Abstract
Peritoneal dialysis (PD) fluid, which contains a high concentration of glucose, is involved in peritoneal damage after long-term use. The mechanisms through which glucose induces damage to the mesothelium have not been clearly elucidated. Although, endoplasmic reticulum (ER) stress response is associated with several diseases, the involvement of ER stress in peritoneal damage has not yet been demonstrated. Primary-cultured rat peritoneal mesothelial cells (RPMCs) and rat PD model were used to investigate the influence of glucose on the peritoneum. Cells treated with glucose were examined for cytotoxicity, induction of apoptosis, and activation of the ER stress pathway. Glucose treatment of RPMCs induced cell death at concentrations higher than 3%. Annexin V positive, that is a feature of apoptosis, occurred in dead cells. Treatment with glucose led to the activation of protein kinase R-like ER kinase (PERK) and eukaryotic translation initiation factor-2α (eIF-2α). Glucose also induced the expression and nuclear translocation of homologous protein C/EBP. Cell death was rescued by the integrated stress response inhibitor, ISRIB, which suppresses the integrated stress response pathway, including ER stress. Glucose in PD fluid induces PERK/eIF-2α-mediated ER stress in RPMCs, resulting in apoptosis. This cellular stress may cause peritoneal damage in patients receiving PD.
Keywords: ER stress, glucose, apoptosis, peritoneal dialysis, integrated stress response inhibitor
I. Introduction
Peritoneal dialysis (PD) is one of the most beneficial treatments for end-stage renal disease. Compared to hemodialysis, PD is a home-based therapy and has many advantages for the patient’s daily life. However, long-term PD has been reported to cause pathological changes in the peritoneum, including abrasion of mesothelial cells, hypertrophy of mesothelium with fibrosis, angiogenesis, and hyalinizing vasculopathy [40, 43].
Peritoneal mesothelial cells (PMCs) function as a dialysis barrier and regulate local defense mechanisms in the peritoneal cavity [15]. During PD therapy, continuous shedding and regeneration of PMCs occurs in response to the PD fluid. PD fluid contains non-physiological materials, such as high concentrations of glucose, lactate-buffered acidic solutions, and glucose degradation products. These materials are believed to be toxic to PMCs and are involved in dysfunction of the peritoneum as a dialysis membrane [1, 5, 29, 42]. In particular, the high concentration of glucose present in PD fluid functions as an osmotic agent causing many adverse effects on PMCs [8, 17]. These damaging effects cause a loss of peritoneal ultrafiltration capacity, and peritoneal disorders lead to cessation of PD therapy [10, 22]. Our previous data indicated that glucose inhibits the regeneration process in the damaged monolayer of PMCs [26]. However, the detailed mechanisms by which glucose induces peritoneal damage have not yet been determined.
The integrated stress response (ISR) is a biological function of cells allowing them to adapt to the environmental stress condition. Four kinases (protein kinase R (PKR), PKR-like endoplasmic reticulum kinase (PERK), heme-regulated inhibitor, and general control non-derepressible 2) detect different stresses as part of the ISR [3, 20]. Although the stress that activates each kinase differs, all kinases phosphorylate eukaryotic translation initiation factor 2α (eIF-2α). eIF-2α is a translation initiation factor and many stresses are eventually integrated into a single eIF-2α response [28]. Phosphorylated eIF-2α inhibits translation to avoid cellular stress. However, specific genes, such as activating transcription factor 4 (ATF4) and C/EBP homologous protein (CHOP), are selectively translated and respond to stress as transcription factors [9].
Endoplasmic reticulum (ER) stress is an ISR caused by over-pooling of unfolded or misfolded proteins in the rough ER. To avoid protein over-accumulation, protein synthesis is suppressed by the unfolded protein response, which is an ER-possessed protein quality control mechanism. This stress is detected by three transmembrane signaling proteins of the ER, namely PERK, inositol-requiring kinase 1, and ATF6. Stress from unfolded proteins stimulates kinase activity, leading to phosphorylation of eIF-2α. If the stress is sufficiently strong to activate ATF4, cells undergo apoptosis that is mediated by the induction of another transcription factor, CHOP [18, 19, 23]. Activated CHOP translocated into nucleus to modulate its function.
To prevent withdrawal from PD therapy, it is important to overcome the peritoneal damage caused by the content of PD fluid through understanding the mechanism. However, the effect of ER stress on PMCs treated with PD fluid has not yet been determined. Therefore, we designed the present study to identify the influence of glucose on rat PMCs (RPMCs) and to investigate the effects of a small molecule inhibitor of ISR (i.e., ISRIB) that inhibits the ISR after phosphorylation of eIF-2α, on mesothelial cell damage.
II. Materials and Methods
Materials
ISRIB was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Cell culture
RPMCs were obtained from 9-week-old Wistar rats (Kyudo, Japan) using a standard digestion method [11, 37, 39]. Cells were cultured in low glucose (0.1%) Dulbecco’s modified Eagle’s medium (Nacalai Tesque, Kyoto, Japan) containing 10% fetal bovine serum (Nichirei Bioscience, Tokyo, Japan), and maintained at 37°C in a humidified atmosphere of 5% CO2 and 95% air. Cells were identified as RPMCs by their cobblestone-like appearance and the expression of mesothelial markers such as HBME-1 and cytokeratin [37].
