Abstract
Background
Liver disease is any disease that negatively affects the normal function of the liver, and it is a major health problem that challenges not only healthcare professionals, but also the pharmaceutical industry and drug regulatory agencies. Similarly, diarrhea is the second leading cause of death among children under five globally next to pneumonia. The available synthetic drugs for the treatment of liver disorders and diarrhoea have limited safety and efficacy.
Objective
To evaluate the in vivo hepatoprotective and antidiarrheal activities of hydroalcoholic leaf and fruit extracts of Schinus molle L. (Anacardiaceae) in mice.
Methods
Hepatoprotective activity of the extracts was evaluated by using CCl4 induced hepatotoxicity in mice model. In this model, mice were divided into groups and treated as follows. The normal control and toxicant control groups were treated with the vehicle used for reconstitution, the positive control was treated with the standard drug (silymarin), and the test groups were treated with different doses of plant extracts daily in the morning for seven days. Additionally, all groups except the normal control were treated with CCl4 (2 mg/kg, IP) on the 4th day of treatment, 30 min post-dose. On the 7th day, blood was collected from each mouse via a cardiac puncture. The collected blood was centrifuged, and serum levels of ALT, AST, and ALP were determined using an automated chemistry analyser. Data were analysed using one-way analysis of variance (ANOVA) followed by Tukey's post-hoc test.
The antidiarrheal activity of the extract was investigated using castor oil-induced diarrhoea, enteropooling, and small intestine transit. The test groups received various doses (100, 200, and 400 mg/kg) of the extract, whereas the positive control received loperamide (3 mg/kg), and the negative control received the vehicle (distilled water, 10 ml/kg).
Result
Hepatoprotective activity: The leaf and fruit crude extracts showed significant improvement in the body weight and liver weight of mice compared to the untreated toxicant control. Additionally, treatment with hydromethanol leaf and fruit extracts caused a significant (P < 0.05) improvement in liver biomarkers compared to the toxicant control. Similarly, the n-butanol and chloroform fractions of the fruit extract caused a significant reduction (P < 0.01) in serum AST, ALT, ALP and Bilirubin levels and a significant (P < 0.001) increase in total protein compared to the toxicant control. However, none of the three solvent fractions (n-butanol, chloroform, and aqueous) of the fruit extract significantly affected (P > 0.05) the level of albumin compared with the toxicant control.
Antidiarrheal activity: In the castor oil-induced diarrheal model, the 80 % methanol extract delayed the onset of defaecation and significantly reduced the number and weight of faeces at all tested doses compared to the negative control. In the enteropooling test, 80 ME significantly (P < 0.001) reduced the weight and volume of intestinal fluid at all tested doses compared with the negative control. Results from the charcoal meal test revealed that the extracts produced a significant anti-motility effect at all tested doses compared with the negative control.
Conclusion
This study confirmed the hepatoprotective and antidiarrheal activities of hydroalcoholic extracts. The highest test dose produced the maximum hepatoprotective and antidiarrheal activities in all models.
Keywords: Antidiarrheal, Hepatoprotective, Castor oil, Loperamide, CCl4, Schinus molle, Leaf and fruit
1. Introduction
There are a growing number of studies purporting hepatoprotective effects with traditional medicines, and based on claims in folkloric use, several medicinal plants have been evaluated scientifically with promising hepatoprotective activity. These plants include Adansonia digitata [1], Alysicarpus vaginalis [2], Bidens pilosa [3], Brassica oleracea [4], Cineraria abyssinica [5], Indigofera barberi [6], Justicia schimperiana [7], Nigella sativa [8], Opuntia monacantha [9], Verbascum sinaiticum [7], Vicia calcarata [10], Ziziphus jujuba [11]. In recent years, hepatoprotective natural antioxidants and compounds with radical-scavenging activity have been identified, including curcumin and ginger [12], Hibiscus species [13] Polyalthia longifolia [14] and Cassia italic [15]. One of the most extensively studied agents is Silybum marianum, which is one of the oldest and most thoroughly researched plants for the treatment of liver disease. The seeds of this plant contain four flavonolignans collectively known as silymarin. Silymarin, a single herbal drug formulation, is mostly used for the treatment of liver disease [16]. Flavonoids with proven antioxidant, antiviral, and anticancer properties, such as glycyrrhizin, phyllanthin, silybin, picroside, and baicalein, can serve as lead compounds for further development of hepatoprotective drugs [17,18].
Experimental studies have shown that many medicinal plants improve diarrhoea through their anti-spasmodic effects, delaying intestinal transit, suppressing gut motility, stimulating water adsorption, and reducing electrolyte secretion [19]. Of the numerous phytochemicals present in active extracts, tannins and flavonoids are thought to be responsible for the antidiarrheal activity by increasing colonic water and electrolyte reabsorption [20]. In addition, many phenolic constituents of medicinal plants may have the ability to inhibit enteropooling and delay gastrointestinal transit, and hence, are very useful in the control of diarrhoea [21]. Different medicinal plants, such as Calpurnia aurea leaf extract [22], Daniellia oliveri leaf extract, Ficus sycomorus leaf extract [23], and Moringa oleifera root extract [24] have been tested for their antidiarrheal activity and found to be effective. In addition, different plants, including Schinus molle L., have been traditionally used for the treatment of diarrhoea, indicating the need for further scientific studies to validate and develop an effective and safe antidiarrheal agent [25,26].
Schinus molle L. is traditionally used to treat hepatic diseases, jaundice, and diarrhoea in Ethiopia. An ethnobotanical survey conducted in Kilte Awulaelo District, Tigray Region of Ethiopia, reported that Schinus molle leaf juice is taken orally to treat jaundice and diarrhoea [27]. Another ethnobotanical survey conducted in the mecha woreda, West Gojjam zone of Ethiopia, reported that the fruit of Schinus molle with S. nigrum leaves is taken orally to treat jaundice [28]. However, the hepatoprotective and antidiarrheal activities of this medicinal plant have not yet been studied scientifically. Additionally, previous experimental studies have reported that Schinus molle has significant in vitro antioxidant, antibacterial, anti-proliferative, and in vivo anti-inflammatory activities [[29], [30], [31], [32]] suggesting that the plant may have hepatoprotective and antidiarrheal effects.
Previous phytochemical studies have shown that S. molle is rich in phytochemicals known to have hepatoprotective and antidiarrheal activities [[30], [32], [33], [34]]. Therefore, this study aimed to scientifically verify the hepatoprotective and antidiarrheal activities of Schinus molle leaf and fruit extracts.
2. Materials and methods
2.1. Chemicals and reagents
The main chemicals that were used include distilled water, 2 % Tween80, 80 % methanol, n-butanol, chloroform, CCl4, 10 % formalin, ether, normal saline, paraffin wax, hematoxylin, eosin, xylol, 2,2-diphenyl-1-picrylhydrazyl (DPPH), the standard drug Silymarin (Silybon-140), castor oil, activated charcoal, loperamide and reagents used for phytochemical screening. Analytical-grade chemicals were used in this study.
2.2. Plant material
The leaves and fruits of Schinus molle were collected from its natural habitat around Kobbo, North Wollo, and Northeast Ethiopia. The identification and authentication of the plant specimens were performed by a taxonomist at the National Herbarium, Department of Biology, Addis Ababa University, where a voucher specimen was deposited for future reference.
2.3. Experimental animals
Healthy adult male and female Swiss albino mice, 6–8 weeks old and weighing 22–30 g, were maintained in an animal house facility at the Department of Pharmacy, Wollo University. Female mice were used for the acute toxicity test and male mice were used for the main study. The mice were housed in polypropylene cages (six mice per cage) under standard environmental conditions and a 12-12 h light-dark cycle, and were kept in the laboratory for a week for acclimatisation. They were provided with laboratory pellet diet and water ad libtum.
