Abstract
Poly(I:C) and R848, synthetic ligands that activate Toll-like receptor 3 (TLR3) and TLR7/8 respectively, have been well-established for their ability to stimulate the immune system and induce antigen-specific immune responses. These ligands are capable of inducing the production of cytokines and chemokines, and hence support the activation and differentiation of B and T cells. We saw the long-lasting and perdurable immune responses by these adjuvants essentially required for an efficacious subunit vaccine. In this study, we investigated the potential of poly(I:C) and R848 to elicit B and T cell responses to the OVA antigen. We assessed the stimulatory effects of these ligands on the immune system, their impact on B and T cell activation, and their ability to enhanced generation of B and T cells. Collectively, our findings contribute to the understanding how poly(I:C) and R848 can be utilized as an adjuvant system to enhance immune responses to protein-based subunit vaccines. In the end, this work provides insights for the development of novel vaccination strategies and improving the vaccine efficacy. Present work shall help formulate newer strategies for subunit vaccines to address the infectious diseases.
Keywords: Poly(I:C), R848, TLR3, Innate immune system, B cells, T cells, Adjuvants, Protein vaccines
1. Introduction
Maintaining immune effector balance is crucial for defending against pathogens. CD8 T cells play a pivotal role in recognizing and eliminating infected cells [1]. Robust and perdurable CD8 T cell responses are essential for effective immunity. Various methods, including vaccination, adoptive transfer, and in vitro expansion, contribute to CD8 T cell generation [2], [3]. Vaccination is the most common approach, where antigens are administered in the body to stimulate the immune system. DNA vaccines and viral vectors are potent strategies [4], but DNA vaccines may induce weaker responses due to suboptimal antigen processing and presentation [5]. Viral vectors efficiently deliver antigens but face limitations from pre-existing immunity, which may reduce their effectiveness, and raises concerns about their safety [4]. Ongoing research focuses on optimizing DNA vaccines and viral vectors for enhanced CD8 T cell responses, addressing limitations and exploring therapeutic potential [6], [7]. However, challenges include the need for adjuvants and specific antigen presentation by antigen-presenting cells (APCs).
The exploration of protein antigens Ag(s) as an alternative source for generating CD8 T cells has gained significant attention and offers a safer approach than traditional methods involving live attenuated or inactivated pathogens [8]. However, eliciting CD8 T cell responses solely against protein antigens is generally less efficient compared to using whole pathogens or viral vectors, resulting in insufficient CD8 T cell activation [9]. The inefficiency is attributed to the necessity for additional signaling molecules to effectively activate CD8 T cells. Ongoing efforts focus on optimizing protein antigen-based approaches for enhanced CD8 T cell generation [9]. Innovative vaccine delivery systems, such as virus-like particles (VLPs) [10] or nanoparticles [11], [12] have been employed to achieve robust CD8 T cell activation, aiming to improve the efficacy of protein Ag-based subunit vaccines.
There is a growing interest in investigating synthetic immunostimulatory agents, or adjuvants, with a few currently approved for human vaccine formulations. Novel adjuvant formulations are crucial for generating augmented immune responses, particularly cellular immune responses [13], [14]. While many subunit vaccines are administered with a single adjuvant, combining adjuvants with different modes of action can increase immunogenicity [15]. Several licensed adjuvants possess immunostimulatory activity [16] and preclinical investigations support various adjuvant combinations, such as the TLR9 agonist CpG (cytidine phosphate guanosine) in combination with aluminium hydroxide [17], alum, DOATP (1,2-dioleoyl-3-trimethylammonium propane), and D35 [18], MPL (monophosphoryl lipid A) and poly(I:C) (polyriboinosinic polyribocytidylic acid) [19]. Poly(I:C) and R848 exhibit immunostimulatory properties, mimicking pathogen-associated molecular patterns (PAMPs) and activating the immune system through pattern recognition receptors (PRRs) [20], [21], [22]. Acting as a TLR3 agonist, poly(I:C) activates B cells, upregulating surface receptors, and inducing cytokine secretion and cellular proliferation. It stimulates the production of key cytokines, including IL-12, IFN-γ (Th1 cytokine) and IL-4 (Th2 cytokine) [23]. This adjuvant system promotes the DC maturation and B cell activation, resulting in robust CD4+ T cell and humoral immune responses [24]. This adjuvant formulation enhances the functionality and abundance of CD8 T cells, promoting the production of IFN-γ crucially required to clear infectious pathogens [25]. Conversely, the synthetic small molecule R848 (resiquimod), a selective agonist for TLR7 and TLR8, induces IFN-α, IL-12, and TNF-α in various immune cells, showing its potential in immunomodulation [26], [27], [28].
Administration of a recombinant subunit vaccine in two or three doses induces a significant IgG and neutralizing antibody response, with negligible differences observed between dosing regimens [29]. Investigations involving a third booster dose demonstrate enhanced immunogenicity and protective efficacy in both human and non-human primate subjects [30], [31], [32]. Studies reveal B cells' involvement in activating CD8+ T cells through distinct mechanisms [33], [34]. Multiple immunizations are speculated to mimic repeated exposure to the protein Ag, mediating efficient antigen presentation. This enhances CD8 T cell activation and expansion, promoting the development of long-lived memory CD8 T cells. Thus, we hypothesize that repeated immunization with protein Ag(s) generating humoral responses may expand memory B cells, improving BCR affinity. Further, high titer antibodies may form immune complexes (IC) with circulating or incoming Ag, captured by antigen-presenting cells through Fc receptors and presented to CD8+ T cells.
Present study investigates the impact of synthetic agent's poly(I:C) and R848 on diverse B and T cell populations in response to the model protein antigen ovalbumin (OVA). We evaluate the ability of these synthetic TLR agonists to elicit antigen-specific immune responses by the ex-vivo and in vivo assays. Repeated immunization with OVA, combined with adjuvants, induces OVA-specific CD8+ T cells in mouse circulation. Ex-vivo stimulation of OVA and OT-I & OT-II peptides similarly stimulate CD8+ T cells in the spleen and LNs. Additionally, the utilized adjuvants elicit B-cell-mediated immune responses, evidenced by OVA-specific antibody production, memory cell development in the spleen, and plasma/antibody-secreting cells (ASCs) in the bone marrow (BM). Our findings contribute to understanding the immunomodulatory potential of poly(I:C) and R848, facilitating the activation of the immune system using OVA antigen. Overall, these results provide insights for designing and developing subunit vaccines to induce specific immune response against protein Ag.
2. Materials and methods
C57BL/6 mice (6-8 weeks old) were procured from Zydus Research Centre (Ahmedabad, India) against the Institutional Animal Ethics Committee (IAEC) approved protocol No.: IS/PHD/30/2022/46 (CPCSEA Reg. No.: 883/PO/ReBi/S/05/CPCSEA). Mice were kept at the Central Animal House Facility of Nirma University, Ahmedabad. They were allowed to acclimatize in the new environment for a week, before the experimental study begins. Appropriate amount of food and water was provided simultaneously proper nesting; veterinary oversight and other required things were taken care of. All the experimental protocols were revised and approved by the IAEC of Nirma University and the experiments were performed at the facility authorized by IAEC. The Handling of mice was done as per the Institutional guidelines.
2.1. Reagents and antibodies
All the reagents used for the experimental work are cell culture tested. Bovine serum albumin (BSA) fraction-V, cell culture tested (Himedia: TC194-100G), goat anti-mouse IgG (H+L) Secondary Ab), HRP (In-vitrogen 62-6520), goat anti-mouse IgG1 Secondary Ab, HRP (In-vitrogen PA1-74421), goat anti-mouse IgG2c, Fc gamma specific Ab, HRP (CST 56970), 3,3′,5,5′-Tetramethylbenzidine (TMB) Liquid Substrate System for ELISA (Sigma T0440-100ML), carbonate-bicarbonate buffer (Sigma C3041-50CAP), eBioscienceTM 10X RBC lysis buffer (Multi-species) (Invitrogen 00-4300-54), OVA-Albumin from chicken egg white, lyophilized powder ≥98% electrophoresis (Sigma A5503-5G), 10X PBS (Himedia ML023-500ML), 10X PBS (Endotoxin Free) (Himedia ML164-100ML), RPMI-1640 (Himedia AL199A-500ML), antibiotic antimycotic solution 100x liquid (Himedia A002-20ML), L-Glutamine 200mM solution (Himedia TCL012-20ML), HEPES-1M Solution Cell culture tested (Himedia TCL021), phosphate buffer saline with 0.05% tween 20, pH 7.4 (Sigma P3563-10PAK), ELISA Plate (Immunoplate Strip Single Well-Genetix-38296 or 96 well, high binding, detachable (HiMedia-EP2-5X10NO)), Poly I:C (LMW-25mg) (InVivogen-tlrl-picw), R848 (Sigma SMLL0196-10MG), BD Cytofix/CytopermTM Plus Fixaion/Permeabilization solution kit with BD GolgiPlugTM (BD 555028). Cell strainer (70 μm nylon; Corning-431751), OVA peptides: OVA257-264 (vac-sin) (H-2Kb restricted OVA MHC class I epitope) & OVA323-339 (vac-isq) (I-Ad restricted OVA MHC class II epitope) (InvivoGen). The antibodies used for flow-cytometry experiments were procured from BD Bioscience/Biolegend/ThermoFisher Scientific (all are anti-mouse). BV510 conjugated anti-CD4 (clone:rm4-5;563106), Alexa-700 conjugated anti-CD8 (clone:53-6.7;557959), APC conjugated anti-IFN-γ (clone:XMG1.2;554413), PE-Cy7 conjugated anti-CD19 (clone:1D3;561739), APC conjugated anti-CD80 (clone:16-10A1;560016), PE conjugated anti-CD273 (clone:TY25;557796), BV510 conjugated anti-IgM (clone:II/41;743324), PE conjugated anti-CD138 (clone:281-2;561070), PE/Percp-Cy 5.5 conjugated anti-CD3(clone:500A2;553240)/(clone:17A2;560527), (BD Biosciences). FITC conjugated anti-CD73 (clone:TY/11.8;Biolegend-127270), Alexa FluorTM 700 conjugated anti-IgG (H+L) (ThermoFisher Scientific-A21036).
2.2. Immunization, blood collection and serum separation
OVA Ag alone or in combination with adjuvants (poly(I:C) and R848) were administered biweekly in C57BL/6 mice subcutaneously (base of the tail) for three times. Blood was collected from retro-orbital plexus of mice before (pre-immune, 3 days prior) and after each antigen/protein booster immunization (preferably two-days prior to booster dose) (Scheme 1). Blood samples were kept undisturbed and incubated at room temperature (RT) (37 ºC) for 3-4 h for the coagulation process to occur. Later, for the separation of serum, samples were centrifuged at 3,000 rpm for 20 min at 4 ºC. The resulting supernatant (upper layer), designated as serum, was collected in fresh eppendorf and stored in -80 ºC until use.
Scheme 1.
The OVA immunization strategy in C57BL/6 mice. Mice were immunized with different combination of OVA antigen (Ag), and endotoxin free PBS used as an experimental control. 2 days prior to the booster dose (on day 12), blood samples were collected. Following the 3 doses, mice were euthanized (on day 14) and Ag specific CD8+ T cell responses and B cell response were determined (n=3).
