Abstract
Background
Stereotactic radiosurgery (SRS) has recently gained space as an accepted non-invasive alternative treatment option for drug resistant Glossopharyngeal neuralgia (GPN). The purpose of this systematic review was to provide an overview of the outcomes of SRS treatment in patients with GPN.
Methods
A literature review until March 2023 was performed. Data about patient's demographics, complications and recurrence rates, additional treatment post procedure as well as pain outcomes in the short and long term were collected. Studies without reported pain outcomes were excluded.
Results
Sixteen studies with a total of 97 patients diagnosed with GPN who had undergone SRS were identified. The mean reported maximal radiation dose ranged from 70 to 88.7 Gy with the glossopharyngeal meatus (GPM) being the most common target in 12/16 studies. The median time from SRS till pain response was between 2 and 120 days. The mean proportion of patients requiring further treatment after SRS ranged from 11.1 to 57.14% in a time frame between 2 and 36 months post procedure. Favourable pain response rates after SRS (BNI-IIIb) ranged from 60% to 100% and 57.1%–100% in short and long term respectively.
Conclusion
SRS for GPN remains a safe alternative to surgery with low complication rates and favourable pain outcomes in both short and long term.
Keywords: Glossopharyngeal neuralgia, Stereotactic radiosurgery, Craniofacial pain, Gamma knife, Frameless image-guided radiosurgery
Abbreviations
- BNI
Barrow Neurological Institute Pain Intensity Score
- CIS
Cisternal part of glossopharyngeal nerve
- CNs
Cranial nerves
- FSRS
Frameless stereotactic radiosurgery
- GKR
Gamma Knife radiosurgery
- GPM
Glossopharyngeal meatus
- GPN
Glossopharyngeal neuralgia
- LINAC
image-guided linear accelerator
- MVD
Microvascular Decompression
- NS
Nerve section
- PRT
Percutaneous radiofrequency thermocoagulation (PRT)
- RHZ
Rhizotomy
- SRS
Stereotactic radiosurgery
1. Introduction
Glossopharyngeal neuralgia (GPN) is a rare craniofacial disorder characterized by brief painful episodes of stabbing pain affecting the sensory distribution of the auricular and pharyngeal branches of the glossopharyngeal (IX) and vagus (X) cranial nerves.1 This includes the ipsilateral external ear canal, base of tongue, tonsil, or the area beneath the angle of the jaw. An episode can last anywhere between 2 s and 2 min and can occur up to 200 times per day.2 Unlike its counterpart trigeminal neuralgia, GPN is a rare facial pain syndrome with an incidence estimated to be between 0.2 and 0.7 per 100,000 individuals per year.3 Occasionally, in a small proportion of cases (2–3%), GPN may be accompanied by severe cardiovascular symptoms, such as life-threatening syncopal episodes, hypotension or bradycardia.4 In the vast majority of GPN patients, no underlying cause or associated neurological deficit is identified and the syndrome is therefore termed “classic” or idiopathic, while a smaller group is “symptomatic”, because of a structural lesion affecting the distribution of the IX and X cranial nerves.5
The treatment strategy is broadly similar to trigeminal neuralgia, with surgery being reserved for drug-resistant cases. First line treatment is typically medical, with anticonvulsant medications such carbamazepine or gabapentin.1 Nowadays, microvascular decompression (MVD) is one of the most widely used surgical options for GPN followed by rhizotomy (RHZ).6 MVD provides high rates of pain relief (up to 80%–90%) and sustained results but at the same time it is associated with complications that include lower cranial nerve damage reported in 8%–19% of cases.7 However, with GPN mostly affecting the elderly population, there are potentially significant risks related to surgery or anaesthesia that need to be considered in the management of GPN.8
Over the last 20 years, similar to trigeminal neuralgia, ablative techniques like stereotactic radiosurgery (SRS) and specifically Gamma Knife radiosurgery (GKR) have become an accepted alternative for patients with GPN.9 The first report of radiosurgery used for the treatment of GPN was by Stieber et al in 2005.10 Since then, given the rarity of the syndrome, only few studies looking at the efficacy of radiosurgery in the treatment of glossopharyngeal neuralgia have been published.
To the best of our knowledge, this is the first systematic review looking at the role of both GKS and frameless SRS in the treatment of drug resistant GPN cases. The aim of this review is to assess the efficacy of SRS as an alternative treatment for GPN and provide an overview of short and long term treatment outcomes.
2. Methods
Data was obtained through a literature search published of PubMed, Scopus, Embase, and Cochrane databases until 9th of March 2023, conducted by two independent reviewers (TS and GA) according to PRISMA guidelines.11 We selected 2005 as a starting date because prior to that, there were no reports on GPN management with SRS. The search query consisted of the following terms: “glossopharyngeal” and “neuralgia” and “Stereotactic radiosurgery or Gamma knife surgery” (The search strategy is available upon request). The reference lists of all relevant studies were also manually checked to identify additional eligible studies that might have been missed during the initial electronic search.
3. Inclusion and exclusion criteria
Eligibility criteria were: 1) full-text, English-language published cohorts, 2) case series, and case reports including patients with a clinical diagnosis of GPN, 3) subsequent management using GKS or frameless SRS, 4) clearly documented treatment outcomes. Exclusion criteria were: 1) no full text (published abstracts), 2) non-English language, 3) neuralgias involving cranial nerves (CNs) other than IX, 4) duplicate patients included in other multi-centre studies. All the articles that were deemed relevant were retrieved, and the full text was reviewed. Studies that met the inclusion criteria were analysed.
