Abstract
Under conditions where glycolate synthesis was inhibited at least 50% in tobacco (Nicotiana tabacum L.) leaf discs treated with glycidate (2,3-epoxypropionate), the ribulose diphosphate carboxylase activity in extracts and the inhibition of the activity by 100% oxygen were unaffected by the glycidate treatment. [1-14C]Glycidate was readily taken into leaf discs and was bound to leaf proteins, but the binding occurred preferentially with proteins of molecular weight lower than ribulose diphosphate carboxylase. Glycidate added to the isolated enzyme did not inhibit ribulose diphosphate carboxylase activity or affect its inhibition by 100% O2. Thus, glycidate did not inhibit glycolate synthesis by a direct effect on ribulose diphosphate carboxylase/oxygenase.
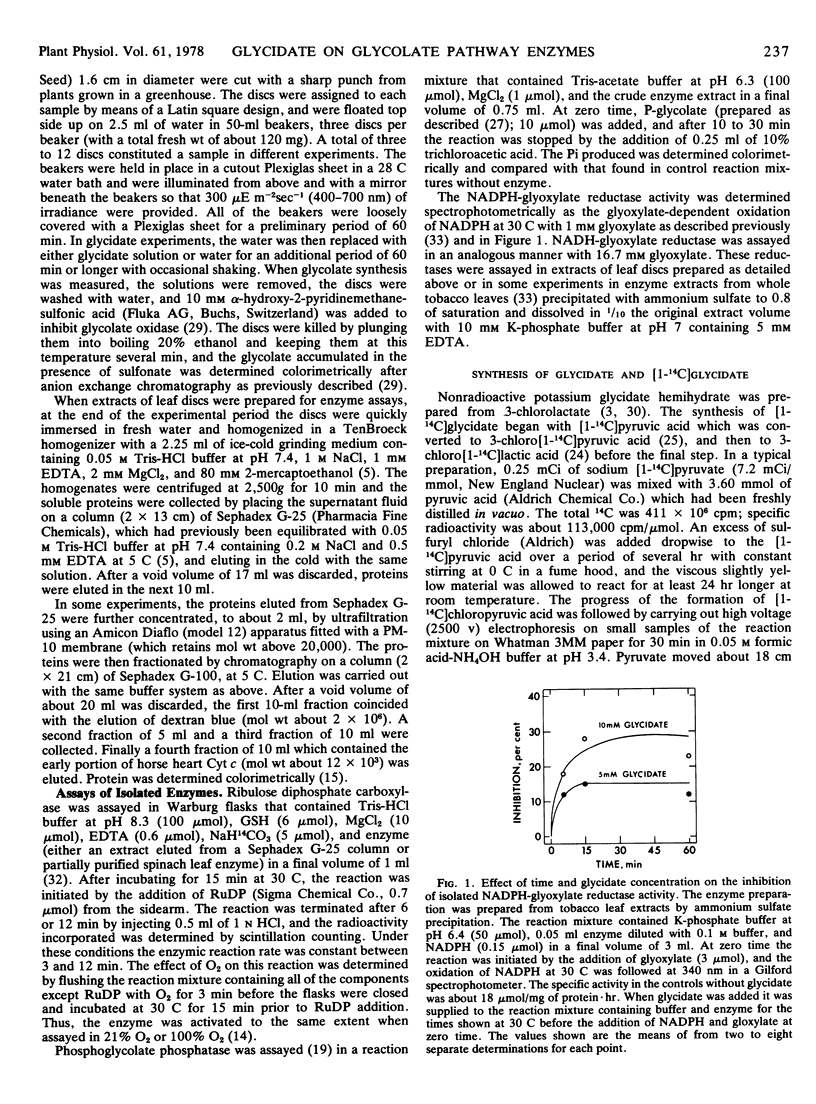
NADH-glyoxylate reductase and phosphoglycolate phosphatase activities were also unaffected in extracts of leaf discs supplied with glycidate, but NADPH-glyoxylate reductase was inhibited about 35% in such extracts. Addition of 10 mm glycidate to isolated NADPH-glyoxylate reductase inhibited the activity about 25% after 15 minutes of reaction.