Measurement of cell viability by the WST-1 assay
Glucose-induced cytotoxicity was analyzed using the WST-1 assay (Roche, Basel, Switzerland). This assay detects the activity of living cells and the absorbance value reflects cell viability. RPMCs grown in 96-well plates were treated with various concentrations of ISRIB and 4% glucose (Nacalai Tesque) for 24 hr. Ten microliters of WST-1 solution were then added to the culture medium in each well, and the cells were incubated for 30 min at 37°C. The absorbance of each sample was measured at 405 nm using a microplate reader (Model 680; Bio-Rad, Hercules, CA, USA).
Flow cytometry analysis
Apoptotic cell death was evaluated using an Annexin V-FITC kit system for detection of apoptosis (Beckman Coulter, Marseille, France). RPMCs treated with various concentrations of glucose were collected and diluted in ice-cold binding buffer to 5 × 105 cells/ml. Cells were stained with 5 μl of 5 μg/ml Annexin V and 2.5 μl of 250 μg/ml propidium iodide (PI) to each 100 μl of cell suspension for 10 min in the dark. The samples were diluted 5-fold with binding buffer, and analyzed with Cell Analyzer EC800 and ec800 version 1.3.6 software (Sony Biotechnology, Tokyo, Japan).
SDS-PAGE and western blotting
RPMCs were serum-starved for 1 hr, then incubated with various concentrations of glucose or glycerol. The cells were washed twice with phosphate-buffered saline (PBS) and scraped into lysis buffer containing protease (cOmplete Mini, Roche) and protein phosphatase inhibitors (Sigma-Aldrich) in PBS. Twelve micrograms of each sample were separated by SDS-PAGE and transferred to Immobilon-P polyvinylidene difluoride membranes (EMD Millipore, Bedford, MA, USA). The membranes were incubated for 2 hr at 20–22°C in a 5% PhosphoBLOCKER solution (Cell Biolab, San Diego, CA, USA) in PBS containing 0.05% Tween-20 (PBS-Tween), then incubated for 16 hr at 4°C in PBS-Tween with specific antibodies. Anti-eIF-2α mouse monoclonal, anti-phospho (Ser-51)-eIF-2α rabbit monoclonal, and anti-CHOP mouse monoclonal antibodies were obtained from Cell Signaling Technology (Boston, MA, USA). Anti-PERK and anti-phospho (Thr981)-PERK rabbit polyclonal antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The anti-β-actin mouse monoclonal antibody was from Sigma-Aldrich. The membranes were washed four times in PBS-Tween for 30 min and incubated for 1 hr at 20–22°C in PBS-Tween containing horseradish peroxidase-conjugated anti-rabbit IgG or anti-mouse IgG secondary antibodies. After the membranes were washed, proteins that interacted with the antibodies were visualized using Amersham ECL Prime detection buffer (Cytiva, Logan, UT, USA). Immunopositive bands were scanned and their intensities quantified using ImageJ software (National Institute of Health, Bethesda, MD, USA).
Immunocytochemistry
For immunocytochemical studies, RPMCs were cultured on coverslips. After appropriate treatments, the coverslips were placed in 3.7% formalin in PBS for 20 min at 4°C and permeabilized in methanol for 20 min at −20°C. The coverslips were blocked with 4% bovine serum albumin (BSA) in PBS (BSA-PBS) for 20 min, then incubated for 45 min at 20–22°C with an anti-CHOP antibody diluted 1:50 in blocking solution. After three washes with PBS for 20 min, they were incubated for 45 min with Alexa Fluor 546-labeled goat anti-mouse IgG (Invitrogen, Carlsbad, CA, USA) diluted 1:150 in 4% BSA-PBS. After three washes with PBS, the coverslips were mounted with a 4',6-diamidino-2-phenylindole (DAPI)-containing mounting solution and analyzed using a fluorescence microscope (Axioskop 2 plus; Carl Zeiss, Göttingen, Germany).
Animal model for PD
A PD rat model was established previously [16]. Briefly, 8-week-old male Wistar rats were instilled intraperitoneally twice a day for 8 weeks with 20 ml of lactate-buffered glucose contained PD fluid (Dianeal® PD-4 4.25; Baxter Healthcare, Tokyo, Japan). The animals were anesthetized by intraperitoneal injection of a mixture of medetomidine, midazolam, and butorphanol, followed by dissection of intact peritoneal tissues for morphological analysis. The research protocol was approved by the Ethics Committee of the University of Occupational and Environmental Health and was carried out in accordance with the University of Occupational and Environmental Health Animal Experimentation Regulation (institutional review board approval number AE-20-010).
Immunohistochemistry
Samples were fixed for 16 hr at 4°C with 4% paraformaldehyde in 0.1 M sodium phosphate buffer (pH 7.4), washed with the same buffer, dehydrated in a graded series of ethanol concentrations followed by xylene, then embedded in paraffin wax (Histosec® pastilles, Merck KGaA, Darmstadt, Germany). Thin sections (5 μm) were cut using a microtome. For detection of eIF-2α, deparaffinized sections were immersed in 10 mM citrate buffer and microwaved for 20 min at 800 W (MicroMed T/T; Milestone, Sorisole, Italy). After antigen retrieval, the sections were blocked with 1% BSA-PBS for 1 hr at 20–22°C. These sections were then incubated for 16 hr at 4°C with anti-eIF-2α and anti-phospho-eIF-2α antibodies diluted 1:100 in 1% BSA-PBS. After three washes with PBS, the indirect immunoperoxidase method (Histofine Simple Stain Rat MAX-PO-Multi; Nichirei Bioscience) was applied to the sections. The sections were then treated for 7 min with a freshly prepared solution of 0.1 mg/ml 3, 3′-diaminobenzidine (DAB) in 50 mM Tris-HCl buffer (pH 7.6) containing 0.05% hydrogen peroxide to visualize eIF-2α and further stained with hematoxylin. The specificity of immunoreactivity was confirmed by replacing the primary antibodies with 1% BSA-PBS.