2.4. Preparation of plant crude extracts
The leaves and fruits of the plant were thoroughly washed with tap water to remove dirt and then cleaned with gauze. They were then dried in the shade separately. The dried plant materials were pulverised using a mortar and pestle and then miller to obtain a coarse powder. The powder was extracted by cold maceration with 80 % methanol for 72 h at room temperature to obtain a crude hydroalcoholic extract. Then, the 80 % methanol liquid extract was evaporated to remove methanol under vacuum at 40 °C using a rotary evaporator, and the extract was frozen in a refrigerator and dried in a lyophiliser. Finally, the extract was transferred into vials and stored in a desiccator until further use.
2.5. Solvent fractionationaton of the crude extract
The crude methanolic extract of S. molle fruit was diluted with distilled water and further fractionated by successive solvent extractions with chloroform and n-butanol. Each fraction was evaporated at 40 °C to dryness under reduced pressure to yield chloroform and butanol fractions, and the remaining water fraction was lyophilised.
2.6. Acute oral toxicity test
Acute oral toxicity tests for leaf and fruit of S. molle were performed according to the Organization for Economic Cooperation and Development guidelines. Mice were fasted for 3–4 h before and 1–2 h after the administration of the extract. Firstly, a sighting study was conducted to determine the starting dose. Female mice were used and each mouse was given 2000 mg/kg of the extract as a single dose by oral gavage. Since mortality was not observed within 24 h, an additional four mice were administered the same dose as mentioned above. The animals were observed continuously for 4 h at 30 min interval and then for 14 consecutive days at an interval of 24 h for general signs and symptoms of toxicity (diarrhoea, weight loss, tremor, lethargy, and paralysis), food and water intake, and mortality. Then, three dose levels were chosen for the extract: a middle dose, which is one-tenth of the limit dose during the acute toxicity study; a low dose, which is half of the middle dose (1/20th of the limit dose); and a high dose which is twice the middle dose (1/5th of the limit dose) [35].
2.7. Phytochemical screening
Phytochemical screening tests were carried out for the extracts using standard procedures to identify the presence of secondary metabolites, such as tannins, saponins, flavonoids, terpenoids, steroids, alkaloids, and cardiac glycosides [36,37].
2.8. Evaluation of hepatoprotective activity
The mice were individually weighed, and their weights were recorded at the beginning of the experiment. Animals were randomly divided into groups of six mice per group. Mice were fasted for 3–4 h before the initiation of the experiment. The normal control was treated with vehicle used for reconstitution (Distilled water), 1 ml/kg orally, daily for seven days. The positive control was treated with the standard drug (silymarin) (100 mg/kg, p. o.) daily for seven days. The toxicant control received the vehicle (1 ml/kg) daily, and the different treatment groups were administered different doses of the extracts daily for seven days. Thirty minutes after treatment with vehicle and extracts, all groups were treated with CCl4 at a dose of 2 ml/kg IP on the 4th day of treatment, except for the normal control group [38].
Blood collection: The mice were weighed and blood samples were collected from each mouse in heparinised sterile centrifuge tubes by cardiac puncture on the 7th day. The tubes were placed in a centrifuge and centrifuged at 2500 rpm for 10 min. Serum samples were collected from each test tube for biochemical liver estimation. The livers of each mouse were extracted for macroscopic examination.
Hepatic biochemical evaluation: The collected serum samples were analysed for liver chemistry and serum ALT, AST, and ALP levels using an automated analyser. Immediately after the final blood collection, the liver of each mouse was harvested for macroscopic investigation.
2.9. Determination of antidiarrheal activity
Animal grouping and dosing: In all models, animals were randomly divided into five groups (negative control, positive control, and three test groups), with six animals per group. The negative controls received distilled water (10 ml/kg), and the positive controls received loperamide (3 mg/kg). The test groups (Groups 3, 4, and 5) received different doses (100, 200, and 400 mg, respectively) of the extract, which were determined based on the acute oral toxicity test and pilot study.
Castor oil induced diarrhoea: The method described by Igboeli et al. [39] was used in this study. Swiss albino mice of either sex were fasted for 18 h with free access to water and were grouped and treated as described in Section 3.8.1. One hour after dosing, each mouse was orally administered castor oil (0.5 mL) to induce diarrhoea and placed individually in cages, in which the floor was lined with white paper. The transparent paper was changed every hour for a total of 4 h. During the observation period, the onset of diarrhoea, number and weight of wet stools, total number of faeces, and total weight of faecal output were recorded. Finally, the percentage of faecal output and diarrhoeal inhibition were calculated using the formulas described below.
where WFC = wet faeces in the control group, and WFT = wet faeces in the test group.
Castor oil induced enteropooling: The effects of the extract on intra-luminal fluid accumulation were determined using the method described by Robert et al. [40]. Animals were fasted for 18 h, grouped, and treated as described in section 3.8.1. After 1 h of treatment, castor oil (0.5 ml of castor oil was administered and the animals were sacrificed by cervical dislocation 1 h following castor oil administration. The abdomen of each animal was then opened, and the small intestine was ligated at both the pyloric sphincter and ileocecal junction and dissected. The dissected small intestine was weighed, the intestinal contents were collected by milking in a graduated tube, and the volume of the contents was measured. The weight of the intestine after milking was measured and the difference between the two weights was recorded. Finally, the percentage reduction in intestinal secretion (volume and weight) was calculated relative to the negative control, using the following formula:
Where, MVIC – Mean Volume of Intestinal Content.
MVICC - Mean Volume of Intestinal Content of Control Group.
MVICT - Mean Volume of Intestinal Content of Test Group
Where, MWIC – Mean Weight of Intestinal Content.
MWICC- Mean Weight of Intestinal Content of Control Group.
MWICT - Mean Weight of Intestinal Content of Test Group.
Gastrointestinal motility test: Animals were fasted for 18h with free access to water, divided, and treated as described in section 3.8.11 h before the administration of castor oil (0.5 ml castor oil. One millilitre of the marker (5%activated charcoal suspension in water) was orally administered 1 h after the castor oil treatment. The animals were sacrificed by cervical dislocation 1 h after charcoal meal, and the small intestine was dissected from the pylorus to the caecum and placed lengthwise on white paper. The distance travelled by the marker and the total length of the intestine were measured. The peristaltic index and percentage of inhibition were calculated using the following formulae [39,41]:
Where, Dc: Mean distance travelled by the charcoal in the control group and.
Dt: Mean distance travelled by the charcoal in the test group
In-vivo antidiarrheal index: The in vivo antidiarrheal index (ADI) for the positive control and different doses of the extract was determined based on the data from the above tests using the formula developed by Aye-Than et al. [41].
where Dfreq is the delay in defaecation time as a percentage of the negative control, Gmeq is the reduction in gut meal travel as a percentage of the negative control, and Pfreq is the reduction in the number of stools as a percentage of the negative control.
2.10. Statistical analysis
Results are expressed as the mean ± standard error of the mean (S.E.M). Statistical Package for Social Science (SPSS) version 23 was used to analyse the results. Statistically significant differences between groups were evaluated using one-way analysis of variance (ANOVA) followed by Tukey's multiple comparison test. The analysis was performed with a 95 % confidence interval, and probabilities less than 0.05 (p < 0.05) was considered significant.
2.11. Ethical consideration
Animals were handled in accordance with the International Animal Care and Welfare Guidelines [42] and the National Institute of Health Guidelines for the Care and Use of Laboratory Animals [43]. Ethical clearance (Reference No, WU15852/05/12) was obtained from the ethical review committee of the College of Medicine and Health Sciences, Wollo University.
