Abstract
Diabetes mellitus (DM) is one of the leading worldwide public health problems. It is characterized by hyperglycemia which induces oxidative stress and inflammation, both involved in the pathogenesis of diabetes. We previously showed that Boswellia dalzielii (BD) and Hibiscus sabdariffa (HS) extracts reduced hyperglycemia and hyperlipidemia in alloxan-induced diabetic rats. In the present study, we evaluated the antioxidant and anti-inflammatory activities of both plants in alloxan-induced diabetic rats. Two sets of experiments were conducted in male Wistar rats subjected to a single intraperitoneal injection of alloxan monohydrate (150 mg/kg, b. w.). Then, diabetic rats were daily administered with either BD (1st set of experiments) or HS (2nd set of experiments) at 100, 200, and 400 mg/kg orally for 21 consecutive days. Glibenclamide (10 mg/kg) was also administered as a reference drug. At the end of the study, the animals were anesthetized, and blood samples were collected from each animal. Then, oxidative stress and inflammatory biomarkers in the serum were determined. We found that treatment with BD and HS significantly reduced malondialdehyde (MDA) and enhanced the levels of reduced glutathione (GSH), superoxide dismutase (SOD), and catalase (CAT). These extracts also significantly decreased the inflammatory markers tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and interleukin-1 beta (IL-1β). From the results obtained, it can therefore be concluded that BD and HS have the potential to being developed as natural sources of antioxidant and anti-inflammatory agents that can be used for the prevention or treatment of DM.
Keywords: Diabetes mellitus, Alloxan, Boswellia dalzielii, Hibiscus sabdariffa, Antioxidant, Anti-inflammatory
Abbreviations
- ANOVA
ANOVA Analysis of variance
- BD
Boswellia dalzielii
- CAT
Catalase
- CV
Coefficient of variation
- DM
Diabetes mellitus
- DTNB
Dithionitrobenzoic acid
- ELISA
Enzyme-linked Immunosorbent Assay
- GSH
Reduced Glutathione
- HRP
Horseradish Peroxidase Conjugate
- HS
Hibiscus sabdariffa
- IDF
International Diabetes Federation
- IL-1β
Interleukin-1 beta
- IL-6
Interleukin-6
- MAPK
Mitogen-activated protein kinases
- MDA
Malondialdehyde
- NFκB
Nuclear factor-kappa B
- Nrf2
Nuclear factor erythroid 2–related factor 2
- ROS
Reactive oxygen species
- SEM
Standard error of the mean
- SOD
Superoxide dismutase
- TBARS
Thiobarbituric acid reactive substance
- TNF-α
Tumor necrosis factor-alpha
1. Introduction
Diabetes mellitus (DM) is a chronic metabolic disorder characterized by elevated levels of glucose in the blood (hyperglycemia) resulting from the inability to produce insulin, or an insufficient response to insulin, or both [1,2]. Diabetes is one of the most common diseases, and it is the leading cause of mortality and morbidity worldwide [3]. According to the International Diabetes Federation (IDF), diabetes affects approximately 537 million persons (20–79 years), and this number is expected to increase to 643 million by 2030 and 783 million by 2045 [4]. In Africa, about 1.24 million adults are living with diabetes, and this number is predicted to increase by 29%–55 million by 2045 (IDF, 2021).
Hyperglycemia is frequently associated with oxidative stress, which plays an important role in the development and progression of diabetes [5]. Oxidative stress is defined as an imbalance between the formation of reactive oxygen species (ROS) and their insufficient degradation by the endogenous antioxidant defense system, which includes superoxide dismutase (SOD), catalase (CAT), and reduced glutathione (GSH) [6]. Under hyperglycemic conditions, ROS overproduction leads to an increase in malondialdehyde (MDA), a highly reactive compound produce by lipid peroxidation used as a biomarker to assess lipid peroxidation of biological oxidative stress [7], as well as a decrease in cellular antioxidants, impairing insulin action and secretion [8].
Hyperglycemia also leads to the activation of nuclear factor-kappa B (NFκB), a pivotal mediator of inflammatory responses that plays an important role in the pathogenesis of diabetes complications [9]. The overproduction of ROS and deregulation of antioxidants SOD, CAT, and GSH by oxidative stress under hyperglycemic conditions promote endothelial dysfunction by damaging DNA, proteins, cell membranes, and plasma lipids, thereby leading to the activation of inflammatory mediators NF-κB [10,11]. The activation of NFκB induces the production of pro-inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), and interleukin-6 (IL-6), and other pro-inflammatory proteins [11]. An increase in the level of these pro-inflammatory cytokines has been observed in both pre-diabetic and diabetic patients [12]. Based on the deleterious role of oxidative stress and inflammation during diabetes, the development of antioxidant and anti-inflammatory strategies is of high interest.
Recently, Boswellia dalzielii (B. dalzielii) and Hibiscus sabdariffa (H. sabdariffa), two medicinal plants widely used in the Cameroonian pharmacopeia for the treatment of various diseases, were shown to reduce fasting blood glucose level, improve glucose tolerance and normalize lipid profiles in alloxan-induced diabetic rats [13,14]. B. dalzielii has been widely reported as anti-microbial, anti-inflammatory, anti-arthritic, anti-convulsant, and against gastrointestinal disorders [[15], [16], [17]], whereas H. sabdariffa has been shown to be anti-hypertensive, hepatoprotective, anti-hyperlipidemic, antioxidant, anti-cancer, anti-inflammatory, anti-microbial, and anti-diabetic properties [18,19]. Antioxidant and anti-inflammatory activities have been proven in bioactive compounds in medicinal plants used to treat diabetes [20]. Previous studies revealed the presence of triterpenes, flavonoids, phenols, tannins, and saponins in various parts of B. dalzielii [13,21], while triterpenes, flavonoids, phenolic compounds, polysaccharides, organic acids, vitamins, and tannins are the major compounds found in the calyces of H. sabdariffa [14,18].
