Abstract
Background
Our objective was to investigate the effect of a day-long exposure to high altitude on peak exercise capacity and safety in stable patients with pulmonary arterial hypertension (PAH) and chronic thromboembolic pulmonary hypertension (CTEPH).
Methods
In a randomised controlled crossover trial, stable patients with PAH or distal CTEPH without resting hypoxaemia at low altitude performed two incremental exercise tests to exhaustion: one after 3–5 h at high altitude (2500 m) and one at low altitude (470 m).
Results
In 27 patients with PAH/CTEPH (44% females, mean±sd age 62±14 years), maximal work rate was 110±64 W at 2500 m and 123±64 W at 470 m (−11%, 95% CI −16– −11%; p<0.001). Oxygen saturation measured by pulse oximetry and arterial oxygen tension at end-exercise were 83±6% versus 91±6% and 6.1±1.9 versus 8.6±1.9 kPa (−8% and −29%; both p<0.001) at 2500 versus 470 m, respectively. Maximal oxygen uptake was 17.8±7.5 L·min−1·kg−1 at high altitude versus 20±7.4 L·min−1·kg−1 at low altitude (−11%; p<0.001). At end-exercise, the ventilatory equivalent for carbon dioxide was 43±9 at 2500 m versus 39±9 at 470 m (9%, 95% CI 2–6%; p=0.002). No adverse events occurred during or after exercise.
Conclusions
Among predominantly low-risk patients with stable PAH/CTEPH, cycling exercise during the first day at 2500 m was well tolerated, but peak exercise capacity, blood oxygenation and ventilatory efficiency were lower compared with 470 m.
Tweetable abstract
In 27 patients with stable PVD, cycling exercise at 2500 m was well tolerated, but exercise capacity was lower compared with 470 m, associated with lower blood oxygenation and increased ventilatory inefficiency https://bit.ly/47ryXTT
Introduction
In the absence of predominant lung disease, the major pre-capillary pulmonary hypertension (PH) groups are pulmonary arterial hypertension (PAH) and chronic thromboembolic PH (CTEPH), hereafter summarised as pulmonary vascular disease (PVD) [1, 2]. PVD leads to reduced exercise capacity and quality of life [3]. Therapeutic advances have improved physical performance, quality of life and life expectancy in these patients. Consequently, there is an increasing desire to participate in ordinary professional or recreational activities, including mountain travel [4, 5]. Many Alpine villages and tourist resorts are located at altitudes between 1000 and 2500 m. Physical activity at high altitude has gained popularity. Worldwide, more than 100 million lowlanders visit areas above 2500 m annually, exposing themselves to hypobaric hypoxia [6]. Among this vast population exposed to a hypoxic environment are many patients with chronic cardiopulmonary disorders, including PVD. At higher altitude, hypoxic pulmonary vasoconstriction may lead to elevated pulmonary arterial pressure [7]. However, in patients with PVD, short exposure to normobaric hypoxia during right heart catheterisation did not relevantly alter pulmonary haemodynamics. This may indicate an insufficient hypoxic stimulus or a blunted hypoxic pulmonary vasoconstriction response [8, 9]. However, adaptive cardiovascular responses to hypoxia, such as increased ventilation or heart rate, may already be present in PVD patients at low altitude due to increased sympathetic activity [10, 11]. Arterial hypoxaemia is aggravated during exercise at high altitude due to impaired ventilation/perfusion matching, accentuated alveolar–arterial oxygen pressure difference and shorter contact times of erythrocytes for gas exchange, all resulting in increased alveolar–arterial oxygen gradients [12, 13]. A first study on 28 patients with PVD indicated a reduced constant work rate exercise time at 2500 m [14]. Although that study provides insights into the aerobic capacity of patients with PVD at high altitude, it is important to note that breath-by-breath parameters were not measured. Furthermore, there is limited knowledge regarding the maximum exercise capacity of patients with PVD at 2500 m, as well as its safety and the physiological mechanisms involved. The aim of this study was, therefore, to investigate the effect of high altitude (2500 m) on maximum incremental ramp cycling exercise capacity in patients with stable pre-capillary PH due to PVD and to study the underlying physiology.
Methods
Study design, randomisation, allocation and subjects
This was a randomised controlled crossover trial conducted between October 2021 and February 2022 at the University Hospital Zurich (Zurich, Switzerland) at 470 m (low altitude) and at mount Säntis (located in the Swiss Alps) at 2500 m. Randomisation was done by software-based blocks with random computed block length and allocation concealment. We recruited adults diagnosed with PAH or distal CTEPH (inoperable or persistent after pulmonary endarterectomy) of all sexes according to current guidelines at the time of their last right heart catheterisation. Participants were under stable conditions with unchanged PH-targeted medication for >4 weeks [1, 2, 15]. A resting arterial oxygen tension (PaO2) <8 kPa in Zurich, travelling to an altitude >1000 m for more than 3 nights within the last 4 weeks before study participation, having any inabilities to follow the study protocol, such as language problems, or other clinically relevant concomitant diseases, pregnancy or breastfeeding were exclusion criteria. Patients agreed to spend 30 h at 2500 m, including an overnight stay, to assess high-altitude-induced adverse events [16]. In the present study, results of incremental cycling exercise testing obtained during the first day at high altitude versus low altitude are reported.
