Summary
A subpopulation of pancreatic beta cells becomes senescent during type 1 diabetes (T1D) progression, and removal of these populations protects against T1D in mice. Here, we present a protocol to measure senescence in murine pancreatic islet cells through analysis of senescence-associated β-galactosidase activity. We describe steps for staining with the fluorogenic substrate C12FDG and analysis by flow cytometry. Increased cell size is another marker of senescence and can also be concurrently measured in the same experiment.
For complete details on the use and execution of this protocol, please refer to Lee et al.1 and Helman et al.2
Subject areas: Cell Biology, Cell culture, Cell isolation, Metabolism, Model Organisms
Graphical abstract

Highlights
-
•
Protocol to measure senescence in islet cells through SA-β-gal activity
-
•
Steps for islet cells isolation, dispersion, and flow analysis
-
•
C12FDG flow-based staining serves as a measurement for SA-β-gal activity
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
A subpopulation of pancreatic beta cells become senescent during type 1 diabetes (T1D) progression, and removal of these populations protects against T1D in mice. Here, we present a protocol to measure senescence in murine pancreatic islet cells through analysis of senescence-associated β-galactosidase activity. We describe steps for staining with the fluorogenic substrate C12FDG and analysis by flow cytometry. Increased cell size is another marker of senescence and can also be concurrently measured in the same experiment.
Before you begin
Senescent beta cells accumulate during the natural progression of T1D in non-obese diabetic (NOD) mice.3 Limiting persistent accumulation of senescent beta cells through immunosurveillance can protect NOD mice against T1D.1 SA-β-gal, encoded by GLB1, is a lysosomal enzyme that is widely used as a marker of senescence.4 SA-β-gal activity is frequently measured by in situ staining using a chromogenic substrate such as X-gal at pH 6.0. Here, we present an alternative protocol that details the staining of dispersed islet cells with a fluorogenic substrate of SA-β-gal (C12FDG) and its analysis via flow cytometry. In principle, this approach can be extended beyond islet cells, yet this will likely require optimization of the conditions.
Institutional permissions
The animal care and experimental procedures were carried out in accordance with the recommendations of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The protocol was approved by the University of Wisconsin-Madison Institutional Animal Care and Use Committee (IACUC). Researchers interested in performing this protocol should acquire prior permissions from their relevant institutions.
Isolation of islets
Timing: 3–6 h
-
1.
Isolate pancreatic islets as described previously in Lee et al.5
Preparing for islet dispersion
Timing: 5 min
-
2.
Set centrifuge (with 5 and 15 mL adapters) temperature to 4°C.
-
3.
Set water bath temperature to 37°C.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| TruStain FcX (anti-mouse CD16/32) antibody (1 μg/106 cells) | BioLegend | Cat# 101320; RRID: AB_1574975 |
| APC anti-mouse CD45 antibody (1:100) | BioLegend | Cat# 147708; RRID: AB_2563539 |
| Biological samples | ||
| Isolated mouse islets | In-house procedure | For information, see Lee et al., 2020 |
| Chemicals, peptides, and recombinant proteins | ||
| Dimethyl sulfoxide | Thermo Fisher Scientific | Cat# BP231-100 |
| C12FDG | Cayman Chemical | Cat# 25583 |
| 7-AAD | BioLegend | Cat# 420404 |
| Accutase | Innovative Cell Technologies | Cat# AT-104 |
| Fetal bovine serum | Thermo Fisher Scientific | Cat# F0926 |
| Cell staining buffer | BioLegend | Cat# 420201 |
| Software and algorithms | ||
| FlowJo v10 | FlowJo, LLC | https://www.flowjo.com |
| Other | ||
| LSRFortessa X-20 | BD Biosciences | |
| 40 μm cell strainer | Falcon | Cat# 352340 |
| Water bath | VWR | Cat# 76308-896 |
Materials and equipment
Mouse islet media
| Reagent | Final concentration | Amount |
|---|---|---|
| RPMI 1640 | 90% | 450 mL |
| Fetal bovine serum | 10% | 50 mL |
| 100X antibiotic/antimycotic | 1× | 5 mL |
| Total | N/A | 505 mL |
Store at 4°C for a maximum of 4 weeks.