Pain response to GKS has been standardized according to the Barrow Neurological Institute Pain Intensity Score (BNI).12 Some studies used the 2-tier scale for pain relief (achieved pain relief or not). BNI Grade I was defined as pain-free without medication; BNI Grade II as occasional pain but not requiring medication, BNI Grade IIIa as no pain but with continued use of medications, BNI Grade IIIb as occasional pain controlled with medication, BNI Grade IV as pain improved but not adequately controlled with medication and BNI Grade V as no pain relief.12 BNI grade I-IIIb was regarded as positive pain response while BNI IV and V as treatment failures. Recurrence was defined as a new painful event after an initial positive response to SRS. RevMan 5.3 was used for statistical analysis (Review Manager (RevMan) [Computer program]. Version 5.4. The Cochrane Collaboration, 2020.)
4. Risk of bias
The risk of bias for each study was evaluated using a domain-based tool from the Newcastle–Ottawa Scale for non-randomised studies.13 Risk of bias assessment was based on the following domains; selection of patients, comparability of cohorts on the basis of design or analysis, and outcomes. Studies are defined as high quality when scoring ≥7. Consecutive or obviously representative series of cases were regarded as low risk of bias in terms of “Selection of patients”. A validated diagnostic tool to assess pain response post treatment with SRS was the main factor that affected the risk of detection. Outcome bias assessment was also affected by whether follow-up was long enough for outcomes to occur, whether there was adequacy of follow-up for cohorts and whether there was blinding of during the outcome assessment.
A quantitative assessment of study quality was performed with the highest quality studies being awarded a maximum of one star for each item.
5. Results
5.1. Risk of bias of included studies
Overall, 11/19 studies (42%)10; 15; 18; 23; 25, 26, 27, 28 were assessed as having low risk of bias based on their NOS score (Table 1). Regarding sample size justification, risk of bias was regarded as low in a minority of studies, with most studies scoring 1 out of 4 stars. This is because given the rarity of GPN, most of the studies were either case reports or case series. Only 8/19 (42%)15, 16; 23; 26, 27, 28 reported a validated diagnostic tool to assess pain response post treatment with SRS. Also, assessment of pain outcomes was not blind in any of the included studies, consequently resulting in high risk of detection bias within this domain. Only two studies collected data prospectively24;25 and the rest of the included studies were retrospective case series.
Table 1.
Risk of bias assessment for the included studies.
| Studies | Selection (out of 4) | Comparability (out of 2) | Outcomes (out of 3) | Total NOS score (out of 9) | Risk of bias |
|---|---|---|---|---|---|
| Kano et al 201615 | 4 | 2 | 3 | 9 | Low |
| Pollock et al 201114 | 2 | 2 | 1 | 5 | High |
| Stanic et al31 | 1 | 2 | 2 | 5 | High |
| Williams et al 201017 | 1 | 2 | 2 | 5 | High |
| Pommier et al 201816 | 1 | 2 | 3 | 6 | High |
| Martinez-Alvarez et al 201418 | 3 | 2 | 2 | 7 | Low |
| Borius et al 201719 | 4 | 2 | 2 | 8 | Low |
| Leveque et al 201120 | 4 | 2 | 2 | 8 | Low |
| Pathmarajah et al 202221 | 1 | 2 | 2 | 5 | High |
| Chua et al 202022 | 1 | 2 | 2 | 5 | High |
| O Connor et al 201323 | 1 | 2 | 3 | 6 | High |
| Almunia et al 202224 | 4 | 2 | 3 | 9 | Low |
| Xiong et al 201525 | 1 | 2 | 3 | 6 | High |
| Yomo et al 200926 | 2 | 2 | 3 | 7 | Low |
| Heroux et al 201527 | 1 | 2 | 2 | 5 | High |
| Ballosier et al 201928 | 4 | 2 | 3 | 9 | Low |
| Chai et al 202129 | 4 | 2 | 3 | 9 | Low |
| Stieber et al 200510 | 1 | 2 | 3 | 6 | High |
| Kaye et al 202030 | 1 | 2 | 3 | 6 | High |
5.2. Patients demographics
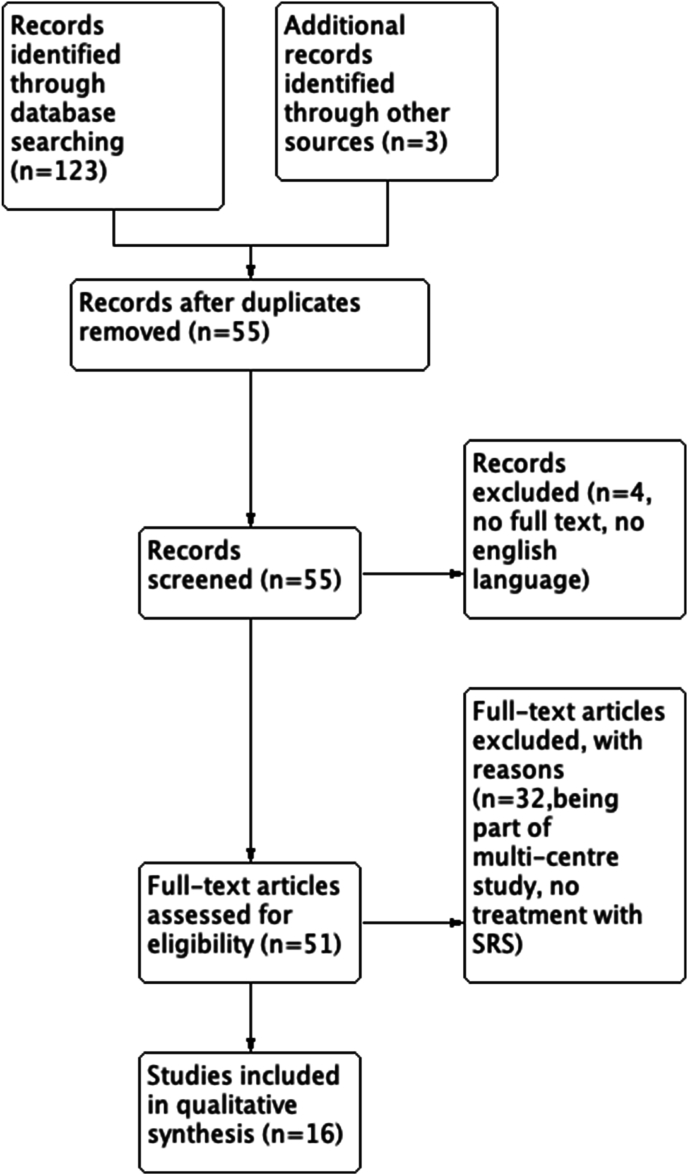
The initial search identified 123 studies. References were screened by viewing their titles and abstracts and 3 additional studies were identified. After the first-level screening and excluding duplicate publications, 55 full texts were assessed for eligibility (Fig. 1). We identified 19 studies14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31 between 2005 and 2023 (Table 2). Three14; 17;31 studies were also part of another multicentre analysis15 so their data was not included in the final analysis. The total number of patients was 97, with the mean age ranging from 47 to 99 years (see Table 3).