Ethics and registration
All patients gave written informed consent for participation and further use of their data for scientific analysis. The study complies with the Declaration of Helsinki and was approved by the local ethical authorities as part of an umbrella study. The study was registered on ClinicalTrials.gov (NCT05107700).
Altitude exposure
After arrival at 2500 m, participants underwent a minimum of 3 h of rest before engaging in cycling activities in the afternoon. Each patient had a control visit in Zurich at 470 m, sequence-randomised either before or after the high altitude exposure, with a washout time of >2 weeks and identical assessments at both locations.
During the altitude exposure, participants’ oxygenation and health condition were frequently monitored. Patients in whom oxygen saturation measured by pulse oximetry (SpO2) dropped to <80% for >30 min or <75% for >15 min were treated with supplemental oxygen for safety reasons and did not participate in exercise testing.
Cycling exercise
Patients performed identical incremental cycling exercise tests to exhaustion on the first day, at least 3 h after arriving at both high and low altitude. After 2 min of quiet rest, patients were instructed to maintain a constant pedalling rate of 60 revolutions·min−1. The first 2 min were unloaded, followed by 10 W·min−1 increments until exhaustion. Incremental cycling exercise tests were performed on Ergoselect 200 cycling ergometers (Ergoline, Bitz, Germany).
Patients were connected to the flow sensor of a metabolic unit via a mouthpiece (Ergostick; Geratherm Medical, Geschwenda, Germany) which was calibrated before each test and measured respiratory gas exchange. The primary outcome was maximal work capacity. Additional outcomes, tidal volume (VT), minute ventilation (V′E), oxygen uptake (V′O2), carbon dioxide production (V′CO2), respiratory exchange ratio (RER) and derived variables, were recorded breath-by-breath. SpO2 was measured continuously by finger clip pulse oximetry. Heart rate was derived from a 12-lead ECG and blood pressure was measured by automated arm-cuff measurements. Maximal voluntary ventilation (MVV) was calculated as forced expiratory volume in 1 s×40 [17] and gas exchange threshold was defined as the first RER >1.0 [18]. At rest as well as end-exercise, arterial blood samples from the radial artery were taken and immediately analysed (ABL90 FLEX; Radiometer, Thalwil, Switzerland). Arterial oxygen content (CaO2) was calculated as SaO2×Hb×1.34+PaO2×0.0031, where SaO2 is arterial oxygen saturation and Hb is haemoglobin concentration. Dead space fraction (dead space volume (VD)/VT) was calculated as (PaCO2−PETCO2)/PaCO2, where PaCO2 is arterial carbon dioxide tension and PETCO2 is end-tidal carbon dioxide tension. After each test, patients reported their leg fatigue and dyspnoea using the Borg CR10 scale.
Variables at end-exercise were defined as the mean over the final 30 s before termination of the exercise, indicated by a drop in cycling rate to <50 revolutions·min−1. Isotime was defined as the final 30 s leading to end-exercise of the shorter test compared with the longer test at the same time-point. Reference values were taken from those reported by Glaser et al. [19].
Data presentation and statistical analysis
To compare the main outcome, i.e. maximum work rate between high and low altitude at end-exercise and isotime, physiological values were averaged over the last 30 s and compared as repeated measures. Data were summarised as mean with standard deviation. A linear mixed model was fitted to the data with intervention (high versus low altitude), period and intervention-by-period interaction as fixed effects and subject as random intercept, thus controlling for carryover (treatment-by-period interaction) and period effects according to the standards of crossover trials. We tested if intervention-by-period interaction could be removed from the model. Model assumptions were tested by visual inspection of the homogeneity and normality of the residuals and the random effects.
The analysis of the secondary outcomes followed the same procedure, but included baseline characteristics as covariates in addition to minimise bias. Missing data were handled by the linear mixed model and intention-to-treat analysis was used for all outcomes.
In all analyses, a 95% confidence interval that excluded the null effect was considered evidence for statistical significance. Analyses were performed using RStudio software version 4.1.0 (https://posit.co).
Results
The patient flow diagram is shown in figure 1. Out of 65 patients with PVD who were assessed for eligibility, 27 could be included and were randomised. One patient received oxygen at high altitude due to hypoxaemia at rest and did not perform the incremental cycling exercise test at high altitude [16]. A total of 27 patients (44% females, 20:7 PAH:CTEPH, age 62±14 years, 24 (89%) low risk, three (11%) intermediate risk) were included in the analysis (for baseline characteristics, see table 1).
FIGURE 1.
Study flowchart. PP: per protocol; ITT: intention to treat.
TABLE 1.