Streptozotocin is commonly used in diabetes research to assess the antioxidant and anti-inflammatory properties of medicinal plants. However, the use of alloxan to assess oxidative stress and inflammation in rats is still poorly understood. The current work sought to assess the antioxidant and anti-inflammatory properties of B. dalzielii stem bark and H. sabdariffa calyces extract in alloxan-induced diabetic rats.
2. Material and methods
2.1. Chemicals and reagents
Alloxan monohydrate and Glibenclamide were purchased from MilliporeSigma (Oakville, ON, Canada). Alloxan was used to induce diabetes in rats, and glibenclamide was used as a standard hypoglycemic drug. Oxidative stress assay kits (MDA, SOD, CAT, and GSH) and inflammatory marker assay kits (TNF-α, IL-6, and IL-1β) were purchased from Elabscience (Houston, TX, USA).
2.2. Preparation of extracts
B. dalzielii and H. sabdariffa extracts were prepared using the methods previously reported [13,14]. In brief, powders of B. dalzielii stem bark or H. sabdariffa calyces were soaked in a mixture solution (70:30) of ethanol/distilled water for 24 h with periodic shaking and stirring. The mixture was then filtered with Whatman paper N° 1 and concentrated using a rotatory evaporator at 40 °C. The aqueous residue was lyophilized and kept in a refrigerator at 4 °C as a powder of B. dalzielii hydroalcoholic extract (BD) or H. sabdariffa hydroalcoholic extract (HS).
2.3. Animals
Healthy adult male Wistar rats (120–160 g), obtained from the animal facility of the Pharmacological Research Laboratory of Medicinal Plants, Department of Life Sciences, Higher Teacher Training College, University of Bertoua (UBe), were kept in polycarbonate cages under standard conditions of temperature (25 ± 2 °C), humidity (55–65%), and light (12-h light/dark cycle), with ad libitum access to food and water. They were acclimatized to experimental conditions for one week before the start of the study. Animal care and experimental procedures described throughout this study were carried out in accordance with the guidelines of the Cameroon National Ethical Committee (FW-IRB00001954).
2.4. Diabetes induction
Rats (except for the control group) were fasted overnight and administered a single intraperitoneal injection of freshly prepared alloxan (150 mg/kg) dissolved in 0.9% saline. Then, they were allowed free access to a solution of 5% glucose for 24 h to overcome the drug-induced hypoglycemia [13]. After 3 days, the fasting blood glucose levels in the rats were assessed to validate the successful induction of diabetes mellitus using a glucometer (Accu-Chek Aviva, Roche, Mannheim, Germany) and compatible blood glucose test strips by collecting blood from the tail vein. Only rats with fasting blood glucose levels greater than 250 mg/dl were considered diabetic and randomly assigned to different groups [14].
2.5. Study design
Two sets of experiments were performed in the present study. A total of 36 rats were randomly assigned into six groups (n = 6) in each experiment. In the first set of experiments, B. dalzielii was used as a treatment, and rats were subdivided as follows: Group 1: Normal rats (NOR) receiving distilled water, Group 2: Alloxan-induced diabetic rats (DIA) receiving distilled water, Groups 3, 4 and 5: Alloxan-induced diabetic rats treated with B. dalzielii at 100 (BD100), 200 (BD200), and 400 (BD400) mg/kg, respectively, Group 6: Alloxan-induced diabetic rats receiving 10 mg/kg of glibenclamide (GLI), a reference standard drug as a positive control. In the second set of experiments, we administered H. sabdariffa as a treatment in Groups 3, 4 and 5 at 100 (HS100), 200 (HS200), and 400 (HS400) mg/kg, respectively. The selection of extract doses (100, 200, and 400 mg/kg) used in this study was based on acute toxicity test results from previous studies [21]. Treatment administration started on the same day as the confirmation of diabetes and was performed via gavage once daily for 21 days. Administration of B. dalzielii and H. sabdariffa in alloxan-induced diabetic rat dose-dependently reduced the fasting blood glucose level and enhanced glucose tolerance. Moreover, B. dalzielii and H. sabdariffa normalized lipid profiles and improved body weight gain.
2.6. Blood sampling and serum analysis
After three weeks of experimentation (day 22), the rats were anesthetized by intraperitoneal administration of the ketamine/xylazine mixture (80/10 mg/kg). Then, blood samples were collected immediately by cardiac puncture and centrifuged at 5000 rpm for 15 min. The resulting serum was separated and used for biochemical analysis.
2.7. Oxidative stress biomarkers
Lipid peroxidation was determined using a MDA colorimetric assay kit (E-BC-K025-S, Elabscience, Houston, TX, USA) by measuring the thiobarbituric acid reactive substance (TBARS) MDA complex, according to the study of Ohkawa [22]. MDA forms a colored complex when reacting with thiobarbituric acid (TBA). The absorbance of the generated colored complex was measured at 532 nm. The intra-assay coefficient of variation (CV) was 3.7% and the inter-assay CV was 6.7%. SOD activity was measured using the SOD activity assay kit (E-BC-K022-S, Elabscience, Houston, TX, USA) according to the method of Giannopolitis and Ries [23]. The color reaction was measured at 550 nm, the intra-assay CV was 3.2% and the inter-assay CV was 7.7%. CAT activity was determined using a CAT activity assay kit (E-BC-K031-S, Elabscience, Houston, TX, USA) according to the method of Aebi [24] CAT activity was measured by the reaction of ammonium molybdate titration with the enzymatic decomposition of H2O2 to generate a yellowish complex at 405 nm. the intra-assay CV was 3.1% and the inter-assay CV was 4%. GSH was determined using the GSH colorimetric assay kit (E-BC-K030-S, Elabscience, Houston, TX, USA) which reacts with dithionitrobenzoic acid (DTNB) to produce thio-nitrobenzoic acid and glutathione disulfide according to the method described by Beutler et al. [25]. The absorbance of the generated colored complex was measured at 420 nm. The intra-assay CV was 2% and the inter-assay CV was 2.7%.