Fig. 1.
Study flow chart.
Table 2.
Patient demographics.
| Studies | Cases | Men % | Mean age | Type | Target | Time of symptoms before treatment (months) | max radiation (Gy) |
|---|---|---|---|---|---|---|---|
| Kano et al 201615 | 22 | 8 (36.3%) | 60 | GKS | GPM | 46 | 80 (80–90) |
| Pollock et al 201114 | 5 | 3 (60%) | 61 | GKS | GPM | 60 | 80 (60–80) |
| Stanic et al31 | 1 | 1 (100%) | 51 | GKS | CIS | 96 | 80 |
|
Williams et al 201017 |
1 |
0 (0%) |
47 |
GKS |
GPM |
NA |
80 |
| Pommier et al 201816 | 9 | 4 (44.4%) | GKS | GPM | 85.3 | 80 (80–90) | |
| Martinez-Alvarez et al 201418 | 5 | 1 (20%) | GKS | GPM | NA | 88 (80–90) | |
| Borius et al 201719 | 21 | 10 (47.6%) | 68.3 | GKS | GPM | 88.8 | 81.4 (60–90) |
| Leveque et al 201120 | 7 | 5 (71.4%) | 62 | GKS | 5 GPM 2 CIS | 28 | 83.6 (60–80) |
| Pathmarajah et al 202221 | 2 | 0 (0%) | 56 | Linac | GPM | NA | 70 |
| Chua et al 202022 | 1 | 0 (0%) | 54 | frame-less SRS | GPM | 48 | 80 |
| O Connor et al 201323 | 1 | 0 (0%) | 99 | GKS | GPM | 18 | 80 |
| Almunia et al 202224 | 8 | 2 (25%) | 59.5 | GKS | GPM | 84 | 88.7 (80–90) |
| Xiong et al 201525 | 3 | 1 (33%) | 65 | GKS | CIS l | 147.6 | 80 |
| Yomo et al 200926 | 2 | 1 (50%) | 66 | GKS | GPM | na | 67.5 (60–75) |
| Heroux et al 201527 | 1 | 1 (100%) | 48 | GKS | GPM | 96 | 80 |
| Ballosier et al 201928 | 6 | 4 (66.6%) | 70.2 | GKS | GPM | 30.5 | 85 (70–90) |
| Chai et al 202129 | 7 | 3 (42.8%) | 69 | GKS | GPM | 82.8 | 82.8 |
| Stieber et al 200510 | 1 | 0 (0%) | NA | GKS | GPM | NA | 80 |
| Kaye et al 202030 | 1 | 1 (100%) | 58 | GKS | CIS | 36 | 80 |
Table 3.
Outcomes after treatment with SRS in patients diagnosed with GPN.
| studies | Last f/u mean (total months) | Median Time to pain response | Months after SRS further that underwent procedure | No of patients that required additional treatment |
|---|---|---|---|---|
| Kano et al 201615 | 45 (6–120) | NA | 2–7 | 10 (45.4%) |
| Pollock et al 201114 | NA | NA | NA | NA |
| Stanic et al31 | 13 (2–19) | 2 days −3 weeks | 2–8 | 2 (MVD)- (40%) |
| Williams et al 201017 | 12 | 4 weeks | 0 | 0 |
| Pommier et al 201816 | 11 | 1 month | na | 0 |
| Martinez-Alvarez et al 201418 | 46 (10–90) | 2–8 weeks | 36 | 1 (2nd GKS) (11.1%) |
| Borius et al 201719 | 43 (14–83) | na | na | 0 |
| Leveque et al 201120 | 62.4 (10.8–145.2) | 25 days | 18 | 4 (1 neurotomy, 3 GKS) (19%) |
| Pathmarajah et al 202221 | 18.2 (7–32) | NA | na | 4 (1 GKS, 1 Thermocoagulation, 1 Cortical stimulator, 1 MVD) (57.1%) |
| Chua et al 202022 | 25.5 (12–39) | 2–4 weeks | na | na |
| O Connor et al 201323 | 24 | 2 weeks | na | 0 |
| Almunia et al 202224 | 18 | 1 month | na | 0 |
| Xiong et al 201525 | 86.64 (12.96–150) | na | na | 3 (37.5%) |
| Yomo et al 200926 | 22 (20–25) | 7 days | na | na |
| Heroux et al 201527 | NA | 3 months | NA | 1 GKS (50%) |
| Ballosier et al 201928 | 48 | <1 week | na | 0 |
| Chai et al 202129 | 12 | 7–120 days | 30m | 2 (33.3%) |
| Stieber et al 200510 | 68 (29–89) | 6–80 days | 29.5 | 2 (GKS) (28.5%) |
| Kaye et al 202030 | 6 | NA | NA | 0 |
The proportion of patients that had undergone prior unsuccessful surgical procedures ranged from 11.1% to 100% cases. MVD was the most frequently performed procedure with the average proportion of patients that had undergone MVD ranging from 33 to 100% among studies. Only 5 patients had undergone rhizotomy and 8 patients had undergone GKS previously. This indicates that repeat GKS can be a treatment option for recurrent cases after failed GKS. Most of the patients reported a chronic history of neuralgia, with the mean duration of symptoms being from 18 months to 12.3 years across studies. The mean reported maximal radiation ranged from 70 to 88.7 Gy, with most of the studies (8/16) using 80Gy as the optimal amount of radiation. With regards to the target of SRS treatment, only in 4 studies (4 patients in total) the cisternal part of the glossopharyngeal nerve (CIS) was targeted, with the rest of the authors choosing the level of the glossopharyngeal meatus (GPM). Xiong et al25 with a case series of 3 patients used CIS as the target of GKS and patients were free of pain during the follow-up. None of them experienced any features of vagus nerve palsy.
