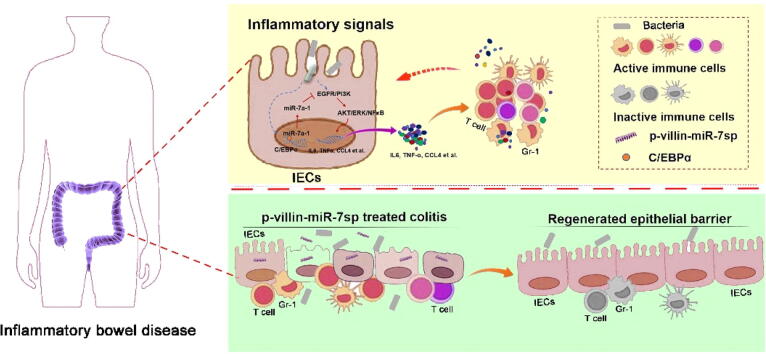
Graphical abstract
The epithelial immunomodulation and regeneration are fundamental and intrinsic critical events against inflammatory bowel disease (IBD). MiR-7 is well implicated as a promising regulator in the pathologies of inflammatory diseases. Herein, we investigate the role of miR-7 in intestinal epithelial cells (IECs) in IBD. We found that miR-7 expression, which is mainly derived from miR-7a-1 operated by transcriptional factor C/EBPα, is increased in IECs from IBD model and Crohn's disease patients. MiR-7 controls the immunomodulation and regeneration of epithelial cells through EGFR/NF-κB/ERK/AKT signaling pathways. Epithelial cell-specific miR-7 silencing alleviates the pathology of IBD model. Therefore, our data reveal the unknown role of miR-7/EGFR axis in IEC immunomodulation and regeneration in IBD and might provide a clue for the development of miRNA-based therapeutic strategy applications in colonic diseases.
Keywords: Inflammatory bowel disease, MiR-7, Immune cells, Intestinal epithelial cells, EGFR
Highlights
-
•
The expression of miR-7, operated by transcriptional factor C/EBPα, is increased in IECs in IBD.
-
•
MiR-7 controls the immunomodulation and regeneration of IECs through EGFR signaling pathways.
-
•
Epithelial cell-specific miR-7 silencing alleviates the pathology of Colitis model.
Abstract
Introduction
The epithelial immunomodulation and regeneration are intrinsic critical events against inflammatory bowel disease (IBD). MiR-7 is well documented as a promising regulator in the development of various diseases including inflammatory diseases.
Objectives
This study aimed to assess the effect of miR-7 in intestinal epithelial cells (IECs) in IBD.
Methods
MiR-7def mice were given dextran sulfate sodium (DSS) to induce enteritis model. The infiltration of inflammatory cells was measured by FCM and immunofluorescence assay. 5′deletion assay and EMSA assays were performed to study the regulatory mechanism of miR-7 expression in IECs. The inflammatory signals and the targets of miR-7 were analyzed by RNA-seq and FISH assay. IECs were isolated from miR-7def, miR-7oe and WT mice to identify the immunomodulation and regeneration capacity. IEC-specific miR-7 silencing expression vector was designed and administered by the tail vein into murine DSS-induced enteritis model to evaluate the pathological lesions of IBD.
Results
We found miR-7 deficiency improved the pathological lesions of DSS-induced murine enteritis model, accompanied by elevated proliferation and enhanced transduction of NF-κB/AKT/ERK signals in colonic IECs, as well as decreased local infiltration of inflammatory cells. MiR-7 was dominantly upregulated in colonic IECs in colitis. Moreover, the transcription of pre-miR-7a-1, orchestrated by transcription factor C/EBPα, was a main resource of mature miR-7 in IECs. As for the mechanism, EGFR, a miR-7 target gene, was downregulated in colonic IECs in colitis model and Crohn's disease patients. Furthermore, miR-7 also controlled the proliferation and inflammatory-cytokine secretion of IECs in response to inflammatory-signals through EGFR/NF-κB/AKT/ERK pathway. Finally, IEC-specific miR-7 silencing promoted the proliferation and transduction of NF-κB pathway in IECs and alleviated the pathological damage of colitis.
Conclusion
Our results present the unknown role of miR-7/EGFR axis in IEC immunomodulation and regeneration in IBD and might provide clues for the application of miRNA-based therapeutic strategies in colonic diseases.
Introduction
Inflammatory bowel disease (IBD) is a chronic inflammatory disease that includes Crohn's disease (CD) and ulcerative colitis (UC), and characterized by accumulation of submucosal inflammatory cells and damage to the epithelial layer [1]. Increasing evidence has shown that the interaction among intestinal epithelial cells (IECs), immune cells and other immune-related cells such as epithelial stem cells and paneth cells in a cellular cross-talk is a major etiology for IBD development [2], [3]. During this biological process, IECs play a central part in regulating mucosal immunity at the local immune reaction sites. In inflammatory condition, IECs secrete many inflammatory cytokines that affect the accumulation and function of a variety of immune cells, such as T cells, CD19+ B cells, and CD11b+ cells as well [4]. Thus, epithelial immunomodulation and regeneration are fundamental and intrinsic critical events against IBD. Studies have shown that multiple signaling pathways, such as AKT, ERK, and NF-κB, control the immunomodulation and proliferation of IECs in IBD. For example, the loss of STAT3 alleviates the epithelial erosions and impairs the regeneration of IECs induced by DSS [5]. Moreover, RORα/HDAC3 controls the balance of inflammatory responses through mediating the attenuation of NF-κB signaling in IECs in IBD [6]. Although certain factors seem to influence immune responses and epithelial regeneration, it is still largely unknown how the signals are regulated in controlling the immunomodulatory and regenerative function of IECs in IBD. This is fundamental for understanding the precise mechanism of IBD development and will provide avenues for therapeutic strategy development.
MIcroRNA-7 (MiR-7), as a distinct member of miRNA family, plays a significant part in the context of organ development and differentiation [7], [8]. Recently, further studies have shown that miR-7 is closely related to the various diseases occurrence and progression, including colon-related diseases. For exemple, Kong et al. suggested that miR-7 was a novel regulator through controlling inflammatory response to promote gastric tumorigenesis [9]. Furthermore, Chen et al. demonstrated that the miR-7 could inhibit the effect of LPS on the activation and function of Macrophage via the TLR4 signal transduction [10]. Our series of studies recently revealed that miR-7 is also involved in inflammatory diseases, including autoimmune hepatitis [11], acute lung injury [12], and brain tissue inflammatory injury [13]. Concerning IBD, Guo et al. suggested that inhibition of miR-7 expression improved the pathological manifestations of TNBS-induced colitis mice [14]. These data raise an interesting issue: whether miR-7 might play a vital part in IBD progression. However, how and to what extent miR-7 contributes to the development of IBD, including the epithelial immunomodulation and regeneration, are not well defined.
In the present work, we aimed to explore the potential biological role of miR-7 in IECs in IBD. We speculated that miR-7 could be a key intrinsic regulator in epithelial immunomodulation and regeneration and promote colitis pathology. Our data showed that miR-7 deficiency could ameliorate the inflammatory response by regulating EGFR signaling pathways within IECs, while concomitantly promoting epithelial cell proliferation and reducing inflammatory cell infiltration in colon tissue in colitis. In turn, we showed that miR-7 expression, operated by transcription factor C/EBPα, was upregulated in IECs in colitis model and IBD patients. Notably, epithelial cell-specific miR-7 silencing could alleviate the pathology of colitis model, which might be valuable for the proceeding of effective clinical IBD gene therapy strategy.
Materials and methods
Ethics statement
All experiments involving animals were evaluated and approved by the Zunyi Medical University Laboratory Animal Care and Use Committee (permit number: SYXK-2021–0004).
Mice and subjects
The miR-7 deficiency (miR-7def) mice [11], [12], [13], and miR-7 overexpression (miR-7oe) mice [15] (Male, 8–10 weeks) have been described in our previous study.
Tissue of Clinical CD patients and healthy controls were purchased from Cybrdi (no. DC-Col00013, Shanxi Chao Ying Biotechnology Co., LTD). The clinical characteristics and pathologic information for the patients were listed (Supplementary Table 1).
Plasmids and luciferase reporter assay
We designed and generated the pGL3.0-Villin1 promoter-miR-7Sponge (p-v-miR-7sp) vector (contained the sequence of 1,799 bp Villin1 promoter and the 140 bp miR-7 sponge), the mouse or human pGL3.0–1000-miR-7, −500-miR-7, −100-miR-7 expression plasmids (contained chemically synthesized 1000 bp, 500 bp, and 100 bp product of the 5′‑flanking region of the mouse and human miR-7a-1 gene), respectively. The sequence of miR-7 inhibitor, negative inhibitor control, miR-7 mimics, and control were obtained from the Guangzhou Ribobio Biological Engineering CO. All recombinant plasmids were confirmed by sequencing.
The luciferase reporter assay was performed to determine whether EGFR was a direct target of miR-7. The putative miR-7 binding sites (560 bp–566 bp, 2858 bp–2864 bp and 6,112 bp–6,118 bp segment) in 3′-UTR of EGFR mRNA were predicted using miRDB database. 3 fragment of 200 bp containing these putative binding sites of EGFR 3′-UTR were directly synthesized (Sangon) and ligated to pEZX-FR02 reporter vector (saved in lab), respectively. Then, the 3 recombinant vector containing different wild-type binding site fragments of EGFR 3′-UTR were co-transfected into murine IECs in 12-well plates with miR-7 mimics or control (200 nM) by Lipofectamine 3000 (Invitrogen), respectively. At 48 h the results were assessed as previously reported [13].
DSS-induced murine colitis model
DSS-treated mice received 2 % (wt/vol) DSS (MP Biomedicals), in their drinking water for seven days, then the mice were given normal drinking water for another three days and were sacrificed on the 10 th day. The EGFR tyrosine kinase inhibitor-Erlotinib was given i.p. three times (50 mg/kg body weight, at days −1, 3, 5, n = 6); p-v-miR-7sp was given i.v. three times (5 mg/kg body weight, at days 2, 4 and 6, n = 6). The percent weight change of mice were detected and calculated. The disease activity index was measured as previously reported (16).
Western blot
Western blot was performed as previously reports [12], [13]. Immunoblotting has performed using a mAb to NF-κB (CST, no.4764), p-NF-κB (CST, no.3039), AKT (CST, no.4691), phos-AKT (CST, no.4060), ERK (CST, no.9012), p-ERK (CST, no.4370), EGFR (Sigma-Aldrich, no.SAB4300481), p-EGFR (CST, no.3777), Ras (Abcam, no.ab.52939), PDK1 (Abcam, no.ab207450), PI3K (Abcam, no.ab191606), p-PI3K (Abcam, no.ab182651), C/EBPα (Abcam, no. ab40764), and GAPDH (Abcam, no.ab9484) at a recommended dilution in a non-fat milk-Tris buffer.
Cell culture and transfection
Human normal colonic epithelial FHC cells (saved in our lab) were cultured in completed RPMI-1640 (GIBCO). FHC cells were transfected using Lipofectamine 3000 reagent with 200 nM of miR-7 inhibitors and the inhibitors-negative control (NC) or miR-7 mimics and mimics-NC according to the previously study (11). Twelve hours later, these cells were treated with LPS (200 ng/ml) for 24 h. Then Erlotinib (5 nM) was administrated for 24 h. The cells were harvested at the indicated times.