2.3. OVA-specific IgG and IgG subclass ELISA
96 well immuno-plates were coated with OVA (2 μg/well) prepared in coating buffer, cover the plate with an adhesive plastic/aluminum foil, and kept at 4 °C for overnight. Unbound antigens were removed and plates were blocked with 2% BSA in PBS (200 μl/well) for 2 h. After 2 h, plates were incubated further with serum samples (diluted in a range of 1:500 to 1:16000) (100 μl/well) at RT for 2 h. Then, plates were washed thrice with wash buffer (phosphate buffer saline with 0.05% tween 20, pH 7.4) (200 μl/well). After washing, antibody, anti-mouse IgG (H+L) HRP-conjugated secondary Ab was diluted 1:2000 in 0.2% BSA and added to the each well (100 μl/well) and incubated for another 1 h at RT. After incubation, the plates were again washed thrice with wash buffer (200 μl/well) and developed with 100 μl TMB substrate solution. The enzymatic reaction was stopped with the addition of 100 μl of 1 M HCl stop solution to each well. The optical density (OD) was measured at 450 nm wavelength (620 nm as a reference wavelength) using microplate reader (Microplate Spectrophotometer-EPOCH-SN). The serum samples were run in duplicates. Experiments were performed minimum two times. The similar method was employed to study the effect of adjuvants against the OVA Ag on the subclass of IgG by using IgG1 (diluted 1: 4000) and IgG2c (1:1000).
2.4. Measurement of antibody avidity index (AI)
The next step was to measure the AI of the same serum samples tested for OVA-specific IgG and subclasses response. Thus, the AI of anti-OVA antibody was measured as described by Fialova et al. [35] and Jelinkova et al. [36] using an ELISA based chaotrope assay. In brief, it follows the standard ELISA protocol as described above. The only difference seen was that following serum incubation, half of the wells were treated with 6M urea for 10 min and remaining half were treated with 1X-PBS as the control. Serum dilutions corresponding to comparable OD value for each group was chosen as control. Additionally, we have also compared the AI between each dose of immunization/group and, we took three different dilutions for each dose. The AI was calculated as the ration of mean OD value of urea treated wells (A6M urea) to control well treated with PBS (Ac); AI=A6M urea/Ac. Multiple dilution of serum were used and all samples were run in duplicate.
2.5. Spleen and lymph node (LN) single cell suspension preparation
On the 14th day following the third dose of immunization, the animals were euthanized through cervical dislocation, after which the spleen, LN, and bone marrow (BM) were harvested. Single cell suspensions of lymphocytes were prepared by mincing spleen cells between the ground ends of two frosted microscopic slides and LN cells by passing it through 70 μm mesh strainers and supplemented with RPMI complete media (10% FCS and 1% antibiotic solutions, 1% L-Glutamine and 1% HEPES buffer). The cells were further pelleted down by spinning at 500 x g for 5 min at 10 ºC and aspirate the supernatant. Spleen samples were further treated with 1X RBC lysis buffer, according to the manufacturer's instructions (eBioscience-Thermo Fisher Scientific). In brief, resuspend the pellet (of spleen cells) in 3 ml of prepared 1X RBC lysis buffer followed by incubation at RT for 4-5 mins. Immediately after incubation, centrifuge the samples at same speed. Decant the supernatant, resuspend the pellet in RPMI complete media and counted using 0.4% trypan blue under the microscope and plated accordingly (1-1.5X106 cells/well). The LN cells were directly resuspended in RPMI complete media after centrifugation, and plated as mentioned for spleen cells.
2.6. Bone marrow single cell suspension
Isolation of immune cell from mouse bone marrow (BM) has been done as per the method described by Liu and Quan et al., [37]. In brief, the mouse (for higher yield of BM cells) was euthanized as per the recommended method and then dissected to take out the femur and tibia bone from hind leg. Muscles and excess tissues were removed from the surrounding of bones with sterile scissors and forceps. Then both the ends of the bones were cut with sharp tools and with the help of 23-gauge needle and a 10cc syringe (1 ml syringe) filled with frozen HBSS (Hank's Balanced Salt Solution) to clear out the BM cells from inside onto a 70 μm nylon cell strainer placed on 50 ml falcon tube. Use up to 10 ml of HBSS for the same or do it until the whole flow through turns white in color. BM cells were smashed out through the filter with the help of plunger of a syringe and washed off with another ~5 ml of HBSS. Later the cells were centrifuged at 1,500 rpm for 7 min at 4 ºC and supernatant was discarded. Then the pellet was resuspended in 1 ml RBC lysis buffer (individually for each mouse) & incubated at 37 ºC for 2 to 5 min at RT. Again, tubes were centrifuged at 1,500 rpm for 7 min at 4 ºC, supernatant was discarded and the pellet was resuspended in RPMI complete media. Further, the cells were counted with the help of hemocytometer for the viability and plated as mentioned earlier for spleen and LN cells. 1-1.5X106 single cell suspensions of spleen and BM organs were seeded in 96-well “V”-bottom plates and stimulated with OVA Ag (10 μg/ml) for 24 h. The unstimulated controls were filled with cell culture medium only (Scheme 2).
Scheme 2.
Ex-vivo stimulation/pulsing of single cell suspension prepared from the spleen and BM with OVA-Ag to study the generation of Ag-specific B cells. 14 days following the third immunization, the spleen and BM were collected aseptically, processed, and single cell suspension was prepared. 1-1.5X106 cells were seeded, and incubated with OVA-Ag for 24 h. The only media was added to the wells served as the unstimulated controls. Post 24 h of incubation, the supernatants were collected and indirect ELISA was performed to detect OVA-specific IgG. The stimulated cells were further stained with B cell markers and characterized using the flow cytometry.
2.7. Multi-parametric flow cytometric analysis
2.7.1. Circulating IFN-γ in peripheral blood of OVA-immunized mice
To study the circulating IFN-γ in OVA-immunized mice, anticoagulant tubes were used to collect blood on 10–12 days post third immunization, processed (lysed using manufacturer's protocol for 1XRBC lysis buffer), and tested for IFN-γ producing cytotoxic T lymphocytes (CTLs) by stimulating with CD8+ T cell–specific OT-I peptide of OVA Ag. Detailed protocol was mentioned in the later section.
2.7.2. Cell surface and intracellular staining (ICS)
1-1.5X106 cells were suspended in FACs buffer. PBS supplemented with 1–2% fetal calf serum (FACs) for cell surface staining and intracellular cytokine staining (ICS). Three to Seven-color staining of spleen and LN cells were performed by using mono-clonal antibodies. The following mouse monoclonal antibodies were used in different combinations as per the requirement of an experiment. BV510 conjugated anti-CD4 (clone:rm4-5;563106), Alexa-700 conjugated anti-CD8 (clone:53-6.7;557959), APC conjugated anti-IFN-γ (clone: XMG1.2;554413), PE-Cy7 conjugated anti-CD19 (clone:1D3;561739), APC conjugated anti-CD80 (clone:16-10A1;560016), PE conjugated anti-CD273 (clone:TY25;557796), BV510 conjugated anti-IgM (clone:II/41;743324), PE conjugated anti-CD138 (clone:281-2;561070), PE/Percp-Cy 5.5 conjugated anti-CD3 (clone:500A2;553240)/(clone:17A2;560527), (BD Biosciences). FITC conjugated anti-CD73 (clone:TY/11.8;Biolegend-127270), Alexa FluorTM 700 conjugated anti-IgG (H+L) (ThermoFisher Scientific-A21036). For cell surface and ICS, 1-1.5×106 cells were resuspended in FACS buffer and incubated for 25 to 30 min at 4 °C with monoclonal antibodies for cell surface markers. Cells were fixed and permeabilized with intracellular (IC) fixation buffer and washed with perm wash buffers. Cells were further incubated with anti-IFN-γ monoclonal antibody for 45 min to 1 h at RT for ICS. Simultaneously, a control sample was kept in which cells were incubated without anti-IFN-γ monoclonal antibody (FMO control). After washing with perm wash buffer and FACs buffer, cells were finally resuspended in 1% paraformaldehyde for cell acquisition by flow cytometer.
For the analysis of the antigen-specific response to the OVA protein (10 μg/ml) and OVA peptides (OT-I & OT-II) (10 μg/ml each), cells were incubated in complete medium for a total of 10 to 12 h and 8 to 10 h, respectively at 37 °C. Brefeldin A (BFA) (BD Bioscience) was added 2 h after initial OVA protein stimulation and incubated further whereas for OVA peptides it was added at the time of peptide stimulation. Cells were acquired using AttenuX flow cytometer (ThermoFisher Scientific) (Scheme 3).
Scheme 3.
Ex-vivo stimulation of single cell suspension prepared from spleen and lymph node (LN) of the OVA immunized mice. Following the three immunizations of B6 mice with OVA-Ag, spleen and lymph node were collected, processed, and single cell suspensions were prepared. V bottom 96-well plates were seeded with 1-1.5X106 cells followed by the stimulation with OVA-Ag (10 μg/ml) (for 10 to 12 h, Brefeldin A (BFA) was added after 2 h of initial incubation for spleen cells), and OT-I and OT-II peptides (10 μg/ml each) (for 8 to 10 h in presence of BFA for LN cells) and culture media only for unstimulated controls. The stimulated cells were stained with cell surface markers (CD4, CD8), fixed, permeabilized, and further stained and incubated for 45 mins-1 h with anti-mouse IFN-γ antibody. The samples were stored in paraformaldehyde and cells (event count=1,00,000/sample) were acquired using flow-cytometry machine.
Statistical analysis
Data are shown as mean ± SD, and statistical analysis was carried out by One-way analysis of variance (ANOVA) with Tukey multiple comparison post-test for endpoint titer analysis and, TWO-way analysis of variance (ANOVA) with Tukey–Kramer multiple comparison post-test for all other experimental results using the GraphPadInStat™ 8 software (GraphPad Software Inc., San Diego, California). ****P<0.0001, ***P=0.0002, **P=0.021, *P=0.0332, ns=0.1234.