5.3. Outcomes results
The median time from SRS till pain response was between 2 and 120 days. The percentage of patients in the included studies excluding case reports requiring further treatment after SRS ranged from 11.1 to 57.14%. This further treatment was in the form of either repeat GKS or surgical treatment with MVD, neurotomy, cortical stimulation, or thermocoagulation. The time frame between SRS and further treatment was 2–36 months. Pain outcomes were assessed in the short term (median follow up 1 week–6 months post procedure) as well as in the long term (median follow up 11 months–86.6 months post procedure). Favourable pain response rates after SRS (BNI-IIIb) ranged from 60% to 100% and 57.1%–100% in short and long term respectively. Poor pain response rates (BNI IV-V) in the short term follow up ranged from 16.6% to 33.3% while in the long term follow up ranged from 11.1% to 57.1% (see Table 4).
Table 4.
Pain outcomes in patients treated with SRS diagnosed with GPN.
| studies | Short term f/u (mean months) | BNI I- IIIb (no of patients) (%) | BNI IV,V (no of patients) (%) | Last f/u (mean months) | BNI (long term f/u (mean months) | BNI I-IIIb (no of patients) (%) | BNI IV-V (no of patients) (%) |
|---|---|---|---|---|---|---|---|
| Kano et al 201615 | 3 | 16 (72.72%) | 6 (27.27%) | 45 | 9 (42.8%) | 3 (14.3%) | 9 (42.8%) |
| Pollock et al 201114 | 6 | 3 (60%) | 2 (40%) | 13 | 0 | 0 | 0 |
| Stanic et al31 | 1 | 1 (100) | 0 | 12 | 0 | 1 (100% | 0 |
| Williams et al 201017 | 1 | 1 (100) | 0 | 11 | 1 (100%) | 0 | 0 |
| Pommier et al 201816 | 6 | 6 (66.6%) | 3 (33.3%) | 46 | 4 (44.4%) | 2 (22.2%) | 3 (33.3%) |
| Martinez-Alvarez et al 201418 | NA | NA | NA | 43 | 3 (60%) | 2 (40%) | 0 |
| Borius et al 201719 | 3 | 18 (85.71%) | 3 (14.28%) | 68.3 | 15 (68.18%) | 5 (22.7%) | 2 (9%) |
| Leveque et al 201120 | 3 | 6 (85.71%) | 1 (14.28%) | 18.2 | 2 (25%) | 2 (25%) | 4 (50%) |
| Pathmarajah et al 202221 | 2 | 2 (100%) | 0 | 25.5 | 0 | 2 (100%) | 0 |
| Chua et al 202022 | 3 | 1 (100%) | 0 | 24 | 1 (100%) | 0 | 0 |
| O Connor et al 201323 | NA | NA | NA | NA | NA | NA | NA |
| Almunia et al 202224 | 3 | 8 (100) | 0 | 86.64 | 3 (37.5%) | 2 (25%) | 3 (37.5%) |
| Xiong et al 201525 | 0.25 | 3 (100) | 0 | 22 | 3 (100%) | 0 | 0 |
| Yomo et al 200926 | NA | 2 (100) | 0 | NA | 1 (50%) | 0 | 1 (50%) |
| Heroux et al 201527 | NA | 1 (100) | 0 | 44 | 1 (100%) | 0 | 0 |
| Ballosier et al 201928 | 3 | 5 (83.3) | 1 (16.6 %) | 12 | 3 (50%) | 2 (33.3%) | 1 (16.6%) |
| Chai et al 202129 | 3 | 7 (100) | 0 | 68 | 4 (57.14%) | 3 (42.8%) | 0 |
| Stieber et al 200510 | 3 | 1 (100) | 0 | NA | 6 | 1 (100%) | 0 |
| Kaye et al 202030 | 0.75 | 1 (100) | 0 | 42 | 1 (100%) | 0 | 0 |
A subgroup of the studies looking at patients that had undergone previous surgery (MVD or rhizotomy) before SRS is shown on Table 5. 72/97 (74.22%) had undergone previous surgical procedure in the past. In this cohort of patients, recurrence rates were reported in 2 studies15; 28 excluding case reports,10 and were calculated at 31.8% and 16.6% respectively. Complications were divided into short term and long term complications. Long term complications were present at the last follow up whereas short term complication had resolved by that time. Kano et al15 and Stieber et al10 are the only studies that comment on long term post operative complication rates (2 patients with hyperesthesia in the palatoglossal arch and 1 patient with hyperesthesia of the pharynx respectively.) Borius19 and Chai29 et al noted that some patients complained of tongue numbness or hyperesthesia in the palatoglossal region which however resolved without any intervention. Regarding the patients that undergone SRS as their primary treatment with any history of any prior surgical management, there were only 4 studies that commented on post operative complication rates.16; 17; 20;21 (Table 6) Interestingly, among these studies there were no adverse events reported. Excluding case reports, Pommier et all was the only study that reported recurrence rates which were calculated at 11.1%.