Intestinal epithelial cell isolation and culture in vitro
Colonic intestinal epithelial cells (IECs) were isolated from suckling mice of WT/miR-7def/miR-7oe mice, and the colonic tract was cut into 1–3 mm pieces, and digested for 45 min at 37 °C in RPMI medium containing 0.5 % FBS, 1 mg/mL of collagenase IV (GIBCO, no. 17104019), and 0.08 mg/mL of DNase I (Solarbio, no. D8071) at 37 °C. These pellets were centrifuged and resuspended in PBS. Then recovered < 100 μm and greater than 40 μm fraction to culture in a plate with completed DMEM/F12 (GIBCO, no. A4192001). 5 days later, these cells were treated with LPS (500 ng/ml) or Erlotinib (5 nM).
Viruses and infections
Colonic IECs were isolated, then 2 × 105 cells were seeded per well of a 6-well plate, and after 24 h infected C/EBPα RNAi and RNAi-NC or C/EBPα overexpression and overexpression-NC virus particles (Shanghai GenePharma Co., Ltd, stock titer ∼ 1 × 108 pfu/ml), 48 h later, these cells were harvested and analyzed.
RNA-seq
RNA-seq was performed by Xiuyue Biol (Jinan, China). Global Gene expression array data are available at the NCBI GEO under accession number GSE174331.
EMSA
EMSA was performed using biotin-labeled oligonucleotide probes for the core promoter region of the miR-7a-1 gene. According to the manufacturer's instructions (20148, Thermo Scientific, USA), the nuclear extracts were prepared from IECs with/without LPS treatment (78833, Thermo Scientific, USA). The biotinylated/un-biotinylated probes (C/EBPα1: forward:5′-CGGATTAAACAAATTCGTCATG-3′, reverse:5′-CATGACGAATTTGTTTAAT-CCG-3′; C/EBPα2: forward: 5′-GCATCAATCAAAATTGATGAAC-3′, reverse:5′-GTTCATCAATTTTGATTG-ATGC-3′; C/EBPα3: forward: 5′-CGCACAGTATTTGCTGCAGAAC-3′, reverse:5′-GTTCTGCAGCAAATAC-TGTGCG-3′; C/EBPα4: forward: 5′-TCTAACTTCTAAATATTCTAAT-3′, reverse:5′-ATTAGAATATTTAGA-AGTTAGA-3′; C/EBPα5: forward: 5′-CCTTAATTTTCTTCTGTTTTCTTACTAAAATGAAGA-3′, reverse:5′-TCTTCATTTTA-GTAAGAAAACAGAAGAAAATTAAGG-3′) were synthesized (Sangon, China).
Statistical analyses
The data were analyzed with GraphPad Prism 7.0 and were presented as the mean ± SD for 3 independent experiments. Student's unpaired t-test was used when two conditions were compared. P-values of < 0.05 were considered to be statistically significant.
For all other Materials and Methods, see the Supplementary Materials.
Results
MiR-7 deficiency ameliorates the pathological damage of IBD and elevates epithelial regeneration
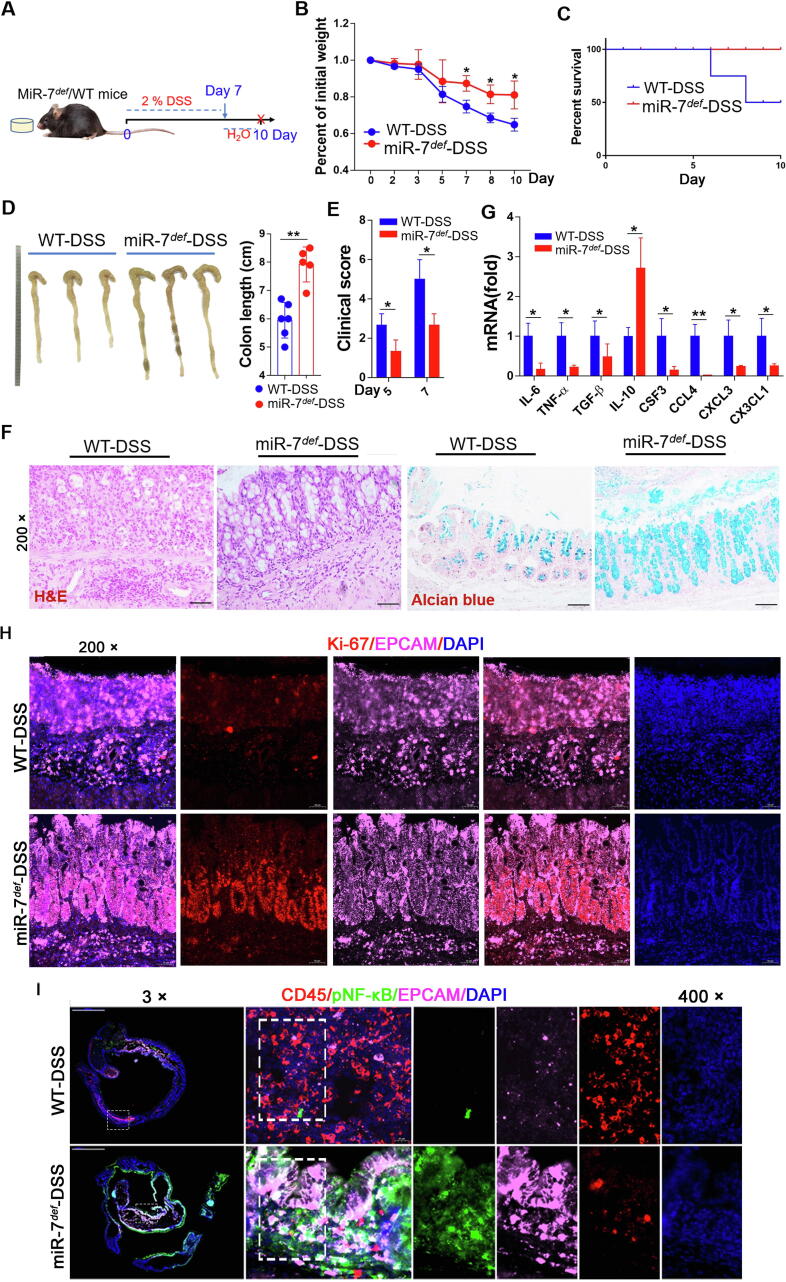
To evaluate the role of miR-7 in the epithelial immunomodulation and regeneration in IBD, we first examined the effect of miR-7 in colitis, we established a DSS-induced experimental model of colitis. Compared with the WT mice of DSS group, the loss of body weight was decreased in the miR-7def mice of DSS group (Fig. 1A and B), along with a marked increase in the survival rate, significantly longer in colon tissue, and dramatically improved clinical disease scores (Fig. 1C–E). Histology assessment and mucous layer analysis further showed a slight inflammatory response and upregulated the number of goblet cells and mucin production (Fig. 1F, Supplementary Fig. 1A and B). Meanwhile, the levels of pro-inflammatory factors TNF-α, TGF-β1, IL-6, CSF3, CCL4, CXCL3 and CX3CL1 decreased obviously, while the anti-inflammatory IL-10 level increased dramatically in the colon tissue from miR-7def mice with DSS (Fig. 1G).
Fig. 1.
MiR-7 deficiency alleviates the pathology of murine IBD and promotes epithelial regeneration. (A) Schematic representation of the establishment of murine DSS-induced IBD. MiR-7def mice and WT mice (8–10 weeks old, n = 8) were given 2% DSS solution for 7 days and changed water to continue feeding for 3 days to induce the IBD model; the mice were sacrificed on the 10th day. The weight loss (B), the survival curve (C) of WT and miR-7def littermates after DSS treatment were observed; Representative gross images of colon and colon length were detected and analyzed (D); The clinical scores were estimated (E); Histopathology was performed by H&E staining and mucin production measured by Alcian blue staining (×200) (F); The relative expression levels of proinflammatory cytokines IL-6, TNF-α, TGF-β, IL-10, CSF4, CCL4, CXCL3, and CX3CL1 were assessed in the colon tissue by Real-time PCR assay (G); Double immunofluorescence for Ki67 and EPCAM in the colon tissue (×200) (H); The expression of CD45, p-NF-kB and EPCAM were determined by multiplexed fluorescent immunohistochemical staining in the colon (×3, ×400) (I). The values are the means ± SD (n = 3). *P < 0.05, **P < 0.01. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Further analysis showed that miR-7 deficiency increased epithelial cell proliferation (Fig. 1H). Multiple signaling pathways, such as ERK, AKT, and NF-κB, are also involved in the pathological lesions of colitis, including epithelial regeneration [[16], [17], [18], [19]]. Expectedly, miR-7 deficiency elevated the levels of p-NF-kB, p-ERK and p-AKT in colon tissue (Supplementary Fig. 2A and B). We further evaluated the expression levels of these molecules in colon tissue–infiltrating inflammatory cells (CD45+ cells) or colonic epithelial cells (CD45− cells) in colitis mice. Data showed that the expression levels of p-NF-kB, p-ERK and p-AKT increased obviously in total colonic tissue cells in DSS-treated miR-7def mice (Supplementary Fig. 2C). Notably, these factors were dominantly expressed in CD45− cells but not in CD45± cells (Supplementary Fig. 2D and E). To verify this result, we detected and analyzed the expression level of p-NF-κB, a representative molecule among these signaling pathways, in IECs. Consistently, p-NF-κB level in IECs in DSS-treated miR7def mice increased obviously (Fig. 1I). Collectively, these data demonstrated that miR-7 deficiency could mitigate the onset of colitis, accompanied by elevated proliferation and altered transduction of inflammatory-related signaling pathways in IECs of colitis mice.