3. Results
3.1. Assessment of OVA specific antibody response in the immunized mice
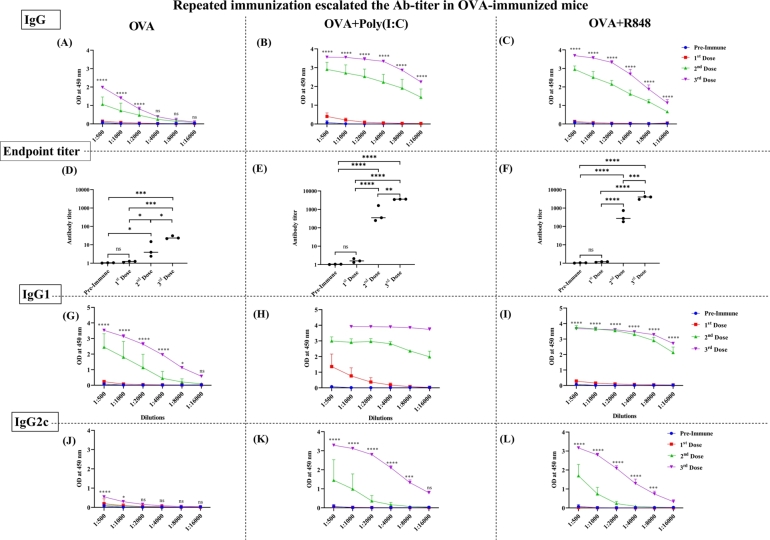
Humoral immune response to the OVA antigen was determined by measuring the antibody IgG and its subclass IgG1 and IgG2c via indirect ELISA. We saw the higher antibody titer in the sera of mice immunized repeatedly (Fig. 1). Mice immunized with OVA and adjuvants (poly(I:C) (OP) or R848 (OR) showed the elevated OVA-specific antibody titer as compared to mice immunized with OVA alone following the booster immunization (Fig. 1B, C). The OVA-only group failed to exhibit a robust Ab titer, even after the booster dose (Fig. 1A). The OP group exhibited a robust OVA-specific IgG response post-priming, which was comparable to the OR group following two booster dose immunization (Fig. 1B & C). Our data suggested that both adjuvants and repeated immunization is required to enhance production of OVA-specific IgG. Additionally, we observed a significant increase in the endpoint titer of OVA-specific IgG in the OP (Fig. 1E) and OR (Fig. 1F) groups, in contract to the OVA-only group (Fig. 1D), which failed to reach the baseline of pre-immune sera. Regarding the IgG1 response, the OP group exhibited a significantly higher titer upon priming (Fig. 1H) when compared to the OVA-only (Fig. 1G) & OR (Fig. 1I) groups showing the sub-sup-optimally lower response. Boosting led to a further increase in the OVA-specific IgG1 response in all groups, reaching a plateau in the OP and OR group (Fig. 1H & I). Analysis of the OVA-specific IgG2c revealed that OVA-only group failed to generate an IgG2c response (Fig. 1J), whereas the OP and OR groups (Fig. 1K & L) showed a significant increase in IgG2c levels after two booster doses. These results suggest that both adjuvants may establish balance between Th1 and Th2-type immune response against the OVA-Ag. Overall, our data highlight the adjuvant-mediated induction of OVA-specific IgG responses following repeated immunization.
Figure 1.
The antibody response following the repeated immunization with OVA when injected with adjuvants. OVA specific IgG response was measured by the indirect ELISA after each immunization (n=3 mice/group) in, (A) immunization with OVA (B) OP (Ovalbumin protein and poly(I:C) (C) OR (Ovalbumin protein and R848). The endpoint titer was measured in (D) OVA, (E) OP and, (F) OR group. Similarly, OVA specific IgG1 and, IgG2c response was measured in (G & J) OVA, (H & K) OP & (I & L) OR group of immunization, respectively. The bar represents mean SD for each dose administered in each group at different dilutions. The P values were determined by comparing means using two-way analysis of variance (ANOVA) test followed by Tukey's multiple comparison test for IgG, IgG1 and IgG2c (between 1st dose and 3rd dose). ****P<0.0001, ***P=0.0002, **P=0.021, *P=0.0332, ns=0.1234. For endpoint titer, p values were determined by comparing means using one-way ANOVA test followed by Tukey's multiple comparison test.
Subsequently, we sought to assess the avidity of IgG, the increased antibody avidity, shown by the avidity index (AI), is indicative of a successful vaccination. AI has been a crucial metric for the long-lasting presence of antibodies in circulation after repeated immunizations. It plays a pivotal role in immune complex (IC) formation, subsequently activating cellular immune responses.
Our findings demonstrated a lower AI with the OVA-only group (SI Fig. 1A and D) compared to the OP (SI Fig. 1B and E) and OR groups (SI Fig. 1C and F). Remarkably, the OR group displayed the highest AI, followed by the OVA and OP. Assessing the individual affinities within each group, the OP group exhibited the highest affinity, succeeded by the OR and OVA-only groups. These findings imply that adjuvants are capable of augment Ab-affinity in the protein-based immunization.
B cells originate in the bone marrow (BM) and undergo maturation in the spleen and lymph nodes (LN). The presence of memory B cells and IgG-producing B cells in the spleen and BM is crucial for the host immunity [38]. Upon re-infection with the same antigen, a recall response is triggered, leading to a robust immune response and subsequent resolution of the infection. To assess the cellular changes following repeated OVA immunization, we measured serum levels of total IgG. Additionally, we decided to investigate the impact of repeated Ag exposure with adjuvants on various B cell subsets, including the memory and plasma B cells. Therefore, ex-vivo stimulation experiments were conducted 14 days post third immunization (Scheme 2); cells were immunophenotyped for the expression of markers by the flow-cytometry
We selected specific memory markers (CD80, PD-L2 [CD273], and CD73), which are indicative of B cells, in addition to the pan-B cell marker CD19. The surface expression of IgG, associated with memory phenotype of B cells, was also investigated (SI Fig 2). Analysis of subsets of memory B cells (MBCs) revealed no significant difference among the experimental groups. However, a minor increase was observed in the single-positive CD80+MBC (SI Fig 3A) within the OR group (SI Fig 3). We hypothesized that CD80-CD273- double negative (DN) population, characterized by the lowest Ag affinity, may contribute to the formation of germinal centers (GCs) following Ag re-exposure or subsequent/booster immunization, predominately expressing IgM+. The expression of IgM+ within the DN population of MBCs was assessed, and a slight increase in IgM+ production from DN MBCs in the OVA and OP groups was observed which did not reach the statistical significance (SI Fig 3D). Based on our findings, we believe that poly(I:C) may have a role in the development of the GC.
The IgG secretion was individually measured for each group to assess the influence of adjuvants on MBCs. IgG is known for its pivotal role in combating a wide range of infectious and inflammatory diseases (SI Fig 4).
The IgG expression on DP (CD80+CD273+) MBCs was observed across all experimental groups. Notably, the OVA and OR groups demonstrated a higher IgG expression compared to other groups. Although the OP-stimulated group exhibited an increased IgG expression, it did not reach the statistical significance (SI Fig 4A). These results suggest that direct exposure to the protein Ag may influence the generation of DP MBCs expressing surface IgG. The quantification of IgG levels through ELISA in the supernatant of ex-vivo stimulated (OVA Ag) spleen cells revealed that the OR group exhibited the highest levels of IgG, followed by OP-immunized group. It is pertinent to note that a substantially higher quantity of IgG may have been secreted into the supernatant upon ex-vivo stimulation (SI Fig 4A). Recent investigations have shown that DP MBCs undergo further differentiate into triple positive (TP) MBCs, characterized by the expression of CD73 (CD80+CD273+CD73+) [39]. Interestingly, TP MBCs producing IgG were found to be increased in the OR and OP groups, with a modest change observed in the OVA group (SI Fig 4B). The % of TP cells in the unstimulated fraction was higher in the OR group compared to the other experimental groups, suggesting the induction of the MBCs. Additionally, upon repeated immunization with OVA, we observed the production of OVA-specific IgG and the expansion of corresponding memory B cells. Furthermore, the higher secretion of IgG in both adjuvant groups indicates that R848 may promote the generation of MBC, in contrast to the poly(I:C).
In the subsequent analysis, the OR group, followed by the OP and OVA immunized groups, exhibited the highest levels of OVA-specific IgG production after stimulation with OVA in the spleen of OVA-immunized mice (SI Fig 5A). The OR and OP groups demonstrated significantly elevated level of OVA-specific IgG production in response to OVA stimulation, implying their active involvement in B cell induction and IgG secretion processes (SI Fig 5A) compared to the control group. Conversely, the OR and OP groups showed comparable IgG production, followed by the OVA-only immunized group upon OVA stimulation in BM (SI Fig 5B).
We speculate that poly(I:C) might not directly induce IgG secretion but could potentially activate OVA-specific B cells upon ex-vivo stimulation with OVA. Plasma cells secrete antibodies across all the experimental groups in the BM. Notably, unstimulated cells from the OR group were observed to secrete antibodies in absence of specific stimulation, indicating that BMB cells in the OR group actively secrete OVA-specific IgG. These observations imply that both spleen and BM cells have the capacity to produce OVA-specific IgG when co-administered with the adjuvants. Furthermore, R848 may play a crucial role, as unstimulated bone marrow B (BMB) cells demonstrate the capability to secrete IgG.
We systematically investigated the influence of adjuvants on the functional aspects of BMB cells, specifically focusing on their role as a source of antibody-secreting cells (ASCs) and the maintenance of functional memory B cells (MBCs) (Scheme 2). Employing a combination of the Pan-B cell marker (CD19), CD138, and IgG markers facilitated the comprehensive characterization of BMB cells (SI Fig. 6). We identified CD19+CD138+B cells as plasma cells, known for their propensity to secrete IgG. Existing studies with human samples suggest that mature plasma cells/ASCs gradually lose CD19 expression while retaining long-term memory and displaying a pro-survival cell phenotype [40]. Accordingly, we classified cells into three distinct CD19 populations (CD19+, CD19low, and CD19-) (SI Fig. 6 and Fig. 2). The quantity of CD19+CD138+ cells (plasma cells) in the BM was significantly elevated in the OR group, followed by the OP and OVA upon ex vivo OVA stimulation (Fig. 2A). Notably, IgG expression was discerned only in the OR group, even in the unstimulated cell fraction of plasma cells (Fig. 2B), indicating the potential involvement of R848 in the production of IgG-containing plasma cells. The diminished CD19 expression during plasma cells/ASC maturation is indicative of the terminal stage of activation. Furthermore, the analysis of CD138 and IgG expression revealed two distinct populations (CD19low, CD19-), with CD19- cells exclusively exhibiting CD138 and IgG expression in the OR group (Fig. 2C). The CD19low population exhibited a similar trend to CD19 expression, with significant CD138 expression observed solely in the OR group (Fig. 2D). Other groups displayed minimal CD138 expression on the ligand-stimulated cells. While the OR and OP groups exhibited comparable IgG expression on CD19low population, statistical significance was however not reached (Fig. 2E). It is probably due to the unknown mechanism taken place during immune response activation.
Figure 2.
CD19 expressing plasma B cells and IgG expression on BM cells following the OVA-immunization and ex-vivo stimulation of BM with OVA antigen. Plasma B cells and IgG expression on different CD19 B cells of CD19+ cells A) CD19+CD138+(B) CD138+IgG+, on CD19- cells (C) CD138+IgG+, and on CD19low cells (D) CD19+CD138+ (E) CD138+IgG+ was determined 14 days following the last immunization in the single cell suspension of BM stimulated with OVA-Ag (10 μg/ml) for 24 h (n=3 mice/group). The bar represents mean SD for each dose administered. The P values were determined by comparing means using two-way analysis of variance (ANOVA) test followed by Tukey's multiple comparison test. ****P<0.0001, ***P=0.0002, **P=0.021, *P=0.0332, ns=0.1234.
Our results propose that R848 may exert a marked influence on the differentiation of BMB cells expressing CD19, promoting IgG expression post OVA-immunization. Furthermore, poly(I:C) displayed the capacity to activate the BM plasma cells, fostering the transition of early plasma cells into late/long-living plasma cells. This observation implies that plasma cells rely on poly(I:C) for longevity and their efficacy in combating infections. Also, our results indicate that the potential role of R848 in sustaining the functionality of spleen and BMB cells. Moreover, the combination of both adjuvants appears to synergistically enhance the humoral immune response. We assessed the supernatant following the OVA-Ag stimulation of spleen and BM cells for the production of OVA-specific IgG using indirect ELISA (SI Fig. 5).
