Abstract
Mephedrone is an illegal drug that is used recreationally. Few studies have been conducted to investigate the mechanisms by which mephedrone is harming cells. In this research, we investigated the effect of mephedrone using toxicology coupled with LC-MS/MS based metabolomics in the two CNS derived cell lines. Methods of assessment such as neutral red (NR) assay, dimethylthiazolyl diphenyltetrazolium bromide (MTT), lactose dehydrogenase (LDH) measurement, and morphology were performed to identify the effect on cell viability and to identify the best concentration to be used in a metabolomics study. A concentration of 100 μM of mephedrone was used in the metabolomic experiment because at this concentration mephedrone had induced several intracellular changes. Although there no clear indicators of cellular damage caused by mephedrone. In astrocytes there was a clear indication that cell membrane function might be impaired by depletion of ether lipids.
Keywords: Mephedrone, Glutathione, Metabolomics, LC-MS/MS
1. Introduction
Mephedrone is an illegal synthetic ring-substituted cathinone, 4-methylmethcathinone (Gibbons and Zloh, 2010). As mephedrone is chemically similar to amphetamines, it would be expected to increase dopamine (DA) and serotonin (5-HT) concentrations in brain in a similar way to which amphetamines affect these compounds (Baumann et al., 2012). In this research toxicity of mephedrone on the human neuronal cells will be assessed using the application of metabolomics. To our knowledge, this is the first study of mephedrone toxicity using a metabolomics approach.
Mephedrone was found to block the reuptake of dopamine and serotonin in rat brain (Baumann et al., 2012, Hadlock et al., 2011), and increased rat locomotor activity (Motbey et al., 2012) and to induce euphoria and stimulate sexual abilities in rats (Kehr et al., 2011). Den Hollander suggested that mephedrone is acting by generating oxidative stress in SH-SY5Y cells (den Hollander et al., 2014).
It is believed that mephedrone has a similar CNS effect to other psychoactive drugs (MDMA and cocaine in particular) (Winstock et al., 2011a). The effects reported by users are summarised as follows: mood boosting, sexual stimulation, excitement, general stimulus effect, decreased aggression, and enhanced cerebral activity (Winstock et al., 2010). It is also believed that mephedrone does increase the ambulatory hyperactivity in mice (Sarefko et al., 2022). In some studies, it was indicated that the binge-like use of mephedrone has a significant role in its effect on memory of mice (Marszalek-Grabska et al., 2022). 60 % of UK clubbers who used mephedrone indicated that mephedrone slightly raised their sexual desire, with 8 % insisting that it was a regular effect of mephedrone (Winstock et al., 2011b).
In a comparison survey in the UK, clubbers who used mephedrone and cocaine were asked about the difference between the effect of both drugs (Winstock et al., 2011b). More than 50 % indicated that mephedrone has a more pronounced euphoria effect than cocaine and more than 60 % said that the euphoria of mephedrone lasted longer than that of cocaine. However, it was noticed that the users who stated that they experienced longer effect were oral route users but not nasal route users (Winstock et al., 2011b).
Drugs that affect the transporters of monoamines are divided into two groups, substrates, or blockers. The substrates stimulate cells to produce more neurotransmitters inside the cell. Production of more neurotransmitters by the increase of substrates will lead, eventually, to insufficiencies in monoamine neurons, neurotransmitter reduction, and decreased efficiency of transporters. Recent research determined that the cathinones such as were butylone, MDPV, and naphyrone were blockers of human monoamines, whereas the substrates were mephedrone, 4-fluoromethcathinone, and methylone. The neurotoxic effect of the substrate group is proposed to act via the inhibition of neurotransmitter uptake (Eshleman et al., 2013). Some previously published papers have studied the potential neurotoxicity of mephedrone. Repetitive dosing of mephedrone to mice or rats had no toxic effect to the cortex and striatum dopamine neural endings, and did not stimulate the striatum microglial cells and astrocytes in mice (Angoa-Pérez et al., 2014). However, mephedrone increased amphetamine (methamphetamine and methyldioxymethamphetamine) neurotoxicity in mouse brain as a mephedrone plus amphetamines mixture decreased serotonin levels more than either of them alone (Angoa-Pérez et al., 2013).
In a study conducted on rats, the authors concluded that when mephedrone is administered in doses mimicking human abuse intake, it decreases weight and causes irregularities of body temperature (López-Arnau et al., 2015). The decrease in weight in rats treated with mephedrone can be explained as a result of appetite suppression caused by the drug use, similar to that observed with other amphetamines use (Angoa-Pérez et al., 2014). However, the loss in weight was temporary and the weight was regained in seven days following the last drug administration. Body temperature was significantly and instantly decreased following the first mephedrone dose.
In López-Arnau’s experiments, it was found that hyperthermia, an increase of body temperature, was detected only after a binge like dosing of mephedrone. This finding is important, since higher body temperature was related to mephedrone binge dosing at both ambient (Baumann et al., 2012) and high temperature (Hadlock et al., 2011, López-Arnau et al., 2015).
Additionally, mephedrone has a noticable effect on DA and 5-HT transporters along with causing a decrease of the enzymes responsible for synthesis of DA and 5-HT. As a result of mephedrone intake in rats, DA transporters were decreased only in the frontal cortex, though, 5-HT transporters were reduced in all of the investigated parts of the rat brain (the striatum, the frontal cortex, and the hippocampus) (López-Arnau et al., 2015).
Mephedrone stability in comparison to other cathinones has been investigated in limited studies (Johnson and Botch-Jones, 2013, Sørensen, 2011, Tsujikawa et al., 2012, Maskell et al., 2013, Busardò et al., 2015). It showed a weaker stability (degraded by 75 % in day 4 and was not detected in days 7 and 14 in blood samples) compared to 3,4-methylenedioxypyrovalerone (MDPV), N-benzylpiperazine (BZP) and 1-[3-(trifluoromethyl)phenyl]piperazine (TFMPP) when stored at room temperature (+22 °C) for different periods of incubation (1, 2, 4, 7 and 8 days) in different matrices (blood, plasma and urine) (Johnson and Botch-Jones, 2013). In the same study mephedrone was significantly degraded at longer periods (7 and 8 days). This previous finding and the fact that, in this research, mephedrone was incubated in the medium at 37 °C for up to 7 days should be taken in consideration when interpreting the results in longer incubations.
An onsite test for mephedrone detection can be carried out by Raman and infrared spectrometers. These can measure only pure mephedrone, and do not allow separation of the drug from biological samples. The identification of mephedrone and its metabolites can be achieved reliably using liquid chromatography with tandem mass spectrometry (LC-MSMS) and gas chromatography-mass spectrometry (GC–MS) (Gibbons and Zloh, 2010, Camilleri et al., 2010, Meyer et al., 2010). However, these methods can only differentiate between the methyl-methcathinone structural isomers if there are standards for reference or through analysis by nuclear magnetic resonance spectroscopy (NMR) (Gibbons and Zloh, 2010, Meyer et al., 2010).
A study conducted by Hollander in 2014 on human neuroblastoma cells (SH-SY5Y), found that mephedrone had a toxic effect on cells at 500 μM and greater (den Hollander et al., 2014). In addition, it also decreased LDH release between 2 and 100 μM. The study also demonstrated that mephedrone has redox electron donor reactivity and displays a relationship of this activity to high pH values and is this is accompanied by formation of an N-acetylated mephedrone. According to Hollander mephedrone decreased mitochondrial respiration. The use of the SH-SY5Y cell line showed that mephedrone yielded cytotoxicity only at high concentrations; while at lower levels it decreased LDH production. It was proposed that oxidative deamination, followed by the oxidative cleavage of mephedrone produces acetic acid, which acetylates mephedrone.
Notably, many in vivo studies have found no sign of toxicity of mephedrone. Variable metabolism reactions and/or inability to penetrate brain and blood barriers could result in this negative finding (Angoa‐Pérez et al., 2012, den Hollander et al., 2013, Motbey et al., 2012).
It was found that mephedrone significantly enhanced methamphetamine dopamine (DA) toxicity (Angoa‐Pérez et al., 2013). Hyperthermia was produced in the treatment of mice with both mephedrone and methamphetamine, and by treatment with any of the drugs separately. The neurotoxicities of amphetamine and 3,4-methylenedioxymethamphetamine on the nerve endings of DA in mice striatum were also enhanced. As mephedrone increases methamphetamine neurotoxicity, this suggests that it has different interaction with the dopamine transporter (DAT) than that observed with other DAT inhibitors. The fact that mephedrone itself has no significant effect on DA nerve endings, could imply a potential high risk caused by its interaction with other drugs, and could lead to neurotoxic complications.
It was found that when mephedrone was co-administered with ethanol in mice the neurotoxicity of mephedrone was enhanced (Ciudad-Roberts et al., 2016). This finding was explained by the authors to be a result of increased oxidative stress in the hippocampus, causing decreased learning, memory, and neurogenesis in the mice.
In another drug interaction study, mephedrone was co-administered with nicotine in mice. The co-administration was found to be effective in reducing general antioxidant function, catalase activity, and antioxidant enzyme activities in the brain, and in increasing lipid peroxidation (Budzynska et al., 2015).