2.8. Inflammatory cytokines markers
Inflammatory factors TNF-α (E-EL-R2856), IL-1β (E-EL-R0012), and IL-6 (E-EL-R0015) were measured in the serum using a sandwich ELISA kit. Samples were incubated with a biotin conjugate solution, followed by streptavidin-HRP. After incubation, the absorbance was read spectrophotometrically at a wavelength of 450 nm. Both intra and inter-CV% are <10%.
2.9. Statistical analysis
The data obtained were presented as mean ± standard error of the mean (SEM) and analyzed using GraphPad Prism 10.1.0 (GraphPad Software LLC, Boston, MA, USA). Comparisons between groups were subjected to one-way analysis of variance (ANOVA), followed by Tukey's multiple comparisons test. Statistical significance was set to P < 0.05.
3. Results
3.1. Effects of B. dalzielii on oxidative stress markers
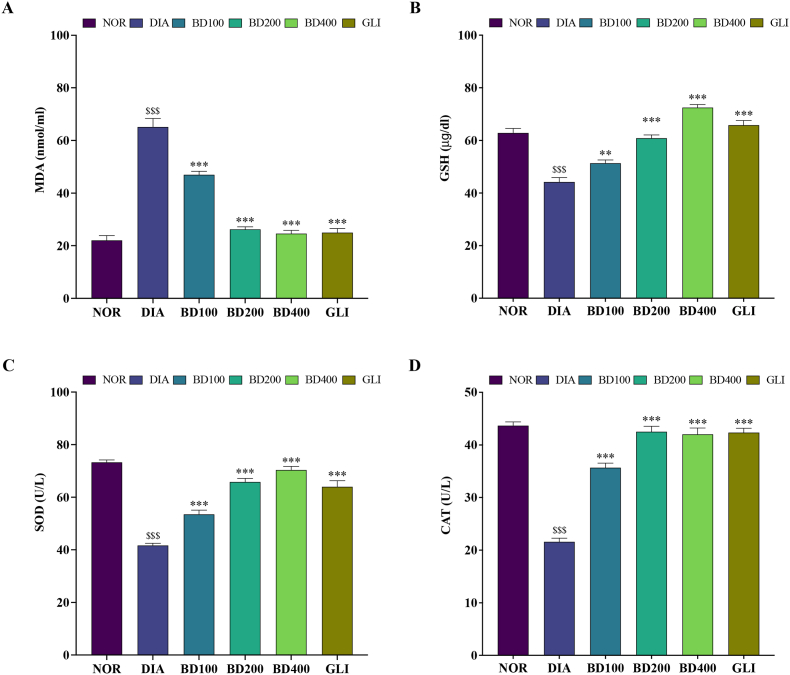
As shown in Fig. 1, all oxidative stress markers were affected by the alloxan injection. The diabetic group showed significantly higher levels of MDA (Fig. 1A) and lower levels of GSH, SOD, and CAT (Fig. 1B, C, and D, respectively) compared to the normal group (p < 0.001). Treatment with BD at 100, 200, and 400 mg/kg significantly reversed the levels of oxidative stress markers. Thus, administration of BD (100, 200, and 400 mg/kg) significantly decreased MDA levels [F (5, 30) = 91.3, P < 0.001; one-way ANOVA] while increasing GSH [F (5, 30) = 50, P < 0.001; one-way ANOVA], SOD [F (5, 30) = 63.3, P < 0.001; one-way ANOVA] and CAT [F (5, 30) = 85.2, P < 0.001; one-way ANOVA] levels.
Fig. 1.
Effects of B. dalzielii extract on antioxidant biomarkers in alloxan-induced diabetic rats. A: MDA, B: GSH, C: SOD and D: CAT. Data are expressed as mean ± SEM (n = 6); $$$: p < 0.001 as compared to normal; **: p < 0.01, ***: p < 0.001 as compared to diabetics. NOR = normal control, DIA = diabetic control, BD100 = extract of B. dalzielii at 100 mg/kg, BD200 = extract of B. dalzielii at 200 mg/kg, BD400 = extract of B. dalzielii at 400 mg/kg, GLI = glibenclamide.
3.2. Effects B. dalzielii extract on inflammatory markers
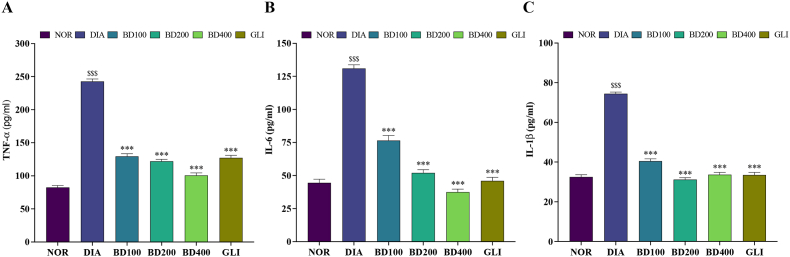
All inflammatory markers were impacted after alloxan injection in rats, as shown in Fig. 2. The diabetic group exhibited significantly greater serum levels of TNFα, IL-6, and IL-1β (Fig. 2A, B, and C, respectively) compared to the normal group P < 0.001). Administration of BD significantly lowered the levels of TNFα [F (5, 30) = 262, P < 0.001; one-way ANOVA], IL-6 [F (5, 30) = 157, P < 0.001; one-way ANOVA] and IL-1β [F (5, 30) = 244, P < 0.001; one-way ANOVA].
Fig. 2.
Effects of B. dalzielii on serum inflammatory markers in alloxan-induced diabetic rats. A: TNFα, B: IL-6 and C: IL-1β. Data are expressed as mean ± SEM (n = 6); $$$: p < 0.001 as compared to normal; ***p < 0.001 as compared to diabetics. NOR = normal control, DIA = diabetic control, BD100 = extract of B. dalzielii at 100 mg/kg, BD200 = extract of B. dalzielii at 200 mg/kg, BD400 = extract of B. dalzielii at 400 mg/kg, GLI = glibenclamide.