3.2. Cell mediated immune (CMI) responses in the OVA-immunized mice
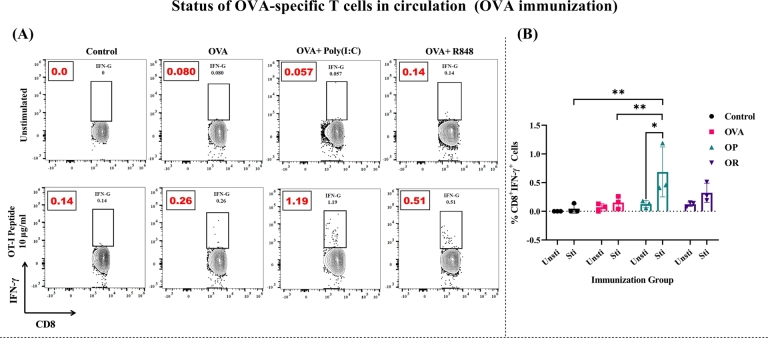
We examined the presence of circulating Ag-specific CD8+ T cells in response to the OVA Ag. Immunization with OVA antigen, in conjunction with adjuvants, facilitated the recognition and reactivation of OVA antigen-specific T cells. These T cells played a crucial role in effectively clearing recurring infection. The assessment of IFN-γ production was conducted through stimulation with the CD8+ T cell specific OT-I peptide of the OVA Ag (SI Fig. 7 & Fig. 3).
Figure 3.
IFN-γproducing CD8+T cell in the circulation following OT-I peptide stimulation (10 μg/ml) in OVA immunized mice with adjuvant: (A) Peripheral blood of OVA immunized mice (10-12 days post last immunization) was collected, processed and stimulated with OVA-Ag specific OT-I peptide (for CD8 T cells) for 6 h in presence of BFA. Intracellular staining (ICS) was performed and the fraction of IFN-γ+CD8+ T cells was determined by flow cytometry (AttenuX -ThermoFisher Scientific) and compared to control group (event count=1,00,000). One representative of each group was presented in the form of contour plots. Dots accounts for events falling outside of the lowest contour and drawn with dots (N=3 mice/group) (B) The analysis of IFN-γ producing CD8+ T cells in circulation. The bar represents mean SD for each dose administered 10-12 days following the last immunization. The P values were determined by comparing means using two-way analysis of variance (ANOVA) test followed by Tukey's multiple comparison test. ****P<0.0001, ***P=0.0002, **P=0.021, *P=0.0332, ns=0.1234.
Our results revealed a significant increase in the percentage of IFN-γ secreting CD8+ T cells in animals immunized with OP compared to other groups (Fig. 3). The group immunized with OR also demonstrated the generation of IFN-γ producing CD8+ T cells, albeit to a lesser magnitude than in the OP group (Fig. 3). These findings suggest that OVA immunization may enhance the production of sizeable IFN-γ secreting CD8+ T cells, with poly(I:C) playing a role in IFN-γ secretion, followed by the R848. In contrast, the OVA-only group did not secret IFN-γ.
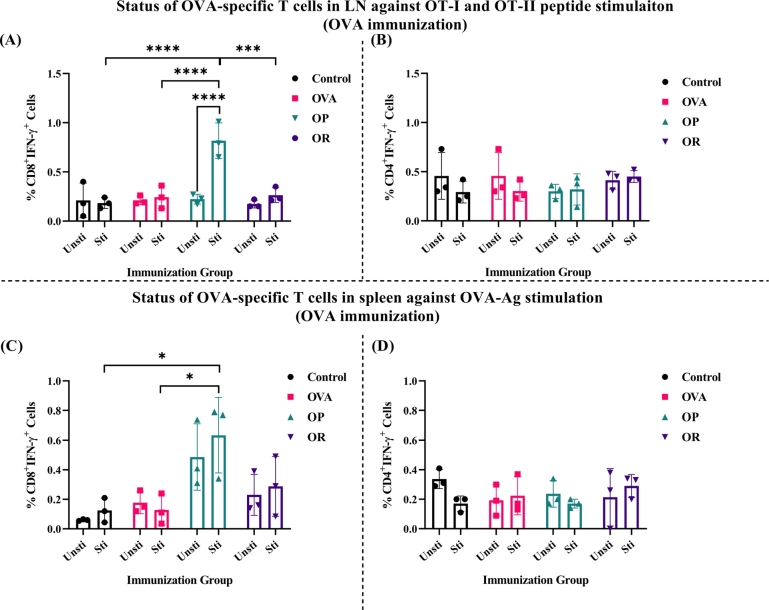
Following the assessment of circulating Ag-specific CD8+ T cells, we investigated whether secondary lymphoid immune organs (SLOs), including spleen and LN, also manifested the Ag-specific immune response. Mice were euthanized 14 days post third immunization, and single cell suspensions of isolated immune organs were ex-vivo stimulated with OVA-Ag and OVA specific peptides (OT-I and OT-II for CD8+ T and CD4+ T cells, respectively) (Scheme 3).
Ex-vivo stimulation with OVA-specific peptides led to the generation of OVA-specific IFN-γ producing CD8+ T cells in the LN of OP group, demonstrating high significance. However, no significant differences were observed in the other tested groups (Fig. 4A and SI Fig. 8A). As expected, the OR group showed a minor change, while the control group did not exhibit cell stimulation. Our results indicate that OVA Ag immunization induces an OVA-specific CD8+ T cell response, with poly(I:C) directly involved. We also examined the activation of IFN-γ+CD4+T cells response to OVA-Ag (Fig. 4B and SI Fig. 8B), but no remarkable activation of OVA-specific CD4+T cells was observed.
Figure 4.
The analysis of IFN-γ producing CD8+ and CD4+ T cells in lymph nodes (LN) (A, B) and spleen (C, D) cells of OVA immunized mice following the OT-I & II peptide and OVA protein stimulation, respectively (n=3 mice/group). The bar indicates SD for each dose administered. The P values were determined by comparing means using two-way analysis of variance (ANOVA) test followed by Tukey's multiple comparison test. ****P<0.0001, ***P=0.0002, **P=0.021, *P=0.0332, ns=0.1234.
In spleen cell suspensions from OVA-immunized mice, stimulation with OVA Ag resulted in the generation of IFN-γ producing CD8+ T cells in both OP and OR groups (Fig. 4C and SI Fig. 9). The OP group showed a substantial increase in IFN-γ+CD8+ T cells, whereas the OR group demonstrated lesser production of IFN-γ, which was not significantly different (Fig. 4C). However, both groups exhibited higher numbers of IFN-γ+CD8+ T cells compared to the OVA-only and control groups, which did not show the generation of IFN-γ+CD8+T cells. No significant difference in the trend of IFN-γ generating CD4+ T cells, compared to the peptide stimulation, was observed (Fig. 4D). Our data suggest that R848 and poly(I:C) may generate CTL responses, and the synergistic effect of both adjuvants could enhance the antigen specific immune response (Fig. 4).
The ex-vivo stimulation experiments provided evidence supporting the capability of the OVA Ag to elicit a specific immune response after three rounds of immunizations. Furthermore, our investigations substantiated that repeated immunization with the protein Ag proficiently triggered a CMI response both in the systemic circulation and within the SLOs.
3.3. Combination of adjuvants [poly(I:C) and R848] augment immune response in OVA-immunized mice
We next decided to determine the synergistic effect of both adjuvants on generation B & T cell responses after OVA Ag immunization. 3 mice/group were immunized with a combination of poly(I:C) & R848 (referred to as OPR), and we analyzed both the humoral and CMI responses. The dosage of both adjuvants was adjusted to 25 μg/mouse of each, and the immunization conditions were same as mentioned earlier (Scheme 1). We assessed the status of B and T cells in circulation and measured circulating Ab responses after each and every immunization.
To assess the OVA-specific IgG and its subclasses (IgG1 and IgG2c), an indirect ELISA was conducted exclusively on the sera samples from the OPR group. The OVA-specific IgG response (Fig. 5A) exhibited a significant increase after the 2nd dose of immunization, displaying a trend similar to that observed with individual adjuvant immunization after the 3rd dose (Fig. 1). This consistent pattern was seen in the response of IgG1 & IgG2c subclasses (Fig. 5B & C). The analysis of the endpoint titer further confirmed the higher response following the 2nd dose of immunization (Fig. 5D). Throughout the immunization process, the avidity index remains unchanged (Fig. 5E & F); however, the AI value estimated was comparatively higher than that observed in the individual adjuvant groups (SI Fig. 1). In summary, our combined adjuvant strategy effectively primed and induced the OVA-specific IgG, IgG1, and IgG2c responses, culminating in enhanced Ab titers compared to the individual adjuvant groups (OP and OR). These findings underscore the potential of the combined adjuvant approach in promoting the IgG secretion.
Figure 5.
Antibody titer and antibody avidity index after the repeated immunization with OVA protein supplied with the combination of adjuvants. OVA specific (A) IgG, (B) IgG1, (C) IgG2c, (D) endpoint titer and, (E & F) antibody avidity index (AI) was measured by the indirect ELISA after each immunization (n=3 mice/group) in immunization with OPR (Ovalbumin protein+ poly(I:C)+R848). The bar represents mean SD for each dose administered in each group at different dilutions. The P values were determined by comparing means using two-way analysis of variance (ANOVA) test followed by Tukey's multiple comparison test for IgG, IgG1 and IgG2c (between 1st dose and 3rd dose). ****P<0.0001, ***P=0.0002, **P=0.021, *P=0.0332, ns=0.1234. For endpoint titer, p values were determined by comparing means using one-way ANOVA test followed by Tukey's multiple comparison test.
In alignment with our prior study of individual adjuvants, we conducted a comprehensive assessment of various MBCs in the context of combined (both)-adjuvant approach. We observed no alternation in the populations of IgM producing MBCs or the DN MBCs (SI Fig. 10) during immunization with the combination of adjuvants. However, a discernible increase in the MBCs was observed when both adjuvants were employed in combination, in contract to their individual use (SI Fig. 3). No significant differences in the IgM-producing cells were observed among the experimental groups (here, OPR, OT-I, and OT-II) (SI Fig. 10D). Notably, the combined adjuvant approach yielded higher IgM levels compared to immunization with individual adjuvants (SI Fig. 3D). Our inference suggests that the combination of adjuvants holds the potential to influence IgM producing MBCs or DN MBCs, thereby contributing to formation of GC responses during recurrent infection or antigen immunization.
Furthermore, the investigation into these MBCs for IgG expression unveiled that the OPR group exhibited higher IgG production compared to the OT-I and OT-II groups (SI Fig. 11). The elevated IgG expression was notably observed in CD80+CD273+DP MBCs of the OPR group compared to other groups (SI Fig. 11A). TP MBCs exhibited increased IgG expression in all groups, with a slightly higher expression in the OPR group compared to OT-I and OT-II peptide-immunized mice (SI Fig. 11B). The heightened levels of IgG were observed in SP MBCs expressing CD80+ and CD273+. Importantly, such increased expression of IgG was not observed within individual adjuvant immunization (SI Fig. 11C and D). Additionally, the immunization of mice with CD8+ and CD4+T cell specific peptides generated the TP MBCs that expressed IgG. Both OT-I and OT-II groups demonstrated greater IgG secretion compared to the OPR group (SI Fig. 13). The prevalence of IgG expressing MBCs outnumbered their counterparts in the study conducted with individual adjuvant immunization (SI Fig. 3). This suggests that combination of adjuvant fosters the generation of IgG antibody-secreting MBCs.