2. Aims and objectives
The primary aim research was to study the toxicity and effect of mephedrone directly on human neuroblastoma and astrocytoma as representative cells for human brain cells. This approach was used to investigate the direct effect of mephedrone on brain when drug users take mephedrone directly (by inhalation). A secondary aim of this section of the research was to investigate the effect of mephedrone on liver using cultured rat primary hepatocytes.
3. Materials and methods
3.1. Mephedrone preparation
Mephedrone hydrochloride (over 98 % purity) was donated by Dr Oliver Sutcliffe from Manchester Metropolitan University, (4.43 mg) was dissolved in 2 % (v/v) acetic acid (0.5 mL) to give 50 mM stock solution of mephedrone, and then diluted in media to obtain the other concentrations. The mephedrone stock solution was stored at −20 °C until the experiments were performed.
3.2. Extraction of cell lysates for metabolomics
Human astrocytoma (U373) and neuroblastoma (SH-SY5Y) cells were purchased from the European Collection of Cell Cultures (catalogues number 08061901, 94030304, respectively). Medium was removed from wells and the cells were washed with 0.5 mL of pre warmed PBS (37 °C) twice. It is important to warm the PBS to decrease the effect on the biochemical reactions in the cells at this stage. A 200 μL volume of pre-cooled extraction solution (50 % of methanol, 30 % of acetonitrile and 20 % water) was added to each well to lyse cells. The use of the pre-cooled mixture is to stop the chemical reactions in the cells in the time of lysis. Cells were scraped off using a small metallic spatula. The extracted cell solution was transferred to 0.5 mL Eppendorf vials. Samples were then shaken at 4 °C for 12 min. Samples were centrifuged at 0 °C and 13000 rpm for 10 min. The supernatant was transferred into glass vials and stored at −80 °C until analysed with LC-MS.
3.3. Cell viability assays
3.3.1. Neutral red (NR) assay
The method was obtained from a previously published method (Inoue et al., 2001). Medium was removed when the incubation with mephedrone was finished, and 100 μl NR solution (0.05 mg/ml of phosphate buffer saline (PBS)) was added to each well of a 96-well plate. The plate was incubated at 37 °C under 5 % CO2 air for 3 h. The solution was removed after incubation and wells were washed with 200 μl of PBS. 100 μl destain (mixture of 50 mL of ethanol, 1 mL of glacial acetic acid and 49 mL of distilled H2O) was added to each well and the plate left on the shaker for 30 min. The absorbance was then measured at 540 nm by plate reader spectrophotometer (Multiskan GO, Thermo Scientific). NR assay is a good indicator of cellular growth.
3.3.2. Dimethylthiazolyl diphenyltetrazolium bromide (MTT) assay
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was carried out following the procedure on (Borenfreund and Puerner, 1985). 10 mM of MTT solution was prepared in PBS and filtered through a 0.2 μM filter. A 50 μl volume of the solution was added to each well when the incubation was complete. A 96-well plate was incubated at 37 °C in presence of 5 % CO2 air for 4 h. MTT solution was removed after incubation and 200 μl of DMSO was added to each well. The solution in each well was mixed to give an even colour, and transferred to a 1 mL cuvette. Absorbance was measured at 540 nm by plate reader spectrophotometer (Multiskan GO, Thermo Scientific). MTT assay is an indicator of cellular metabolism.
3.3.3. Lactate dehydrogenase (LDH) assay
Lactate dehydrogenase (LDH) assay is based on the ability of LDH to reduce nicotinamide adenine dinucleotide (NAD) to NADH. It is a good indicator to assess membrane damage in the cell. NADH will reduce the tetrazolium salt 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-phenyl-2H-tetrazolium (INT) to red formazan. Two solutions were prepared. First, to prepare 0.1 M sodium phosphate buffer (NaPi buffer at pH 7.6) Na2HPO4 (14.2 g) was weighed and dissolved in 500 mL of distilled H2O. Another solution was prepared by dissolving 2.4 g of NaH2PO4 in 100 mL of distilled H2O. These two solutions were mixed to give a sodium phosphate buffer, 0.1 M at pH 7.6 (NaPi buffer). The second solution which was a mixture of 3 mg pyruvic acid and 3 mg of NADH dissolved in 1 mL of NaPi buffer. This was prepared immediately before use. For each well measurement, 0.86 mL of 0.1 M sodium phosphate buffer (pH 7.6) and 40 μl of pyruvic acid (3 mg in 1 mL NaPi buffer)/NADH solution were added to a cuvette (1 mL). Then 100 μl of medium from each well was added. The change in absorbance was recorded at 340 nm by a UV-spectrophotometer (UV-2401PC, Shimadzu) over 60 s at room temperature.
3.3.4. Morphology
Cells were imaged after 6 days incubation with and without 100 μM of mephedrone by using a Motic AE31 microscope-20 power dry lenses. Any change in the cell membrane or intracellular components would be monitored.
4. Results
4.1. The effects of mephedrone on viability measured by MTT, NR and LDH viability assays
4.1.1. MTT assay
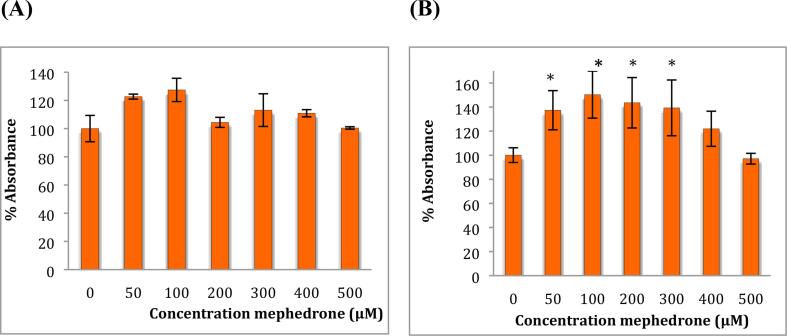
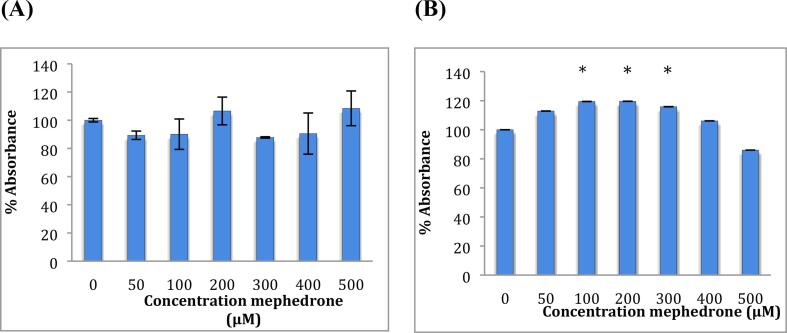
To study the effect of mephedrone on cell metabolic activity, an MTT assay was performed on both cell lines (neuroblastoma and astrocytoma) after treatment with a range of concentrations of mephedrone for 48 h see Fig. 1.
Fig. 1.
MTT assay measured in (A) SH-SY5Y cells and in (B) U-373 cells after 48 h. Results are percentage values (Mean ± SD, n = 6) where 100 % corresponds to control values. Cells treated with mephedrone in culture medium throughout the experiment. Data were analysed using one-way ANOVA followed by Tukey test. * Data were significantly different from their relevant control P value < 0.05.
The MTT assay has shown that mephedrone at a low concentration (100 μM) raised metabolism up to 127 % and 150 % in SH-SY5Y and U-373 cells, respectively. This is thought to be an adaptive response in response to mephedrone-induced stress on the cells similar to that observed with exposure to other substances (e.g. Chromium) in previous studies (Zijlstra et al., 2012, Posada et al., 2015). With increased concentrations of mephedrone the activity drops again to reach levels around 100 %.
4.1.2. NR assay
Both cell lines (neuroblastoma and astrocytoma) were treated with a range of concentrations of mephedrone for 48 h, and NR assay was performed on the cells to see the effect on the cell count see Fig. 5 below.
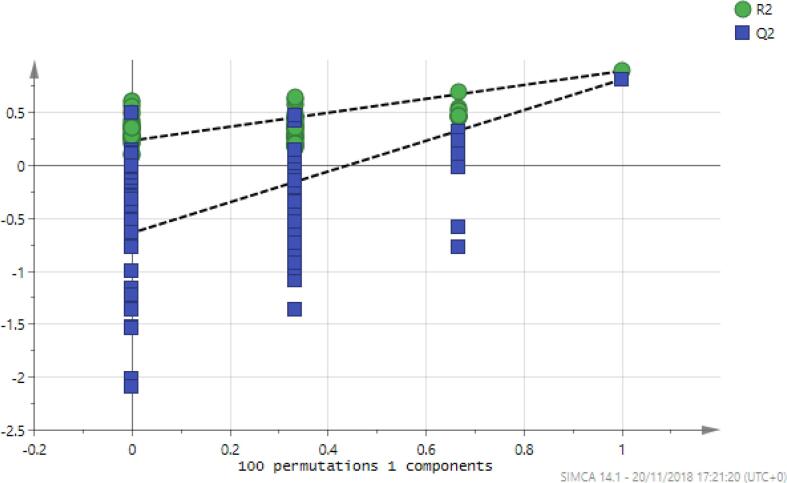
Fig. 5.
Permutation analysis of the OPLSDA model for mephedrone treated and non-treated astrocytoma cells. Validation of the model was performed with 100 permutation tests. R2 represent the goodness of the fit and Q2 represent the predictive capability.