MiR-7 deficiency reduces the infiltration of inflammatory cells in colon in IBD
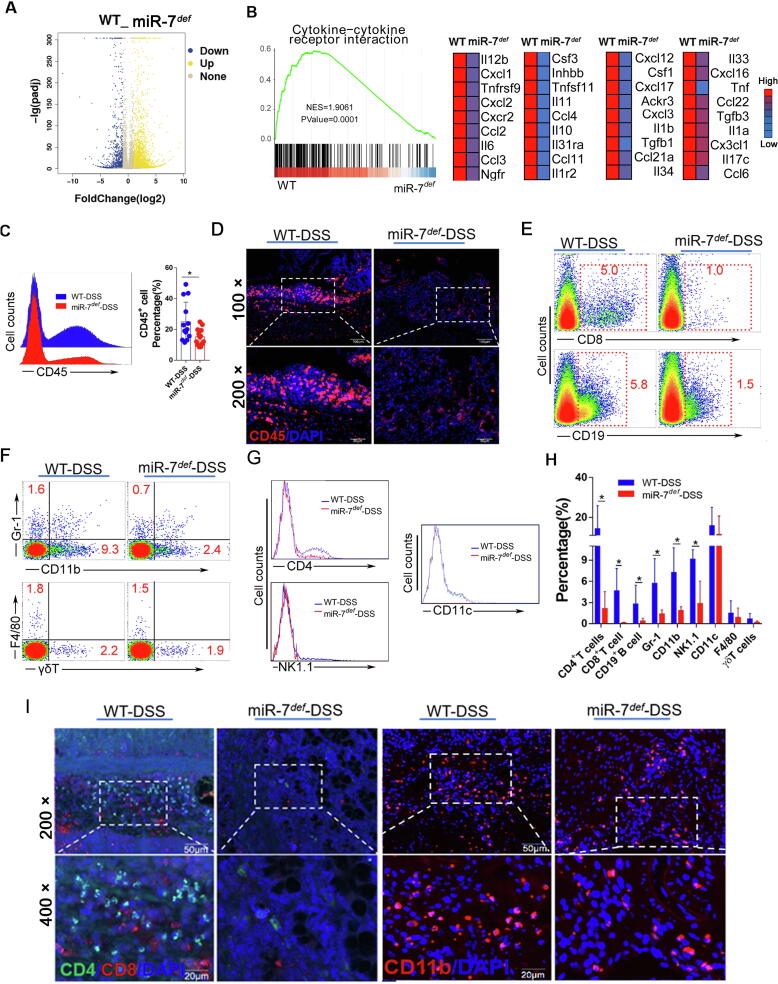
The local infiltration of inflammatory cells, mediated by chemokines secreted by epithelial cells, plays a critical part in the pathogenesis and progression of colitis [20]. Therefore, we further evaluated the change of global genes in colon tissue in DSS-treated miR-7def mice using RNA-seq assay and confirmed that miR-7 deficiency reduced the enrichment of the cytokine-cytokine receptor interaction, leukocyte transendothelial migration and TCR signaling pathway (Fig. 2A and B, Supplementary Fig. 3A and B). Furthermore, the levels of chemokines, such as CCL4, CXCL3, and CX3CL1, which are pivotal to mediate inflammatory cell migration, decreased obviously in DSS-treated miR7def mice (Fig. 2 B). Consistent with these findings, the local infiltration of CD45± inflammatory cells in the colon tissue decreased significantly (Fig. 2C and D). Next, we analyzed the possible changes in the proportion of the innate immune cell populations in colon tissue. Even though the percentage of CD11c+ DCs, γδ+ T cells and F4/80±Mφ in colon tissue did not change significantly, the proportion of NK1.1± T cells, Gr-1± neutrophil cells and CD11b± cells was reduced in miR7def IBD mice (Fig. 2E–H). Consistently, the proportions of adaptive immune cells CD8+ T cells, CD19+ B cells and CD4+ T cells were always obviously reduced (Fig. 2E, G and H). Finally, immunofluorescence assay also confirmed the decreased local infiltration of CD11b± cells, CD4+ T cells, and CD8+ T cells in the colon tissue of miR7def IBD mice (Fig. 2I). These results illustrated that miR-7 deficiency could reduce the local infiltration of multiple inflammatory related-cells in colonic tissue, which is positively correlated with the improved histopathological damage in mice with enteritis.
Fig. 2.
MiR-7 deficiency reduces the infiltration of inflammatory cells in colon tissue in IBD. (A–B) The gene-expression profiles in the colorectal tissues of miR-7def and WT IBD mice were performed and analyzed using RNA-seq assay. GSEA plots from RNA-seq data observed the inflammatory-related genes. (C) The proportion of CD45+ cells were detected and analyzed in the colon tissue by FCM; The expression of CD45 was determined by IF (D); The expression of CD4+ T cells, CD8+ T cells, CD19+ B cells, Gr-1+ cells, CD11b+ cells, NK1.1+ T cells, CD11c+ cells, F4/80+ cells and γδ+ T cells in CD45+ cells were analyzed and calculated by FCM (E–H); The expression of CD4+ T cells, CD8+ T cells, and CD11b+ cells were detected by Multiplexed fluorescent immunohistochemical staining (I). The values are the means ± SD (n = 3). *P < 0.05, **P < 0.01.
MiR-7 expression in colonic epithelial cells in IBD
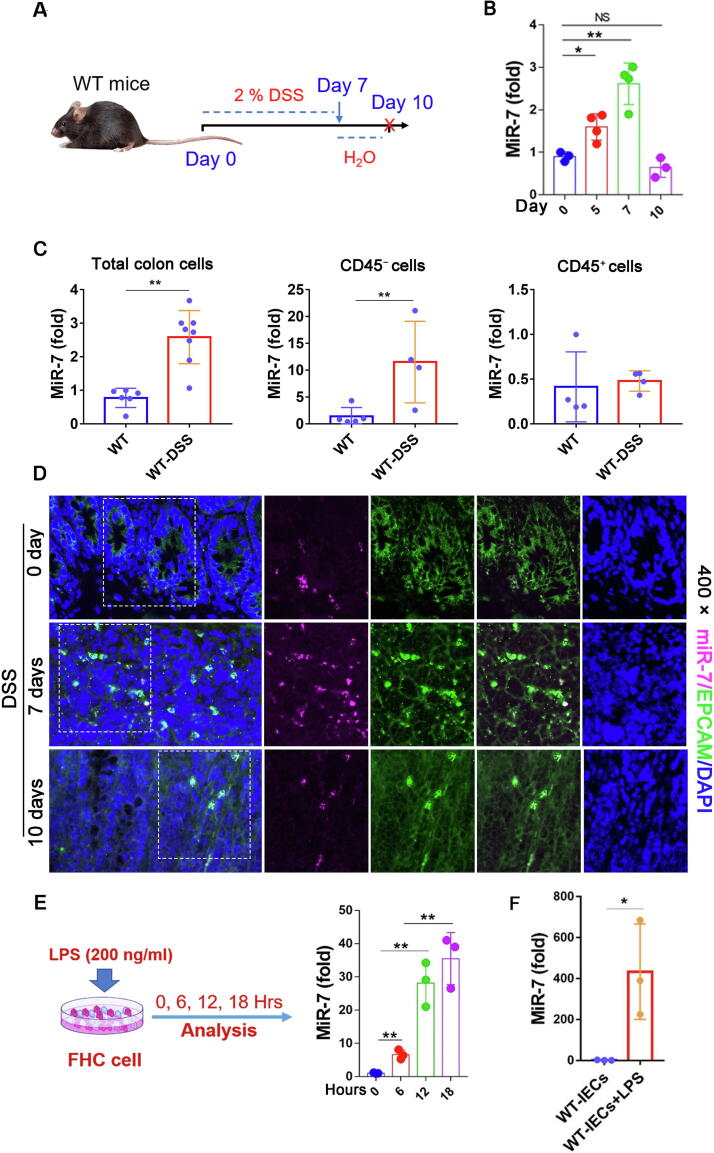
Next, we further monitored miR-7 expression pattern in the IBD model. The levels of proinflammatory cytokines TNF-α, CXCL3, CSF3, and CX3CL1 increased significantly in the colonic tissue from mice following DSS treatment for 7 days (Supplementary Fig. 4A and B). Conversely, the anti-inflammatory cytokines TGF-β and IL-10 levels downregulated obviously (Supplementary Fig. 4C). Histopathological analysis indicated notable intestinal defects, including mucosal edema and the tissue content of mucins in colonic tissue, concomitantly with infiltration of CD45+ inflammation cells (Supplementary Fig. 4D–F). Expectedly, the expression level of miR-7 steadily upregulated in the colonic tissue of IBD mice (Fig. 3A and B). Next, the expression levels of TNF-α, CSF3, CXCL3, and CX3CL1 decreased significantly in colonic tissue, whereas the expression levels of TGF-β and IL-10 increased obviously in 3 days after withdrawal of DSS treatment (Supplementary Fig. 4B and C). Consistently, the pathological change of colon tissues improved and the infiltration of CD45+ inflammation cells decreased (Supplementary Fig. 4D–F). Notably, miR-7 expression decreased obviously (Fig. 3B), indicating a positive connection with a pathological change of colon tissues. Furthermore, we noticed that the expression level of miR-7 was increased dominantly in CD45− cells (mainly epithelial cells) but not in CD45+ infiltrated inflammatory cells in colonic tissue in IBD mice (Fig. 3C). Consistent with previous data, FISH assay confirmed that miR-7, dominantly expressed in EPCAM+ IECs, was upregulated following DSS treatment for 7 days and decreased in 3 days after withdrawal of DSS treatment (Fig. 3D). These results demonstrated that the expression level of miR-7 in IECs was upregulated at the acute phase and decreased at the resolution phase of IBD. Finally, in vitro analysis also revealed that the expression level of miR-7 increased obviously in human/mouse colonic IECs induced by LPS (Fig. 3E and F).
Fig. 3.
MiR-7 expression is upregulated in colonic epithelial cells of mouse colitis. (A) Schematic representation of the establishment of murine DSS-induced IBD. WT mice (8–10 weeks old, n = 8) were given 2% DSS solution for 7 days and changed water to continue feeding for 3 days to induce IBD model; MiR-7 expression was detected in the colon tissue at the indicated time points by Real-time PCR (B). (C) CD45+ inflammatory cells in colorectal tissue and CD45− colonic epithelial cells of colorectal tissue were purified by MACS, then the levels of miR-7 were detected using Real-time PCR assay. (D) The expression and localization of miR-7 in EPCAM+ IECs of colorectal tissue was verified by FISH. (E) Human colonic epithelium cells FHC cells were treated with LPS (200 ng/ml), and the expression of miR-7 at different time points (0, 6, 12, and 18 h) was detected and analyzed by Real-time PCR. (F) IECs from WT mice were treated by LPS (500 ng/ml); 48 h later, miR-7 expression was analyzed by Real-time PCR. The values are the means ± SD (n = 3). *P < 0.05, **P < 0.01.