-
•
10% FBS in RPMI 1640: add 50 mL of FBS in 450 mL RPMI 1640.
Store at 4°C for a maximum of 1 week.
Step-by-step method details
Islet dispersion
Timing: 1 h
Since pancreatic islets are clusters of cells aggregated together, it is necessary to disperse them into a single cell suspension before performing the flow cytometry experiments. In this section, it is crucial to obtain a high percentage of single cells with high viability.
-
1.
Put islets from each mouse into a 60 mm plate with 5 mL of mouse islet media.
-
2.
Allow to rest from 1 h to overnight (16 h) in a 5% CO2 incubator at 37°C.
-
3.Move islets from 60 mm plate into a 15 mL tube.
-
a.Rinse with 5 mL of PBS to ensure all islets are moved.
-
a.
-
4.
Centrifuge at 200 g for 3 min at room temperature (20°C–24°C) and remove supernatant with aspirator.
-
5.
Add 5 mL of PBS to wash the mouse islet media away. Centrifuge at 200 g for 3 min and remove supernatant completely.
-
6.
Add 2 mL of Accutase, a cell dissociation solution, in the tube and incubate in a 37°C water bath.
-
7.
Set a timer for 30 min. After 15 min, pipet up and down 10x every 5 min, for a total of 4 times.
-
8.
Add 10 mL of prewarmed 10% FBS in RPMI 1640 to stop the reaction.
-
9.Filter the cell suspension using a 40 μm cell strainer into a 50 mL tube.Note: This will get rid of larger aggregates, and potentially separate loosely associated doublets/triplets into single cells.
-
a.Rinse filter with additional media to minimize cell loss.
-
a.
-
10.
Spin at 300 g for 5 min at 4°C, and aspirate supernatant.
-
11.Wash cells by adding 2 mL of cell staining buffer.
-
a.Spin at 300 g for 5 min at 4°C, and aspirate supernatant.
-
b.Resuspend pellet in 1 mL cell staining buffer.
-
a.
Preparation for flow cytometry
Timing: 2 h
C12FDG staining serves as a measurement for SA-β-gal activity. In this section, cells will be stained with APC-CD45 and 7-AAD in addition to C12FDG, allowing for exclusion of dead cells and leukocytes in our flow analysis.
-
12.
Add 1.8 μL of C12FDG solution (stock: 16.5 mM) to the tube.
-
13.
Incubate the tube in a 37°C water bath for 1 h.
-
14.
Spin at 300 g for 5 min at 4°C, and aspirate supernatant.
-
15.Add 1 mL of cell staining buffer, and transfer cells to a 5 mL tube.
-
a.Rinse tube with 1 mL of additional cell staining buffer to minimize cell loss.
-
a.
-
16.
Spin at 300 g for 5 min at 4°C, and remove supernatant by decanting.
Note: around 100 μL of solution is left after decanting.
-
17.Add anti-CD16/32 antibody (1.0 μg per 106 cells) to the tube to block FC receptors.
-
a.Incubate for 10 min.
-
a.
-
18.
Add 1 μL of APC anti-CD45 to the tube and incubate on ice for 30 min.
Note: it is not necessary to wash away the anti-CD16/32 antibody after step 17.
-
19.
Wash cells by adding 1 mL of cell staining buffer, and spin at 300 g for 5 min at 4°C.
-
20.
Decant supernatant and resuspend cells in 250–500 μL of cell staining buffer.
-
21.
Add 5 μL of 7-AAD solution to the tube and incubate for 10 min before flow analysis.
-
22.Analyze sample with a flow cytometer.