Table 5.
Studies that included patients that had undergone prior surgical procedures before SRS.
| Studies | No of patients | No of patients with prior unsuccessful surgical procedures (%) | Short term complications (%) | Long term Complications (%) | Recurrence since pain free |
|---|---|---|---|---|---|
| Kano et al 201615 | 22 | 3 (2MVD, 1 Balloon Decompression) (13.63%) | NA | 2 (Hyperesthesia of Palatoglossal arch (9%) | 7 (31.8%) |
| Martinez-Alvarez et al 201418 | 5 | 3 (2 MVD, 1 Rhizotomy) (60%) | NA | NA | NA |
| Borius et al 201719 | 21 | 3 (MVD) (14.28%) | 1 (4.8%) Tongue Numbness | NA | |
| Chua et al 202022 | 1 | 1 (MVD) (100%) | NA | NA | 0 |
| O Connor et al 201323 | 1 | 1 (SPA block) (100%) | NA | NA | 0 |
| Almunia et al 202224 | 8 | 6 (2MVD, 4 Rhizotomy) (75%) | NA | NA | NA |
| Ballosier et al 201928 | 6 | 6 (GKR) (100%) | NA | 1 (Pharyngeal Hypoesthesia) (16.6%) | 1 (16.6%) |
| Chai et al 202129 | 7 | 7 (MVD, 2 Repeat GKS (100%) | 1 (14.3%) (hyperesthesia in the palatoglossal region) | NA | |
| Stieber et al 200510 | 1 | 1 (MVD) (100%) | NA | NA | 1 (100%) |
Table 6.
Studies that included patients that had SRS as their primary treatment for GPN without history of surgical management in the past.
| studies | No of patients | Short term Complications (%) | Long term Complications (%) | Recurrence since pain free (%) |
|---|---|---|---|---|
| Pollock et al 201114 | 5 | NA | NA | 0 |
| Pommier et al 201816 | 9 | NA | 0 | 1 (11.1%) |
| Williams et al 201017 | 1 | NA | 0 | NA |
| Leveque et al 201120 | 7 | NA | 0 | NA |
| Pathmarajah et al 202221 | 2 | NA | 0 | NA |
| Xiong et al 201525 | 3 | NA | NA | 0 |
| Yomo et al 200926 | 2 | NA | NA | NA |
| Heroux et al 201527 | 1 | NA | NA | 0 |
| Stanic et al 2012 | 1 | NA | NA | NA |
| Kaye et al 202030 | 1 | NA | NA | 1 |
6. Discussion
Because GPN is a relative rare condition, this systematic review aimed to provide an overview of the outcomes of patients with GPN treated with SRS. MVD is considered to be the first line of surgical treatment in medically refractive GPN. In a recent study of 427 cases, long-term pain relief was achieved in 84.7% of cases 4.9 years after MVD on average, with a complication rate of 13.2%.32 The most commonly described complications after MVD are transient hoarseness and dysphagia. RHZ alone shows an instant pain relief in 85–100% of the patients, but the rate of long-term pain relief is lower when compared to MVD.33 RHZ is not associated with increased risk of complications such as cranial nerve dysfunction.34
Given the non-invasive nature of SRS, the incidence of postoperative complications was unsurprisingly low. In the included studies, the mean complication rates ranged from 9 to 16.6%. It is interesting to note that complication rates were only reported in the group of patients that had previously undergone a surgical procedure to treat GPN. There were no reported complications in the cohort of patients that underwent primary SRS. Also, there were no neurological deficits post procedure and the complications were only limited to hyperesthesia over the pharyngeal region. This is particularly important for patients with significant medical comorbidities that are not suitable for surgical management. For example, Pollock et al14 and Benerjee et al described 3 cases with refractory cardiac dysfunction associated with painful attacks of GPN that were managed with SRS. This presents an important argument in favour of its consideration as a treatment modality for refractory cases especially in patients who are poor surgical candidates.35
Over the past 15 years, SRS has become an accepted alternative to surgery for patients with trigeminal neuralgia. Likewise, for GPN SRS is regarded as a less invasive option than posterior fossa surgery for patients. Nonetheless, radiosurgical targeting for GPN is more difficult than in trigeminal neuralgia mainly due to the complex anatomy of the lower cranial nerves and jugular foramen.36 Also, similar to trigeminal neuralgia, the low morbidity observed to date with GPN, SRS makes dose escalation higher than 80 Gy reasonable in the hope of providing better pain outcomes. Identifying the optimal radiation dose for GKS is an important challenge that must combine the effectiveness of the procedure together with the minimization of morbidity, especially related to the vagus nerve and the brainstem. In our review, the mean max radiation dose was 70–88.7 Gy, with only few studies using doses higher than 80 Gy.15,16,18,19,24,28
Recurrence of symptoms is one of the anticipated complications of SRS. Spina et al33 showed that recurrence was encountered in 41.6% of the patients with GPN after GKS. In our systematic review, the proportion of patients that needed further procedures due to recurrence of the symptoms ranged from 11.1 to 57.14%. Interestingly, in our cohort of patients, recurrence rates ranged from 16.6% to 31.8% in the group with a background of previous surgical treatment whereas that figure was 11.1% in the group of patients that underwent SRS as primary treatment for GPN. MVD was the most frequently used treatment modality for recurrent cases. There are no precise treatment guidelines for recurrent GPN after the initial SRS. Recently, there have been reports of management of recurrent cases with repeat SRS. Kaye et al30 reported a review of recurrent cases treated with repeat SRS. The median time to recurrence after the initial SRS was 12 months. In total, 9 patients (75%) had a favourable pain response at their final follow-up. Ballosier et al28 reported a case of resistant GPN that benefited from a third GKR.