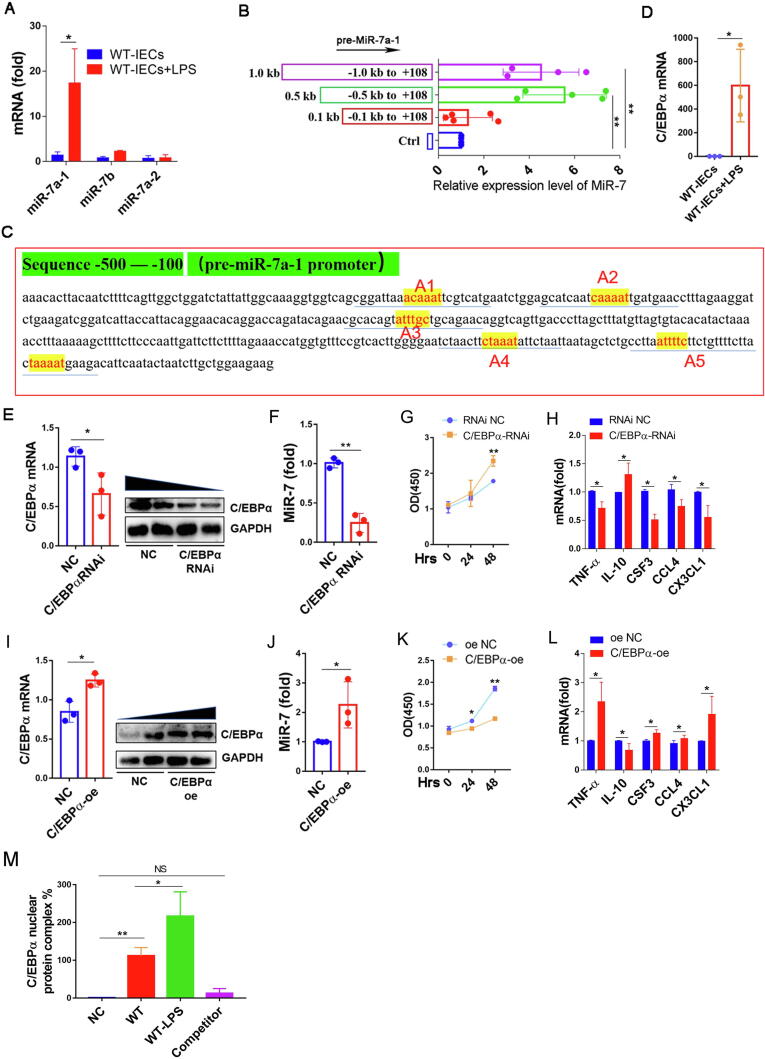
Above studies have consistently suggested that miR-7 is highly expressed in IECs in colitis. So, we next assessed the potential mechanism of miR-7 expression-transcriptional regulation in IECs. The expression levels of precursor sequences from murine miR-7, including pri-miR-7a-1, pri-miR-7a-2, and pri-miR-7b, was assessed. Unexpectedly, even though pri-miR-7a-2 expression in CD45− cells slightly increased, pri-miR-7a-1 expression was upregulated obviously in colonic tissue, especially in CD45− cells (Supplementary Fig. 5A–C). Consistently, pri-miR-7a-1expression was significantly increased, whereas pri-miR-7b and pri-miR-7a-2 expression did not change in IECs treated by LPS in vitro (Fig. 4A). These results indicated that pri-miR-7a-1 was the leading resource of mature miR-7 in IECs in response to inflammatory stimulation. 5′deletion assay further showed that the sequence from −500 site to −100 site, was miR-7a-1 promoter core region (Fig. 4B). Our recent study showed that the transcription factor C/EBPα can regulate miR-7 expression in T cell population [11]. Similarly, screening analysis of CISTER database and TRANSFAC database revealed 5 putative binding sites (from −500 bp to −100 bp) for C/EBPα at this region of the miR-7a-1 promoter (Fig. 4C). Moreover, C/EBPα expression was sharply increased in IECs in response to LPS stimulation (Fig. 4D). Further analysis showed that C/EBPα silencing reduced miR-7 expression in IECs in response to LPS stimulation (Fig. 4E and F), accompanied by a significantly increased proliferation capacity (Fig. 4G). Importantly, the levels of proinflammatory factors CSF3, TNF-α, CCL4, and CX3CL1 decreased significantly, while the level of anti-inflammatory cytokine IL-10 upregulated remarkedly (Fig. 4H). Conversely, after C/EBPα overexpression, the expression level of miR-7 was obviously upregulated in IECs, accompanied by decreased proliferation capacity, increased levels of TNF-α, CSF3, CCL4, and CX3CL1 and decreased level of IL-10 (Fig. 4I–L). Finally, EMSA assay confirmed that C/EBPα could bind to the core promoter region (from −159 site to −154 site, A5) of miR-7a-1 gene (Fig. 4M).
Fig. 4.
C/EBPα is an upstream regulator of murine miR-7 expression in IECs. (A) WT-IECs were isolated, cultured and stimulated with LPS (500 ng/ml) or a control. 24 h later, Real-time PCR analysis for pri-miR-7a-1, pri-miR-7a-2, and pri-miR-7b; (B) WT-IECs transferred with different plasmids (p-1000-miR-7, p-500-miR-7, or p-100-miR-7) were cultured as described previously. 48 h later, Real-time PCR analysis for mature miR-7. (C) Biological analysis showed the potential binding sites (from − 500 to − 100 site) for transcription factor C/EBPα in the miR-7a-1 promoter sequence (yellow A1-A5). (D) WT-IECs were stimulated with LPS or a control. 24 h later, Real-time PCR analysis for C/EBPα; (E-F, I-J) WT-IECs were infected with C/EBPα RNAi and RNAi-NC or C/EBPα overexpression and oe-NC virus particle, 48 h later, Immunoblot and Real-time PCR analysis of C/EBPα, and Real-time PCR analysis for miR-7; the cell proliferation capacity was observed at the indicated time points by CCK8 (G, K). Real-time PCR analysis for inflammatory cytokines TNF-α, IL-10, CSF4, CCL4 and CX3CL1 (H, L). (M) EMSA analysis showed the ability of C/EBPα binding to the core promoter sequence (A5) of the miR-7a-1 gene. The values are the means ± SD (n = 3). *P < 0.05, **P < 0.01, NS no significant. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Next, we analyzed the role of C/EBPα on miR-7 expression in human colonic IECs. Interestingly, 5′deletion assay showed that the sequence from the −500 site to −100 site was also the core region of the miR-7a-1 promoter for mature miR-7 in human IECs (Supplementary Fig. 6A). Moreover, the homology rate reached 90% in the sequence from −500 site to −100 site of human and mouse miR-7a-1 promoter (Supplementary Fig. 6B). Further analysis revealed 5 putative binding sites for C/EBPα in this region of human miR-7a-1 promoter (Supplementary Fig. 6C). Similarly, C/EBPα silencing also reduced the expression level of miR-7 in IECs in response to LPS stimulation (Supplementary Fig. 6D, E), together with a significant increase in proliferative capacity (Supplementary Fig. 6F). And, the levels of proinflammatory factors CSF3, TNF-α, IL-6, TGF-β, CCL4, and CX3CL1 downregulated remarkedly (Supplementary Fig. 6G). All of these results proved that C/EBPα is an important upstream transcription regulator in operating the expression of miR-7 in IECs in IBD.
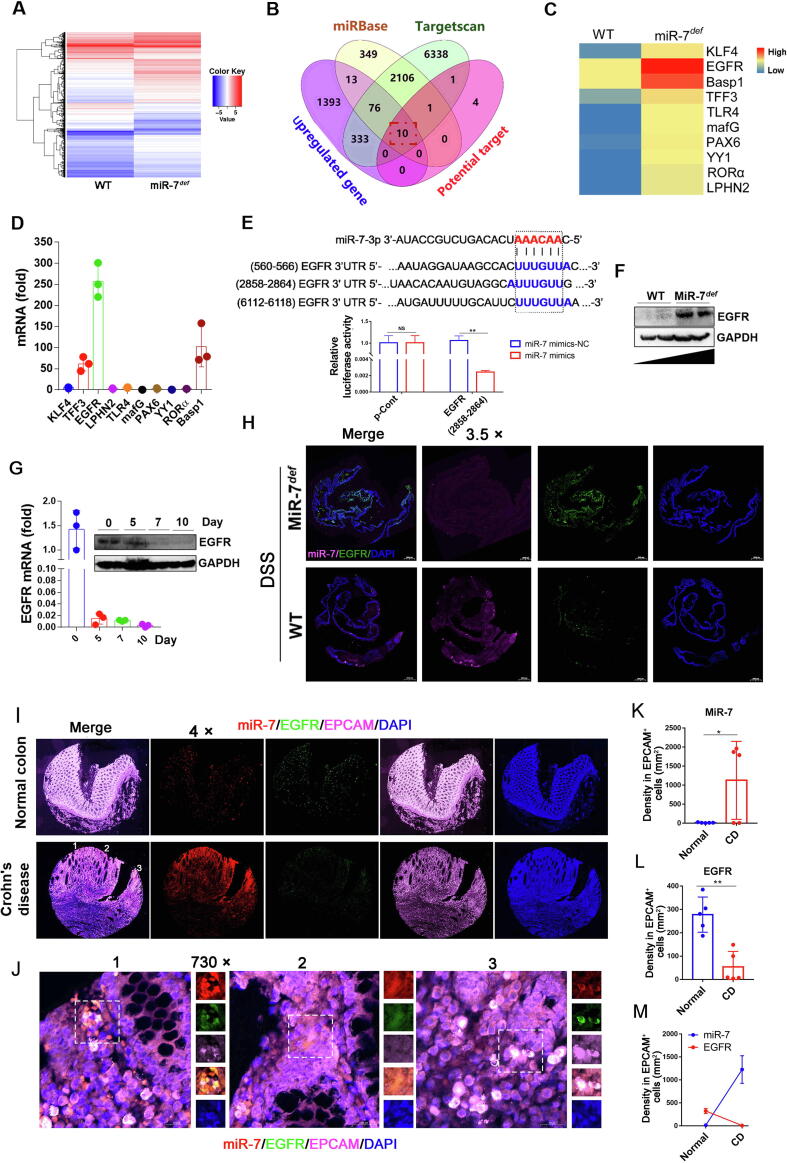
EGFR is a distinct target of miR-7 in the IBD model
To further elucidate the underlying molecular mechanism of miR-7 in IBD, we conducted RNA-seq analyses in colon tissues of the WT and miR-7def colitis model, and then used the miRbase, Targetscan databases, Venny analysis software to screen out 10 putative target genes of miR-7, including TFF3[21], KLF4[22], EGFR[23], LPHN2[24], TLR4[25], MAFG[26], PAX6[27], YY1[28], RORα[13], Basp1[29] (Fig. 5A–C), which always were closely related to inflammatory related-reaction according to the previous literature. Unexpectedly, we identified the changes of these genes expression levels and found that EGFR and Basp1, among all predicted target genes of miR-7, were markedly increased by more than 100-fold in the colonic tissue of DSS-treated miR-7def IBD mice (Fig. 5D). Recent studies have shown that EGFR is a vital target of miR-7 in various diseases [30]. Importantly, EGFR is also a critical protective regulator in the DSS-induced inflammatory response through the activation of the NF-κB pathway [31], [32]. BLAST analysis further showed 3 putative binding sites of miR-7 in the 3′UTR-region of EGFR, and luciferase gene reporter assay suggested that miR-7 could negatively control the expression level of EGFR through directly binding to 3′UTR-region (2858–2864 bp site) (Fig. 5E), but not other regions (560–566 bp and 6112–6118 bp sites) (data not shown). Western blot analysis showed a higher expression in the EGFR protein level in colon tissue from miR-7def mice induced by DSS compared with WT mice induced by DSS (Fig. 5F).
Fig. 5.
EGFR is a target of MiR-7. (A) The gene-expression profiles in the colorectal tissues of miR-7def and WT IBD mice were performed and analyzed using RNA-seq; (B–C) Wenny analysis of miR-7 target genes, whole-genome gene-expression analysis, and related literature; the overlapping 10 genes were screened out as the potential interesting genes. (D) Real-time PCR analysis for KLF4, EGFR, Basp1, TFF1, TLR4, mafG, PAX6, YY1, RORα, and LPHN2 in murine colitis tissue. (E) Putative miR-7 binding sites in the 3′UTR of EGFR from murine species (up); luciferase activity of miR-7 mimics vs mimics NC in WT murine IECs for EGFR (2858–2864 bp site) (down). (F) The protein level of EGFR was detected in the miR-7def and WT murine colitis tissue. (G). The mRNA and protein expression of EGFR in the WT murine colitis tissue were analyzed at indicated time points. (H) The expression of miR-7 and EGFR was detected in colorectal tissues by FISH. The values are the means ± SD (n = 3). (I, K-M) The expression of miR-7 and EGFR were detected in the colon specimens from Normal colon (n = 5) and CD (n = 5) by FISH, then the level and relation of miR-7 and EGFR in EPCAM+ IECs from these specimens were analyzed. Statistical analysis was performed using an unpaired t-test. (J) Representative images of FISH (1, 2, 3) from the CD in Figure I. *P < 0.05, **P < 0.01.