The small inhibition of NADPH-glyoxylate reductase by glycidate may help to explain the increase in glyoxylate concentration found in glycidate-treated leaf discs. Increasing the glyoxylate pool size in leaf discs has been shown to effectively block glycolate synthesis and photorespiration and increase net photosynthesis. Thus, the similar effects brought about by glycidate in leaf discs can be attributed to indirect effects of metabolic regulation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badger M. R., Andrews T. J. Effects of CO2, O2 and temperature on a high-affinity form of ribulose diphosphate carboxylase-oxygenase from spinach. Biochem Biophys Res Commun. 1974 Sep 9;60(1):204–210. doi: 10.1016/0006-291x(74)90192-2. [DOI] [PubMed] [Google Scholar]
- Bowes G., Ogren W. L., Hageman R. H. Phosphoglycolate production catalyzed by ribulose diphosphate carboxylase. Biochem Biophys Res Commun. 1971 Nov 5;45(3):716–722. doi: 10.1016/0006-291x(71)90475-x. [DOI] [PubMed] [Google Scholar]
- Chan P. H., Sakano K., Singh S., Wildman S. G. Crystalline fraction I protein: preparation in large yield. Science. 1972 Jun 9;176(4039):1145–1146. doi: 10.1126/science.176.4039.1145. [DOI] [PubMed] [Google Scholar]
- Chollet R., Anderson L. L. Regulation of ribulose 1,5-bisphosphate carboxylase-oxygenase activities by temperature pretreatment and chloroplast metabolites. Arch Biochem Biophys. 1976 Sep;176(1):344–351. doi: 10.1016/0003-9861(76)90173-9. [DOI] [PubMed] [Google Scholar]
- Chollet R. Effect of glycidate on glycolate formation and photosynthesis in isolated spinach chloroplasts. Plant Physiol. 1976 Feb;57(2):237–240. doi: 10.1104/pp.57.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinman J. P. The interaction of an epoxide with yeast alcohol dehydrogenase: evidence for binding and the modification of two active site cysteines by styrene oxide. Biochemistry. 1975 Jun 17;14(12):2568–2574. doi: 10.1021/bi00683a002. [DOI] [PubMed] [Google Scholar]
- Kohn L. D., Warren W. A., Carroll W. R. The structural properties of spinach leaf glyoxylic acid reductase. J Biol Chem. 1970 Aug 10;245(15):3821–3830. [PubMed] [Google Scholar]
- Kung S. Tobacco fraction 1 protein: a unique genetic marker. Science. 1976 Feb 6;191(4226):429–434. doi: 10.1126/science.1108201. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lawyer A. L., Zelitch I. Inhibition of glutamate:glyoxylate aminotransferase activity in tobacco leaves and callus by glycidate, an inhibitor of photorespiration. Plant Physiol. 1978 Feb;61(2):242–247. doi: 10.1104/pp.61.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorimer G. H., Badger M. R., Andrews T. J. The activation of ribulose-1,5-bisphosphate carboxylase by carbon dioxide and magnesium ions. Equilibria, kinetics, a suggested mechanism, and physiological implications. Biochemistry. 1976 Feb 10;15(3):529–536. doi: 10.1021/bi00648a012. [DOI] [PubMed] [Google Scholar]
- Randall D. D., Tolbert N. E., Gremel D. 3-Phosphoglycerate Phosphatase in Plants: II. Distribution, Physiological Considerations, and Comparison with P-Glycolate Phosphatase. Plant Physiol. 1971 Oct;48(4):480–487. doi: 10.1104/pp.48.4.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schray K. J., O'Connell E. L., Rose I. A. Inactivation of muscle triose phosphate isomerase by D- and L-glycidol phosphate. J Biol Chem. 1973 Mar 25;248(6):2214–2218. [PubMed] [Google Scholar]
- Tolbert N. E., Yamazaki R. K., Oeser A. Localization and properties of hydroxypyruvate and glyoxylate reductases in spinach leaf particles. J Biol Chem. 1970 Oct 10;245(19):5129–5136. [PubMed] [Google Scholar]
- Walsh C. T., Schonbrunn A., Abeles R. H. Studies on the mechanism of action of D-amino acid oxidase. Evidence for removal of substrate -hydrogen as a proton. J Biol Chem. 1971 Nov 25;246(22):6855–6866. [PubMed] [Google Scholar]
- Walsh C., Lockridge O., Massey V., Abeles R. Studies on the mechanism of action of the flavoenzyme lactate oxidase. Oxidation and elimination with beta-chlorolactate. J Biol Chem. 1973 Oct 25;248(20):7049–7054. [PubMed] [Google Scholar]
- Wildner G. F., Henkel J. Specific inhibition of the oxygenase activity of ribulose-1,5-bisphosphate carboxylase. Biochem Biophys Res Commun. 1976 Mar 8;69(1):268–275. doi: 10.1016/s0006-291x(76)80302-6. [DOI] [PubMed] [Google Scholar]