Baseline characteristics at low altitude
| Participants | 27 |
| Sex | |
| Female | 12 (44) |
| Male | 15 (56) |
| Age (years) | 62±14 |
| Pulmonary arterial hypertension | 20 |
| Chronic thromboembolic pulmonary hypertension | 7 |
| New York Heart Association Functional Class | |
| I | 7 (26) |
| II | 19 (70) |
| III | 1 (4) |
| ESC/ERS multipoint three-strata risk assessment [1, 2, 15] | |
| Low risk | 24 (89) |
| Intermediate risk | 3 (11) |
| Body mass index (kg·m−2) | 25.1±4.0 |
| SpO2 at rest (%) | 94±4 |
| Oxygen uptake (mL·min−1·kg−1) | 18.6±4.8 |
| Data from last right heart catheterisation | |
| Mean pulmonary arterial pressure (mmHg) | 42±14 |
| Pulmonary arterial wedge pressure (mmHg) | 11±4 |
| Pulmonary vascular resistance (WU) | 5.9±2.7 |
| Cardiac output (L·min−1) | 5.2±1.6 |
| Cardiac index (L·min−1·m−2) | 2.7±0.7 |
| PH-targeted medication | |
| Endothelin receptor antagonist | 20 (74) |
| Phosphodiesterase 5 inhibitor | 11 (41) |
| Intravenous prostacylins | 3 (11) |
| Soluble guanylate cyclase stimulator | 6 (22) |
| Selexipag | 3 (11) |
| Calcium channel blocker | 3 (11) |
| Combination therapy | 15 (56) |
Data are presented as n, n (%) or mean±sd. ECS: European Society of Cardiology; ERS: European Respiratory Society; SpO2: oxygen saturation measured by pulse oximetry; PH: pulmonary hypertension.
Cycling exercise: end-exercise
Table 2 shows the results of incremental cycling exercise tests comparing high versus low altitude. There were no adverse events during exercise at either high or low altitude. The primary outcome, maximum work rate at end-exercise, was reduced by −13 W (95% CI −16– −11 W; p<0.001) at high compared with low altitude.
TABLE 2.
Cardiorespiratory parameters measured at end-exercise or gas exchange threshold
|
Low altitude (Zurich, 470 m) |
High altitude (Säntis, 2500 m) |
Mean change (95% CI) | p-value | |
| Load (W) | 123±64 | 110±64 | −13 (−16– −11) | <0.001* |
| SpO2 (%) | 91±6 | 83±5.8 | −8 (−10– −7) | <0.001* |
| Peak heart rate (beats·min−1) | 135±36 | 131±36 | −4 (−11–4) | 0.367 |
| SBP (mmHg) | 165±36 | 164±36 | +1.2 (−15–18) | 0.884 |
| DBP (mmHg) | 76±15 | 71±15 | −5 (−13–3) | 0.235 |
| Peak V′O2 (L·min−1) | 1.5±0.6 | 1.3±0.6 | −0.2 (−0.2– −0.1) | <0.001* |
| At gas exchange threshold | 1.2±0.3 | 1.0±0.4 | −0.2 (−0.3– −0.07) | 0.002* |
| Peak V′O2 per kg (L·min−1·kg−1) | 20±7.4 | 17.8±7.5 | −2.2 (−3.1– −1.3) | <0.001* |
| At gas exchange threshold | 16.5±3.3 | 13.9±3.9 | −2.6 (−4.1– −1.2) | 0.001* |
| V′CO2 (L·min−1) | 1.67±0.76 | 1.58±0.76 | −0.09 (−1.16–0.01) | 0.031* |
| O2 pulse (mL·beat−1) | 11.2±3.0 | 9.9±3.1 | −1.3 (−1.9– −0.8) | <0.001* |
| V′E/V′CO2 | 39±9 | 43±9 | +4 (2–6) | 0.002* |
| V′E/V′CO2 slope | 35±6 | 39±7 | +4 (2–6) | <0.001* |
| V′E (L·min−1) | 71±33 | 68±33 | −3 (−1–6) | 0.146 |
| V′E/MVV | 0.72±0.2 | 0.73±0.2 | 0.01 (−0.02–0.06) | 0.257 |
| VT (L) | 2±1 | 2±1 | +0 (−0.0–0.1) | 0.365 |
| Dead space fraction (VD/VT) | 0.32±0.1 | 0.32±0.1 | 0.00 (−0.07–0.06) | 0.907 |
| RER | 1.12±0.17 | 1.18±0.17 | +0.06 (0.03–0.09) | <0.001* |
| Borg (CR10) leg fatigue | 6±3 | 6±3 | 0 (−0.5–0.5) | 0.787 |
| Borg (CR10) dyspnoea | 6±2 | 6±2 | 0 (−0.3–0.9) | 0.370 |
| PaO2 (kPa (mmHg)) | 8.6±1.9 (65±14) | 6.1±1.9 (46±14) | −2.5 (−3– −2) | <0.001* |
| PaCO2 (kPa (mmHg)) | 4.5±0.6 (34±5) | 4.0±0.6 (30±5) | −0.5 (−0.6– −0.3) | <0.001* |
| SaO2 (%) | 92±6 | 82±6 | −10 (−11– −8) | <0.001* |
| Arterial pH | 7.39±0.05 | 7.42±0.05 | 0.03 (0.02–0.05) | <0.001* |
| Lactate (mmol·L−1) | 7.1±3.7 | 7.2±3.9 | +0.1 (−0.9–0.1) | 0.883 |
| Haematocrit (%) | 49±2 | 49±4 | 0 (0–1) | 0.452 |
| CaO2 (mL·dL−1) | 19.9±1.8 | 17.9±1.7 | −2.0 (−3.0– −1.42) | <0.001* |
| PaCO2–PETCO2 gradient (kPa (mmHg)) | 1.45±0.63 (11±5) | 0.27±0.62 (2±5) | −0.18 (−0.57–0.21) | 0.356 |
Data are presented as mean±sd, unless otherwise stated. SpO2: peripheral oxygen saturation; SBP: systolic blood pressure; DBP: diastolic blood pressure; V′O2: oxygen consumption; V′CO2: carbon dioxide production; V′E: minute ventilation; V′E/V′CO2 slope: regression slope of V′E and V′CO2 over the entire exercise; MVV: maximal voluntary ventilation: VT: tidal volume; VD: dead space volume; RER: respiratory exchange rate; PaO2: arterial oxygen tension; PaCO2: arterial carbon dioxide tension; SaO2: arterial oxygen saturation; CaO2: arterial oxygen content; PETCO2: end-tidal carbon dioxide tension. *: p<0.05.