Analyzing the plasma/ASCs within the BMB cell pool after stimulation with the OVA Ag (Scheme 2), followed by immunization with the combination of adjuvants, we observed a transition from CD19+ to CD19-. CD138 expression was observed in all immunized groups, with OT-II and OPR suggesting enhanced expression across all CD19 populations (SI Fig. 12). All groups, except the control, exhibited higher IgG expression. CD138 expression in the early stage of CD19 transition was slightly higher in the OT-II group but was not statistically significant (SI Fig. 12A). The frequency of CD138 expression was higher in the OPR group compared to groups individually stimulated with poly(I:C) & R848 (Fig. 2A). Both the OPR and OT-II groups exhibited a higher propensity for IgG expression in activated plasma (SI Fig. 12B). Functionally mature ASCs lacking CD19 expression indicated that OPR showed the most substantial difference in IgG expression (SI Fig. 12C). The combination approach using both adjuvants played a crucial role in ASCs development, as the experimental groups (OPR, OT-I, and OT-II) exhibited IgG expression. CD138 expression on the CD19low population was driven by OT-II and OPR, mirroring the pattern observed in CD19+ cells (SI Fig. 12D). The distinct pattern of individual IgG expression further highlights the marked difference of the OPR group compared to the other groups (SI Fig. 12E). Our hypothesis is that the co-administration of poly(I:C) and R848 could enhance the generation of plasma cells or ASCs undergoing terminal differentiation, thereby promoting the production of IgG Abs.
The ex-vivo stimulation of spleen and BM cells from mice immunized with OVA (OPR and other groups), and the collected supernatant were then analyzed for OVA-specific IgG levels using indirect ELISA. We observed significant production of IgG in the stimulated cells of the experimental groups, particularly in OT-II group (SI Fig. 13). Notably, the detection of IgG in the supernatant from the spleen and BM of OT-II mice was significant. IgG expression was also observed to be higher in other groups, such as OT-I and OPR. Interestingly, the OPR group exhibited Ab expression similar to the OR group in the individual adjuvant immunization study in BM (SI Fig. 5B), but seen lesser in the spleen (SI Fig. 5A).
Our data suggested that the combination of adjuvants has an impact on the humoral immune response in the spleen and BM (SI Fig. 10 to 13). And, it seems to stimulate the signal to generate the MBCs, IgG antibodies, and terminally differentiated ASCs. The combined used of these adjuvants could produce the robust expression of IgG as compared to the immunization (against the OVA Ag) with adjuvant individually (in case of OVA, OP, and OR) (Fig. 2, and SI Fig. 3 to 5).
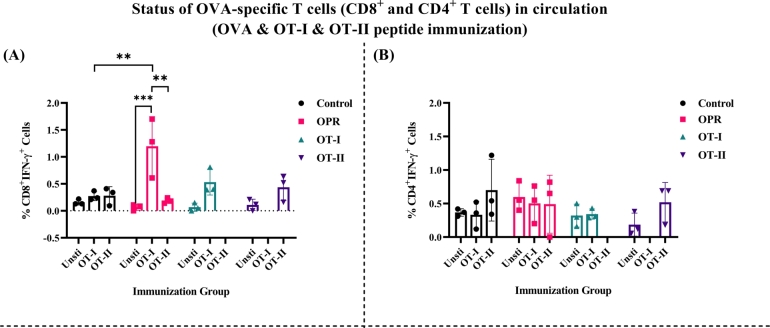
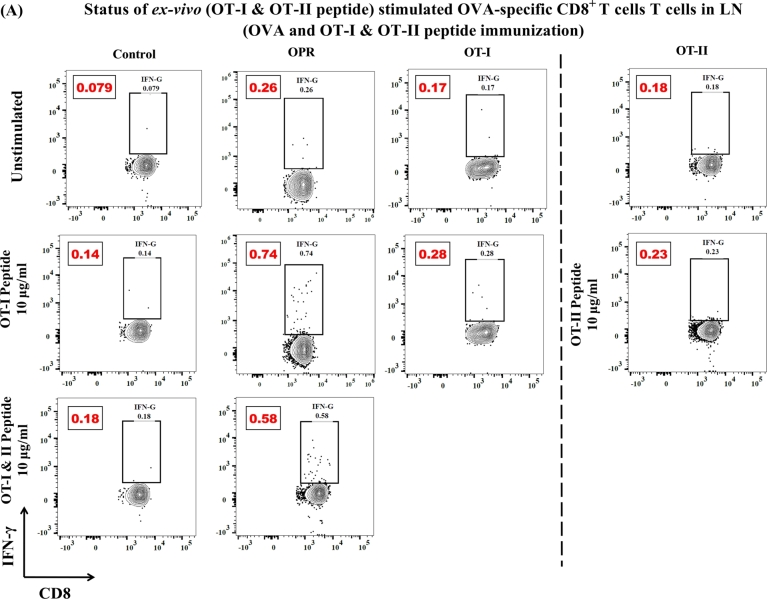
Here, we investigated the immune response of mice immunized with OPR and stimulated with peptides OT-I and OT-II, and found that upon stimulation with OT-I peptide, IFN-γ+CD8+ T cells in the peripheral blood was observed, whereas OT-II peptide failed to activate (SI Fig. 14A). Compared to the control and OT-I groups, a significant increase in IFN-γ producing CD8+ T cells was observed in the OPR group (Fig. 6A and SI Fig. 14A). Additionally, mice immunized with OT-II peptide showed a phenotypically similar IFN-γ+CD8+ T cells to those in the OT-I immunized group (Fig. 6A). However, there was minimal difference in IFN-γ+CD8+ T cells between mice receiving OT-I immunization and those further stimulated with OT-I peptide ex vivo (SI Fig. 14A and Fig. 6A). The percentage of IFN-γ+CD4+T cells showed minimal variation among all experimental groups (Fig. 6B and SI Fig. 14B), with the control group showing some response, possibly due to environmental exposure.
Figure 6.
The generation of IFN-γproducing CTLs following the OVA and OT-I and OT-II peptide immunization: The circulating IFN-γ producing CTLs specific to OVA-Ag was measured through ICS by stimulating with OVA specific OT-I & OT-II peptide. OVA-specific IFN-γ producing A) CD8+ T cells, B) CD4+ T cells were seen in the periphery. The peripheral cells of OT-I and OT-II group were stimulated with their respective peptide (n=3 mice/group). The bar represents mean SD in each dose of the group) 10-12 days post last immunization. The P values were determined by comparing means using two-way analysis of variance (ANOVA) test followed by Tukey's multiple comparison test. ****P<0.0001, ***P=0.0002, **P=0.021, *P=0.0332, ns=0.1234.
Moreover, we observed a substantial elevation in the OPR group, revealing significantly heightened levels of circulating IFN-γ producing CD8+ T cells specific to the OT-I peptide. Both OT-I and OT-II immunized groups showed activation against their respective peptides (Fig. 6A). The peptide-specific response of CD8+ T cells was higher in the OPR group when compared to individual adjuvant immunization (Fig. 3). Consistent outcomes were observed with IFN-γ producing CD4+ T cells (Fig. 6B). Thus, our results demonstrate that the combined adjuvant approach effectively activates both IFN-γ producing CD8+ and CD4+ T cells.
Having substantiated the heightened presence of circulating B and T cells, along with their Ag-specificity, our subsequent investigation focused on dealing the OVA-specific immune response within the SLOs. This aspect is pivotal for mounting an effective defense against potential reinfection/challenge. Mice were euthanized 14-day post third immunization, and ex-vivo stimulation with OVA and its peptides (OT-I and OT-II) was performed to assess the Ag-specific T cell responses (Scheme 3) (Fig. 7, SI Fig. 15 to 17).
Figure 7.
A: IFN-γproducing CD8+T cell in the LN of OVA and OT-I & OT-II peptide immunized mice following OVA-peptide (OT-I alone and OT-I & OT-II combined) stimulation: Single cell suspension of LN cells (14 days post last immunization) was prepared as mentioned earlier, and seeded with 1-1.5X106 cells/well in 96-well ‘V’ bottom plate. Cells were stimulated with OVA-Ag specific OT-I peptide (10 μg/ml) alone and peptide combination of OT-I & OT-II peptide (10 μg/ml each) for 8 to 10 h in presence of BFA. Intracellular staining (ICS) was performed and the fraction of IFN-γ+CD8+T cells was determined by flow cytometry (AttenuX -ThermoFisher Scientific) and compared to control group (event count=1,00,000). One representative of each group was presented in the form of contour plots. Dots accounts for events falling outside of the lowest contour and drawn with dots (N=3 mice/group). B: IFN-γproducing CD8+T cell in the spleen following OVA-peptide (OT-I alone and OT-I & OT-II combined) stimulation (10 μg/ml) in OVA and OT-I & OT-II peptide immunized mice: Spleen cells of OVA & OT-I and OT-II peptide immunized mice (after 3rd dose) were stimulated with OT-I peptide (10 μg/ml) alone and peptide combination of OT-I & OT-II peptide (10 μg/ml each) for 8 to 10 h in presence of BFA and intracellular staining (ICS) was performed to check the proliferation of IFN-γ with compared to control group (event=1,00,000/sample).
Our results revealed that the OPR group exhibited a significantly higher IFN-γ+CD8+T cells compared to other groups, with OT-I (only) peptide stimulation alone inducing the observed immune response (Fig. 7A and 8A).
Figure 8.
The analysis of IFN-γproducing T cells (CD8+& CD4+) in LN (A, B) spleen (C, D) cells of OVA immunized mice following OT-I & II peptide and OVA protein stimulation (n=3 mice/group): The bar indicates data in the form of mean SD in each dose administered in all groups. The P values were determined by comparing means using two-way analysis of variance (ANOVA) test followed by Tukey's multiple comparison test. ****P<0.0001, ***P=0.0002, **P=0.021, *P=0.0332, ns=0.1234.
Interestingly, in a combinational approach, the IFN-γ+CD8+T cell response within the OPR group remained comparable to that observed in the groups subjected to individual adjuvant immunizations (Fig. 4A). Stimulation with OVA-Ag failed to generate any IFN-γ producing CD8+ T cells in most groups (10 μg/ml; stimulation for a total of 12 h, BFA treatment for last 10 h) (Fig. 8A and SI Fig. 15), except for a slight change observed in the OT-II group. However, upon stimulation of LN cells with OVA, an OVA-specific response targeting to CD8+ T cells was evident, particularly in the OT-II peptide immunization group (Fig. 6A). Mice immunized with the OT-I peptide also generated non-significant CTLs responses. As anticipated, the OPR group consistently generated IFN-γ+CD8+T cells, signifying the activation of CD8 T cells by the protein Ag. This activation process involves the processing and presentation of the Ag, and triggering the induction of the CTL responses. Furthermore, we scrutinized the CTL response in splenocytes upon stimulation with OVA-Ag and OVA-specific peptides (Fig. 7B, 8C, and 8D; SI Fig. 16 & 17).
It demonstrated efficient activation of IFN-γ secreting CD8+ T cells in the OPR group (Fig. 8C and SI Fig. 16), mirroring the outcome of individual adjuvant immunization (Fig. 4C). The OT-I and OT-II peptide immunized groups exhibited Ag-specific T cell responses with negligible variations. Additionally, we assessed the impact of OVA-specific peptides (OT-I and OT-II), and stimulation with OT-I peptide alone induced a noteworthy Ag-specific CD8+ T cell response in the OPR group (Fig. 8C). A comparable CTL response was evident in the OT-I group, where mice were immunized with the OT-I peptide (Fig. 8C).