Statistical analysis
Data are expressed as means ± standard deviation. Statistical differences were assessed using the Dunnett’s test. Statistical significance was set at p < 0.05.
III. Results
Glucose induces cytotoxicity on RPMCs
Cytotoxicity was measured using the WST-1 cell viability assay to examine the influence of glucose on RPMCs. Figure 1 shows that treatment with up to 5% glucose induced cytotoxicity in RPMCs in a concentration-dependent manner. With 5% glucose treatment, cell viability was reduced to 48% of that in the control cultures. We measured the pH of the cell culture medium and found no significant differences between the various treatments (data not shown).
Fig. 1.
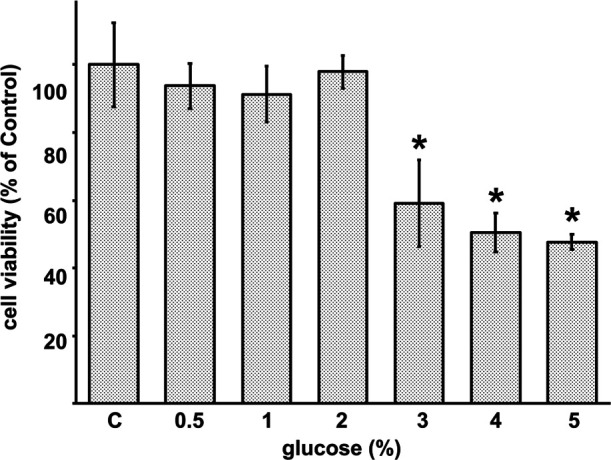
Influence of glucose on rat peritoneal mesothelial cell (RPMC) viability. RPMCs were treated with various concentrations of glucose for 24 hr and cell viability was determined using the WST-1 assay. Results are expressed as percentages of the control (means ± SD; n = 6). *Significantly different from the control; p < 0.05.
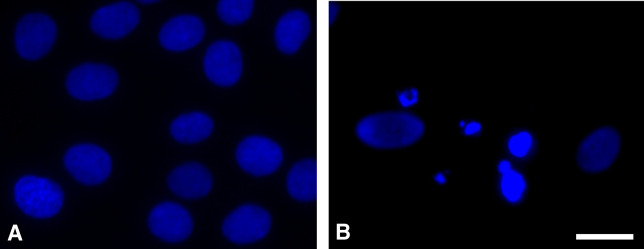
To determine whether glucose-induced cytotoxicity could be attributed to apoptosis, we examined RPMCs stained with DAPI after 24 hr of glucose treatment. RPMCs in the control culture had round nuclei with homogenous chromatin (Fig. 2A). In the culture treated with 4% glucose, chromatin condensation and micronuclei formation occurred (Fig. 2B). Cell size was evaluated using forward scattered light and PI staining analysis with flowcytometry (Fig. 3A). The number of cells with intact cell membranes, negative PI, and small FS values indicating small cell size increased from 45.2% in control to 77.5% in cells treated with glucose 4%.
Fig. 2.
Nuclear fragmentation revealed by 4',6-diamidino-2-phenylindole (DAPI) staining. Rat peritoneal mesothelial cells were left untreated (A) or treated with 4% glucose for 24 hr (B). The cells were then stained with DAPI. Glucose treatment resulted in nuclear fragmentation. Bar = 10 μm.
Fig. 3.
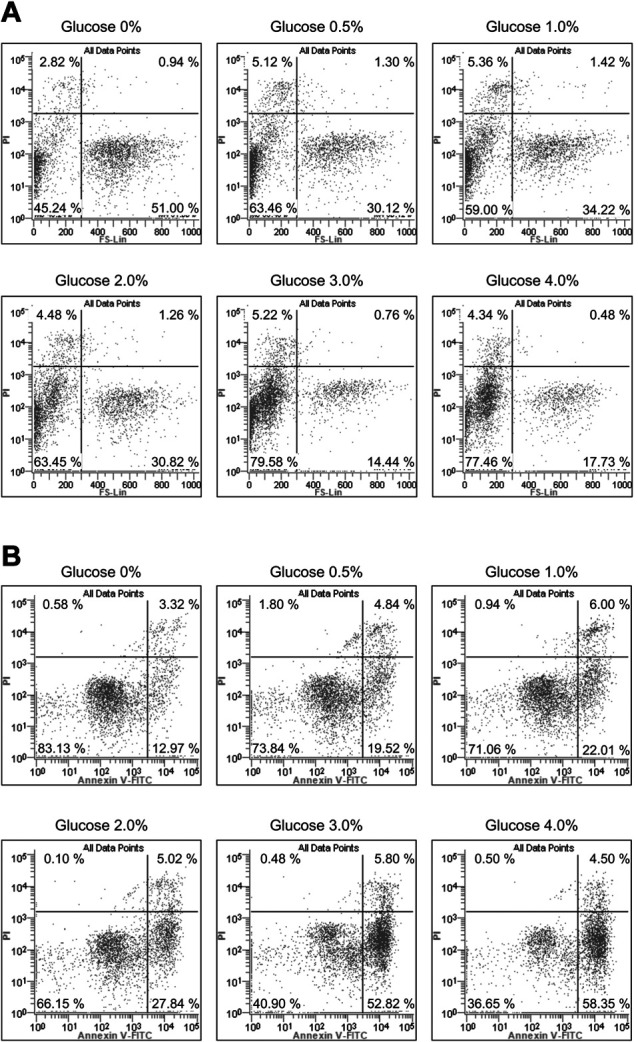
Analysis of glucose treated RPMCs using Flow cytometry. (A) The cell size was evaluated using forward scatter (FS) and propidium iodide (PI) staining analysis. (B) Detection of apoptosis with annexin V and PI staining.