3.3. Effects of H. sabdariffa on oxidative stress markers
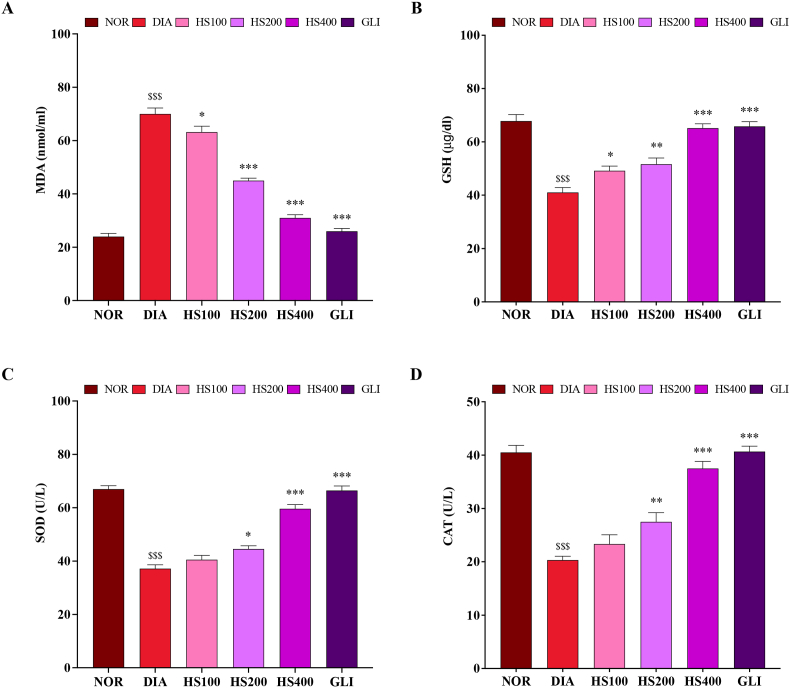
Oxidative stress was observed in rats following the injection of alloxan, as illustrated in Fig. 3. The diabetic group exhibited significantly higher levels of MDA (Fig. 3A) and lower levels of GSH, SOD, and CAT (Fig. 3B, C and D, respectively) compared to the normal group (p < 0.001). Treatment with HS at 100, 200, and 400 mg/kg significantly restored alloxan-induced oxidative stress. Thus, treatment with HS (100, 200, and 400 mg/kg) significantly decreased MDA levels [F (5, 30) = 157, P < 0.001; one-way ANOVA] while increasing the levels of GSH [F (5, 30) = 31.8, P < 0.001; one-way ANOVA], SOD [F (5, 30) = 82.6, P < 0.001; one-way ANOVA] and CAT [F (5, 30) = 43.9, P < 0.001; one-way ANOVA].
Fig. 3.
Effects of H. sabdariffa extract on antioxidant biomarkers in alloxan-induced diabetic rats. A: MDA, B: GSH, C: SOD and D: CAT. Data are expressed as mean ± SEM (n = 6); $$$: p < 0.001 as compared to normal; *: p < 0.05, **: p < 0.01, ***: p < 0.001 as compared to diabetics. NOR = normal control, DIA = diabetic control, HS100 = extract of H. sabdariffa at 100 mg/kg, HS200 = extract of H. sabdariffa at 200 mg/kg, HS400 = extract of H. sabdariffa at 400 mg/kg, GLI = glibenclamide.
3.4. Effects H. sabdariffa extract on inflammatory markers
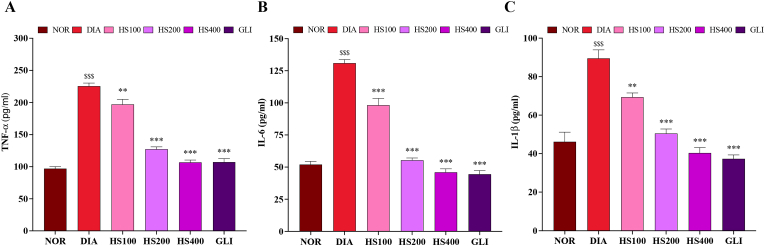
Fig. 4 shows inflammatory responses in rats following injection of alloxan. Diabetic group showed significantly higher serum levels of TNFα, IL-6, and IL-1β (Fig. 4A, B, and C, respectively) compared to the normal group P < 0.001). Treatment with HS significantly lowered levels of TNFα [F (5, 30) = 121, P < 0.001; one-way ANOVA], IL-6 [F (5, 30) = 127, P < 0.001; one-way ANOVA] and IL-1β [F (5, 30) = 36.8, P < 0.001; one-way ANOVA].
Fig. 4.
Effects of H. sabdariffa on serum inflammatory markers in alloxan-induced diabetic rats. A: TNFα, B: IL-6 and C: IL-1β. Data are expressed as mean ± SEM (n = 6); $$$: p < 0.001 as compared to normal; **p < 0.01 as compared to diabetics, ***p < 0.001 as compared to diabetics. NOR = normal control, DIA = diabetic control, HS100 = extract of H. sabdariffa at 100 mg/kg, HS200 = extract of H. sabdariffa at 200 mg/kg, HS400 = extract of H. sabdariffa at 400 mg/kg, GLI = glibenclamide.
4. Discussion
Herbal medicines are used to treat diabetes since they are less toxic and have fewer side effects than pharmacological or manufactured medications. In the current study, we assessed the antioxidant and anti-inflammatory properties of B. dalzielii and H. sabdariffa, two medicinal plants often utilized in Cameroon's pharmacopeia. The toxicity investigations of these plants indicated that they are safe at a level of 2000 mg/kg after oral administration [17,26]. Our previous studies demonstrated that the hydroethanolic extracts of B. dalzielii and H. sabdariffa possessed hypoglycemic and hypolipidemic properties in alloxan-induced diabetic rats [13,14], notably through reducing blood glucose, improving glucose tolerance, and normalizing lipid profiles. These plants and their phytochemical compounds might act by several mechanisms, such as enhancing insulin secretion by stimulating the β-cell of the Langerhans islets or regenerating the insulin-producing pancreatic β-cell population in the islets of Langerhans [27]. We evaluated the antioxidant and anti-inflammatory properties of B. dalzielii and H. sabdariffa in alloxan-induced diabetic rats. Alloxan monohydrate has been frequently used in recent years to induce stable hyperglycemia in diabetic animal models [28]. It has been proven that alloxan administration produced ROS by cyclic redox reactions of dialuric acid, which acts as a free radical generator and damages the pancreas, liver, and kidney [29]. Damage to the pancreas led to a reduction or loss of β-cell population in the islets of Langerhans, resulting hyperglycemia [30].