4.1.3. LDH assay
LDH assay results of neuroblastoma and astrocytoma cells after treatment with 100 μM of mephedrone for 6 days are shown in Table 1.
Table 1.
LDH release 6 days after treatment of SH-SY5Y and U-373cells with 100 μM mephedrone (n = 3). Data represent absorbance ± standard error.
|
SH-SY5Y |
Treatment |
LDH activity (mol/ml/min) ±STD |
| 100 μM of mephedrone | 5.98 ± (0.01) | |
| Control | 5.74 ± (0.006) | |
|
U-373 |
Treatment |
LDH activity (mol/ml/min) ±STD |
| 100 μM of mephedrone | 4.01 ± (0.01) | |
| Control | 4.00 ± (0.007) |
From the results in the Table 1 we can conclude that there were no significant differences in LDH activity between the 100 μM mephedrone treated and the non-treated cells in either cell type.
4.1.4. Effect on morphology
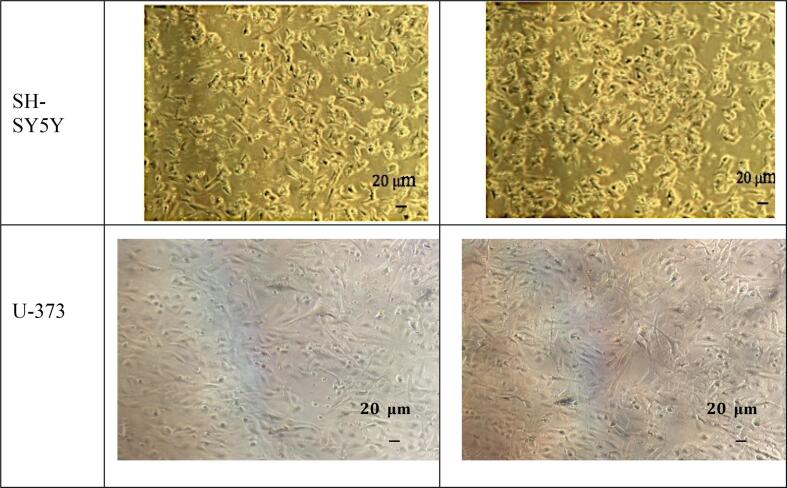
The visual examination by microscope for both cell types (neuroblastoma and astrocytoma) before and after treatment with 100 μM of mephedrone for 6 days, is shown in Fig. 3.
Fig. 3.
Morphology of SH-SY5Y (A and B) and U373 (C and D) cells after 6 days incubation without mephedrone treatment (A and C), and with mephedrone (B and D). Pictures were taken by the Motic AE31 microscope-20 power dry lenses.
Examination of the morphology (see Fig. 5) for both cell types showed no sign of abnormal proliferation after treatment with 100 μM mephedrone.
4.2. Metabolomic analysis
The metabolomic analysis of the treated cells showed significant changes in metabolites inside both cell types after mephedrone treatment as shown in Fig. 4, Fig. 5 and Table 2, Table 3.
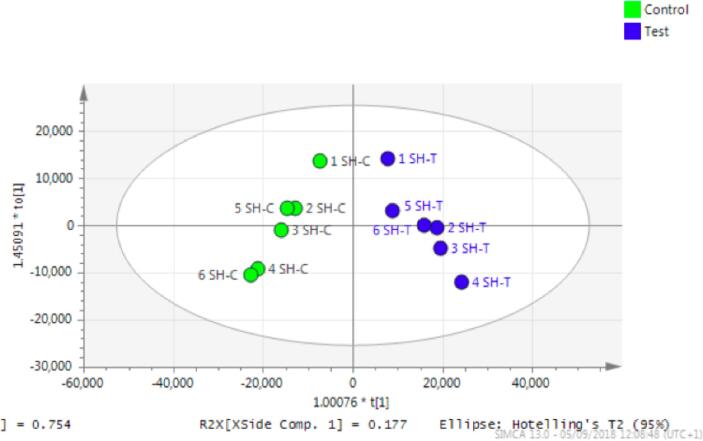
Fig. 4.
OPLS-DA score plot of neuroblastoma (SH-SY5Y) treated with mephedrone (blue) and non-treated (green). Test stands for treated samples and control stands for non-treated samples.
Table 2.
Metabolomic results of SH-SY5Y cell line after incubation with 100 μM mephedrone for 6 days (n = 6). Values indicate ratio of (treated/non-treated) peak areas of metabolites. * Mean peak area of samples treated with mephedrone only as mean peak area value of control samples was zero.
|
Rt (min) |
M.Wt | Structure | Metabolite name | Meph/c | P- value |
|---|---|---|---|---|---|
| 15.1 | 119.05824 | C4H9NO3 | L-Threonine | 1.27 | 0.038 |
| 10.5 | 122.04801 | C6H6N2O | Nicotinamide | 1.35 | 0.045 |
| 17.9 | 130.02661 | C5H6O4 | Mesaconate | 1.98 | 0.044 |
| 15.5 | 129.04259 | C5H7NO3 | Oxoproline | 2.01 | 0.039 |
| 11.6 | 131.09463 | C6H13NO2 | L-Leucine | 1.53 | 0.041 |
| 15.6 | 146.06914 | C5H10N2O3 | L-Glutamine | 1.65 | 0.005 |
| 15.6 | 148.07356 | C6H12O4 | Methyl,hydroxymethyldihydroxy-pentanoic acid | 1.40 | 0.020 |
| 12.1 | 149.05105 | C5H11NO2S | *L-Methionine | 1.49 | 0.043 |
| 21.2 | 155.06948 | C6H9N3O2 | L-Histidine | 1.44 | 0.046 |
| 16.5 | 158.06914 | C6H10N2O3 | 4-Methylene-L-glutamine | 1.33 | 0.038 |
| 12.3 | 204.08988 | C11H12N2O2 | L-Tryptophan | 1.43 | 0.040 |
| 10.3 | 217.13141 | C10H19NO4 | O-Propanoylcarnitine | 1.36 | 0.018 |
| 4.5 | 222.08921 | C12H14O4 | Dodecatetraenedioic acid | 0.46 | 0.035 |
| 4.4 | 244.16746 | C13H24O4 | Tridecanedioic acid | 2.07 | 0.008 |
| 23.2 | 264.10448 | C12H17N4OS | Thiamine | 1.90 | 0.041 |
| 5.1 | 287.28243 | C17H37NO2 | Heptadecasphinganine | 0.02 | 0.037 |
| 4.3 | 351.31373 | C22H41NO2 | Eicosadienoyl-ethanolamine | 1.47 | 0.025 |
| 4.3 | 353.32938 | C22H43NO2 | Eicosaenoyl)-ethanolamine | 2.29 | 0.015 |
| 17.3 | 426.08791 | C13H22N4O8S2 | S-glutathionyl-L-cysteine |
-(130050) * |
0.027 |
Table 3.
CVANOVA statistics for the OPLS-DA model for astrocytoma cells treated and non-treated with mephedrone.
| SS | DF | MS | F | P-value | SD | |
|---|---|---|---|---|---|---|
| Total correlation | 11 | 11 | 1 | 1 | ||
| Regression | 8.96 | 4 | 2.24 | 7.697 | 0.0105 | 1.497 |
| Residual | 2.038 | 7 | 0.29 | 0.54 |
The metabolomic effects on neuroblastoma cells after treatment with 100 μM of mephedrone for 6 days are shown in Table 2.
Orthogonal partial least squares-discriminant analysis (OPLS-DA) score plot exhibited in (Fig. 4) shows that there was a geometrical separation between treated and non-treated samples. OPLS-DA is a good parameter to diagnose the difference between two groups using multivariate analysis. The permutation analysis (Fig. 5) and the CVANOVA statistics (Table 3) show the validity of the model. It can be concluded that treatment with mephedrone has produced significant changes in the metabolome of the treated samples compared with the metabolome of the non-treated samples.
In SH-SY5Y mephedrone treated cells, there was a large increase in cysteine glutathione disulfide, which is produced as a result of oxidative stress. Oxidation of glutathione results in formation of mixed disulfides with protein thiol groups, and initiating reversible S-glutathionylation. S-glutathionylation is vital to transfer signals of oxidants (Lim et al., 2003), by uncoupling the enzyme endothelial nitric oxide sythase (eNOS) to adjust it to produce O2•− instead of NO (La Quaglia and Manchester, 1996).
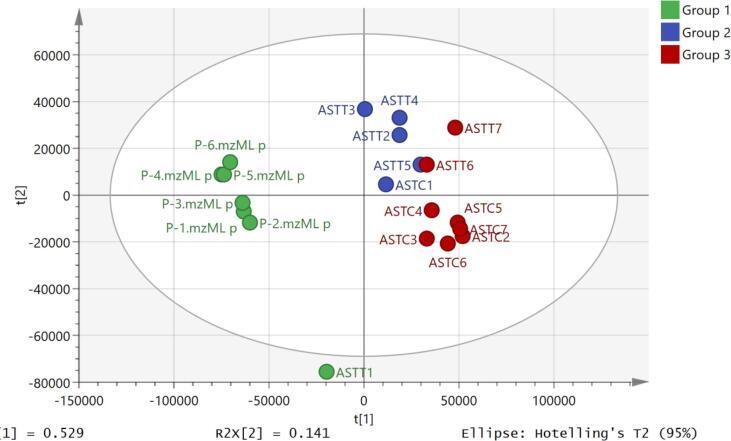
Fig. 6 shows a PCA model of the control and mephedrone (100 μM) treated astrocytes. The pooled samples, although not in the centre are quite well clustered showing technical stability. The pooled sample is made of equal small aliquot (20 μl) from each sample to assess the technical procedure.