To further characterize apoptotic cell death, we assessed the staining of Annexin V and PI with flowcytometry (Fig. 3B). The number of cells with intact plasma membrane, negative PI, and positive Annexin V, due to phosphatidylserine translocation to the outer layer of the plasma membrane, increased from 13.0% in control cells to 58.4% in cells treated with glucose of 4%.
Glucose stimulates the phosphorylation of PERK and eIF-2α
The PERK-eIF-2α cascade is a pathway controlled by its phosphorylation state and is associated with stress-induced apoptosis in the ER. To investigate whether the levels of active PERK were increased by glucose treatment, we examined the activated (i.e., phosphorylated) form of PERK by western blotting using an anti-phospho-PERK antibody. Treatment with glucose concentrations higher than 2% for 24 h increased phosphorylation of PERK (Fig. 4).
Fig. 4.
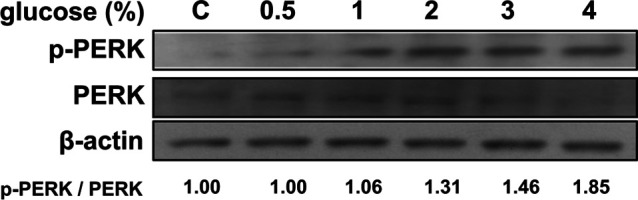
Protein kinase R-like ER kinase (PERK) expression and phosphorylation after glucose treatment. The expression of PERK and its phosphorylated form were analyzed in glucose-treated rat peritoneal mesothelial cells. Cells were treated with glucose for 24 hr at the indicated concentrations. The intensities of the immune positive bands from the blot were quantified and compared with those of the control using ImageJ software.
The phosphorylation level of eIF-2α was also analyzed by western blotting using an anti-phospho-eIF-2α antibody. After incubating the RPMCs for 24 hr with up to 4% glucose, the phosphorylation level of eIF-2α increased, while that of the non-phosphorylated form decreased (Fig. 5A). Thus, the ratio of phosphorylated to non-phosphorylated eIF-2α increased. In RPMCs subjected to osmotic loading by glycerol, eIF-2α phosphorylation was oppositely decreased (Fig. 5B).
Fig. 5.
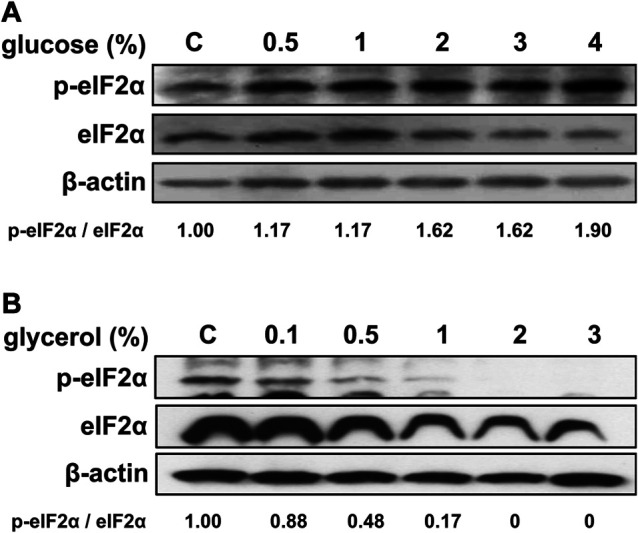
Eukaryotic translation initiation factor-2α (eIF-2α) expression and phosphorylation after treatment. The expression of eIF-2α and its phosphorylated form were analyzed in rat peritoneal mesothelial cells. Cells were incubated with various concentrations of glucose (A) or glycerol (B) for 24 hr. Intensities of the immune-positive bands of the blot were quantified and compared to the control using ImageJ software.
Glucose induces CHOP translocalization
To examine whether glucose treatment altered CHOP expression, we incubated RPMCs with various concentrations of glucose for 24 hr. CHOP protein expression was shown to increase in a concentration-dependent manner by western blotting (Fig. 6A). To examine whether treatment with glucose altered the cellular localization of CHOP, we incubated RPMCs with 4% glucose for 24 hr and detected this protein with immunofluorescence using an anti-CHOP antibody. In control cells, CHOP was observed mostly in the cytoplasm, with only a weak reaction in nuclei (Fig. 6B). In contrast, cells treated with 4% glucose showed intense fluorescence in nuclei (Fig. 6C).
Fig. 6.