In our study, administration of alloxan monohydrate (150 mg/kg) in rats resulted in the development of oxidative stress as evidenced by a significant increase in MDA levels and a significant decrease in GSH, SOD, and CAT activities, indicating a decline in antioxidant enzyme defense mechanisms, which could be due to the chemical reduction of alloxan to dialuric acid, which induces oxidative damage through the generation of free radicals [30,31]. Our finding was consistent with studies conducted in experiments on animals using alloxan to induce diabetes [32,33] and in the clinic with diabetic patients [34]. Furthermore, multiple investigations have shown that overproduction of MDA and a decrease in the activity of the antioxidants SOD, CAT, and GSH play a significant role in the etiology of diabetes [35,36].
Administration of BD and HS for 21 days to diabetic rats significantly improved oxidative stress. Thus, both extracts significantly reduce MDA levels while significantly increasing GSH, CAT, and SOD levels, similar to glibenclamide, as compared to the diabetic group of rats. Glibenclamide has been found to possess antioxidant effects [37,38]. The ability of our extracts to reduce the imbalance between ROS generation and enzymatic antioxidant activity in diabetic rats may explain the improvement in oxidative stress indicators. These findings showed that both extracts protect diabetic rats from oxidative stress, which was consistent with several research examining the antioxidant activity of medicinal plants in animal models of diabetes produced by streptozotocin or alloxan [32,39,40]. H. sabdariffa has been shown to lower MDA levels in diabetic rats [41,42] and to increase the activity of antioxidant enzymes such as SOD, CAT, and GSH [43,44]. There have been very few investigations on B. dalzielii antioxidant properties. Experiments on streptozotocin-induced diabetic mice [45] and rats [15] revealed that treatment with B. dalzielii increased levels of SOD and CAT while decreasing MDA.
The pharmacological activities of plant extracts have been attributed to the presence of phytochemical compounds. Previous studies revealed the presence of triterpenes, flavonoids, phenols, tannins, and saponins in B. dalzielii extract [13], as well as triterpenes, flavonoids, phenols, and tannins in H. sabdariffa extract [14]. As a result, these bioactive compounds may be responsible for the antioxidant properties of BD or HS extracts. Phenolic and flavonoids compounds have been shown to possess antioxidant effects due to their high antioxidant capacity [46,47]. Saponins have been demonstrated to significantly reduce lipid peroxidation and enhance antioxidant enzyme levels [48]. Tannins and their derivatives are phenolic compounds considered to be important antioxidants or free radical scavengers [49].
Inflammation, like oxidative stress, plays an important role in DM as hyperglycemia is always linked with the release of pro-inflammatory cytokines [50,51]. Inflammation is known to be a protective response of the host against infections and tissue damages, which can prevent the spread of pathogens or promote tissue repair [52,53]. NF-κB is a transcriptional factor that regulates inflammation and cytokine production, including TNF-α, IL-6, and IL-1β [52]. In diabetes, the cytokines are responsible of mediating an autoimmune response by activating NF-κB which causes destruction in pancreatic β-cells.
As expected in the present study, the expression levels of the inflammatory cytokines of TNF-α, IL-6, and IL-1β in the serum of diabetic rats were significantly higher than in normal rats following injection of alloxan monohydrate in rats. This indicated that alloxan induced inflammation in rats due to an excess of ROS. Our findings supported previous studies indicating that chronic inflammation plays a significant role in the development of diabetes [9,50]. Treatment with BD and HS extracts for 21 days in the diabetic rats significantly reduced the levels of serum inflammatory markers TNF-α, IL-6, and IL-1β compared to diabetic rats. This effect is similar to that of glibenclamide, which has been proven to exhibit anti-inflammatory properties [54,55]. Our findings were also consistent with previous studies on the inflammatory benefits of B. dalzielii [15,45] and H. sabdariffa [43,53] in animal models of diabetes. These anti-inflammatory effects could be attributed to the presence of phytochemical compounds in both extracts, as demonstrated in our previous studies [13,14]. Triterpenoid has anti-inflammatory actions on macrophages through the NF-κB, Mitogen-activated protein kinases (MAPK) and nuclear factor erythroid 2–related factor 2 (Nrf2) signaling pathways [56]. It also decreases TNF-α and IL-6 production [57]. Flavonoids and phenol have been found to be major modulators of pro-inflammatory cytokines [58,59]. Saponins are effective at combating inflammation by directly targeting pro-inflammatory cytokines like TNF-α and IL-6 [60].
The current study, like many others that use animal models to examine diabetes, has few limitations. First, while our extracts demonstrated antioxidant and anti-inflammatory properties in alloxan-induced diabetic rats, a well-known diabetogenic agent widely used to induce type 1 diabetes in animals, we did not conduct a study on diabetic rats induced by streptozotocin, another popular diabetogenic agent used for the induction of type 2 diabetes, to compare the effects of our extracts on the two types of diabetes. It may also be interesting to investigate another alternative model of diabetes in rodents, such as obesity (high-fat diet). Second, the current study used crude extracts and investigations to determine the mechanism of the extract remains unclear. As a result, more research is needed to isolate and identify the primary bioactive components found in these two plants that are responsible for antioxidant and anti-inflammatory properties. It is also necessary to determine the exact mechanism by which B. dalzielii and H. sabdariffa act as antioxidants and anti-inflammatory agents. Finally, we did not measure the insulin. However, these limitations do not affect the quality of the present study, and additional research and studies to better understand the antioxidant and anti-inflammatory properties will be explored.