Fig. 6.
PCA model of the control (brown), mephedrone treated (blue) astrocytes and pooled samples (green).
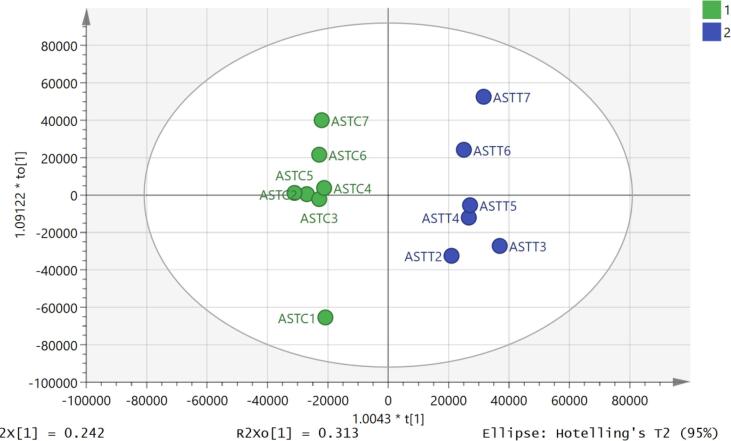
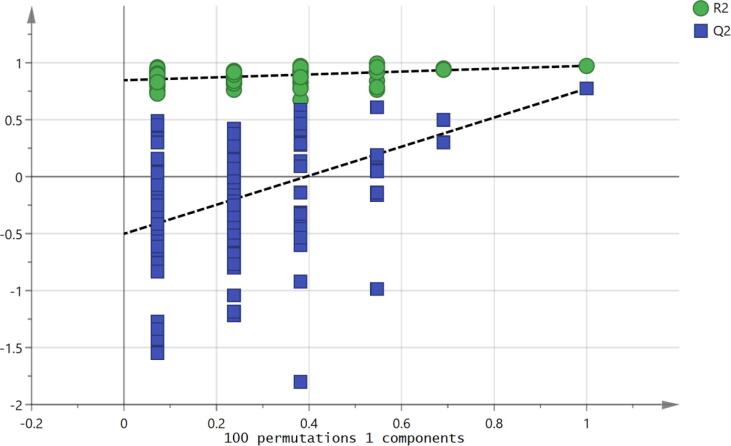
Using an OPLSDA model it was possible to separate the treated and control samples (Fig. 7) and the model was valid according to the cross-validation test (Fig. 8) and the CVANOVA statistics (Table 4)Fig 9.
Fig. 7.
OPLS-DA score plot of astrocytoma (U-373) treated with mephedrone (blue) and non-treated (green) without pooled samples. C represents the control (non-treated) samples, and T represents the test (treated) samples.
Fig. 8.
Permutation analysis of the OPLS-DA model for mephedrone treated and non-treated astrocytoma cells. Validation of the model was performed with 100 permutation tests. R2 represent the goodness of the fit and Q2 represent the predictive capability.
Table 4.
CVANOVA of the OPLS-DA model for mephedrone treated and non-treated astrocytoma.
| SS | DF | MS | F | P-value | SD | |
|---|---|---|---|---|---|---|
| Total correlation | 11 | 11 | 1 | 1 | ||
| Regression | 10.098 | 4 | 2.52 | 19.59 | 0.0007 | 1.59 |
| Residual | 0.902 | 7 | 0.129 | 0.36 |
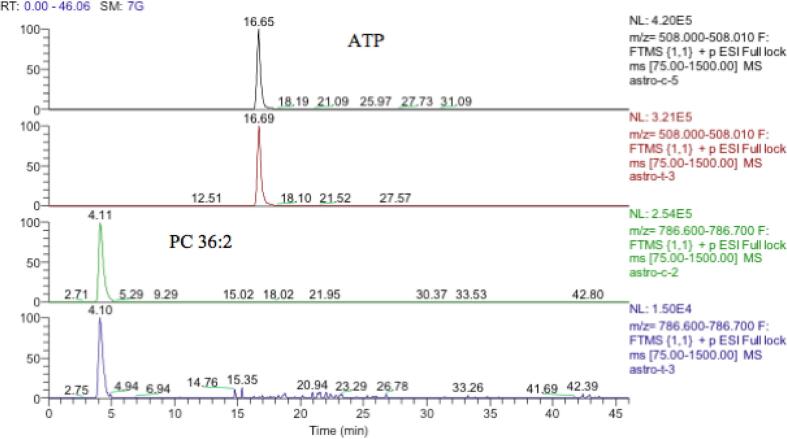
Fig. 9.
Extracted ion traces showing similar levels of ATP in treated and untreated extracts but a large reduction of the amount of a major cellular lipid (PC 36:2) in treated astrocytoma cells.
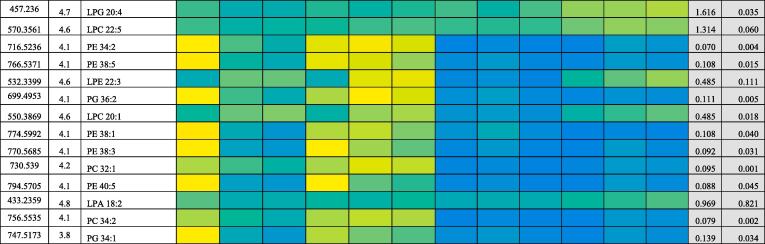
Metabolomic analysis results of astrocytoma cells after treatment with 100 μM of mephedrone for 6 days, is shown in Table 5.
Table 5.
Non-lipid metabolite changes in U-373 astrocytes following treatment with 100 μM mephedrone for 6 days (n = 6). Values indicate ratio of metabolites compared with controls (average of mephedrone treated peak area/control peak area). M/z is the mass to charge ratio and Rt is the retention time. Ratio represent the treatment concentration of the metabolite divided by control concentration.
| M/z | Rt (min) | Structure | Metabolite | Ratio | P-Value |
|---|---|---|---|---|---|
| 316.248 | 4.1 | C17H33NO4 | O-decanoyl-R-carnitine | 0.836 | 0.044 |
| 311.294 | 4.2 | C20H38O2 | Eicosenoic acid | 1.737 | 0.033 |
| 155.107 | 4.8 | C9H14O2 | Nonadienoic acid | 0.561 | 0.004 |
| 353.231 | 4.4 | C20H32O5 | Trihydroxy eicosatetraenoic acid | 1.999 | 0.038 |
| 365.342 | 3.8 | C24H46O2 | Tetracosenoic acid | 0.305 | 0.032 |
| 173.117 | 4.9 | C9H16O3 | Oxononanoic acid | 0.555 | 0.001 |
| 283.192 | 4.3 | C16H28O4 | Dihydroxy hexdecadienoic acid | 1.511 | 0.016 |
| 337.311 | 3.9 | C22H42O2 | Docosenoic acid | 0.487 | 0.031 |
| 309.28 | 3.9 | C20H38O2 | Eicosenoic acid | 0.548 | 0.046 |
| 282.279 | 5.6 | C18H35NO | Octadecenamide | 0.387 | 0.021 |
| 145.014 | 15.6 | C5H6O5 | 2-Oxoglutarate | 0.407 | 0.027 |
| 162.076 | 11.4 | C6H11NO4 | L-2-Aminoadipate | 1.633 | 0 |
| 258.038 | 14.8 | C6H14NO8P | D-Glucosamine 6-phosphate | 1.62 | 0.035 |
| 190.051 | 11.3 | C10H9NO3 | 5-Hydroxyindoleacetate | 0.665 | 0.04 |
| 206.081 | 13.6 | C11H11NO3 | Indolelactate | 0.763 | 0.031 |
| 402.011 | 17.3 | C9H15N3O11P2 | CDP | 0.479 | 0.048 |
| 503.162 | 17.5 | C18H32O16 | Glycogen | 1.905 | 0.037 |
| 665.214 | 18.2 | C24H42O21 | Glycogen | 3.03 | 0.044 |
| 133.061 | 15.8 | C4H8N2O3 | L-Asparagine | 0.555 | 0.013 |
| 336.14 | 16.4 | C12H21N3O8 | N4-(Acetyl-beta-D-glucosaminyl) asparagine | 0.482 | 0.001 |
| 368.999 | 18.6 | C7H16O13P2 | D-Sedoheptulose 1,7-bisphosphate | 0.5 | 0.032 |
| 664.117 | 13.7 | C21H29N7O14P2 | NADH | 0.553 | 0.03 |
| 123.055 | 7.5 | C6H6N2O | Nicotinamide | 1.442 | 0.005 |
| 162.113 | 13.8 | C7H15NO3 | L-Carnitine | 0.6 | 0.039 |
| 230.139 | 5.1 | C11H19NO4 | Butenylcarnitine | 0.828 | 0.035 |
| 248.149 | 7.4 | C11H21NO5 | Hydroxybutyrylcarnitine | 0.687 | 0.023 |
| 229.144 | 5.1 | C12H22O4 | Dodecanedioic acid | 1.332 | 0.045 |
| 216.063 | 16.1 | C5H14NO6P | Sn-glycero-3-Phosphoethanolamine | 0.652 | 0.049 |
OPLS-DA score plot exhibited in Fig. 5 shows that there was greater separation, than that for neuroblastoma, between treated and non-treated samples. It can be concluded that treatment with mephedrone has produced a more significant change in the metabolome of treated astrocytoma samples compared with the metabolome of the non-treated astrocytoma samples. The effects in this cell line are much clearer than in the neuroblastoma cells. Since most of the metabolites affected are decreased it is important to check that there is not some bias in the treatment and in Table 6 it can be seen that some important intracellular metabolites such as glutathione and ATP are no different between treatment and control. This can also be seen in the raw data shown in Fig. 10 where ATP is unaffected by treatment. If there was general toxic reaction producing cell lysis one might expect ATP and glutathione to be largely lost from the samples since they do not occur in the cell medium.