3. Result
3.1. Acute oral toxicity test
The acute oral toxicity test of 80 ME of leaves as well as fruits of Schinus molle L. leaves and fruits indicated that the extracts did not cause gross behavioural changes or mortality within 24 h or in the next 14 days, indicating that the LD50 of the extracts was greater than 2000 mg/kg in mice.
3.2. Preliminary phytochemical screening
Preliminary phytochemical screening of 80 ME revealed the presence of all tested constituents except steroids, alkaloids, and glycosides (Table 1).
Table 1.
Preliminary phytochemical screening of the 80 % methanol extract of the leaf and fruit of Schinus Molle.
| Constitutes | 80 % methanol leaf Extract | 80 % methanol fruit extract |
|---|---|---|
| Flavonoids | + | + |
| Alkaloids | + | + |
| Saponins | – | – |
| Steroids | – | – |
| Tannins | + | + |
| Terpenoids | – | – |
-, absence; +, precense
3.3. Hepatoprotective activity
Effect on Body Weight and Liver Weight of mice with CCl4 induced liver toxicity: Carbon tetrachloride caused a significant (P < 0.01) reduction in body and liver weights compared to the normal control, as shown in Table 2. The crude leaf and fruit extracts significantly improved the body weight and liver weight of mice compared to the untreated control (Table 2).
Table 2.
Effect of 80 % Methanol Extract of the Leaves and fruits of Schinus Molle on Body Weight, Change in Body Weight and Liver Weight of Mice with CCl4 induced hepatotoxicity.
| Group | Body weight (g) |
% Change in body weight | Liver weight (g) | |
|---|---|---|---|---|
| Initial (day 1) | Final (day 8) | |||
| DW (NC) | 29.81 ± 0.68 | 31.47 ± 0.77 | 5.57 % | 2.19 ± 0.12 |
| DW + CCl4 (TC) | 30.25 ± 1.01 | 27.39 ± 0.99a** | −9.45 % | 3.39 ± 0.24a** |
| MEL100 + CCl4 | 29.93 ± 0.92 | 27.64 ± 0.89 | −7.65 % | 3.07 ± 0.19 |
| MEL200 + CCl4 | 29.97 ± 0.88 | 30.62 ± 1.02b* | 2.17 % | 2.51 ± 0.14 |
| MEL400 + CCl4 | 30.41 ± 0.98 | 31.98 ± 1.10b** | 5.16 % | 2.20 ± 0.20b* |
| MEF100 + CCl4 | 30.25 ± 0.69 | 2p9.14 ± 0.95 | −3.67 % | 3.05 ± 0.18 |
| MEF200 + CCl4 | 30.12 ± 0.77 | 32.01 ± 0.65b** | 6.27 % | 2.34 ± 0.17b* |
| MEF400 + CCl4 | 29.87 ± 1.05 | 32.14 ± 0.79b** | 7.59 % | 2.19 ± 0.16b* |
| Silymarin + CCl4 | 30.13 ± 0.92 | 33.01 ± 1.1b*** | 9.56 % | 2.25 ± 0.13 |
Each value represents the mean ± S.E.M; n = 6; aagainst DW-treated group; bAgainst DW and CCl4 treated group; cAgainst standard (silymarin); *p < 0.05, **p < 0.01, ***p < 0.001.
Abbreviations: MEL100, 80 % methanol leaf extract 100 mg/kg; MEL200, 80 % methanol leaf extract 200 mg/kg; MEL400, 80 % methanol leaf extract 400 mg/kg; MEF100, 80 % methanol fruit extract 100 mg/kg; MEF200, 80 % methanol fruit extract 200 mg/kg; MEF400, 80 % methanol fruit extract 400 mg/kg; silymarin, silymarin 100 mg/kg; CCl4, carbon tetrachloride 2 mL/kg; DW, distilled water 10 ml/kg.
Effect of the crude extracts on liver biomarkers: As shown in Table 3, CCl4 caused a significant (P < 0.001) elevation in serum AST, ALT, ALP and Bilirubin levels and a significant (P < 0.05) reduction in total protein and albumin levels. However, treatment with hydromethanol leaf and fruit extracts caused a significant (P < 0.05) improvement in liver biomarkers compared to the toxicant control (Table 3).
Table 3.
Effect of 80 % Methanol Extract of the Leaves and fruits of Schinus Molle on Liver biomarkers of Mice with CCl4 induced hepatotoxicity.
| Group | AST (IU/L) | ALT (IU/L) | ALP (IU/L) | Total protein (g/dL) | Albumin (mg/dL) | Bilirubin (mg/dL) |
|---|---|---|---|---|---|---|
| DW (NC) | 84.25 ± 2.13 | 95.24 ± 2.33 | 150.23 ± 3.56 | 5.44 ± 0.45 | 2.35 ± 0.14 | 1.15 ± 0.21 |
| DW + CCl4 (TC) | 211.32 ± 9.13a*** | 223.14 ± 7.45a*** | 354.22 ± 8.99a*** | 3.19 ± 0.33a** | 1.19 ± 0.22a* | 3.90 ± 0.41a*** |
| MEL100 + CCl4 | 192.23 ± 7.26 | 197.25 ± 6.35 | 346.89 ± 6.44 | 3.32 ± 0.23 | 1.22 ± | 3.85 ± 0.39 |
| MEL200 + CCl4 | 115.14 ± 4.12b*** | 120.14 ± 5.12b*** | 198.68 ± 4.22b*** | 4.14 ± 0.25 | 2.05 ± 0.19 | 2.01 ± 0.25b** |
| MEL400 + CCl4 | 102.88 ± 3.20b*** | 107.32 ± 2.50b*** | 170.45 ± 3.88b*** | 5.01 ± 0.44b* | 2.12 ± 0.23b* | 1.65 ± 0.21b*** |
| MEF100 + CCl4 | 189.65 ± 6.17 | 194.33 ± 4.99 | 348.95 ± 5.44 | 3.55 ± 0.26 | 1.25 ± 0.19 | 3.83 ± 0.42 |
| MEF200 + CCl4 | 109.55 ± 2.9b*** | 114.29 ± 3.00b*** | 189.47 ± 3.74b*** | 4.58 ± 0.35b* | 2.14 ± 0.21 | 1.95 ± 0.22b** |
| MEF400 + CCl4 | 98.65 ± 2.77b*** | 103.12 ± 2.88b*** | 160.19 ± 3.22b*** | 5.15 ± 0.41b* | 2.32 ± 0.18b* | 1.20 ± 0.18b*** |
| Silymarin + CCl4 | 91.23 ± 2.64b*** | 96.99 ± 3.97b*** | 156.82 ± 2.98b*** | 5.18 ± 0.42b* | 2.31 ± 0.25b* | 1.23 ± 0.23b** |
Each value represents the mean ± S.E.M; n = 6; aagainst DW-treated group; bAgainst DW and CCl4 treated group; cAgainst standard (silymarin); *p < 0.05, **p < 0.01, ***p < 0.001.
Abbreviations: MEL100, 80 % methanol leaf extract 100 mg/kg; MEL200, 80 % methanol leaf extract 200 mg/kg; MEL400, 80 % methanol leaf extract 400 mg/kg; MEF100, 80 % methanol fruit extract 100 mg/kg; MEF200, 80 % methanol fruit extract 200 mg/kg; MEF400, 80 % methanol fruit extract 400 mg/kg; silymarin, silymarin 100 mg/kg; CCl4, carbon tetrachloride 2 mL/kg; DW, distilled water 10 ml/kg.