We further showed that the expression level of EGFR steadily decreased in colonic tissue from WT IBD mice (Fig. 5G), which was negative correlation with miR-7 expression (Fig. 3B). Next, the FISH assay always revealed that the EGFR expression was remarkable increase in colon with DSS-treated miR-7def mice (Fig. 5H). Furthermore, the expression of EGFR was downregulated observably in colonic tissue, dominantly in CD45− cells, but not in CD45+ infiltrated inflammatory cells in IBD mice (Supplementary Fig. 7A). A FISH assay suggested that the expression level of miR-7 increased dramatically. Meanwhile, the expression level of EGFR decreased significantly in colon tissue from DSS-treated WT mice; More importantly, EGFR and miR-7 were dominantly colocalized in IECs from DSS-treated WT mice (Supplementary Fig. 7B). Finally, Immunofluorescence assay suggested that the expression level of miR-7 in EPCAM+ epithelial cells also increased obviously in clinical CD, accompanied by decreased level of EGFR (Fig. 5I–M), displaying a significant negative correlation. Together, these results revealed that EGFR is one of the key targets of miR-7 in IECs in IBD.
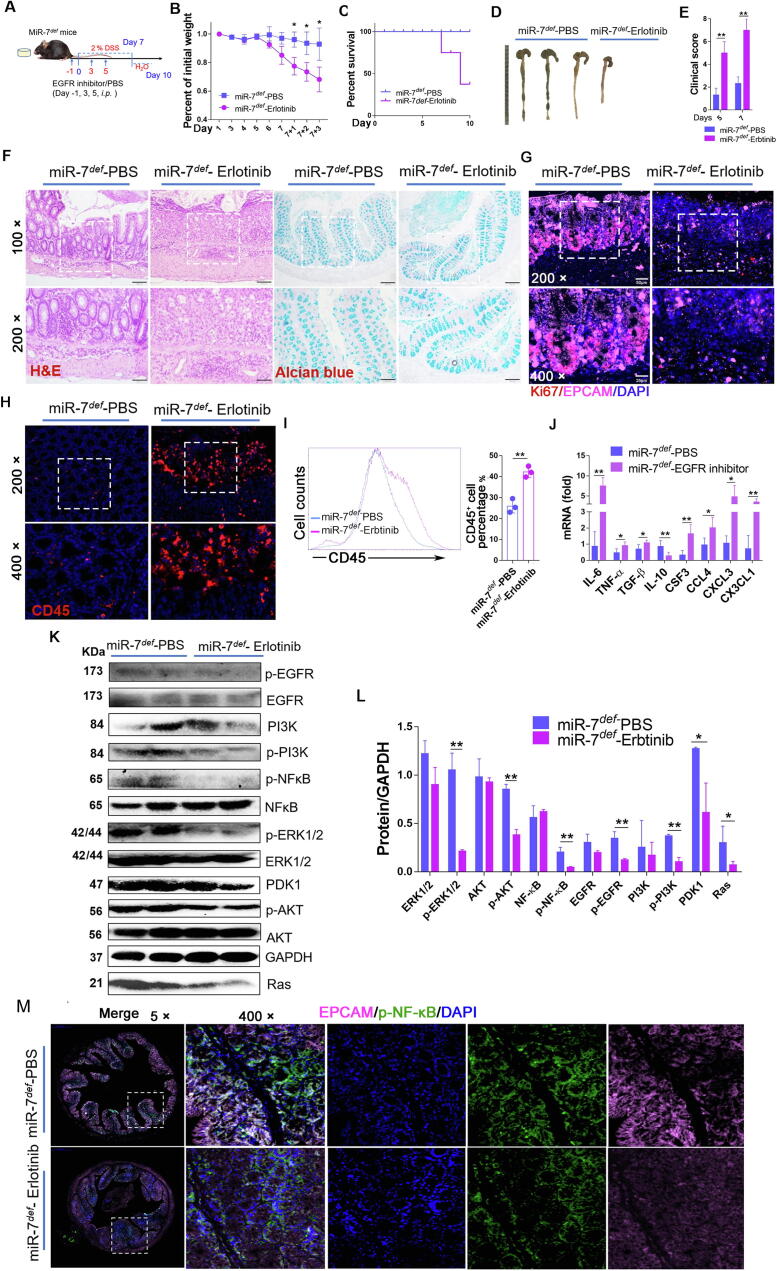
Downregulation of EGFR signaling aggravates the pathologies of the miR-7def IBD model
To next investigate the potential role of EGFR in the effect of miR-7 deficiency on IBD, we silenced the EGFR signaling pathway in miR-7def mice using Erlotinib, an inhibitor of EGFR tyrosine kinase, and then established colitis model, as in the description of Fig. 6A: miR-7def mice were injected with Erlotinib (50 mg/kg, i.p.) on DSS induction for −1, 3 and 5 days, and colitis model was established by feeding mice with 2 % DSS for 7 days and then changing water for 3 days. Erlotinib treatment resulted in considerably more weight loss (Fig. 6B), significantly lower survival (Fig. 6C) and shorter colon length (Fig. 6D), as well as higher clinical disease scores (Fig. 6E), as accompanied by a severe inflammatory response and reduced the number of goblet cells and mucin production, compared with control treatment (Fig. 6F). Further Western Blot analysis revealed that the levels of p-EGFR, p-PI3K, PDK1, Ras, p-NF-κB, p-ERK and p-AKT also decreased visibly in colon tissue (Fig. 6K and L). These results revealed that inhibition of EGFR signaling could reverse the effect of miR-7 deficiency on pathological damage in colitis.
Fig. 6.
Downregulated EGFR signaling aggravates the pathology of the miR-7def IBD model. (A) Schematic representation of the in vivo experiment. MiR-7def mice (8–10 W, n = 6) were injected intraperitoneally with Erlotinib (50 mg/kg) on DSS for −1, 3 and 5 days, and the IBD model was established as described previously (The mice were injected intraperitoneally with cosolvent PBS for contrast); The body weight loss (B), the survival curve (C), representative gross images of colon (D), and the clinical scores (E) were analyzed; Histopathology was performed by H&E staining and mucin production detected by Alcian blue staining (×100, ×200) (F); The expression of Ki67 and EPCAM (G) or CD45 (H) were detected by IF (×200, ×400); FCM analysis of CD45+ cells in colon (I); Real-time PCR analysis of inflammatory cytokines IL-6, TNF-α, TGF-β, IL-10, CSF4, CCL4, CXCL3 and CX3CL1 in the colon (J); Immunoblot analysis of p-EGFR, EGFR, PI3K, p-PI3K, NF-kB, p-NF-kB, AKT, p-AKT, ERK, p-ERK, Ras and PDK1 in colorectal tissues (K–L); The expression of p-NF-kB and EPCAM were determined by multiplexed fluorescent immunohistochemical staining in colon (×5, ×400). The values are the means ± SD (n = 6). *P < 0.05, **P < 0.01. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Expectedly, regenerative response in epithelial cells in colon tissue was obviously impaired (Fig. 6G), accompanied by a decreased level of p-NF-κB (Fig. 6M). Meanwhile, IF and FCM analysis also showed CD45+ inflammatory cell infiltration was elevated significantly (Fig. 6H and I), accompanied with elevated levels of some pro-inflammatory cytokines such as IL-6, TNF-α, and CSF3 and so on, as well as a decreased level of anti-inflammatory cytokine IL-10 (Fig. 6J). Together with these results suggested that EGFR plays a crucial role in miR-7 deficiency on the epithelial immunomodulatory and regeneration of IECs in IBD.
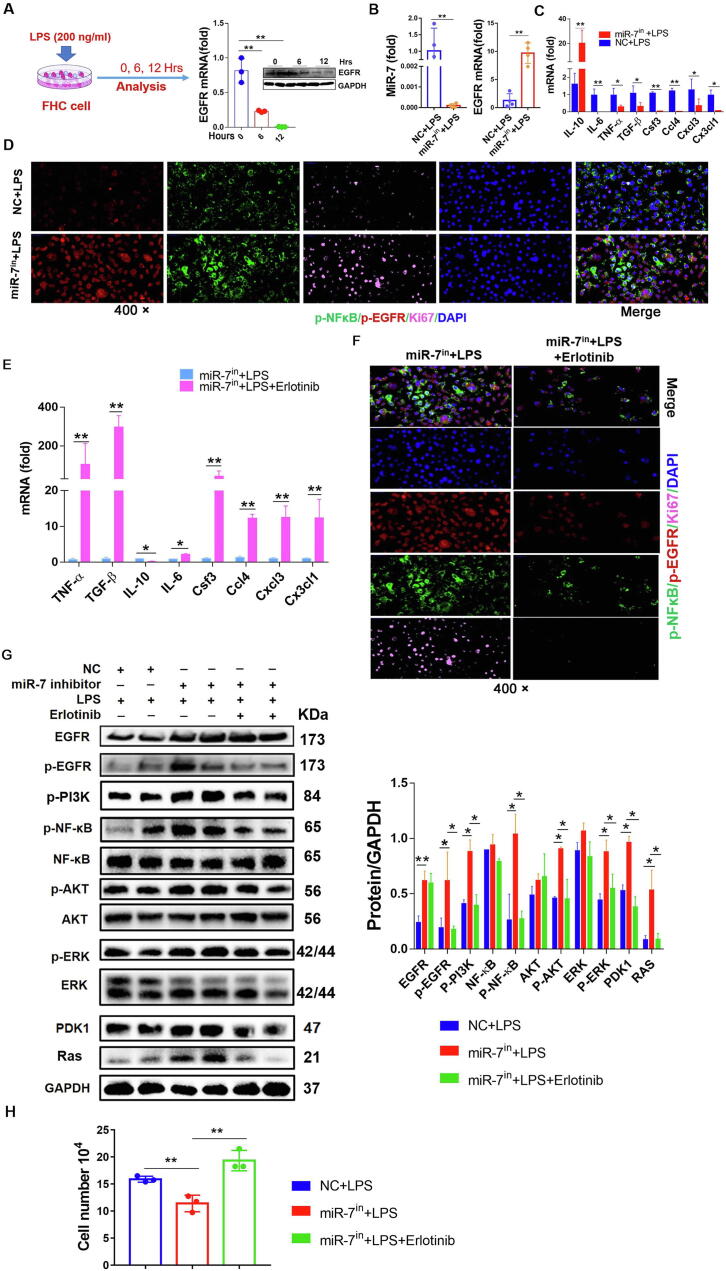
MiR-7 controls the epithelial immunomodulation and regeneration via EGFR in vitro
To confirm the role of the MiR-7/EGFR axis in the immunomodulation and regeneration of IECs, we further observed the expression of EGFR in human colonic IECs in response to inflammatory stimulation. As the Fig. 7A showed that, after LPS stimulation, the expression level of EGFR decreased distinctly in colonic IECs, displaying an opposite pattern to miR-7 expression (Fig. 3E). Then, we silenced miR-7 expression using a miR-7 inhibitor to observe the possible change of EGFR expression. After miR-7 inhibitor treatment, EGFR expression increased dramatically (Fig. 7B) in IECs in response to LPS stimulation, accompanied with decreased levels of proinflammatory factors such as TGF-β, TNF-α, IL-6, and chemokines CSF3 and so on and increased anti-inflammatory factor IL-10 level (Fig. 7C). In line with these findings, the migration capacity of IECs was enhanced significantly in miR-7 inhibitor treatment group (Supplementary Figure 13A). Immunofluorescence assay also confirmed the elevated expression of EGFR and upregulated levels of Ki-67 and p-NF-κB (Fig. 7D). In addition, we further evaluated the change of global genes using RNA-seq. Expectedly, miR-7 inhibitor treatment group's transcriptome profile was lowly enriched in cytokine/chemokines (Supplementary Fig. 8A–B). Moreover, the capacity of the supernatants of miR-7 inhibitor treatment group to attract CD45+ immune cells also decreased (Fig. 7H).