Lu et al recently conducted a meta-analysis of the GPN treatment outcomes after NS, MVD, and GKS, looking at a total number of 792 cases. The authors suggested that short-term pain relief was lower after GKS than after NS.37 In a 2017 systematic review of 42 cases, Spina et al.33 reported an overall rate of favourable pain response in 78.6% of patients with GPN after GKS, with an average follow up time of 27 months. In our results, a favourable pain response ranged from 60% to 100% and 57.1%–100% in short and long term respectively, with a mean final follow up duration ranging between 1 and 7 years. It has been advocated that it takes at least one year for maximum pain relief for patients with trigeminal neuralgia after undergoing treatment with SRS. This was not reflected in our results for patients with GPN whose median time to pain response ranged from 2 days to 4 months.38 Given the non-invasive nature of SRS, it can be considered as an alternative treatment option for frail patients suffering from GPN. A recent large multicentre study looking at 26 patients with GPN undergoing MVD found that pre-operative frailty is associated with worse surgical outcomes.39 Berckemeyer et al suggest in a recent systematic review that SRS is an alternative treatment option for high-risk surgical patients or after a failed attempt with MVD40
Over the last years, with the advances in image-guided linear accelerator (LINAC)-based systems, there have been reports of GPN cases managed with frameless SRS (fSRS).21;22 GKS requires a headframe to allow patient immobilization by maintaining rigid head fixation in order to ensure precise radiation delivery. fSRS on the other hand is fitted with a custom thermoplastic mask molded to the face that secures the head. This technique provides a quicker and less invasive experience for the patient. fSRS was first described for the management of refractory trigeminal neuralgia, with a reported satisfactory pain relief being achieved in 75%–95.7% of cases.41 Pathmarajah21 and Chua22 reported 3 cases of GPN which were managed with fSRS and all had favorable pain outcomes at 2 years follow up.
Percutaneous radiofrequency thermocoagulation (PRT) is a well-accepted alternative treatment for GPN who respond poorly to pharmacological treatment. In a series of 80 patients with GPN treated with PRT, 63% remained at “good” pain relief condition 3 years after the procedure42 However, PRT was associated with several complications including dysphagia, dysesthesias, and diminished gag reflex43
7. Limitations
There are a few important limitations of this review. First of all, the literature on SRS for GPN remains relatively limited, consisting mainly of small retrospective case series or case reports. Therefore, the sample size was quite small due to the novelty of the treatment, limiting our ability to draw meaningful conclusions. Additionally, given the heterogeneity in reporting outcomes data between different studies, it was difficult to conduct a thorough comparative analysis with some studies using BNI pain scale and others the 2-tier scale (pain or no pain). As a result, conducting a meta-analysis with pooled results was not possible.
8. Conclusion
SRS is an emerging minimally invasive option for the treatment of drug-resistant GPN. In our data, high pain control outcomes as well as low complications rate were noticed. Further prospective studies with longer follow up periods are needed to assess the optimal radiosurgical strategy and the long-term results.
Funding
None.
CRediT authorship contribution statement
Timoleon Siempis: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. Roberta Rehder: Data curation, Investigation, Writing – original draft. Spyridon Voulgaris: Data curation, Investigation, Methodology, Validation. George A. Alexiou: Conceptualization, Methodology, Supervision.
Declaration of competing interest
None.
Contributor Information
Roberta Rehder, Email: Robertarehder1@gmail.com.
George A. Alexiou, Email: galexiou@uoi.gr, alexiougr@gmail.com.