Moreover, a similar response to OT-I peptide stimulation was observed in the OPR group when both OT-I & OT-II peptides were used in the study. However, OT-II peptide alone failed to activate the CMI in the same OPR group (Fig. 7B and 8C). Likewise, when OT-II peptide immunized mice were stimulated with OT-II peptide, producing Ag-specific T cell that suggested the specificity of OT-II peptide to the CD4+ T cells.
Furthermore, we assessed IFN-γ producing CD4+ T cells (Fig. 8B & D, SI Fig. 17) and found minimal differences in LN between OVA- and OVA-peptide immunized mice (Fig. 8B). Splenocytes from the OPR, OT-I, and OT-II groups demonstrated the generation of IFN-γ producing CD4+ T cells compared to the unstimulated controls. However, this was not observed in the individual adjuvant approach (Fig. 4). Additionally, mice immunized with individual OT-I and OT-II peptides produced IFN-γ+CD4+ T cells (Fig. 8D and SI Fig. 17). The OPR group demonstrated the ability to generate Ag-specific CD4+ T cell responses regardless of the presence of OVA-Ag or its peptides alone or in combination. In summary, our study illustrated that the combination of poly(I:C) and R848 activates both CD4+ and CD8+ T cells, thereby eliciting cell-mediate immunity in the OVA Ag stimulated cells.
4. Discussion
B and T cells play pivotal roles in the immune response against infectious and inflammatory conditions. Cytotoxic T lymphocytes (CTLs) are crucial for eradicating intracellular infections like viruses or parasites and mobilize immune effectors in recurring infections. While cell-mediated immune (CMI) responses are essential for pathogen protection, vaccine trials have traditionally focused on eliciting neutralizing antibodies through B cell responses [19]. Overcoming challenges in vaccine development, the discovery of synthetic ‘adjuvants’ has emerged, resulting in the licensure of various adjuvants for vaccine formulations [13], [14].
Combining TLR7/8 agonists with TLR4 has shown improved immune responses, including heightened IL-12 production in in vitro models [41] and enhanced memory CD8 T-cell responses against a peptide cancer antigen [42]. The combination of TLR7/8 and TLR9 agonists increased antibody titters against the protein immunogen of HIV-1 envelope gp140 in nonhuman primates [43]. Alum, DOTAP (1,2-dioleoyl-3-trimethylammonium propane), and D35, when used in combination, induce a potent T cell and antibody responses against a protein Ag [18]. MPL and poly(I:C) demonstrated the induction of CMI by generating Ag-specific T cell responses, enhancing APC stimulation, and improving antibody production [19].
These findings highlight the potential synergistic interactions among different TLR agonists to enhance immune responses, emphasizing the need for careful selection of adjuvants that can activate both humoral and cell-mediated immune responses against intracellular viral pathogens. Poly(I:C) adjuvanted vaccines exhibited promising results in the preclinical and clinical trials, showing the enhanced antibody and improved cellular immune responses [44]. R848, known to activate B cells, promotes their growth, differentiation, and antibody production [45]. It induces the production of IL-6, IL-10, and IL-12 through the interaction between B and T cells. Additionally, R848 stimulates B-cell division and differentiation in mouse splenic cultures and purified human B cells, with a significant impact on various B cell subsets [45]. Through APCs (DCs and macrophages), R848 indirectly stimulates T cells, enhancing antigen-specific responses [46]. Clinical trials are underway to evaluate R848-adjuvanted vaccines, demonstrating efficacy in preclinical models [47]. Considering various vaccine types, subunit, recombinant, and protein vaccines are generally safe but may require adjuvants, multiple doses, or booster shots for long-lasting immunity [48], [49].
The combination of poly(I:C) and R848 has the potential to exhibit synergistic effects, particularly enhancing both HMI and CMI when applied to a protein-Ag based subunit vaccine. This study highlights notable findings related to the development of typical B cells, ASCs, CD138+ plasma cells, and the generation of antigen-specific IFN-γ+ producing CD8+ T cells. The OVA protein is employed as a model antigen in this study due to its ability to engage various immune mechanisms [50], [51], [52], [53].
The generation of antigen-specific antibody (Ab) responses is pivotal for effective vaccine development. Our investigation into OVA-specific IgG across various adjuvant settings, including individual adjuvants (OVA, OP, OR) and the combination of OP and OR (OPR), revealed that all adjuvant settings induced OVA-specific IgG (Fig. 1), with the combination showing higher Ab titer (Fig. 5). Moreover, it also suggested that repeated immunizations successfully generated OVA-specific IgG titres in the adjuvant-based group, required for a successful vaccine [19], [54]. As other's [55], [56], [57], our data on Ab-avidity indicated that the combined adjuvants drive the higher Ab titers and AI, indicating an augmented immune response. A subset of GC B cells can differentiate into MBCs or long-lived plasma cells (LLPCs) when their affinities for the Ag is sufficiently high [58]. MBCs can be identified by the expression of CD80, PD-L2 (CD273), and CD73, rather than their BCR isotypes [39], [59], [60]. To investigate the effect on cells in the spleen and BM, known as B cell reservoirs, we performed ex vivo OVA-Ag stimulation post 14-day of third immunization. No significant changes were observed in any of the MBC subsets in the spleen (SI Fig. 3, and 10). While the MBCs remain unchanged, the frequency of all MBCs was higher in the OPR group, potentially due to the synergistic action of both adjuvants used (SI Fig. 10). CD80-CD273- DN MBCs, which predominantly express IgM and have a low affinity for the Ag, are thought to play a role in the formation of GC during reinfection or subsequent (booster) immunization [39], [61]. Therefore, we investigated the status of IgM-expressing DN MBCs and observed a similar pattern to that of other MBCs (SI Fig. 3D and 10D). A slight increase was observed in the OVA-only and OP groups, while a larger frequency was seen in the combination (OPR) settings. This is of importance as poly(I:C) may play a role in the establishment of GC. Repeated immunization, particularly with the OPR settings, resulted in the generation of IgM-expressing DN MBCs, which have been shown to confer protection against malaria infection when expressing granzyme B [62]. Furthermore, these IgM-expressing MBCs are capable of producing the class-switched antibodies upon re-challenge with Plasmodium berghei infection [63], and generating merozoite-specific IgM, targeting the asexual blood stage infection of P. falciparum [64]. Considering the importance of IgG in clearing infectious pathogens, it was imperative to assess its expression on MBCs. Our data indicated that the combination of adjuvants (OPR) increased IgG expression on all types of MBCs (SI Fig. 11), including SP MBCs (CD80+IgG+ & CD273+IgG+), compared to individual adjuvants (OP and OR) (SI Fig. 3). These results support previous studies suggesting that DP and TP MBCs differentiate into plasma cells expressing IgG [39], [60], [61]. Our data confirmed that ex-vivo stimulation of cells from mice receiving the combination of adjuvants (OPR) immunization resulted in the development of IgG expressing MBCs.
LLPCs are pivotal in sustaining enduring long-lasting antibody levels, crucial for host defense against infectious pathogens. LLPCs continuously secrete antibodies, while MBCs necessitate reactivation for optimal antibody production. LLPCs, with a lifespan spanning months to decades in the BM [40], [65] were evaluated via ex-vivo stimulation of cells collected from immunized animals. Recent human studies propose that stable and pro-survival mature phenotypes of LLPCs lack CD19 expression (CD19-) compared to the CD19+, potentially elucidating the long-term memory retention of memory plasma cells [66], [67]. Hence, we assessed LLPCs/ASCs expressing CD138 and IgG across distinct CD19 populations (CD19+, CD19low and CD19-) [68]. CD138 and IgG expression affirmed the influence of R848 in individual adjuvant studies; with the OR group demonstrating higher expression than other groups (Fig. 2). Furthermore, the combination of adjuvants (OPR) exhibited significant differences in IgG expression in fully developed ASCs and the CD19low cells (SI Fig. 12). CD138 expression was observed across various CD19 populations in peptide-immunized mice, suggesting the potential importance of T and B cell interaction in LLPCs development. Specific TLRs may play specialised roles in CD40 signalling or with BCR, which are necessary for the expression of MBC markers such as CD73 and CD80 [61], [69]. Combinations of TLR3, TLR4, or TLR9 promote activation and proliferation, whereas TLR4 & TLR7 favour ASCs generation [70]. These findings suggest that IgM+ and IgG+ MBCs persist as quiescent cells indefinitely [71], and naïve B cells with high-avidity Ag receptors can produce persistent IgM+ and transient IgG+ MBC [72]. This supports the generation of MBCs, their IgG expression, and the formation of LLPCs/ASCs in the spleen and BM of immunized mice.
To assess IgG secretion, we analysed the supernatant of ex-vivo stimulated cells from immunized mice. Our data revealed that both individual adjuvants (SI Fig. 5) and their combination (SI Fig. 13) facilitated IgG secretion. Moreover, peptide-immunized mice demonstrated the capacity for IgG secretion, indicating the potential importance of peptide immunization in Ag-specific B cell development. Similar to others [73], we propose that the immune-complex (IC) may stimulate and differentiate naïve B cells into antibody-producing plasma cells.
CD8+ T cells are reported to defend the host from the attack emanated from a variety of diseases in rodents, primates, and humans [74], [75], [76]. Thus, we first investigated the presence of circulating INF-γ producing CD8+T cells in mouse periphery. Our result revealed the pivotal role of poly(I:C) in generating INF-γ+CD8+T cells in the OP group (Fig. 3). Additionally, the combination of adjuvants (OPR) exhibited significant T cell expression when stimulation with OT-I peptide (Fig. 6A and SI Fig. 14A). Consistent to other's findings [19], [25], [77], our data suggests that poly(I:C) may induce CD8+ T cells. Surprisingly, OT-II peptide, non-specific to CD8 T cells, elicited the production of INF-γ+CD8+T cells in OT-II immunized mice. Analysis of INF-γ+CD4+T cells showed insignificant differences in LN between OVA- and OVA-peptide immunized mice (Fig. 6B and SI Fig. 14B). Our data indicated the presence of OVA-specific IFN-γ+CD4+ T in the circulation of OVA immunized mice. However, the OT-I peptide did not directly stimulate CD4+ T cells due to its specificity for CD8+ T cells. Ex-vivo stimulation of LN and spleen cells effectively generated Ag-specific INF-γ producing CD8+ T cells in response to OT-I & OT-II peptides (Fig. 4A, SI Fig. 8A) and OVA stimulation (Fig. 4C, SI Fig. 9), respectively, in the OP group. Stimulation with both peptides (OPR group) showed a response akin to that seen with the OT-I peptide, while stimulation with OVA protein failed to induce CD8+T cells in the LN (Fig. 7A, 18A, and SI Fig. 15). The CD8+T cells specific to OVA-Ag in the spleen exhibited elevated level of IFN-γ production in the OPR group compared to other groups (Fig. 7B, 18C, and SI Fig. 14). The reported protective role of IFN-γ against malaria [78], [79] and viral infections [80] as well as cancer [76] underscores its significance. The presence of memory T cells, similar to the B cells, is crucial for the durable and robust immune response [81]. It is believed that some CD8 T cells may have memory like phenotypes among IFN-γ+ CD8+ T cells and IFN-γ may be their source [81], [82].