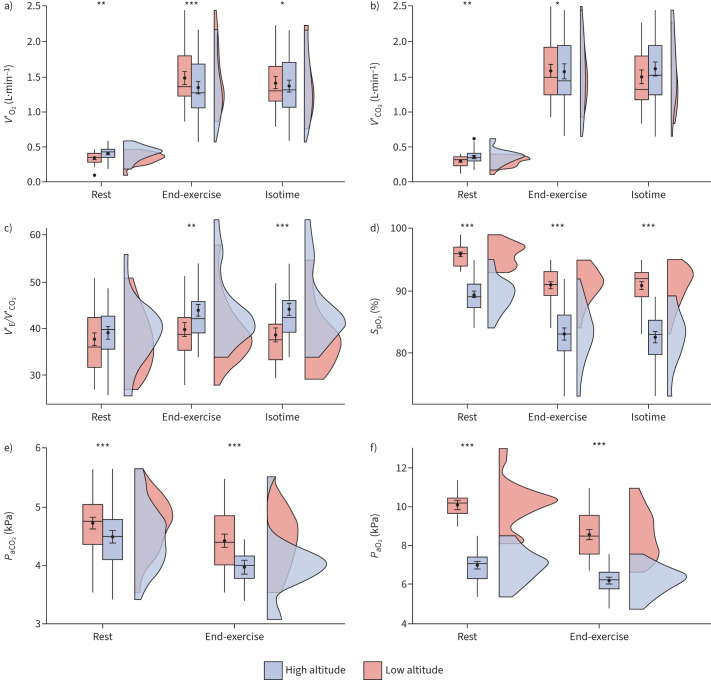
Furthermore, SpO2 at end-exercise was reduced at high compared with low altitude by −8%, SaO2 by −10%, PaO2 by −2.5 kPa and PaCO2 by −0.5 kPa (all four p<0.001) (for 95% confidence intervals and partial pressures in mmHg, see table 2). The PaCO2–PETCO2 gradient was unchanged at end-exercise.
We found that peak V′O2 was lower at high compared with low altitude, both by −0.2 L·min−1 at end-exercise (p<0.001), as well as at gas exchange (anaerobic) threshold (p=0.002). Oxygen pulse (O2 pulse) was reduced by −1.3 mL·beat−1 (p<0.001) and V′CO2 by −0.09 L·min−1 (p=0.031) at high versus low altitude. The ventilatory equivalent for carbon dioxide (V′E/V′CO2) and V′E/V′CO2 slope were both increased at high altitude by 4 (p=0.002 and p<0.001), while VT and V′E, as well as V′E/MVV, remained unchanged (for 95% confidence intervals, see table 2 and figure 2).
FIGURE 2.
Physiological secondary outcomes at rest and at end-exercise, at high versus low altitude: a) oxygen uptake (V′O2), b) carbon dioxide production (V′CO2), c) ventilatory equivalent for carbon dioxide (minute ventilation (V′E)/V′CO2), d) oxygen saturation measured by pulse oximetry (SpO2), e) arterial carbon dioxide tension (PaCO2) and f) arterial oxygen tension (PaO2). Box plots represent the medians with corresponding standard deviation; the black dots represent the means with corresponding standard error of measurement. The cloud plots to the right of the box plots represent the data distribution. Statistical significance between high and low altitude: *: p<0.05; **: p<0.01; ***: p<0.001.
RER was increased at high altitude at end-exercise by a mean change of 0.06 (p<0.001), whereas lactate did not differ.
There was no statistically significant difference in heart rate, blood pressure or haematocrit (table 2). Moreover, the Borg CR10 scale scores for leg fatigue and dyspnoea also did not differ at end-exercise.
Cycling exercise: isotime
The mean isotime was determined at 110 W. At isotime, SpO2 was reduced at high compared with low altitude by −8% (p<0.001). Furthermore, V′O2 and O2 pulse were both lower at high altitude at isotime by −0.1 L·min−1 (p=0.047) and −1.2 mL·beat−1 (p=0.003), respectively. V′CO2 did not differ between high and low altitude at isotime, while V′E and V′E/V′CO2 were increased at high altitude by 13 L·min−1 and 6 (both p<0.001), respectively.