Table 6.
Major intracellular metabolites in astrocytoma cells that are not affected by mephedrone treatment.
| M/z |
Rt (min) |
Structure | Metabolite name | Ratio | P-Value |
|---|---|---|---|---|---|
| 232.1545 | 9.1 | C11H21NO4 | O-Butanoylcarnitine | 1.001 | 0.994 |
| 118.0863 | 13.0 | C5H11NO2 | L-Valine | 0.995 | 0.962 |
| 88.04019 | 15.3 | C3H7NO2 | L-Alanine | 1.009 | 0.955 |
| 579.0266 | 19.1 | C15H22N2O18P2 | UDP-glucuronate | 0.988 | 0.948 |
| 132.1019 | 11.5 | C6H13NO2 | Leucine | 0.983 | 0.900 |
| 189.1599 | 23.1 | C9H20N2O2 | N6,N6,N6-Trimethyl-L-lysine | 1.018 | 0.893 |
| 130.0621 | 15.2 | C4H9N3O2 | Creatine | 1.050 | 0.868 |
| 402.9948 | 15.3 | C9H14N2O12P2 | UDP | 0.953 | 0.814 |
| 608.0888 | 15.3 | C17H27N3O17P2 | UDP-N-acetyl-D-glucosamine | 0.958 | 0.806 |
| 104.107 | 21.1 | C5H13NO | Choline | 1.071 | 0.806 |
| 124.0394 | 7.6 | C6H5NO2 | Nicotinate | 0.953 | 0.805 |
| 282.0843 | 13.1 | C10H13N5O5 | Guanosine | 1.046 | 0.801 |
| 147.1128 | 25.2 | C6H14N2O2 | L-Lysine | 0.930 | 0.785 |
| 523.9981 | 19.4 | C10H16N5O14P3 | GTP | 0.958 | 0.777 |
| 133.0142 | 16.1 | C4H6O5 | (S)-Malate | 1.051 | 0.777 |
| 505.9881 | 16.7 | C10H16N5O13P3 | ATP | 0.881 | 0.285 |
| 482.9611 | 18.0 | C9H15N2O15P3 | UTP | 0.711 | 0.345 |
| 308.091 | 14.6 | C18H13NO4 | Glutathione | 0.897 | 0.616 |
Fig. 10.
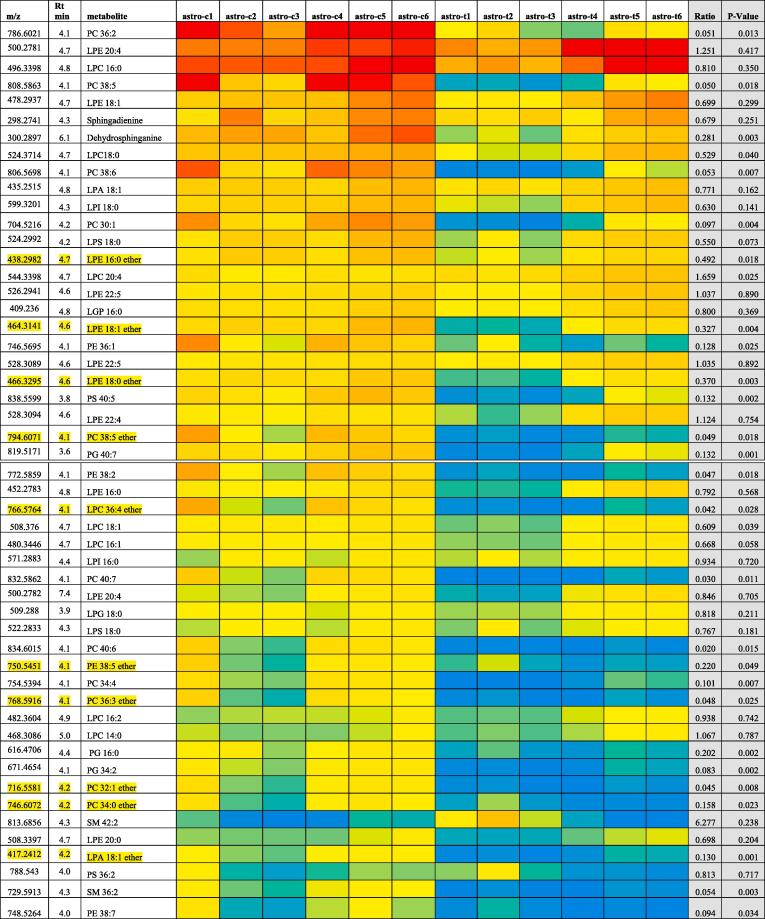
Lipids in astrocytes sorted by abundance before and after treatment with mephedrone. Red = 30 % of maximum value, yellow = 2 % of maximum value and blue = 0. SM = sphingomyelin, CER = ceramide.
In addition to the low molecular weight metabolites shown in Table 5 there are a lot of changes in lipids within the astrocytes promoted by mephedrone treatment Fig. 10. Many lipids most of which are decreased in the cells by the treatment. This can be seen in the heatmap where even some very abundant lipids are changed.
The similarity of the levels of ATP in both treated and control cells indicates that the comparison of both cells were reliable. Consequently, the comparison of the PC 36:2 levels was genuine.
Table 6 above shows no significant change was observed in the levels of the major intracellular metabolites in the astrocytoma cells after treatment with mephedrone at this concentration.
Separation between astrocytoma samples and between control and test groups is shown in Fig. 10.
Some of the most marked effects on the astrocytes are on ether lipids which are not particularly abundant in cells but have important effects on membrane fluidity (Lohner, 1996) and protective effects toward myelin against oxidative stress. Some of the ether lipids (LPE 18:1 ether, PC 38:5 ether, LPC 36:4 ether, PC 36:3 ether and PC 32:1 ether) in the cells are almost completely depleted. Mice and human patients with deficiency in ether lipids developed problems in myelination in CNS (da Silva et al., 2012). Thus, it is proposed here that mephedrone could affect myelination
Several PC and PE lipids: PE 34:1, PC 38:2, LPC 18:2, PC 36:2, PC 28:0, PC 30:0, PC 30:1, PC 30:2, PC 32:1, PC 32:2, PC 32:3, PC 32:4, PC 34:1, PC 38:5 and PC 40:7 and PC ether lipids: PC 36:3 ether, PC 32:0, PC 32:1 ether, PC 36:3 ether, PC 38:3 ether and PC 40:5 ether were all decreased. Glycerophospholipids are the main component of the cellular membrane (Hannun and Obeid, 2002). It is believed that cells change the cell membrane as a defensive reaction when attacked by extracellular toxic compounds (Montealegre et al., 2014). Glycerophosphocholines control the balance between the solute concentration inside and outside the cell (Hannun and Obeid, 2002). A previous study showed that PC levels were decreased in mouse brain after treatment with antidepressant drugs (paroxetine and maprotiline) (Lee et al., 2009). In another study, a decrease in glycerophospholipids was associated with oxidative stress and changes in the lipid membrane in the cell (Kahle et al., 2015). It is believed that oxidative stress has affected the cellular membrane composition in mephedrone treated cells. Interestingly, oxidative stress as a result of chronic unpredictable stress was shown to disturb the phospholipids levels in mouse brain (Faria et al., 2014). In another study conducted on the hippocampus of rat prefrontal cortex, 4 weeks of chronic unpredictable stress caused significant decrease in PE levels (Oliveira et al., 2016). Oxidative stress causes lipid peroxidation which leads to alteration in lipid balance, which was related with neurological disorders (Black, 2002). Oxidation of lipids causes destabilisation of membrane of the cell and then cell death (Tyurin et al., 2009). This finding suggest that mephedrone may be reducing glycerophosphocholine by oxidative stress as the effects of mephedrone were previously related to oxidative stress (den Hollander et al., 2014).
Other altered membrane lipids include glycerophospholipids (PG) phosphatidylserines (PS) and sphingomyelins (SM) which all decreased. All these lipids are part of cellular membrane, thus decrease in these lipids is a sign of membrane damage. Several lyso phospholipids were altered. As lyso phospholipids are known to have a neuroprotective role (Casado and Ascher, 1998) and any decrease will have an impact on the protective ability of neurons.
In addition, several sphingomyelins are affected by the treatment. Sphingomyelin 36:2 has decreased, and it is a type of sphingolipid located in the myelin sheath of the membrane which covers the axon of the nerve cell.