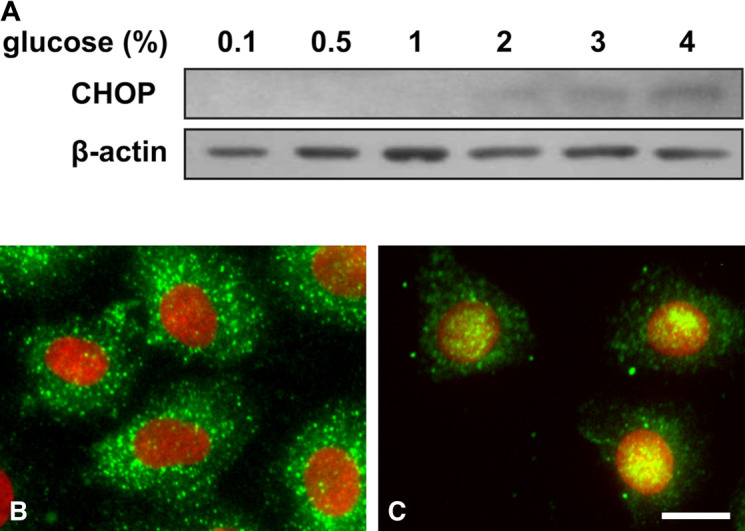
Homologous protein C/EBP (CHOP) expression after glucose treatment. (A) Rat peritoneal mesothelial cells (RPMCs) were incubated for 24 hr with various concentrations of glucose. CHOP expression levels were analyzed by western blotting. Subcellular localization of CHOP in RPMCs incubated for 24 hr without (B) or with (C) 4% glucose was analyzed by immunocytochemistry using antibodies against CHOP (Bar = 10 μm). CHOP (green) and 4',6-diamidino-2-phenylindole (DAPI) (blue) staining were merged. The blue channel was replaced with the red channel to improve visualization.
Effects of an ISR inhibitor on the cytotoxicity of glucose
Figure 7 shows that the treatment of cells with ISRIB ameliorated the glucose-induced cytotoxicity in a concentration-dependent manner. The viability of cells treated with glucose alone was 60% of the control cells, while further addition of 100 nM ISRIB increased the viability to approximately 94% of control cells.
Fig. 7.
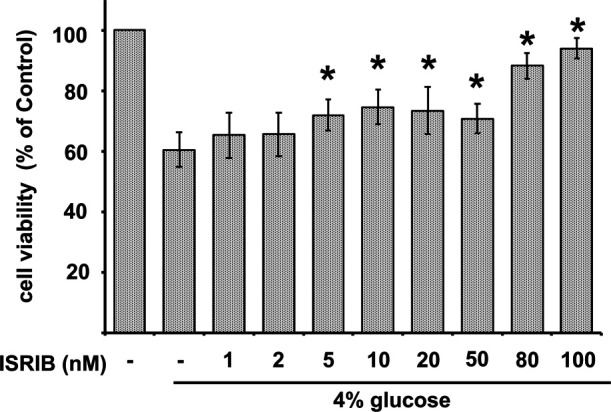
Effect of ISRIB on the cytotoxicity of glucose. Rat peritoneal mesothelial cells treated with 4% glucose were also added with various concentrations of ISRIB for 24 hr. Cell viability was determined using the WST-1 assay. Results are expressed as percentages of the control (means ± SD; n = 6). *Significantly different from the control; p < 0.05.
In vivo activation of eIF-2α
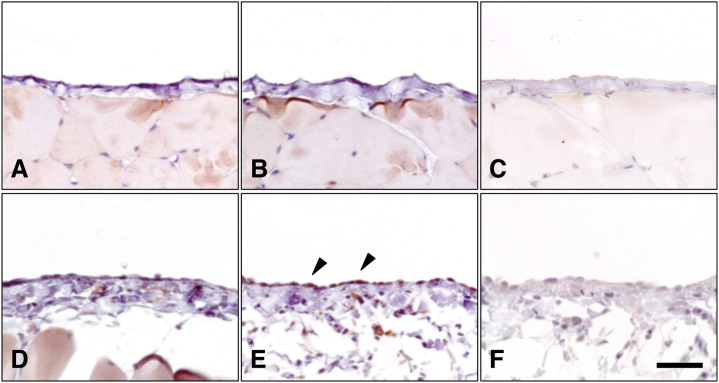
To determine the role of eIF-2α in the mesothelium in vivo, we performed an immunohistochemical analysis of activated eIF-2α in a PD model rats. As shown in Figure 8, eIF-2α was expressed in mesothelial cells and localized in the thin cytoplasm (Fig. 8A). To investigate whether the level of active eIF-2α could be increased by glucose contained PD fluid treatment, we examined the phosphorylated form of eIF-2α by immunohistochemistry. PD fluid induced mesothelial cell heightening and hyperplasia of sub-mesothelial connective tissue (Fig. 8 D-F). The glucose contained PD fluid treatment increased the expression of eIF-2α phosphorylated form (Fig. 8 E arrowheads) in mesothelial cells. Phosphorylation of eIF-2α in peritoneal mesothelial cells was weak in icodextrin contained PD fluid treatment (Supplemental Fig. 1). These in vivo results indicate that eIF-2α is expressed in mesothelial cells and is phosphorylated after treatment with glucose contained PD fluid.
Fig. 8.