Fig. 7.
MiR-7 controls epithelial immunomodulation and regeneration via EGFR in vitro. (A) The normal colonic epithelial cell FHC cells were treated with LPS (200 ng/ml) in vitro, and the mRNA and protein expression of EGFR was detected and analyzed at different time points. (B–C) FHC cells were transfected with miR-7 inhibitors/NC (200 nM) following LPS treatment, 48 h later, Real-time PCR analysis for miR-7, EGFR, and inflammatory cytokines IL-6, TNF-α, TGF-β, IL-10, CSF4, CCL4, CXCL3, and CX3CL1. The expression of phos-NF-kB, phos-EGFR, and Ki-67 were observed by multiplexed fluorescent immunohistochemical staining (D). (E) FHC cells were transfected with miR-7 inhibitors / miR-7 inhibitors plus Erlotinib (5 nM) following LPS treatment, the relative expression of inflammatory cytokines IL-6, TNF-α, TGF-β, IL-10, CSF4, CCL4, CXCL3, and CX3CL1 was detected and analyzed by Real-time PCR; and the expression of phos-NF-kB, phos-EGFR, and Ki-67 was observed by multiplexed fluorescent immunohistochemical staining (F); (G) Immunoblot analysis of p-EGFR, EGFR, PI3K, p-PI3K, NF-kB, p-NF-kB, AKT, p-AKT, ERK, p-ERK, Ras and PDK1 in the cells of each group; (H) The cell migration was assessed by transwell assay. The values are the means ± SD (n = 3). *P < 0.05, **P < 0.01.
Furthermore, our analysis also displayed that miR-7 mimics obviously suppressed the proliferation of human colonic IECs in response to LPS stimulation, accompanied by the increased levels of TNF-α, IL-6, TGF-β, CSF3, CCL4 and CX3CL1 and the decreased level of IL-10. Meanwhile, the related signaling pathway including EGFR, p-PI3K, PDK1, Ras, p-NF-κB, p-ERK1/2 and p-AKT also decreased significantly (Supplementary figure 9A–F). Moreover, we also observed miR-7 expression in colonic epithelial cells derived from miR-7 overexpression transgenic mice (miR-7oe mice). Data showed that the expression of miR-7 increased dramatically in miR-7oe colonic epithelial cells stimulated by LPS (Supplementary Figure 10A). In addition, the expression level of EGFR decreased significantly (Supplementary Figure 10A). Consistent with the above findings, the proliferation of miR-7oe colonic epithelial cells decreased obviously (Supplementary Figure 10B). Furthermore, the expression levels of inflammatory factor TNF-α, TGF-β, IL-6, CSF3, CCL4, and CX3CL1 increased significantly (Supplementary Figure 10C), while IL-10 level decreased remarkedly (Supplementary Figure 10C). Finally, the levels of EGFR, p-PI3K, PDK1, Ras, p-NF-κB, p-ERK1/2, and p-AKT in miR-7oe colonic epithelial cells decreased significantly (Supplementary Figure 10D and E).
To next confirm the role of EGFR in the effect of miR-7 inhibition colonic IECs, we silenced the EGFR signaling pathway of miR-7 inhibited colonic IECs using Erlotinib. As expected, Erlotinib treatment resulted in increased levels of TNF-α, IL-6 and so on, whereas decreased IL-10 level (Fig. 7E). Moreover, the expression levels of p-NF-κB and Ki-67 were dramatically downregulated (Fig. 7F). RNA-seq results also revealed that Erlotinib treatment group's transcriptome profile was highly enriched in cytokine/chemokines (Supplementary Figure 11A and B). Consistently, the capacity of the Erlotinib treatment group's supernatants on attractive migration of CD45+ immune cells was significantly elevated (Fig. 7H). Furthermore, the levels of p-EGFR, EGFR, p-PI3K, PDK1, Ras, p-AKT, p-ERK1/2, and p-NF-κB in the miR-7-inhibitor-transfection alone group increased visibly compared with those in the control group (Fig. 7G). Importantly, Erlotinib treatment reversed the levels of these signaling factors (Fig. 7G).
Consistently, we found that EGFR expression decreased distinctly in mouse colonic IECs in response to inflammatory stimulation. And the miR-7 deficiency colonic IECs with LPS treatment always had a higher EGFR expression (Supplementary Figure 12A–C). Furthermore, miR-7 deficiency promoted the proliferation of murine colonic IECs with LPS treatment, accompanied with the increased levels of inflammatory signals such as p-NF-κB, p-ERK and so on, and decreased levels of pro-inflammatory cytokines such as IL-6, TNF-α, and CSF3, etc., (Supplementary Figure 12A–F); Meanwhile, the migration capacity of IECs was also significantly enhanced (Supplementary Figure 13B). Notably, Erlotinib treatment also could reverse the effect of miR-7 deficiency on the proliferation, inflammatory signals (p-PI3K, PDK1, Ras, p-NF-κB, p-ERK and p-AKT, etc.), and inflammatory cytokines (CSF3 and IL6, etc.) secretion of murine IECs stimulated by LPS (Supplementary Figure 12G–I). Finally, the capacity of the supernatants to attract CD45+ immune cells also was elevated (Supplementary Figure 12 J), which was in keeping with our above data (Fig. 7H). Together, these observations demonstrated that miR-7 could control the immunomodulation and regeneration of colonic IECs through EGFR, which was positively correlated with the transduction of the NF-κB, AKT, and ERK signaling pathways.
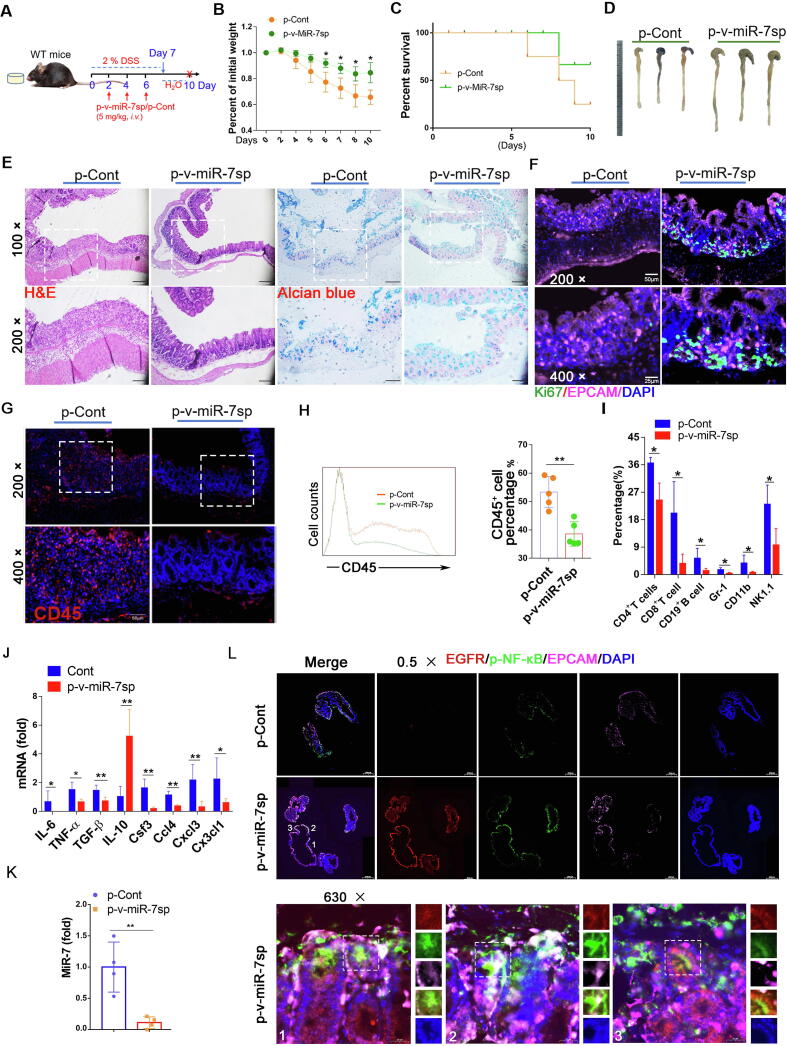
Intestinal epithelial cell-specific miR-7 silencing alleviates the pathology of murine IBD model
To explore the potential value of targeting miR-7 in IECs in therapy against IBD, we designed a Villin1-promoter-operating miR-7 sponge expression vector. We monitored the possible effect of targeting silence of miR-7 in IECs on the pathology injury of colitis (Fig. 8A). Compared with the p-Cont NC group, p-v-miR-7sp treatment caused a slower body weight loss, a higher survival rate, and decreased colon shortening (Fig. 8B–D). Histology assessment and mucous layer analysis showed alleviated damage and elevated mucin production in colon tissue (Fig. 8E). Importantly, epithelial cell proliferation increased obviously (Fig. 8F), accompanied by the reduced infiltration of inflammatory cell populations and the altered expression levels of inflammatory cytokines such as IL-6, TNF-α, CCL4, and so on (Fig. 8G–J). Expectedly, the miR-7 level also dominantly decreased significantly in the colon tissue (Fig. 8K), indicating that Villin1 promoter might effectively operate miR-7 sponge expression to reduce miR-7 level in colon tissue. Importantly, the levels of p-AKT, p-ERK, and p-NF-KB were elevated in colon tissue (Supplementary Figure 14A–B). To further validate these results, we analyzed the level of p-NF-KB in IECs in colon tissue and found that the p-NF-κB level in IECs also increased obviously (Fig. 8L), consistent with our above results (Fig. 1I). These combined data indicated that the targeting silence of miR-7 in IECs could significantly alleviate the pathology damage of DSS-induced colitis.
Fig. 8.