References
- 1.Han, A., Montgomery, C., Zamora, A., Winder, E., Kaye, A., Carroll, C., Aquino, A., Kakazu, J., Kaye, A.D., n.d. Glossopharyngeal Neuralgia: Epidemiology, Risk factors, Pathophysiology, Differential diagnosis, and Treatment Options. Health Psychol Res 10, 36042. 10.52965/001c.36042. [DOI] [PMC free article] [PubMed]
- 2.Tepper S.J. Cranial neuralgias. Continuum. 2018;24:1157–1178. doi: 10.1212/CON.0000000000000637. [DOI] [PubMed] [Google Scholar]
- 3.Wang X., Tang Y., Zeng Y., Ni J. Long-term outcomes of percutaneous radiofrequency thermocoagulation for glossopharyngeal neuralgia: a retrospective observational study. Medicine (Baltim) 2016;95 doi: 10.1097/MD.0000000000005530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen J., Sindou M. Vago-glossopharyngeal neuralgia: a literature review of neurosurgical experience. Acta Neurochir. 2015;157:311–321. doi: 10.1007/s00701-014-2302-7. ; discussion 321. [DOI] [PubMed] [Google Scholar]
- 5.Headache Classification Subcommittee of the International Headache Society The international classification of headache disorders: 2nd edition. Cephalalgia. 2004;24(Suppl 1):9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 6.Franzini Andrea, Messina G., Franzini Angelo, et al. Treatments of glossopharyngeal neuralgia: towards standard procedures. Neurol Sci. 2017;38:51–55. doi: 10.1007/s10072-017-2909-6. [DOI] [PubMed] [Google Scholar]
- 7.Patel A., Kassam A., Horowitz M., Chang Y.-F. Microvascular decompression in the management of glossopharyngeal neuralgia: analysis of 217 cases. Neurosurgery. 2002;50:705–710. doi: 10.1097/00006123-200204000-00004. ; discussion 710-711. [DOI] [PubMed] [Google Scholar]
- 8.Wang H.-B., Fan Z.-M., Han J., Li K.-Y., Fan Z. Serious complications of the microvascular decompression in cerebellopontine angle. Zhonghua er bi yan hou tou jing wai ke za zhi. 2005;40:352–356. [PubMed] [Google Scholar]
- 9.Régis J., Metellus P., Hayashi M., Roussel P., Donnet A., Bille-Turc F. Prospective controlled trial of gamma knife surgery for essential trigeminal neuralgia. J Neurosurg. 2006;104:913–924. doi: 10.3171/jns.2006.104.6.913. [DOI] [PubMed] [Google Scholar]
- 10.Stieber V.W., Bourland J.D., Ellis T.L. Glossopharyngeal neuralgia treated with gamma knife surgery: treatment outcome and failure analysis. Case report. J Neurosurg. 2005;102(Suppl):155–157. doi: 10.3171/jns.2005.102.s_supplement.0155. [DOI] [PubMed] [Google Scholar]
- 11.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. W64. [DOI] [PubMed] [Google Scholar]
- 12.Han P., Shetter A.G., Rogers C.L., et al. Gamma knife radiosurgery for trigeminal neuralgia: the initial experience at the Barrow neurological Institute. Neurosurgery. 1999;45(3):723. doi: 10.1159/000029771. 723. [DOI] [PubMed] [Google Scholar]
- 13.Wells G.A., Shea B., O’connell D., et al. Ottawa Hospital Research Institute; Ottawa, ON: 2009. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Accessed March, 2023. [Google Scholar]
- 14.Pollock B.E., Boes C.J. Stereotactic radiosurgery for glossopharyngeal neuralgia: preliminary report of 5 cases. J Neurosurg. 2011;115:936–939. doi: 10.3171/2011.5.JNS1133. [DOI] [PubMed] [Google Scholar]
- 15.Kano H., Urgosik D., Liscak R., et al. Stereotactic radiosurgery for idiopathic glossopharyngeal neuralgia: an international multicenter study. J Neurosurg. 2016;125:147–153. doi: 10.3171/2016.7.GKS161523. [DOI] [PubMed] [Google Scholar]
- 16.Pommier B., Touzet G., Lucas C., Vermandel M., Blond S., Reyns N. Glossopharyngeal neuralgia treated by Gamma Knife radiosurgery: safety and efficacy through long-term follow-up. J Neurosurg. 2018;128:1372–1379. doi: 10.3171/2017.3.JNS162542. [DOI] [PubMed] [Google Scholar]
- 17.Williams B.J., Schlesinger D., Sheehan J. Glossopharyngeal neuralgia treated with gamma knife radiosurgery. World Neurosurg. 2010;73:413–417. doi: 10.1016/j.wneu.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Martínez-Álvarez R., Martínez-Moreno N., Kusak M.E., Rey-Portolés G. Glossopharyngeal neuralgia and radiosurgery. J Neurosurg. 2014;121(Suppl):222–225. doi: 10.3171/2014.8.GKS141273. [DOI] [PubMed] [Google Scholar]
- 19.Borius P.-Y., Tuleasca C., Muraciole X., et al. Gamma Knife radiosurgery for glossopharyngeal neuralgia: a study of 21 patients with long-term follow-up. Cephalalgia. 2018;38:543–550. doi: 10.1177/0333102417698961. [DOI] [PubMed] [Google Scholar]
- 20.Lévêque M., Park M.C., Melhaoui A., Yomo S., Donnet A., Régis J. Gamma knife radiosurgery for glossopharyngeal neuralgia: marseille experience. J Radiosurg SBRT. 2011;1:41–46. [PMC free article] [PubMed] [Google Scholar]
- 21.Pathmarajah T., Katipally R.R., Flores-Martinez E., et al. Linear accelerator-based stereotactic radiosurgery for glossopharyngeal neuralgia is safe and effective – report of two cases. J Radiosurg SBRT. 2022;8:151–153. [PMC free article] [PubMed] [Google Scholar]
- 22.Chua E., Pappas C.T., Chen A.Y. Successful frameless radiosurgery for glossopharyngeal neuralgia — case report. J Radiosurg SBRT. 2020;7:85–87. [PMC free article] [PubMed] [Google Scholar]