Eukaryotic translation initiation factor-2α (eIF-2α) expression in rat peritoneum. Immunohistochemical analysis were performed on sections of the control (A–C) and glucose contained peritoneal dialysis fluid-treated (D–F) rat peritoneum. Sections were analyzed by immunohistochemistry using antibodies against eIF-2α (A, D) or phospho-eIF-2α (B, E). For negative control, the primary antibodies replaced with 1% BSA-PBA (C, F). Bars = 40 μm.
IV. Discussion
In the present study, we demonstrated that treating RPMCs with glucose induced apoptotic cell death mediated by the ER stress pathway. Three pathways are activated by ER stress, the PERK, ATF6, and inositol-requiring kinase 1 pathways. We focused on the PERK pathway in this study.
ER stress is associated with the development of various diseases such as diabetes mellitus, liver disease, neurodegenerative diseases, rheumatic disease, and atherosclerosis [19, 24, 25, 27, 36, 38]. ER stress is also associated with renal diseases involving glomerular, tubular, ischemic, and drug injuries [14, 21, 30, 41]. Complement component 5–9 complex assembled with glomerular epithelial cells stimulates ER stress and leads to glomerular epithelial cell injury in an experimental passive Heymann nephritis model of membranous nephropathy [6]. The signal is also activated in a rodent model of mesangial proliferative glomerulonephritis, anti-thy1 nephritis [13]. Although high glucose concentrations and glycoxidation end products induce apoptosis and inhibit growth in peritoneal cells [2, 12], the clinical significance of ER stress generated after the use of glucose-contained PD fluids remains unknown.
In this study, RPMCs were cultured in high glucose-containing medium, and the phosphorylation levels of PERK and eIF-2α were analyzed by western blotting. Expression of the activated form of PERK (phospho-PERK) and CHOP were increased by treatment with glucose at concentrations that induced apoptosis in RPMCs subsequently. We also used glycerol to reveal the effect of osmotic pressure. Glycerol 2.5% produces an osmotic pressure of approximately 540 mOsm/kg, higher than the osmotic pressure of 486 mOsm/kg produced by Dianeal D 4,25% [7]. The treatment of RPMCs with glycerol did not cause phosphorylation of eIF-2α. In vivo experiment, icodextrin contained PD fluid induced less eIF-2α phosphorylation comparing to glucose contained PD fluid-treated rats. The induction of ER stress by high concentrations of glucose was thought to be due to the chemical properties of glucose, not osmotic pressure. These data suggest that ER stress mediated by the PERK-eIF-2α pathway plays a key role in the process of glucose-induced apoptotic cell death in peritoneal mesothelium.
ISRIB is a small-molecule compound found to be a potent ISR inhibitor [33]. ISRIB blocks the ISR by stabilizing eIF2B subunits and inhibiting the binding of phospho-eIF-2α to eIF2B [35, 44]. With this mechanism, ISRIB inhibits the downstream reaction of activated eIF-2α. This substance improves long-term memory and has an inhibitory effect on the assembly of stress granules [31, 34]. ISRIB also improves several diseases such as Alzheimer’s disease, traumatic brain injury, and vanishing white matter disease [4, 34].
Excessive amounts of phospho-eIF-2α induce CHOP expression resulting in the induction of apoptosis. In the present study, ISRIB prevented cell death, even when eIF-2α phosphorylation was capable of inducing apoptosis. ISRIB alone did not induce cytotoxicity in RPMCs (unpublished data), but inhibited glucose-induced apoptosis through an eIF-2α phosphorylation-dependent mechanism.
Our study provides evidence that glucose induces apoptosis in PMCs through ER stress; specifically, by activating the PERK-eIF-2α pathway and inducing CHOP expression. Our data also show that ISRIB suppresses glucose-induced ER stress-mediated cell death. Although we did not examine an effect of ISRIB in vivo animal model, the suppression would explain the efficacy of ER stress inhibitors in reducing peritoneal cell damage during PD therapy. Modulation of ER stress ameliorates the epithelial-to-mesenchymal transition and cell death in the mesothelium [32]. In the present study, ISRIB suppressed glucose-induced cell death. The recovery suggesting that inhibition of glucose-induced ER stress might benefit patients receiving PD therapy through preservation of the peritoneum.
V. Conflicts of Interest
The authors have no financial conflicts of interest and other potential competing interest to declare.
VI. Acknowledgments
This work is dedicated to the late Dr. Yoshiaki Doi.
VII. Funding Statement
This study was supported by a grant from the Grant-in-Aid for the Promotion of Occupational Health for the Post Graduate Student of the University of Occupational and Environmental Health, Japan (JN) and from JSPS KAKENHI grant numbers 21K07927 (HM) and 17K00903 (RB).