-
a.Gating strategy: exclusion of debris, inclusion of single cells, exclusion of dead cells (negative for 7-AAD or other live/dead marker used), and exclusion of leukocytes (CD45-negative).
-
b.Lasers used: 7-AAD with 561 nm laser; APC-CD45 with 640 nm laser, C12FDG with 488 nm laser. This set-up may be different depending on the live/dead marker and other fluorophores used.
-
a.
Expected outcomes
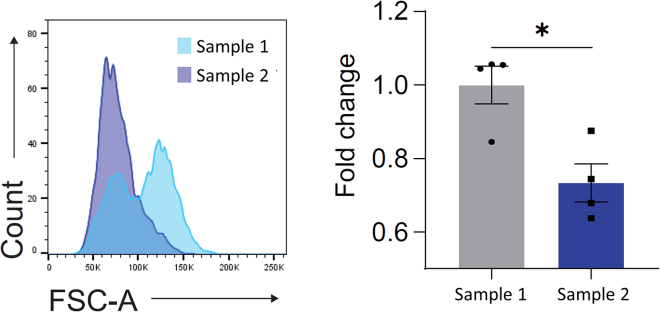
The activity of SA-β-gal is a hallmark of senescence and can be measured by staining cells with C12FDG followed by flow cytometry analysis. C12FDG is a substrate of SA-β-gal and can be cleaved to form a detectable fluorescent product.6 During the analysis, after gating for single, viable, and non-leukocyte cells, the comparison of the percentage of positively stained cells from the histogram of C12FDG from each sample can be used to compare the amount of SA-β-gal+ cells in each sample (Figure 1). Alternatively, SA-β-gal activity levels between samples can be compared by calculating median fluorescence intensity (MFI) from the C12FDG+ gated population. With the same gating strategy, cell size can also be compared by creating a histogram for forward scatter, with cells undergoing senescence having a greater cell size (Figure 2).
Figure 1.
Measurement of SA-β-gal activity in islet cells through C12FDG analysis, related to step 22
(A) Gating strategy for analysis of single and viable cells that are negative for CD45. (B) Representative histogram of C12FDG, where sample 1 exhibits increased senescence compared to sample 2. Fold change calculation for samples is based on median fluorescence intensity (RFI). Data are represented as means ± SEM. ∗∗p<0.01. Unpaired two tail t-test was applied. Figure adapted from Lee et al.1
Figure 2.
Comparison of islet cell size between samples, related to step 22
Representative histogram of forward scatter, where sample 1 has a subpopulation of cells that are larger. Fold change calculation for samples is based on median forward scatter. Data are represented as means ± SEM. ∗p<0.05. Unpaired two tailed t-test was applied. Figure adapted from Lee et al.1
Limitations
This protocol aims to quantify SA-β-gal activity as a phenotype of late-stage senescence in non-immune islet cells during T1D progression in the NOD mouse model. However, islet cells are heterogeneous and consist of multiple cell types such as alpha, beta, and delta cells. Therefore, the results of the analysis would also consist of cells other than beta cells. In addition, there are non-endocrine cells in the islet that could be stained, such as endothelial cells. It should also be noted that this protocol will not distinguish between senescent islet cells that arise during aging and those that result from a stress response as SA-β-gal activity can be high in both.
Troubleshooting
Problem 1
Poor viability during flow analysis, related to step 22.
Potential solution
-
•
Determine if the viability issues are stemming from the dispersion step or the staining step. This can be done with trypan blue staining and cell counting after the dispersion step, or through live/dead dye with flow cytometry.
-
•
Be wary of being too harsh during islet dispersion with Accutase. If cell viability is low after dispersion, use less force during pipetting or reduce incubation time. Alternatively, a non-enzymatic PBS or HBSS based dissociation buffer can be used (e.g., Thermo Fisher Cat #13150016).
-
•
Make sure cells are on ice when they are not incubating at 37°C, and ensure appropriate media is prewarmed before adding to cells.