Three sphinganines decreased and they have a role in blocking esterification of low-density lipoprotein of cholesterol to yield accumulation of unesterified cholesterol in perinuclear vesicles (Deguchi et al., 2004). Sphinganines are an effective signaling lipid or lipid messenger that are attached to target protein receptor, which will facilitate the function of the lipid on particular responses of the cell (Hannun and Obeid, 2008). We can conclude that a decrease in sphinganines will affect lipid signalling in the cell.
Nine fatty acids were affected (Table 5), two increased and seven decreased. Astrocytes (represented by astrocytoma cells) are responsible for transferring fatty acids from plasma to the brain (Spector, 1988, Magret et al., 1996) and they are the only cells in the brain that can oxidise fatty acids and actually prefer them as their fuel (Magret et al., 1996). Any malfunction in astrocytes will lead to a disturbance in fatty acids levels in the brain and any decrease in fatty acids levels will lead to a decrease in astrocyte function. Mephedrone may cause a disturbance in the fatty acid levels in astrocytoma. Since, astrocytes provide other brain cells with glucose and ketone bodies through their ability to oxidise fatty acids (Flynn and Wecker, 1987), thus any effect on them will lead to decrease in energy supply for other brain cells.
Three carnitine metabolites were decreased which are l-carnitine, butenylcarnitine and hydroxybutyrylcarnitine. Carnitines have a very important role in providing cells with energy and they are vital for metabolism of fatty acids (Olpin, 2005).
Menaquinol-8 or vitamin K2 has decreased which has a role in regulating blood clotting. Vitamin K vitamins have the same methylated naphthoquinone ring structure, and differ only in the type of the side aliphatic chain linked at site 3 (Shearer and Newman, 2014).
Two glycogens are increased by the treatment. Glycogen is the main storage product of glucose in human cells. Glycogen is located in granular structures in the cytosol (Zderic et al., 2004). It is proposed that cells have produced more glycogen in order to compensate for the loss in energy supply in the face of membrane damage. In the brain glycogen is found predominantly in the astrocytes where it acts as an energy source to protect the brain from hypoglycemia (Walls et al., 2009).
Other metabolites associated with glutamate metabolism such as 2-oxoglutarate, l-2-aminoadipate and d-glucosamine 6-phosphate were altered. The oxidised glutamate (oxoglutarate) was decreased by 60 % and the others were increased. Aminoadipate was upregulated and it is an antagonist for glutamate excitatory effect and is a metabolite of lysine (Wu et al., 1995). Glutamate is an important neurotransmitter and responsible for fast excitation effect in the brain (Smith, 2000). L-Asparagine was decreased and it is a metabolite of glutamate (Avramis and Panosyan, 2005). However, we cannot conclude any effect on glutamate, as its level was not altered significantly.
Two metabolites related to the tryptophan and serotonin pathway were decreased. 5-Hydroxyindoleacetate is a metabolite of the neurotransmitter serotonin (Owens and Nemeroff, 1994). Indolelactate was also decreased which is a metabolite of tryptophan, the precursor for serotonin and melatonin (Morita et al., 1990). However, we could not detect serotonin or tryptophan in our method.
Nicotinamide and NADH were also altered with significant p values. NADH was decreased and nicotinamide was increased. Nicotinamide plays an important role in the protection of neurons (Fricker et al., 2018) as the pretreatment with nicotinamide reversed hypoxia in hippocampus of rats (Shetty et al., 2014). Thus, we conclude that in one of its defence mechanisms, astrocytoma cells produced nicotinamide to protect themselves and neuronal cells against oxidative stress caused by mephedrone treatment.
5. Discussion
In the MTT assay, both cell types increased metabolic activity after mephedrone treatment. The SH-SY5Y cells did not show any increase in NR assay in the 100 μM wells. In contrast, in the U-373 cells, the increased activity was observed for both assays (NR and MTT), with a higher increase in the metabolism activity. This can be explained by the additional protective ability of U-373 to increase cell numbers, as they represent astrocytes, which play a role in protecting neuronal cells (La Quaglia and Manchester, 1996).
The NR assay showed no significant effect of mephedrone, over a range of concentrations, on the growth rate of SH-SY5Y cells as shown in Fig. 5. For the U-373 cells there was gradual increase in cell number as we increased the concentration of mephedrone up to 300 μM. Cell growth started to decline at the 400 μM and 500 μM concentrations of mephedrone.
LDH release measurement is a good indicator of cellular damage and the integrity of cell membrane. Since the results showed no significant increase in both cell types, we can conclude that mephedrone at this concentration and time of exposure did not have a strong impact on the integrity of the cell membrane in these cells.
We can conclude from the morphology examination that there was no significant change in the shape of the cells as a result of mephedrone treatment at this concentration and time of exposure in SH-SY5Y and U-373 cells. This finding contradicts with the above mentioned study of Hollander finding especially in the SH-SY5Y cells at the concentration of 500 μM. However, the methodology used by Hollander study was different (WST-1 assay) (den Hollander et al., 2014). This can be also interpreted by the difference in the passage numbers as Hollander conducted the study between 6 and 20 passages, whereas in our study we used passages above 35.
The overall the impression after the metabolomics analysis, is that there is no strong pattern of effect of mephedrone on the SH-SY5Y cells in these concentrations. Either the mephedrone is targeting a specific type of cell other than neuroblastoma or that this concentration of mephedrone is not able to cause a potent effect on this cell line. The metabolite alterations are in a random selection of metabolites, which do not fall in any particular pathway and the fold changes are generally small.
It is also noteworthy to mention that these cell lines (SH-SY5Y) lose their dopaminergic abilities after 20 passages (Biedler et al., 1978), and the analysis was performed at 35 and above passages, which makes these cells less representative of neuronal cells. The rise in the metabolism seen in the MTT results (Fig. 1) at this concentration can be explained as a possible increase in specific proteins that are not detectable by metabolomics analysis.
In the astrocytoma cells, the major pathway affected was the lipid pathway particularly cell membrane lipids. This finding can open the way to understand the effect of mephedrone on the brain particularly the brain-barrier (astrocytes). Affecting the astrocytes as an important part of the brain barrier will affect the neurons as the astrocytes known to have protective role towards the neuronal cells (La Quaglia and Manchester, 1996). However, mephedrone was not strongly cytotoxic at this concentration and even though it induced changes in the CNS cell metabolome, these are not responsible for its toxicity in the body as cell numbers were not reduced as shown in the results of the NR assay (Fig. 2).
Fig. 2.
Neutral red assay measured in (A) SH-SY5Y cells and in (B) U-373 cells after 48 h. Results are percentage values (Mean ± SD, n = 6) where 100 % corresponds to control values. Cells treated with mephedrone in culture medium throughout the experiment. Data were analysed using one-way ANOVA plus Tukey test. * data were significantly different from their relevant control P value < 0.05.
The metabolomic analysis has proved that there was a change in the metabolism of SH-SY5Y and U373 cells after treatment with mephedrone at 100 μM, which was consistent with MTT assay findings. Both cell types metabolomic results proposed a rise in glutamine concentration after treatment with mephedrone. The appearance of cysteineglutathione disulfide in SH-SY5Y after treatment with mephedrone suggests an oxidative stress occurs. The effect of mephedrone was much more marked on the U373 cells.
6. Conclusion
From the analysis of different cell types, it is clear that although mephedrone did not kill or decrease the growth of cells; but it did produce marked intracellular metabolic changes. It is believed that even a small change in brain cell homeostasis can lead to neurological disease (Budzynska et al., 2015, López-Arnau et al., 2015). Although there no clear indicators of cellular damage caused by mephedrone. In astrocytes there was a clear indication that cell membrane function might be impaired by depletion of ether lipids. Higher concentrations of mephedrone on same cell lines is recommended to be used in a future metabolomic study to better investigate the toxic effect of mephedrone.
CRediT authorship contribution statement
Ibrahim M. Alanazi: Conceptualising, Original draft, Writing, Reviewing, Editing, Formal analysis. Abdullah R. Alzahrani: Review and editing. Mohammad A. Alsaad: Review and editing. Abdulaziz L. Moqeem: Formal analysis. Abdulmohsen M. Hamdi: Formal analysis. Mohiuddin M. Taher: Review and editing. David G. Watson: Supervision. M. Helen Grant: Supervision.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Ibrahim M. Alanazi, Email: imanzi@uqu.edu.sa.
Mohammad A. Alsaad, Email: masaad@uqu.edu.sa.
Abdulaziz L. Moqeem, Email: abdulaziz9394@hotmail.com.
Abdulmohsen M. Hamdi, Email: ammzh7@yahoo.com.
Mohiuddin M. Taher, Email: tmmohiuddin@uqu.edu.sa.
David G. Watson, Email: D.G.Watson@strath.ac.uk.