Measurement at rest: high altitude versus low altitude
Table 3 shows the cardiorespiratory parameters measured at rest. Oxygen saturation was lower at high compared with low altitude, corresponding to a SpO2 reduction by −7% (p<0.001) and SaO2 reduction by −8% (p<0.001). In accordance, PaO2 was reduced by −3.3 kPa (p<0.001) at high compared with low altitude. PaCO2 was reduced by −0.3 kPa (p<0.001).
TABLE 3.
Cardiorespiratory parameters measured at rest
|
Low altitude (Zurich, 470 m) |
High altitude (Säntis, 2500 m) |
Mean change (95% CI) | p-value | |
| Load (W) | 0 | 0 | ||
| SpO2 (%) | 96±6 | 89±6 | −7 (−7– −6) | <0.001* |
| Heart rate (beats·min−1) | 77±35 | 79±36 | 2 (−1–5) | 0.165 |
| SBP (mmHg) | 115±37 | 117±36 | 2 (−8–13) | 0.660 |
| DBP (mmHg) | 70±15 | 67±15 | −3 (−9–2) | 0.251 |
| V′O2 (L·min−1) | 0.34±0.61 | 0.41±0.61 | 0.07 (0.02–0.12) | 0.008* |
| V′O2 per kg (L·min−1·kg−1) | 4.67±7.43 | 5.44±7.46 | 0.84 (0.2–1.47) | 0.011* |
| V′CO2 (L·min−1) | 0.29±0.75 | 0.36±0.76 | 0.07 (0.02–0.12) | 0.009* |
| V′E/V′CO2 | 41±9 | 39±9 | −2 (−9–4) | 0.476 |
| V′E (L·min−1) | 14±33 | 16±33 | 2 (1–4) | 0.004* |
| VT (L) | 1±1 | 1±1 | 0 (−0.1–0.2) | 0.195 |
| Dead space fraction (VD/VT) | 0.35±0.13 | 0.32±0.08 | −0.03 (−0.08–0.01) | 0.132 |
| RER | 0.9±0.2 | 0.9±0.2 | 0 (−0.03–0.1) | 0.681 |
| PaO2 (kPa (mmHg)) | 10.2±1.9 (77±14) | 6.9±1.9 (52±14) | −3.3 (−3.8– −2.9) | <0.001* |
| PaCO2 (kPa (mmHg)) | 4.8±0.6 (36±5) | 4.5±0.6 (34±5) | −0.3 (−0.4– −0.1) | <0.001* |
| SaO2 (%) | 96±6 | 88±6 | −8 (−9– −6) | <0.001* |
| Arterial pH | 7.46±0.05 | 7.48±0.05 | 0.02 (0.01–0.03) | <0.001* |
| Lactate (mmol·L−1) | 0.93±3.7 | 0.94±3.7 | 0.01 (−0.27–0.29) | 0.942 |
| Haematocrit (%) | 45±5 | 45±5 | 0 (−1–1) | 0.361 |
| P aCO2 –PETCO2 gradient (kPa (mmHg)) | 1.81±0.90 (14±7) | 1.42±0.43 (11±3) | −0.39 (−0.77–0.03) | 0.059 |
Data are presented as mean±sd, unless otherwise stated. SpO2: peripheral oxygen saturation; SBP: systolic blood pressure; DBP: diastolic blood pressure; V′O2: oxygen consumption; V′CO2: carbon dioxide production; V′E: minute ventilation; VT: tidal volume; VD: dead space volume; RER: respiratory exchange rate; PaO2: arterial oxygen tension; PaCO2: arterial carbon dioxide tension; SaO2: arterial oxygen saturation; PETCO2: end-tidal carbon dioxide tension. *: p<0.05.
We found that both V′O2 and V′CO2 were higher at rest at high altitude (0.41 versus 0.34 L·min−1 (p=0.008) and 0.36 versus 0.29 L·min−1 (p=0.009), respectively). Additionally, V′E was also increased at rest at high compared with low altitude by 2.2 L·min−1 (p=0.004), but V′E/V′CO2 was unchanged (for 95% confidence intervals and partial pressures in mmHg, see table 3).
Discussion
The current trial in patients with PAH or distal CTEPH showed that maximal cycling exercise at 2500 m was generally well tolerated. However, the peak work rate was reduced compared with 470 m, along with lower arterial oxygenation and increased ventilation and ventilatory inefficiency.
The finding of reduced work rates at high compared with low altitude concurs with previous studies that reported a reduction in exercise performance with increasing altitude in healthy individuals and, to an even greater extent, in patients with COPD or coronary heart disease [20–22]. Challenges in achieving comparability between studies arise from differences in exercise protocols, chosen altitudes and the definition of high altitude. While a broad range from 1500 to 3500 m is generally considered high altitude [23], the risk for acute altitude-related illness starts with an ascent above 2000 m in healthy subjects, although significant problems typically manifest only above 3000 m for the majority. Nonetheless, individuals with chronic medical conditions face risks even above 1500 m. Hence, we designated the altitude of 2500 m, where the current PVD cohort was examined, as high altitude [24].