Problem 2
Lack of C12FDG+ stained population, related to step 22.
Potential solution
-
•
C12FDG was stored improperly: Ensure stocks are made of 16.5 mM in DMSO and stored at -20°C and not subject to repeated freeze-thaw cycles.
-
•
C12FDG concentration was too low: Ensure concentration is ∼33 μM in final solution with cells.
Problem 3
All cells stain C12FDG+, related to step 22.
Potential solution
-
•
C12FDG concentration was too high: Ensure concentration is ∼33 μM in final solution with cells.
-
•
Incubation with C12FDG too long: Ensure cells are incubated for 1 h at 37°C in a water bath.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Feyza Engin (fengin@wisc.edu).
Technical contact
Technical questions on executing this protocol should be directed to and will be answered by the technical contact, Hugo Lee (hslee5@wisc.edu).
Materials availability
This protocol did not generate new unique reagents.
Data and code availability
This study did not generate new datasets or code.
Acknowledgments
H.L. was supported by NIH T32 GM007215 and a UW-SCRMC graduate fellowship. P.J.T. was supported by grants from the University of Manitoba (URGP), the Children’s Hospital Research Institute of Manitoba (CHRIM OG-2021-05), and the Canadian Institutes of Health Research (CIHR PJT-479641). F.E. is supported by grants from the NIH (DK130919 and DK128136), the JDRF (3-SRA2023-1315-S-B and 5-CDA-2014-184-A-N), the Greater Milwaukee Foundation, and startup funds from UW-Madison. Graphical abstract was created using BioRender.com.
Author contributions
H.L. performed the experiments and wrote the manuscript. P.J.T. optimized the protocol and revised the manuscript. F.E. supervised and supported the project and revised the manuscript.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Hugo Lee, Email: hslee5@wisc.edu.
Feyza Engin, Email: fengin@wisc.edu.
References
- 1.Lee H., Sahin G.S., Chen C.W., Sonthalia S., Cañas S.M., Oktay H.Z., Duckworth A.T., Brawerman G., Thompson P.J., Hatzoglou M., et al. Stress-induced beta cell early senescence confers protection against type 1 diabetes. Cell Metab. 2023;35:2200–2215.e9. doi: 10.1016/j.cmet.2023.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Helman A., Klochendler A., Azazmeh N., Gabai Y., Horwitz E., Anzi S., Swisa A., Condiotti R., Granit R.Z., Nevo Y., et al. p16(Ink4a)-induced senescence of pancreatic beta cells enhances insulin secretion. Nat. Med. 2016;22:412–420. doi: 10.1038/nm.4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson P.J., Shah A., Ntranos V., Van Gool F., Atkinson M., Bhushan A. Targeted Elimination of Senescent Beta Cells Prevents Type 1 Diabetes. Cell Metab. 2019;29:1045–1060.e10. doi: 10.1016/j.cmet.2019.01.021. [DOI] [PubMed] [Google Scholar]
- 4.Lee B.Y., Han J.A., Im J.S., Morrone A., Johung K., Goodwin E.C., Kleijer W.J., DiMaio D., Hwang E.S. Senescence-associated β-galactosidase is lysosomal β-galactosidase. Aging Cell. 2006;5:187–195. doi: 10.1111/j.1474-9726.2006.00199.x. [DOI] [PubMed] [Google Scholar]
- 5.Lee H., Engin F. Preparing Highly Viable Single-Cell Suspensions from Mouse Pancreatic Islets for Single-Cell RNA Sequencing. STAR Protoc. 2020;1 doi: 10.1016/j.xpro.2020.100144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Debacq-Chainiaux F., Erusalimsky J.D., Campisi J., Toussaint O. Protocols to detect senescence-associated beta-galactosidase (SA-betagal) activity, a biomarker of senescent cells in culture and in vivo. Nat. Protoc. 2009;4:1798–1806. doi: 10.1038/nprot.2009.191. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate new datasets or code.