Intestinal epithelial cell-specific miR-7 silencing alleviates the pathology of IBD. (A) Schematic representation of the establishment of WT IBD mice challenged with the p-v-miR-7sp vector. p-v-miR-7sp vector was injected into WT mice (8–10 W, n = 6/per group) via tail vein at an interval of 5 mg/kg, every 2 days, for 3 times, and establish IBD model, then the body weight loss (B) and the survival curve (C) were analyzed; Representative gross images of colon were observed (D); Histopathology was performed by H&E staining and mucin production was measured by Alcian blue staining (×100, ×200) (E); IF analysis of CD45+ cell and Ki-67 expression in colorectal tissue (×200, ×400) (F–G); FCM analysis of CD45+ cells and CD4+ T cells, CD8+ T cells, CD19+ B cells, Gr-1+ cells, CD11b+ cells and NK1.1+ T cells in CD45+ cells (H–I); Real-time PCR analysis of cytokines IL-6, TNF-α, TGF-β, IL-10, CSF4, CCL4, CXCL3 and CX3CL1 in colon (J); the relative expression of miR-7 was detected by Real-time PCR in colon tissue (K); IF analysis for p-NF-κB, EGFR and EPCAM in colon (×0.5, ×630) (L). The values are the means ± SD (n = 6). *P < 0.05, **P < 0.01. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Discussion
In this study, we first found that miR-7 deficiency could elevate the proliferation and transduction of inflammatory-related NF-κB/AKT/ERK signaling pathways of IECs in the DSS-induced colitis model, accompanied with decreased expression profiles of proinflammatory cytokines and infiltration of various immune cells in colon tissue. Moreover, the expression of miR-7, mainly operated by C/EBPα which binds to the core sequence of the promoter of miR-7a-1, was upregulated in IECs in the murine colitis model and CD patients. Mechanistically, miR-7 controlled the immunomodulation and regeneration of colonic IECs through EGFR/NF-κB/AKT/ERK pathway. Notably, IEC-specific miR-7 silencing alleviated the pathology of colonic tissue in IBD model.
Recently, accumulating evidence have shown that miR-7 plays a promising controller in regulating the biological process of various diseases, including inflammatory diseases [33]. In our recent study, we revealed that miR-7 could also regulate the pathologies of autoimmune hepatitis [11], acute lung injury [12], and brain tissue inflammatory injury [13], displaying that miR-7 might be a novel intrinsic regulator in inflammatory-related diseases. Concerning IBD, one recent study has reported that RNF183, a target of miR-7, promoted intestinal inflammation in TNBS-induced IBD, indicating that miR-7 has a protective effect on the pathological process of IBD [34]. However, Guo et al. suggested that miR-7 expression was upregulated in the colon tissue and inhibition of miR-7 could reduce the pathological lesion of TNBS-induced colitis inflammation [14]. In line with these findings, in this study, we revealed that miR-7 deficiency could relieve the symptoms and pathological injury of DSS-induced colitis. Importantly, we extended previous findings by illustrating that miR-7 deficiency promoted the local proliferation of IECs in colon tissue. Furthermore, we revealed that the expression of miR-7, dominantly expressed by IECs except for inflammatory cells in colon tissue, increased at the acute phase of colitis and decreased at the resolution phase. Our recent study has shown that the transcription factor C/EBPαbind to the promoter of miR-7b to regulate the expression of miR-7 in CD4+ T cells [11]. Different from our previous findings [11], we herein found that the expression of miR-7 was dominantly derived from pri-miR-7a-1, but not pri-miR-7a-2 in human and murine IECs. Furthermore, C/EBPα could bind to the core sequence of the promoter of pri-miR-7a-1 to control miR-7 expression, thereby affecting the proliferation and secretion of inflammatory cytokines in IECs. These results further demonstrate the vital effect of miR-7 in inflammation diseases. Finally, because of the complex etiology of IBD and the limitation of clinical specimens in present study, we believe that further studies on miR-7 expression in the pathological damage process of colitis, especially in the different phases, might be valuable for the ultimate exploration of the exact role of miR-7 in colitis development.
More and more evidence has suggested that the IECs immunomodulatory capacity is well involved in the pathogenesis and progression of multiple colon diseases [35], [36]. In response to inflammatory signals, IECs secrete many inflammatory cytokines/chemokines to affect the local accumulation of lymphoid cells, such as CD19+ B cells, T cells, NKTs, and γδ+ T cells, as well. Subsequently affecting the pathologic damage of colon diseases [20]. In the current study, we revealed that the miR-7 deficiency altered the expression pattern of inflammatory cytokines/chemokines, such as IL-6 and TNF-α as well. were significantly decreased in the colitis model. Moreover, the proportions of innate immune cells, such as Gr-1 and NK1.1+ T cells, and adaptive immune cells, including CD8+ T cells, CD4+ T cells, and CD19+ B cells, in colon tissue also decreased dramatically. Importantly, the altered miR-7 expression also affected the levels of pro-inflammatory cytokines/chemokines and the proliferation capacity of colon IECs stimulated by LPS. These results indicated that, under the condition of inflammation stimuli, miR-7 could regulate the immunomodulatory capacity and regeneration of IECs, thereby remodeling the local infiltration of immune cells in colon tissues and subsequently affecting the pathological process of colitis. In addition, in our most recent works, we reveal that miR-7 can control the CD4+ T cells activation and function in AIH [11]. Unfortunately, it is regretful that the exact roles of miR-7 in other immune cells remain largely unknown. Given the fact that the inflammatory cytokines or other bioactive molecules secreted by colonic IECs can activate CD19+ B cells, CD4+ T cells, and other immune cells [37], [38], our current data still support the important associations between colonic IECs and immune cells in colitis. Finally, the potential roles of miR-7 in these immune cells and other immune-related cells such as epithelial stem cells and paneth cells in IBD remain to be further elucidated, which might helpful to further explore the cellular mechanism of IBD pathogenesis. Herein, our present data might provide valuable data for future research on the interactions among epithelial cells, immune and immune-related cells, which should help understand the role of miR-7 in the pathogenesis of IBD.
EGFR is a transmembrane glycoprotein with tyrosine kinase activity by activating the transduction of the AKT/ERK/NF-κB signaling pathways; it is widely recognized for its importance in organ development and disease [39], [40]. Accumulating evidence has shown that EGFR signaling plays an essential role in regulating the biological function and immune-regulation of colonic IECs and the response to injury and inflammation [32]. Similarly, we found that EGFR expression decreased in the colonic IECs in murine colitis model and clinical IBD patients. In addition, some factors such as TFF3, which are regulated by miR-7, have been documented play roles in the development of colitis model and clinical IBD patients [41]. Of note, the repression of EGFR signaling could decrease the epithelial regeneration and exacerbate the colitis pathology of miR-7 deficiency mice, accompanied by increased inflammatory cell infiltration, elevated transduction of the NF-κB signaling pathway in the colonic IECs. Furthermore, EGFR and miR-7 were dominantly co-located in colonic IECs, but not CD45+ inflammatory cells in colon tissue in IBD. Importantly, in the inhibition of miR-7, silencing of EGFR signaling encouraged the production of pro-inflammatory cytokines/chemokines of colonic IECs induced by LPS, which was closely related to changes in the NF-κB signal transduction pathway. Hence, these data revealed the vital role of the MiR-7/EGFR axis in the immunomodulatory capacity and regeneration of IECs in pathologies of colitis. Actually, we also noticed that the other possible targets such as Basp1 and TFF3, also were upregulated in colitis tissue in the absence of miR-7. Moreover, blockade of EGFR pathway did not totally abrogate the effect of miR-7 in IECs, indicating that these factors, which did not be verified in current study, also might contribute to the effect of miR-7 in IECs.
Gene targeted expression techniques, such as the different delivery systems and specific gene-promoter-operating gene expression, are vital strategies for gene therapy of multiple inflammatory diseases [37]. However, in IBD gene therapy, studies on the underlying significance of the targeted expression of protector genes through operating promoters remain scarce. The Villin1 gene is dominantly expressed in colon tissue and used for the spatiotemporal control of gene expression in the colon IECs [37]. In the current study, we aimed at design and built a eukaryotic vector encoding a miR-7 sponge, which was operated by the Villin1 promoter, to reduce miR-7 expression in colon IECs in colitis. Interestingly, we found that the Villin1-promoter-operating miR-7 down-expression could dramatically decrease the level of miR-7 in colon tissue in colitis model. Importantly, the pathology of DSS-induced colitis in mice was obviously alleviated, accompanied by the promotion of colonic epithelial regeneration and the reduction of the infiltration of inflammatory cells, as well as elevated EGFR/AKT/ERK/NF-κB signaling. Together, all the above data revealed that miR-7 might be an attractive candidate for therapeutic strategies for IBD gene therapy, which was beneficial to the development of miRNA-based molecule intervention in clinical IBD.
In summary, we demonstrated that miR-7, mainly derived from miR-7a-1 operated by C/EBPα, could control the immunomodulation and regeneration of epithelial cells, thereby orchestrating the pathologies of DSS-induced IBD through EGFR, altered the transduction of the NF-κB, ERK and AKT signals (Supplementary Figure 16A). Furthermore, IEC-specific miR-7 silencing using Villin1 promoter driving the miR-7 sponge expression could alleviate the pathology of IBD (Supplementary Figure 16B). Therefore, our current data reveal the vital value of miR-7/EGFR axis in the immunomodulation and regeneration of epithelial cells in colonic diseases and might provide a light on the development of miRNA-based therapeutic strategy applications in inflammation colonic diseases.