Cycling athletes demonstrated a decreased load of −13.8% when cycling at 2340 m compared with sea level, a reduction comparable to that observed in the current PVD cohort, although the absolute values were notably lower [23].
In 2018, Furian et al. [20] investigated the effect of 1650 versus 470 m on incremental cycling exercise tests in 40 patients with COPD. Consistent with our data, they reported an impaired peak exercise performance by −10 W (−11%). However, conclusive and comparable studies on exercise performance at high altitude in patients with PVD are scarce [24]. In 2021, we investigated the aerobic capacity in constant work rate exercise tests at 2500 m in 28 patients with PVD. The intensity was set at 60% of their maximal work rate and we observed a reduced cycling time of −6.4 min (−22.9%) [14]. To the best of our knowledge, the present trial is the first study investigating the decrement in maximum exercise capacity with incremental cycling exercise tests, including metabolic measurements by ergospirometry, at high altitude in patients with PVD.
The exposure to high altitude was associated with a decrease in arterial oxygenation at rest, end-exercise and isotime, which may lead to tissue hypoxia, augmented lactic acid loading and hyperventilation. Therefore, hypoxaemia may have significantly contributed to earlier exercise cessation at 2500 m, given that the maximal volitional effort was comparable in both tests, as indicated by a RER >1.1 and lactate >4 mmol·L−1, and similar Borg scale scores at end-exercise at both high and low altitude. In healthy subjects, the decrease in exercise capacity at moderate altitude was also mainly due to the decreased CaO2 [13].
Previous research suggests that the primary compensatory mechanism mitigating the decline in PaO2 involves an elevation in ventilation, subsequently resulting in a reduction in PaCO2 [21, 25–27]. In line with this, our current study found that PaCO2 at end-exercise was lower at high compared with low altitude. Despite concluding the exercise with a lower work rate at high altitude, there was no statistically significant difference in V′E and VT at end-exercise, indicating comparable ventilation at the point of exercise termination. At isotime, which corresponded to the identical time-point during tests at both altitudes, V′E was increased at high altitude, signifying adaptive hyperventilation. In patients with PVD at low altitude, increased physiological dead space, ventilation/perfusion mismatch and chemoreceptor response were described as mechanisms triggering hyperventilation during exercise [28].
There was a greater ventilatory inefficiency at high altitude, evidenced by the increased V′E/V′CO2 at end-exercise as well as at isotime and the higher V′E/V′CO2 slope compared with low altitude. This can be attributed to the hypoxia-induced stimulation of ventilation, aligning with observations in healthy mountaineers. However, it manifests at higher altitudes compared with patients with PVD [29]. The accelerated ventilatory drive in the hypoxic environment at high altitude contrasts with the demonstration in PVD patients breathing oxygen-enriched air at low altitude [11]. The PaCO2–PETCO2 gradient was already elevated at low altitude in this PVD collective, as described in relation to both increased chemosensitivity and increased dead space fraction (VD/VT) [30]. However, the PaCO2–PETCO2 gradient, as well as dead space fraction, remained unchanged at rest and end-exercise when comparing low versus high altitude, suggesting an altitude-independent, exercise-related increase in ventilatory inefficiency. At end-exercise, patients revealed a preserved ventilatory reserve, as expressed in V′E/MVV; however, this parameter remained unchanged between high and low altitude. The reduced peak V′O2 at end-exercise at high compared with low altitude, in accordance with healthy subjects, was primarily driven by the reduced CaO2 [13, 23]. Correspondingly, V′O2 at the gas exchange threshold was reduced by 16% at high versus low altitude. Recent evidence suggests that, during exercise, even far beyond the gas exchange threshold, anaerobic energy metabolism of working muscles is not the driver of increased lactate production [18, 31]. In line with this, despite increased hypoxaemia at high altitude, blood lactate in this study remained unchanged at end-exercise compared with low altitude. V′CO2 was reduced at high altitude only at end-exercise, whereas it was similar at isotime, most likely explained by hyperventilation.
Previous studies have shown an acceleration of heart rate in hypoxia, thereby increasing cardiac output and preserving systemic oxygen delivery [9, 14, 25]. In the present study, heart rate at rest and at end-exercise did not differ statistically significant between low and high altitude. Nevertheless, O2 pulse during exercise was diminished at high altitude. This could be attributed to patients with PVD already exhibiting a degree of sympathetic activation, leading to an increased heart rate at low altitude, which does not undergo further exaggeration at high altitude [10]. Of interest, the systemic blood pressure at end-exercise was similar at high and low altitude.
Schneider et al. [14] reported that dyspnoea at end-exercise of constant work rate exercise tests remained unaltered at an altitude of 2500 m. Consistent with these findings, patients with PVD in the present study reported similar Borg CR10 leg fatigue and dyspnoea scale scores at high compared with low altitude at end-exercise. In contrast to Schneider et al. [14], participants in the current trial showed no statistically significant difference in the exercise-induced rise of blood lactate concentration at high altitude, indicating that anaerobic metabolism at end-exercise at high altitude was similar to that at low altitude. This difference may be attributed to the typically shorter exercise duration in incremental cycling exercise tests compared with submaximal constant work rate tests.