5. Conclusions
This study showed that the alloxan administration resulted in the generation of ROS, leading to oxidative stress and inflammation. We also found that B. dalzielii and H. sabdariffa extracts significantly ameliorated lipid peroxidation and antioxidants by decreasing MDA levels and improving the enzymatic antioxidants GSH, CAT, and SOD. Moreover, both extracts significantly decrease the expression of pro-inflammatory cytokines TNF-α, IL-6, and IL-1β. These pharmacological properties could be due to the presence of active compounds such as phenols, flavonoids, tannins, and saponins in BD and HS. Therefore, both extracts have the potential to being developed as a natural source of antioxidant and anti-inflammatory that can be used for the prevention or even the treatment of DM.
CRediT authorship contribution statement
Adjia Hamadjida: Writing – review & editing, Writing – original draft, Supervision, Project administration, Investigation, Conceptualization. Rigobert Espoir Ayissi Mbomo: Writing – review & editing, Investigation. Stéphane Essono Minko: Writing – review & editing, Investigation. Fidèle Ntchapda: Writing – review & editing, Formal analysis. Jean Pierre Kilekoung Mingoas: Writing – review & editing, Supervision, Formal analysis. Nga Nnanga: Writing – review & editing, Formal analysis.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.American Diabetes Association (ADA) Standards of medical care in diabetes-2020. Diabetes Care. 2020;43:1–212. [Google Scholar]
- 2.Kerner W., Bruckel J. Definition, classification and diagnosis of diabetes mellitus. Exp Clin Endocrinol Diabetes. 2014;122:384–386. doi: 10.1055/s-0034-1366278. [DOI] [PubMed] [Google Scholar]
- 3.Aslan M., Deliorman Orhan D., Orhan N., Sezik E., Yesilada E. In vivo antidiabetic and antioxidant potential of Helichrysum plicatum ssp. plicatum capitulums in streptozotocin-induced-diabetic rats. J Ethnopharmacol. 2007;109:54–59. doi: 10.1016/j.jep.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 4.International I.D.F. tenth ed. 2021. Diabetes atlas. [Google Scholar]
- 5.Dos Santos J.M., Tewari S., Mendes R.H. The role of oxidative stress in the development of diabetes mellitus and its complications. J Diabetes Res. 2019;2019:1–3. doi: 10.1155/2019/4189813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matés J.M. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology. 2000;153:83–104. doi: 10.1016/s0300-483x(00)00306-1. [DOI] [PubMed] [Google Scholar]
- 7.Zakaria F.R., Prangdimurti E., Damanik R. The effect of roselle extract (Hibiscus sabdariffa linn.) on blood glucose level and total antioxidant level on diabetic rat induced by streptozotocin. IOSR J Pharm. 2014;4:8–16. [Google Scholar]
- 8.Singh A., Kukreti R., Saso L., Kukreti S. Mechanistic insight into oxidative stress-triggered signaling pathways and type 2 diabetes. Molecules. 2022;27:950. doi: 10.3390/molecules27030950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yulianti E., Sunarti, Wahyuningsih M.S.H. The effect of Kappaphycus alvarezii active fraction on oxidative stress and inflammation in streptozotocin and nicotinamide-induced diabetic rats. BMC Complementary Medicine and Therapies. 2022;22 doi: 10.1186/s12906-021-03496-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta S.C., Sundaram C., Reuter S., Aggarwal B.B. Inhibiting NF-κB activation by small molecules as a therapeutic strategy. Biochimica et Biophysica Acta (BBA) - Gene Regulatory Mechanisms. 2010;1799:775–787. doi: 10.1016/j.bbagrm.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suryavanshi S.V., Kulkarni Y.A. NF-Κβ: a potential target in the management of vascular complications of diabetes. Front Pharmacol. 2017;8 doi: 10.3389/fphar.2017.00798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Araújo L.S., Da Silva M.V., Da Silva C.A., Borges M.D.F., Palhares H.M.D.C., Rocha L.P., et al. Analysis of serum inflammatory mediators in type 2 diabetic patients and their influence on renal function. PLoS One. 2020;15 doi: 10.1371/journal.pone.0229765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamadjida A., Tchuisseu Tchiengang F.D., Metechie L.C., Ndji Otto G.L., Ndogo Eteme O., Kilekoung Mingoas J.P., et al. Hypoglycemic and hypolipidemic effects of hydroethanolic stem bark extract of Boswellia dalzielii in alloxan-induced diabetic rats. World J Pharm Pharmaceut Sci. 2023;12:1910–1929. [Google Scholar]
- 14.Hamadjida A., Laurilan Channelle M., Florey Dotrice Tchuisseu T., Gustave Lebeau Ndji O., Olivier Ndogo E., Nicolas Yanou N., et al. Antidiabetic potential of Hibiscus sabdariffa extract in alloxan-induced diabetic rats. GSC Biological and Pharmaceutical Sciences. 2023;23:193–203. [Google Scholar]
- 15.Mbiantcha M., Almas J., Atsamo A.D., Ateufack G., Shabana S.U., Bomba Tatsinkou D.F., et al. Anti-inflammatory and anti-arthritic effects of methanol extract of the stem bark of Boswellia dalzielii Hutch (Burseraceae) in rats. Inflammopharmacology. 2018;26:1383–1398. doi: 10.1007/s10787-018-0505-x. [DOI] [PubMed] [Google Scholar]
- 16.Nwinyi F.C., Binda L., Ajoku G.A., Aniagu S.O., Enwerem N.M., Orisadipe A., et al. Evaluation of the aqueous extract of Boswellia dalzielii stem bark for antimicrobial activities and gastrointestinal effects. Afr J Biotechnol. 2004;3:284–288. [Google Scholar]
- 17.Tahir A.T., Hauwau A., Aminu U.K., Suleiman Y., Katagum Y.M. The anticonvulsant potential of Boswellia dalzielii in mice – pilot study. Advances in Pharmacology and Pharmacy. 2023;11:239–244. [Google Scholar]