Effect of solvent fractions on liver biomarkers: The effect of the solvent fractions of 80 % methanol fruit extract on liver biomarkers was evaluated. As shown in Table 4, the n-butanol and chloroform fractions caused a significant reduction (P < 0.01) in the levels of serum AST, ALT, ALP and Bilirubin, and a significant (P < 0.001) increase in total protein compared to the toxicant control. However, none of the three solvent fractions of the fruit extract significantly affected (P > 0.05) the level of albumin compared with the toxicant control.
Table 4.
Effect of solvent fractions of Schinus Molle fruits on Liver biomarkers of Mice with CCl4 induced hepatotoxicity.
| Group | AST (IU/L) | ALT (IU/L) | ALP (IU/L) | Total protein (g/dL) | Albumin (mg/dL) | Bilirubin (mg/dL) |
|---|---|---|---|---|---|---|
| DW | 84.25 ± 2.13 | 95.24 ± 2.33 | 150.23 ± 3.56 | 5.44 ± 0.45 | 2.35 ± 0.14 | 1.15 ± 0.21 |
| DW + CCl4 | 211.32 ± 9.13a*** | 223.14 ± 7.45a*** | 354.22 ± 8.99a*** | 3.19 ± 0.33a** | 1.19 ± 0.22a* | 3.90 ± 0.41a*** |
| BFF100 + CCl4 | 203.54 ± 4.98 | 217.23 ± 4.66 | 339.23 ± 7.55 | 3.77 ± 0.25 | 1.53 ± 0.34 | 3.85 ± 0.31 |
| BFF200 + CCl4 | 119.53 ± 3.97b*** | 121.92 ± 3.45b*** | 199.67 ± 6.14b*** | 4.52 ± 0.14b*** | 2.21 ± 0.41 | 1.67 ± 0.25b*** |
| BFF400 + CCl4 | 113.38 ± 3.86b*** | 110.14 ± 3.01b*** | 170.88 ± 5.55b*** | 4.92 ± 0.22b*** | 2.30 ± 0.29 | 1.25 ± 0.22b*** |
| CFF100 + CCl4 | 204.22 ± 5.52 | 219.27 ± 5.25 | 350.12 ± 6.36 | 3.66 ± 0.32 | 1.52 ± 0.34 | 3.75 ± 0.25 |
| CFF200 + CCl4 | 193.47 ± 3.91 | 201.95 ± 4.77 | 330.62 ± 5.42 | 4.41 ± 0.29 | 1.98 ± 0.42 | 3.22 ± 0.29 |
| CFF400 + CCl4 | 117.99 ± 4.13b*** | 139.13 ± 2.44b*** | 210.25 ± 4.33b*** | 4.81 ± 0.34b*** | 2.19 ± 0.38 | 1.61 ± 0.19b** |
| AFF100 + CCl4 | 209.98 ± 4.66 | 222.21 ± 4.98 | 352.14 ± 8.22 | 3.22 ± 0.33 | 1.34 ± 0.37 | 3.70 ± 0.51 |
| AFF200 + CCl4 | 211.68 ± 5.22 | 219.33 ± 3.22 | 342.66 ± 7.15 | 3.29 ± 0.41 | 1.41 ± 0.28 | 3.65 ± 0.48 |
| AFF400 + CCl4 | 207.26 ± 6.74 | 211.14 ± 4.45 | 337.17 ± 7.18 | 3.30 ± 0.40 | 1.39 ± 0.31 | 3.15 ± 0.49 |
| Silymarin + CCl4 | 91.23 ± 2.64b*** | 96.99 ± 3.97b*** | 156.82 ± 2.98b*** | 5.18 ± 0.42b* | 2.31 ± 0.25b* | 1.23 ± 0.23b** |
Each value represents the mean ± S.E.M; n = 6; aagainst DW-treated group; bAgainst DW and CCl4 treated group; cAgainst standard (silymarin); *p < 0.05, **p < 0.01, ***p < 0.001.
Abbreviations: BFF, n-butanol fruit fraction; CFF, chloroform fruit fraction; AFF, aqueous fruit fraction; silymarin, silymarin 100 mg/kg; CCl4, carbon tetrachloride 2 mL/kg; DW, distilled water.
3.4. Antidiarrheal activity
Effects of 80 % methanol extract on castor oil induced diarrhoea: In the castor oil-induced diarrhoea model, 80 ME of leaves of Schinus molle L. leaves delayed the onset of defaecation and reduced the frequency of defaecation at all tested doses (100, 200, and 400 mg/kg) significantly (P < 0.001) compared to the negative control. The results from the experiment also revealed that all tested doses of 80 ME significantly (p < 0.001) reduced the weight of faeces (wet faeces and total faeces) when compared with the negative control (Table 5).
Table 5.
Effects of methanol leaf extract of S. molle on castor oil induced diarrhoea in mice.
| Group | Onset of diarrhoea (min) | No of wet faces | Total no of faces | Weight of wet faces | Weight of total faces |
|---|---|---|---|---|---|
| Control | 13.2 ± 1.08 | 6.6 ± 0.33 | 9.7 ± 0.33 | 0.39 ± 0.01 | 0.46 ± 0.01 |
| 80ME100 | 35.7 ± 1.50a3b3c3d3 | 3.1 ± 0.31a3b2c2d3 | 6.8 ± 0.31a3b3c3d3 | 0.24 ± 0.02a3b3c3d3 | 0.34 ± 0.02a3b3c3d3 |
| 80ME200 | 68.3 ± 2.10a3b3d3 | 1.6 ± 0.21a3 | 4.5 ± 0.22a3b3d3 | 0.14 ± 0.02a3d3 | 0.22 ± 0.02a3b3d3 |
| 80ME400 | 185.3 ± 3.72a3b3 | .8 ± 0.17a3 | 2.3 ± 0.21a3b1 | 0.04 ± 0.01a3 | 0.08 ± 0.01a3 |
| Loperamide | 93.3 ± 2.40a3b3 | 1.5 ± 0.22a3 | 3.5 ± 0.22a3 | 0.08 ± 0.01a3 | 0.13 ± 0.01a3 |
Data are expressed as mean ± SEM (n = 6); analysis was performed with One-Way ANOVA followed by Tukey's test; a compared to negative control; b compared to loperamide, 3 mg/kg; c compared to 200 mg/kg; d compared to 400 mg/kg; 1p < 0.05, 2p < 0.01, 3p < 0.001; 80 ME, 80 % methanol extract, negative controls received distilled water.
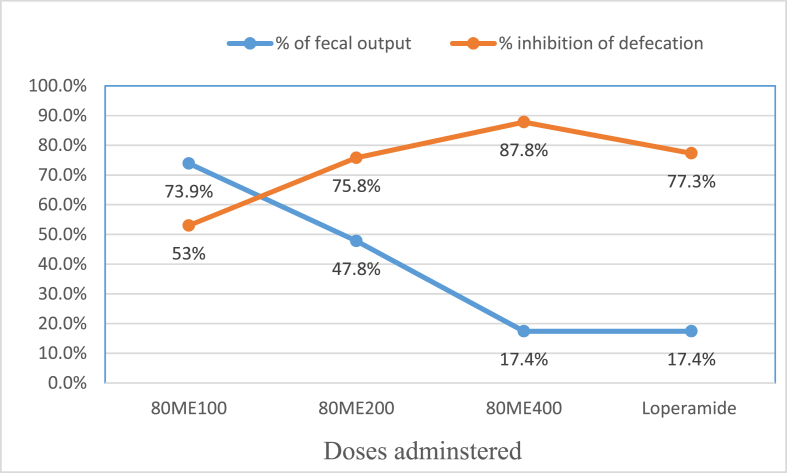
Moreover, there was a significant difference between the tested doses of the extract in delaying the onset of defaecation and reducing the frequency of defaecation. The highest tested dose of 80 ME (400 mg/kg) showed the maximum percentage inhibition of defaecation and the lowest percentage of mean faecal output when compared with the tested doses of the extract and positive control, as shown in Fig. 1.