Our study demonstrates that the combination of the adjuvants is more effective than the individual adjuvants (Fig. 4B and D, SI Fig. 8B) in activating the Ag-specific CD4+ T cells in the spleen (Fig. 8D, SI Fig. 17). Stimulation with OT-I peptide resulted in the generation of CD8+T cells from splenocytes of OT-I immunized mice, while OT-II stimulation did not induce CD8+T cell production (Fig. 8B). However, both groups exhibited successful activation of CD4+ T cells upon OVA-Ag stimulation (Fig. 8D), suggesting cooperation among CD4 T cells drive their activation [83]. Various factors, including ex-vivo and in-vitro stimulation conditions and cytokine accumulation, may influence IFN-γ/multifunctional CD4 T cell production [84], contributing to the observed reduction in CD4+ T cell response.
Understanding adjuvant efficacy in protein/subunit vaccine is crucial for unravelling the role of B cells in activating CD8+ T cell during malaria infection. While subunit vaccines hold an importance for infectious pathogens such as malaria, investigating the interplay between B and T cells is imperative.
Ag characteristics (monovalent or multivalent, folding stability) [85], [86] and the mouse model used (C57BL/6 and Balb/C) [87], [88] should be considered as they can impact adjuvant performance. The immunophenotyping of B cells based solely on CD138 and IgG markers can be considered as a limitation of this study since it may not provide a comprehensive characterization of different B cell subsets. The addition of new surface markers could differentiate the B cell populations. We did not observe any harmful effects, but a thorough analysis of potential local and systemic toxicity associated with the adjuvant combinations is indeed imperative. Additionally, a comprehensive comparison of Th1, Th2, and Th17 responses with different adjuvant combinations is required for a better understanding of immune status in infectious disease pathogenesis.
5. Conclusions
Significance of poly(I:C) and R848 as “adjuvants” to enhance B and T cell responses against the protein Ag could be important for eliciting immune response. The combination of these adjuvants could be important to develop an effective vaccine for the infectious diseases such as malaria. The compounded impact of poly(I:C) and R848 in combination could elicit the robust and enduring immune response. These adjuvants may be useful for designing the vaccines for inducing robust and long-lasting protection. In the end, we believe this adjuvant system may be used to stimulate the malaria antigens specific immune response.
CRediT authorship contribution statement
Nikunj Tandel: Writing – original draft, Validation, Formal analysis, Data curation, Conceptualization. Digna Patel: Methodology, Investigation, Data curation. Mansi Thakkar: Writing – original draft, Software, Methodology, Investigation, Data curation. Jagrut Shah: Writing – original draft, Investigation, Data curation. Rajeev K. Tyagi: Writing – review & editing, Resources, Formal analysis. Sarat K. Dalai: Writing – original draft, Validation, Supervision, Software, Resources, Methodology, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank all the present members of our laboratory for their contributions to the work described herein. We would like to acknowledge Nirma Education and Research Foundation (NERF), Ahmedabad for financial support to SKD lab. Prof. Sarat K. Dalai would like to thank the Department of Biotechnology (DBT), New Delhi, Govt. of India for funding part of this study (PRR No.: BT/PR3445/MED/29/1482/2019). Nikunj Tandel would like to thank the Indian Council of Medical Research (ICMR) for providing the fellowship to carry out his research (ICMR award letter No.: 2020-7623/CMB-BMS).
Footnotes
Supplementary material related to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e26887.
Contributor Information
Rajeev K. Tyagi, Email: rajeevtyagi@imtech.res.in, rajeev.gru@gmail.com.
Sarat K. Dalai, Email: sarat.dalai@nirmauni.ac.in.
Appendix A. Supplementary material
The following is the Supplementary material related to this article.
SI figures.
Data availability statement
All figures have been given in the manuscript and no other data is left to be disclosed.
References
- 1.Sun L., et al. T cells in health and disease. Signal Transduct. Targeted Ther. 2023;8(1):235. doi: 10.1038/s41392-023-01471-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hont A.B., et al. The generation and application of antigen-specific T cell therapies for cancer and viral-associated disease. Mol. Ther. 2022;30(6):2130–2152. doi: 10.1016/j.ymthe.2022.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rotrosen E., Kupper T.S. Assessing the generation of tissue resident memory T cells by vaccines. Nat. Rev. Immunol. 2023;23(10):655–665. doi: 10.1038/s41577-023-00853-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Travieso T., et al. The use of viral vectors in vaccine development. npj Vaccines. 2022;7(1):75. doi: 10.1038/s41541-022-00503-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qin F., et al. A guide to nucleic acid vaccines in the prevention and treatment of infectious diseases and cancers: from basic principles to current applications. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.633776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rauch S., et al. New vaccine technologies to combat outbreak situations. Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.01963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu Z., et al. Harnessing recent advances in synthetic DNA and electroporation technologies for rapid vaccine development against COVID-19 and other emerging infectious diseases. Front. Med. Technol. 2020;2 doi: 10.3389/fmedt.2020.571030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pollet J., Chen W.H., Strych U. Recombinant protein vaccines, a proven approach against coronavirus pandemics. Adv. Drug Deliv. Rev. 2021;170:71–82. doi: 10.1016/j.addr.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hou Y., et al. Advanced subunit vaccine delivery technologies: from vaccine cascade obstacles to design strategies. Acta Pharm. Sin. B. 2023;13(8):3321–3338. doi: 10.1016/j.apsb.2023.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tariq H., et al. Virus-like particles: revolutionary platforms for developing vaccines against emerging infectious diseases. Front. Microbiol. 2022:12. doi: 10.3389/fmicb.2021.790121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Badten A.J., et al. Protein nanoparticle-mediated delivery of recombinant influenza hemagglutinin enhances immunogenicity and breadth of the antibody response. ACS Infect. Dis. 2023;9(2):239–252. doi: 10.1021/acsinfecdis.2c00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bezbaruah R., et al. Nanoparticle-based delivery systems for vaccines. Vaccines. 2022;10(11) doi: 10.3390/vaccines10111946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pulendran B., P S.A., O'Hagan D.T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 2021;20(6):454–475. doi: 10.1038/s41573-021-00163-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verma S.K., et al. New-age vaccine adjuvants, their development, and future perspective. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1043109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jia Y., et al. The synergistic effects of sulfated lactosyl archaeol archaeosomes when combined with different adjuvants in a murine model. Pharmaceutics. 2021;13(2) doi: 10.3390/pharmaceutics13020205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan J., et al. Advances in infectious disease vaccine adjuvants. Vaccines. 2022;10(7) doi: 10.3390/vaccines10071120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu F., et al. Formulation of aluminum hydroxide adjuvant with TLR agonists poly(I:C) and CpG enhances the magnitude and avidity of the humoral immune response. Vaccine. 2019;37(14):1945–1953. doi: 10.1016/j.vaccine.2019.02.033. [DOI] [PubMed] [Google Scholar]
- 18.Haseda Y., et al. Development of combination adjuvant for efficient T cell and antibody response induction against protein antigen. PLoS ONE. 2021;16(8) doi: 10.1371/journal.pone.0254628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahn S.Y., Le C.T.T., Ko E.J. Monophosphoryl lipid a and poly I: C combination adjuvant promoted ovalbumin-specific cell mediated immunity in mice model. Biology. 2021;10(9) doi: 10.3390/biology10090908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hänel G., et al. Blood DCs activated with R848 and poly(I:C) induce antigen-specific immune responses against viral and tumor-associated antigens. Cancer Immunol. Immunother. 2022;71(7):1705–1718. doi: 10.1007/s00262-021-03109-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ko C.-N., et al. Nanocarriers for effective delivery: modulation of innate immunity for the management of infections and the associated complications. J. Nanobiotechnol. 2022;20(1):380. doi: 10.1186/s12951-022-01582-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomai M., Vasilakos J. Immunopotentiators in Modern Vaccines. Elsevier; 2017. Toll-like receptor 7 and 8 agonists for vaccine adjuvant use; pp. 149–162. [Google Scholar]
- 23.Weir G.M., et al. Combination of poly I: C and Pam3CSK4 enhances activation of B cells in vitro and boosts antibody responses to protein vaccines in vivo. PLoS ONE. 2017;12(6) doi: 10.1371/journal.pone.0180073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tewari K., et al. Poly(I:C) is an effective adjuvant for antibody and multi-functional CD4+ T cell responses to Plasmodium falciparum circumsporozoite protein (CSP) and αDEC-CSP in non human primates. Vaccine. 2010;28(45):7256–7266. doi: 10.1016/j.vaccine.2010.08.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salem M.L., et al. The TLR3 agonist poly(I:C) targets CD8+ T cells and augments their antigen-specific responses upon their adoptive transfer into naïve recipient mice. Vaccine. 2009;27(4):549–557. doi: 10.1016/j.vaccine.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van L.P., et al. Treatment with the TLR7 agonist R848 induces regulatory T-cell-mediated suppression of established asthma symptoms. Eur. J. Immunol. 2011;41(7):1992–1999. doi: 10.1002/eji.201040914. [DOI] [PubMed] [Google Scholar]
- 27.Makris S., Johansson C. R848 or influenza virus can induce potent innate immune responses in the lungs of neonatal mice. Mucosal Immunol. 2021;14(1):267–276. doi: 10.1038/s41385-020-0314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levy O., et al. Selective impairment of TLR-mediated innate immunity in human newborns: neonatal blood plasma reduces monocyte TNF-alpha induction by bacterial lipopeptides, lipopolysaccharide, and imiquimod, but preserves the response to R-848. J. Immunol. 2004;173(7):4627–4634. doi: 10.4049/jimmunol.173.7.4627. [DOI] [PubMed] [Google Scholar]
- 29.Hu Z., et al. A two-dose optimum for recombinant S1 protein-based COVID-19 vaccination. Virology. 2022;566:56–59. doi: 10.1016/j.virol.2021.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ai J., et al. Recombinant protein subunit vaccine booster following two-dose inactivated vaccines dramatically enhanced anti-RBD responses and neutralizing titers against SARS-CoV-2 and Variants of Concern. Cell Res. 2022;32(1):103–106. doi: 10.1038/s41422-021-00590-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liao Y., et al. Safety and immunogenicity of heterologous recombinant protein subunit vaccine (ZF2001) booster against COVID-19 at 3-9-month intervals following two-dose inactivated vaccine (CoronaVac) Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.1017590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He Q., et al. Immunogenicity and protective efficacy of a recombinant protein subunit vaccine and an inactivated vaccine against SARS-CoV-2 variants in non-human primates. Signal Transduct. Targeted Ther. 2022;7(1):69. doi: 10.1038/s41392-022-00926-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chua B.Y., et al. Soluble proteins induce strong CD8+ T cell and antibody responses through electrostatic association with simple cationic or anionic lipopeptides that target TLR2. J. Immunol. 2011;187(4):1692–1701. doi: 10.4049/jimmunol.1100486. [DOI] [PubMed] [Google Scholar]