In the present study, a day-long exposure to high altitude including maximal cycling exercise was found to be safe for the vast majority of stable and predominantly low-risk patients with PVD. These individuals, who remained non-hypoxic during exercise at low altitude, were mostly classified in New York Heart Association Functional Class I/II. Following predefined safety criteria, only one participant out of 27 received oxygen therapy at rest within the initial 3 h at high altitude. No adverse events occurred during incremental cycling exercise tests. During a subsequent overnight stay at high altitude, which was not the focus of this trial, 10 out of 27 patients revealed asymptomatic severe hypoxaemia (SpO2 <80% for >30 min) and were treated with oxygen for safety [16].
Conclusions
Among 27 stable, non-hypoxaemic patients with PAH and distal CTEPH, who were mostly in New York Heart Association Functional Class I/II and predominantly assessed as low risk, incremental cycling to exhaustion at an altitude of 2500 m on the first day was found to be safe and well tolerated. However, exercise capacity and ventilatory efficiency were lower at 2500 m compared with 470 m.
Shareable PDF
Footnotes
This article has an editorial commentary: https://doi.org/10.1183/13993003.00111-2024
This study is registered at ClinicalTrials.gov with identifier number NCT05107700. Individual participant data reported in this randomised controlled trial will be available after de-identification by contacting the corresponding author.
Ethics statement: The study complies with the Declaration of Helsinki and was approved by the local ethical authorities (KEK Project-ID 2021-00243).
Author contributions: All authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors. All authors contributed to the production of the final manuscript with revision for important intellectual content.
Conflict of interest: None of the authors have any conflicts of interest in relation to this manuscript.
Support statement: The trial is publicly funded by the Swiss National Science Foundation (SNSF, grant number 32003B_197706). Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2023; 61: 2200879. doi: 10.1183/13993003.00879-2022 [DOI] [PubMed] [Google Scholar]
- 2.Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 2022; 43: 3618–3731. doi: 10.1093/eurheartj/ehac237 [DOI] [PubMed] [Google Scholar]
- 3.McGoon MD, Ferrari P, Armstrong I, et al. The importance of patient perspectives in pulmonary hypertension. Eur Respir J 2019; 53: 1801919. doi: 10.1183/13993003.01919-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saxer S, Lichtblau M, Berlier C, et al. Physical activity in incident patients with pulmonary arterial and chronic thromboembolic hypertension. Lung 2019; 197: 617–625. doi: 10.1007/s00408-019-00248-x [DOI] [PubMed] [Google Scholar]
- 5.Hoeper MM, Apitz C, Grunig E, et al. Targeted therapy of pulmonary arterial hypertension: updated recommendations from the Cologne Consensus Conference 2018. Int J Cardiol 2018; 272S: 37–45. doi: 10.1016/j.ijcard.2018.08.082 [DOI] [PubMed] [Google Scholar]
- 6.Mallet RT, Burtscher J, Richalet JP, et al. Impact of high altitude on cardiovascular health: current perspectives. Vasc Health Risk Manag 2021; 17: 317–335. doi: 10.2147/VHRM.S294121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swenson ER. Hypoxic pulmonary vasoconstriction. High Alt Med Biol 2013; 14: 101–110. doi: 10.1089/ham.2013.1010 [DOI] [PubMed] [Google Scholar]
- 8.Groth A, Saxer S, Bader PR, et al. Acute hemodynamic changes by breathing hypoxic and hyperoxic gas mixtures in pulmonary arterial and chronic thromboembolic pulmonary hypertension. Int J Cardiol 2018; 270: 262–267. doi: 10.1016/j.ijcard.2018.05.127 [DOI] [PubMed] [Google Scholar]
- 9.Carta AF, Lichtblau M, Berlier C, et al. The impact of breathing hypoxic gas and oxygen on pulmonary hemodynamics in patients with pulmonary hypertension. Front Med 2022; 9: 791423. doi: 10.3389/fmed.2022.791423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hildenbrand FF, Fauchère I, Huber LC, et al. A low resting heart rate at diagnosis predicts favourable long-term outcome in pulmonary arterial and chronic thromboembolic pulmonary hypertension. A prospective observational study. Respir Res 2012; 13: 76. doi: 10.1186/1465-9921-13-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ulrich S, Hasler ED, Saxer S, et al. Effect of breathing oxygen-enriched air on exercise performance in patients with precapillary pulmonary hypertension: randomized, sham-controlled cross-over trial. Eur Heart J 2017; 38: 1159–1168. doi: 10.1093/eurheartj/ehx099 [DOI] [PubMed] [Google Scholar]