- 23.O'Connor J.K., Bidiwala S. Effectiveness and safety of Gamma Knife radiosurgery for glossopharyngeal neuralgia. SAVE Proc. 2013;26:262–264. doi: 10.1080/08998280.2013.11928976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lara-Almunia M., Moreno N.E.M., Sarraga J.G., Alvarez R.M. Gamma Knife radiosurgery and refractory glossopharyngeal neuralgia: a single-center series with long-term follow-up. Neurosurg Rev. 2022;45:525–531. doi: 10.1007/s10143-021-01557-7. [DOI] [PubMed] [Google Scholar]
- 25.Xiong N.-X., Tan D., Fu P., Zhao H.-Y. Gamma knife radiosurgery for glossopharyngeal neuralgia by targeting the medial cisternal segment of the glossopharyngeal nerve: report of 3 cases. Stereotact Funct Neurosurg. 2015;93:292–296. doi: 10.1159/000375176. [DOI] [PubMed] [Google Scholar]
- 26.Yomo S., Arkha Y., Donnet A., Régis J. Gamma Knife surgery for glossopharyngeal neuralgia. J Neurosurg. 2009;110:559–563. doi: 10.3171/2008.8.17641. [DOI] [PubMed] [Google Scholar]
- 27.Héroux F., Mathieu D. Treatment of glossopharyngeal neuralgia by gamma knife radiosurgery. Can J Neurol Sci. 2015;42:350–352. doi: 10.1017/cjn.2015.58. [DOI] [PubMed] [Google Scholar]
- 28.Balossier A., Tuleasca C., Muracciole X., Donnet A., Levivier M., Régis J. The outcomes of a second and third Gamma Knife radiosurgery for recurrent essential glossopharyngeal neuralgia. Acta Neurochir. 2020;162:271–277. doi: 10.1007/s00701-019-04124-8. [DOI] [PubMed] [Google Scholar]
- 29.Chai S., Xu H., Xiao D., et al. Salvage gamma knife surgery for recurrent glossopharyngeal neuralgia following microvascular decompression: a retrospective case series. Acta Neurochir. 2021;163:1021–1026. doi: 10.1007/s00701-020-04654-6. [DOI] [PubMed] [Google Scholar]
- 30.Kaye J., Daggubati L.C., Zeller S., McInerney J. Repeat gamma knife radiosurgery for recurrent glossopharyngeal neuralgia: a systematic review and our initial experience. Stereotact Funct Neurosurg. 2020;98:324–330. doi: 10.1159/000508541. [DOI] [PubMed] [Google Scholar]
- 31.Stanic S., Franklin S.D., Pappas C.T., Stern R.L. Gamma knife radiosurgery for recurrent glossopharyngeal neuralgia after microvascular decompression. SFN. 2012;90:188–191. doi: 10.1159/000338089. [DOI] [PubMed] [Google Scholar]
- 32.Rey-Dios R., Cohen-Gadol A.A. Current neurosurgical management of glossopharyngeal neuralgia and technical nuances for microvascular decompression surgery. Neurosurg Focus. 2013;34:E8. doi: 10.3171/2012.12.FOCUS12391. [DOI] [PubMed] [Google Scholar]
- 33.Spina A., Boari N., Gagliardi F., et al. The emerging role of gamma knife radiosurgery in the management of glossopharyngeal neuralgia. Neurosurg Rev. 2019;42:31–38. doi: 10.1007/s10143-017-0886-0. [DOI] [PubMed] [Google Scholar]
- 34.Wang L., Liu Q., Dong X., Wang J. Comparative analysis of MVD and RHZ in the treatment of primary glossopharyngeal neuralgia: a clinical report on 61 cases. Front Neurol. 2023;14 doi: 10.3389/fneur.2023.1024142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Banerjee C., Viers A., Vender J. Glossopharyngeal neuralgia/neuropathy with hemodynamic instability and associated syncope treated with stereotactic radiosurgery. World Neurosurg. 2020;139:314–317. doi: 10.1016/j.wneu.2020.04.130. [DOI] [PubMed] [Google Scholar]
- 36.Pollock B.E., Phuong L.K., Foote R.L., Stafford S.L., Gorman D.A. High-dose trigeminal neuralgia radiosurgery associated with increased risk of trigeminal nerve dysfunction. Neurosurgery. 2001;49:58–62. doi: 10.1097/00006123-200107000-00008. discussion 62-64. [DOI] [PubMed] [Google Scholar]
- 37.Lu V.M., Goyal A., Graffeo C.S., Perry A., Jonker B.P., Link M.J. Glossopharyngeal neuralgia treatment outcomes after nerve section, microvascular decompression, or stereotactic radiosurgery: a systematic review and meta-analysis. World Neurosurg. 2018;120:572–582.e7. doi: 10.1016/j.wneu.2018.09.042. [DOI] [PubMed] [Google Scholar]
- 38.Wolf A., Kondziolka D. Gamma knife surgery in trigeminal neuralgia. Neurosurgery clinics of north America. Trigeminal Neuralgia. 2016;27:297–304. doi: 10.1016/j.nec.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 39.Estes E., Rumalla K., Dicpinigaitis A.J., et al. Preoperative frailty predicts worse outcomes after microvascular decompression for trigeminal neuralgia, hemifacial spasm, and glossopharyngeal neuralgia: a multicenter analysis of 1,473 patients from a prospective surgical registry. Stereotact Funct Neurosurg. 2023;101:188–194. doi: 10.1159/000529763. [DOI] [PubMed] [Google Scholar]
- 40.Berckemeyer M.A., Suarez-Meade P., Carcelen M.F.V., et al. Current advances in the surgical treatment of glossopharyngeal neuralgia. Neurosurg Rev. 2023;46:47. doi: 10.1007/s10143-023-01948-y. [DOI] [PubMed] [Google Scholar]
- 41.De La Peña N.M., Singh R., Anderson M.L., et al. High-dose frameless stereotactic radiosurgery for trigeminal neuralgia: a single-institution experience and systematic review. World Neurosurgery. 2022;167:e432–e443. doi: 10.1016/j.wneu.2022.08.038. [DOI] [PubMed] [Google Scholar]
- 42.Varela-Lema L., Lopez-Garcia M., Maceira-Rozas M., Munoz-Garzon V. Linear accelerator stereotactic radiosurgery for trigeminal neuralgia. Pain Physician. 2015;18:15–27. [PubMed] [Google Scholar]
- 43.Wang X., Tang Y., Zeng Y., Ni J. Long-term outcomes of percutaneous radiofrequency thermocoagulation for glossopharyngeal neuralgia: a retrospective observational study. Medicine. 2016;95 doi: 10.1097/MD.0000000000005530. [DOI] [PMC free article] [PubMed] [Google Scholar]