Compliance with Ethics Requirements
All the mice were housed under specific pathogen-free (SPF) conditions at Zunyi Medical University, according to the guidelines for the Care and Use of Laboratory Animals (Ministry of Health, China, 1998). The experimental procedures were approved by the Zunyi Medical University Laboratory Animal Care and Use Committee (permit number SYXK-2021-00048).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This manuscript was supported by the National Natural Science Foundation of China (82272812, 32160178), the collaborative Innovation Center of Chinese Ministry of Education (2020-39), the Program for Excellent Young Talents of Zunyi Medical University (15ZY-001), the Project of Guizhou Provincial Department of Science and Technology (QKHZC-2020-4Y156, QKH-JC-ZK-2022-623), and the Guizhou Province Graduate Research Fund (YJSCXJH-2020-093), the Program for Science and Technology Joint Fund Project in Zunyi Science and Technology Bureau and Zunyi Medical University (ZSKH-RPT-2020-6, ZSKH-HZ-2021-276, ZSKH-HZ-2021-193), Guizhou Province Science and Technology Planning Project (QKPTRC-2019-052), Science and technology Fund project of Guizhou Provincial Health Commission (gzwkj2022-271). Doctoral Research Foundation project of Zunyi Medical University (QHKPTRC-2020-033). “12345 Future Talent Training Plan” of Zunyi Medical University- Future science and technology Elite talent project (ZYSE-2022-04). We thank LetPub (www.letpub.com) for its linguistic assistance and scientific consultation during the preparation of this manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2023.04.011.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Shen Q., Huang Z., Yao J., Jin Y. Extracellular vesicles-mediated interaction within intestinal microenvironment in inflammatory bowel disease. J Adv Res. 2021;37:221–233. doi: 10.1016/j.jare.2021.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jayme TS, Leung G, Wang A, Workentine ML, Rajeev S, Shute A, et al. Human interleukin-4-treated regulatory macrophages promote epithelial wound healing and reduce colitis in a mouse model. Sci Adv 2020;6:eaba4376. [DOI] [PMC free article] [PubMed]
- 3.Corbin AL, Gomez-Vazquez M, Berthold DL, Attar M, Arnold IC, Powrie FM, et al. IRF5 guides monocytes toward an inflammatory CD11c(+) macrophage phenotype and promotes intestinal inflammation. Sci Immunol 2020;5. [DOI] [PMC free article] [PubMed]
- 4.Neurath M.F. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14:329–342. doi: 10.1038/nri3661. [DOI] [PubMed] [Google Scholar]
- 5.Pickert G., Neufert C., Leppkes M., Zheng Y., Wittkopf N., Warntjen M., et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med. 2009;206:1465–1472. doi: 10.1084/jem.20082683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oh S.K., Kim D., Kim K., Boo K., Yu Y.S., Kim I.S., et al. RORα is crucial for attenuated inflammatory response to maintain intestinal homeostasis. Proc Natl Acad Sci U S A. 2019;116:21140–21149. doi: 10.1073/pnas.1907595116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li X., Cassidy J.J., Reinke C.A., Fischboeck S., Carthew R.W. A microRNA imparts robustness against environmental fluctuation during development. Cell. 2009;137:273–282. doi: 10.1016/j.cell.2009.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Memczak S., Jens M., Elefsinioti A., Torti F., Krueger J., Rybak A., et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–338. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- 9.Kong D., Piao Y.S., Yamashita S., Oshima H., Oguma K., Fushida S., et al. Inflammation-induced repression of tumor suppressor miR-7 in gastric tumor cells. Oncogene. 2012;31:3949–3960. doi: 10.1038/onc.2011.558. [DOI] [PubMed] [Google Scholar]
- 10.Chen H., Guo M., Yue D., Zhao J., Ding T., Zhou Y., et al. MicroRNA-7 Negatively Regulates Toll-Like Receptor 4 Signaling Pathway through FAM177A. Immunology. 2021;162(1):44–57. doi: 10.1111/imm.13252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao J., Chu F., Xu H., Guo M., Shan S., Zheng W., et al. C/EBPα/miR-7 Controls CD4(+) T-Cell Activation and Function and Orchestrates Experimental Autoimmune Hepatitis in Mice. Hepatology. 2021;74:379–396. doi: 10.1002/hep.31607. [DOI] [PubMed] [Google Scholar]
- 12.Zhao J., Chen C., Guo M., Tao Y., Cui P., Zhou Y., et al. MicroRNA-7 Deficiency Ameliorates the Pathologies of Acute Lung Injury through Elevating KLF4. Front Immunol. 2016;7:389. doi: 10.3389/fimmu.2016.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yue D., Zhao J., Chen H., Guo M., Chen C., Zhou Y., et al. MicroRNA-7, synergizes with RORalpha, negatively controls the pathology of brain tissue inflammation. J Neuroinflammation. 2020;17:28. doi: 10.1186/s12974-020-1710-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo J., Yang L.J., Sun M., Xu L.F. Inhibiting microRNA-7 Expression Exhibited a Protective Effect on Intestinal Mucosal Injury in TNBS-Induced Inflammatory Bowel Disease Animal Model. Inflammation. 2019;42:2267–2277. doi: 10.1007/s10753-019-01091-1. [DOI] [PubMed] [Google Scholar]
- 15.Hu L., Zhou Y., Yang J., Zhao X., Mao L., Zheng W., et al. MicroRNA-7 overexpression positively regulates the CD8(+) SP cell development via targeting PIK3R1. Exp Cell Res. 2021;407 doi: 10.1016/j.yexcr.2021.112824. [DOI] [PubMed] [Google Scholar]
- 16.He C., Shi Y., Wu R., Sun M., Fang L., Wu W., et al. miR-301a promotes intestinal mucosal inflammation through induction of IL-17A and TNF-alpha in IBD. Gut. 2016;65:1938–1950. doi: 10.1136/gutjnl-2015-309389. [DOI] [PubMed] [Google Scholar]
- 17.Zhou R, Qiu P, Wang H, Yang H, Yang X, Ye M, et al. Identification of microRNA-16-5p and microRNA-21-5p in feces as potential noninvasive biomarkers for inflammatory bowel disease. Aging (Albany NY) 2021;12:4634–4646. doi: 10.18632/aging.202428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jardine S., Anderson S., Babcock S., Leung G., Pan J., Dhingani N., et al. Drug Screen Identifies Leflunomide for Treatment of Inflammatory Bowel Disease Caused by TTC7A Deficiency. Gastroenterology. 2020;158:1000–1015. doi: 10.1053/j.gastro.2019.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weber C.K., Liptay S., Wirth T., Adler G., Schmid R.M. Suppression of NF-kappaB activity by sulfasalazine is mediated by direct inhibition of IkappaB kinases alpha and beta. Gastroenterology. 2000;119:1209–1218. doi: 10.1053/gast.2000.19458. [DOI] [PubMed] [Google Scholar]
- 20.Scheibe K., Backert I., Wirtz S., Hueber A., Schett G., Vieth M., et al. IL-36R signalling activates intestinal epithelial cells and fibroblasts and promotes mucosal healing in vivo. Gut. 2017;66:823–838. doi: 10.1136/gutjnl-2015-310374. [DOI] [PubMed] [Google Scholar]
- 21.Belle N.M., Ji Y., Herbine K., Wei Y., Park J., Zullo K., et al. TFF3 interacts with LINGO2 to regulate EGFR activation for protection against colitis and gastrointestinal helminths. Nat Commun. 2019;10:4408. doi: 10.1038/s41467-019-12315-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim Y.C., Jung H., Seok S., Zhang Y., Ma J., Li T., et al. MicroRNA-210 Promotes Bile Acid-Induced Cholestatic Liver Injury by Targeting Mixed-Lineage Leukemia-4 Methyltransferase in Mice. Hepatology. 2020;71:2118–2134. doi: 10.1002/hep.30966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song J., Liu Y., Wan J., Zhao G.N., Wang J.C., Dai Z., et al. SIMPLE Is an Endosomal Regulator That Protects Against NAFLD by Targeting the Lysosomal Degradation of EGFR. Hepatology. 2021 doi: 10.1002/hep.32075. [DOI] [PubMed] [Google Scholar]
- 24.Yasinska IM, Sakhnevych SS, Pavlova L, Teo Hansen Selnø A, Teuscher Abeleira AM, Benlaouer O, Gonçalves Silva I, et al. The Tim-3-Galectin-9 Pathway and Its Regulatory Mechanisms in Human Breast Cancer. Front Immunol 2019;10:1594. [DOI] [PMC free article] [PubMed]
- 25.Zhang XD., Fan QY, Qiu Z, Chen S. MiR-7 alleviates secondary inflammatory response of microglia caused by cerebral hemorrhage through inhibiting TLR4 expression. Eur Rev Med Pharmacol Sci. 2018;22:5597–5604. doi: 10.26355/eurrev_201809_15824. [DOI] [PubMed] [Google Scholar]
- 26.Vera O., Jimenez J., Pernia O., Rodriguez-Antolin C., Rodriguez C., Sanchez Cabo F., et al. DNA Methylation of miR-7 is a Mechanism Involved in Platinum Response through MAFG Overexpression in Cancer Cells. Theranostics. 2017;7:4118–4134. doi: 10.7150/thno.20112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin Y., Zhang B., Lu J., Song Y., Wang W., Zhang W., et al. Long noncoding RNA PM maintains cerebellar synaptic integrity and Cbln1 activation via Pax6/Mll1-mediated H3K4me3. PLoS Biol. 2021;19:e3001297. doi: 10.1371/journal.pbio.3001297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang P., Li J., Peng C., Tan Y., Chen R., Peng W., et al. TCONS_00012883 promotes proliferation and metastasis via DDX3/YY1/MMP1/PI3K-AKT axis in colorectal cancer. Clin Transl Med. 2020;10:e211. doi: 10.1002/ctm2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu S., Tang L., Liu Z., Luo C., Cheng Q. Hypoxia-Related lncRNA Correlates With Prognosis and Immune Microenvironment in Lower-Grade Glioma. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.731048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weng W., Wei Q., Toden S., Yoshida K., Nagasaka T., Fujiwara T., et al. Circular RNA ciRS-7-A Promising Prognostic Biomarker and a Potential Therapeutic Target in Colorectal Cancer. Clin Cancer Res. 2017;23:3918–3928. doi: 10.1158/1078-0432.CCR-16-2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCole D.F., Rogler G., Varki N., Barrett K.E. Epidermal growth factor partially restores colonic ion transport responses in mouse models of chronic colitis. Gastroenterology. 2005;129:591–608. doi: 10.1016/j.gastro.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Dubé P.E., Yan F., Punit S., Girish N., McElroy S.J., Washington M.K., et al. Epidermal growth factor receptor inhibits colitis-associated cancer in mice. J Clin Invest. 2012;122:2780–2792. doi: 10.1172/JCI62888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lei L., Chen C., Zhao J., Wang H., Guo M., Zhou Y., et al. Targeted Expression of miR-7 Operated by TTF-1 Promoter Inhibited the Growth of Human Lung Cancer through the NDUFA4 Pathway. Mol Ther Nucleic Acids. 2017;6:183–197. doi: 10.1016/j.omtn.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu Q., Zhang S., Chao K., Feng R., Wang H., Li M., et al. E3 Ubiquitin ligase RNF183 Is a Novel Regulator in Inflammatory Bowel Disease. J Crohns Colitis. 2016;10:713–725. doi: 10.1093/ecco-jcc/jjw023. [DOI] [PubMed] [Google Scholar]
- 35.Chen J., Zhao B.C., Dai X.Y., Xu Y.R., Kang J.X., Li J.L. Drinking alkaline mineral water confers diarrhea resistance in maternally separated piglets by maintaining intestinal epithelial regeneration via the brain-microbe-gut axis. J Adv Res. 2022;17:S2090–1232(22),:00287–00289. doi: 10.1016/j.jare.2022.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spalinger M.R., Sayoc-Becerra A., Santos A.N., Shawki A., Canale V., Krishnan M., et al. PTPN2 Regulates Interactions Between Macrophages and Intestinal Epithelial Cells to Promote Intestinal Barrier Function. Gastroenterology. 2020;159:1763–1777.e1714. doi: 10.1053/j.gastro.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tian Y., Xu J., Li Y., Zhao R., Du S., Lv C., et al. MicroRNA-31 Reduces Inflammatory Signaling and Promotes Regeneration in Colon Epithelium, and Delivery of Mimics in Microspheres Reduces Colitis in Mice. Gastroenterology. 2019;156:2281–2296.e2286. doi: 10.1053/j.gastro.2019.02.023. [DOI] [PubMed] [Google Scholar]
- 38.Yang J.Y., Jie Z., Mathews A., Zhou X., Li Y., Gu M., et al. Intestinal Epithelial TBK1 Prevents Differentiation of T-helper 17 Cells and Tumorigenesis in Mice. Gastroenterology. 2020;159:1793–1806. doi: 10.1053/j.gastro.2020.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song J., Liu Y., Wan J., Zhao G.N., Wang J.C., Dai Z., et al. SIMPLE Is an Endosomal Regulator That Protects Against NAFLD by Targeting the Lysosomal Degradation of EGFR. Hepatology. 2021;74(6):3091–3109. doi: 10.1002/hep.32075. [DOI] [PubMed] [Google Scholar]
- 40.Hu W., Zheng S., Guo H., Dai B., Ni J., Shi Y., et al. PLAGL2-EGFR-HIF-1/2α Signaling Loop Promotes HCC Progression and Erlotinib Insensitivity. Hepatology. 2021;73(2):674–691. doi: 10.1002/hep.31293. [DOI] [PubMed] [Google Scholar]
- 41.Guo J., Sun M., Teng X., Xu L. MicroRNA-7-5p regulates the expression of TFF3 in inflammatory bowel disease. Mol Med Rep. 2017;16:1200–1206. doi: 10.3892/mmr.2017.6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.