Fig. 1.
Percentage inhibition of defecation and % of fecal output of the 80 % methanol leaf extract of Schinus molle L. on castor oil induced diarrhoea in mice.
Effects of 80 % methanol extract on castor oil-induced enteropooling: In the gastrointestinal enteropooling test, the 80 ME of leaves of Schinus molle L. leaves significantly reduced the weight and volume of intestinal content at all tested doses of the extract (P < 0.001) when compared to the negative control. The highest effect on both the weight and volume of intestinal content was achieved with the highest dose of the extract (400 mg), as shown in Table 6.
Table 6.
Effects of 80 % methanol leaf extract of Schinus molle L. on castor oil induced enteropooling in mice.
| Group | Mean weight of small intestinal content (gm) | % of inhibition | Mean volume of small intestinal content(ml) | % of inhibition |
|---|---|---|---|---|
| Control | 0.65 ± 0.170 | – | 0.50 ± 0.026 | – |
| 80ME100 | 0.41 ± 0.134a3b3c3d3 | 36.9 | 0.30 ± 0.133a3b3c3d3 | 40 |
| 80ME200 | 0.24 ± 0.129a3b2d3 | 63.1 | 0.18 ± 0.019a3d3 | 64 |
| 80ME400 | 0.07 ± 0.004a3b3 | 89.2 | 0.06 ± 0.034a3 | 88 |
| Loperamide | 0.16 ± 0.073a3 | 75.4 | 0.12 ± 0.071a3 | 76 |
Data are expressed as mean ± SEM (n = 6); analysis was performed with One-Way ANOVA followed by Tukey's test; a compared to negative control; b compared to loperamide, 3 mg/kg; c compared to 200 mg/kg; d compared to 400 mg/kg; 1p < 0.05, 2p < 0.01, 3p < 0.001; 80 ME, 80 % methanol extract, negative controls received distilled water.
Effect of 80 % methanol extract on gastrointestinal motility: The 80 % methanol extract significantly reduced normal gastrointestinal motility and castor oil-induced movement at all doses (p < 0.001) compared with the control, as shown in Table 7, Table 8, respectively, and the maximum effect was observed at 400 mg/kg on castor oil-induced gastrointestinal movement (73.7).
Table 7.
Effects of 80 % methanol leaf extract of Schinus molle L. on normal gastrointestinal motility in mice.
| Group | Mean length of small intestine (cm) | Mean distance travelled by charcoal meal (cm) | Peristaltic index(PI | % of inhibition |
|---|---|---|---|---|
| Control | 55.6 ± 0.9 | 47.1 ± 0.9 | 84.7 ± 0.4 | – |
| 80ME100 | 55.8 ± 1.0 | 36.1 ± 0.6a3b3d3e3 | 62.4 ± 0.6a3b3c3d3 | 23.4 |
| 80ME200 | 57.0 ± 0.8 | 22.7 ± 0.5a3b3 | 39.8 ± 0.7a3b3d3 | 51.8 |
| 80ME400 | 55.1 ± 1.7 | 15.9 ± 0.4a3 | 28.9 ± 1.0a3 | 66.2 |
| Loperamide | 55.8 ± 1.3 | 16.7 ± 0.4a3 | 30.0 ± 0.8a3 | 64.5 |
Data are expressed as mean ± SEM (n = 6); analysis was performed with One-Way ANOVA followed by Tukey's test; a compared to negative control; b compared to loperamide, 3 mg/kg; c compared to 200 mg/kg; d compared to 400 mg/kg; 1p < 0.05, 2p < 0.01, 3p < 0.001; 80 ME, 80 % methanol extract, negative controls received distilled water.
Table 8.
Effects of 80 % methanol leaf extract of Schinus molle L. on castor oil induced gastrointestinal motility in mice.
| Group | Mean length of small intestine (cm) | Mean distance travelled by charcoal meal (cm) | Peristaltic index (PI) | % of inhibition |
|---|---|---|---|---|
| Control | 55.9 ± 1.3 | 38.0 ± 0.3 | – | – |
| 80ME100 | 55.3 ± 1.0 | 25.0 ± 0.5a3b3c3d3 | 68.2 ± 1.4a3b3c3d3 | 34.2 |
| 80ME200 | 54.9 ± 1.1 | 15.5 ± 0.5a3b3 | 28.2 ± 1.1a3b3d3 | 59.2 |
| 80ME400 | 56.4 ± 0.8 | 10.0 ± 0.3a3 | 17.8 ± 0.4a3 | 73.7 |
| Loperamide | 55.4 ± 0.8 | 10.4 ± 0.4a3 | 18.5 ± 0.6a3 | 72.6 |
Data are expressed as mean ± SEM (n = 6); analysis was performed with One-Way ANOVA followed by Tukey's test; a compared to negative control; b compared to loperamide, 3 mg/kg; c compared to 200 mg/kg; d compared to 400 mg/kg; 1p < 0.05, 2p < 0.01, 3p < 0.001; 80 ME, 80 % methanol extract, negative controls received distilled water.
In vivo anti-diarrheal index: Results from the determination of in vivo ADI revealed that the ADI increased with dose for each fraction, and the highest tested dose had the maximum ADI when compared with other tested doses of the extract and the positive control (loperamide), as shown in Table 9.
Table 9.
In vivo anti-diarrheal index of 80 % methanol leaf extract of Schinus molle L.
| Group | Delay in defecation time (%) | Gut meal travel reduction (%) | No of faces reduction (%) | Anti-diarrheal index (ADI) |
|---|---|---|---|---|
| 80ME100 | 170.5 | 23.4 | 53.0 | 59.57 |
| 80ME200 | 417.4 | 51.8 | 75.8 | 117.91 |
| 80ME400 | 1303.8 | 66.2 | 87.8 | 196.42 |
| Loperamide | 606.8 | 64.5 | 77.3 | 144.63 |
80 ME, 80 % methanol extract, Negative controls received distilled water.
4. Discussion
Chronic liver disease is one of the foremost health problems worldwide, with liver cirrhosis and drug-induced liver injury accounting for the ninth leading cause of death in western and developing countries [44]. It is a well-known fact that the available synthetic drugs to treat liver disorders also cause further damage to the liver [45]. Most drugs used for the management of hepatic diseases are ineffective and associated with different side effects.
Inflammatory response and oxidative stress are the potential pathophysiological mechanisms involved in different types of liver disease including non-alcoholic fatty liver disease, and addressing inflammation and oxidative stress is crucial in managing liver diseases [46,47]. Previous experimental studies have reported that Schinus molle has significant in vitro antioxidant, antibacterial, antiproliferative, and in vivo anti-inflammatory activities [[29], [30], [31], [32]]. Thus, the antioxidant and anti-inflammatory effects of this plant may contribute to its hepatoprotective activity. Studies have identified glycosides, flavonoids, triterpenes and phenolic compounds as classes of phytochemicals with hepatoprotective effect [33,34], and previous phytochemical studies showed Schinus molle is rich in these compounds known to have hepatoprotective activity [30,32]. Therefore, it is suggested that the hepatoprotective activity of S. molle leaves and fruits Schinus molle is due to the presence of glycosides, flavonoids, triterpenes, and phenolic compounds.