Supplementary Material
Materials and Methods
VIII. References
- 1.Bartosova, M. and Schmitt, C. P. (2018) Biocompatible Peritoneal Dialysis: The Target Is Still Way Off. Front Physiol. 9; 1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boulanger, E., Wautier, M. P., Gane, P., Mariette, C., Devuyst, O. and Wautier, J. L. (2004) The triggering of human peritoneal mesothelial cell apoptosis and oncosis by glucose and glycoxydation products. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association—European Renal Association. 19; 2208–2216. [DOI] [PubMed] [Google Scholar]
- 3.Chesnokova, E., Bal, N. and Kolosov, P. (2017) Kinases of eIF2a Switch Translation of mRNA Subset during Neuronal Plasticity. Int. J. Mol. Sci. 18; 2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chou, A., Krukowski, K., Jopson, T., Zhu, P. J., Costa-Mattioli, M., Walter, P., et al. (2017) Inhibition of the integrated stress response reverses cognitive deficits after traumatic brain injury. Proc. Natl. Acad. Sci. U S A. 114; E6420–E6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coester, A. M., Smit, W., Struijk, D. G., Parikova, A. and Krediet, R. T. (2014) Longitudinal analysis of peritoneal fluid transport and its determinants in a cohort of incident peritoneal dialysis patients. Peritoneal dialysis international: journal of the International Society for Peritoneal Dialysis. 34; 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cybulsky, A. V. (2010) Endoplasmic reticulum stress in proteinuric kidney disease. Kidney International. 77; 187–193. [DOI] [PubMed] [Google Scholar]
- 7.Gastaldello, K., Husson, C., Dondeyne, J. P., Vanherweghem, J. L. and Tielemans, C. (2008) Cytotoxicity of mononuclear cells as induced by peritoneal dialysis fluids: insight into mechanisms that regulate osmotic stress-related apoptosis. Peritoneal dialysis international: journal of the International Society for Peritoneal Dialysis. 28; 655–666. [PubMed] [Google Scholar]
- 8.Ha, H. and Lee, H. B. (2000) Effect of high glucose on peritoneal mesothelial cell biology. Peritoneal dialysis international: journal of the International Society for Peritoneal Dialysis. 20 Suppl 2; S15–18. [PubMed] [Google Scholar]
- 9.Harding, H. P., Zhang, Y., Zeng, H., Novoa, I., Lu, P. D., Calfon, M., et al. (2003) An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Molecular Cell 11; 619–633. [DOI] [PubMed] [Google Scholar]
- 10.Heimburger, O., Waniewski, J., Werynski, A., Tranaeus, A. and Lindholm, B. (1990) Peritoneal transport in CAPD patients with permanent loss of ultrafiltration capacity. Kidney International 38; 495–506. [DOI] [PubMed] [Google Scholar]
- 11.Hjelle, J. T., Golinska, B. T., Waters, D. C., Steidley, K. R., McCarroll, D. R. and Dobbie, J. W. (1989) Isolation and propagation in vitro of peritoneal mesothelial cells. Peritoneal dialysis international: journal of the International Society for Peritoneal Dialysis. 9; 341–347. [PubMed] [Google Scholar]
- 12.Hung, K. Y., Liu, S. Y., Yang, T. C., Liao, T. L. and Kao, S. H. (2014) High-dialysate-glucose-induced oxidative stress and mitochondrial-mediated apoptosis in human peritoneal mesothelial cells. Oxid. Med. Cell Longev. 2014; 642793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inagi, R., Kumagai, T., Nishi, H., Kawakami, T., Miyata, T., Fujita, T., et al. (2008) Preconditioning with endoplasmic reticulum stress ameliorates mesangioproliferative glomerulonephritis. Journal of the American Society of Nephrology: JASN. 19; 915–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inagi, R. (2009) Endoplasmic reticulum stress in the kidney as a novel mediator of kidney injury. Nephron. Exp. Nephrol. 112; e1–9. [DOI] [PubMed] [Google Scholar]
- 15.Isaza-Restrepo, A., Martin-Saavedra, J. S., Velez-Leal, J. L., Vargas-Barato, F. and Riveros-Duenas, R. (2018) The Peritoneum: Beyond the Tissue—A Review. Front. Physiol. 9; 738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishimatsu, N., Miyamoto, T., Ueno, H., Hasegawa, E., Kuma, A., Fujimoto, Y., et al. (2016) High glucose concentration-induced expression of pentraxin-3 in a rat model of continuous peritoneal dialysis. Histol. Histopathol. 31; 1251–1258. [DOI] [PubMed] [Google Scholar]
- 17.Ito, T. and Yorioka, N. (2008) Peritoneal damage by peritoneal dialysis solutions. Clinical and Experimental Nephrology. 12; 243–249. [DOI] [PubMed] [Google Scholar]
- 18.Iurlaro, R. and Munoz-Pinedo, C. (2016) Cell death induced by endoplasmic reticulum stress. FEBS J. 283; 2640–2652. [DOI] [PubMed] [Google Scholar]
- 19.Kim, I., Xu, W. and Reed, J. C. (2008) Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nature Reviews. Drug discovery. 7; 1013–1030. [DOI] [PubMed] [Google Scholar]
- 20.Kimball, S. R. (1999) Eukaryotic initiation factor eIF2. Int. J. Biochem. Cell Biol. 31; 25–29. [DOI] [PubMed] [Google Scholar]
- 21.Kitamura, M. (2008) Endoplasmic reticulum stress in the kidney. Clinical and Experimental Nephrology. 12; 317–325. [DOI] [PubMed] [Google Scholar]
- 22.Krediet, R. T. (2018) Ultrafiltration Failure Is a Reflection of Peritoneal Alterations in Patients Treated With Peritoneal Dialysis. Front. Physiol. 9; 1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Logue, S. E., Cleary, P., Saveljeva, S. and Samali, A. (2013) New directions in ER stress-induced cell death. Apoptosis: an international journal on programmed cell death. 18; 537–546. [DOI] [PubMed] [Google Scholar]
