med Sci. 2020;17:224–233. doi: 10.7150/ijms.39252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hou XS, Zhao JP, Wang N, Sun LY, Qu SH, Meng X, Wang GQ. Acupuncture for secondary dysmenorrhea of adenomyosis: a prospective case-series study. Zhongguo Zhen Jiu. 2020;40:834–8. doi: 10.13703/j.0255-2930.20190630-0002. [DOI] [PubMed] [Google Scholar]
- 16.Du L, Du DH, Chen B, Ding Y, Zhang T, Xiao W. Anti-inflammatory activity of Sanjie Zhentong capsule assessed by network pharmacology analysis of adenomyosis treatment. Drug Des Devel Ther. 2020;14:697–713. doi: 10.2147/DDDT.S228721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schrager S, Yogendran L, Marquez CM, Sadowski EA. Adenomyosis: diagnosis and management. Am Fam Physician. 2022;105:33–38. [PubMed] [Google Scholar]
- 18.Zhu L, Yang X, Cao B, Tang S, Tong J. The suture fixation of levonorgestrel-releasing intrauterine device using the hysteroscopic cold-knife surgery system: an original method in treatment of adenomyosis. Fertil Steril. 2021;116:1191–1193. doi: 10.1016/j.fertnstert.2021.05.113. [DOI] [PubMed] [Google Scholar]
- 19.Yang H, Wang S, Fu X, Lan R, Gong H. Effect of modified levonorgestrel-releasing intrauterine system in human adenomyosis with heavy menstrual bleeding. J Obstet Gynaecol Res. 2022;48:161–168. doi: 10.1111/jog.15031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ying P, Li H, Jiang Y, Yao Z, Lu S, Yang H, Zhu Y. Qiu’s Neiyi recipe regulates the inflammatory action of adenomyosis in mice via the MAPK signaling pathway. Evid Based Complement Alternat Med. 2021;2021:9791498. doi: 10.1155/2021/9791498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donnez J, Donnez O, Tourniaire J, Brethous M, Bestel E, Garner E, Charpentier S, Humberstone A, Loumaye E. Uterine adenomyosis treated by linzagolix, an oral gonadotropin-releasing hormone receptor antagonist: a pilot study with a new ‘Hit Hard First and then Maintain’ regimen of administration. J Clin Med. 2021;10:5794. doi: 10.3390/jcm10245794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou Y, Peng Y, Xia Q, Yan D, Zhang H, Zhang L, Chen Y, Zhao X, Li J. Decreased Indian hedgehog signaling activates autophagy in endometriosis and adenomyosis. Reproduction. 2021;161:99–109. doi: 10.1530/REP-20-0172. [DOI] [PubMed] [Google Scholar]
- 23.Clemenza S, Vannuccini S, Capezzuoli T, Meleca CI, Pampaloni F, Petraglia F. Is primary dysmenorrhea a precursor of future endometriosis development? Gynecol Endocrinol. 2021;37:287–293. doi: 10.1080/09513590.2021.1878134. [DOI] [PubMed] [Google Scholar]
- 24.Zhang W, Han B, Ma C, Qiao J. Effect of GnRH-a pretreatment before frozen-thawed embryo transfer on pregnancy outcome of adenomyosis-associated infertile patients with 56 cm3 ≤ uterine volume ≤100 cm3 . Ann Transl Med. 2022;10:509. doi: 10.21037/atm-21-6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu B, Chen Y, Guo M, Zhang C, Huang L, Pan Q, Lin T, Lu Y, Shen X, Zhang H. Berberine attenuates hyperalgesia in mice with adenomyosis. Arch Gynecol Obstet. 2022;306:115–125. doi: 10.1007/s00404-022-06438-y. [DOI] [PubMed] [Google Scholar]