Liver is the main site of protein synthesis, especially albumin [48]. In this study, the levels of total protein and albumin were measured to assess liver synthetic capability. The leaf and fruit crude extracts significantly increased total protein and albumin. Likewise, the fruit solvent fractions increased total protein and albumin which demonstrates the hepatoprotective activity of the plant.
Medicinal plants have been used to treat various disorders, including diarrhoea and related gastrointestinal disorders, although their safety and efficacy profiles have not been well addressed. Therefore, it is important to properly evaluate the safety and efficacy of medicinal plants used in traditional medicine. The need for newer, more effective, cheaper, and safer antidiarrheal drugs has become a paramount concern for safe and cost-effective therapeutic alternatives [49].
The present study aimed to evaluate the antidiarrheal activity of the hydroalcoholic leaf extract of Schinus molle L using different experimental models of diarrhoea in mice. In all models, diarrhoea was induced by administering castor oil to the mice. Castor oil causes diarrhoea owing to its active metabolite, ricinoleic acid which is liberated by the action of lipases in the upper part of the small intestine. It facilitates the accumulation of fluid in the intestine and alters the motility of GI smooth muscles [50].
In the castor oil-induced diarrhoea model, the extract had a significant effect on all measured parameters: onset of diarrhoea, number of wet and total stools, and weight of wet stools. This result is in agreement with a report on the methanol fraction of the leaves of L. camara [51], aqueous steam extract of L. camara [51], 80 % Methanolic Leaf Extract of J. schimperiana [52], and Hydromethanolic Root Extract [53].
Phytochemical analysis of the extracts revealed the presence of various bioactive compounds. Among the secondary metabolite identified flavonoids and phytosterols are known to modify the production of cyclooxygenase 1 and 2 (COX-1, COX-2) and lipooxygenase (LOX) thereby inhibiting prostaglandin production [52]. Tannins present in the extract precipitate the proteins in the intestinal mucosa by forming the protein tannates, which make the intestinal mucosa more resistance to chemical alteration and hence reduce the peristaltic movements and intestinal secretion. Therefore, the anti-diarrheal activity of S. Mole crude extract observed in this study may be attributed to the presence of flavonoids, alkaloids, tannins, and phytosterols in the crude extract.
To determine the antidiarrheal activity of S. molle leaves, the possible mechanism of action was tested using intestinal motility and enteropooling models. The enteropooling model was designed to assess the antisecretory effect of the hydromethanolic leaf extract of S. molle, in which the extract significantly reduced intraluminal fluid accumulation compared to the negative control. This result is in line with those of other studies [[50], [51], [52], [53]]. The active metabolite of castor oil, ricinoleic acid, induces irritation and inflammation of the intestinal mucosa, leading to the release of prostaglandins. The prostaglandins thus released stimulate secretion by preventing reabsorption of sodium chloride and water. Thus, it is possible that the extract significantly inhibited gastrointestinal hypersecretion and enteropooling by increasing the reabsorption of electrolytes and water or by inhibiting the induced intestinal accumulation of fluid [53]. The anti-enteropooling activity of the extract may also be related to the presence of phytochemical constituents including flavonoids, steroids, and tannins [[53], [54], [55]].
In the castor oil-induced gastrointestinal motility model, the extract significantly suppressed the movement of the charcoal marker at all tested doses of the extract (100, 200, and 400 mg/kg) compared to the negative control. The higher percentage of inhibition (66.2 %, p < 0.001) of the marker perceived at the maximum dose was comparable to that of loperamide (64.5 %, p < 0.001 at a dose of 3 mg/kg). This finding showed that the extract could influence the peristaltic movement of the intestine, thereby indicating intestinal antimotility activity. Several plants have shown antidiarrheal activity by reducing gastrointestinal motility and secretion [[50], [51], [52], [53], [54], [55]].
Several studies have suggested that the anti-motility properties of herbs are mostly due to flavonoids; inhibiting the release of autacoids and prostaglandins results in the inhibition of motility and hydro-electrolytic secretions induced by ricinoleic acid. Tannins may also show an anti-motility effect by reducing intracellular Ca2+ by decreasing the Ca2+ inward current or increasing calcium outflow, resulting in reduced peristaltic movement and intestinal secretions due to the induction of muscle relaxation [54]. Pre-treatment with 80 % methanol extract significantly reduced peristaltic movements, as evidenced by the decrease in the distance travelled by a charcoal meal in the GIT, showing that these crude extracts could have anti-motility activity due to their flavonoid and tannin constituents.
Similar to the castor oil-induced and enteropooling diarrhoeal models, the maximum effect was observed with the highest dose of the extract rather than the standard drug in the charcoal meal test. This may be due to the different secondary metabolites in the extract, which may prolong the time for the absorption of water and electrolytes by hampering the peristaltic movement of the intestine.
Clinically, diarrhoea may result from disturbed bowel function, impaired intestinal absorption, excessive intestinal secretion of water and electrolytes, and rapid bowel transit [51]. In vivo, the ADI is a measure of the combined effects of different components of diarrhoea, including purging frequency, onset of diarrhoeal stools, and frequency of intestinal movement. In addition, a higher ADI value is a measure of the effectiveness of an extract in curing diarrhoea. The ADI value increased with dose, suggesting the dose dependency of this parameter. The highest selected dose of the extract, with the highest ADI value, was associated with the best antidiarrheal activity when compared with the other selected doses, as indicated in the above results.
As limitation, this study didn't include effect of the plant extracts on liver histopathology of mice. Moreover, this study didn't isolate and identify the active phytochemicals responsible for hepatoprotective and antidiarrheal activities of the plant.
5. Conclusion
The results of this study revealed that the hydroalcoholic leaf and fruit extracts of S. molle have significant hepatoprotective and antidiarrheal activities. The hepatoprotective and antidiarrheal activities of the extracts may be attributed to the presence of phytochemicals, including glycosides, flavonoids, triterpenes, phenols, tannins, alkaloids, saponins, and phytosterols, which act individually or collectively. These findings provide scientific support for the traditional use of S. molle leaves and fruits as a remedy for hepatic and diarrhoeal diseases.
Ethics approval and consent to participate
The experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals [56], and the study was approved by the Ethical Review Committee of the College of Medicine and Health Sciences, Wollo University (reference number: WU/1137/05/12).
Consent to publish
Not applicable.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
Funding
This study was funded by Wollo University. The funder provided the drugs, chemicals, and laboratory animals required for the study. Additionally the funder provided financial support to cover the per diem costs of the investigators.
CRediT authorship contribution statement
Yaschilal Muche Belayneh: Writing – review & editing, Writing – original draft, Supervision, Methodology, Data curation, Conceptualization. Getnet Mengistu: Writing – original draft, Visualization, Validation, Software, Formal analysis, Data curation. Kidan Hailay: Writing – original draft, Validation, Formal analysis, Data curation.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgement
We thank Wollo University for funding this study.
Contributor Information
Yaschilal Muche Belayneh, Email: yaschilal.muche19@gmail.com.
Getnet Mengistu, Email: mgetnet12@gmail.com.
Kidan Hailay, Email: kid403813@gmail.com.