M. Helen Grant, Email: m.h.grant@strath.ac.uk.
References
- Angoa-Pérez M., Kane M.J., Briggs D.I., Francescutti D.M., Sykes C.E., Shah M.M., Thomas D.M., Kuhn D.M. Mephedrone does not damage dopamine nerve endings of the striatum, but enhances the neurotoxicity of methamphetamine, amphetamine, and MDMA. J. Neurochem. 2013;125(1):102–110. doi: 10.1111/jnc.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angoa-Pérez M., Kane M.J., Herrera-Mundo N., Francescutti D.M., Kuhn D.M. Effects of combined treatment with mephedrone and methamphetamine or 3, 4-methylenedioxymethamphetamine on serotonin nerve endings of the hippocampus. Life Sci. 2014;97(1):31–36. doi: 10.1016/j.lfs.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avramis V.I., Panosyan E.H. Pharmacokinetic/pharmacodynamic relationships of asparaginase formulations: the past, the present and recommendations for the future. Clin. Pharmacokinet. 2005;44(4):367–393. doi: 10.2165/00003088-200544040-00003. [DOI] [PubMed] [Google Scholar]
- Baumann M.H., Ayestas M.A., Partilla J.S., Sink J.R., Shulgin A.T., Daley P.F., Brandt S.D., Rothman R.B., Ruoho A.E., Cozzi N.V. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology. 2012;37(5):1192–1203. doi: 10.1038/npp.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedler J.L., Roffler-Tarlov S., Schachner M., Freedman L.S. Multiple neurotransmitter synthesis by human neuroblastoma cell lines and clones. Cancer Res. 1978;38(11 Pt 1):3751–3757. [PubMed] [Google Scholar]
- Black P.H. Stress and the inflammatory response: a review of neurogenic inflammation. Brain Behav. Immun. 2002;16(6):622–653. doi: 10.1016/s0889-1591(02)00021-1. [DOI] [PubMed] [Google Scholar]
- Borenfreund E., Puerner J.A. Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol. Lett. 1985;24(2–3):119–124. doi: 10.1016/0378-4274(85)90046-3. [DOI] [PubMed] [Google Scholar]
- Budzynska B., Boguszewska-Czubara A., Kruk-Slomka M., Kurzepa J., Biala G. Mephedrone and Nicotine: Oxidative Stress and Behavioral Interactions in Animal Models. Neurochem. Res. 2015;40(5):1083–1093. doi: 10.1007/s11064-015-1566-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busardò F.P., Kyriakou C., Tittarelli R., Mannocchi G., Pantano F., Santurro A., Zaami S., Baglìo G. Assessment of the stability of mephedrone in ante-mortem and post-mortem blood specimens. Forensic Sci. Int. 2015;256:28–37. doi: 10.1016/j.forsciint.2015.07.021. [DOI] [PubMed] [Google Scholar]
- Camilleri A., Johnston M.R., Brennan M., Davis S., Caldicott D.G. Chemical analysis of four capsules containing the controlled substance analogues 4-methylmethcathinone, 2-fluoromethamphetamine, alpha-phthalimidopropiophenone and N-ethylcathinone. Forensic Sci Int. 2010;197(1–3):59–66. doi: 10.1016/j.forsciint.2009.12.048. [DOI] [PubMed] [Google Scholar]
- Casado M., Ascher P. Opposite modulation of NMDA receptors by lysophospholipids and arachidonic acid: common features with mechanosensitivity. J. Physiol. 1998;513(Pt 2):317–330. doi: 10.1111/j.1469-7793.1998.317bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciudad-Roberts A., Duart-Castells L., Camarasa J., Pubill D., Escubedo E. The combination of ethanol with mephedrone increases the signs of neurotoxicity and impairs neurogenesis and learning in adolescent CD-1 mice. Toxicol. Appl. Pharmacol. 2016;293:10–20. doi: 10.1016/j.taap.2015.12.019. [DOI] [PubMed] [Google Scholar]
- da Silva T.F., Sousa V.F., Malheiro A.R., Brites P. (2012) 'The importance of ether-phospholipids: a view from the perspective of mouse models'. BBA. 2012;9:1501–1508. doi: 10.1016/j.bbadis.2012.05.014. [DOI] [PubMed] [Google Scholar]
- Deguchi H., Yegneswaran S., Griffin J.H. Sphingolipids as bioactive regulators of thrombin generation. J. Biol. Chem. 2004;279(13):12036–12042. doi: 10.1074/jbc.M302531200. [DOI] [PubMed] [Google Scholar]
- den Hollander B., Rozov S., Linden A.-M., Uusi-Oukari M., Ojanperä I., Korpi E.R. Long-term cognitive and neurochemical effects of “bath salt” designer drugs methylone and mephedrone. Pharmacol. Biochem. Behav. 2013;103(3):501–509. doi: 10.1016/j.pbb.2012.10.006. [DOI] [PubMed] [Google Scholar]
- den Hollander B., Sundström M., Pelander A., Ojanperä I., Mervaala E., Korpi E.R., Kankuri E. Keto amphetamine toxicity-focus on the redox reactivity of the cathinone designer drug mephedrone. Toxicol. Sci. 2014;141(1):120–131. doi: 10.1093/toxsci/kfu108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshleman A.J., Wolfrum K.M., Hatfield M.G., Johnson R.A., Murphy K.V., Janowsky A. Substituted methcathinones differ in transporter and receptor interactions. Biochem. Pharmacol. 2013;85(12):1803–1815. doi: 10.1016/j.bcp.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria R., Santana M.M., Aveleira C.A., Simões C., Maciel E., Melo T., Santinha D., Oliveira M.M., Peixoto F., Domingues P., Cavadas C., Domingues M.R. Alterations in phospholipidomic profile in the brain of mouse model of depression induced by chronic unpredictable stress. Neuroscience. 2014;273:1–11. doi: 10.1016/j.neuroscience.2014.04.042. [DOI] [PubMed] [Google Scholar]
- Flynn C.J., Wecker L. Concomitant increases in the levels of choline and free fatty acids in rat brain: evidence supporting the seizure-induced hydrolysis of phosphatidylcholine. J. Neurochem. 1987;48(4):1178–1184. doi: 10.1111/j.1471-4159.1987.tb05644.x. [DOI] [PubMed] [Google Scholar]
- Fricker R.A., Green E.L., Jenkins S.I., Griffin S.M. The Influence of Nicotinamide on Health and Disease in the Central Nervous System. Int. J. Tryptophan Res. 2018;11 doi: 10.1177/1178646918776658. 1178646918776658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons S., Zloh M. An analysis of the 'legal high' mephedrone. Bioorg. Med. Chem. Lett. 2010;20(14):4135–4139. doi: 10.1016/j.bmcl.2010.05.065. [DOI] [PubMed] [Google Scholar]
- Hadlock G.C., Webb K.M., McFadden L.M., Chu P.W., Ellis J.D., Allen S.C., Andrenyak D.M., Vieira-Brock P.L., German C.L., Conrad K.M., Hoonakker A.J., Gibb J.W., Wilkins D.G., Hanson G.R., Fleckenstein A.E. 4-Methylmethcathinone (mephedrone): neuropharmacological effects of a designer stimulant of abuse. J. Pharmacol. Exp. Ther. 2011;339(2):530–536. doi: 10.1124/jpet.111.184119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannun Y.A., Obeid L.M. The Ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. J. Biol. Chem. 2002;277(29):25847–25850. doi: 10.1074/jbc.R200008200. [DOI] [PubMed] [Google Scholar]
- Hannun Y.A., Obeid L.M. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 2008;9(2):139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- Inoue H., Yokota H., Makino T., Yuasa A., Kato S. Bisphenol a glucuronide, a major metabolite in rat bile after liver perfusion. Drug Metab. Dispos. 2001;29(8):1084–1087. [PubMed] [Google Scholar]
- Johnson R.D., Botch-Jones S.R. The stability of four designer drugs: MDPV, mephedrone, BZP and TFMPP in three biological matrices under various storage conditions. J. Anal. Toxicol. 2013;37(2):51–55. doi: 10.1093/jat/bks138. [DOI] [PubMed] [Google Scholar]
- Kahle M., Schäfer A., Seelig A., Schultheiß J., Wu M., Aichler M., Leonhardt J., Rathkolb B., Rozman J., Sarioglu H., Hauck S.M., Ueffing M., Wolf E., Kastenmueller G., Adamski J., Walch A., Hrabé de Angelis M., Neschen S. High fat diet-induced modifications in membrane lipid and mitochondrial-membrane protein signatures precede the development of hepatic insulin resistance in mice. Molecular Metabolism. 2015;4(1):39–50. doi: 10.1016/j.molmet.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehr J., Ichinose F., Yoshitake S., Goiny M., Sievertsson T., Nyberg F., Yoshitake T. Mephedrone, compared with MDMA (ecstasy) and amphetamine, rapidly increases both dopamine and 5-HT levels in nucleus accumbens of awake rats. Br. J. Pharmacol. 2011;164(8):1949–1958. doi: 10.1111/j.1476-5381.2011.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Quaglia M.P., Manchester K.M. A comparative analysis of neuroblastic and substrate-adherent human neuroblastoma cell lines. J. Pediatr. Surg. 1996;31(2):315–318. doi: 10.1016/s0022-3468(96)90025-1. [DOI] [PubMed] [Google Scholar]