- 18.Da-Costa-Rocha I., Bonnlaender B., Sievers H., Pischel I., Heinrich M. Hibiscus sabdariffa L. - a phytochemical and pharmacological review. Food Chem. 2014;165:424–443. doi: 10.1016/j.foodchem.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Lans C.A. Ethnomedicines used in Trinidad and Tobago for urinary problems and diabetes mellitus. J Ethnobiol Ethnomed. 2006;2:45. doi: 10.1186/1746-4269-2-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abduh M.S., Saghir S.A.M., Al Hroob A.M., Bin-Ammar A., Al-Tarawni A.H., Murugaiyah V., et al. Averrhoa carambola leaves prevent dyslipidemia and oxidative stress in a rat model of poloxamer-407-induced acute hyperlipidemia. Front Pharmacol. 2023:14. doi: 10.3389/fphar.2023.1134812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ibrahim A.A., Abdussalami M.S., Appah J., Umar A.H., Muhammad A.U., Haruna S., et al. Evaluation of antihyperglycemic activity of aqueous stem bark extract of Boswellia dalzielii in alloxan-induced diabetic Wistar rats. Future Journal of Pharmaceutical Sciences. 2023;9 [Google Scholar]
- 22.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 23.Giannopolitis C.N., Ries S.K. Superoxide dismutases. Plant Physiol. 1977;59:309–314. doi: 10.1104/pp.59.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 25.Beutler E., Duron O., Kelly B.M. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;61:882–888. [PubMed] [Google Scholar]
- 26.Njinga N.S., Kola-Mustapha A.T., Quadri A.L., Atolani O., Ayanniyi R.O., Buhari M.O., et al. Toxicity assessment of sub-acute and sub-chronic oral administration and diuretic potential of aqueous extract of Hibiscus sabdariffa calyces. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e04853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Desta G.T., Ferede Y.A., Zewdu W.S., Adugna B.Y., Arega T., Alemu M.A. Validation of antidiabetic and antihyperlipidemic effects of 80% methanolic extract of the lonchocarpus laxiflorus leaves in streptozotocin-induced diabetic Swiss albino mice. Evid base Compl Alternative Med. 2022;2022:1–9. doi: 10.1155/2022/8411851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ndarubu A.T., Onukogu S.C., Suleiman A., Mustapha A., Osuigwe E.C., Dannana L.W., et al. Phytochemicals, hypoglycemic and hypolipidemic effects of methanol leaf extract of Hibiscus sabdariffa in alloxan induced diabetic rats. GSC Biological and Pharmaceutical Sciences. 2019;8:70–78. [Google Scholar]
- 29.Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 2008;51:216–226. doi: 10.1007/s00125-007-0886-7. [DOI] [PubMed] [Google Scholar]
- 30.Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res. 2001;50:536–546. [PubMed] [Google Scholar]
- 31.Ojo O.A., Osukoya O.A., Ekakitie L.I., Ajiboye B.O., Oyinloye B.E., Agboinghale P.E., et al. Gongronema latifolium leaf extract modulates hyperglycaemia, inhibits redox imbalance and inflammation in alloxan-induced diabetic nephropathy. J Diabetes Metab Disord. 2020;19:469–481. doi: 10.1007/s40200-020-00533-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mannir R.A., Kano A.M., Nuhu I.A., Salisu A., Imrana I., Malami M.A., et al. In vivo evaluation of antidiabetic effects of some polyherbal formulations in alloxan-induced diabetic wistar rats. Tropical Journal of Natural Product Research. 2022;6:818–825. [Google Scholar]
- 33.Fatima N., Anwar F., Saleem U., Khan A., Ahmad B., Shahzadi I., et al. Antidiabetic effects of Brugmansia aurea leaf extract by modulating the glucose levels, insulin resistance, and oxidative stress mechanism. Front Nutr. 2022;9 doi: 10.3389/fnut.2022.1005341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao L.Y., Lan Q.J., Huang Z.C., Ouyang L.J., Zeng F.H. Antidiabetic effect of a newly identified component of Opuntia dillenii polysaccharides. Phytomedicine. 2011;18:661–668. doi: 10.1016/j.phymed.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 35.Idris A.E., Seke Etet P.F., Saeed A.A., Farahna M., Satti G.M.H., AlShammari S.Z., et al. Evaluation of metabolic, antioxidant and anti-inflammatory effects of Garcinia kola on diabetic rats. Saudi J Biol Sci. 2020;27:3641–3646. doi: 10.1016/j.sjbs.2020.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rains J.L., Jain S.K. Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med. 2011;50:567–575. doi: 10.1016/j.freeradbiomed.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nunes P.R., Bueno Pereira T.O., Bertozzi Matheus M., Grandini N.A., Siqueira J.S., Correa C.R., et al. Glibenclamide increases nitric oxide levels and decreases oxidative stress in an in vitro model of preeclampsia. Antioxidants. 2022;11:1620. doi: 10.3390/antiox11081620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chukwunonso Obi B., Chinwuba Okoye T., Okpashi V.E., Nonye Igwe C., Olisah Alumanah E. Comparative study of the antioxidant effects of metformin, glibenclamide, and repaglinide in alloxan-induced diabetic rats. J Diabetes Res. 2016;2016:1–5. doi: 10.1155/2016/1635361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Balogun F.O., Ashafa A.O.T. Aqueous root extracts of Dicoma anomala (Sond.) extenuates postprandial hyperglycaemia in vitro and its modulation on the activities of carbohydrate-metabolizing enzymes in streptozotocin-induced diabetic Wistar rats. South Afr J Bot. 2017;112:102–111. [Google Scholar]