- 34.Klarquist J., et al. B cells promote CD8 T cell primary and memory responses to subunit vaccines. Cell Rep. 2021;36(8) doi: 10.1016/j.celrep.2021.109591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fialová L., Petráčková M., Kuchař O. Comparison of different enzyme-linked immunosorbent assay methods for avidity determination of antiphospholipid antibodies. J. Clin. Lab. Anal. 2017;31(6) doi: 10.1002/jcla.22121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jelínková L., et al. An epitope-based malaria vaccine targeting the junctional region of circumsporozoite protein. npj Vaccines. 2021;6(1):13. doi: 10.1038/s41541-020-00274-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu X., Quan N. Immune cell isolation from mouse femur bone marrow. Bio Protoc. 2015;5(20) doi: 10.21769/bioprotoc.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slifka M.K., Matloubian M., Ahmed R. Bone marrow is a major site of long-term antibody production after acute viral infection. J. Virol. 1995;69(3):1895–1902. doi: 10.1128/jvi.69.3.1895-1902.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He J.S., et al. IgG1 memory B cells keep the memory of IgE responses. Nat. Commun. 2017;8(1):641. doi: 10.1038/s41467-017-00723-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khodadadi L., et al. The maintenance of memory plasma cells. Front. Immunol. 2019;10:721. doi: 10.3389/fimmu.2019.00721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Napolitani G., et al. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat. Immunol. 2005;6(8):769–776. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldinger S.M., et al. Nano-particle vaccination combined with TLR-7 and -9 ligands triggers memory and effector CD8+ T-cell responses in melanoma patients. Eur. J. Immunol. 2012;42(11):3049–3061. doi: 10.1002/eji.201142361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moody M.A., et al. Toll-like receptor 7/8 (TLR7/8) and TLR9 agonists cooperate to enhance HIV-1 envelope antibody responses in rhesus macaques. J. Virol. 2014;88(6):3329–3339. doi: 10.1128/JVI.03309-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pavlick A., et al. Combined vaccination with NY-ESO-1 protein, poly-ICLC, and montanide improves humoral and cellular immune responses in patients with high-risk melanoma. Cancer Immunol. Res. 2020;8(1):70–80. doi: 10.1158/2326-6066.CIR-19-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tomai M.A., et al. The immune response modifiers imiquimod and R-848 are potent activators of B lymphocytes. Cell. Immunol. 2000;203(1):55–65. doi: 10.1006/cimm.2000.1673. [DOI] [PubMed] [Google Scholar]
- 46.Shen Y., et al. Resiquimod (R848) has more stronger immune adjuvantivity than other tested TLR agonists. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2017;33(5):591–596. [PubMed] [Google Scholar]
- 47.Bhagchandani S., Johnson J.A., Irvine D.J. Evolution of Toll-like receptor 7/8 agonist therapeutics and their delivery approaches: from antiviral formulations to vaccine adjuvants. Adv. Drug Deliv. Rev. 2021;175 doi: 10.1016/j.addr.2021.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cid R., Bolívar J. Platforms for production of protein-based vaccines: from classical to next-generation strategies. Biomolecules. 2021;11(8) doi: 10.3390/biom11081072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pollard A.J., Bijker E.M. A guide to vaccinology: from basic principles to new developments. Nat. Rev. Immunol. 2021;21(2):83–100. doi: 10.1038/s41577-020-00479-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dölen Y., et al. Nanovaccine administration route is critical to obtain pertinent iNKt cell help for robust anti-tumor T and B cell responses. Oncoimmunology. 2020;9(1) doi: 10.1080/2162402X.2020.1738813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kanagaratham C., Sallis B.F., Fiebiger E. Experimental models for studying food allergy. Cell. Mol. Gastroenterol. Hepatol. 2018;6(3):356–369.e1. doi: 10.1016/j.jcmgh.2018.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martel B.C., et al. Translational animal models of atopic dermatitis for preclinical studies. Yale J. Biol. Med. 2017;90(3):389–402. [PMC free article] [PubMed] [Google Scholar]
- 53.Korupalli C., et al. Single-injecting, bioinspired nanocomposite hydrogel that can recruit host immune cells in situ to elicit potent and long-lasting humoral immune responses. Biomaterials. 2019;216 doi: 10.1016/j.biomaterials.2019.119268. [DOI] [PubMed] [Google Scholar]
- 54.Zhou C.-X., et al. Resiquimod and polyinosinic–polycytidylic acid formulation with aluminum hydroxide as an adjuvant for foot-and-mouth disease vaccine. BMC Vet. Res. 2014;10(1):2. doi: 10.1186/1746-6148-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Budroni S., et al. Antibody avidity, persistence, and response to antigen recall: comparison of vaccine adjuvants. npj Vaccines. 2021;6(1):78. doi: 10.1038/s41541-021-00337-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dobaño C., et al. Concentration and avidity of antibodies to different circumsporozoite epitopes correlate with RTS,S/AS01E malaria vaccine efficacy. Nat. Commun. 2019;10(1):2174. doi: 10.1038/s41467-019-10195-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khurana S., et al. AS03-adjuvanted H5N1 vaccine promotes antibody diversity and affinity maturation, NAI titers, cross-clade H5N1 neutralization, but not H1N1 cross-subtype neutralization. npj Vaccines. 2018;3:40. doi: 10.1038/s41541-018-0076-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luo W., Yin Q. B cell response to vaccination. Immunol. Invest. 2021;50(7):780–801. doi: 10.1080/08820139.2021.1903033. [DOI] [PubMed] [Google Scholar]
- 59.Tomayko M.M., et al. Cutting edge: hierarchy of maturity of murine memory B cell subsets. J. Immunol. 2010;185(12):7146–7150. doi: 10.4049/jimmunol.1002163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zuccarino-Catania G.V., et al. CD80 and PD-L2 define functionally distinct memory B cell subsets that are independent of antibody isotype. Nat. Immunol. 2014;15(7):631–637. doi: 10.1038/ni.2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koike T., et al. The quantity of CD40 signaling determines the differentiation of B cells into functionally distinct memory cell subsets. eLife. 2019;8 doi: 10.7554/eLife.44245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Parra M., et al. Memory CD73+IgM+ B cells protect against Plasmodium yoelii infection and express Granzyme B. PLoS ONE. 2020;15(9) doi: 10.1371/journal.pone.0238493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brown S.L., et al. IgM(+) and IgM(-) memory B cells represent heterogeneous populations capable of producing class-switched antibodies and germinal center B cells upon rechallenge with P. yoelii. J. Leukoc. Biol. 2022;112(5):1115–1135. doi: 10.1002/JLB.4A0921-523R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boyle M.J., et al. IgM in human immunity to Plasmodium falciparum malaria. Sci. Adv. 2019;5(9) doi: 10.1126/sciadv.aax4489. eaax4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brynjolfsson S.F., et al. Long-lived plasma cells in mice and men. Front. Immunol. 2018;9:2673. doi: 10.3389/fimmu.2018.02673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Malkiel S., et al. Plasma cell differentiation pathways in systemic lupus erythematosus. Front. Immunol. 2018;9:427. doi: 10.3389/fimmu.2018.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pabst R. The bone marrow is not only a primary lymphoid organ: the critical role for T lymphocyte migration and housing of long-term memory plasma cells. Eur. J. Immunol. 2018;48(7):1096–1100. doi: 10.1002/eji.201747392. [DOI] [PubMed] [Google Scholar]
- 68.Pracht K., et al. A new staining protocol for detection of murine antibody-secreting plasma cell subsets by flow cytometry. Eur. J. Immunol. 2017;47(8):1389–1392. doi: 10.1002/eji.201747019. [DOI] [PubMed] [Google Scholar]
- 69.D'Souza L., et al. CD73 expression identifies a subset of IgM(+) antigen-experienced cells with memory attributes that is T cell and CD40 signalling dependent. Immunology. 2017;152(4):602–612. doi: 10.1111/imm.12800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boeglin E., et al. Toll-like receptor agonists synergize with CD40L to induce either proliferation or plasma cell differentiation of mouse B cells. PLoS ONE. 2011;6(10) doi: 10.1371/journal.pone.0025542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jones D.D., Wilmore J.R., Allman D. Cellular dynamics of memory B cell populations: IgM+ and IgG+ memory B cells persist indefinitely as quiescent cells. J. Immunol. 2015;195(10):4753–4759. doi: 10.4049/jimmunol.1501365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pape K.A., et al. Naive B cells with high-avidity germline-encoded antigen receptors produce persistent IgM+ and transient IgG+ memory B cells. Immunity. 2018;48(6):1135–1143.e4. doi: 10.1016/j.immuni.2018.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goins C.L., et al. Immune complex-mediated enhancement of secondary antibody responses. J. Immunol. 2010;184(11):6293–6298. doi: 10.4049/jimmunol.0902530. [DOI] [PubMed] [Google Scholar]
- 74.Kurup S.P., Butler N.S., Harty J.T. T cell-mediated immunity to malaria. Nat. Rev. Immunol. 2019;19(7):457–471. doi: 10.1038/s41577-019-0158-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee H., Jeong S., Shin E.-C. Significance of bystander T cell activation in microbial infection. Nat. Immunol. 2022;23(1):13–22. doi: 10.1038/s41590-021-00985-3. [DOI] [PubMed] [Google Scholar]
- 76.Raskov H., et al. Cytotoxic CD8+ T cells in cancer and cancer immunotherapy. Br. J. Cancer. 2021;124(2):359–367. doi: 10.1038/s41416-020-01048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mandraju R., et al. Differential ability of surface and endosomal TLRs to induce CD8 T cell responses in vivo. J. Immunol. 2014;192(9):4303–4315. doi: 10.4049/jimmunol.1302244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Espinosa D.A., et al. Robust antibody and CD8(+) T-cell responses induced by P. falciparum CSP adsorbed to cationic liposomal adjuvant CAF09 confer sterilizing immunity against experimental rodent malaria infection. npj Vaccines. 2017;2 doi: 10.1038/s41541-017-0011-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lefebvre M.N., Harty J.T. You shall not pass: memory CD8 T cells in liver-stage malaria. Trends Parasitol. 2020;36(2):147–157. doi: 10.1016/j.pt.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mallajosyula V., et al. CD8(+) T cells specific for conserved coronavirus epitopes correlate with milder disease in COVID-19 patients. Sci. Immunol. 2021;6(61) doi: 10.1126/sciimmunol.abg5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kambayashi T., et al. Memory CD8+ T cells provide an early source of IFN-gamma. J. Immunol. 2003;170(5):2399–2408. doi: 10.4049/jimmunol.170.5.2399. [DOI] [PubMed] [Google Scholar]
- 82.de Araújo-Souza P.S., et al. Differential interferon-γ production by naive and memory-like CD8 T cells. J. Leukoc. Biol. 2020;108(4):1329–1337. doi: 10.1002/JLB.2AB0420-646R. [DOI] [PubMed] [Google Scholar]
- 83.Peters N.C., et al. CD4 T cell cooperation is required for the in vivo activation of CD4 T cells. Int. Immunol. 2009;21(11):1213–1224. doi: 10.1093/intimm/dxp085. [DOI] [PubMed] [Google Scholar]
- 84.Kaveh D.A., Whelan A.O., Hogarth P.J. The duration of antigen-stimulation significantly alters the diversity of multifunctional CD4 T cells measured by intracellular cytokine staining. PLoS ONE. 2012;7(6) doi: 10.1371/journal.pone.0038926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Scheiblhofer S., et al. Influence of protein fold stability on immunogenicity and its implications for vaccine design. Expert Rev. Vaccines. 2017;16(5):479–489. doi: 10.1080/14760584.2017.1306441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Saylor K., et al. Designs of antigen structure and composition for improved protein-based vaccine efficacy. Front. Immunol. 2020;11:283. doi: 10.3389/fimmu.2020.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Klein S.L., Flanagan K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016;16(10):626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 88.Sellers R.S. Translating mouse models: immune variation and efficacy testing. Toxicol. Pathol. 2017;45(1):134–145. doi: 10.1177/0192623316675767. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SI figures.
Data Availability Statement
All figures have been given in the manuscript and no other data is left to be disclosed.