- Lee L.H., Shui G., Farooqui A.A., Wenk M.R., Tan C.H., Ong W.Y. Lipidomic analyses of the mouse brain after antidepressant treatment: evidence for endogenous release of long-chain fatty acids? Int. J. Neuropsychopharmacol. 2009;12(7):953–964. doi: 10.1017/S146114570900995X. [DOI] [PubMed] [Google Scholar]
- Lim A., Prokaeva T., McComb M.E., Connors L.H., Skinner M., Costello C.E. Identification of S-sulfonation and S-thiolation of a novel transthyretin Phe33Cys variant from a patient diagnosed with familial transthyretin amyloidosis. Protein Sci. 2003;12(8):1775–1785. doi: 10.1110/ps.0349703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohner K. Is the high propensity of ethanolamine plasmalogens to form non-lamellar lipid structures manifested in the properties of biomembranes? Chem. Phys. Lipids. 1996;81(2):167–184. doi: 10.1016/0009-3084(96)02580-7. [DOI] [PubMed] [Google Scholar]
- López-Arnau R., Martínez-Clemente J., Rodrigo T., Pubill D., Camarasa J., Escubedo E. Neuronal changes and oxidative stress in adolescent rats after repeated exposure to mephedrone. Toxicol. Appl. Pharmacol. 2015;286(1):27–35. doi: 10.1016/j.taap.2015.03.015. [DOI] [PubMed] [Google Scholar]
- Magret V., Elkhalil L., Nazih-Sanderson F., Martin F., Bourre J.M., Fruchart J.C., Delbart C. Entry of polyunsaturated fatty acids into the brain: evidence that high-density lipoprotein-induced methylation of phosphatidylethanolamine and phospholipase A2 are involved. Biochem. J. 1996;316(Pt 3):805–811. doi: 10.1042/bj3160805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marszalek-Grabska M., Zakrocka I., Budzynska B., Marciniak S., Kaszubska K., Lemieszek M.K., Winiarczyk S., Kotlinska J.H., Rzeski W., Turski W.A. Binge-like mephedrone treatment induces memory impairment concomitant with brain kynurenic acid reduction in mice. Toxicol. Appl. Pharmacol. 2022;454 doi: 10.1016/j.taap.2022.116216. [DOI] [PubMed] [Google Scholar]
- Maskell P.D., Seetohul L.N., Livingstone A.C., Cockburn A.K., Preece J., Pounder D.J. Stability of 3,4-methylenedioxymethampetamine (MDMA), 4-methylmethcathinone (mephedrone) and 3-trifluromethylphenylpiperazine (3-TFMPP) in formalin solution. J. Anal. Toxicol. 2013;37(7):440–446. doi: 10.1093/jat/bkt051. [DOI] [PubMed] [Google Scholar]
- Meyer M.R., Wilhelm J., Peters F.T., Maurer H.H. Beta-keto amphetamines: studies on the metabolism of the designer drug mephedrone and toxicological detection of mephedrone, butylone, and methylone in urine using gas chromatography-mass spectrometry. Anal Bioanal Chem. 2010;397(3):1225–1233. doi: 10.1007/s00216-010-3636-5. [DOI] [PubMed] [Google Scholar]
- Montealegre C., Verardo V., Luisa Marina M., Caboni M.F. Analysis of glycerophospho- and sphingolipids by CE. Electrophoresis. 2014;35(6):779–792. doi: 10.1002/elps.201300534. [DOI] [PubMed] [Google Scholar]
- Morita I., Kawamoto M., Hattori M., Eguchi K., Sekiba K., Yoshida H. Determination of tryptophan and its metabolites in human plasma and serum by high-performance liquid chromatography with automated sample clean-up system. J. Chromatogr. 1990;526(2):367–374. doi: 10.1016/s0378-4347(00)82520-7. [DOI] [PubMed] [Google Scholar]
- Motbey C.P., Hunt G.E., Bowen M.T., Artiss S., McGregor I.S. Mephedrone (4-methylmethcathinone, 'meow'): acute behavioural effects and distribution of Fos expression in adolescent rats. Addict. Biol. 2012;17(2):409–422. doi: 10.1111/j.1369-1600.2011.00384.x. [DOI] [PubMed] [Google Scholar]
- Oliveira T.G., Chan R.B., Bravo F.V., Miranda A., Silva R.R., Zhou B., Marques F., Pinto V., Cerqueira J.J., Di Paolo G., Sousa N. The impact of chronic stress on the rat brain lipidome. Mol. Psychiatry. 2016;21(1):80–88. doi: 10.1038/mp.2015.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olpin S.E. Fatty acid oxidation defects as a cause of neuromyopathic disease in infants and adults. Clin. Lab. 2005;51(5–6):289–306. [PubMed] [Google Scholar]
- Owens M.J., Nemeroff C.B. Role of serotonin in the pathophysiology of depression: focus on the serotonin transporter. Clin. Chem. 1994;40(2):288–295. [PubMed] [Google Scholar]
- Posada O.M., Tate R.J., Grant M.H. Effects of CoCr metal wear debris generated from metal-on-metal hip implants and Co ions on human monocyte-like U937 cells. Toxicol. In Vitro. 2015;29(2):271–280. doi: 10.1016/j.tiv.2014.11.006. [DOI] [PubMed] [Google Scholar]
- Serefko A., Bielecka-Papierz G., Talarek S., Szopa A., Skałecki P., Szewczyk B., Radziwoń-Zaleska M., Poleszak E. Central Effects of the Designer Drug Mephedrone in Mice—Basic Studies. Brain Sci. 2022;12:189. doi: 10.3390/brainsci12020189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer M.J., Newman P. Recent trends in the metabolism and cell biology of vitamin K with special reference to vitamin K cycling and MK-4 biosynthesis. J. Lipid Res. 2014;55(3):345–362. doi: 10.1194/jlr.R045559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty P.K., Galeffi F., Turner D.A. Nicotinamide pre-treatment ameliorates NAD(H) hyperoxidation and improves neuronal function after severe hypoxia. Neurobiol. Dis. 2014;62:469–478. doi: 10.1016/j.nbd.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Q.R. Transport of glutamate and other amino acids at the blood-brain barrier. J. Nutr. 2000;130(4S Suppl):1016S–S1022. doi: 10.1093/jn/130.4.1016S. [DOI] [PubMed] [Google Scholar]
- Sørensen L.K. 'Determination of cathinones and related ephedrines in forensic whole-blood samples by liquid-chromatography-electrospray tandem mass spectrometry'. J. Chromatogr. B, Anal. Technol. Biomed. Life Sci. 2011;879(11–12):727–736. doi: 10.1016/j.jchromb.2011.02.010. [DOI] [PubMed] [Google Scholar]
- Spector R. Fatty acid transport through the blood-brain barrier. J. Neurochem. 1988;50(2):639–643. doi: 10.1111/j.1471-4159.1988.tb02958.x. [DOI] [PubMed] [Google Scholar]
- Tsujikawa K., Mikuma T., Kuwayama K., Miyaguchi H., Kanamori T., Iwata Y.T., Inoue H. Degradation pathways of 4-methylmethcathinone in alkaline solution and stability of methcathinone analogs in various pH solutions. Forensic Sci. Int. 2012;220(1–3):103–110. doi: 10.1016/j.forsciint.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Tyurin V.A., Tyurina Y.Y., Jung M.Y., Tungekar M.A., Wasserloos K.J., Bayir H., Greenberger J.S., Kochanek P.M., Shvedova A.A., Pitt B., Kagan V.E. 'Mass-spectrometric analysis of hydroperoxy- and hydroxy-derivatives of cardiolipin and phosphatidylserine in cells and tissues induced by pro-apoptotic and pro-inflammatory stimuli', Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences. 2009;877(26):2863–2872. doi: 10.1016/j.jchromb.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstock A., Mitcheson L., Marsden J. Mephedrone: still available and twice the price. Lancet. 2010;376(9752):1537. doi: 10.1016/S0140-6736(10)62021-1. [DOI] [PubMed] [Google Scholar]
- Winstock A., Mitcheson L., Ramsey J., Davies S., Puchnarewicz M., Marsden J. Mephedrone: use, subjective effects and health risks. Addiction. 2011;106(11):1991–1996. doi: 10.1111/j.1360-0443.2011.03502.x. [DOI] [PubMed] [Google Scholar]
- Winstock A.R., Mitcheson L.R., Deluca P., Davey Z., Corazza O., Schifano F. Mephedrone, new kid for the chop? Addiction. 2011;106(1):154–161. doi: 10.1111/j.1360-0443.2010.03130.x. [DOI] [PubMed] [Google Scholar]
- Wu H.Q., Ungerstedt U., Schwarcz R. L-alpha-aminoadipic acid as a regulator of kynurenic acid production in the hippocampus: a microdialysis study in freely moving rats. Eur. J. Pharmacol. 1995;281(1):55–61. doi: 10.1016/0014-2999(95)00224-9. [DOI] [PubMed] [Google Scholar]
- Zderic T.W., Schenk S., Davidson C.J., Byerley L.O., Coyle E.F. 'Manipulation of dietary carbohydrate and muscle glycogen affects glucose uptake during exercise when fat oxidation is impaired by beta-adrenergic blockade', American Journal of Physiology. Endocrinol. Metab. 2004;287(6):E1195–E1201. doi: 10.1152/ajpendo.00302.2004. [DOI] [PubMed] [Google Scholar]
- Zijlstra W.P., Bulstra S.K., van Raay J.J., van Leeuwen B.M., Kuijer R. Cobalt and chromium ions reduce human osteoblast-like cell activity in vitro, reduce the OPG to RANKL ratio, and induce oxidative stress. J. Orthop. Res. 2012;30(5):740–747. doi: 10.1002/jor.21581. [DOI] [PubMed] [Google Scholar]