- 24.Malhi, H. and Kaufman, R. J. (2011) Endoplasmic reticulum stress in liver disease. J. Hepatol. 54; 795–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marciniak, S. J. and Ron, D. (2006) Endoplasmic reticulum stress signaling in disease. Physiol. Rev. 86; 1133–1149. [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto, M., Tamura, M., Miyamoto, T., Furuno, Y., Kabashima, N., Serino, R., et al. (2012) Impacts of icodextrin on integrin-mediated wound healing of peritoneal mesothelial cells. Life Sciences 90; 917–923. [DOI] [PubMed] [Google Scholar]
- 27.Navid, F. and Colbert, R. A. (2017) Causes and consequences of endoplasmic reticulum stress in rheumatic disease. Nat. Rev. Rheumatol. 13; 25–40. [DOI] [PubMed] [Google Scholar]
- 28.Pakos-Zebrucka, K., Koryga, I., Mnich, K., Ljujic, M., Samali, A. and Gorman, A. M. (2016) The integrated stress response. EMBO Rep. 17; 1374–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perl, J., Nessim, S. J. and Bargman, J. M. (2011) The biocompatibility of neutral pH, low-GDP peritoneal dialysis solutions: benefit at bench, bedside, or both? Kidney International. 79; 814–824. [DOI] [PubMed] [Google Scholar]
- 30.Ricciardi, C. A. and Gnudi, L. (2019) Endoplasmic Reticulum stress in chronic kidney disease. New molecular targets from bench to the bedside. G. Ital. Nefrol. 36; 2019-vol 6. [PubMed] [Google Scholar]
- 31.Sharma, V., Sood, R., Khlaifia, A., Eslamizade, M. J., Hung, T. Y., Lou, D., et al. (2020) eIF2alpha controls memory consolidation via excitatory and somatostatin neurons. Nature 586; 412–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shin, H. S., Ryu, E. S., Oh, E. S. and Kang, D. H. (2015) Endoplasmic reticulum stress as a novel target to ameliorate epithelial-to-mesenchymal transition and apoptosis of human peritoneal mesothelial cells. Lab. Invest. 95; 1157–1173. [DOI] [PubMed] [Google Scholar]
- 33.Sidrauski, C., Acosta-Alvear, D., Khoutorsky, A., Vedantham, P., Hearn, B. R., Li, H., et al. (2013) Pharmacological brake-release of mRNA translation enhances cognitive memory. Elife. 2; e00498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sidrauski, C., McGeachy, A. M., Ingolia, N. T. and Walter, P. (2015) The small molecule ISRIB reverses the effects of eIF2alpha phosphorylation on translation and stress granule assembly. Elife. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sidrauski, C., Tsai, J. C., Kampmann, M., Hearn, B. R., Vedantham, P., Jaishankar, P., et al. (2015) Pharmacological dimerization and activation of the exchange factor eIF2B antagonizes the integrated stress response. Elife. 4; e07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sokka, A. L., Putkonen, N., Mudo, G., Pryazhnikov, E., Reijonen, S., Khiroug, L., et al. (2007) Endoplasmic reticulum stress inhibition protects against excitotoxic neuronal injury in the rat brain. The Journal of Neuroscience: the official journal of the Society for Neuroscience. 27; 901–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tamura, M., Osajima, A., Nakayamada, S., Anai, H., Kabashima, N., Kanegae, K., et al. (2003) High glucose levels inhibit focal adhesion kinase-mediated wound healing of rat peritoneal mesothelial cells. Kidney International. 63; 722–731. [DOI] [PubMed] [Google Scholar]
- 38.Wang, S. and Kaufman, R. J. (2012) The impact of the unfolded protein response on human disease. J. Cell Biol. 197; 857–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watanabe, Y., Tamura, M., Osajima, A., Anai, H., Kabashima, N., Serino, R., et al. (2003) Integrins induce expression of monocyte chemoattractant protein-1 via focal adhesion kinase in mesangial cells. Kidney International. 64; 431–440. [DOI] [PubMed] [Google Scholar]
- 40.Williams, J. D., Craig, K. J., Topley, N., Von Ruhland, C., Fallon, M., Newman, G. R., et al. (2002) Morphologic changes in the peritoneal membrane of patients with renal disease. Journal of the American Society of Nephrology: JASN. 13; 470–479. [DOI] [PubMed] [Google Scholar]
- 41.Yan, M., Shu, S., Guo, C., Tang, C. and Dong, Z. (2018) Endoplasmic reticulum stress in ischemic and nephrotoxic acute kidney injury. Ann. Med. 50; 381–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zareie, M., Hekking, L. H., Welten, A. G., Driesprong, B. A., Schadee-Eestermans, I. L., Faict, D., et al. (2003) Contribution of lactate buffer, glucose and glucose degradation products to peritoneal injury in vivo. Nephrology, Dialysis, Transplantation: official publication of the European Dialysis and Transplant Association—European Renal Association. 18; 2629–2637. [DOI] [PubMed] [Google Scholar]
- 43.Zhou, Q., Bajo, M. A., Del Peso, G., Yu, X. and Selgas, R. (2016) Preventing peritoneal membrane fibrosis in peritoneal dialysis patients. Kidney International. 90; 515–524. [DOI] [PubMed] [Google Scholar]
- 44.Zyryanova, A. F., Weis, F., Faille, A., Alard, A. A., Crespillo-Casado, A., Sekine, Y., et al. (2018) Binding of ISRIB reveals a regulatory site in the nucleotide exchange factor eIF2B. Science. 359; 1533–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Materials and Methods