- 12.Woorons X, Richalet JP. Modelling the relationships between arterial oxygen saturation, exercise intensity and the level of aerobic performance in acute hypoxia. Eur J Appl Physiol 2021; 121: 1993–2003. doi: 10.1007/s00421-021-04667-8 [DOI] [PubMed] [Google Scholar]
- 13.Faoro V, Deboeck G, Vicenzi M, et al. Pulmonary vascular function and aerobic exercise capacity at moderate altitude. Med Sci Sports Exerc 2017; 49: 2131–2138. doi: 10.1249/MSS.0000000000001320 [DOI] [PubMed] [Google Scholar]
- 14.Schneider SR, Mayer LC, Lichtblau M, et al. Effect of a day-trip to altitude (2500 m) on exercise performance in pulmonary hypertension: randomised crossover trial. ERJ Open Res 2021; 7: 00314-2021. doi: 10.1183/23120541.00314-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015; 46: 903–975. doi: 10.1183/13993003.01032-2015 [DOI] [PubMed] [Google Scholar]
- 16.Schneider SR, Müller J, Bauer M, et al. Overnight exposure to high altitude in pulmonary hypertension: adverse events and effect of oxygen therapy. Eur Heart J 2024; 45: 309–311. doi: 10.1093/eurheartj/ehad789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radtke T, Crook S, Kaltsakas G, et al. ERS statement on standardisation of cardiopulmonary exercise testing in chronic lung diseases. Eur Respir Rev 2019; 28: 180101. doi: 10.1183/16000617.0101-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poole DC, Rossiter HB, Brooks GA, et al. The anaerobic threshold: 50+ years of controversy. J Physiol 2021; 599: 737–767. doi: 10.1113/JP279963 [DOI] [PubMed] [Google Scholar]
- 19.Glaser S, Ittermann T, Schaper C, et al. Referenzwerte für die Spiroergometrie – Ergebnisse der Study of Health in Pomerania (SHIP). [The Study of Health in Pomerania (SHIP) reference values for cardiopulmonary exercise testing.] Pneumologie 2013; 67: 58–63. doi: 10.1055/s-0032-1325951 [DOI] [PubMed] [Google Scholar]
- 20.Furian M, Flueck D, Latshang TD, et al. Exercise performance and symptoms in lowlanders with COPD ascending to moderate altitude: randomized trial. Int J Chron Obstruct Pulmon Dis 2018; 13: 3529–3538. doi: 10.2147/COPD.S173039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Latshang TD, Turk AJ, Hess T, et al. Acclimatization improves submaximal exercise economy at 5533 m. Scand J Med Sci Sports 2013; 23: 458–467. doi: 10.1111/j.1600-0838.2011.01403.x [DOI] [PubMed] [Google Scholar]
- 22.de Vries ST, Komdeur P, Aalbersberg S, et al. Effects of altitude on exercise level and heart rate in patients with coronary artery disease and healthy controls. Neth Heart J 2010; 18: 118–121. doi: 10.1007/BF03091749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schuler B, Thomsen JJ, Gassmann M, et al. Timing the arrival at 2340 m altitude for aerobic performance. Scand J Med Sci Sports 2007; 17: 588–594. doi: 10.1111/j.1600-0838.2006.00611.x [DOI] [PubMed] [Google Scholar]
- 24.Ulrich S, Lichtblau M, Schneider SR, et al. Clinician's corner: counseling patients with pulmonary vascular disease traveling to high altitude. High Alt Med Biol 2022; 23: 201–208. doi: 10.1089/ham.2022.0051 [DOI] [PubMed] [Google Scholar]
- 25.Siebenmann C, Lundby C. Regulation of cardiac output in hypoxia. Scand J Med Sci Sports 2015; 25: Suppl. 4, 53–59. doi: 10.1111/sms.12619 [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez-Garcia M, Aguirre-Franco CE, Vargas-Ramirez L, et al. Effect of pulmonary hypertension on exercise capacity and gas exchange in patients with chronic obstructive pulmonary disease living at high altitude. Chron Respir Dis 2022; 19: 14799731221104095. doi: 10.1177/14799731221104095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.West JB. The physiologic basis of high-altitude diseases. Ann Intern Med 2004; 141: 789–800. doi: 10.7326/0003-4819-141-10-200411160-00010 [DOI] [PubMed] [Google Scholar]
- 28.Farina S, Bruno N, Agalbato C, et al. Physiological insights of exercise hyperventilation in arterial and chronic thromboembolic pulmonary hypertension. Int J Cardiol 2018; 259: 178–182. doi: 10.1016/j.ijcard.2017.11.023 [DOI] [PubMed] [Google Scholar]
- 29.Ulrich S, Schneider SR, Bloch KE. Effect of hypoxia and hyperoxia on exercise performance in healthy individuals and in patients with pulmonary hypertension: a systematic review. J Appl Physiol 2017; 123: 1657–1670. doi: 10.1152/japplphysiol.00186.2017 [DOI] [PubMed] [Google Scholar]
- 30.Naeije R, van de Borne P. Clinical relevance of autonomic nervous system disturbances in pulmonary arterial hypertension. Eur Respir J 2009; 34: 792–794. doi: 10.1183/09031936.00091609 [DOI] [PubMed] [Google Scholar]
- 31.Hogan MC. What Wasserman wrought: a celebratory review of 50 years of research arising from the concept of an ‘anaerobic threshold’. J Physiol 2021; 599: 1005. doi: 10.1113/JP280980 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This one-page PDF can be shared freely online.
Shareable PDF ERJ-01001-2023.Shareable (536.8KB, pdf)