Abbreviations
- ADI
Anti-diarrheal Index
- AFF
Aqueous fruit fraction
- ANOVA
Analysis Of Variance
- BFF
n-Butanol fruit fraction
- CFF
Chloroform fruit fraction
- DW
Distilled water
- GIT
Gastro-Intestinal Tract
- MEF
Methanol fruit extract
- MEL
Methanol Leaf extract
- WFC
wet faeces in the Control group
- WFT
wet faeces in the Test group
- 80 ME
80 % methanol extract
References
- 1.Hanafy A., Aldawsari H.M., Badr J.M., Ibrahim A.K., Abdel-Hady S.E.-S. Evaluation of hepatoprotective activity of Adansonia digitata extract on acetaminophen-induced hepatotoxicity in rats. Evid base Compl Alternative Med. 2016;2016 doi: 10.1155/2016/4579149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rathi M.A., Meenakshi P., Gopalakrishnan V.K. Hepatoprotective activity of ethanolic extract of Alysicarpus vaginalis against nitrobenzene-induced hepatic damage in rats. South Indian Journal of Biological Sciences. 2015;1(2):60–65. [Google Scholar]
- 3.Abdel-Ghany R.H., Barakat W.M., Shahat A.A., Abd-Allah W.E.-S., Ali E.A. In vitro and in vivo hepatoprotective activity of extracts of aerial parts of Bidens pilosa L (Asteraceae) Trop J Pharmaceut Res. 2016;15(11):2371–2381. [Google Scholar]
- 4.El-Baz F.K., Salama Z.A., Gaafar A.A. Evaluation of hepatoprotective effect of broccoli extract against ccl^ sub 4^ in rats. Int J Med Biol Front. 2012;18(7):521. [Google Scholar]
- 5.Sintayehu B., Bucar F., Veeresham C., Asres K. Hepatoprotective and free radical scavenging activities of extracts and a major compound isolated from the leaves of Cineraria abyssinica Sch. Bip. exA. Rich. Pharmacognosy Journal. 2012;4(29) [Google Scholar]
- 6.Rao A.L., Aminabee S., Eswariah M.C. 2017. Evaluation of hepatoprotective activity of Indigofera barberi in Rats against Paracetamol induced hepatic injury. [Google Scholar]
- 7.Umer S., Asres K., Veeresham C. Hepatoprotective activities of two Ethiopian medicinal plants. Pharmaceut Biol. 2010;48(4):461–468. doi: 10.3109/13880200903173593. [DOI] [PubMed] [Google Scholar]
- 8.Mudie K., Seifu D., Challa F., Abebe A., Debella A., Gebregzabher A. Hepatoprotective activity of aqueous seed extract of Nigella sativa against highly active antiretroviral therapy induced hepatotoxicity in rats. Pharmacol Online. 2014;3:11–21. [Google Scholar]
- 9.Saleem M., Irshad I., Baig M.K., Naseer F. Evaluation of hepatoprotective effect of chloroform and methanol extracts of Opuntia monacantha in paracetamol-induced hepatotoxicity in rabbits. Bangladesh J Pharmacol. 2015;10:16–20. [Google Scholar]
- 10.Singab A.N.B., Youssef D.T., Noaman E., Kotb S. Hepatoprotective effect of flavonol glycosides rich fraction from egyptianVicia calcarata desf. Against CCI 4-induced liver damage in rats. Arch Pharm Res (Seoul) 2005;28(7):791–798. doi: 10.1007/BF02977344. [DOI] [PubMed] [Google Scholar]
- 11.Rajopadhye A., Upadhye A.S. Evidence-based Complementary and alternative medicine. 2016. 2016. Estimation of bioactive compound, maslinic acid by HPTLC, and evaluation of hepatoprotective activity on fruit pulp of Ziziphus jujuba Mill. cultivars in India. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poorrostami A., Farokhi F., Heidari R. Effect of hydroalcoholic extract of ginger on the liver of epileptic female rats treated with lamotrigine. Avicenna journal of phytomedicine. 2014;4(4):276. [PMC free article] [PubMed] [Google Scholar]
- 13.Salem M.Z., Olivares-Pérez J., Salem A. Studies on biological activities and phytochemicals composition of Hibiscus species-A review. Life Sci J. 2014;11(5):1–8. [Google Scholar]
- 14.Jothy S.L., Aziz A., Chen Y., Sasidharan S. Antioxidant activity and hepatoprotective potential of Polyalthia longifolia and Cassia spectabilis leaves against paracetamol-induced liver injury. Evid base Compl Alternative Med. 2012;2012 doi: 10.1155/2012/561284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nadro M., Onoagbe I. Protective effects of aqueous and ethanolic extracts of the leaf of Cassia italica in CCl4–induced liver damage in rats. Amer J Res Comm. 2014;2:122–130. [Google Scholar]
- 16.Ramadan S.I., Shalaby M., Afifi N., El-Banna H. Hepatoprotective and antioxidant effects of Silybum marianum plant in rats. Int J Agro Vet Med Sci (IJAVMS) 2011;5(6):541–547. [Google Scholar]
- 17.Vargas-Mendoza N., Madrigal-Santillán E., Morales-González Á, Esquivel-Soto J., Esquivel-Chirino C., y González-Rubio M.G.-L., et al. Hepatoprotective effect of silymarin. World J Hepatol. 2014;6(3):144. doi: 10.4254/wjh.v6.i3.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freitag A.F., Cardia G.F.E., da Rocha B.A., Aguiar R.P., Silva-Comar FMdS., Spironello R.A., et al. Hepatoprotective effect of silymarin (Silybum marianum) on hepatotoxicity induced by acetaminophen in spontaneously hypertensive rats. Evid base Compl Alternative Med. 2015;2015 doi: 10.1155/2015/538317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sarin R., Bafna P. Herbal antidiarrhoeals: a review. Int J Res Pharmaceut Biomed Sci. 2012;3:637–649. [Google Scholar]
- 20.Palombo E.A. Phytochemicals from traditional medicinal plants used in the treatment of diarrhoea: modes of action and effects on intestinal function. Phytother Res: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives. 2006;20(9):717–724. doi: 10.1002/ptr.1907. [DOI] [PubMed] [Google Scholar]
- 21.Njume C., Goduka N.I. Treatment of diarrhoea in rural African communities: an overview of measures to maximise the medicinal potentials of indigenous plants. Int J Environ Res Publ Health. 2012;9(11):3911–3933. doi: 10.3390/ijerph9113911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Umer S, Tekewe A, Kebede N. Antidiarrhoeal and antimicrobial activity of Calpurnia aurea leaf extract. BMC Compl Alternative Med.13(1):21. [DOI] [PMC free article] [PubMed]
- 23.Ahmadua A., Zezi A., Yaro A. Anti-diarrheal activity of the leaf extracts of Daniellia oliveri hutch and Dalz (Fabaceae) and ficus sycomorus Miq (Moraceae) Afr J Tradit, Complementary Altern Med. 2007;4(4):524–528. [PMC free article] [PubMed] [Google Scholar]
- 24.Saralaya MG, Patel P, Roy MPSP, Patel AN. Research article antidiarrheal activity of methanolic extract of Moringa oleifera lam roots in experimental animal models. International Journal of Pharmaceutical Research.2(2) .