- 40.Ojo O.A., Oni A.I., Grant S., Amanze J., Ojo A.B., Taiwo O.A., et al. Antidiabetic activity of elephant grass (Cenchrus purpureus (Schumach.) Morrone) via activation of PI3K/AkT signaling pathway, oxidative stress inhibition, and apoptosis in wistar rats. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.845196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adeyemi D.O., Adewole O.S. Hibiscus sabdariffa renews pancreatic β-cells in experimental type 1 diabetic model rats. Morphologie. 2019;103:80–93. doi: 10.1016/j.morpho.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 42.Mahmoud A., Wahba N., Mahmoud M., Zakaria M. Hibiscus sabdariffa extract augments the renoprotective effect of lisinopril against streptozotocin-induced diabetic nephropathy in rats. Zagazig Journal of Pharmaceutical Sciences. 2016;25:47–67. [Google Scholar]
- 43.Janson B., Prasomthong J., Malakul W., Boonsong T., Tunsophon S. Hibiscus sabdariffa L. calyx extract prevents the adipogenesis of 3T3-L1 adipocytes, and obesity-related insulin resistance in high-fat diet-induced obese rats. Biomed Pharmacother. 2021;138 doi: 10.1016/j.biopha.2021.111438. [DOI] [PubMed] [Google Scholar]
- 44.Ajiboye T.O., Raji H.O., Adeleye A.O., Adigun N.S., Giwa O.B., Ojewuyi O.B., et al. Hibiscus sabdariffa calyx palliates insulin resistance, hyperglycemia, dyslipidemia and oxidative rout in fructose-induced metabolic syndrome rats. J Sci Food Agric. 2016;96:1522–1531. doi: 10.1002/jsfa.7254. [DOI] [PubMed] [Google Scholar]
- 45.Mbiantcha M., Khalid R., Atsamo D.A., Njoku I.S., Mehreen A., Ateufack G., et al. Anti-hypernociceptive effects of methanol extract of Boswellia dalzielii on STZ-induced diabetic neuropathic pain. Advances in Traditional Medicine. 2020;20:405–417. [Google Scholar]
- 46.Algonaiman R., Alharbi H.F., Barakat H. Antidiabetic and hypolipidemic efficiency of lactobacillus plantarum fermented oat (Avena sativa) extract in streptozotocin-induced diabetes in rats. Fermentation. 2022;8:267. doi: 10.3390/antiox11061122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hameed A., Akhtar N. Comparative chemical investigation and evaluation of antioxidant and tyrosinase inhibitory effects of Withania somnifera (L.) Dunal and Solanum nigrum (L.) berries. Acta Pharm. 2018;68:47–60. doi: 10.2478/acph-2018-0007. [DOI] [PubMed] [Google Scholar]
- 48.Nakitto A.M.S., Muyonga J.H., Byaruhanga Y.B., Wagner A.E. Solanum anguivi lam. Fruits: their potential effects on type 2 diabetes mellitus. Molecules. 2021;26:2044. doi: 10.3390/molecules26072044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ul Haq M.N., Shah G.M., Gul A., Foudah A.I., Alqarni M.H., Yusufoglu H.S., et al. Biogenic synthesis of silver nanoparticles using phagnalon niveum and its in vivo anti-diabetic effect against alloxan-induced diabetic wistar rats. Nanomaterials. 2022;12:830. doi: 10.3390/nano12050830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Naqvi F., Dastagir N., Jabeen A. Honey proteins regulate oxidative stress, inflammation and ameliorates hyperglycemia in streptozotocin induced diabetic rats. BMC Complementary Medicine and Therapies. 2023:23. doi: 10.1186/s12906-023-03837-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taïlé J., Bringart M., Planesse C., Patché J., Rondeau P., Veeren B., et al. Antioxidant polyphenols of antirhea borbonica medicinal plant and caffeic acid reduce cerebrovascular, inflammatory and metabolic disorders aggravated by high-fat diet-induced obesity in a mouse model of stroke. Antioxidants. 2022;11:858. doi: 10.3390/antiox11050858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun S.-C. The non-canonical NF-κB pathway in immunity and inflammation. Nat Rev Immunol. 2017;17:545–558. doi: 10.1038/nri.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patil S.B., Munoli S. Evaluation of anti - inflammatory activity of aqueous extract of leaves of hibiscus sabdariffa in albino rats. Int J Basic Clin Pharmacol. 2017;6:1155. [Google Scholar]
- 54.Zhang G., Lin X., Zhang S., Xiu H., Pan C., Cui W. A protective role of glibenclamide in inflammation-associated injury. Mediat Inflamm. 2017;2017:1–11. doi: 10.1155/2017/3578702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moureq, Amal Ahmed, Mohammed Hatem. Salim. In vivo assessment of combined effects of glibenclamide and losartan in diabetic rats. Med Princ Pract. 2019;28:178–185. doi: 10.1159/000496104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Han Y., Yuan C., Zhou X., Han Y., He Y., Ouyang J., et al. Anti-inflammatory activity of three triterpene from hippophae rhamnoides L. In lipopolysaccharide-stimulated RAW264.7 cells. Int J Mol Sci. 2021;22 doi: 10.3390/ijms222112009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Almeida P.D.O., Boleti A.P.D.A., Rüdiger A.L., Lourenço G.A., Da Veiga Junior V.F., Lima E.S. Anti-inflammatory activity of triterpenes isolated from protium paniculatum oil-resins. Evid base Compl Alternative Med. 2015;2015:1–10. doi: 10.1155/2015/293768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leyva-López N., Gutierrez-Grijalva E., Ambriz-Perez D., Heredia J. Flavonoids as cytokine modulators: a possible therapy for inflammation-related diseases. Int J Mol Sci. 2016;17:921. doi: 10.3390/ijms17060921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Perez-Ramirez I.F., Castano-Tostado E., Ramirez-de Leon J.A., Rocha-Guzman N.E., Reynoso-Camacho R. Effect of stevia and citric acid on the stability of phenolic compounds and in vitro antioxidant and antidiabetic capacity of a roselle (Hibiscus sabdariffa L.) beverage. Food Chem. 2015;172:885–892. doi: 10.1016/j.foodchem.2014.09.126. [DOI] [PubMed] [Google Scholar]
- 60.Passos F.R.S., Araújo-Filho H.G., Monteiro B.S., Shanmugam S., Araújo AAdS., Almeida JRGdS., et al. Anti-inflammatory and modulatory effects of steroidal saponins and sapogenins on cytokines: a review of pre-clinical research. Phytomedicine. 2022;96 doi: 10.1016/j.phymed.2021.153842. [DOI] [PubMed] [Google Scholar]