- 25.Teklay A., Abera B., Giday M. An ethnobotanical study of medicinal plants used in Kilte Awulaelo District, Tigray Region of Ethiopia. J Ethnobiol Ethnomed. 2013;9(1):65. doi: 10.1186/1746-4269-9-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woldeab B., Regassa R., Alemu T., Megersa M. Medicinal plants used for treatment of diarrhoeal related diseases in Ethiopia. Evid base Compl Alternative Med. 2018;2018 doi: 10.1155/2018/4630371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teklay A, Abera B, Giday M. An ethnobotanical study of medicinal plants used in Kilte Awulaelo District, Tigray Region of Ethiopia. J Ethnobiol Ethnomed.9(1):65. [DOI] [PMC free article] [PubMed]
- 28.Getaneh G., Zemede A., Abiyu E., Nagappan r. Ethnobotanical study of traditional medicinal plants and their conservation status in Mecha woreda, west Gojam zone of Ethiopa. Iternational journal of pharmaceuticals and health care research. 2014;2(3):8. [Google Scholar]
- 29.do Rosário Martins M, Arantes S, Candeias Ft, Tinoco MT, Cruz-Morais Jl. Antioxidant, antimicrobial and toxicological properties of Schinus molle L. essential oils. J Ethnopharmacol.151(1):485-492. [DOI] [PubMed]
- 30.Dãaz C., Quesada S., Brenes O., Aguilar G., Cicció J.F. Chemical composition of Schinus molle essential oil and its cytotoxic activity on tumour cell lines. Nat Prod Res. 2008;22(17):1521–1534. doi: 10.1080/14786410701848154. [DOI] [PubMed] [Google Scholar]
- 31.Yueqin Z., Recio M.C., Mã¡Ã±ez S., Giner R.M., Cerdá-Nicolás M., Rãos J.-L. Isolation of two triterpenoids and a biflavanone with anti-inflammatory activity from Schinus molle fruits. Planta Med. 2003;69(10):893–898. doi: 10.1055/s-2003-45096. [DOI] [PubMed] [Google Scholar]
- 32.Bendaoud H, Romdhane M, Souchard JP, Cazaux S, Bouajila J. Chemical composition and anticancer and antioxidant activities of Schinus molle L. and Schinus terebinthifolius Raddi berries essential oils. Journal of food Science.75(6):C466-C472. [DOI] [PubMed]
- 33.Adewusi E., Afolayan A.J. A review of natural products with hepatoprotective activity. J Med Plants Res. 2010;4(13):1318–1334. [Google Scholar]
- 34.Kumar C.H., Ramesh A., Kumar J.S., Ishaq B.M. A review on hepatoprotective activity of medicinal plants. Int J Pharmaceut Sci Res. 2017;2(3):501. [Google Scholar]
- 35.Guideline O.O. 425: acute oral toxicity—up-and-down procedure. OECD Guidel Test Chem. 2001;2:12–16. [Google Scholar]
- 36.Hossain M., AL-Raqmi K., AL-Mijizy Z., Weli A., Al-Riyami Q., Mohammad A., et al. Study of total phenol, flavonoids contents and phytochemical screening of various leaves crude extracts of locally grown Thymus vulgaris. Asian Pac J Trop Biomed. 2013;3(9):705–710. doi: 10.1016/S2221-1691(13)60142-2. 10.1016. S2221-1691 (13).60142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Esatu H., Alemayehu I., Haile E., Tadesse S., Mammo F., Dekebo A., et al. Phenolic glycosides from roots of Clerodendrum myricoides. American Journal of Essential Oils and Natural Products. 2015;2(5):1–6. [Google Scholar]
- 38.Javed S., Ahsan W., Kohli K. Pharmacological influences of natural products as bioenhancers of silymarin against carbon tetrachloride-induced hepatotoxicity in rats. Clinical Phytoscience. 2018;4(1):18. [Google Scholar]
- 39.Igboeli N., Onyeto C.A., Okorie A.N., Mbaoji F.N., Nwabunike I.A., Alagboso D.I. Antidiarrheal activity of methanol leaf extract of lophira lanceolata tiegh (ochnaeceae) Merit Res J Environ Sci Toxicol. 2015;3(4):59–64. [Google Scholar]
- 40.Robert A., Nezamis J., Lancaster C., Hanchar A., Klepper M. Enteropooling assay: a test for diarrhea produced by prostaglandins. Prostaglandins. 1976;11(5):809–828. doi: 10.1016/0090-6980(76)90189-1. [DOI] [PubMed] [Google Scholar]
- 41.Than A., Kulkarni H.J., Hmone W., Tha S. Anti-diarrhoeal efficacy of some Burmese indigenous drug formulations in experimental diarrhoeal test models. Int J Crude Drug Res. 1989;27(4):195–200. [Google Scholar]
- 42.Vogel H.G., Vogel W.H. Springer Science & Business Media; 2013. Drug discovery and evaluation: pharmacological assays. [Google Scholar]
- 43.Council N.R. National Academies Press; 2010. Guide for the care and use of laboratory animals. [Google Scholar]
- 44.Saleem T.M., Chetty C.M., Ramkanth S., Rajan V., Kumar K.M., Gauthaman K. Hepatoprotective herbs–a review. International Journal of Research in Pharmaceutical Sciences. 2010;1(1):1–5. [Google Scholar]
- 45.Chen C.-J., Deng A.-J., Liu C., Shi R., Qin H.-L., Wang A.-P. Hepatoprotective activity of Cichorium endivia L. extract and its chemical constituents. Molecules. 2011;16(11):9049–9066. doi: 10.3390/molecules16119049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Asrih M., Jornayvaz F.R. Inflammation as a potential link between nonalcoholic fatty liver disease and insulin resistance. J Endocrinol. 2013;218(3):R25–R36. doi: 10.1530/JOE-13-0201. [DOI] [PubMed] [Google Scholar]
- 47.Wong S.K., Chin K.-Y., Ahmad F., Ima-Nirwana S. Regulation of inflammatory response and oxidative stress by tocotrienol in a rat model of non-alcoholic fatty liver disease. J Funct Foods. 2020;74 [Google Scholar]
- 48.Meharie B.G., Amare G.G., Belayneh Y.M. Evaluation of hepatoprotective activity of the crude extract and solvent fractions of clutia abyssinica (euphorbiaceae) leaf against CCl4-induced hepatotoxicity in mice. J Exp Pharmacol. 2020:137–150. doi: 10.2147/JEP.S248677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sisay M., Engidawork E., Shibeshi W. Evaluation of the antidiarrheal activity of the leaf extracts of Myrtus communis Linn (Myrtaceae) in mice model. BMC Compl Alternative Med. 2017;17(1):1–11. doi: 10.1186/s12906-017-1625-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mengistu G., Engidawork E., Nedi T. Evaluation of the antidiarrhoeal activity of 80% methanol extract and solvent fractions of the leaves of Lantana camara linn (Verbenaceae) in mice. Ethio Pharm J. 2015;31:107–121. [Google Scholar]
- 51.Tadesse E., Engidawork E., Nedi T., Mengistu G. Evaluation of the anti-diarrheal activity of the aqueous stem extract of Lantana camara Linn (Verbenaceae) in mice. BMC Compl Alternative Med. 2017;17(1):1–8. doi: 10.1186/s12906-017-1696-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mekonnen B., Asrie A.B., Wubneh Z.B. Antidiarrheal activity of 80% methanolic leaf extract of Justicia schimperiana. Evid base Compl Alternative Med. 2018;2018 doi: 10.1155/2018/3037120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zayede D., Mulaw T., Kahaliw W. Antidiarrheal activity of hydromethanolic root extract and solvent fractions of Clutia abyssinica jaub. & spach. (Euphorbiaceae) in mice. Evid base Compl Alternative Med. 2020;2020 doi: 10.1155/2020/5416749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gudeta B.M., Taye G.M., Abula T., Gadisa D.A. Evaluation of anti-diarrheal activity of 80% methanol extracts of vernonia amygdalina delile (asteraceae) leaves in mice. J Exp Pharmacol. 2020;12:455. doi: 10.2147/JEP.S282669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Degu A., Kefale B., Alemayehu D., Tegegne G.T. Evaluation of the antidiarrheal activity of hydromethanol crude extracts of Ruta chalepensis and Vernonia amygdalina in mice. Evid base Compl Alternative Med. 2020:2020. doi: 10.1155/2020/8318713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clark J., Baldwin R., Bayne K., Brown M., Gebhart G., Gonder J., et al. vol. 125. Institute of Laboratory Animal Resources, National Research Council; Washington, DC: 1996. (Guide for the care and use of laboratory animals). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article and its supplementary information files.